Abstract
This study aimed to investigate the cytotoxicity of a cluster of differentiation 70 antibody‐drug conjugate (CD70‐ADC) against ovarian cancer in in vitro and in vivo xenograft models. CD70 expression was assessed in clinical samples by immunohistochemical analysis. Western blotting and fluorescence‐activated cell sorting analyses were used to determine CD70 expression in the ovarian cancer cell lines A2780 and SKOV3, and in the cisplatin‐resistant ovarian cancer cell lines A2780cisR and SKOV3cisR. CD70 expression after cisplatin exposure was determined in A2780 cells transfected with mock‐ or nuclear factor (NF)‐κB‐p65‐small interfering RNA. We developed an ADC with an anti‐CD70 monoclonal antibody linked to monomethyl auristatin F and investigated its cytotoxic effect. We examined 63 ovarian cancer clinical samples; 43 (68.3%) of them expressed CD70. Among patients with advanced stage disease (n = 50), those who received neoadjuvant chemotherapy were more likely to exhibit high CD70 expression compared to those who did not (55.6% [15/27] vs 17.4% [4/23], P < .01). CD70 expression was confirmed in A2780cisR, SKOV3, and SKOV3cisR cells. Notably, CD70 expression was induced after cisplatin treatment in A2780 mock cells but not in A2780‐NF‐κB‐p65‐silenced cells. CD70‐ADC was cytotoxic to A2780cisR, SKOV3, and SKOV3cisR cells, with IC50 values ranging from 0.104 to 0.341 nmol/L. In A2780cisR and SKOV3cisR xenograft models, tumor growth in CD70‐ADC treated mice was significantly inhibited compared to that in the control‐ADC treated mice (A2780cisR: 32.0 vs 1639.0 mm3, P < .01; SKOV3cisR: 232.2 vs 584.9 mm3, P < .01). Platinum treatment induced CD70 expression in ovarian cancer cells. CD70‐ADC may have potential therapeutic implications in the treatment of CD70 expressing ovarian cancer.
Keywords: CD70, neoadjuvant chemotherapy, NF‐κB‐p65, ovarian cancer, xenograft model
The immunohistochemical analysis of ovarian cancer specimens revealed that cluster of differentiation 70 (CD70) is expressed in approximately 70% of patients who received platinum‐based neoadjuvant chemotherapy. CD70 is induced in cisplatin‐treated ovarian cancer cells and is strongly expressed in platinum‐resistant cells. The CD70 antibody‐drug conjugate is effective against CD70‐expressing ovarian cancer both in vitro and in vivo. Our translational research indicates that the effectiveness of CD70 antibody‐drug conjugate merits further investigation as a novel therapeutic strategy for women with ovarian cancer.

Abbreviations
- ADC
antibody‐drug conjugate
- CD70
cluster of differentiation 70
- FACS
fluorescence‐activated cell sorting
- MMAF
monomethyl auristatin F
- mRNA
messenger ribonucleic acid
- NACT
neoadjuvant chemotherapy
- NHL
non‐Hodgkin lymphoma
- OvCA
ovarian cancer
- PBS
phosphate‐buffered saline
- PDS
primary debulking surgery
- PHH3
phosphor‐histone H3
- RCC
renal clear cell carcinoma
- RCT
randomized‐controlled trial
- siRNA
small interfering RNA
- WB
western blot
1. INTRODUCTION
Ovarian cancer is the fifth leading cause of cancer‐related deaths among women in the United States, with 13,940 registered ovarian cancer deaths in 2020.1 Platinum agents are key chemotherapeutic drugs currently used as a first‐line OvCA treatment2, 3; however, women with recurrent OvCA treated with second‐line or later‐line therapeutic agents have a median survival of 9‐12 months with a response rate of approximately 20% for subsequent therapies.4, 5, 6 Several chemotherapeutic and target therapy agents have been evaluated in clinical trials for recurrent OvCA, including cytotoxic agents, target therapy, poly (adenosine diphosphate‐ribose) polymerase inhibitors, and immune checkpoint inhibitors; however, these agents exhibit only modest clinical responses.6, 7, 8, 9, 10, 11
Antibody‐drug conjugates are novel therapeutic agents that enhance the therapeutic indices of cytotoxic anticancer agents by combining the antigen selectivity of a monoclonal antibody with the cytotoxicity of an anticancer drug.12 The pace of clinical trials for ADC is accelerating, with the number of agents evaluated in clinical trials being increased by more than three times in the last 5 years.13 To date, more than 150 ADCs have been investigated in clinical trials and more than 35 ongoing clinical trials for treatment‐refractory solid tumors have been published to date;12, 13, 14 however, the currently available ADCs demonstrate modest survival effects in patients with OvCA.15
Cluster of differentiation 70 is a member of the tumor necrosis factor superfamily and the ligand for CD27.16, 17 It was first identified in activated T‐ and B‐lymphocytes. The binding of CD70 to CD27 on activated lymphocytes signals the co‐stimulation of T, B, and natural killer cells,18, 19 and also regulates cell differentiation and T‐helper 1/2 switching.20 CD70 is frequently expressed in various malignant diseases, including renal clear cell carcinoma (RCC 60%) and non‐Hodgkin lymphoma (NHL 87%),21, 22 but is minimally expressed in normal tissues.23
Previous studies have shown that anti‐CD70 monoclonal antibodies (CD70 mAb) and CD70‐ADC (CD70 mAb conjugate with MMAF) exhibit antitumor effects in xenograft models of CD70 malignant diseases, such as lymphoma, NHL, and RCC.21, 24, 25, 26 Based on the positive results of preclinical studies, a phase‐I study of CD70‐ADC in patients with CD70‐positive relapsed/refractory NHL or metastatic RCC was conducted; however, CD70‐ADC exhibited modest effects on these diseases with tolerable side effects.22
Studies that examined OvCA cells/clinical samples27, 28, 29 have reported that CD70 is expressed in OvCA cells and is a potential predictor of platinum resistance.30 Given that the effectiveness of CD70‐ADC therapy in patients with OvCA has been poorly studied, we examined the antitumor activity of CD70‐ADC in OvCA cases.
2. MATERIALS AND METHODS
Detailed information is provided in Document S1.
2.1. Patient and tissue samples
Sixty‐three high‐grade serous OvCA surgical specimens were obtained from women who had undergone surgical treatment between 2012 and 2020 at Osaka University Hospital (Table S1). The histopathological analysis and diagnosis of the tissues were performed by board‐certified gynecologic pathologists. Informed consent was obtained from the patients prior to sample collection and examination. Tumor staging was based on the 2014 International Federation of Gynecology and Obstetrics staging system.31 The study was approved by the Institutional Review Board of Osaka University Hospital (Approval No. 18236).
Among 63 OvCA surgical specimens, there were 29 women who received NACT before cytoreductive surgery. Of those, nine women underwent surgical biopsy before NACT in our institution and three women were excluded from the investigation as the samples were too small to perform immunohistochemistry. Thus, the expression of CD70 was compared before and after NACT in six patients (Table S2).
2.2. Immunohistochemistry
All clinical samples were paraffin‐embedded, fixed in 10% neutral buffered formalin, sliced into 4‐µm tissue sections, deparaffinized, and rehydrated in graded alcohols. Immunostaining was performed using mouse monoclonal anti‐CD70 antibody (MAB2738, 1:100; R&D Systems) as the primary antibody, followed by the Vectastain ABC kit (Vector Laboratories) as previously described.23, 32, 33, 34, 35 The immunostained sections were photographed using a Visualix Pro2 camera (Olympus).
Three cases with advanced renal cell cancer (clear cell carcinoma) were immune‐stained as positive controls,36 and three healthy ovary specimens were used as negative controls28 (formalin‐fixed, paraffin‐embedded sections were obtained from surgical treatment cases at Osaka University Hospital).
The intensity and density of positive staining were scored by three independent gynecological pathologists (SM, RN, and MS) who were blinded to clinico‐pathological information, as described in the Supporting Information Materials and Methods section.
2.3. Cell lines and culture
The human ovarian carcinoma cell lines A2780 and SKOV3 were obtained from the European Collection of Authenticated Cell Culture (Salisbury, Scotland) and the American Type Culture Collection (Manassas, VA, USA), respectively.37 The human ovarian carcinoma cell lines OVISE, OVTOKO, and RMG‐I were purchased from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). RMG‐1 was cultured in Ham's F12 medium (Thermo Fisher Scientific Inc.) containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin (Nacalai Tesque). A2780, SKOV3, OVTOKO, and OVISE were cultured in RPMI 1640 medium (FUJIFILM Wako Pure Chemical Corporation, Ltd) containing 10% FBS (Hyclone Laboratories), 100 U/mL penicillin, and 100 µg/mL streptomycin at 37°C in a humidified atmosphere with 5% CO2. The identity of each cell line was confirmed by DNA fingerprinting using short tandem repeat profiling.32, 33, 38
2.4. Establishment of cisplatin (platinum)‐resistant cells
To develop platinum‐resistant OvCA cell lines, A2780 and SKOV3 cells were treated with increasing concentrations of cisplatin;39 these cells were designated A2780cisR and SKOV3cisR. A2780 paclitaxel‐resistant cell lines were generated using a similar method. Platinum‐resistant cell lines were designated as A2780cisR and SKOV3cisR, and the paclitaxel‐resistant cell line was designated as A2780pacR.
2.5. Western blotting
The cells were prepared as described previously.23, 32, 33, 34, 38 The proteins were transferred to polyvinylidene difluoride membranes and incubated with goat polyclonal anti‐CD70 antibody (1:500, sc‐1741; Santa Cruz Biotechnology). Subsequently, the western blotting (WB) samples were incubated with donkey anti‐goat horseradish peroxidase (HRP)‐conjugated secondary antibodies (1:10 000, sc‐2020; Santa Cruz Biotechnology).
In an investigation of CD70, NFkB‐p65, and AP‐1 expression after cisplatin exposure, rabbit monoclonal anti‐NFkB‐p65 antibody (1:1000, #3034, Cell Signaling Technology), rabbit monoclonal anti‐phosphorylation NFkB‐p65 (1:1000, #3033, Cell Signaling Technology) and rabbit monoclonal anti‐c‐Jun antibody (1:1000, #9165, Cell Signaling Technology) were used. Donkey anti‐rabbit HRP‐conjugated secondary antibodies (1:5000, GE Healthcare Bio‐Sciences) were used for subsequent visualization.
2.6. Fluorescence‐activated cell sorting analysis
The cells were washed in phosphate‐buffered saline (PBS) (Nacalai Tesque) and were detached with 0.02% ethylenediaminetetraacetic acid solution (Nacalai Tesque).23, 33, 35 The cells were washed twice with fluorescence‐activated cell sorting (FACS) buffer (PBS with 1% FBS and 0.1% sodium azide) and then incubated with mouse monoclonal antihuman CD70 antibody (1:50, clone Ki‐24; BD Biosciences) as the primary antibody.
2.7. Preparation and transfection of siRNA
To silence CD70 expression, siRNA‐CD70 (Hs_TNFSF7_1; _3; Qiagen) against CD70 was transfected using Lipofectamine RNAiMAX reagent (#13778150; Invitrogen) as previously described.32, 38 Selective silencing of CD70 was confirmed by WB analysis. For cytotoxicity assays, the cells were seeded onto 96‐well plates after a 4‐h transfection, and measurements were performed on day 7.
For NFkB‐p65 knockdown, siRNA‐NFkB‐p65 (#6261, Cell Signaling Technology) against NFkB‐p65 was transfected as aforementioned. NFkB‐p65‐silencing cells were designated as A2780‐NFkB‐p65‐silencing cells. Selective silencing of NFkB‐p65 expression was confirmed by WB analysis.
2.8. CD70 expression after cisplatin exposure
After transfection of siRNA‐NkB‐p65 and control siRNA for 48 h, the A2780 cells were incubated with nontreated cells and 20 µmol/L cisplatin for 1, 4, 8, and 12 h, followed by the preparation of protein extracts. The prepared protein extract was analyzed by WB analysis using CD70, c‐Jun, NF‐κB‐p65, and phospho‐NF‐κB‐p65 antibodies.
2.9. CD70 confirmation after paclitaxel exposure
The expression of CD70 was determined in A2780 cells by WB using the protein extracts at 1, 4, 8, and 12 h after administration of 10 nmol/L paclitaxel and 10 nmol/L paclitaxel exposing cells followed by the preparation of protein extracts.
2.10. Real‐time quantitative reverse transcription PCR
A2780‐NF‐kB‐p65‐silenced cells were incubated with 5 μM cisplatin for 0, 1, or 2 h, followed by RNA extraction for real‐time quantitative RT‐PCR. CD70 expression in A2780 cells was determined using RT‐PCR. Total RNA extraction and cDNA synthesis were performed according to the manufacturer's protocol.23, 34
PCR was performed using TB Green Premix Ex Taq II (#RR820A, Takara Bio, Shiga, Japan) and specific primers. PCR primers were also purchased from Takara Bio. The sequences of the primers used were as follows: human CD70 (180 bp), forward primer 5‐GCCCTATGGGTGCGTCCTGC‐3 and reverse primer 5‐AGCCTGGGGTCCTGCTGAGG‐3; β‐actin (174 bp), 5‐AGCCTCGCCTTTGCCGA‐3 (forward), and 5‐CTGGTGCCTGGGGCG‐3 (reverse).
2.11. ADC development
Using previously described methods,23 commercially available anti‐human‐CD70 mAb (vorsetuzumab, TAB‐H76; Creative Biolabs)40 and isotype control human‐IgG1 mAb (clone QA16A12; BioLegend) were used to generate CD70‐ADC and control‐ADC, respectively. MMAF was used as a payload for ADCs, and the conjugation was performed by Moradec LLC. The mAbs were conjugated with MMAF using a maleimide‐cysteine method, wherein the mAb inter‐chain disulfide bonds were first reduced with tris(2‐carboxyethyl) phosphine at 37°C and the maleimide moiety of the drug was subsequently linked to the reduced cysteines.
Conjugated ADCs were separated from the free drug on Sephadex G‐50 columns. Next, the buffer was replaced with PBS, and the resultant ADC containing solution was filtered. The drug distribution was examined using hydrophobic interaction chromatography.
2.12. Cytotoxicity assay
Cytotoxicity assays of anti‐CD70 mAb, human‐IgG1‐isotype control antibody, CD70‐ADC, control‐ADC, and MMAF were performed. The cells were evaluated using a flat‐bottomed 96‐well white polystyrene plate (Thermo Fisher Scientific Inc.) and 1000 cells were seeded per well in RPMI containing 10% FBS. After 24 h of incubation, anti‐CD70 mAb, human‐ IgG1‐isotype control antibody, CD70‐ADC, control‐ADC, and MMAF were added at different concentrations in hexaplicate wells in a humidified 5% CO2 atmosphere. After incubation for 6 days at 37°C, cell viability was determined using the CellTiter‐Glo Luminescent Cell Viability Assay (Promega) and luminescence values were measured using a microplate reader (Lumat3 XS LB9508; Berthold Technologies).
The results are reported as the IC50, defined as the concentration of a compound needed to reduce the viability by 50% compared with the control cells (control = 100%).33
2.13. In vivo efficacy
All animal experiments were conducted according to the institutional ethical guidelines for animal experimentation of Osaka University (registered protocol number 28‐032‐013).23 The in vivo treatment schedule is presented in Figure S1.
A2780cisR and SKOV3cisR xenografted mice were randomly divided into three groups (n = 6) when the mean tumor size reached approximately 90 mm3. CD70‐ADC (3 mg/kg), control‐ADC (3 mg/kg), or PBS was intravenously administered in 100 µL of PBS every 4 days for a total of four days (days 0, 4, 8, and 12). The xenografted tumors were removed and weighed on day 28.
2.14. Phospho‐histone H3 staining
To investigate the pharmacologic effect of CD70‐ADC at the cellular level, PHH3, which is a mitotic marker, was examined.3, 11, 12, 13, 14 A2780cisR xenograft mice were intravenously administered with PBS, control‐ADC (3 mg/kg), or CD70‐ADC (3 mg/kg), and the tumors were excised 24 h later. The tumor sections were sliced into 4‐μm formalin‐fixed paraffin‐embedded tissue sections.
PHH3 (Ser10) staining was recorded as the ratio of PHH3 positive cells to all tumor cells in five fields (×400 magnification).23, 41
2.15. Statistical analysis
The results are expressed as means with standard deviations for the in vitro data and as means with standard errors of the mean for the in vivo experiments. Student's t test, Mann‐Whitney U test, Kruskal‐Wallis H test, Pearson's chi‐square test, and Fisher's exact test were used to assess the difference between groups, as appropriate. All statistical analyses were based on two‐tailed hypothesis. JMP 13 (SAS Institute) was used for statistical analysis. P values <.05 were considered significant.
3. RESULTS
3.1. CD70 was highly expressed in patients with OvCA who received NACT
CD70 expression in OvCA clinical samples was evaluated by immunohistochemistry (Table 1). There were 63 OvCA samples and all samples were of high‐grade serous type. Of those, 22 (34.9%) and 21 (33.3%) were in the CD70‐high and CD70‐low groups, respectively (Figure 1A,B). On univariate analysis, CD70 expression was not associated with age, stage, residual disease, or serum CA‐125 levels (all P > .05). The median follow‐up duration was 33.0 (interquartile range [IQR] 19.5‐52) months (Table S1). Women in the CD70‐high group were more likely to receive NACT compared to those in the CD70‐low/negative group (72.7% [16/22] vs 29.3% [12/41], P < .01; Table 1).
TABLE 1.
Correlation between CD70 expression levels and clinicopathological characteristics in serous ovarian carcinoma
| Characteristics |
CD70 high |
CD70 low/negative |
P value |
|---|---|---|---|
| No. (%) | n = 22 | n = 41 | |
| Median age (years) | 61.5 (52‐70) | 61 (52‐70.5) | .76 |
| FIGO stage | |||
| I | 2 (9.1%) | 7 (17.1%) | .99 |
| II | 1 (4.6%) | 3 (7.3%) | |
| III | 16 (72.7%) | 25 (61.0%) | |
| IV | 3 (13.6%) | 6 (14.6%) | |
| Residual disease* | |||
| Yes | 9 (40.9%) | 15 (36.6%) | .79 |
| No | 13 (59.1%) | 26 (63.4%) | |
| Serum CA‐125 level | |||
| Normal | 3 (13.6%) | 7 (17.1%) | .99 |
| Abnormal | 19 (86.4%) | 34 (82.9%) | |
| NACT | |||
| Yes | 16 (72.7%) | 12 (29.3%) | <.01 |
| No | 6 (27.3%) | 29 (70.7%) | |
| NACT regimen | |||
| TC | 9 (56.2%) | 5 (41.7%) | |
| TC+Bev | 7 (43.8) | 6 (50.0%) | |
| DC | 0 (0%) | 1 (8.3%) | |
| Median FU (months) | 45 (26.3‐61.3) | 30 (17‐46) | .03 |
Median (IQR) or number (percentage per column) is shown. Chi‐square test or Student's t test was performed for univariate analysis. Mann‐Whitney U test was used to compare two groups with nonparametric data. Significant P values are given in bold font.
Abbreviations: Bev, bevacizumab; CD70, cluster of differentiation 70; DC, docetaxel and carboplatin; FIGO, International Federation of Gynecology and Obstetrics; FU, follow‐up; IQR, interquartile range; NACT, neoadjuvant chemotherapy; TC, paclitaxel and carboplatin.
Residual disease after cytoreductive surgery.
FIGURE 1.
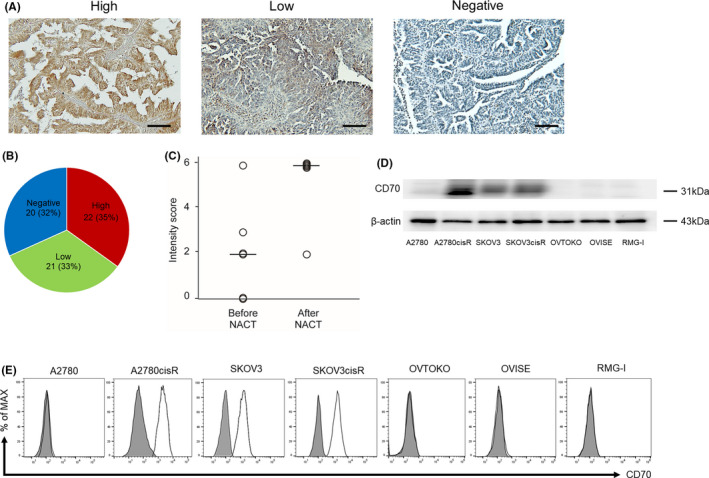
Confirmation of cluster of differentiation 70 (CD70) expression in serous ovarian carcinoma cells and surgical specimens. A, Representative CD70 staining in clinical samples. The immunohistochemistry score divided clinical samples into three groups: high (>4 points), low (1‐3 points), and negative (0 points). Scale bar: 100 μm. B, Among the 63 samples, 22 (34.9%), 21 (33.3%), and 20 (31.7%) represented the CD70‐high, CD70‐low, and CD70‐negative groups, respectively. C, The comparison of CD70 expression before and after neoadjuvant chemotherapy is shown. CD70 staining was assessed according to the intensity score. The black bar indicates the median score of intensity. Significantly higher scores are observed in the after neoadjuvant chemotherapy (NACT) group compared to those in the before NACT group (P =.03). (Mann‐Whitney U test was used to compare the groups). D, CD70 expression was determined using western blotting analysis in four serous ovarian carcinoma cell lines and three ovarian clear cell carcinoma cell lines. Strong CD70 expression was observed in A2780cisR and SKOV3cisR cells, moderate CD70 expression was observed in SKOV3 cells, and no CD70 expression was observed in A2780, OVTOKO, OVISE, and RMG‐I cells. E, In fluorescence‐activated cell sorting analysis, CD70 expression was detected in A2780cisR, SKOV3, and SKOV3cisR cells using an anti‐CD70 monoclonal antibody. The gray‐shaded areas show isotype control
Among patients with stage III‐IV (advanced) diseases (n = 50), 34 (68.0%) patients showed CD70 expression. Of those, 19 (38.0%) and 15 (30.0%) showed high and low CD70 expression. Among the patients with advanced OvCA, those in the NACT group were more likely to show high CD70 expression compared to those in the non‐NACT group (55.6% [15/27] vs 17.4% [4/23], odds ratio 3.2, 95% confidence interval 1.2‐8.3, P < .01; Table S3).
Nine out of 29 women underwent surgical biopsy in the NACT group and three women were excluded from the investigation as the samples were too small to perform immunohistochemistry. Of those (n = 6), the expression of CD70 was examined before and after NACT in the same patients. Significantly higher intensity score was observed in the after NACT group compared to that in the before NACT group (Figure 1C; median score 6 vs 2, P = .03). These findings suggested that platinum‐based NACT had the potential to induce CD70 expression in OvCA cells.
3.2. Platinum‐resistant OvCA cells overexpressed CD70
CD70 expression in OvCA cells was examined using WB and FACS. The cisplatin toxicity IC50 values in the cisplatin‐resistant models (A2780cisR and SKOV3cisR cells) were significantly higher than those in their parental counterparts (344.1 vs 0.62 µmol/L and 45.6 vs 3.7 µmol/L, respectively [all P < .01]) (Table S4). WB results showed strong CD70 expression in A2780cisR and SKOV3cisR cells, moderate expression in SKOV3 cells, and no expression in A2780 cells (Figure 1D). CD70 overexpression in A2780cisR, SKOV3, and SKOV3cisR cells was confirmed using FACS (Figure 1E).
3.3. Silencing CD70 did not affect cisplatin sensitivity or cell proliferation
To explore the CD70 expression effect on platinum sensitivity and cell proliferation in OvCA cells, CD70 expression was silenced using siRNA (Figures 2A and S2). On cytotoxicity analysis, the IC50 values of cisplatin were similar in two A2780cisR‐CD70‐silenced (363.1 and 382.6 µmol/L) and A2780cisR mock cells (372.7 µmol/L, P = .28 and P = .13, respectively). Similarly, no significant change in IC50 was observed in two variants of SKOV3cisR‐CD70‐silenced (74.0 and 77.3 µmol/L) and SKOV3cisR mock cells (72.9 µmol/L, P = .51 and P = .83, respectively) (Figure 2B). The proliferation rates of CD70‐silenced A2780cisR, SKOV3, and SKOV3cisR cells were similar (Figure 2C).
FIGURE 2.
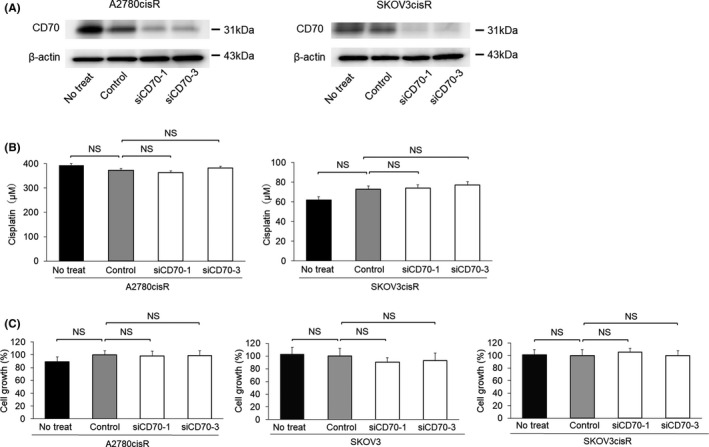
Silencing cluster of differentiation 70 (CD70) expression was not associated with platinum resistance and proliferation. A, CD70 expression was silenced by CD70 small interfering RNA (siRNA) transfection in A2780cisR and SKOV3cisR cells. Silencing of CD70 expression was confirmed by western blotting analysis. B, IC50 values for cisplatin were determined in A2780cisR and SKOV3cisR cells. IC50 values of cisplatin in A2780cisR‐siCD70‐1 and A2780cisR‐siCD70‐3 cells were similar to those in A2780cisR‐C cells. IC50 values of cisplatin were not significantly different among SKOV3cisR‐C, SKOV3cisR‐siCD70‐1, and SKOV3cisR‐siCD70‐3 cells. Kruskal‐Wallis H test was used to compare groups. C, Proliferation assays were performed in mock cells and two types of CD70‐silenced cells in A2780cisR, SKOV3, and SKOV3cisR cells. No significant difference in proliferation between mock cells and CD70‐silenced cells was observed in each cell line. Kruskal‐Wallis H test was used to compare groups. Abbreviations: No treat, no treatment; NS, not significant
3.4. Cisplatin exposure induces CD70 expression
To explore the mechanism of CD70 induction after cisplatin administration, the expression levels of c‐Jun and NF‐κB‐p65 were determined. The WB results revealed positive NF‐κB‐p65 and negative c‐Jun expressions in A2780 cells (Figure 3A).
FIGURE 3.
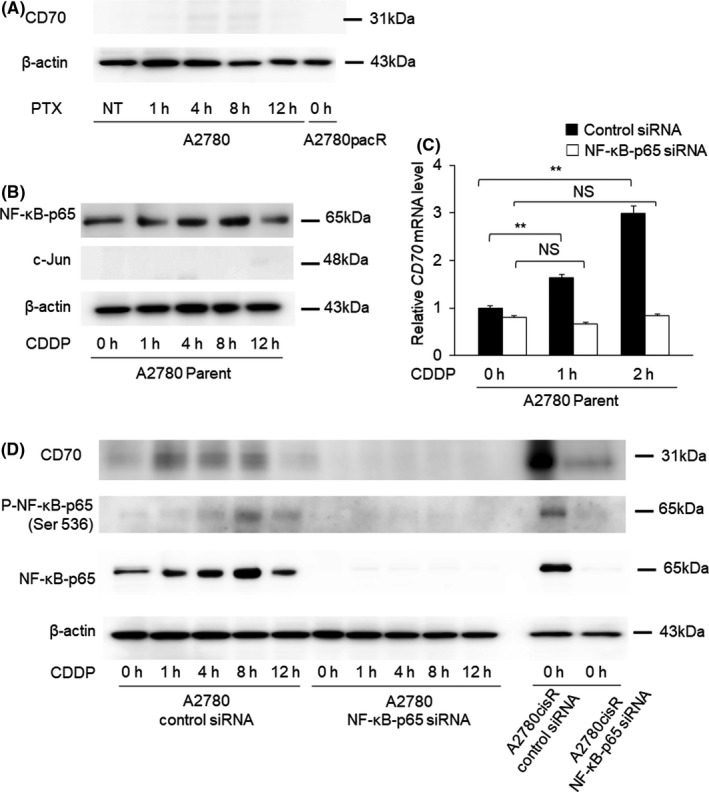
Cluster of differentiation 70 (CD70) expression after cisplatin and paclitaxel exposure. A, The expression of AP‐1 (c‐Jun) was determined in groups with no treatment and after 1, 4, 8, and 12 h of cisplatin exposure. c‐Jun expression was not observed in any of the groups. B, mRNA level of CD70 expression was examined in A2780 mock cells and A2780‐NF‐κB‐p65‐silenced cells 1 and 2 h after cisplatin exposure. The expression levels of CD70 mRNA were corrected with those of GAPDH mRNA. CD70 expression was induced by short‐term exposure to cisplatin in A2780 mock cells, whereas CD70 was not induced on cisplatin exposure in A2780‐NF‐κB‐p65‐silenced cells. C, The expression of CD70, NF‐κB‐p65, and phospho‐NF‐κB‐p65 was determined in A2780 mock cells and A2780‐NF‐κB‐p65‐silenced cells at 0 h and after 1, 4, 8, and 12 h of cisplatin exposure. Knockdown of NF‐κB‐p65 and phospho‐NF‐κB‐p65 was observed in A2780‐NF‐κB‐p65‐silenced cells. In A2780 mock cells, the expression of NF‐κB‐p65 and phospho‐NF‐κB‐p65 was induced after cisplatin exposure and CD70 expression was also induced after cisplatin exposure. In A2780cisR cells, NF‐κB‐p65 suppression led to a substantial decrease in CD70 expression. D, The expression of CD70 was determined at 0 h and after 1, 4, 8, and 12 h of paclitaxel exposure in A2780 cells and at 0 h in A2780pacR cells. The expression of CD70 was not observed in any of the cells. Abbreviations: CDDP, cisplatin; mRNA, messenger ribonucleic acid; pacR, paclitaxel resistant cells; P‐NF‐κB, phospho‐NF‐κB; PTX, paclitaxel
To examine the role of NF‐κB‐p65 in CD70 induction, CD70 messenger ribonucleic acid (mRNA) was measured in A2780 mock and A2780‐NF‐κB‐p65‐silenced cells (Figure 3B). The A2780 mock cells expressed progressively higher CD70 mRNA expression at 1 h (1.63, P = .04) and 2 h (2.99, P < .01) of treatment compared to the corresponding untreated cells. Conversely, CD70 mRNA expression after cisplatin treatment did not increase in A2780‐NF‐κB‐p65‐silenced cells, relative to that in the untreated cells (P = .06; Figure 3B).
CD70 expression was compared between the A2780 mock and A2780‐NF‐κB‐p65‐silenced cells after cisplatin treatment (Figures 3C and S3). The knockdown of NF‐κB‐p65 and phosphorylated NF‐κB‐p65 expression was confirmed by WB analysis. The expression of NF‐κB‐p65 and phospho‐NF‐κB‐p65 continuously increased after 1, 4, 8, and 12 h of cisplatin exposure in A2780 mock cells. CD70 expression gradually increased in A2780 mock cells starting at 1 h of cisplatin exposure, whereas no significant change in CD70 expression was observed in A2780‐NF‐κB‐p65‐silenced cells. Concerning the paclitaxel exposure, CD70 was not induced in A2780 parent cells after 1, 4, 8, and 12 h of paclitaxel exposure and CD70 was not expressed in A2780‐paclitaxel resistant cells (Figure 3D).
3.5. The anti‐CD70 mAb did not inhibit cell proliferation
As shown in Figure 4A, neither the anti‐CD70 mAb nor the anti‐human‐IgG1‐isotype control antibody inhibited the cell proliferation of A2780, A2780cisR, SKOV3, and SKOV3cisR cell lines.
FIGURE 4.

In vitro cell growth activity. A, A2780, A2780cisR, SKOV3, and SKOV3cisR cells were exposed to anti‐cluster of differentiation 70 (CD70) monoclonal antibody or anti‐human‐IgG1‐isotype control antibody for 144 h. Cytotoxicity assays showed no significant growth inhibition in these cell lines. The y axis shows relative growth compared with that in the no treatment group. B, The structure of CD70‐antibody‐drug conjugate (ADC) consists of an anti‐CD70 antibody conjugated to the monomethyl auristatin F (MMAF) payload. Reused data from Nakae et al23, Figure 2B Copyright (2020) with permission from Elsevier. C, Cells were exposed to CD70‐ADC and human‐IgG1‐control‐ADC. Compared with the control ADC, CD70‐ADC significantly inhibited the growth of CD70‐positive A2780cisR, SKOV3, and SKOV3cisR cells. Cytotoxicity assays showed no significant growth inhibition in the CD70‐negative A2780 cell line. The y axis shows relative growth compared with that in the no treatment group. Abbreviations: ADC, antibody‐drug conjugate, mAb; monoclonal antibody
3.6. Bioengineering of CD70‐ADC
Each of the anti‐CD70 mAb and the isotype control antibody was directly conjugated to MMAF (Figure 4B), with drug‐to‐antibody ratios of 3.59 and 3.64, respectively.
3.7. CD70‐ADC showed cytotoxicity in CD70‐expressing OvCA cells
MMAF demonstrated cytotoxicity in OvCA cell lines, including those that were cisplatin‐resistant; IC50 values ranged from 19.5 to 67.8 nmol/L (Table S4). As MMAF has poor cell membrane permeability, CD70 mAb conjugated MMAF was more likely to be sensitive compared to unconjugated MMAF in CD70‐expressing cells. CD70‐ADC showed a concentration‐dependent inhibition of tumor growth among the CD70‐expressing cell lines A2780cisR (IC50: 0.104 nmol/L), SKOV3 (IC50: 0.205 nmol/L), and SKOV3cisR cells (IC50: 0.341 nmol/L), whereas no such inhibition was observed in the CD70‐nonexpressing cell line A2780 and in the control‐ADC treated cells (Figure 4C and Table S5).
3.8. Cisplatin sensitivity of tumors in mouse xenograft models
To determine the platinum sensitivity of tumors derived from A2780, A2780cisR, SKOV3, and SKOV3cisR cells, xenograft models were established for each cell line (Figure 5A‐D). In A2780 and SKOV3 xenograft mice, the tumor growth rate was significantly lower in the cisplatin‐treated than in the PBS‐treated group (A2780: 841.9 vs 1991.5 mm3, P < .05; SKOV3: 254.4 vs 706.4 mm3, P < .01) (Figure 5A,B). In the SKOV3 xenograft mice, the tumor weight was significantly lower in the cisplatin than in the PBS group (263.3 vs 503.3 mg, P < .05) (Figure 5E).
FIGURE 5.
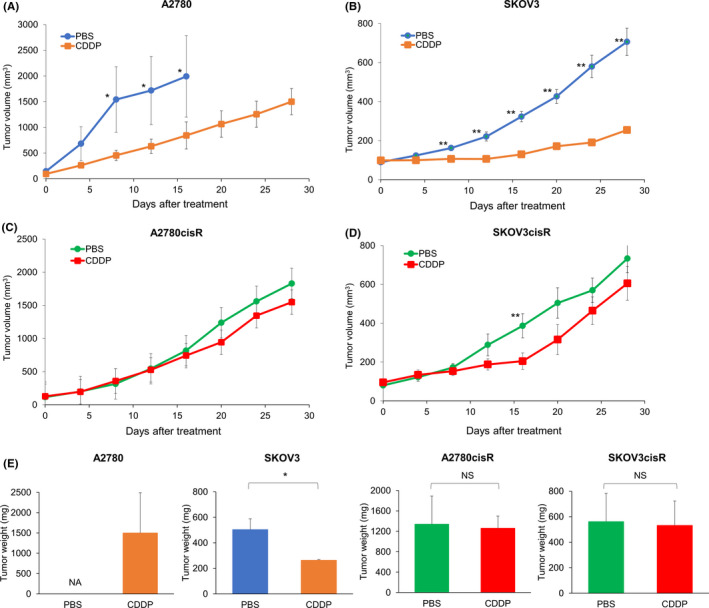
Antitumor activity of cisplatin in A2780, A2780cisR, SKOV3, and SKOV3cisR xenograft mouse models. Antitumor efficacy of cisplatin was investigated in the A2780, A2780cisR, SKOV3, and SKOV3cisR xenograft mouse models. Tumor‐bearing mice were administered PBS or cisplatin (3 mg/kg) twice weekly (days 0, 4, 8, and 12) four times. Dots represent observed values and bars represent SEM. NS, not significant, *P < .05, **P < .01; Mann‐Whitney U test was used to compare the groups). A and B, A2780 xenografted mice treated with PBS were sacrificed on day 16 after drug administration because of the tumor size. In the A2780 xenograft model, tumor size in the cisplatin treatment group was significantly more suppressed than that in the PBS group on day 16 after drug administration (841.9 vs 1991.5 mm3, P < .05). Similarly, tumor size in the cisplatin treatment group was more inhibited than that in the PBS group (254.4 vs 706.4 mm3, P < .01) in the SKOV3 xenograft model on day 28. C and D, In A2780cisR and SKOV3cisR xenograft models, tumor growth in the cisplatin group was similar to that in the PBS group (A2780cisR: 1548.4 vs 1829.8 mm3, P = .10 and SKOV3cisR: 605.0 vs 733.4 mm3, P = .45). Platinum resistance was confirmed in A2780cisR and SKOV3cisR xenograft models. Mice in the PBS group were the same as those used to determine the effect of the cluster of differentiation 70‐antibody drug conjugate. E, In A2780 xenograft mice treated with PBS, the mice were euthanized on day 16 due to the excessive tumor size. In the SKOV3 xenograft mice, the tumor weight was significantly lower in the cisplatin group than in the PBS group (263.3 vs 503.3 mg, P < .05). In A2780cisR and SKOV3cisR xenograft mice, the tumor weight on day 28 post‐administration (A2780cisR: 1260 vs 1340 mg, P = .75, SKVO3cisR: 531.7 vs 561.7 mg, P = .52) were similar in the cisplatin and PBS‐treated groups. The bar graph shows the average tumor weight with SEM. Abbreviations: PBS, phosphate‐buffered saline; CDDP, cisplatin; NA, not applicable; NS, not significant
In A2780cisR and SKOV3cisR xenograft mice, the growth rate of tumors (A2780cisR: 1548.4 vs 1829.8 mm3, P = .10; SKOV3cisR: 605.0 vs 733.4 mm3, P = .45) and weight on day 28 post‐administration (A2780cisR: 1260 vs 1340 mg, P = .75, SKVO3cisR: 531.7 vs 561.7 mg, P = .52) were similar in the cisplatin and PBS‐treated groups (Figure 5C‐E).
3.9. CD70‐ADC significantly inhibited tumor growth in A2780cisR and SKOV3cisR xenograft models
The effects of CD70‐ADC were evaluated in the A2780cisR‐ and SKOV3cisR‐based xenograft models. SKOV3‐xenografted tumors were not assessed because of the absence of CD70 expression (Figure S1). In the A2780cisR xenograft model, the tumor size (32.0 vs 1639.0 mm3, P < .01) and weight (140 vs 983.3 mg, P < .01) on day 28 after administration were significantly lower in the CD70‐ADC than in the control‐ADC group (Figure 6A,B), with no significant total body weight loss in any group (Figure 6C). Likewise, in SKOV3cisR xenograft models, the tumor size (232.2 vs 584.9 mm3, P < .01) and weight (260 vs 476.7 mg, P < .01) were significantly lower in the CD70‐ADC than in the control‐ADC group (Figure 6D,E), with no significant difference in body weight across all groups (Figure 6F). Similar tumor sizes were observed between the control‐ADC and PBS groups (A2780cisR, P = .92; SKOV3cisR, P = .20) (Figure 6B,E).
FIGURE 6.
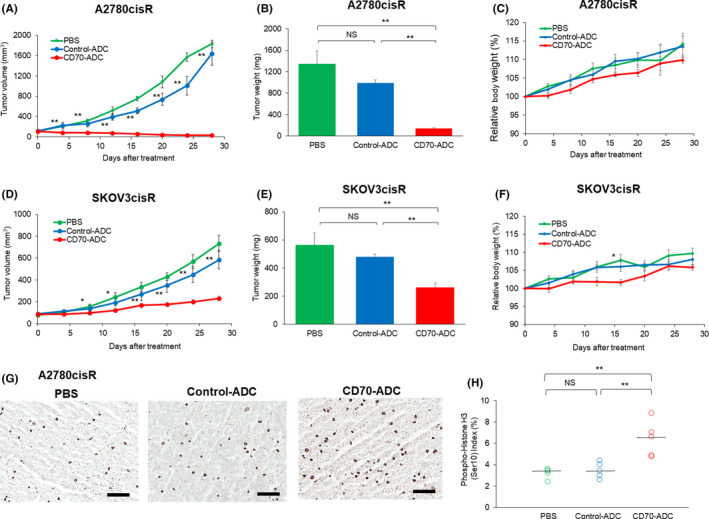
Antitumor activity of cluster of differentiation 70‐antibody‐drug conjugate (CD70‐ADC) in vivo. Antitumor efficacy of CD70‐ADC was investigated in the A2780cisR (A‐C) and SKOV3 cisR (D‐F) xenograft mouse models (n = 6). Tumor‐bearing mice were intravenously administered PBS, control‐ADC (3 mg/kg), or CD70‐ADC (3 mg/kg) twice weekly (days 0, 4, 8, and 12) four times. Dots represent observed values and bars represent SEM. *P < .05, **P < .01; Kruskal‐Wallis H test was used to compare the groups). In (A) and (D), * or ** indicates the significant difference between control‐ADC group vs PBS treatment group. A‐C, The average tumor volume at each time point is shown in the graph. Similar tumor sizes were observed between the control ADC and PBS groups (P = .92). Tumor size in the CD70‐ADC treatment group was significantly suppressed relative to that in the control ADC group. Tumors were resected and weighed 28 days after implantation in the A2780cisR xenograft mouse model. The bar graph shows the average tumor weight with SEM. Relative bodyweight changes are shown in the right panel. D‐F, The average tumor volume at each time point is shown in the graph. The average tumor volume at each time point is shown in the graph. Similar tumor sizes were observed between the control ADC and PBS groups (P = .2). Tumor size in the CD70‐ADC treatment group was significantly suppressed relative to that in the control ADC group. Tumors were resected and weighed on day 28 after implantation in the SKOV3cisR xenograft mouse model. The bar graph shows the average tumor weight with SEM. Relative bodyweight changes are shown in the right panel. G, A single dose of PBS, control‐ADC, or CD70‐ADC was administered to A2780cisR xenograft mice. The percentage of mitotic cells after CD70‐ADC administration significantly increased relative to that after control‐ADC administration. Scale bar: 100 μm. H, Phospho‐histone H3 (Ser10) staining was assessed quantitatively as the ratio of mitotic cells to the total number of tumor cells in five fields (×400 magnification). The black bar indicates the median rate of mitotic cells. **P < .01; Kruskal‐Wallis H test was used to compare groups. Abbreviations: ADC, antibody‐drug conjugate; PBS, phosphate‐buffered saline; NS, not significant; SEM, standard error of the mean
To assess the pharmacological effect of CD70‐ADC, immunohistochemistry was performed to assess the expression of the mitotic marker PHH3 (Ser10). A significantly higher percentage of mitotic cells was observed in the CD70‐ADC‐treated than in the control‐ADC group (6.40% [IQR 4.85%‐7.0%] vs 3.48% [IQR 3.0%‐4.0%], P < .01) (Figure 6G, H). Conjugation with anti‐CD70 mAb led to the successful delivery of MMAF into OvCA cells, where it induced mitotic arrest via the inhibition of tubulin polymerization.
4. DISCUSSION
4.1. Principal findings
The key findings of this study were as follows: (i) CD70 expression was induced when the OvCA cells were treated with cisplatin (this appeared to be mediated by the NF‐κB transcription pathway); (ii) a significantly higher CD70 expression was observed in the NACT group compared to that in the non‐NACT group; and (iii) CD70‐ADC exhibited a significant inhibitory effect in CD70‐expressing OvCA cells, both in vitro and in vivo.
4.2. Results
Recently, NACT use for advanced OvCA has been significantly increased.42, 43, 44, 45 Approximately 10%‐30% of women with advanced OvCA received NACT in the early 2000s, and the rate of NACT use has nearly doubled in the following decade.42, 44 RCTs that demonstrated that NACT was noninferior concerning the overall survival compared to PDS may have influenced the adoption of NACT.46, 47 Although previous RCTs have demonstrated the noninferior overall survival of NACT, some studies have shown that the recurrence risk (platinum‐resistant OvCA) is higher with NACT use than with PDS.48, 49 NACT use has a potential to induce the expression of CD70, and CD70‐ADC may show an antitumor effect even in platinum‐resistant OvCA when the tumor showed CD70 expression. Therefore, CD70‐ADC may be an attractive agent for women with OvCA treated with NACT.
ADCs constitute a novel technology for targeting rapidly proliferating cells by combining monoclonal antibodies that are highly specific for tumor antigens with effective cytotoxic agents.50 Progress has been made in the clinical application of ADCs in several cancer types, and there are seven FDA‐approved ADCs;51 however, none of them is indicated for OvCA cases.
IMGN853 is an ADC designed to target the folate receptor alpha, which is moderately or strongly expressed in 66% of patients with OvCA.52 This ADC consists of a humanized anti‐folate receptor alpha mAb attached via a cleavable disulfide linker to the cytotoxic maytansinoid DM4, which inhibits tubulin polymerization. Based on its favorable tolerability and activity in a phase 1/2 study,53 a randomized phase 3 trial (FORWARD I) was conducted to compare the safety and efficacy of IMGN853 and single‐agent chemotherapy in women with medium or high folate receptor alpha expression. Although IMGN853 is the most advanced ADC for OvCA that is presented under clinical development for OvCA, IMGN853 showed a modest improvement in progression‐free survival (hazard ratio 0.98, P = .897) and overall survival rates (hazard ratio 0.81, P = .248).13
Several CD70‐ADCs, including SGN‐75 (MMAF‐conjugated), SGN‐70A (pyrrolobenzodiazepine‐conjugated), and MDX‐1203 (duocarmycin‐conjugated), have been evaluated clinically to date.22, 54, 55 For targeting platinum‐resistant OvCA via ADC, acquired cross‐resistance between platinum drugs and other chemotherapeutic agents should be noted. Several studies have demonstrated that there is no cross‐resistance between platinum drugs and antimicrotubule agents.56, 57 Consistent with the findings in previous studies, MMAF was effective in A2780cisR and SKOV3cisR cells (Table S4). Based on this finding, a CD70‐ADC conjugated with MMAF was generated in this study. CD70‐ADC significantly inhibited the proliferation of CD70‐expressing OvCA cells in vitro and in vivo and the tolerability of CD70‐ADC has been widely investigated in previous studies.21, 22, 25, 58 Clinical trials are warranted to examine the efficacy of CD70‐ADC in recurrent OvCA.
4.3. Clinical implication
To the best of our knowledge, the mechanism of CD70 induction after cisplatin exposure has not been examined. Some transcription factors related to proliferation and platinum resistance may facilitate cell survival during cisplatin exposure; however, they seem not to induce CD70.
The transcription factors c‐Jun and NF‐κB have been reported to induce CD70 expression.59 As c‐Jun was not expressed after cisplatin exposure in A2780 cells, we focused on NF‐κB. Notably, quantitative RT‐PCR assays showed that CD70 mRNA was not elevated in A2780‐NF‐κB‐p65‐silenced cells, whereas CD70 mRNA was elevated in A2780 mock‐transfected cells. These results suggested that NF‐κB‐p65 expression may play a role in inducing CD70. Moreover, CD70 expression following platinum drug administration is an NF‐κB‐mediated pathway that may not be specific to OvCA cells, therefore this finding is crucial as it could be applicable to other malignancies.
4.4. Research implications
Although CD70 was not found to be associated with proliferation, previous studies involving murine fibrosarcoma, melanoma, and colon cancer models have shown that CD70‐CD27 signaling increases the number of CD4+‐CD25+‐Treg cells and promotes tumor growth and metastasis in vivo.60 To investigate the effect of CD70 on OvCA tumor prognosis in vivo, further investigation is warranted.
Our study focused on the inhibitory effect of CD70‐ADC in OvCA cells. Based on our immunohistochemistry data, we hypothesized that platinum administration may induce CD70 expression in women with OvCA and this finding was confirmed in vitro. These findings suggested that platinum‐based NACT followed by CD70‐ADC administration may be effective therapies. Therefore, CD70‐ADC could ultimately be effective for approximately 70% of patients with advanced OvCA (CD70‐high/low).
Targeting platinum drug‐induced membrane proteins via ADC is a novel concept. The relative and absolute quantification and comparison of membrane proteins in OvCA cells using methods such as isobaric tags before and after platinum treatment35, 61, 62 would be important to establish and design new treatment modalities for this disease.
4.5. Strengths and limitations
To our knowledge, this is the first study to demonstrate that CD70 is induced in OvCA cells treated with cisplatin. Moreover, CD70‐ADC demonstrated a therapeutic effect against CD70‐expressing OvCA cells.
However, several limitations should be noted. First, CD70 expression was not examined in platinum‐resistant OvCA clinical samples because they could not be obtained, therefore we could not determine the efficacy of CD70‐ADC in a patient‐derived xenograft mouse model of platinum‐resistant OvCA. Second, as a human antibody was used to generate CD70‐ADC, the adverse effects of CD70‐ADC in xenograft models could not be investigated. As similar CD70‐ADCs have been reported to be tolerable in clinical trials, ours may also be safe. Third, a multivariate analysis could not be performed between the CD70‐high and CD70‐low/negative groups because of the small sample size, therefore further studies including a larger number of cases are needed to assess the role of CD70 in OvCA prognosis.
Fourth, although CD70 was not associated with platinum resistance in vitro examination, the association between platinum/chemo resistance and the CD70 expression could not be determined because of the limited samples and various NACT regimens. Further studies that would examine the effect of CD70 expression on chemo resistance in clinical OvCA samples are warranted.
5. CONCLUSION
CD70‐ADC inhibits the proliferation of CD70 expressing OvCA cells both in vitro and in vivo. As the tolerability of CD70‐ADC has been demonstrated in phase‐1/2 clinical trials for treatment refractory malignancies, data from our preclinical OvCA models merit further investigation for OvCA in a clinical trial setting.
6. ETHICAL COMMITTEE APPROVAL
Osaka University Institutional Review Board (No. 18236).
DISCLOSURE
Honorarium, Chugai, textbook editorial expense, Springer, investigator meeting attendance expense, VBL Therapeutics (KM), research grant, MSD (Sh.M.). Other authors report no conflicts of interest concerning the materials or methods used in this review or the findings specified in this paper. The authors have no competing financial interests related to this study.
Supporting information
Fig S1‐S3
Table S1‐S5
Document S1
Shiomi M, Matsuzaki S, Serada S, et al. CD70 antibody‐drug conjugate: A potential novel therapeutic agent for ovarian cancer. Cancer Sci. 2021;112:3655–3668. 10.1111/cas.15027
Funding information
This work was supported by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (17K11278 to SM, 16K11139 to TK).
Contributor Information
Shinya Matsuzaki, Email: zacky@gyne.med.osaka-u.ac.jp.
Satoshi Serada, Email: serada@kochi-u.ac.jp.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7‐30. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin LA, Huang B, Miller RW, et al. Ten‐year relative survival for epithelial ovarian cancer. Obstet Gynecol. 2012;120:612‐618. [DOI] [PubMed] [Google Scholar]
- 3.Matsuzaki S, Serada S, Morimoto A, et al. Annexin A4 is a promising therapeutic target for the treatment of platinum‐resistant cancers. Expert Opin Ther Targets. 2014;18:403‐414. [DOI] [PubMed] [Google Scholar]
- 4.Markman M, Rothman R, Hakes T, et al. Second‐line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991;9:389‐393. [DOI] [PubMed] [Google Scholar]
- 5.Davis A, Tinker AV, Friedlander M. "Platinum resistant" ovarian cancer: what is it, who to treat and how to measure benefit? Gynecol Oncol. 2014;133:624‐631. [DOI] [PubMed] [Google Scholar]
- 6.Bogani G, Lopez S, Mantiero M, et al. Immunotherapy for platinum‐resistant ovarian cancer. Gynecol Oncol. 2020;158:484‐488. [DOI] [PubMed] [Google Scholar]
- 7.Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti‐PD‐1 antibody, nivolumab, in patients with platinum‐resistant ovarian cancer. J Clin Oncol. 2015;33:4015‐4022. [DOI] [PubMed] [Google Scholar]
- 8.Pujade‐Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum‐resistant recurrent ovarian cancer: The AURELIA open‐label randomized phase III trial. J Clin Oncol. 2014;32:1302‐1308. [DOI] [PubMed] [Google Scholar]
- 9.Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69:280‐304. [DOI] [PubMed] [Google Scholar]
- 10.Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495‐2505. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo K, Lin YG, Roman LD, Sood AK. Overcoming platinum resistance in ovarian carcinoma. Expert Opin Investig Drugs. 2010;19:1339‐1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birrer MJ, Moore KN, Betella I, Bates RC. Antibody‐drug conjugate‐based therapeutics: state of the science. J Natl Cancer Inst. 2019;111:538‐549. [DOI] [PubMed] [Google Scholar]
- 13.Lee EK, Liu JF. Antibody‐drug conjugates in gynecologic malignancies. Gynecol Oncol. 2019;153:694‐702. [DOI] [PubMed] [Google Scholar]
- 14.U. S. National Library of Medicine . ClinicalTrials.gov. https://clinicaltrials.gov/ (accessed 9/10/2020).
- 15.Polakis P. Antibody drug conjugates for cancer therapy. Pharmacol Rev. 2016;68:3‐19. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin RG, Alderson MR, Smith CA, et al. Molecular and biological characterization of a ligand for CD27 defines a new family of cytokines with homology to tumor necrosis factor. Cell. 1993;73:447‐456. [DOI] [PubMed] [Google Scholar]
- 17.Hintzen RQ, Lens SM, Koopman G, Pals ST, Spits H, van Lier RA . CD70 represents the human ligand for CD27. Int Immunol. 1994;6:477‐480. [DOI] [PubMed] [Google Scholar]
- 18.Grewal IS. CD70 as a therapeutic target in human malignancies. Expert Opin Ther Targets. 2008;12:341‐351. [DOI] [PubMed] [Google Scholar]
- 19.Borst J, Hendriks J, Xiao Y. CD27 and CD70 in T cell and B cell activation. Curr Opin Immunol. 2005;17:275‐281. [DOI] [PubMed] [Google Scholar]
- 20.Wajant H. Therapeutic targeting of CD70 and CD27. Expert Opin Ther Targets. 2016;20:959‐973. [DOI] [PubMed] [Google Scholar]
- 21.McEarchern JA, Smith LM, McDonagh CF, et al. Preclinical characterization of SGN‐70, a humanized antibody directed against CD70. Clin Cancer Res. 2008;14:7763‐7772. [DOI] [PubMed] [Google Scholar]
- 22.Tannir NM, Forero‐Torres A, Ramchandren R, et al. Phase I dose‐escalation study of SGN‐75 in patients with CD70‐positive relapsed/refractory non‐Hodgkin lymphoma or metastatic renal cell carcinoma. Invest New Drugs. 2014;32:1246‐1257. [DOI] [PubMed] [Google Scholar]
- 23.Nakae R, Matsuzaki S, Serada S, et al. CD70 antibody‐drug conjugate as a potential therapeutic agent for uterine leiomyosarcoma. Am J Obstet Gynecol. 2021;224:197.e1‐197.e23. [DOI] [PubMed] [Google Scholar]
- 24.Israel BF, Gulley M, Elmore S, Ferrini S, Feng WH, Kenney SC. Anti‐CD70 antibodies: a potential treatment for EBV+ CD70‐expressing lymphomas. Mol Cancer Ther. 2005;4:2037‐2044. [DOI] [PubMed] [Google Scholar]
- 25.Law CL, Gordon KA, Toki BE, et al. Lymphocyte activation antigen CD70 expressed by renal cell carcinoma is a potential therapeutic target for anti‐CD70 antibody‐drug conjugates. Cancer Res. 2006;66:2328‐2337. [DOI] [PubMed] [Google Scholar]
- 26.McEarchern JA, Oflazoglu E, Francisco L, et al. Engineered anti‐CD70 antibody with multiple effector functions exhibits in vitro and in vivo antitumor activities. Blood. 2007;109:1185‐1192. [DOI] [PubMed] [Google Scholar]
- 27.Bayat P, Taghdisi SM, Rafatpanah H, Abnous K, Ramezani M. In vitro selection of CD70 binding aptamer and its application in a biosensor design for sensitive detection of SKOV‐3 ovarian cells. Talanta. 2019;194:399‐405. [DOI] [PubMed] [Google Scholar]
- 28.Ryan MC, Kostner H, Gordon KA, et al. Targeting pancreatic and ovarian carcinomas using the auristatin‐based anti‐CD70 antibody‐drug conjugate SGN‐75. Br J Cancer. 2010;103:676‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michie CO, Sakala M, Rivans I, Strachan MW, Clive S. The frequency and severity of capecitabine‐induced hypertriglyceridaemia in routine clinical practice: a prospective study. Br J Cancer. 2010;103:617‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aggarwal S, He T, Fitzhugh W, et al. Immune modulator CD70 as a potential cisplatin resistance predictive marker in ovarian cancer. Gynecol Oncol. 2009;115:430‐437. [DOI] [PubMed] [Google Scholar]
- 31.Prat J, Oncology FCoG. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124:1‐5. [DOI] [PubMed] [Google Scholar]
- 32.Kakuda M, Matsuzaki S, Ueda Y, et al. Copper ions are novel therapeutic agents for uterine leiomyosarcoma. Am J Obstet Gynecol. 2020;222(64):e1–64 e16. [DOI] [PubMed] [Google Scholar]
- 33.Matsuzaki S, Serada S, Hiramatsu K, et al. Anti‐glypican‐1 antibody‐drug conjugate exhibits potent preclinical antitumor activity against glypican‐1 positive uterine cervical cancer. Int J Cancer. 2018;142:1056‐1066. [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama T, Enomoto T, Serada S, et al. Plasma membrane proteomics identifies bone marrow stromal antigen 2 as a potential therapeutic target in endometrial cancer. Int J Cancer. 2013;132:472‐484. [DOI] [PubMed] [Google Scholar]
- 35.Hiramatsu K, Serada S, Enomoto T, et al. LSR antibody therapy inhibits ovarian epithelial tumor growth by inhibiting lipid uptake. Cancer Res. 2018;78:516‐527. [DOI] [PubMed] [Google Scholar]
- 36.Jilaveanu LB, Sznol J, Aziz SA, Duchen D, Kluger HM, Camp RL. CD70 expression patterns in renal cell carcinoma. Hum Pathol. 2012;43:1394‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4:2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuzaki S, Enomoto T, Serada S, et al. Annexin A4‐conferred platinum resistance is mediated by the copper transporter ATP7A. Int J Cancer. 2014;134:1796‐1809. [DOI] [PubMed] [Google Scholar]
- 39.Loh SY, Mistry P, Kelland LR, Abel G, Harrap KR. Reduced drug accumulation as a major mechanism of acquired resistance to cisplatin in a human ovarian carcinoma cell line: circumvention studies using novel platinum (II) and (IV) ammine/amine complexes. Br J Cancer. 1992;66:1109‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Creative Biolabs . CD70 Therapeutic Antibody (Vorsetuzumab) https://www.creativebiolabs.net/Anti‐Human‐CD70‐Therapeutic‐Antibody‐vorsetuzumab‐13746.htm (accessed on 05/29/2021)
- 41.Nishigaki T, Takahashi T, Serada S, et al. Anti‐glypican‐1 antibody‐drug conjugate is a potential therapy against pancreatic cancer. Br J Cancer. 2020;122:1333‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuo K, Matsuzaki S, Nusbaum DJ, et al. Possible candidate population for neoadjuvant chemotherapy in women with advanced ovarian cancer. Gynecol Oncol. 2021;160:32‐39. [DOI] [PubMed] [Google Scholar]
- 43.Meyer LA, He W, Sun CC, et al. Neoadjuvant chemotherapy in elderly women with ovarian cancer: Rates of use and effectiveness. Gynecol Oncol. 2018;150:451‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer LA, Cronin AM, Sun CC, et al. Use and effectiveness of neoadjuvant chemotherapy for treatment of ovarian cancer. J Clin Oncol. 2016;34:3854‐3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rauh‐Hain JA, Melamed A, Wright A, et al. Overall survival following neoadjuvant chemotherapy vs primary cytoreductive surgery in women with epithelial ovarian cancer: analysis of the National Cancer Database. JAMA Oncol. 2017;3:76‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943‐953. [DOI] [PubMed] [Google Scholar]
- 47.Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open‐label, randomised, controlled, non‐inferiority trial. Lancet. 2015;386:249‐257. [DOI] [PubMed] [Google Scholar]
- 48.Matsuo K, Eno ML, Im DD, Rosenshein NB. Chemotherapy time interval and development of platinum and taxane resistance in ovarian, fallopian, and peritoneal carcinomas. Arch Gynecol Obstet. 2010;281:325‐328. [DOI] [PubMed] [Google Scholar]
- 49.Rauh‐Hain JA, Nitschmann CC, Worley MJ Jr, et al. Platinum resistance after neoadjuvant chemotherapy compared to primary surgery in patients with advanced epithelial ovarian carcinoma. Gynecol Oncol. 2013;129:63‐68. [DOI] [PubMed] [Google Scholar]
- 50.Stewart D, Cristea M. Antibody‐drug conjugates for ovarian cancer: current clinical development. Curr Opin Obstet Gynecol. 2019;31:18‐23. [DOI] [PubMed] [Google Scholar]
- 51.Gemtuzumab Ozogamicin Makes a Comeback. Cancer Discov. 2017;7:1208. [DOI] [PubMed] [Google Scholar]
- 52.Kalli KR, Oberg AL, Keeney GL, et al. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol. 2008;108:619‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore KN, Martin LP, O'Malley DM, et al. Safety and activity of mirvetuximab soravtansine (IMGN853), a folate receptor alpha‐targeting antibody‐drug conjugate, in platinum‐resistant ovarian, fallopian tube, or primary peritoneal cancer: a phase I expansion study. J Clin Oncol. 2017;35:1112‐1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Owonikoko TK, Hussain A, Stadler WM, et al. First‐in‐human multicenter phase I study of BMS‐936561 (MDX‐1203), an antibody‐drug conjugate targeting CD70. Cancer Chemother Pharmacol. 2016;77:155‐162. [DOI] [PubMed] [Google Scholar]
- 55.Pal SK, Forero‐Torres A, Thompson JA, et al. A phase 1 trial of SGN‐CD70A in patients with CD70‐positive, metastatic renal cell carcinoma. Cancer. 2019;125:1124‐1132. [DOI] [PubMed] [Google Scholar]
- 56.Sonego M, Pellizzari I, Dall'Acqua A, et al. Common biological phenotypes characterize the acquisition of platinum‐resistance in epithelial ovarian cancer cells. Sci Rep. 2017;7:7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Busschots S, O'Toole S, O'Leary JJ, Stordal B. Carboplatin and taxol resistance develops more rapidly in functional BRCA1 compared to dysfunctional BRCA1 ovarian cancer cells. Exp Cell Res. 2015;336:1‐14. [DOI] [PubMed] [Google Scholar]
- 58.Oflazoglu E, Stone IJ, Gordon K, et al. Potent anticarcinoma activity of the humanized anti‐CD70 antibody h1F6 conjugated to the tubulin inhibitor auristatin via an uncleavable linker. Clin Cancer Res. 2008;14:6171‐6180. [DOI] [PubMed] [Google Scholar]
- 59.Sanchez‐Perez I, Benitah SA, Martinez‐Gomariz M, Lacal JC, Perona R. Cell stress and MEKK1‐mediated c‐Jun activation modulate NFkappaB activity and cell viability. Mol Biol Cell. 2002;13:2933‐2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Claus C, Riether C, Schurch C, Matter MS, Hilmenyuk T, Ochsenbein AF. CD27 signaling increases the frequency of regulatory T cells and promotes tumor growth. Cancer Res. 2012;72:3664‐3676. [DOI] [PubMed] [Google Scholar]
- 61.Hara H, Takahashi T, Serada S, et al. Overexpression of glypican‐1 implicates poor prognosis and their chemoresistance in oesophageal squamous cell carcinoma. Br J Cancer. 2016;115:66‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hiramatsu K, Yoshino K, Serada S, et al. Similar protein expression profiles of ovarian and endometrial high‐grade serous carcinomas. Br J Cancer. 2016;114:554‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S3
Table S1‐S5
Document S1


