Abstract
Chimeric antigen receptors (CARs) have a unique facet of synthetic biology and offer a paradigm shift in personalized medicine as they can use and redirect the patient's immune cells to attack cancer cells. CAR‐natural killer (NK) cells combine the targeted specificity of antigens with the subsequent intracellular signaling ability of the receptors to increase their anti‐cancer functions. Importantly, CAR‐NK cells can be utilized as universal cell‐based therapy without requiring human leukocyte antigen (HLA) matching or earlier contact with tumor‐associated antigens (TAAs). Indeed, CAR‐NK cells can be adapted to recognize various antigens, hold higher proliferation capacity, and in vivo persistence, show improved infiltration into the tumors, and the ability to overcome the resistant tumor microenvironment leading to sustained cytotoxicity against tumors. Accumulating evidence from recent in vivo studies rendering CAR‐NK cell anti‐cancer competencies renewed the attention in the context of cancer immunotherapy, as these redirected effector cells can be used in the development of the “off‐the‐shelf” anti‐cancer immunotherapeutic products. In the current review, we focus on the therapeutic efficacy of CAR‐NK cell therapies for treating various human malignancies, including hematological malignancies and solid tumors, and will discuss the recent findings in this regard, with a special focus on animal studies.
Keywords: cancer, CAR‐NK, chimeric antigen receptors, immunotherapy, natural killer cells
In the current review, we focus on the therapeutic efficacy of CAR‐NK cell therapies for treating various human malignancies, including hematological malignancies and solid tumors, and will discuss the recent findings in this regard, with a special focus on animal studies.
1. INTRODUCTION
In recent years, natural killer (NK) cells, first defined by Herberman in 1976,1 have been described as effector cells like cytotoxic T lymphocytes (CTLs), which elicit natural cytotoxicity toward primary malignant cells and metastatic cells by suppressing the proliferation and migration of tumor cells.2 Regardless of their cytotoxic attributes, NK cells can produce several cytokines, in particular interferon‐γ (IFN‐γ), to moderate adaptive immune responses and contribute to other associated axis.2, 3 Most importantly, NK cells can distinguish malignant cells from healthy ones, providing a more selective anti‐tumor cytotoxicity and mitigating off‐target problems. Indeed, throughout development in bone marrow (BM), a process called education selects for NK cells that engage their immunoreceptor tyrosine‐based inhibitory motifs (ITIMs) upon detection of major histocompatibility complex I (MHC‐I), and shapes NK functional maturation and self‐tolerance.4 Cancer cells, which downregulate MHC‐I molecules as an immune evasion mechanism, can still be targeted by NK cells. NK cells respond to tumor cells, which usually express non‐classical MHC molecules such as MHC class I polypeptide‐related sequence A/B (MICA/MICB) and UL16‐binding proteins (ULBP), thereby sustaining the NK cell's use as an attractive anti‐cancer approach.5
Although NK cells can detect and eliminate most cancer cells, some cancer cells advance their mechanisms to avoid detection by NK cells or block NK cell activities. For instance, cancer cells secrete immunosuppressive molecules (eg, interleukin‐10 [IL‐10] and transforming growth factor‐beta [TGF‐β]), which suppress NK cell function.6 Furthermore, either attenuation of the expression of tumor‐associated antigens (TAAs) or downregulation of MHC‐I have been seen as other mechanisms applied by tumor cells for evasion from immune surveillance.7 A myriad of in vitro and in vivo studies has shown that NK cell engineering to express a chimeric antigen receptor (CAR) may defeat immune escape.8, 9, 10 Undoubtedly, the progress of “off‐the‐shelf” CAR‐modified NK cells is an exclusively encouraging and unique strategy by which to evolve a new generation of anti‐cancer immunotherapeutic products.10 CAR‐NK cells kill cancer cells not only via CAR, which selectively identifies TAAs, but also via NK cell receptors themselves. Recent preclinical and clinical studies have supported the idea that CAR‐NK cell therapy can exert the desired outcomes in both human hematological and solid malignancies, signifying their broad clinical applications.11, 12, 13 Here, we will highlight the importance of the development of CAR‐NK cell therapy as an innovative strategy in the context of cancer immunotherapy, and provide a brief overview of the recent findings on CAR‐NK cell therapy in human malignancies, focusing on preclinical studies.
2. NK CELL‐MEDIATED IMMUNOSURVEILLANCE OF CANCER
NK cell identification of cancerous cells is a firmly controlled procedure comprising the communication of particular ligands on the cancerous cells with NK cell receptors and resulting elicitation of signals derived from these receptors in the effector NK cells.14 Direct cancer cell elimination by NK cells is believed to be mainly perforin dependent, as widely evidenced in a variety of experimental model systems. Nonetheless, NK cells can also eradicate tumor cells by death receptor‐mediated axes including tumor necrosis factor (TNF)‐related apoptosis‐inducing ligand (TRAIL) and Fas ligand (FasL).15 Moreover, effector NK cells secrete various cytokines and chemokines, which trigger other innate and adaptive immune cells responses. NK cell‐mediated elimination of primary acute myeloid leukemia (AML) blasts has been shown with several NK cell preparations such as in vitro‐expanded allogeneic NK cells.16, 17, 18 It seems that killer cell immunoglobulin‐like receptors (KIR) mismatching with their ligand (KIR‐L) largely contributes to cancer cell lysis. The elimination of AML cells by NK cells is predominantly demonstrated in monoblastic cells that express natural killer group 2 member D (NKG2D) ligands, while cells that miss the corresponding ligands are resistant to NK eradication.17 Most studies supporting the concept that NK cells contribute to the lysis of cancer cells have been carried out by inserting syngeneic cancerous cells in mice that either were genetically deficient in NK cell activation or had exhausted NK cells due to the injection of antibodies.19 For instance, Kim et al found that despite having the functionally normal B, T, and natural killer T (NKT) cells, transgenic mice with defective natural killing and specific lack of NK1.1 (CD161)‐positive CD3‐negative cells, could not abrogate the growth of cancerous cells, suggesting that NK1.1‐positive CD3‐negative cells are liable to undergo acute tumor rejection.20
Regardless of the supportive role of NKG2D in NK cell‐mediated immunosurveillance of human cancers, its role in NK cell proliferation and development is debatable. Some studies in NKG2D‐deficient mice revealed that NKG2D is not required for NK cell proliferation but that it is crucial for immunosurveillance of epithelial and lymphoid malignancies in transgenic models. In addition, in a prostate tumor model, aggressive tumors established in NKG2D‐deficient mice showed more prominent levels of NKG2D ligands compared with similar tumors in wild‐type mice, indicating a lack of selective pressure by NKG2D‐mediated responses.21, 22, 23, 24 Other reports delivered proof for the importance of NKG2D in NK cell growth and suggested that the lack of the NKG2D resulted in stunted proliferation of NK cells, and further reinforced their susceptibilities to apoptosis.25
3. NK CELLS DYSFUNCTIONALITY IN CANCERS
Tumor cells, tumor‐associated fibroblasts (TAF), macrophages, dendritic cells (DCs), T cells and also other types of cell can generate immunosuppressive molecules or promote expression of a myriad of receptors, which facilitate moderation of the activation and anti‐tumor function of NK cells; however, this suppressive effect can be defeated using CAR‐NK cells (Figure 1).26 Regulatory T cells (Treg cells) and myeloid‐derived suppressor cells (MDSC) can constrain NK cell activation by various mechanisms. Correspondingly, the MDSCs can shape a tumor permissive environment, and also affect tumor onset, angiogenesis, and development by negative regulation of NK cells.27 The MDSCs from cancer patients can suppress NK cell Fc receptors (FcRs)‐mediated activities, encompassing antibody‐dependent cellular cytotoxicity (ADCC), cytokine releases, and signal transduction.28 As well, during tumor progression, the expression of activating NK cell receptors such as natural killer protein 30 (NKp30), NKG2D, and CD16 is decreased; whereas the expression of inhibitory receptors such as NKG2A is augmented. NKG2A can robustly abrogate NK cell activities, most markedly cytotoxicity. Nonetheless, some splicing of NKp30 could function as inhibitory receptor. Conversely, various stromal‐derived factors such as TGF‐β1 participate in tumor‐mediated suppression of NK cells.29, 30
FIGURE 1.
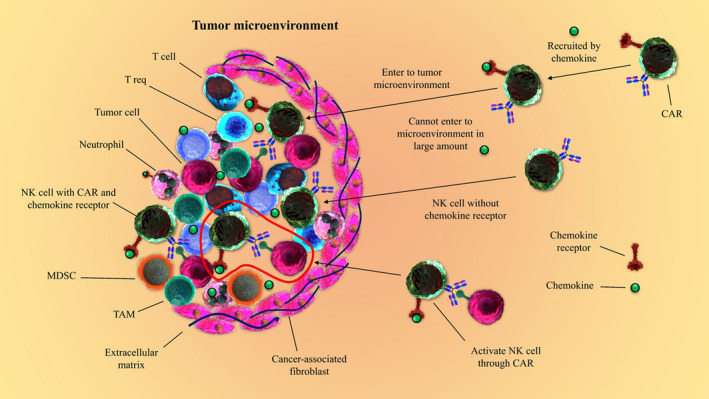
NK cell dysfunction in the tumor microenvironment (TME). Varied immunosuppressive molecules secreted by tumor cells, tumor‐associated macrophages (TAM), myeloid‐derived suppressor cells (MDSC), regulatory T‐cells (Treg), dendritic cells (DC), and T cells and also other types of cells presented in TME can stimulate suppressive effects on NK cell functions and proliferation. Nonetheless, using chimeric antigen receptor‐natural killer (CAR‐NK) cells can result in overcoming the NK cell dysfunction in TME. Moreover, engineered CAR‐NK cells to overexpress specific chemokine receptors can support the CAR‐NK cell more proficient homing to tumor tissue and accordingly may lead to more reliable therapeutic outcomes
Toll‐like receptor 4 (TLR4) activation in cancer cells enables the secretion of various soluble factors and proteins including IL‐6, IL‐12, inducible nitric oxide synthase (iNOS), B7‐H1, and B7‐H2, which leads to cancer cell resistance against CTL attack. Similarly, the weakened suppressive effects of tumor cells on NK cells both in vitro and in vivo following blockage of TLR4, suggested that TLR signaling could induce a cascade sustaining tumor evasion from immunosurveillance.31
Furthermore, the evasion from immunosurveillance may result from the higher expression of C–C chemokine receptor type 4 (CCR4) on the surface of cancer cells. In non‐small‐cell lung carcinoma (NSCLC) patients, CCR4 expression in a tumor microenvironment enables CCR4 communication with chemokines CCL17 and CCL22 that may improve Treg cells recruitment to tumor sites and subsequently hinder NK cell function.32
Interestingly, it has been shown that tumor‐infiltrating NK cells in human liver tumors have small and fragmented mitochondria, while NK cells outside tumors and also peripheral NK cells have normal mitochondria, suggesting that the NK cell dysfunction in the tumor area can be ensued from their phenotypic impairments obstructing corresponding anti‐tumor function. Mitochondrial fragmentation may occur due to the hypoxic tumor microenvironment that commonly induces activation of the mechanistic target of rapamycin‐GTPase dynamin‐related protein 1 (mTOR‐Drp1) in NK cells. This is evident by amelioration of mitochondrial fragmentation, survival, and anti‐tumor competencies of NK cells following the blockade of mTOR‐Drp1.33
Lastly, the generation of soluble lactate dehydrogenase 5 (LDH5) by malignant cells, such as glioblastoma cells, has been found to induce NKG2D ligands on the surface of healthy myeloid cells. Expression of NKG2D ligands by healthy myeloid cells may barricade activation of NKG2D receptor on NK cells, therefore averting identification of NKG2D ligand‐bearing tumors and modifying the anti‐tumor response.34
4. CARS STRUCTURE
A CAR is a synthetic hybrid antigen receptor typically consisting of variable regions of an antibody accompanying by a constant region of a T‐cell receptor (TCR), enabling the immune cell to selectively recognize TAA expressed on tumor cells. A classic CAR has a signal peptide, single‐chain antibody variable fragment (scFv), transmembrane (TM) and intracellular signaling domains.35 The scFvs are derived from a tumor‐specific antibody and can interact with that specific antigen presented on the cancer cell surface, while the intracellular signaling domains are derived from immunoreceptor tyrosine‐based activation motifs (ITAMs) of TCR or other stimulating receptor's cytoplasmic domains.36
Due to inability of first‐generation CARs containing only CD3ζ to effectively stimulate tumor eradication upon T‐cell activation, second‐generation CARs encompassing T‐cell co‐stimulatory molecules, including CD28, 4‐1BB (CD137), ICOS, or OX40 (CD134) in addition to CD3ζ were established.37 After that, third‐generation CARs including 3 signaling domains, for instance, CD3ζ and 2 co‐stimulatory domains were designed and developed. Although there is no reliable proof to signify an enhanced performance in the third—in comparison with the second—generation CARs, it has been suggested that CARs containing 4‐1BB co‐activation can exert better T‐cell memory features, while CD28 co‐activation elicits superior T‐cell activation and expansion.38
CAR constructs are typically inserted into a retrovirus‐ or lentivirus‐based expression vector, which was subsequently applied for transduction of primary NK cells or NK cell lines, most commonly NK‐92, an interleukin‐2 (IL‐2)‐dependent NK cell line isolated from peripheral blood mononuclear cells (PBMCs) (Figure 2).39 As current trials based on NK cell immunotherapy request high NK cell doses and commonly multiple administrations, the achievement of the optimized protocol for NK cell expansion is of paramount importance. In addition, several complicated NK cell expansion approaches have recently been established. NK cell expansion using either IL‐2 or IL‐15 or both to produce 1000‐fold expansion needs a cultivation duration of up to 3 mo.40 However, one feeder cell‐based NK expansion strategy using genetically modified K562 cells provided large numbers of NK cells after a 2‐wk expansion period.40
FIGURE 2.
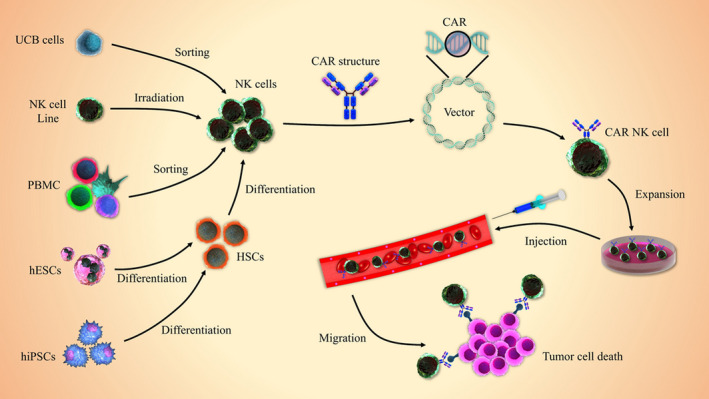
Generation process of CAR‐NK cells. Isolated or established NK cells from diverse sources can be modified with CAR‐expressing vectors (eg, lentivirus or retrovirus) and then expanded in NK cell‐specific expansion media. The established CAR‐NK cells are commonly injected via an intravenous route to selectively kill tumor cells. Umbilical cord blood (UCB), peripheral blood (PB), human induced pluripotent stem cell (hiPSCs), human embryonic stem cell (hESC), hematopoietic stem cell (HSC), chimeric antigen receptor‐natural killer (CAR‐NK) cells
5. CAR‐NK CELLS IN HEMATOLOGICAL MALIGNANCIES
5.1. Leukemia
Malignant leukemic cells can evade NK cell responses as a result of both NK cell abnormalities and immunosuppressive properties of leukemic cells. It has been found that NK‐92 cells engineered with anti‐CD33 CAR (CD33‐CAR‐NK‐92) cells have advantages over CAR‐T cells in terms of cytotoxicity against CD33‐positive leukemic cells.13 Additionally, CD33‐CAR‐NK‐92 cells demonstrated superior toxicity over parental NK‐92 cells against the human AML cell line, HL‐60, and also were safe for patients who were suffering from relapsed and refractory AML.13 In this regard, Salman et al found that CD4‐CAR‐NK cells injected into a systemic AML implanted murine model, although experiencing shorter in vivo persistence, could robustly stimulate anti‐leukemic effects.41 Also, a study in the FMS‐like tyrosine kinase 3 (FLT3)‐specific CAR‐NK cells respecting common overexpression of FLT3 in B‐ALL demonstrated that the injection of redirected NK cells, but not parental NK‐92 cells, markedly abrogated disease development in a B‐ALL NOD‐SCID IL‐2Rγnull mice model, and thereby signified that B‐ALL could be targeted successfully by FLT3‐CAR‐NK cells.42
In a B‐cell lymphoma (BCL) model in NOD‐SCID IL‐2Rγnull mice, CD19‐CAR‐NK‐92 cells obstructed leukemia development, signifying the feasibility of generating CAR‐engineered NK‐92 cells with effective and targeted anti‐tumor activity.11 Similarly, the investigation of the anti‐cancer effects of redirected NK cells from adult peripheral blood (PB) and umbilical cord blood (UCB) against CD19‐positive leukemic cells revealed that CD19‐CAR‐PB‐NK cells were significantly better compared with CD19‐CAR‐UCB‐NK cells at the elimination of leukemic cells.43
CD7‐CAR‐NK‐92 presented selective and significant anti‐tumor activities toward T‐cell acute lymphoblastic leukemia (T‐ALL) cell lines and xenograft mouse models of T‐ALL, possibly mediated by the secretion of higher levels of granzyme B and IFN‐γ, supporting the idea that CAR‐NK cell therapy could be effective for the treatment of T‐cell‐related disorders as well.44
5.2. Myeloma and lymphoma
In addition to leukemia, redirected CAR‐NK cells can also support favorable outcomes in lymphomas and myelomas. Correspondingly, CD19‐CAR memory‐like (ML)‐NK cells exhibited potent anti‐tumor activities, evident by boosted IFN‐γ production, degranulation, and selective lysis, toward NK‐resistant lymphoma cell lines in vitro. Moreover, CD19‐CAR ML‐NK cells established from lymphoma patients demonstrated ameliorated responses against their autologous lymphomas, and also exhibited diminished lymphoma burden and promoted the overall survival rate in human xenograft models.45 In addition, CAR‐NK cells specific for CS1 (CD319), a surface protein largely expressed on multiple myeloma (MM) cells, improved specific CS1+MM cell elimination and IFN‐γ secretion in vitro. They also suppressed the proliferation of human IM9 MM cells and also extended the overall survival rate in an orthotopic MM xenograft murine model in vivo.12 Similarly, examination of the possible therapeutic benefits of the CD38‐specific nanobody‐based CAR (Nb‐CARs) showed that Nb‐CAR‐NK‐92 cells could trigger cytotoxicity against primary patient‐derived CD38‐expressing MM cells. In addition, redirected cells selectively killed CD38‐positive but not CD38‐deficient tumor cells, and also proficiently exhausted CD38‐positive MM cells in primary human BM samples.46
Similar to MM, CAR‐NK cells have shown promising results in the treatment of various types of non‐Hodgkin's lymphomas (NHLs), such as peripheral T‐cell lymphomas (PTCLs). In this regard, studies have demonstrated that CD4‐CAR‐NK‐92 cells could selectively lyse various CD4+ human T‐cell leukemia and lymphoma cell lines (eg, KARPAS‐299 and CCRF‐CEM) as well as patient samples ex vivo. More significantly, CD4‐CAR‐NK‐92 cells efficiently eliminated KARPAS‐299 cells in vivo and significantly prolonged the survival rate of xenograft mice,47 highlighting the importance of CD4 as a potential TAAs for T–cell‐related disorders. In another study, 2 specific CARs consisting of the low‐affinity Fc receptor CD16 or the high‐affinity Fc receptor CD64, with the addition of the CD8a extracellular domain, CD28 TM domains, 2 co‐stimulatory domains CD28 and 4‐1BB, and the signaling domain from CD3ζ were constructed and called CD16‐BB‐ζ and CD64‐BB‐ζ. Then, they were used to establish NK‐92MI cells expressing the corresponding CARs, named NK‐92MIhCD16 and NK‐92MIhCD64 cells. Markedly, both NK‐92MIhCD16 and NK‐92MIhCD64 cells induced higher cytotoxicity toward CD20‐expressing NHL cells when used in combination with rituximab, compared with rituximab treatment alone, thereby implying that CAR‐expressing NK‐92MI cells, an IL‐2‐independent NK cell line derived from the NK‐92 cell line by transfection, can sustain the clinical responses to conventional anti‐cancer monoclonal antibodies.48
Recently, phase 1 and phase 2 clinical trials were carried out using CD19‐CAR CB‐NK cells in 11 patients with relapsed or refractory CD19+ cancers (eg, NHL and chronic lymphocytic leukemia [CLL]). Based on observation, CAR‐NK cell infusion was not related to the incidence of cytokine release syndrome (CRS), neurotoxicity, or graft‐versus‐host disease (GVHD) commonly observed in CAR‐T‐cell therapy. Moreover, the majority of the patients showed a response to the treatment with CAR‐NK cells;49 however, to conclude on efficacy, execution of further trials is of paramount importance.
5.3. CAR‐NK cells in solid tumors
The EGF receptor (EGFR) is a well known tyrosine kinase receptor and remains at the front of targeted therapies toward tumors. In addition, reports have shown that EGFR‐specific CAR‐NK‐92 cells and also primary NK cells‐exerted notable cytotoxicity and IFN‐γ production in vitro upon exposure to breast cancer cell lines MDA‐MB‐231, MDA‐MB‐468, and MCF‐7, while EGFR‐specific CAR‐NK‐92 showed better therapeutic efficacy compared with primary NK cells. In a xenograft model of breast tumor, intra‐tumoral injection of either EGFR‐CAR‐NK‐92 cells or oncolytic herpes simplex‐1 (oHSV‐1), abrogated tumor development, while combination therapy with both EGFR‐CAR‐NK‐92 cells and oHSV‐1, caused more evident elimination of tumor cells and prolonged survival of the transplanted mice.50 In addition, EGFR‐CAR‐NK‐92 cell administration resulted in regression of triple‐negative breast cancer (TNBC) in breast cancer cell line‐derived xenograft (CLDX) and patient‐derived xenograft (PDX) mouse models.51 Similarly, in orthotopic glioblastoma (GB) xenograft mouse models, intracranial injection of EGFR‐CAR‐NK‐92 cells robustly repressed tumor development and consequently extended the overall survival rate of tumor‐bearing mice, introducing redirected NK cells a capable strategy to treat GB.52 Other studies have indicated that further genetic modification of these EGFR‐CAR‐NK cells with the chemokine receptor CXCR4, could improve chemotaxis to stromal cell‐derived factor 1 (SDF1) generating U87‐MG glioblastoma cells in vitro, and promoted survival in xenografts mice in comparison with treatment with EGFR‐CAR‐NK cells.53
Conversely, specific targeting of folate receptor alpha (αFR), which is overexpressed in 90% of ovarian cancers, through using the αFR‐specific CAR‐NK‐92 cells led to the selective lysis of αFR+ tumor cells in vitro and in a mouse xenograft model of ovarian cancer. Moreover, comparative analysis indicated that third‐generation αFR‐CAR‐NK‐92 cells were superior to first‐ and second‐generation CARs in terms of effective cytotoxicity against αFR+ tumor cells.54 Furthermore, mesothelin‐specific CAR‐NK cells could selectively eliminate mesothelin‐positive ovarian cancer cells (OVCAR‐3 and SK‐OV‐3), while they were non‐reactive against mesothelin‐negative cells (SK‐HEP‐1) in vitro. More importantly, redirected NK cells efficiently eradicated ovarian cancer cells in both subcutaneous and intraperitoneal tumor models, suggesting that mesothelin could be a feasible target for ovarian cancer targeted therapy in addition to αFR.55
Recently, other findings have suggested that co‐expression of chemokine receptor CXCR1 and CAR may deliver an innovative strategy to improve the therapeutic efficacy of NK cells in human ovarian tumors through enhancing tumor infiltration of effector immune cells. For example, administration of NK cells genetically modified to express CXCR1 and a CAR targeting NKG2D ligands demonstrated better migration against tumor cells in vivo in xenograft models. Furthermore, the CAR‐NK cell‐exerted cytotoxicity was not negatively affected by CXCR1 transgene expression, and ameliorated tumor trafficking of the administered cells led to boosted anti‐tumor reactions in xenografts.56
Ganglioside GD2‐specific CAR‐NK cells were found to significantly induce higher cytotoxicity toward GD2+ neuroblastoma (NB) cells compared with parental NK cells. In addition to the marked trafficking of GD2‐CAR‐NK cells into 3D tumor spheroids, which resulted in a reduced growth rate as evidenced by live‐cell imaging analysis, GD2‐CAR‐NK treatment repressed tumor development in mice,. Also, further analysis indicated that combination therapy with redirected NK cells and either histone deacetylase inhibitors (HDACi) or programmed cell death protein 1 (PD‐1)/programmed cell death ligand 1 (PD‐L1) blocker may serve to be more effective anti‐cancer functions.57
CAR‐modified NK cells are currently suggested as a reliable immunotherapeutic modality for hepatocellular carcinoma (HCC) treatment. Accordingly, glypican‐3 (GPC3)‐specific CAR‐NK‐92 cells co‐cultured with GPC3+ HCC cells caused noticeable in vitro cytotoxicity and cytokine release, and also stimulated anti‐tumor in vivo effects in HCC xenografts expressing GPC3 at high and low levels, but not in those without GPC3 expression. Correspondingly, abrogated tumor development concurrently enhanced tumor apoptosis in the GPC3+ HCC xenografts.58
Concerning the TGF‐β competence to suppress NK cell function, Wang et al genetically engineered NK‐92 cells to express a chimeric receptor with TGF‐β type II receptor extracellular and transmembrane domains concomitant with the intracellular domain of NKG2D, named as TN chimeric receptor. They found that TN‐expressing NK‐92 cells exhibited significant resistance against TGF‐β‐induced suppressive signaling, and also stimulated higher killing capacity and IFN‐γ generation against HCC cells in vitro.59 In addition, TN‐expressing NK‐92 cells prominently trafficked into TGF‐β‐expressing malignant tumors, and suppressed tumor progress in an HCC xenograft murine model, suggesting that the TN chimeric receptor could be applied to improve anti‐tumor efficacy in NK cell adoptive therapy.59
6. CONCLUSION AND PROSPECT
Based on collective evidence, CAR‐NK cell‐based cancer therapy has become an encouraging and advanced research area. Accordingly, a wide spectrum of animal studies has been carried out (Table 1) and several clinical trials are being accomplished (Table 2) to evaluate CAR‐NK cell clinical safety and efficacy in human cancers. However, the execution of large‐scale clinical studies is required to address the feasibility, safety, and efficacy of the CAR‐NK cell application in human malignancies, such as leukemia, myeloma, and lymphoma, and also solid tumors. Significantly, CRS and neurotoxicity are less likely to happen in CAR‐NK cell‐based immunotherapy compared with CAR‐T cells mainly because activated NK cells largely secrete IFN‐γ and GM‐CSF, while CAR‐T cells mostly stimulate cytokines, including IL‐1, IL‐2, IL‐6, TNF‐α, IL‐8, IL‐10, and IL‐15, which generally support CRS and severe neurotoxicity.10 In addition, the attenuated risk for GVHD occurrence even with allogeneic NK cells possibly permits CAR‐NK cells to be established from several sources, such as NK92 cell lines, PBMCs, UCB, and also pluripotent stem cells.60 Although a great deal of progress has been achieved in NK cell immunotherapy, clinical efficacy is still limited due to the short lifespan and low cytotoxicity of NK cells in vivo, highlighting the importance of finding a novel approach for overcoming this challenge. However, the risk of on‐target/off‐tumor toxicity to normal tissues is comparatively low because of the restricted lifespan of CAR‐NK cells in the circulation.61
TABLE 1.
CAR‐NK cell‐based therapy for human cancers (in vitro and in vivo reports)
| Condition | Target antigen | Results (ref) |
|---|---|---|
| Hematological malignancies | ||
|
ALL AML |
CD7 | Induction of specific targeting effect against CD7‐positive leukemic cells in vitro62 |
| MM | CS1 |
Induction of MM cytolysis and IFN‐γ production in vitro12 Inhibition of the growth of human IM9 MM cells leading to the prolonged survival rate of a xenograft mice model12 |
|
MM BL |
CD38 | Cytotoxicity against CD38‐positive cells in vitro9 |
| AML | CD33 | Verifying the feasibility and safety of CD33‐CAR‐NK cells in patients with AML13 |
| B‐ALL | CD19 |
NK cell degranulation and selective cytotoxicity toward leukemic cells in vitro11, 63 Inhibition of B‐ALL progress in NOD‐SCID IL2R γnull mice11 |
| AML | CD4 |
Eradication of CD4‐expressing AML cell lines in vitro41 Potent anti‐leukemic activities in a systemic AML murine model41 |
| B‐ALL | FLT3 | Suppression of B‐ALL development in a xenograft model in NOD‐SCID IL2R γnull mice8 |
| NHL | CD20 | Promoted cytotoxicity against CD20‐positive NHL cells in vitro48 |
| Solid tumors | ||
|
Breast cancer Gastric cancer Glioblastoma |
EGFR family |
Suppression of breast tumor cell proliferation in mice models51 Eradication of small but not larger gastric tumor xenografts64 Anti‐tumors activities in glioblastoma xenograft models in NSG mice65 |
| Colorectal cancer | EpCAM | Inhibition of tumor development in a xenograft model when used in combination with regorafenib66 |
|
Ovarian cancer Liver cancer |
Glypican‐3 |
Improvement of the overall survival of the mouse xenograft model of ovarian cancer67 Reduction in liver tumor growth and promotion of tumor cells apoptosis in liver tumor xenograft models58 |
| Ovarian cancer | αFR | Improved survival of tumor‐bearing mice by inhibition of tumor growth54 |
| Liver cancer | CD147 | Stimulation of the specific elimination of CD147‐positive tumor cells in transgenic mice model68 |
| Pancreatic cancer | Robo1 | Eliciting of specific anti‐tumor effects in an orthotopic nude mouse model69 |
|
Ovarian cancer Lung cancer Liver cancer |
NKG2D ligand |
Induction of anti‐tumor functions in ovarian cancer xenografts56 Showing the synergistic therapeutic efficacy in combination with CD73 targeting toward CD73‐positive lung cancer xenograft models70 Inhibition of tumor growth in liver cancer xenografts59 |
Abbreviations: ALL, Acute lymphoblastic leukemia; AML, Acute myeloid leukemia; BL, Burkitt Lymphoma; CAR‐NK, Chimeric antigen receptor‐expressing natural killer cell; EGFR, Epidermal growth factor receptor; EpCAM, Epithelial cell adhesion molecule; FLT3, FMS‐like tyrosine kinase 3; IFNγ, Interferon gamma; MM, Multiple myeloma; NHL, Non‐Hodgkin lymphoma; NKG2D, Natural killer group 2 member D; Robo1, Roundabout homolog 1; αFR, Folate receptor alpha.
TABLE 2.
Clinical trials based on CAR‐NK cell therapy for human cancers registered in ClinicalTrials.gov (March 2021)
| Condition | Dose | Antigen | Phase | Location | Participant number | NCT number |
|---|---|---|---|---|---|---|
| Hematological malignancies | ||||||
| NHL |
2 × 106/kg 6 × 106/kg 2 × 107/kg |
CD19 | Early 1 | China | 9 | NCT04639739 |
| BCL |
50 × 103/kg 600 × 103/kg |
CD22 | Early 1 | China | 9 | NCT03692767 |
| BCL |
50 × 103/kg 600 × 103/kg |
CD19 | Early 1 | China | 9 | NCT03690310 |
| BCL |
50 × 103/kg 600 × 103/kg |
CD19 CD22 |
Early 1 | China | 10 | NCT03824964 |
|
MCL DLBCL NHL FL |
NA | CD19 | 1/2 | USA | 0 | NCT03579927 (Withdrawn) |
| Solid tumors | ||||||
| Prostate Cancer |
0.5 × 107/kg 3 × 107/kg |
PSMA | Early 1 | China | 9 | NCT03692663 |
| Neuroblastoma |
3 × 106/kg 1 × 108/kg |
GD2 | 1 | USA | 0 | NCT02439788 (Withdrawn) |
| Ovarian cancer |
0.5 × 107/kg 3 × 107/kg |
Mesothelin | Early 1 | China | 30 | NCT03692637 |
| Various tumors | NA | Robo1 | 1/2 | China | 20 | NCT03940820 |
| Metastatic tumors | NA | NKG2D | 1 | China | 30 | NCT03940820 |
Abbreviations: BCL, B‐cell lymphoma; CAR‐NK, Chimeric antigen receptor‐expressing natural killer cell; DLBCL, Diffuse large B‐cell lymphoma; FL, Follicular lymphoma; GD2, Ganglioside GD2; MCL, Mantle cell lymphoma; NA, Not available; NHL, Non‐Hodgkin lymphoma; NKG2D, Natural killer group 2 member D; PSMA, Prostate‐specific membrane antigen; Robo1, Roundabout homolog 1.
In sum, it is speculated that the development of the CAR construction for superior NK cell‐elicited cytotoxicity, improving redirected cell infiltration into tumor microenvironments (TME), concomitant with developing CAR‐NK cells with memory phenotypes to offer prolonged immune surveillance, can lead to the desired anti‐cancer outcomes.
CONFLICT OF INTERESTS
There is no conflict of interests.
Marofi F, Abdul‐Rasheed OF, Rahman HS, et al. CAR‐NK cell in cancer immunotherapy; A promising frontier. Cancer Sci. 2021;112:3427–3436. 10.1111/cas.14993
REFERENCES
- 1.Herberman RB, Holden HT, Ting C‐C, Lavrin DL, Kirchner H. Cell‐mediated immunity to leukemia virus‐and tumor‐associated antigens in mice. Cancer Res. 1976;36(2 Part 2):615‐621. [PubMed] [Google Scholar]
- 2.Sivori S, Pende D, Quatrini L, et al. NK cells and ILCs in tumor immunotherapy. Mol Aspects Med. 2020;21:100870. [DOI] [PubMed] [Google Scholar]
- 3.Cheng M, Zhang J, Jiang W, Chen Y, Tian Z. Natural killer cell lines in tumor immunotherapy. Front Med. 2012;6(1):56‐66. [DOI] [PubMed] [Google Scholar]
- 4.Brodin P, Kärre K, Höglund P. NK cell education: not an on‐off switch but a tunable rheostat. Trends Immunol. 2009;30(4):143‐149. [DOI] [PubMed] [Google Scholar]
- 5.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor‐associated expression and recognition by tumor‐derived γδ T cells of MICA and MICB. Proc Natl Acad Sci. 1999;96(12):6879‐6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellone G, Turletti A, Artusio E, et al. Tumor‐associated transforming growth factor‐β and interleukin‐10 contribute to a systemic Th2 immune phenotype in pancreatic carcinoma patients. Am J Pathol. 1999;155(2):537‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villalba M, Rathore MG, Lopez‐Royuela N, Krzywinska E, Garaude J, Allende‐Vega N. From tumor cell metabolism to tumor immune escape. Int J Biochem Cell Biol. 2013;45(1):106‐113. [DOI] [PubMed] [Google Scholar]
- 8.Burger MC, Zhang C, Harter PN, et al. CAR‐Engineered NK Cells for the Treatment of Glioblastoma: Turning Innate Effectors Into Precision Tools for Cancer Immunotherapy. Front Immunol. 2019;10:2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hambach J, Riecken K, Cichutek S, et al. Targeting CD38‐Expressing Multiple Myeloma and Burkitt Lymphoma Cells In Vitro with Nanobody‐Based Chimeric Antigen Receptors (Nb‐CARs). Cells. 2020;9(2):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegler EL, Zhu Y, Wang P, Yang L. Off‐the‐shelf CAR‐NK cells for cancer immunotherapy. Cell Stem Cell. 2018;23(2):160‐161. [DOI] [PubMed] [Google Scholar]
- 11.Oelsner S, Friede ME, Zhang C, et al. Continuously expanding CAR NK‐92 cells display selective cytotoxicity against B‐cell leukemia and lymphoma. Cytotherapy. 2017;19(2):235‐249. [DOI] [PubMed] [Google Scholar]
- 12.Chu J, Deng Y, Benson DM, et al. CS1‐specific chimeric antigen receptor (CAR)‐engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28(4):917‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang X, Yang L, Li Z, et al. First‐in‐man clinical trial of CAR NK‐92 cells: safety test of CD33‐CAR NK‐92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res. 2018;8(6):1083. [PMC free article] [PubMed] [Google Scholar]
- 14.Liu P, Chen L, Zhang H. Natural killer cells in liver disease and hepatocellular carcinoma and the NK Cell‐based immunotherapy. J Immunol Res. 2018;2018:1206737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malmberg KJ, Carlsten M, Björklund A, Sohlberg E, Bryceson YT, Ljunggren HG. Natural killer cell‐mediated immunosurveillance of human cancer. Semin Immunol. 2017;31:20‐29. [DOI] [PubMed] [Google Scholar]
- 16.Liu LL, Béziat V, Oei VYS, et al. Ex vivo expanded adaptive NK cells effectively kill primary acute lymphoblastic leukemia cells. Cancer Immunol Res. 2017;5(8):654‐665. [DOI] [PubMed] [Google Scholar]
- 17.Diermayr S, Himmelreich H, Durovic B, et al. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK‐cell lines with single KIR‐HLA class I specificities. Blood. 2008;111(3):1428‐1436. [DOI] [PubMed] [Google Scholar]
- 18.Baba MA, Bouchriti Y, Achbani A, Kharbach A, Sine H, Naciri A. Risk of COVID‐19 for patients with cancer: A narrative overview. Eur J Med Educ Technol. 2020;13(3):em2008. [Google Scholar]
- 19.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27(45):5932‐5943. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell‐deficient mice. Proc Natl Acad Sci. 2000;97(6):2731‐2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerra N, Tan YX, Joncker NT, et al. NKG2D‐deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28(4):571‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikbakht‐Dastjerdi M, Rashidi I, Valiani A, Khanahmad H. Belinostat effects on expression of RBM5 tumor suppressor gene and inhibits prostate cancer cell line (PC3) proliferation. Electro J Gen Med. 2018;15(3):297–302. [Google Scholar]
- 23.Arı F, Çelikler S, Karakaş D, Cevatemre B, Fırat M, Ulukaya E. Total Phenolic Content, Antioxidant and Cyto‐/Genotoxic Activities of Pelargonium Quercetorum Agnew in Human Breast Cancer Cells. J Clin Exp Investig. 2017;8(1). [Google Scholar]
- 24.Zephaniah H, Asbestos AS. A silent potent killer. Eur J Environ Public Health. 2018;3(2):1078–1086. [Google Scholar]
- 25.Zafirova B, Mandarić S, Antulov R, et al. Altered NK cell development and enhanced NK cell‐mediated resistance to mouse cytomegalovirus in NKG2D‐deficient mice. Immunity. 2009;31(2):270‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An Y, Liu F, Chen Y, Yang Q. Crosstalk between cancer‐associated fibroblasts and immune cells in cancer. J Cell Mol Med. 2020;24(1):13‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruno A, Mortara L, Baci D, Noonan DM, Albini A. Myeloid derived suppressor cells interactions with natural killer cells and pro‐angiogenic activities: Roles in tumor progression. Front Immunol. 2019;10:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruno A, Mortara L, Baci D, Noonan DM, Albini A. Myeloid Derived Suppressor Cells Interactions With Natural Killer Cells and Pro‐angiogenic Activities: Roles in Tumor Progression. Front Immunol. 2019;10:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mamessier E, Sylvain A, Thibult ML, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Investig. 2011;121(9):3609‐3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamar G, Larkey LK, Howe N, Nidhi G. Coping with Women's Cancer and Perceived Providers' Support: Does Type of Cancer Make a Difference? Online J Commun Media Technol. 2015;5(1):41. [Google Scholar]
- 31.Huang B, Zhao J, Li H, et al. Toll‐like receptors on tumor cells facilitate evasion of immune surveillance. Can Res. 2005;65(12):5009‐5014. [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Wei X, Li L, et al. CCR4 mediated chemotaxis of regulatory T cells suppress the activation of T cells and NK cells via TGF‐β pathway in human non‐small cell lung cancer. Biochem Biophys Res Comm. 2017;488(1):196‐203. [DOI] [PubMed] [Google Scholar]
- 33.Zheng X, Qian Y, Fu B, et al. Mitochondrial fragmentation limits NK cell‐based tumor immunosurveillance. Nat Immunol. 2019;20(12):1656‐1667. [DOI] [PubMed] [Google Scholar]
- 34.Crane CA, Austgen K, Haberthur K, et al. Immune evasion mediated by tumor‐derived lactate dehydrogenase induction of NKG2D ligands on myeloid cells in glioblastoma patients. Proc Natl Acad Sci USA. 2014;111(35):12823‐12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. J Gene Med. 2012;14(6):405‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frigault MJ, Lee J, Basil MC, et al. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol Res. 2015;3(4):356‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ingegnere T, Mariotti FR, Pelosi A, et al. Human CAR NK Cells: A New Non‐viral Method Allowing High Efficient Transfection and Strong Tumor Cell Killing. Front Immunol. 2019;10:957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ajina A, Maher J. Strategies to Address Chimeric Antigen Receptor Tonic Signaling. Mol Cancer Ther. 2018;17(9):1795‐1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu L, Li XJ, Kalimuthu S, et al. Natural Killer Cell (NK‐92MI)‐Based Therapy for Pulmonary Metastasis of Anaplastic Thyroid Cancer in a Nude Mouse Model. Front Immunol. 2017;8:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lapteva N, Szmania SM, van Rhee F , Rooney CM. Clinical grade purification and expansion of natural killer cells. Crit Rev Oncogenes. 2014;19(1–2):121‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salman H, Pinz KG, Wada M, et al. Preclinical targeting of human acute myeloid leukemia using CD4‐specific chimeric antigen receptor (CAR) T cells and NK cells. J Cancer. 2019;10(18):4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oelsner S, Waldmann A, Billmeier A, et al. Genetically engineered CAR NK cells display selective cytotoxicity against FLT3‐positive B‐ALL and inhibit in vivo leukemia growth. Int J Cancer. 2019;145(7):1935‐1945. [DOI] [PubMed] [Google Scholar]
- 43.Herrera L, Santos S, Vesga M, et al. Adult peripheral blood and umbilical cord blood NK cells are good sources for effective CAR therapy against CD19 positive leukemic cells. Sci Rep. 2019;9(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.You F, Wang Y, Jiang L, et al. A novel CD7 chimeric antigen receptor‐modified NK‐92MI cell line targeting T‐cell acute lymphoblastic leukemia. Am J Cancer Res. 2019;9(1):64. [PMC free article] [PubMed] [Google Scholar]
- 45.Gang M, Marin ND, Wong P, et al. CAR‐modified memory‐like NK cells exhibit potent responses to NK‐resistant lymphomas. Blood, J Am Soc Hematol. 2020;136(20):2308‐2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hambach J, Riecken K, Cichutek S, et al. Targeting CD38‐expressing multiple myeloma and burkitt lymphoma cells in vitro with nanobody‐based chimeric antigen receptors (Nb‐CARs). Cells. 2020;9(2):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinz KG, Yakaboski E, Jares A, et al. Targeting T‐cell malignancies using anti‐CD4 CAR NK‐92 cells. Oncotarget. 2017;8(68):112783‐112796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, You F, Jiang L, et al. Gene‐modified NK‐92MI cells expressing a chimeric CD16‐BB‐ζ or CD64‐BB‐ζ receptor exhibit enhanced cancer‐killing ability in combination with therapeutic antibody. Oncotarget. 2017;8(23):37128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu E, Marin D, Banerjee P, et al. Use of CAR‐transduced natural killer cells in CD19‐positive lymphoid tumors. N Engl J Med. 2020;382(6):545‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X, Han J, Chu J, et al. A combinational therapy of EGFR‐CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget. 2016;7(19):27764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, Zhou Y, Huang KH, et al. Targeting epidermal growth factor‐overexpressing triple‐negative breast cancer by natural killer cells expressing a specific chimeric antigen receptor. Cell Prolif. 2020;53(8):e12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han J, Chu J, Chan WK, et al. CAR‐engineered NK cells targeting wild‐type EGFR and EGFRvIII enhance killing of glioblastoma and patient‐derived glioblastoma stem cells. Sci Rep. 2015;5(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Müller N, Michen S, Tietze S, et al. Engineering NK cells modified with an EGFRvIII‐specific chimeric antigen receptor to overexpress CXCR4 improves immunotherapy of CXCL12/SDF‐1α‐secreting glioblastoma. J Immunother. 2015;38(5):197‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ao X, Yang Y, Li W, et al. Anti‐αFR CAR‐engineered NK‐92 Cells Display Potent Cytotoxicity Against αFR‐positive Ovarian Cancer. J Immunother. 2019;42(8):284‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao B, Liu M, Wang L, et al. Use of chimeric antigen receptor NK‐92 cells to target mesothelin in ovarian cancer. Biochem Biophys Res Comm. 2020;524(1):96‐102. [DOI] [PubMed] [Google Scholar]
- 56.Ng YY, Tay JCK, Wang S. CXCR1 Expression to Improve Anti‐Cancer Efficacy of Intravenously Injected CAR‐NK Cells in Mice with Peritoneal Xenografts. Mol Ther Oncol. 2020;16:75‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen S, Ferrell K, Chaudhry K, Vu A, Nordon R, O'Brien T. Modulation of anti‐tumour activity of ex vivo expanded NK and GD2CAR‐NK cells against neuroblastoma. Cytotherapy. 2019;21(5):S20. [Google Scholar]
- 58.Yu M, Luo H, Fan M, et al. Development of GPC3‐specific chimeric antigen receptor‐engineered natural killer cells for the treatment of Hepatocellular Carcinoma. Mol Therap. 2018;26(2):366‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Z, Guo L, Song Y, et al. Augmented anti‐tumor activity of NK‐92 cells expressing chimeric receptors of TGF‐βR II and NKG2D. Cancer Immunol Immunother. 2017;66(4):537‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang W, Jiang J, Wu C. CAR‐NK for tumor immunotherapy: Clinical transformation and future prospects. Cancer Lett. 2020;472:175‐180. [DOI] [PubMed] [Google Scholar]
- 61.Mehta RS, Rezvani K. Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front Immunol. 2018;9:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu XY, Liu X, Wang XB, et al. Killing Effect of A CD7 Chimeric Antigen Receptor‐Modified NK‐92MI Cell Line on CD7‐Positive Hematological Malignant Cells. Zhongguo shi yan xue ye xue za zhi. 2020;28(4):1367‐1375. [DOI] [PubMed] [Google Scholar]
- 63.Herrera L, Santos S, Vesga MA, et al. Adult peripheral blood and umbilical cord blood NK cells are good sources for effective CAR therapy against CD19 positive leukemic cells. Sci Rep. 2019;9(1):18729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu X, Huang S. HER2‐specific chimeric antigen receptor‐engineered natural killer cells combined with apatinib for the treatment of gastric cancer. Bull Cancer. 2019;106(11):946‐958. [DOI] [PubMed] [Google Scholar]
- 65.Zhang C, Burger MC, Jennewein L, et al. ErbB2/HER2‐Specific NK Cells for Targeted Therapy of Glioblastoma. J Natl Cancer Inst. 2016;108(5). [DOI] [PubMed] [Google Scholar]
- 66.Zhang Q, Zhang H, Ding J, et al. Combination therapy with EpCAM‐CAR‐NK‐92 Cells and Regorafenib against Human Colorectal Cancer Models. J Immunol Res. 2018;2018:4263520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ueda T, Kumagai A, Iriguchi S, et al. Non‐clinical efficacy, safety and stable clinical cell processing of induced pluripotent stem cell‐derived anti‐glypican‐3 chimeric antigen receptor‐expressing natural killer/innate lymphoid cells. Cancer Sci. 2020;111(5):1478‐1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tseng HC, Xiong W, Badeti S, et al. Efficacy of anti‐CD147 chimeric antigen receptors targeting hepatocellular carcinoma. Nat Commun. 2020;11(1):4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li C, Yang N, Li H, Wang Z. Robo1‐specific chimeric antigen receptor natural killer cell therapy for pancreatic ductal adenocarcinoma with liver metastasis. J Cancer Res Therap. 2020;16(2):393‐396. [DOI] [PubMed] [Google Scholar]
- 70.Wang J, Lupo KB, Chambers AM, Matosevic S. Purinergic targeting enhances immunotherapy of CD73(+) solid tumors with piggyBac‐engineered chimeric antigen receptor natural killer cells. J Immunother Cancer. 2018;6(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]


