Abstract
Tazemetostat is a selective, reversible, small‐molecule inhibitor of the histone methyltransferase enzyme, enhancer of zest homolog 2 (EZH2). In this multicenter, open‐label, phase II study, we assessed the efficacy and safety of tazemetostat in Japanese patients with relapsed or refractory (R/R) B‐cell non‐Hodgkin lymphoma harboring the EZH2 mutation. Tazemetostat (800 mg twice daily) was given orally (28‐day cycle) until disease progression or unacceptable toxicity. Among the 20 eligible patients, 17 were enrolled in cohort 1 (follicular lymphoma [FL]), and three were enrolled in cohort 2 (diffuse large B‐cell lymphoma). At data cut‐off, the objective response rate in cohort 1 was 76.5%, including six patients (35.3%) with complete response and seven patients (41.2%) with partial response (PR). All three patients in cohort 2 achieved PR. In cohort 1, median progression‐free survival (PFS) was not reached at the median follow‐up of 12.9 months. The estimated PFS rate at 12 and 15 months was 94.1% and 73.2%, respectively. The most common grade 3 treatment‐emergent adverse event (TEAE) was lymphopenia (n = 2). Grade 4 TEAEs included hypertriglyceridemia and pneumonia aspiration (n = 1 each), which were not related to tazemetostat. Treatment‐emergent adverse events leading to study drug discontinuation were reported in four of the 20 patients, indicating that the safety profile of tazemetostat was acceptable and manageable. Tazemetostat 800 mg twice daily showed encouraging efficacy in patients with R/R EZH2 mutation‐positive FL with a manageable safety profile in the overall population. Thus, tazemetostat could be a potential treatment for R/R EZH2 mutation‐positive FL.
Keywords: efficacy, enhancer of zeste homolog 2, follicular lymphoma, non‐Hodgkin lymphoma, safety, tazemetostat, trimethylation of lysine 27 in histone 3
Percentage change from baseline in sum of the product of the perpendicular diameters of target lesions based on independent reviewer assessment.
Abbreviations
- AE
adverse event
- BOR
best overall response
- CI
confidence interval
- CR
complete response
- DLBCL
diffuse large B‐cell lymphoma
- DOR
duration of response
- ECOG PS
Eastern Cooperative Oncology Group performance status
- EZH2
enhancer of zest homolog 2
- FDA
Food and Drug Administration
- FL
follicular lymphoma
- IRA
independent reviewer assessment
- ORR
objective response rate
- PD
progressive disease
- PR
partial response
- R/R
relapsed or refractory
- SAE
serious adverse event
- SPD
sum of the products of the perpendicular diameters
- TEAE
treatment‐emergent adverse event
- TRAE
treatment‐related adverse event
- TTR
time to response
1. INTRODUCTION
Follicular lymphoma is the most common subtype of indolent B‐cell lymphoma.1, 2 Patients with advanced‐stage FL and a high tumor burden are generally treated with immunochemotherapy, and most of them show response to treatment. However, relapse is common in patients with FL. For patients with R/R FL, several treatment options are available, including rituximab monotherapy, chemoimmunotherapy, radioimmunotherapy, lenalidomide plus rituximab, and stem cell transplantation. Moreover, several novel agents, including PI3K inhibitors, are being developed for R/R FL. However, the response duration and survival progressively shorten with the subsequent line of therapy.3 Thus, there is a need for a new therapeutic option for patients with FL.
Tazemetostat (E7438) is a selective, reversible, small molecule inhibitor of EZH2, a histone methyltransferase enzyme. A catalytic subunit of polycomb repressive complex 2, EZH2 is responsible for the methylation of histone H3K27. It plays a role in the epigenetic regulation of various genes. Studies have shown that tazemetostat specifically decreases histone H3K27 methylation in different cell lines. Incubation with tazemetostat significantly inhibited the proliferation of cancer cells such as DLBCL cell lines bearing EZH2 mutations, INI1 (SMARCB1)‐negative malignant rhabdoid tumor cell lines, and INI1‐deficient synovial sarcoma cell lines.4, 5 Tazemetostat, given orally, showed significant antitumor effects in human xenograft model mice, including DLBCL cell lines with EZH2 mutations, ranging from tumor growth inhibition to complete and durable tumor regression.5 In addition, tazemetostat exposure led to a dose‐ and time‐dependent decrease in intracellular trimethylate at H3K27 in both tumor and selected nontumor tissues. Therefore, on the basis of the above results, a global phase I/II study of tazemetostat in patients with advanced solid tumors, including INI1‐ or SMARCA4‐negative tumors or with B‐NHLs (including DLBCL) and FL, was undertaken outside Japan.6 The results from this phase I study indicated a favorable safety profile and provided evidence of the antitumor activity of tazemetostat in patients with refractory B‐NHLs and advanced solid tumors including epithelioid sarcomas. The recommended dose was 800 mg BID.
Tazemetostat received accelerated approval by the US FDA in January 2020 for the treatment of adults and adolescents aged 16 years and over with locally advanced or metastatic epithelioid sarcoma not eligible for complete resection, based on the ORR and DOR observed in a phase II study.7 In the phase II study, the ORR of tazemetostat was 69% (95% CI, 53‐82; 31 of 45 patients) in the EZH2 mutant FL cohort and 35% (95% CI, 23‐49; 19 of 54 patients) in the EZH2 WT FL cohort.8 Based on the results of this study, tazemetostat received an accelerated approval from the FDA in June 2020 for the treatment of adult patients with R/R EZH2‐positive FL (as detected by an FDA‐approved test), patients who have received at least two prior systemic therapies, and those with R/R FL who have no satisfactory alternative treatment options. A phase I study of tazemetostat in Japanese patients with B‐NHLs, including FL and DLBCL, was carried out. The study reported that tazemetostat 800 mg BID showed a manageable safety profile, and 800 mg BID was determined as a recommended phase II dose.9 The present study was undertaken to evaluate the efficacy and safety of tazemetostat in Japanese patients with B‐cell NHL including FL and DLBCL harboring the EZH2 mutation.
2. MATERIALS AND METHODS
2.1. Study design
This multicenter, open‐label, phase II study was undertaken to assess the efficacy and safety of tazemetostat in Japanese patients with FL with the EZH2 mutation (cohort 1) and DLBCL with the EZH2 mutation (cohort 2) (ClinicalTrials.gov: NCT03456726). This study was carried out in accordance with the principles of the World Medical Association Declaration of Helsinki, the Good Clinical Practice guidelines, and local regulations. The study protocol was approved by the institutional review board of each institution. Written informed consent was obtained from the patients for participation in screening using the tumor EZH2 mutation test and enrollment to the clinical trial.
2.2. Patients
Adult patients (20 years of age and older) with a histological diagnosis of FL or DLBCL (including primary mediastinal B‐cell lymphoma and transformed FL), for which no standard therapy existed, were eligible for screening. Patients were eligible for the study treatment only if the EZH2 mutation of tumor assessed with the Cobas EZH2 Mutation Test (Roche Molecular Systems) was confirmed by the central laboratory. Other major inclusion criteria were previous therapy with systemic chemotherapy or Ab therapy, presence of a measurable lesion by computed tomography scan, an ECOG PS score of 0 or 1, and adequate renal, liver, and bone marrow functions. The major exclusion criteria included a history of allogeneic stem cell transplantation, prior exposure to EZH2 inhibitor, venous thrombosis or pulmonary embolism within the last 3 months, and continued use of potent inhibitors or inducers of cytochrome P450 3A.
2.3. Treatments
Tazemetostat 800 mg BID (1600 mg total daily dose) was given orally in 28‐day continuous cycles. The treatment was continued until disease progression, development of unacceptable toxicity, or patient request to discontinue. The dose of tazemetostat was determined based on the safety and efficacy data from a previous study.6, 8 Treatment with tazemetostat was interrupted when patients experienced intolerable grade 2 or higher toxicity or neutropenia with an absolute neutrophil count of less than 0.75 × 109/L, except for grade 3 thrombocytopenia, anemia, and grade 3 or 4 leukopenia. After recovery, the study drug was resumed at a reduced dose based on the previous doses in the order of 600 and 400 mg BID (1200 and 800 mg total daily dose, respectively).
2.4. Outcomes and assessments
Efficacy end‐points were assessed by an independent radiological review committee according to the revised response criteria for malignant lymphoma (IWG‐2007).10 The primary end‐point was the ORR, defined as the rate of CR or PR assessed as the best response of the individual patient. The secondary end‐points were PFS, defined as the time from the first dose of study treatment to confirmed PD or death from any cause, DOR, defined as the time from initial confirmation of response to the first documented evidence of PD, TTR, defined as the time from the first dose to the time of first response. and safety. The exploratory end‐points included the plasma concentration of tazemetostat and the frequency of EZH2 mutations in B‐cell NHL.
Response was assessed every 8 weeks up to 32 weeks, every 12 weeks thereafter, and at discontinuation. Furthermore, [18F] fluorodeoxyglucose PET scans were used to confirm CR as the BOR. Follow‐up was carried out for 30 days after the final treatment with tazemetostat or until the initiation of a new therapy. Blood samples for determining tazemetostat concentration were collected at predose of the first treatment with the study drug for 2 days at the steady state.
The severity of AEs was classified according to the Common Terminology Criteria for AEs (version 4.03).
2.5. Statistical analysis
Efficacy and safety were evaluated in all patients who received at least one dose of the study drug. For ORR, two‐sided 95% exact CI was calculated using the Clopper‐Pearson method. Time‐to‐event end‐points were estimated using the Kaplan‐Meier method. The TTR was summarized using descriptive statistics of the responders.
In cohort 1, eight efficacy‐evaluable FL patients with the EZH2 mutation were required to detect the lower limit of the 90% CI that exceeded the 10% threshold in ORR (primary end‐point of the study), with an expected ORR of 50% and a power of approximately 80%. In cohort 2, 13 efficacy‐evaluable DLBCL patients with the EZH2 mutation were required to detect the lower limit of the 90% CI that exceeded the 10% threshold in ORR, with an expected ORR of 40% and a power of approximately 80%. Statistical analyses were undertaken using SAS for Windows (version 9.2 or later).
3. RESULTS
3.1. Patients and treatment
This study was initiated in April 2018 at 28 sites in Japan. The data cut‐off point was December 2019, by which all patients were followed for at least 12 months or discontinued treatment. Based on the sponsor’s decision not to pursue the development of this agent for DLBCL, new enrollment for the DLBCL cohort was stopped from August 2018, and only three patients were enrolled in this cohort.
One hundred patients were enrolled for screening by the tumor EZH2 mutation test. Of these, 20 were positive for the EZH2 mutation (Figure 1). Among the 20 eligible patients, 17 were enrolled in the FL cohort and three in the DLBCL cohort. The baseline characteristics of the patients are shown in Table 1. The median age of the 20 patients was 69.5 (range, 46‐83) years. All patients had received previous chemotherapy and two (10.0%) patients had previous radiotherapy; no patients had autologous stem‐cell transplantation or other medications. In cohort 2, two patients were diagnosed with transformed FL and one patient was diagnosed with DLBCL. The median number of prior systemic chemotherapy regimens was two (range, one to five). The most common types of prior systemic therapy among the 20 patients included cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP; 80%), rituximab (80%), and bendamustine (40%) used alone or in combination.
FIGURE 1.
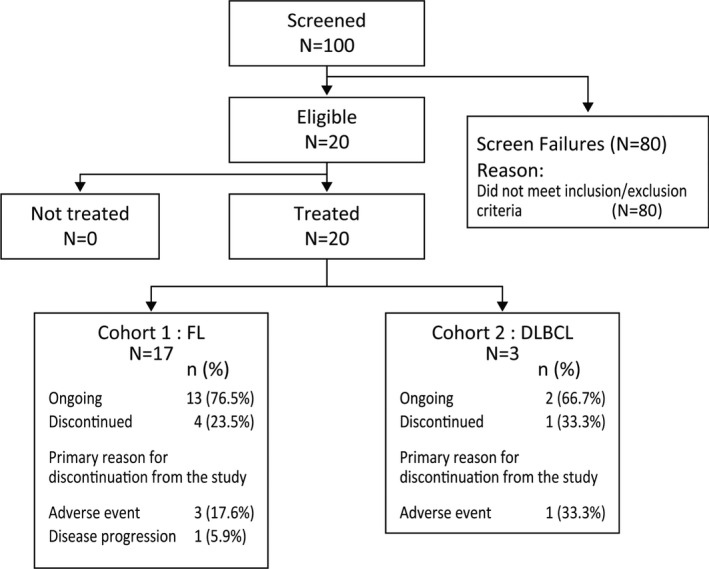
Patient disposition among participants in this phase II study of tazemetostat for relapsed or refractory B‐cell non‐Hodgkin lymphoma with EZH2 mutation. DLBCL, diffuse large B‐cell lymphoma; FL, follicular lymphoma
TABLE 1.
Demographics and baseline characteristics of Japanese patients with relapsed or refractory B‐cell non‐Hodgkin lymphoma with EZH2 mutation treated with tazemetostat
| Category | FL | DLBCL | Total |
|---|---|---|---|
| (N = 17) | (N = 3) | (N = 20) | |
| n (%) | n (%) | n (%) | |
| Age, y; median (range) | 66 (46, 81) | 71 (70, 83) | 69.5 (46, 83) |
| Age group | |||
| <65 y | 8 (47.1) | 0 | 8 (40.0) |
| ≥65 y | 9 (52.9) | 3 (100.0) | 12 (60.0) |
| Sex | |||
| Male | 8 (47.1) | 1 (33.3) | 9 (45.0) |
| Female | 9 (52.9) | 2 (66.7) | 11 (55.0) |
| ECOG PS | |||
| 0 | 16 (94.1) | 3 (100.0) | 19 (95.0) |
| 1 | 1 (5.9) | 0 | 1 (5.0) |
| No. of prior systemic chemotherapy regimens | |||
| Mean (SD) | 2.5 (1.01) | 3.3 (2.08) | 2.7 (1.18) |
| Median (range) | 2 (1, 4) | 4 (1, 5) | 2 (1, 5) |
| No. of prior systemic chemotherapy regimens | |||
| 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 1 | 2 (11.8) | 1 (33.3) | 3 (15.0) |
| 2 | 8 (47.1) | 0 (0.0) | 8 (40.0) |
| 3 | 3 (17.6) | 0 (0.0) | 3 (15.0) |
| 4 | 4 (23.5) | 1 (33.3) | 5 (25.0) |
| ≥5 | 0 (0.0) | 1 (33.3) | 1 (5.0) |
Abbreviations: DLBCL, diffuse large B‐cell lymphoma; FL, follicular lymphoma; PS, performance status.
As of the data cut‐off date, 15 (75.0%) of the 20 patients continued treatment in this study, including 13 (76.5%) of the 17 patients with FL and two (66.7%) of the three patients with DLBCL. The primary reasons for treatment discontinuation were AEs (four patients) and disease progression (one patient).
The median duration of exposure was 14.5 (range, 2.8‐18.6) months. The median dose intensity per patient was 1567.2 (range, 975.0‐1600.0) mg/d. Of the 20 treated patients, two (10.0%) received 100% of their planned starting dose and 16 (80.0%) received at least 90% of the planned dose. Tazemetostat was interrupted in nine (45.0%) patients, whereas four (20.0%) patients experienced dose reduction, with a median time to the first dose reduction of 2.2 months.
3.2. Efficacy
Among the 17 patients in the FL cohort, the ORR based on the IRA was 76.5% (90% CI, 53.9‐91.5), with six (35.3%) patients achieving CR and seven (41.2%) achieving PR at the data cut‐off (Table 2), thereby meeting the primary end‐point of the study. The BOR was SD in three (17.6%) patients and PD in one (5.9%) patient in the FL cohort. As shown in Figure 2, except one patient with FL, all showed at least some reduction in tumor volume as defined by the sum of the products of the perpendicular diameters.
TABLE 2.
Summary of tumor response to tazemetostat in Japanese patients with relapsed or refractory B‐cell non‐Hodgkin lymphoma with EZH2 mutation, based on independent reviewer assessment
| Response category | FL | DLBCL |
|---|---|---|
| (N = 17) | (N = 3) | |
| n (%) | n (%) | |
| BOR | ||
| CR | 6 (35.3) | 0 (0.0) |
| PR | 7 (41.2) | 3 (100.0) |
| SD | 3 (17.6) | 0 (0.0) |
| PD | 1 (5.9) | 0 (0.0) |
| NE | 0 (0.0) | 0 (0.0) |
| Objective response rate (CR+PR), n (%) | 13 (76.5) | 3 (100.0) |
Abbreviations: BOR, best overall response; CR, complete response; DLBCL, diffuse large B‐cell lymphoma; FL, follicular lymphoma; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
FIGURE 2.
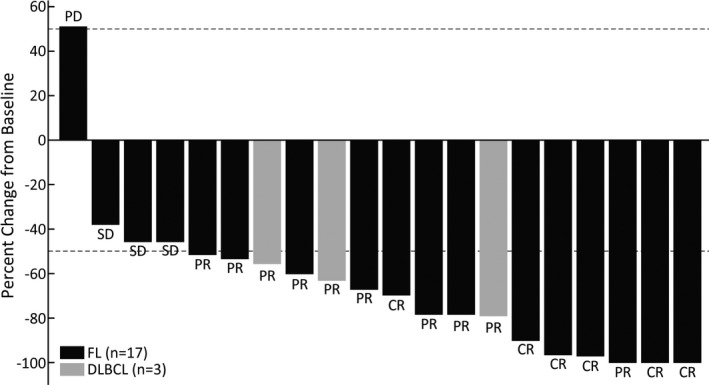
Percentage change from baseline in the sum of the product of the perpendicular diameters (SPD) of target lesions, based on independent reviewer assessment. Study participants were treated with tazemetostat for relapsed or refractory B‐cell non‐Hodgkin lymphoma with EZH2 mutation. This plot represents each subject percentage change from baseline in SPD of target lesions at post‐baseline nadir. CR, complete response; DLBCL, diffuse large B‐cell lymphoma; FL, follicular lymphoma; PR, partial response; SD, stable disease
In the DLBCL cohort, the ORR (based on the IRA) was 100% with three (100%) patients achieving PR at the data cut‐off.
3.3. Survival
In the FL cohort, three (17.6%) of the 17 patients had PFS events, as determined using the IRA. With a median follow‐up duration of 12.9 months for surviving patients, the median PFS was not reached, and the Kaplan‐Meier‐estimated PFS rate at 12 and 15 months was 94.1% and 73.2%, respectively (Figure 3). The median DOR was also not reached, and the Kaplan‐Meier‐estimated DOR rate at 9 and 12 months was 100.0% and 80.0%, respectively, in the FL cohort (Figure 4). The median TTR was 3.64 (range, 1.8‐10.1) and 3.7 (range, 1.9‐7.4) months in the FL and DLBCL cohorts, respectively.
FIGURE 3.
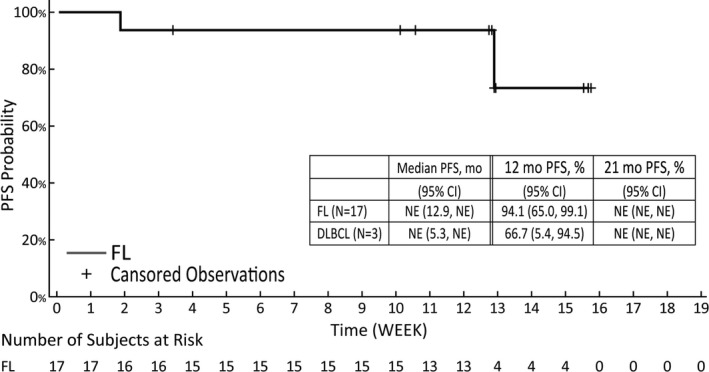
Progression‐free survival (PFS) among Japanese patients with relapsed or refractory B‐cell non‐Hodgkin lymphoma with EZH2 mutation treated with tazemetostat. The tumor assessment is based on the revised response criteria for malignant lymphoma (IWG‐2007) criteria. Confidence intervals (CIs) were calculated using the Kaplan‐Meier estimate and Greenwood’s formula. DLBCL, diffuse large B‐cell lymphoma; FL, follicular lymphoma; NE, not evaluable
FIGURE 4.
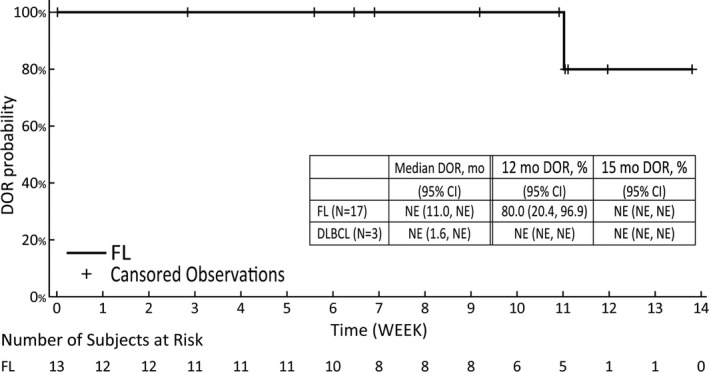
Kaplan‐Meier‐estimated duration of response (DOR) among Japanese patients with relapsed or refractory B‐cell non‐Hodgkin lymphoma with EZH2 mutation treated with tazemetostat. The tumor assessment is based on the revised response criteria for malignant lymphoma (IWG‐2007) criteria. Confidence intervals (CIs) were calculated using the Kaplan‐Meier estimate and Greenwood’s formula. DLBCL, diffuse large B‐cell lymphoma; FL, follicular lymphoma; NE, not evaluable
The duration of treatment and response are shown in Figure S1. The treatment duration for all six patients who achieved CR and four of the seven patients with FL who achieved PR as the BOR was more than 12 months. Six of the 10 patients who achieved PR in the FL cohort experienced an improvement of response to CR.
3.4. Safety
A summary of AEs is provided in Table 3. All the 20 patients experienced at least one TEAE. The most commonly reported TEAE was dysgeusia (n = 10, 50.0%), followed by nasopharyngitis (n = 7, 35.0%), lymphopenia, increased blood creatine phosphokinase (n = 5 each, 25.0%), constipation, and upper respiratory tract infection (n = 4 each, 20.0%).
TABLE 3.
Treatment‐emergent adverse events (TEAEs) occurring in two or more patients and treatment‐related adverse events (TRAEs) in Japanese patients with relapsed or refractory B‐cell non‐Hodgkin lymphoma with EZH2 mutation treated with tazemetostat
| MedDRA system organ class | TEAE | TR‐TEAE | ||
|---|---|---|---|---|
| Preferred term | (N = 20) | (N = 20) | ||
| n (%) | n (%) | |||
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Subjects with any TEAE | 20 (100.0) | 8 (40.0) | 20 (100.0) | 6 (30.0) |
| Dysgeusia | 10 (50.0) | 0 (0.0) | 10 (50.0) | 0 (0.0) |
| Nasopharyngitis | 7 (35.0) | 0 (0.0) | 2 (10.0) | 0 (0.0) |
| Lymphopenia | 5 (25.0) | 2 (10.0) | 5 (25.0) | 2 (10.0) |
| Blood creatine phosphokinase increased | 5 (25.0) | 0 (0.0) | 1 (5.0) | 0 (0.0) |
| Constipation | 4 (20.0) | 0 (0.0) | 2 (10.0) | 0 (0.0) |
| Upper respiratory tract infection | 4 (20.0) | 0 (0.0) | 1 (5.0) | 0 (0.0) |
| Neutropenia | 3 (15.0) | 1 (5.0) | 3 (15.0) | 1 (5.0) |
| Thrombocytopenia | 3 (15.0) | 0 (0.0) | 3 (15.0) | 0 (0.0) |
| Nausea | 3 (15.0) | 0 (0.0) | 2 (10.0) | 0 (0.0) |
| Stomatitis | 3 (15.0) | 0 (0.0) | 3 (15.0) | 0 (0.0) |
| Weight decreased | 3 (15.0) | 0 (0.0) | 2 (10.0) | 0 (0.0) |
| Alopecia | 3 (15.0) | 0 (0.0) | 3 (15.0) | 0 (0.0) |
| Rash | 3 (15.0) | 0 (0.0) | 1 (5.0) | 0 (0.0) |
| Anemia | 2 (10.0) | 0 (0.0) | 2 (10.0) | 0 (0.0) |
| Fatigue | 2 (10.0) | 0 (0.0) | 1 (5.0) | 0 (0.0) |
| Malaise | 2 (10.0) | 0 (0.0) | 2 (10.0) | 0 (0.0) |
| Herpes simplex infection | 2 (10.0) | 0 (0.0) | 1 (5.0) | 0 (0.0) |
| Pneumonia | 2 (10.0) | 1 (5.0) | 1 (5.0) | 1 (5.0) |
| Alanine aminotransferase increased | 2 (10.0) | 0 (0.0) | 2 (10.0) | 0 (0.0) |
| Amylase increased | 2 (10.0) | 0 (0.0) | 2 (10.0) | 0 (0.0) |
| Aspartate aminotransferase increased | 2 (10.0) | 0 (0.0) | 2 (10.0) | 0 (0.0) |
| Blood creatinine increased | 2 (10.0) | 0 (0.0) | 2 (10.0) | 0 (0.0) |
| Electrocardiogram QT prolonged | 2 (10.0) | 0 (0.0) | 2 (10.0) | 0 (0.0) |
| Hypophosphatemia | 2 (10.0) | 0 (0.0) | 2 (10.0) | 0 (0.0) |
| Eczema | 2 (10.0) | 0 (0.0) | 2 (10.0) | 0 (0.0) |
Grade 3 or higher TEAEs occurred in eight (40.0%) patients. The most commonly reported grade 3 TEAE was lymphopenia (n = 2, 10.0%). Grade 4 TEAEs were hypertriglyceridemia and pneumonia aspiration (n = 1 each, 5.0%). The incidence of grade 3 or higher AEs and TEAEs in the FL cohort (47.1%) were similar to that in the total patient population.
All patients experienced at least one TRAE, as determined by the investigator. The most commonly reported TRAEs were dysgeusia (n = 10, 50.0%) and lymphopenia (n = 5, 25.0%). Treatment‐related AEs of grade 4 or 5 were not reported.
Serious adverse events occurred in six (30.0%) patients and included mechanical ileus, atypical pneumonia, Pneumocystis jirovecii pneumonia, pneumonia, traumatic intracranial hemorrhage, non‐small‐cell lung cancer, pneumonia aspiration, and upper respiratory tract inflammation (n = 1 each). Three patients (17.6%) experienced SAEs related to the study drug, including atypical pneumonia, P. jirovecii pneumonia, pneumonia, and upper respiratory tract inflammation. Four (20.0%) patients discontinued the study drug due to the following TEAEs: atypical pneumonia, traumatic intracranial hemorrhage and muscle spasticity (two events in the same patient), non‐small‐cell lung cancer, or dysgeusia. Of these TEAEs, TRAEs were muscle spasticity, atypical pneumonia, and dysgeusia. Treatment‐emergent AEs led to dose reduction of the study drug in four (20.0%) patients and interruption of the study drug in nine (45.0%) patients. The most frequently reported TEAEs leading to study drug interruption were dysgeusia and influenza. In patients with DLBCL, TEAEs leading to study drug dose reduction included supraventricular tachycardia and prolonged electrocardiogram QT in one patient.
3.5. Pharmacokinetics
A summary of the trough concentration of tazemetostat at the steady state is shown in Table S1. The mean plasma tazemetostat concentration of cycle 2 day 1 was 106 ng/mL, which was slightly higher than 76.7 ng/mL of cycle 1 day 15 in patients with FL and DLBCL; however, there was no significant difference between these values.
4. DISCUSSION
This was a multicenter, open‐label, phase II study of tazemetostat in Japanese patients with R/R B‐cell NHL with EZH2 mutation. It consisted of two cohorts of patients with FL and DLBCL. Only three patients were enrolled in the DLBCL cohort due to termination of development of tazemetostat monotherapy, and the efficacy results of this study focused on the FL cohort consisting of 17 patients. The safety of the drug in all the treated patients was evaluated. Among the 17 patients in the FL cohort, the ORR based on the IRA was 76.5% with six patients (35.3%) achieving CR at the data cut‐off. These results were consistent with those of the global phase II study, in which the ORR based on IRA in 45 patients with EZH2‐mutant FL treated with at least two prior therapies was 68.9%, with six patients (13.3%) achieving CR.8 Our safety data were also consistent with those of the global phase II study.8
The median PFS and median DOR were not reached at the data cut‐off date. The median TTR was 3.64 months, as determined using the IRA. Although the median DOR was not reached due to immaturity of the data, the Kaplan‐Meier‐estimated DOR rates (assessed by independent reviewers) at 9 and 12 months (100.0% and 80.0%, respectively) were similar to or higher than the DOR rates at 6 and 12 months (77.1% and 38.7%, respectively) in the global phase II study (follicular lymphoma mutant cohort). With respect to the demographics and baseline characteristics of the patients, there was no significant difference between this study and the global phase II study, except for the higher number of patients with ECOG PS of 1 or higher (Table 2). Although the two studies cannot be compared directly, both studies showed potential clinical benefits of tazemetostat in patients with R/R FL with EZH2 mutation.
In this study, all patients were treated with 800 mg BID. The most commonly reported TEAEs were dysgeusia, nasopharyngitis, lymphopenia, increased blood creatine phosphokinase, constipation, and upper respiratory tract infection. The major TEAEs were hematological events or infections. The specific TEAE profile of tazemetostat reported in patients with FL or DLBCL (eg, nasopharyngitis, constipation, and upper respiratory tract infection) was consistent with that observed in other phase I/II studies on tazemetostat.6, 7 Dysgeusia was the most common TEAE in this study, as in the phase I study on tazemetostat in Japan.9 Dysgeusia was also reported by previous studies on tazemetostat with an incidence of more than 10%.9 Therefore, no new or unexpected safety signals were observed in this study. Additionally, the incidence of grade 3 or higher neutropenia was considerably lower (n = 1, 5%) in this study than in patients treated with chemoimmunotherapy or the recently approved combination of lenalidomide and rituximab, although we need to be cautious in cross‐trial comparison.11, 12, 13, 14 These findings indicated that the safety profile of tazemetostat (800 mg BID) was manageable and that the drug was generally well tolerated in patients with R/R B‐NHL.
Our study had some potential limitations. First, the number of patents was limited. Second, we did not compare the efficacy and toxicity of tazemetostat with those of other treatment options, because there were no comparators in this study. Finally, the follow‐up period was short, and therefore, a longer follow‐up is needed to obtain long‐term efficacy and safety data.
In conclusion, the results of this phase II study suggested that tazemetostat shows a clinically meaningful efficacy in patients with R/R FL with EZH2 mutation, and the safety profile of tazemetostat was favorable in patients with FL and DLBCL.
DISCLOSURE
KI has received grants, honoraria, and research funding from Eisai. MN has received research grants from Eisai., and received honoraria from SymBio Pharmaceuticals, Janssen, Celgene, Sanofi, Sumitomo Dainippon, Bristol‐Myer Squib, Bayer, Nippon Shinyaku, Kyowa Kirin, Chugai, and Eisai. HS has received honoraria from Eisai, Takeda, Ono, Novartis, Celgene (BMS), Janssen, and Chugai and received research funding from Eisai, Janssen, Ono, Celgene, Novartis, Sanofi, AstraZeneca, AbbVie, and Chugai. JK has received grants from AbbVie, Asahi Kasei, Bristol‐Myers Squibb, Celgene, Chugai Pharmaceutical, Daiichi Sankyo, Sumitomo Dainippon Pharma, Eisai, Fujimoto Pharmaceutical, Kyowa Kirin, MSD, Nippon Shinyaku, Ono Pharmaceutical, Otsuka Pharmaceutical, Sanofi, Shionogi, Sysmex, Taiho Pharmaceutical, and Takeda. He has received research support from Bristol‐Myers Squibb, Celgene, Ono Pharmaceutical, and Sysmex, served as a consultant to AbbVie, Bristol‐Myers Squibb, Celgene, Ono Pharmaceutical, Sanofi, and SymBio Pharmaceuticals, received honoraria or advisory boards from AbbVie, Astellas Pharma, Bristol‐Myers Squibb, Celgene, Chugai Pharmaceutical, Daiichi Sankyo, Sumitomo Dainippon Pharma, Eisai, Fujimoto Pharmaceutical, Janssen Pharmaceutical, Kyowa Kirin, Nippon Shinyaku, Ono Pharmaceutical, Otsuka Pharmaceutical, Sanofi, Takeda Pharmaceutical, and Novartis. KK has received honoraria from Takeda, MSD, Kyowa‐Kirin, Janssen, Celgene, Ono, Mundi, Dainippon‐Sumitomo, and Bristol‐Myers Squibb, research funding from Chugai, Takeda, Kyowa Kirin, AbbVie, Novartis, Eisai, Janssen, Celgene, Ono, Novartis, and Daiichi Sankyo, and served as a consultant to AbbVie, AstraZeneca, Celgene, Chugai, Eisai, Janssen, Novartis, Daiichi Sankyo, and Ono. YI has received honoraria from Bristol‐Myers Squibb, Celgene, Chugai Pharmaceutical, Eisai, Kyowa Kirin, Sanofi, and SymBio Pharmaceuticals. KN has received research support from Kyowa‐Kirin and Chugai Pharmaceutical, and served as a consultant to Kyowa‐Kirin. RS has received honoraria from Janssen, Nippon Shinyaku, Ono Pharmaceuticals, Eisai, Takeda, and Chugai Pharmaceutical, and received research support from Chugai Pharmaceutical, Kyowa Kirin, and Taiho Pharma. SH and TN are employees of Eisai. The other authors have no conflict of interest.
Supporting information
Figure S1
Table S1
ACKNOWLEDGMENTS
We thank the patients who participated in this study as well as their families and the investigators, physicians, nurses, and clinical research coordinators who helped this study. We would also like to thank Dr Kiyohiko Hatake (International University of Health and Welfare, Mita Hospital) as the independent efficacy and safety adviser and Dr Akira Tomonari (Eisai Co., Ltd.) as the medical adviser of the sponsor. We thank Ms Mari Kikuchi and Mr Satoru Ito (Eisai Co., Ltd.) for their study management, and Dr Kenzo Muramoto and Dr Michiko Sugawara (Eisai Co., Ltd.) for their help in preparing this manuscript. This study was funded and supported by Eisai Co., Ltd.
Izutsu K, Ando K, Nishikori M, et al. Phase II study of tazemetostat for relapsed or refractory B‐cell non‐Hodgkin lymphoma with EZH2 mutation in Japan. Cancer Sci. 2021;112:3627–3635. 10.1111/cas.15040
Funding information
Eisai Co., Ltd.
REFERENCES
- 1.Casulo C. How I manage patients with follicular lymphoma. Br J Haematol. 2019;186:513‐523. [DOI] [PubMed] [Google Scholar]
- 2.Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies. Blood. 2016;127:2055‐2063. [DOI] [PubMed] [Google Scholar]
- 3.Rivas‐Delgado A, Magnano L, Moreno‐Velázquez M, et al. Response duration and survival shorten after each relapse in patients with follicular lymphoma treated in the rituximab era. Br J Haematol. 2019;184:753‐759. [DOI] [PubMed] [Google Scholar]
- 4.Knutson SK, Warholic NM, Wigle TJ, et al. Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc Natl Acad Sci USA. 2013;110:7922‐7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knutson SK, Kawano S, Minoshima Y, et al. Selective inhibition of EZH2 by EPZ‐6438 leads to potent antitumor activity in EZH2‐mutant non‐Hodgkin lymphoma. Mol Cancer Ther. 2014;13:842‐854. [DOI] [PubMed] [Google Scholar]
- 6.Italiano A, Soria J‐C, Toulmonde M, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B‐cell non‐Hodgkin lymphoma and advanced solid tumours: a first‐in‐human, open‐label, phase 1 study. Lancet Oncol. 2018;19:649‐659. [DOI] [PubMed] [Google Scholar]
- 7.Gounder M, Schöffski P, Jones RL, et al. Tazemetostat in advanced epithelioid sarcoma with loss of INI1/SMARCB1: an international, open‐label, phase 2 basket study. Lancet Oncol. 2020;21:1423‐1432. [DOI] [PubMed] [Google Scholar]
- 8.Morschhauser F, Tilly H, Chaidos A, et al. Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open‐label, single‐arm, multicentre, phase 2 trial. Lancet Oncol. 2020;21:1433‐1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munakata W, Shirasugi Y, Tobinai K, et al. Phase 1 study of tazemetostat in Japanese patients with relapsed or refractory B‐cell lymphoma. Cancer Sci. 2021;112:1123‐1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579‐586. [DOI] [PubMed] [Google Scholar]
- 11.Ohmachi K, Ando K, Ogura M, et al. Multicenter phase II study of bendamustine for relapsed or refractory indolent B‐cell non‐Hodgkin lymphoma and mantle cell lymphoma. Cancer Sci. 2010;101:2059‐2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobinai K, Watanabe T, Ogura M, et al. Phase II study of oral fludarabine phosphate in relapsed indolent B‐Cell non‐Hodgkin's lymphoma. J Clin Oncol. 2006;24:174‐180. [DOI] [PubMed] [Google Scholar]
- 13.Leonard JP, Trneny M, Izutsu K, et al. AUGMENT: a phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol. 2019;37:188‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine‐rituximab or R‐CHOP/R‐CVP in first‐line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944‐2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1


