Abstract
Little is known about chemical contaminant exposures of office workers in buildings globally. Complex mixtures of harmful chemicals accumulate indoors from building materials, building maintenance, personal products, and outdoor pollution. We evaluated exposures to 99 chemicals in urban office buildings in the USA, UK, China, and India using silicone wristbands worn by 251 participants while they were at work. Here, we report concentrations of polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs) and other brominated flame retardants (BFRs), organophosphate esters (OPEs), phthalates and phthalate alternatives, pesticides, and polycyclic aromatic hydrocarbons (PAHs). First, we found major differences in office worker chemical exposures by country, some of which can be explained by regulations and use patterns. For example, exposures to several pesticides were substantially higher in India where there are fewer restrictions and unique malaria challenges, and exposures to flame retardants tended to be higher in the USA and UK where there were historic, stringent furniture flammability standards. Higher exposures to PAHs in China and India could be due to high levels of outdoor air pollution that penetrates indoors. Second, some office workers were still exposed to legacy PCBs, PBDEs, and pesticides, even decades after bans or phase-outs. Third, we identified exposure to a contemporary PCB that is not covered under legacy PCB bans due to its presence as an unintentional byproduct in materials. Fourth, exposures to novel BFRs, OPEs, and other chemicals commonly used as substitutes to previously phased-out chemicals were ubiquitous. Fifth, some exposures were influenced by individual factors, not just countries and buildings. Phthalate exposures, for example, were related to personal care product use, country restrictions, and building materials. Overall, we found substantial country differences in chemical exposures and continued exposures to legacy phased-out chemicals and their substitutes in buildings. These findings warrant further research on the role of chemicals in office buildings on worker health.
Graphical Abstract

1. Introduction
Complex mixtures of harmful chemicals accumulate in the dust and air of our indoor environments due to building materials, maintenance practices, personal care products, and the infiltration of outdoor pollution (Lucattini et al., 2018; Weschler and Nazaroff, 2008). Office buildings play an important role in defining indoor exposures of workers. Typical office workers spend about one quarter of their week inside office buildings (U.S. Bureau of Labor Statistics, 2013). In fact, semi-volatile organic compounds (SVOCs) are often detected at higher levels in offices than homes (Bi et al., 2015; D’Hollander et al., 2010; Harrad et al., 2006; Xiong et al., 2019; Zhang et al., 2011). Many of the hormone-disrupting SVOCs are undisclosed ingredients that readily travel out of materials and expose occupants via inhalation, incidental dust ingestion, and dermal absorption (Lucattini et al., 2018; Mitro et al., 2016).
Polychlorinated biphenyls (PCBs) were historically used in joint sealants, caulking, and other building materials (Erickson and Kaley, 2011; Kohler et al., 2005). PCBs have been classified as known human carcinogens as a group (Lauby-Secretan et al., 2013) and are linked to hormone disruption and impaired brain development (Brouwer et al., 1999; Encarnacao et al., 2019; Klocke et al., 2020). Phthalate plasticizers are used in polyvinyl chloride (PVC) flooring, wiring, plastic building materials, food packaging, cosmetics, and personal care products (US FDA, 2018; Wittassek et al., 2011). Phthalate exposure is associated with negative impacts on sperm quality, pregnancy outcomes, fetal development, and potentially cardiometabolic health (Casals-Casas and Desvergne, 2011; Hauser et al., 2006; James-Todd et al., 2012, 2016; Mariana et al., 2016; Messerlian et al., 2018a).
Brominated flame retardants (BFRs), including polybrominated diphenyl ethers (PBDEs) and organophosphate esters (OPEs), have been added as flame retardants in foam furniture, carpet, electronics, and building insulation (Abbasi et al., 2016; Allen et al., 2008; Cooper et al., 2016; Hammel et al., 2017; Jinhui et al., 2017; Kemmlein et al., 2003; Stapleton et al., 2012). OPEs have additional uses as plasticizers in furniture, floor finishes, plastic, rubber, paints, coatings, and wallpaper (van der Veen and de Boer, 2012; Wang et al., 2017). Research has found human exposure to be associated with adverse effects on the thyroid, fertility, pregnancy outcomes, and brain development for both PBDEs (Allen et al., 2016; Boas et al., 2012; Choi et al., 2019; Czerska et al., 2013; Linares et al., 2015; Mumford et al., 2015; Vuong et al., 2018) as well as OPEs (Carignan et al., 2017; Doherty et al., 2019; Meeker and Stapleton, 2010; Messerlian et al., 2018b; Preston et al., 2017).
Pesticides are applied indoors and outdoors for management of pests, vector-borne diseases, and crop protection. They are sometimes impregnated in textiles, carpets, and treated wood to control pests and fungi (Butte, 2004; Cooper et al., 2020; Handford et al., 2015). Exposures to some organochlorine, organophosphate, and pyrethroid pesticides are associated with cancer, neurodegenerative disease, impaired brain development, thyroid disease, respiratory symptoms, and diabetes (Burke et al., 2017; Evangelou et al., 2016; Kim et al., 2017; Mostafalou and Abdollahi, 2013; Shrestha et al., 2018). Polycyclic aromatic hydrocarbons (PAHs) are formed during incomplete burning of organic substances from certain indoor home appliances, smoking, cooking, traffic, coal burning, natural gas extraction, power plants, waste incineration, and forest fires (Kim et al., 2013; Ravindra et al., 2008). In addition, certain PAHs like naphthalene are used in moth repellents and building materials (Jia and Batterman, 2010). PAH exposure has been linked to increased risk of cancer, respiratory distress, and impaired development (Kim et al., 2013; Miller et al., 2004; Padula et al., 2015; Perera and Herbstman, 2011; Rota et al., 2014; Singh et al., 2018).
Silicone wristbands have recently emerged as novel personal passive samplers of exposures to chemical mixtures (Anderson et al., 2017; Hammel et al., 2016; O’Connell et al., 2014). Wristbands adsorb SVOCs from air, dust, and products that people interact with, capturing inhalation and dermal pathways of exposure (Hammel et al., 2018; S. Wang et al., 2019b). Chemical concentrations in wristbands are significantly correlated with both serum and urinary biomarkers of exposure, demonstrating their usefulness as external indicators of internal dose for compounds with short or long biological half-lives. Wristbands also reflect internal dose better than purely external samples such as dust, air, and hand wipes (Dixon et al., 2018; Gibson et al., 2019a; Hammel et al., 2020, 2018, 2016; Levasseur et al., 2021). Compared to traditional urine or blood biomarkers, wristbands are simple and non-invasive and reflect external exposures without dietary contributions (Hammel et al., 2016). Most importantly for this study, wristbands can isolate external exposures to one microenvironment and exposure time window of interest. For example, participants could wear wristbands only in the office building and only during one work week.
The objectives of this study were to: 1) evaluate exposures of 251 office workers to 99 SVOCs in their office buildings in the USA, UK, India, and China using silicone wristbands; and 2) investigate the impact of country, building factors, and individual activities on indoor chemical exposures. To our knowledge, this was the first study to use silicone wristbands to pinpoint personal chemical exposures specifically isolated to office buildings, which are often understudied compared to homes (Lucattini et al., 2018). We also studied buildings in China and especially India to address major gaps in research about human exposures to SVOCs in low- and middle-income countries (LMICs), which contain 84% of the global population (Goodman et al., 2020; Lucattini et al., 2018; Saillenfait et al., 2018).
2. Methods
2.1. Study Population
We recruited office worker participants from 36 buildings of the preexisting Global CogFx Study cohort of 43 office buildings. This convenience sample consisted of urban buildings occupied by knowledge work companies in the USA, UK, India, and China, as well as Mexico and Thailand which were not included in our sub-study of chemical exposures. None of the buildings permitted smoking indoors. Participants in the larger cohort were required to be full-time permanent employees, aged 18 to 65, non-smoking, and not color blind; this sub-study did not have additional eligibility exclusions. More information on recruitment, eligibility, and building locations are provided in the Supplementary Material.
In total, 255 office workers from 36 study buildings in the USA, UK, India, and China participated in this sub-study. One wristband was lost during storage and three participants were excluded because they did not report the amount of time that they wore the wristband. Of the final 251 included participants, the sample sizes were 85 in the USA, 42 in the UK, 54 in India, and 70 in China.
Table S1 summarizes participant information, workstation characteristics, building factors, and indoor air quality parameters. The 251 participants worked across 15 buildings in the USA, six in the UK, nine in India, and six in China. The average number of participants per building was 7.0 with a range of 2–15 (USA: 5.7 [3–8]; UK: 7.0 [6–8]; India: 6.0 [2–10]; China: 12 [6–15]).
2.2. Study Design
During a predefined study week in 2019 for each building, participants were instructed to wear their provided silicone wristbands on either wrist for four consecutive days, Monday–Thursday, only during work hours. We did not have them wear the wristbands on Friday so that that day could be used to ship the wristbands before the weekend. Some participants skipped certain sampling days when out of the office. Most participants (80%) wore the wristband for four workdays, and 13% wore it for three. The median number of hours the participants wore the wristbands for each country was between 33 and 34 hours [range 6–56] (Table S1). The sampling duration did not significantly differ by country (p=0.27 in ANOVA test).
For each day during the study period, participants were told to put on their wristbands when they arrived at their workplace and then to wear it continuously until leaving the office building after their work shift. Participants filled out the exact times they put on and removed their wristbands on a paper time log sheet and via electronic daily surveys, which we cross-checked against each other to ensure compliance with the sampling protocol. Participants wore the wristbands continuously during any lunch breaks or external meetings since exposures during those times may be ‘brought back’ to the building on clothes, skin, and other materials. Overnight, the participants stored their wristbands wrapped in aluminum foil and sealed in plastic bags at their desk.
Most buildings within a country were sampled during the same study week. The UK buildings were sampled in February 2019, most USA buildings were sampled in late April (except one in January), most buildings in India were sampled in May (except one in June), and the buildings in China were sampled in July. Sampling weeks were scheduled based on logistics and already-planned in-person visits to the UK and China. For all buildings, wristbands were shipped back to Boston and stored at −13°C within two weeks after the end of the study week but usually (94%) within one week. SVOCs have been experimentally shown to be stable on wristbands at high temperatures (30°C) in transport for at least one month (Anderson et al., 2017).
Participants took one baseline survey about their workstation (such as the presence of foam furniture and flooring) and a daily survey about their behaviors while wearing their wristband the day before (such as personal care product uses). These Qualtrics-based surveys were administered through email or the custom-developed mobile app developed for the Global CogFx study (ForHealth App, AppLab, Boston, MA, USA). All study materials were translated to Chinese by native and fluent members of the research team. In India, the sampled offices used English as the language at work and all participants were fluent. A few study point people from the buildings in India or China reviewed the surveys in advance to check for understanding and terminology.
We also had access to surveys of building managers about building characteristics such as age of construction and cleaning practices, which were almost always reported to be daily. Global CogFx participants were provided with low-cost air quality sensors at their workstations, which provided real-time data on indoor temperature, relative humidity, carbon dioxide, and in many cases, fine particulate matter (PM2.5). More information on the sensors is in Jones et al. 2021 (Citation TBD).
In the laboratory, we analyzed 21 field blanks from different buildings and countries. Field blanks were wristbands prepared in the same way as the sample kits and shipped to and from buildings in the same packages, but not opened or worn by participants. The study protocols were approved by the Institutional Review Board of the Harvard T.H. Chan School of Public Health.
2.3. Wristband Preparation and Laboratory Analysis
Our wristband sample preparation, storage, and analysis followed previously published protocols (Hammel et al., 2020, 2018, 2016). Red silicone wristbands were purchased from 24hourwristbands.com (Houston, TX, USA), pre-cleaned using solvent extraction (overnight soxhlet with 1:1 acetate:hexane then repeated with 1:1 in ethyl acetate:methanol), wrapped in pre-cleaned (combusted at 400°C) aluminum foil, and double sealed in two plastic Ziploc bags. We sent each participant an envelope with the wristband sample bag, instructions, and extra foil.
Wristbands were analyzed at the Michael and Annie Falk Foundation Exposomics Laboratory at Duke University. The following were quantified in wristband samples based on previously described methods (Reddam et al., 2020; Wise et al., 2020) and mostly using GC-MS/MS: 11 PCBs, 13 BFRs, 31 OPEs, 10 phthalates and their alternatives, 12 pesticides, and 20 PAHs. The 13 BFRs included 10 PBDEs and 3 novel BFRs. Abbreviations for the chemicals described in detail in the discussion include: 2-ethyl hexyl-2,3,4,5-tetrabromobenzoate (EHTBB), bis (2-ethyl hexyl)-2,3,4,5-tetrabromophthalate (BEHTBP), tris (1-chloro-isopropyl) phosphate (TCIPP), tris (2,4-dichloro-isopropyl) phosphate (TDCIPP), tris (2-chloro-ethyl) phosphate (TCEP), triphenyl phosphate (TPHP), 2-ethylhexyl diphenyl phosphate (EHDPP), tris(2-ethylhexyl) phosphate (TEHP), tri-iso-butyl phosphate (TiBP), tri-n-butyl phosphate (TnBP), tri-p-cresyl phosphate (TpCP), 2-isopropylphenyl diphenyl phosphate (2IPPDPP), 4-tert-butylphenyl diphenyl phosphate (4tBPDPP), bis(4-tert-butylphenyl) phenyl phosphate (B4tBPPP), bis (2-ethlyhexyl) phthalate (DEHP), di-n-butyl phthalate (DnBP), di-isobutyl phthalate (DiBP), di-isononyl phthalate (DINP), benzyl butyl phthalate (BzBP), di-methyl phthalate (DMP), bis (2-ethylhexyl) adipate (DEHA), bis (2-ethylhexyl) terephthalate (DEHT), and trioctylmetallitate (TOTM). Table S2 provides a full list of analyte abbreviations and CAS numbers. Wristbands from the first eight participants (3.2%), including three from the USA and five from the UK, were not measured for some analytes, including one PCB, ten OPEs, five pesticides, and all PAHs (Table S2).
Information on laboratory methods, quality assurance and quality control, blanks, and recoveries (Table S3) are in the Supplementary Material. Method detection limits (MDLs) were calculated as three times the standard deviation of field and lab blank responses (Table S2).
2.4. Statistical Analyses
We blank-corrected chemical concentrations by subtracting average field blank concentrations. Because each participant wore their wristband for a different reported sampling duration, we scaled detected chemical concentrations to a 32-hour time period (equivalent to four 8-hour workdays). After scaling, we substituted non-detect values with one-half the MDL of the sample batch (Hornung and Reed, 1990).
To identify groupings for certain chemical classes, we conducted principal component analysis among scaled and centered concentrations of analytes detected in over one-third of samples in a country. We evaluated principal components (PCs) that explained a cumulative variance of over 70% and each had eigenvalues of at least one. We calculated Spearman correlation coefficients among the same analytes, with statistically significant correlations evaluated at αBH_C=0.032 (based on the Benjamini-Hochberg procedure to decrease false discovery rates) (Benjamini and Hochberg, 1995).
We developed multilevel regression models of natural logs of chemical concentrations (due to non-normality in histograms and Shapiro-Wilk tests), with building-specific random intercepts. For each modeled chemical, only data from countries with at least 5% detections were included. Final sample sizes in statistical models ranged from n=233 to n=243 (except lower when only a subset of countries had enough detections to analyze), as explained in Supplementary Material. Model estimates were transformed to percent differences because we used log-transformed outcomes.
For models of phthalates and their alternatives, we conducted latent class analysis (LCA) to identify personal care product user types and to avoid problems with multicollinearity and high data dimensionality from including variables for each product. The variables inputted into LCA included reported use at any time on any study day of aftershave; body or hand lotion; deodorant; hair oil, grease, or leave-in conditioner; hair spray; makeup foundation, blush, or eye shadow; nail polish; and perfume or cologne. Latent classes were inputted as variables in models and interpreted as user types defined by the profile of products used. More information on LCA and model covariates are described in Supplementary Material.
To avoid multiple testing issues that could arise from statistical models on 99 chemicals, for models we selected key chemicals based on detection rates or tracer chemicals identified in principal component analysis (see Supplementary Material and Figure S1). We did not calculate summations of all chemicals in a class due to differences in physical-chemical properties that would influence chemical uptake efficiency into wristbands. We did not conduct models on PAHs since they have high contributions from outdoor sources that we did not evaluate or that had too much missing data. Due to the targeted selection of only a subset of chemicals for these analyses, statistical significance in models was evaluated at α=0.05 with suggestive evidence at α=0.10.
In sensitivity analyses, we conducted the same regression models with additional control for average indoor temperature, relative humidity, fine particulate matter (PM2.5), and air exchange rate (outdoor ventilation estimated from CO2 measurements) during each building’s four-day study period during business hours (9:00–17:00). The measurements were not available for 4 of 36 study buildings (32 participants; 9%) due to sensor technological issues, so we did not include these parameters in main models. All analyses were conducted in R (version 3.6.1).
3. Results and Discussion
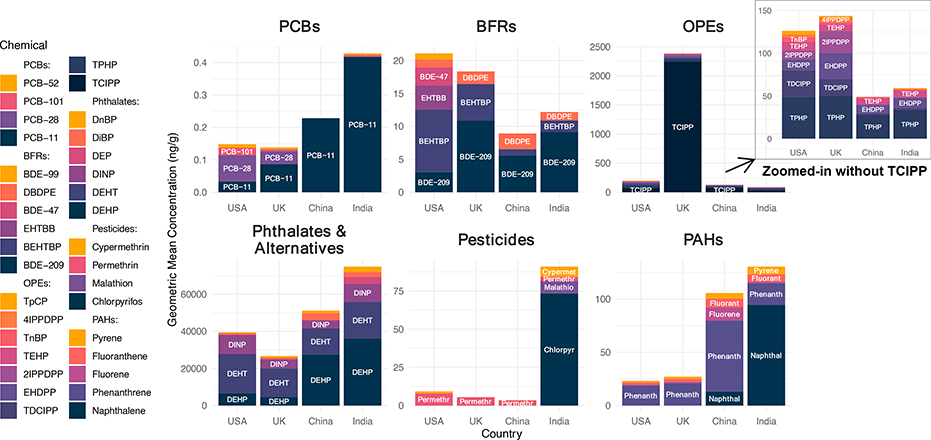
Ninety-four of the 99 measured SVOCs across six target chemical classes were detected in at least one silicone wristband sample (Table S4). We found considerable differences in chemical exposures by country (Figure 1). India or China tended to have higher exposures to most phthalates, PAHs, and pesticides, indicative of lower restrictions, higher fuel combustion sources, and unique malaria challenges (in India), respectively. On the other hand, the UK and USA tended to have higher exposures to most BFRs and OPEs, suggestive of the more historically strict flammability standards necessitating the use of chemical flame retardants. Detections of banned legacy PCBs were highest in the USA, likely due to the older ages of buildings in this study.
Figure 1.
Profiles of chemical classes in wristband samples worn by 251 office workers in the USA, UK, China, and India. Note: only chemicals contributing to more than 1% of the height of the tallest bar in the chemical class (in at least one country) are shown. Chemical classes are presented on different y-axis scales. Caveat: the different physical-chemical properties of the chemicals may influence their respective sampling uptakes and thus concentrations on the wristbands, so comparisons should focus on country differences within an individual chemical, not comparisons between concentrations of different chemicals.
Overall, we found that legacy chemicals still exposed building occupants even decades after they were banned in a country. Exposures to certain legacy chemicals were also higher in some countries that did not implement the same bans. Even when exposures to legacy chemicals were low, harmful substitute chemicals ubiquitously exposed office workers.
Further details on each chemical class are provided in the following sections, and the Supplementary Material includes tables of summary statistics (Tables S4–S5), additional boxplots of each common chemical by country (Figures S2–S7), and bar charts comparing our results to previous wristband studies with concentrations scaled to the same exposure duration (Figures S8–S12).
3.1. Polychlorinated Biphenyls
Office workers in this study were exposed to several legacy PCBs that were banned in the USA by 1979, banned in the UK by 1986, and added to the global Stockholm Convention in 2004 (Conolly et al., 2009; The People’s Republic of China, 2007; US Environmental Protection Agency, 1979). In particular, PCB-28 and PCB-101 were found in the wristbands at higher detection frequencies and geometric mean concentrations in the USA compared to other countries. These two PCBs were detected in 0% of samples from China and less than 4% from India, compared to 33–36% in the USA and 5–19% in the UK (Table S4). The sampled buildings in China and India were all constructed after 2003, which likely explains the relatively lower detections of legacy PCBs compared to the USA and UK. By contrast, the USA workers occupied the oldest buildings in our study, dating back to 1898 (eight of the 15 buildings were constructed in or before the 1979 ban), and three of the six study buildings in the UK were constructed between 1987–1990 (only right after the 1986 ban). In regression models, a 10-year decrease in construction age among buildings in the USA was associated with 19% lower PCB-101 concentrations in wristbands (95% confidence interval [CI]: −31– −4%; p=0.029), adjusted for hand-washing frequency and wristbands worn under clothing sleeve (Table 1). The detections of legacy PCBs were likely a result of PCB-containing materials in older buildings constructed before bans, since pre-existing materials were allowed to remain in use and often have not been tested for PCBs. Several studies have similarly documented the continued presence of legacy PCBs in old buildings in the USA and Europe due to their historic use in materials such as joint sealants (Frederiksen et al., 2012; Hazrati and Harrad, 2006; Heinzow et al., 2007; Herrick et al., 2004; Jartun et al., 2009; Kohler et al., 2005).
Table 1.
Results of multilevel linear regression models of log concentrations of key PCB, BFR, and OPE chemicals (as determined by principal component analysis) in silicone wristbands worn by office workers in the USA, UK, China, and India.
| Variable | Polychlorinated Biphenyls | Brominated Flame Retardants | ||||||||||
| PCB-11 | PCB-101 | BDE-47 | BDE-209 | BEHTBP | ||||||||
| % Change | p | % Change | p | % Change | p | % Change | p | % Change | p | |||
| Country (Ref: United States) | ||||||||||||
| United Kingdom | 170% | 0.15 | Excluded | −98.2% *** | <0.0001 | 273% ** | 0.0023 | −26.70% | 0.31 | |||
| China | 942% ** | 0.0022 | Excluded | Excluded | 72.60% | 0.22 | −88.3% *** | <0.0001 | ||||
| India | 1730% *** | <0.0001 | Excluded | Excluded | 227% ** | 0.0063 | −74.6% *** | 0.00012 | ||||
| Year of Building Construction | −0.35% | 0.68 | −2.02% * | 0.029 | −0.13% | 0.84 | −0.14% | 0.77 | −0.55% | 0.15 | ||
| Carpeting in Workstation | – | – | 129% | 0.3 | −22.90% | 0.4 | −23.50% | 0.24 | ||||
| Foam Chair at Workstation | – | – | −22.90% | 0.59 | 15.30% | 0.5 | 23.30% | 0.36 | ||||
| Average # Times Hands Washed | 1.59% | 0.87 | −54.1% * | 0.034 | −10.60% | 0.44 | 0.51% | 0.93 | −2.53% | 0.74 | ||
| Wore Wristband Under Sleeve | 23.80% | 0.38 | 105%. | 0.09 | −22.10% | 0.47 | −33.7% ** | 0.0085 | 7.67% | 0.69 | ||
| Variable | Organophosphate Esters | OPEs: TBPPs | OPEs: ITPs | |||||||||
| EHDPP | TiBP | TnBP | TpCP | 4tBPDPP | 2IPPDPP | |||||||
| % Change | p | % Change | p | % Change | p | % Change | p | % Change | p | % Change | p | |
| Country (Ref: United States) | ||||||||||||
| United Kingdom | 206% * | 0.019 | 113% ** | 0.0081 | −49.4%. | 0.092 | −41.90% | 0.75 | −76.9% * | 0.045 | 93.10% | 0.38 |
| China | 15.70% | 0.77 | −41.2%. | 0.069 | −73.8% ** | 0.0047 | −98.8% * | 0.023 | −96.4% *** | 0.00026 | −61.60% | 0.25 |
| India | 62.60% | 0.3 | −47.7% * | 0.024 | −67.7% ** | 0.0079 | −57.10% | 0.62 | −91.2% ** | 0.0018 | −89.3% ** | 0.0058 |
| Year of Building Construction | −0.64% | 0.27 | −0.02% | 0.96 | 0.01% | 0.99 | −1.17% | 0.57 | −0.50% | 0.57 | −0.03% | 0.98 |
| Carpeting in Workstation | 16.70% | 0.66 | 23.90% | 0.29 | 39.30% | 0.26 | −6.70% | 0.96 | 328% ** | 0.0079 | 571% ** | 0.002 |
| Foam Chair at Workstation | −22.90% | 0.3 | 7.62% | 0.68 | 29.10% | 0.15 | 267%. | 0.088 | 25% | 0.46 | 0.50% | 0.99 |
| Average # Times Hands Washed | −1.16% | 0.87 | −1.76% | 0.75 | −4.60% | 0.34 | −17.10% | 0.39 | 3.80% | 0.66 | −13.10% | 0.25 |
| Wore Wristband Under Sleeve | −17.70% | 0.29 | 7.51% | 0.61 | 12.90% | 0.33 | 105% | 0.2 | −4.38% | 0.84 | −3.78% | 0.9 |
OPEs = organophosphate esters; ITPs = isopropylated triaryl phosphates; TBPPs = tertbutylated triaryl phosphates. Excluded = fewer than 5% of samples in the country had detectable levels so were excluded from model.
Note: Model outcomes were log-transformed before analysis, so model estimates were transformed to percent differences.
PCB-11, on the other hand, is a contemporary PCB congener that was not historically added to commercial mixtures at appreciable levels and has been an emerging concern in buildings (Frame et al., 1996; Hu and Hornbuckle, 2010; Rodenburg et al., 2010). In this study, PCB-11 was the most frequently detected PCB in 73% of wristbands from all countries (Table S4). PCB-11 was detected at significantly higher concentrations and in at least 89% of wristbands from China and India, where the buildings were all constructed more recently (after 2007 in China and after 2002 in India) (Figure S2). Specifically, PCB-11 levels were estimated to be 942% higher in wristbands worn by office workers in China (95% CI: 184–3,720%; p=0.0022) and 1,730% higher in India (95% CI: 437–6,140%; p<0.001) compared to the USA (Table 1). In addition, PCB-11 was negatively correlated with legacy congeners PCB-101 (Spearman r=−0.21; p=0.0012) and PCB-28 (r=−0.14; p=0.03), whereas those two legacy PCBs were positively correlated with each other (r=0.28, p<0.001) (Figure S8). And, PCB-11 levels were not significantly associated with building age, unlike PCB-101 (Table 1). These findings are supported by previous research showing that PCB-11 has non-legacy sources indoors. Not covered under PCB manufacturing bans, PCB-11 is an unintentional byproduct in certain pigments used in wall paint, product packaging, textiles, and other pigmented materials (Anezaki et al., 2014; Guo et al., 2014; Hu et al., 2008; Rodenburg et al., 2010; Shang et al., 2014; Vorkamp, 2016). Recent studies also identified unexpectedly high PCB-11 emissions in outdoor air and water that were not driven by dechlorination of heavier congeners and continued even as other PCBs declined (Hazrati and Harrad, 2006; Hites, 2018; Hu et al., 2008; Rodenburg et al., 2010).
3.2. Brominated Flame Retardants
Similar to PCBs, legacy PBDEs still exposed office workers despite being phased out (Figure 2). A large majority of wristband samples (87%) had at least one detectable PBDE. Even with the phase-out of PBDEs, novel emerging BFRs and OPEs have been frequently used as flame-retardant substitutes in the same product applications as PBDEs (Cooper et al., 2016; Saillenfait et al., 2018; Stapleton et al., 2012; van der Veen and de Boer, 2012). About 63% of office workers in this study were exposed to at least one of the three measured novel BFRs, and all but one (99.6%) were exposed to an OPE.
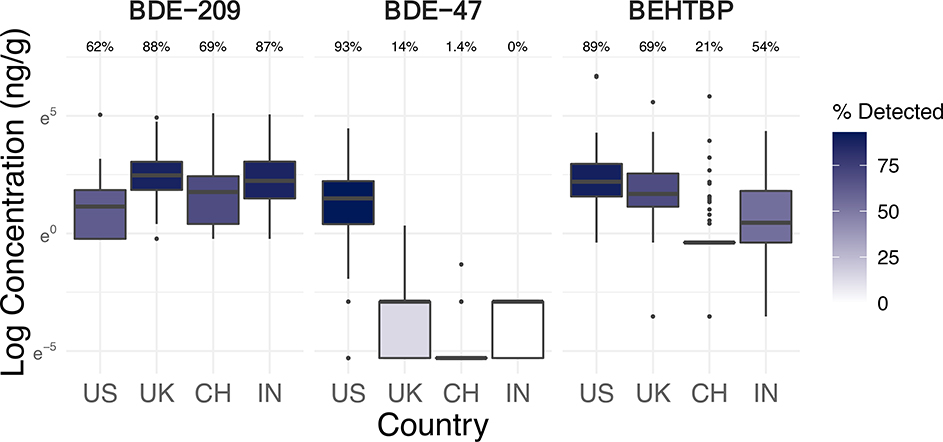
Figure 2.
Log concentrations (ng/g-wristband, scaled to 32 hours of sampling) and detection frequencies of select brominated flame retardants in silicone wristbands worn by 251 office workers in the USA, UK, China, and India (see Figure S3 for boxplots of more chemicals).
3.2.1. Polybrominated Diphenyl Ethers: PentaBDE
PBDE congeners 47, 99, and 100 were detected almost exclusively in wristbands from the USA (at 93%, 84%, and 39% respectively) and UK (14%, 19%, and 2%), while not in any from China or India except one wristband (Table S4). Most other PBDEs (except BDE-209) were rarely detected. There were evident country-level differences in the magnitude of concentrations of PBDEs in wristbands. The boxplots and models demonstrate that the USA had the highest concentrations of BDE-47, BDE-99, and BDE-100 in wristbands, even compared to the UK (Figure 2; Figure S3; Table 1).
BDE-47, BDE-99, and BDE-100 were components of the historically common commercial flame-retardant mixture PentaBDE (La Guardia et al., 2006). In 2004, PentaBDE was banned in the UK and voluntarily phased out in the USA (followed by California’s 2006 ban) (Dodson et al., 2012; European Parliament, 2003a; State of California, 2004). Thus, office workers in the USA and UK were exposed to PentaBDE chemicals 15 years after their phase-out and likely from legacy sources. By contrast, the absence of PentaBDE in samples from China and India was possibly explained by the contrasting lack of similar flammability regulations (which were mandated over 30 years ago in the USA and UK) and/or the more recent construction of study buildings (2009 or later in China; 2003 or later in India) (Petreas et al., 2016; UK Statutory Instruments, 1988).
As expected, given their co-presence in PentaBDE mixtures, concentrations of PBDE congeners 47, 99, and 100 in our wristbands were strongly positively correlated with each other (r=0.58–0.84; p<0.001) and were the primary chemicals of the third principal component (Figure S1; Figure S13). They were also positively correlated with legacy PCB-101 (r=0.14–0.42; p<0.05) and negatively correlated with contemporary PCB-11 (r=−0.41– −0.27; p<0.001), indicating that they have legacy sources.
3.2.2. Polybrominated Diphenyl Ethers: DecaBDE
Unlike PentaBDE chemicals, BDE-209, the main component of DecaBDE, was found in 74% of wristbands overall, and the lowest detection frequencies occurred in the USA (USA: 62%; UK: 88%; China: 69%; India: 87%) (Table S4). BDE-209 detections and concentrations in the wristbands were highest in the UK and India, followed by China (Figure 2). There were estimated 273% and 227% higher concentrations of BDE-209 in wristbands from the UK (95% CI: 80–670%; p=0.0023) and India (95% CI: 54–587%; p=0.0063), respectively, compared to the USA, adjusted for building age, carpeting, foam chair, handwashing, and wearing wristband under clothing sleeve (Table 1). The higher presence, and difference in trends, of BDE-209 compared to other PBDEs could have resulted from its more recent restrictions or differences in physical-chemical properties. While DecaBDE was voluntarily phased out in the USA by 2013, the UK only banned it in 2019 after our study ended (and in 2008 ended its PBDE exemption in electronics restrictions) (European Court of Justice, 2008; European Parliament, 2017, 2003b). India has restricted PBDEs in electronics since 2014, but not in other products to our knowledge (Government of India, 2011). In China, DecaBDE is not restricted and remains a high production-volume flame retardant (Chinese Ministry of Industry and Information Technology, 2016; Chung and Zhang, 2011; Dou and Sarkis, 2013; Ni et al., 2013). In addition, BDE-209 was negatively correlated with legacy BDE-47 (r=−0.34; p<0.001) and PCB-101 (r=−0.17; p=0.007) (Figure S13), suggesting that BDE-209 is a more contemporary chemical that has not yet declined in use as much as phased-out PBDEs and PCBs. BDE-209, along with two novel substitute BFRs (EHTBB and BEHTBP), also loaded onto different principal components than PentaBDE congeners (Figure S1).
The elevated BDE-209 levels in wristbands from the UK align with previous research reporting high use and detection of BDE-209 in the UK compared to other countries (Harrad, 2015). Our findings of BDE-209 in wristbands worn in office buildings across the four countries reveal the lags between regulations and exposure reductions as well as limitations of restrictions that do not treat chemicals as an entire class.
3.2.3. Novel Brominated Flame Retardants
Novel BFRs are often used in commercial flame-retardant mixtures as PBDE replacements (Covaci et al., 2011; Stapleton et al., 2008). BEHTBP was found in over half of all wristbands (59%), with the highest detection frequencies and concentrations occurring within the USA (89%), followed by the UK (69%) (Table S4; Figure 2). Compared to the USA, BEHTBP concentrations were 88% lower in wristbands from China (95% CI: −94– −79%; p<0.001) and 74.6% lower in India (95% CI: −86– −54%; p=0.00012), adjusted for building age, carpeting, foam chair, handwashing, and wearing wristband under sleeve (Table 1). By contrast, EHTBB was detected primarily (and at much higher magnitudes) in samples from the USA (79%), while rarely in the UK (7%), India (4%), and China (1%) (Figure 2). This suggests that in our study, the USA may be the only country with appreciable contributions from commercial flame-retardant mixtures containing both BEHTBP and EHTBB, such as Firemaster 550, BZ-54, and Firemaster 600 (Li et al., 2017; Ma et al., 2012; Qi et al., 2014; Stapleton et al., 2008). Reflective of these mixtures, BEHTBP and EHTBB were positively correlated with each other in wristbands (r=0.26 overall, 0.49 in USA; p<0.001) (Figure S13) and contributed heavily to the same distinct principal component (Figure S1). The other countries may have higher relative use of other commercial mixtures, such as DP-45, which consists exclusively of BEHTBP (Davis and Stapleton, 2009; Ma et al., 2012; Qi et al., 2014).
3.3. Organophosphate Esters
The office workers were ubiquitously exposed to OPEs, but often at significantly different detection frequencies and concentrations across countries. Four OPEs were detected in at least 95% of samples: TCIPP, TPHP, EHDPP, and TEHP (Table S4). Similar to legacy PBDEs and novel BFRs, India and China typically had the lowest levels of OPEs in wristbands compared to the USA or UK, which aligns with previous research of OPEs in dust from India and China compared to industrialized countries with stricter flammability standards (W. Li et al., 2019). For example, the detections and magnitudes of EHDPP, TiBP, TnBP, ITPs (such as 2IPPDPP), and TBPPs (such as 4tBPDPP) were higher in the USA and UK than India or China (Figure S4). In regression models, compared to the USA, wristbands from India had 48% lower TiBP levels (95% CI: −68– −14%; p=0.024), 68% lower TnBP (95% CI: −84– −34%; p=0.0079), 89% lower 2IPPDPP (95% CI: −97– −56%; p=0.0058), and 91% lower 4tBPDPP (95% CI: −98– −69%; p=0.0018), adjusted for building age, carpeting, foam chair, handwashing, and wearing wristband under sleeve (Table 1). Similarly, wristbands from China had 74% lower TnBP (95% CI: −88– −43%; p=0.0047), 99% lower TpCP (95% CI: −100– −65%; p=0.023), and 96% lower 4tBPDPP (95% CI: −99– −86%; p=0.00026) compared to the USA.
Our regression results corroborate the likely use of OPEs as flame retardants in carpet and foam furniture, among other applications. There were 571% higher 2IPPDPP levels (95% CI: 133–1,810%; p=0.0020) and 328% higher 4tBPDPP levels (95% CI: 62–977%; p=0.0079) for participants that had carpeting compared to no carpeting in their workstation (Table 1). We found suggestive evidence that participants with a foam chair had 267% higher exposures to TpCP compared to those without a foam chair (95% CI: −18–1,440%; p=0.088).
Of particular note among chlorinated OPEs, TCIPP concentrations in wristbands from the UK were orders of magnitude higher than for any other country or any other OPE, even though it was widely detected in all countries (Figure 1; Figure S4). In addition, we observed higher geometric means of TCIPP than eight previous wristband-based studies, for which we scaled concentrations to a comparable sampling duration (Figure S9) (Gibson et al., 2019b; Hammel et al., 2020, 2016; Kile et al., 2016; Reddam et al., 2020; Travis et al., 2020; S. Wang et al., 2019a; Wang et al., 2020). These findings align with previous research that found preferential use of TCIPP in the UK compared to other countries (Brommer and Harrad, 2015; Harrad et al., 2016). Furthermore, TDCIPP levels were higher in wristbands from the USA and UK compared to India or China (Figure S4) (Cooper et al., 2016; Stapleton et al., 2009). By contrast, TCEP was much higher and more frequently detected in wristbands from China (77%) than other countries (50–58%) (Figure S4). This result is consistent with previous research reporting that TCEP comprises a larger proportion of OPEs in indoor dust from China and other Asian countries compared to the USA and European countries where TCEP dropped in production (California Environmental Protection Agency, 2020; W. Li et al., 2019). These three chlorinated OPEs are most often used as flame retardants in polyurethane foam applications but can also be added as plasticizers to PVC, plastic, wallpaper, textiles, coatings, and paints like other OPEs (Wang et al., 2017).
The OPE concentrations also demonstrated different potential profiles of flame-retardant mixtures across countries. TPHP did not load onto the same principal component as BEHTBP and EHTBB (Figure S1), and TPHP was only slightly correlated with those two chemicals (Figure S13). This indicates that FM 550 (of which TPHP is another component) may not be the only or primary mixture used (Knudsen et al., 2016; Phillips et al., 2017; Stapleton et al., 2008). Instead, EHTBB and BEHTBP were highly correlated with two TBPPs (4tBPDPP and B4tBPPP; r=0.29–0.65; p<0.001), which points to FM 600, at least in the USA and UK where there were significantly higher TBPP wristband concentrations (Figures S13 and S4; Table 1) (Knudsen et al., 2016; Phillips et al., 2017). On the other hand, TPHP was significantly correlated with ITPs such as 2IPPDPP (r=0.64; p<0.001) and together they tightly formed the first principal component, suggesting prevalent use of the so-called ITP flame retardant mixture consisting of TPHP and ITPs (Phillips et al., 2017) (Figure S1; Figure S13).
3.4. Phthalates and Phthalate Alternatives
Phthalates were the most abundant class of SVOCs we measured in wristbands (Figure 1; Table S4). Every office worker was exposed to phthalates, and four phthalates were detected in at least 97% of wristbands: DEHP, DnBP, DiBP, and DINP. The phthalate alternatives DEHT, DEHA, and TOTM were also common: 99% of participants were exposed to at least one and 95% were exposed to all three. Office workers in India tended to have the highest, or at least comparable, exposures to most phthalates and phthalate alternatives except for BzBP and DMP (Figure 3; Figure S5; Table S4). Elevated exposures in India may reflect potential differences in office building materials and the lack of restrictions for phthalates compared to the USA, UK, and later in China (The People’s Republic of China, 2014; Y. Wang et al., 2019).
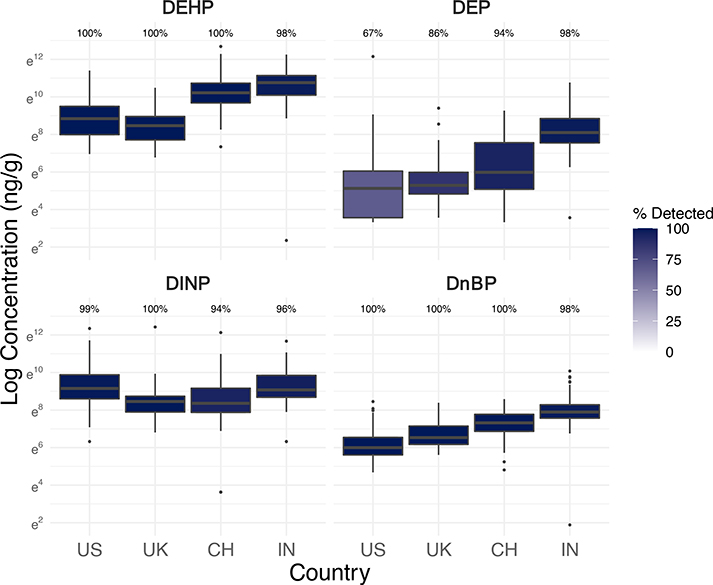
Figure 3.
Log concentrations (ng/g-wristband, scaled to 32 hours of sampling) and detection frequencies of select phthalates in silicone wristbands worn by 251 office workers in the USA, UK, China, and India (see Figure S5 for boxplots of more phthalates and phthalate alternatives).
The European Union (EU), which the UK was a member state of during our study, has restricted six phthalates in children’s toys starting since 1999, including DINP, DEHP, DnBP, and BzBP (Y. Wang et al., 2019; Wittassek et al., 2011). DEHP, DnBP, and BzBP were also prohibited in cosmetics in 2009 by the EU (European Union, 2016). The USA followed the EU in restricting the same phthalates in children’s toys in 2008 (US Consumer Product Safety Commission, 2008), and China passed a similar standard effective 2016 (The People’s Republic of China, 2014). Even though specific to children’s products, these regulations may have increased awareness and reduced total use of phthalates in other applications (Sackmann et al., 2018).
In our study, office worker exposures to DEHP and DnBP in the UK and USA were all much lower than exposures in China and especially in India, as expected given differences in phthalate regulations and their timing by country (Figure 3). However, DINP exposures were actually the lowest in both the UK and China compared to the USA and India. In regression models of key phthalates selected from principal component analysis (Figure S1), DINP levels in wristbands were 59% lower in the UK (95% CI: −76– −30%; p=0.0044) and 60% lower in China (95% CI: −77– −31%; p=0.0049) compared to the USA, adjusted for building age, vinyl flooring, scented clothes, product user type, handwashing, and wearing wristband under sleeve (Table 2). In this study, China may not have had higher DINP exposures than the UK, despite the much earlier implementation of the UK’s restriction, because DEHP exposures in China are still higher (Figure 3) and DINP is a common DEHP substitute. A previous study found clear trends in urinary metabolite levels of DEHP declining while DINP increased in the USA general population (Zota et al., 2014). Both of these high molecular weight phthalates are used in PVC materials and plastisol applications including food packaging, cables and wiring, flooring, and furniture (Wittassek et al., 2011). DnBP, a lower molecular weight phthalate, is used in cosmetics, food packaging, and some PVC materials (Zota et al., 2014).
Table 2.
Results of multilevel linear regression models of log concentrations of key phthalates and phthalate alternatives (as determined by principal component analysis) in silicone wristbands worn by office workers in the USA, UK, China, and India.
| Variable | Phthalates | Phthalate Alternatives | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DiBP | DINP | DEP | DEHA | DEHT | TOTM | |||||||
| % Change | p | % Change | p | % Change | p | % Change | p | % Change | p | % Change | p | |
| Country (Ref: United States) | ||||||||||||
| United Kingdom | −1.48% | 0.95 | −58.6% ** | 0.0044 | 10.10% | 0.84 | −40.4%. | 0.096 | −16.60% | 0.59 | 50.70% | 0.25 |
| China | 289% *** | <0.0001 | −60.3% ** | 0.0049 | 45.40% | 0.45 | −67.7% ** | 0.0015 | −13.20% | 0.7 | −57.5% * | 0.028 |
| India | 254% *** | <0.0001 | −12.90% | 0.65 | 1420% *** | <0.0001 | 6.96% | 0.83 | 36.90% | 0.37 | 97.3%. | 0.068 |
| Year of Building Construction | −0.07% | 0.81 | 0.10% | 0.79 | 1.12%. | 0.062 | 0.01% | 0.97 | −0.61% | 0.16 | −0.26% | 0.56 |
| Vinyl Flooring in Workstation | 127% * | 0.011 | −6.91% | 0.86 | −22% | 0.63 | 24.10% | 0.55 | −36.80% | 0.3 | 33.10% | 0.56 |
| Wore Clothes With Scented Wash | 9.67% | 0.46 | 19.60% | 0.31 | 0.71% | 0.97 | 55.1% ** | 0.0017 | 33.6%. | 0.1 | 34.40% | 0.15 |
| Latent Class (Ref: Minimal Product Users) | ||||||||||||
| Deodorant Users | −1.38% | 0.91 | −17.50% | 0.28 | 52.4% * | 0.026 | 4.53% | 0.74 | −8.81% | 0.59 | −30.5%. | 0.079 |
| Perfume and Makeup Users | 14.80% | 0.35 | −21% | 0.26 | 83.9% ** | 0.0066 | −0.64% | 0.97 | −5.96% | 0.76 | −41.9% * | 0.027 |
| Average # Times Hands Washed | 4.52% | 0.34 | 1.04% | 0.87 | −6.01% | 0.38 | 2.20% | 0.66 | −4.02% | 0.52 | −10% | 0.17 |
| Wore Wristband Under Sleeve | −6.10% | 0.58 | −7.85% | 0.62 | 14.10% | 0.45 | −6.18% | 0.61 | −20.90% | 0.15 | −13.40% | 0.45 |
Note: Model outcomes were log-transformed before analysis, so model estimates were transformed to percent differences.
DEP, an unrestricted, low molecular weight phthalate commonly used in perfume, makeup, and other personal care products, had significantly lower levels and detection frequencies in wristbands from the USA (67%) compared to other countries (UK: 86%; China: 95%; India: 98%) (Figure 3) (Y. Wang et al., 2019). In fact, wristbands from India had 1,420% higher DEP levels than the USA (95% CI: 549–3,450%; p<0.001) (Table 2). The lower exposures in the USA, even compared to the UK, may be partially explained by extensive advocacy efforts in the USA to reduce phthalates in cosmetics, a reason why urinary metabolite levels of DEP in the USA general population declined more than other phthalates with legislative measures (Zota et al., 2014). The wristband levels of DEP in our study were much higher in India than China (Figure 3), which aligns with previous research showing that the predominant urinary metabolite in India was DEP, unlike in China (Guo et al., 2011; Y. Wang et al., 2019). In contrast to DEP, BzBP was restricted in cosmetics in the EU (European Union, 2016), which could theoretically extend to voluntarily phase-outs in other products, such as its substantial use in vinyl flooring (Hammel et al., 2019). The comparative lack of regulatory restriction of BzBP in any products in the USA may help explain the higher concentrations and detections of BzBP in wristbands from the USA than the UK (Figure S5).
Our regression models identified that phthalate exposures were related to product user types defined from latent class analysis (Figure S14). Perfume/makeup users had an estimated 84% higher levels of DEP in wristbands (95% CI: 21–186%; p=0.0066) and deodorant users had 52% higher levels (95% CI: 7–123%; p=0.026) compared to minimal product users (Table 2). This association has also been observed previously for urinary metabolite levels of DEP (Braun et al., 2013; Just et al., 2010; Nassan et al., 2019) and is consistent with common DEP use in cosmetic and personal care products. Our calculated product user types did capture potential gender differences and still showed significant country associations (such as higher DEP in India) despite some differences in gender breakdowns by country (Table S1). Among participants who reported gender (n=217), most males (73%) were classified as minimal product users, whereas only 32% of females were.
On the other hand, vinyl flooring in workstations was associated with 127% higher DiBP levels (95% CI: 26–325%; p=0.011) in wristbands than workstations without vinyl flooring (Table 2). PVC flooring is a common application of phthalates, although not thought to be a main use for DiBP (Wittassek et al., 2011; Zota et al., 2014). However, in our study DiBP loaded strongly onto the first principal component along with DEHP, suggesting potentially similar sources such as PVC (Figure S1).
Office workers in all four countries had ubiquitous exposures to understudied plasticizers used as phthalate alternatives, especially in place of DEHP in food packaging and other PVC materials (Table S4; Figure S5). DEHT, detected in 99% of wristbands and at appreciable concentrations compared to other phthalates (Figure S5), is a structural isomer to DEHP and major non-phthalate substitute (Frederiksen et al., 2020). DEHA is another alternative plasticizer detected in 95% of wristbands (Table S4). Finally, we detected TOTM in 99% of wristbands. By contrast, a previous study of German children only found TOTM metabolites in less than 5% of urine samples and TOTM in less than 15% of 122 house dust samples, and the authors hypothesized that TOTM exposure in the general population might not be expected yet due to its main use in medical devices (Murawski et al., 2020). The geometric mean levels of each of these three phthalate alternatives in wristbands were also higher in our study compared to scaled levels in a previous wristband-based study of children in the USA in 2015 (Figure S10) (Hammel et al., 2020). Our study’s different findings in the common exposure to TOTM may be partially due to later sampling years, sampling of adults in office buildings and in other countries, and the additional use of TOTM in electrical cords which may be more prevalent in offices (Murawski et al., 2020).
Interestingly, we found that wearing clothes washed with a scented detergent/conditioner/softener product was associated with 55.1% significantly higher exposures to DEHA (95% CI: 19–102%; p=0.0017) and 33.6% higher exposures to DEHT (95% CI: −5– −86%; p=0.10) compared to office workers who did not, adjusted for country, building age, vinyl flooring, product user type, handwashing, and wearing wristband under sleeve (Table 2). This finding suggests that DEHA and DEHT may not just be used in PVC materials but also fragranced products; similarly the more traditional phthalate DEHP has been found in many perfumes although at lower levels than DEP (Al-Saleh and Elkhatib, 2016). By contrast, deodorant users were estimated to have 42% lower TOTM exposures (95% CI: −64– −6%; p=0.027) than minimal product users, an unusual result perhaps because other phthalates or alternatives are more common in personal care products or because other behaviors are associated with deodorant users (Table 2). Although the phthalate alternatives are hypothesized to be less toxic than DEHP, more human toxicological and epidemiological research is needed to determine potential health effects, population exposures, and sources of these understudied chemicals (Frederiksen et al., 2020; Murawski et al., 2020).
3.5. Pesticides
Previous research has focused on outdoor and occupational exposures to pesticides more than indoor exposures. Our use of silicone wristbands shows that office workers are commonly exposed to pesticides inside office buildings, whether they are directly applied in buildings or carried in from outdoors or the home (Figure 4). We found that exposures to certain banned pesticides have persisted, and that exposures to organophosphate and pyrethroid alternative pesticides were common. Higher exposures to some pesticides in India may reflect malaria control challenges.
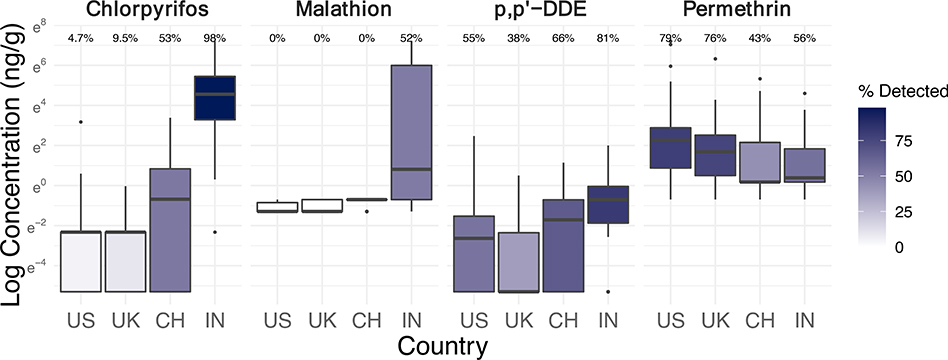
Figure 4.
Log concentrations (ng/g-wristband, scaled to 32 hours of sampling) and detection frequencies of select pesticides in silicone wristbands worn by n=243–251 office workers in the USA, UK, China, and India (see Figure S6 for boxplots of more pesticides).
P,p’-DDE is a breakdown product of the legacy organochlorine pesticide dichloro-diphenyl-trichloroethane (DDT) (Qiu et al., 2004). In our study, office workers in India experienced significantly higher exposures to DDE than any other country (Figure 4). Compared to the USA, DDE levels in wristbands were 540% higher in India (95% CI: 98–1,970%; p=0.0054), adjusted for building age, indoor pesticide application in last year, eating raw produce at desk, handwashing, and wearing wristband under sleeve (Table 3). The global distribution of DDT compounds (including DDE) in human milk has been highly explained by tropical countries (such as India) where malaria is still an important public health challenge (M. van den Berg et al., 2017). In fact, India has the highest ongoing production and use of DDT (H. van den Berg et al., 2017). By contrast, technical DDT was banned in the USA in 1972, the EU in 1983, and China for agricultural use in 1983 (Qiu et al., 2004; Wong et al., 2005). DDT production in China stopped in 2008 (H. van den Berg et al., 2017). Despite decades-old measures to ban DDT, its breakdown product DDE was still detected in 55% of samples from the USA, 38% of samples from the UK, and 66% of samples from China, highlighting continued issues with extremely persistent legacy chemicals (Table S4).
Table 3.
Results of multilevel linear regression models of log concentrations of commonly detected pesticides in silicone wristbands worn by office workers in the USA, UK, China, and India.
| Variable | Chlorpyrifos | Cypermethrin | Malathion | p,p’-DDE | Permethrin | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Change | p | % Change | p | % Change | p | % Change | p | % Change | p | |
| Country | ||||||||||
| United States | Excluded | Excluded | Excluded | Reference | Reference | |||||
| United Kingdom | Reference | Excluded | Excluded | −39% | 0.39 | −41.80% | 0.19 | |||
| China | 300% | 0.39 | −98.7% *** | <0.0001 | Excluded | 131% | 0.18 | −64.5% * | 0.029 | |
| India | 396000% *** | <0.0001 | Reference | – | 540% ** | 0.0054 | −63.2% * | 0.037 | ||
| Year of Building Construction | 1.33% | 0.83 | 60.1% *** | 0.00042 | 27% | 0.45 | −0.61% | 0.39 | 0.33% | 0.53 |
| Pesticide Applied in Last Year | −30.90% | 0.73 | 517% * | 0.042 | 580% | 0.55 | 60.10% | 0.29 | −19.90% | 0.5 |
| Ate Raw Produce At Desk | 62.10% | 0.18 | −47.10% | 0.38 | −12.30% | 0.7 | −27.50% | 0.28 | 3.58% | 0.87 |
| Average # Times Hands Washed | −1.18% | 0.92 | 25.60% | 0.39 | −32% * | 0.021 | −5% | 0.66 | 0.81% | 0.93 |
| Wore Wristband Under Sleeve | 15.40% | 0.68 | 286% * | 0.048 | −15.90% | 0.46 | 0.38% | 0.99 | −19.50% | 0.31 |
Excluded = fewer than 5% of samples in the country had detectable levels so were excluded from model.
Note: Model outcomes were log-transformed before analysis, so model estimates were transformed to percent differences.
Chlorpyrifos is an organophosphate pesticide developed as a DDT substitute (N’Guessan et al., 2010) with again substantially higher exposures in office workers from India compared to any other country (Figure 4). Chlorpyrifos levels in wristbands were 393,000% higher in India than the UK (95% CI; 26,700–5,690,000%; p<0.001) (Table 3). Chlorpyrifos was detected in nearly all (98%) wristbands from India, in about half (53%) of those from China, and very rarely in those from the UK (10%) and USA (5%) (Table S4). The pesticide was banned for all but one use in the EU effective 2016, was voluntarily phased out in certain applications in the USA since 2000, and was restricted on vegetables effective 2016 in China (EU Health and Safety Executive, 2016; The People’s Republic of China, 2013; US EPA, 2020). The higher exposures in participants from India reflects the comparative lack of chlorpyrifos restrictions in India (Government of India, 2020). The use of chlorpyrifos as a substitute pesticide has reportedly increased in India after the ban on organochlorine pesticide endosulfan in 2011 (Bonvoisin et al., 2020).
Malathion, another organophosphate insecticide with diverse applications, was exclusively detected in wristbands from India (52%) even though the pesticide is approved for use in all four countries (Figure 4) (Guyton et al., 2015; Handford et al., 2015; United Nations, 2015). Again, malaria control in India likely explains this exposure difference, especially since malathion is applied to interior walls and roofs of buildings in India for that purpose (Guyton et al., 2015).
Although office workers from India still had the highest exposure levels and detections of cypermethrin (56%), those from China did have appreciable detections (36%) unlike the UK (0%) or USA (4%) (Table S4; Figure 4). Cypermethrin is a pyrethroid pesticide commonly used in agricultural settings (Tang et al., 2018) and that does have approved uses in the studied countries (European Union, 2005; United Nations, 2015). Cypermethrin exposures were 99% lower in China compared to India (95% CI: −100– −95%; p<0.001) (Table 3). Among buildings in India and China, cypermethrin levels in wristband samples were also 517% higher (95% CI: 52–2,410%; p=0.042) in buildings that reportedly had applied chemical pesticide in the last year compared to those who had not, indicating a potential indoor source. Sixty-seven percent of buildings in India (6 of 9), 67% of buildings in China (4 of 6), 27% of buildings in the USA (4 of 15), and none in UK reported pesticide applications in the last year (Table S1). Interestingly, a one-year decrease in building age was associated with an estimated 60% increase in exposures to cypermethrin in India and China (95% CI: 36–89%; p=0.00042) (Table 3). This model included buildings constructed between 2003 and 2017 in India and China (Table S1). Although the reason for newer buildings having higher cypermethrin exposures is not clear, newer buildings may have had differences in pesticide applications, garden care, pesticide-impregnated building materials, or nearby agricultural use.
In contrast to the other pesticides, permethrin was widely detected in all countries, with higher concentrations and detection frequencies in the USA (79%) and UK (76%) compared to India (56%) or China (43%) (Figure 4). This finding is consistent with the fact that permethrin is another common pyrethroid chemical used more in urban non-agricultural settings and found at high levels in indoor environments (Tang et al., 2018). Permethrin levels in wristbands were 65% lower in China (95% CI: −85– −18%; p=0.029) and 63% lower in India (95% CI: (−85– −13%; p=0.037) compared to the USA (Table 3). Permethrin was actually not approved for use as a plant protection product in the EU (European Union, 2000), so indoor pest control may be more likely to influence exposures in the studied buildings. Previous studies have found that permethrin and cypermethrin are some of the most commonly detected pesticides in homes in the USA and have been linked to home pesticide treatments (Deziel et al., 2015; Lu et al., 2013).
Other less frequently detected pesticides were not as well explained by regulation. Lindane was mostly found in wristbands from UK office workers (Figure S5), even though the pesticide has not been approved for most uses by the EU (Handford et al., 2015). By contrast, India did not have elevated lindane concentrations, likely because of the 2013 national ban (Bonvoisin et al., 2020). On the other hand, chlordane had appreciable detections in only wristbands from the USA (39%) even though the legacy pesticide was banned in 1988 (US EPA, n.d.); it was also banned in India in 1996 and has not been approved by the EU or China (Bonvoisin et al., 2020; United Nations, 2015). The findings on chlordane and lindane highlight persistent exposures to legacy pesticides. Azoxystrobin, an unrestricted pesticide (United Nations, 2015), had some detections in samples from the USA (31%) and UK (38%) but not China (0%) or India (2%) (Table 1). In addition to its use as a fungicide in agriculture and landscaping, azoxystrobin has been shown to be in mold-resistant dry wall and migrate out into dust (Cooper et al., 2020). Other uncommon pesticides in wristbands included atrazine, chlorfenapyr, fipronil, and trifloxystrobin (Table 1). Season would not explain away country differences in pesticide exposures, which were similar in the UK and USA despite different seasons (February versus mostly April).
3.6. Polycyclic Aromatic Hydrocarbons
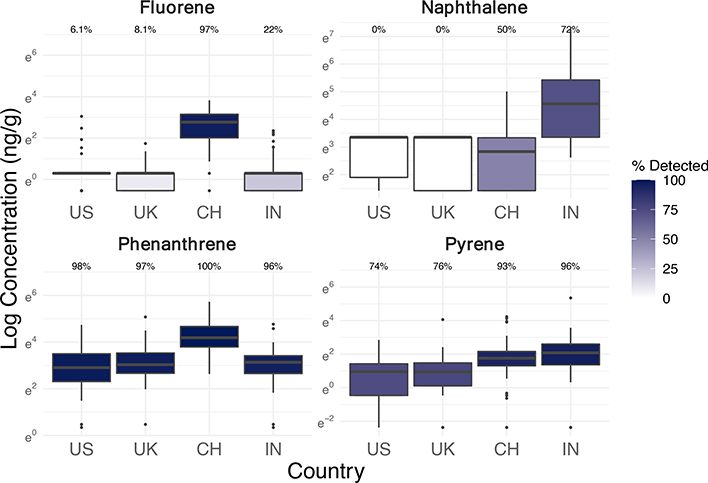
Exposures of office workers to PAHs differed considerably by country, with the highest levels in China and India (Figure 5). Most of the detected PAHs were lower molecular weight compounds, including phenanthrene, pyrene, fluoranthene, fluorene, naphthalene, and acenaphthene (Table S4) (Ravindra et al., 2008). All of these six PAHs had the highest detection frequencies and concentrations in wristbands from China and India compared to the USA or UK (Table S4; Figure S12). These detection frequencies align with inventories that attribute 50% of global PAH emissions to naphthalene and another 38% to other low molecular weight PAHs (Zhang and Tao, 2009). The country disparities align with previous research finding that China and India have the highest inventoried PAH emissions across the world, some of the highest emission densities, and higher PAH environmental and indoor dust contamination than high-income countries (Ma and Harrad, 2015; Ramesh et al., 2012; Shen et al., 2013; Zhang and Tao, 2009). PAH concentrations in ambient air tend to increase longitudinally in China and India towards urban areas of denser populations, traffic, and industrial activities (Hong et al., 2016). Whereas PAH concentrations in the USA have decreased in indoor air and dust over the last few decades, they have not done so in Asia (Ma and Harrad, 2015). All the office buildings in this study were located in cities.
Figure 5.
Log concentrations (ng/g-wristband, scaled to 32 hours of sampling) and detection frequencies of select PAHs in silicone wristbands worn by 243 office workers in the USA, UK, China, and India (see Figure S7 for boxplots of more PAHs).
Of particular note in our study, fluorene was found in 97% of wristbands in China with much higher concentrations than any other country, whereas it was only detected in 22% from India and less than 10% from the USA and UK (Figure S7; Table 1). However, China and India had comparable concentrations and detections (93–96%) of pyrene in wristbands. By comparing concentrations of these two chemicals across countries, we can hypothesize that PAH exposures in China may have had higher contributions from diesel traffic (higher relative fluorene) and India from gasoline traffic (higher relative pyrene), based on previously studied diagnostic ratios (Ravindra et al., 2008). We did not evaluate other PAH diagnostic ratios or ratios within a wristband or within a country due to insufficient detections of certain compounds and differences in sampling rates (i.e. octanol-air partition coefficients) of each PAH onto wristbands, which limits comparisons across chemicals but not across countries.
Naphthalene was only detected in wristbands from India (72%) and China (50%), and levels were still much higher in India than China (Figure 5). This trend for naphthalene did not match country differences for any other commonly detected PAH, which suggests a potentially unique emission source for naphthalene (Figure S7). Naphthalene was actually the only PAH reportedly emitted from consumer product usage according to global inventories (Zhang and Tao, 2009). Other than fossil fuel combustion, a major source of naphthalene are moth preventatives, and other uses include toilet deodorizers, fumigants, pesticides, caulking, and carpet pads (Jia and Batterman, 2010; Zhang and Tao, 2009). One study found that the vast majority of naphthalene in indoor air from non-smoking homes in China was attributed to mothball emissions (Zhu et al., 2009). In addition, the significant correlation of naphthalene levels in wristbands with several pesticides suggests pesticide products are a contributing indoor or outdoor source (chlorpyrifos: r=0.69, p<0.001; cypermethrin: r=0.64, p<0.001) (Table S13).
Although only 3% of participants reportedly smoked cigarettes during sampling, more participants from India (30%) and China (27%) spent time around another smoker than in the UK (19%) and USA (14%) (Table S1). Secondhand smoke exposure may have slightly influenced some PAH exposures, but for naphthalene, cigarettes are only a minor source indoors (Batterman et al., 2012).
Several frequently detected PAHs in our study were positively correlated with average indoor PM2.5 concentrations in the buildings during the study week (available for 87% of participants): pyrene (Spearman r=0.32; p<0.001), fluoranthene (r=0.32; p<0.001), fluorene (r=0.25; p<0.001), phenanthrene (r=0.22; p=0.0014), and acenaphthene (r=0.20; p=0.0043). They were even more correlated with average outdoor PM2.5 over the study week, based on nearest hourly measurements within 50 km from government-approved outdoor sensors (via OpenAQ) as described in Jones et al., 2021 (data available for 82% of participants, none from India): pyrene (r=0.38; p<0.001), fluoranthene (r=0.41; p<0.001), fluorene (r=0.57; p<0.001), phenanthrene (r=0.47; p<0.001), and acenaphthene (r=0.37; p<0.001). Thus, outdoor emissions of many PAHs likely contribute to indoor exposures. On the other hand, naphthalene was only negatively correlated with average outdoor PM2.5 (r=−0.17; p=0.020). Naphthalene was also not significantly correlated with average indoor PM2.5 (r=0.12; p=0.084), and only negatively so in India (r=−0.12; p=0.40), where exposures to naphthalene were the highest, which again suggests a major indoor source for naphthalene in the buildings.
3.7. Building Variability, Sampling Factors, and Indoor Air Quality Measurements
In our models with random intercepts for the building (Tables 1–3), the majority of variability in log concentrations of most chemicals was attributed to differences between individuals within buildings, as opposed to differences between buildings (Table S6). This may indicate that building materials and practices vary even within a space and that personal product uses are also important factors. Figure S15 shows that more variation in many chemical exposures occurs at the country level when compared to the building level.
Handwashing and clothing may have influenced participant’s sampled chemical exposures. A one-unit increase in the average daily number of times someone washed their hands was associated with 32% lower levels of the pesticide malathion in wristbands in India (95% CI: −50– −8%; p=0.021), adjusted for building age, recent pesticide application, raw produce at desk, and wearing wristband under sleeve (Table 3). We also found 54% lower PCB-101 levels in wristbands among USA participants for a one-unit increase in hand-washing frequency (95% CI: −78– −8%; p=0.034), adjusted for building age and wearing wristband under sleeve (Table 1). This finding is consistent with previous research showing lower contamination of pesticides and other SVOCs after simple handwashing (Marquart et al., 2002; Nielsen, 2010; Watkins et al., 2011). We do not expect handwashing to significantly affect wristband sampling efficiencies, as chemicals absorb into the material.
Furthermore, wearing wristbands underneath clothing sleeves at least one study day was associated with 286% higher cypermethrin levels among participants in China and India (95% CI: 7–1,320%; p=0.048) and 105% higher PCB-101 levels in the USA (95% CI: −11–356%; p=0.09) compared to those who did not (Table 3; Table 1). Clothes can act as reservoirs and mediators of exposure to certain airborne chemicals, including PCBs, and thus extend dermal exposures beyond the time spent in contaminated buildings (Licina et al., 2019; Morrison et al., 2018). On the other hand, we found 34% lower BDE-209 levels for those wearing wristbands underneath sleeves (95% CI: −51– −10%; p=0.0085) (Table 1), which suggests that clothes may act as barriers against airborne compounds that are less volatile and are primarily particle-bound (like BDE-209) (Watkins et al., 2013).
In sensitivity analyses, we assessed the impact of indoor environmental measurements on results of our primary statistical models. We added covariates for average indoor air exchange rate (calculated from CO2), PM2.5, temperature, and relative humidity during the study week based on sensors located at participant workstations. We did not find appreciable differences in the results of our primary covariates except for changes in the precision of some estimates but negligible changes in actual point estimates. Decreases in precision were likely due to the smaller sample size (32 participants could not be included due to sensor issues) (Table S7).
3.8. Public Health Implications
This study found several limitations in market and regulatory approaches that should be addressed to reduce indoor chemical exposures in buildings. First, chemical restrictions do not always occur, or apply equally, in every country. For example, exposures to DDE were much higher in India, where the parent pesticide is still used for malaria control even though it was banned in 1972 in the USA, in 1983 in the EU, and in 1983 for agricultural uses in China. The flame retardant BDE-209 was phased out in the USA in 2013 (where exposures were the lowest), but it still does not have restrictions in China, has only very limited restrictions in India, and was only recently phased out in the UK in 2019. Exposures to phthalate plasticizers DEHP and DnBP were lower in the USA and UK where there have been more (or earlier) restrictions compared to India (or China). Even though the phthalate restrictions mostly applied to children’s toys or cosmetics, they may extend to voluntary elimination in other products. Given substantial country-level differences in chemical exposures and regulations, this study highlights the importance of conducting more research about exposure disparities in understudied LMICs (Goodman et al., 2020).
Second, even when chemicals are eliminated, many buildings still contain the legacy chemicals for decades. This is especially evident for office workers exposed to PCBs and PBDEs from building materials present in older buildings in the USA and UK despite bans implemented decades ago. Several other legacy pesticides, including DDE and chlordane, exposed some office workers even in the USA where they had been banned over three decades ago.
Third, chemical classes phased out from certain uses may appear in other applications. PCB-11, a contemporary byproduct in pigmented materials, was widely detected in the studied office buildings despite bans on intentional uses of legacy PCBs. Similarly, the recycling of discarded products, such as PBDE-containing plastic electronics, could cause phased-out chemicals to carry over into the manufacturing of new products, although we did not study this specific source (Y. Li et al., 2019; Turner, 2018; Turner and Filella, 2017).
Fourth, phased-out chemicals are frequently replaced with similar, harmful substitutes (Zimmerman and Anastas, 2015). We observed common exposures to organophosphate or pyrethroid pesticides often used as substitutes to organochlorine pesticides. Furthermore, this study found significant exposures of office workers to replacements to legacy PBDEs in several countries, including novel BFRs and possibly OPEs (also used as plasticizers). We found widespread exposures to emerging phthalates and phthalate alternatives used to substitute traditional phthalates. Exposures to certain flame-retardant and plasticizer chemicals were significantly associated with the presence of foam furniture, carpet, or vinyl flooring in the building, and may be related to other types of products we did not survey. As a result, building materials represent an important point of intervention. Eliminating entire classes of toxic chemicals (such as flame retardants) from materials to prevent harmful ingredient substitution has been shown to substantially reduce chemical levels in building dust (Young et al., 2020).
Fifth, personal care products within buildings influences exposures to certain chemicals, not just the materials, building, country, or outdoor environment. Our results showed that cosmetics (such as perfume, makeup, and deodorant) influenced exposures to the phthalate DEP, and scented laundry products influenced exposures to two phthalate substitutes.
3.9. Strengths and Limitations
A unique strength of this study was the use of silicone wristbands as simple, non-invasive samplers of chemical exposures in office buildings across the world, including the USA, UK, and two understudied LMICs: India and China. Our study design allowed us to isolate exposures specifically in workplaces and to collect extensive data about building factors, workstation characteristics, personal behaviors, sampling factors, and indoor air quality. This information and our large sample size allowed us to conduct statistical modelling of predictors of chemical exposures with wristbands and to control for air quality factors in sensitivity analyses, both of which have largely been unavailable to previous wristband-based studies. In addition, we reported on concentrations of a large set of 99 analytes, and the wristbands were able to capture both primarily gas-phase (e.g. BDE-47) and particle-bound chemicals (e.g. BDE-209).
Limitations include that our convenience sample of office buildings occupying commercial real estate cannot generalize to all types of office environments in these countries. Due to unknowns about different sampling rates of chemicals onto wristbands, we could not convert chemical masses in wristbands to air or dust concentrations, and we could not accurately compare concentrations across chemicals. However, we did have internal validity within each chemical, so could compare a chemical’s concentrations by country and other factors. Finally, sampling was conducted in different months in each country, but we do not anticipate this significantly affected our takeaways because the study was conducted on indoor climate-controlled environments, and our sensor data allowed us to control for minor differences in temperature and humidity; the sensitivity analyses showed that our results were robust (with some reductions in statistical power).
4. Conclusions
The use of novel silicone wristband samplers allowed us to isolate and measure chemical exposures inside office buildings across the USA, UK, China, and India. We found much higher exposures of office workers in India and/or China to many phthalates, pesticides, and PAHs, likely related to the fewer or later chemical restrictions, the malaria challenges in India, and higher outdoor sources of PAHs from fuel combustion. By contrast, there were higher exposures to many BFRs and OPEs in the USA or UK, reflective of more stringent historic flammability standards that necessitated chemical flame retardant use. In addition, we found that building occupants are still exposed to legacy chemicals many years after they were banned, including PBDEs, pesticides, and PCBs (which have contemporary unintentional PCB sources not covered under the ban). Or, if other countries did not implement the same bans, office workers often experienced even higher exposures. Furthermore, exposures to substitutes to certain phased-out PBDEs, phthalates, and pesticides were widespread, which motivates the need for careful toxicological evaluation of any chemical substitutes before use, the need to avoid entire classes of toxic chemicals (e.g. flame retardants, phthalates) in products instead of addressing chemicals one at a time, and the need to use non-chemical methods when possible (e.g. integrated pest management). The decades-long life span of building materials and the chemicals in them urges the need for forward-looking decisions on healthier materials.
Supplementary Material
Highlights.
Silicone wristbands captured worker exposures to 99 chemicals in office buildings
Wristbands were worn for four days only at work and reflected external exposures
Indoor chemical exposures varied substantially across the USA, UK, India, and China
Workers were exposed to certain chemicals years after bans and to their substitutes
5. Acknowledgments
This research was made possible by NIOSH Grant T42 OH008416, NIEHS Grant ES000002, NIEHS T32 ES007069, the Marilyn Hoffman Program on Chemicals and Health at Harvard, and a gift from Carrier Global Corporation (Carrier). Financial support for wristband analyses was provided by a donation to Heather Stapleton’s laboratory by the Michael and Annie Falk Foundation. Additional support was provided by Jones Lang LaSalle, Inc. (JLL) and by several companies that participated in this study, whose identities we cannot disclose because it could inadvertently breach participant confidentiality and who provided funds to cover the costs of study equipment for their building(s). JLL, Carrier, and the companies that participated in the study were not involved in study design, data collection, data analysis, data interpretation, data presentation, or drafting of the manuscript. We would like to thank Maya Bliss, Skye Flanigan, Jose Vallarino, Xiaodong Cao, Sydney Robinson, and Duncan Hay for their help with the study.
Footnotes
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbasi G, Saini A, Goosey E, Diamond ML, 2016. Product screening for sources of halogenated flame retardants in Canadian house and office dust. Sci. Total Environ. 545–546, 299–307. 10.1016/j.scitotenv.2015.12.028 [DOI] [PubMed] [Google Scholar]
- Al-Saleh I, Elkhatib R, 2016. Screening of phthalate esters in 47 branded perfumes. Environ. Sci. Pollut. Res. 23, 455–468. 10.1007/s11356-015-5267-z [DOI] [PubMed] [Google Scholar]
- Allen JG, Gale S, Zoeller RT, Spengler JD, Birnbaum L, McNeely E, 2016. PBDE flame retardants, thyroid disease, and menopausal status in U.S. women. Environ. Health 15, 60. 10.1186/s12940-016-0141-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JG, McClean MD, Stapleton HM, Webster TF, 2008. Linking PBDEs in House Dust to Consumer Products using X-ray Fluorescence. Environ. Sci. Technol. 42, 4222–4228. 10.1021/es702964a [DOI] [PubMed] [Google Scholar]
- Anderson KA, Points GL 3rd, Donald CE, Dixon HM, Scott RP, Wilson G, Tidwell LG, Hoffman PD, Herbstman JB, O’Connell SG, 2017. Preparation and performance features of wristband samplers and considerations for chemical exposure assessment. J. Expo. Sci. Environ. Epidemiol. 27, 551–559. 10.1038/jes.2017.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anezaki K, Kannan N, Nakano T, 2014. Polychlorinated biphenyl contamination of paints containing polycyclic- and Naphthol AS-type pigments. Environ. Sci. Pollut. Res. Int. 22. 10.1007/s11356-014-2985-6 [DOI] [PubMed] [Google Scholar]
- Batterman S, Chin J-Y, Jia C, Godwin C, Parker E, Robins T, Max P, Lewis T, 2012. Sources, concentrations, and risks of naphthalene in indoor and outdoor air. Indoor Air 22, 266–278. 10.1111/j.1600-0668.2011.00760.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 57, 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Bi X, Yuan S, Pan X, Winstead C, Wang Q, 2015. Comparison, association, and risk assessment of phthalates in floor dust at different indoor environments in Delaware, USA. J. Environ. Sci. Health. A. Tox. Hazard. Subst. Environ. Eng. 50, 1428–1439. 10.1080/10934529.2015.1074482 [DOI] [PubMed] [Google Scholar]
- Boas M, Feldt-Rasmussen U, Main KM, 2012. Thyroid effects of endocrine disrupting chemicals. Mol. Cell. Endocrinol. 355, 240–248. 10.1016/j.mce.2011.09.005 [DOI] [PubMed] [Google Scholar]
- Bonvoisin T, Utyasheva L, Knipe D, Gunnell D, Eddleston M, 2020. Suicide by pesticide poisoning in India: a review of pesticide regulations and their impact on suicide trends. BMC Public Health 20, 251. 10.1186/s12889-020-8339-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R, 2013. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J. Expo. Sci. Environ. Epidemiol. 24, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brommer S, Harrad S, 2015. Sources and human exposure implications of concentrations of organophosphate flame retardants in dust from UK cars, classrooms, living rooms, and offices. Environ. Int. 83, 202–207. 10.1016/j.envint.2015.07.002 [DOI] [PubMed] [Google Scholar]
- Brouwer A, Longnecker MP, Birnbaum LS, Cogliano J, Kostyniak P, Moore J, Schantz S, Winneke G, 1999. Characterization of potential endocrine-related health effects at low-dose levels of exposure to PCBs. Environ. Health Perspect. 107, 639–649. 10.2307/3434557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RD, Todd SW, Lumsden E, Mullins RJ, Mamczarz J, Fawcett WP, Gullapalli RP, Randall WR, Pereira EFR, Albuquerque EX, 2017. Developmental neurotoxicity of the organophosphorus insecticide chlorpyrifos: from clinical findings to preclinical models and potential mechanisms. J. Neurochem. 142 Suppl, 162–177. 10.1111/jnc.14077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte W, 2004. Sources and Impacts of Pesticides in Indoor Environments BT - Air Pollution: Indoor Air Pollution, in: Pluschke P (Ed.),. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 89–116. 10.1007/b94832 [DOI] [Google Scholar]
- California Environmental Protection Agency, 2020. Tris (2-chloroethyl) phosphate [WWW Document]. URL https://oehha.ca.gov/chemicals/tris2-chloroethyl-phosphate
- Carignan CC, Mínguez-Alarcón L, Butt CM, Williams PL, Meeker JD, Stapleton HM, Toth TL, Ford JB, Hauser R, 2017. Urinary concentrations of organophosphate flame retardant metabolites and pregnancy outcomes among women undergoing in vitro fertilization for the EARTH study team. Environ. Health Perspect. 125, 8. 10.1289/EHP1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals-Casas C, Desvergne B, 2011. Endocrine disruptors: from endocrine to metabolic disruption. Annu. Rev. Physiol. 73, 135–162. 10.1146/annurev-physiol-012110-142200 [DOI] [PubMed] [Google Scholar]
- Chinese Ministry of Industry and Information Technology, 2016. Administrative Measures for the Restriction of the Use of Hazardous Substances in Electrical and Electronic Products. [Google Scholar]
- Choi G, Wang Y-B, Sundaram R, Chen Z, Barr DB, Buck Louis GM, Smarr MM, 2019. Polybrominated diphenyl ethers and incident pregnancy loss: The LIFE Study. Environ. Res. 168, 375–381. 10.1016/j.envres.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S-S, Zhang C, 2011. An evaluation of legislative measures on electrical and electronic waste in the People’s Republic of China. Waste Manag. 31, 2638–2646. 10.1016/j.wasman.2011.07.010 [DOI] [PubMed] [Google Scholar]
- Conolly C, Davis R, Dore C, Gardner M, Horn J, Wenborn M, Whiting R, Xu Y, 2009. Review and Update of the UK Source Inventories of Dioxins, Dioxin-Like Polychlorinated Biphenyls and Hexachlorobenzene for Emissions to Air, Water and Land: Report to the Department for Environment Food and Rural Affairs. [Google Scholar]
- Cooper EM, Kroeger G, Davis K, Clark CR, Ferguson PL, Stapleton HM, 2016. Results from Screening Polyurethane Foam Based Consumer Products for Flame Retardant Chemicals: Assessing Impacts on the Change in the Furniture Flammability Standards. Environ. Sci. Technol. 50, 10653–10660. 10.1021/acs.est.6b01602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EM, Rushing R, Hoffman K, Phillips AL, Hammel SC, Zylka MJ, Stapleton HM, 2020. Strobilurin fungicides in house dust: is wallboard a source? J. Expo. Sci. Environ. Epidemiol. 30, 247–252. 10.1038/s41370-019-0180-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covaci A, Harrad S, Abdallah MA-E, Ali N, Law RJ, Herzke D, de Wit CA, 2011. Novel brominated flame retardants: A review of their analysis, environmental fate and behaviour. Environ. Int. 37, 532–556. 10.1016/j.envint.2010.11.007 [DOI] [PubMed] [Google Scholar]
- Czerska M, Zielinski M, Kaminska J, Ligocka D, 2013. Effects of polybrominated diphenyl ethers on thyroid hormone, neurodevelopment and fertility in rodents and humans. Int. J. Occup. Med. Environ. Health 26, 498–510. 10.2478/s13382-013-0138-7 [DOI] [PubMed] [Google Scholar]
- D’Hollander W, Roosens L, Covaci A, Cornelis C, Reynders H, Campenhout K. Van, Voogt P. de, Bervoets L, 2010. Brominated flame retardants and perfluorinated compounds in indoor dust from homes and offices in Flanders, Belgium. Chemosphere 81, 478–487. 10.1016/j.chemosphere.2010.07.043 [DOI] [PubMed] [Google Scholar]
- Davis EF, Stapleton HM, 2009. Photodegradation Pathways of Nonabrominated Diphenyl Ethers, 2-Ethylhexyltetrabromobenzoate and Di(2-ethylhexyl)tetrabromophthalate: Identifying Potential Markers of Photodegradation. Environ. Sci. Technol. 43, 5739–5746. 10.1021/es901019w [DOI] [PubMed] [Google Scholar]
- Deziel NC, Colt JS, Kent EE, Gunier RB, Reynolds P, Booth B, Metayer C, Ward MH, 2015. Associations between self-reported pest treatments and pesticide concentrations in carpet dust. Environ. Heal. 14, 27. 10.1186/s12940-015-0015-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon HM, Scott RP, Holmes D, Calero L, Kincl LD, Waters KM, Camann DE, Calafat AM, Herbstman JB, Anderson KA, 2018. Silicone wristbands compared with traditional polycyclic aromatic hydrocarbon exposure assessment methods. Anal. Bioanal. Chem. 410, 3059–3071. 10.1007/s00216-018-0992-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA, 2012. After the PBDE Phase-Out: A Broad Suite of Flame Retardants in Repeat House Dust Samples from California. Environ. Sci. Technol. 46, 13056–13066. 10.1021/es303879n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty BT, Hoffman K, Keil AP, Engel SM, Stapleton HM, Goldman BD, Olshan AF, Daniels JL, 2019. Prenatal exposure to organophosphate esters and cognitive development in young children in the Pregnancy, Infection, and Nutrition Study. Environ. Res. 169, 33–40. 10.1016/j.envres.2018.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Y, Sarkis J, 2013. A multiple stakeholder perspective on barriers to implementing China RoHS regulations. Resour. Conserv. Recycl. 81, 92–104. 10.1016/j.resconrec.2013.10.004 [DOI] [Google Scholar]
- Encarnacao T, Pais AA, Campos MG, Burrows HD, 2019. Endocrine disrupting chemicals: Impact on human health, wildlife and the environment. Sci. Prog. 102, 3–42. 10.1177/0036850419826802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MD, Kaley RG, 2011. Applications of polychlorinated biphenyls. Environ. Sci. Pollut. Res. 18, 135–151. 10.1007/s11356-010-0392-1 [DOI] [PubMed] [Google Scholar]
- EU Health and Safety Executive, 2016. Changes to authorisations for products containing chlorpyrifos [WWW Document]. URL https://www.hse.gov.uk/pesticides/news/information-update-0316.htm
- European Court of Justice, 2008. Joined Cases C-14/06 and C-295/06: Judgment of the Court (Grand Chamber) of 1 April 2008 — European Parliament (C-14/06), Kingdom of Denmark (C-295/06) v Commission of the European Communities. Off. J. Eur. Union 2. [Google Scholar]
- European Parliament, 2017. Commission Regulation (EU) 2017/227 of 9 February 2017 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards. Off. J. Eur. Union 6. [Google Scholar]
- European Parliament, 2003a. Directive 2003/11/EC of the European Parliament and of the Council of 6 February 2003 amending for the 24th time Council Directive 76/769/EEC relating to restrictions on the marketing and use of certain dangerous substances and preparations (pentabromodip. Off. J. Eur. Union 45–46. [Google Scholar]
- European Parliament, 2003b. Directive 2002/95/EC of the European Parliament and of the Council of 27 January 2003 on the restriction of the use of certain hazardous substances in electrical and electronic equipment. Off. J. Eur. Union L37, 21. [Google Scholar]
- European Union, 2016. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. [Google Scholar]
- European Union, 2005. Review report for the active substance cypermethrin (Directive 91/414/EEC). [Google Scholar]
- European Union, 2000. Review report for the active substance permethrin (Directive 91/414/EEC). [Google Scholar]
- Evangelou E, Ntritsos G, Chondrogiorgi M, Kavvoura FK, Hernández AF, Ntzani EE, Tzoulaki I, 2016. Exposure to pesticides and diabetes: A systematic review and meta-analysis. Environ. Int. 91, 60–68. 10.1016/j.envint.2016.02.013 [DOI] [PubMed] [Google Scholar]
- Frame GM, Cochran JW, Bøwadt SS, 1996. Complete PCB congener distributions for 17 aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener-specific analysis. J. High Resolut. Chromatogr. 19, 657–668. 10.1002/jhrc.1240191202 [DOI] [Google Scholar]
- Frederiksen H, Nielsen O, Koch HM, Skakkebaek NE, Juul A, Jørgensen N, Andersson A-M, 2020. Changes in urinary excretion of phthalates, phthalate substitutes, bisphenols and other polychlorinated and phenolic substances in young Danish men; 2009–2017. Int. J. Hyg. Environ. Health 223, 93–105. 10.1016/j.ijheh.2019.10.002 [DOI] [PubMed] [Google Scholar]
- Frederiksen M, Meyer HW, Ebbehøj NE, Gunnarsen L, 2012. Polychlorinated biphenyls (PCBs) in indoor air originating from sealants in contaminated and uncontaminated apartments within the same housing estate. Chemosphere 89, 473–479. 10.1016/j.chemosphere.2012.05.103 [DOI] [PubMed] [Google Scholar]
- Gibson EA, Stapleton HM, Calero L, Holmes D, Burke K, Martinez R, Cortes B, Nematollahi A, Evans D, Anderson KA, Herbstman JB, 2019a. Differential exposure to organophosphate flame retardants in mother-child pairs. Chemosphere 219, 567–573. 10.1016/j.chemosphere.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EA, Stapleton HM, Calero L, Holmes D, Burke K, Martinez R, Cortes B, Nematollahi A, Evans D, Herbstman JB, 2019b. Flame retardant exposure assessment: findings from a behavioral intervention study. J. Expo. Sci. Environ. Epidemiol. 29, 33–48. 10.1038/s41370-018-0049-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D, Arisco N, Jaacks LM, 2020. Synthetic Chemical Trade as a Potential Driver of Global Health Disparities and Data Gaps on Synthetic Chemicals in Vulnerable Populations. Curr. Environ. Heal. Reports. 10.1007/s40572-020-00261-w [DOI] [PubMed] [Google Scholar]
- Government of India, 2020. List of pesticides which are banned, refused registration and restricted in use. [Google Scholar]
- Government of India, 2011. Notification: S.O.1035(E). Ministry of Environment and Forests. Gaz. India Extraordinary. [Google Scholar]
- Guo J, Capozzi SL, Kraeutler TM, Rodenburg LA, 2014. Global Distribution and Local Impacts of Inadvertently Generated Polychlorinated Biphenyls in Pigments. Environ. Sci. Technol. 48, 8573–8580. 10.1021/es502291b [DOI] [PubMed] [Google Scholar]
- Guo Y, Alomirah H, Cho H-S, Minh TB, Mohd MA, Nakata H, Kannan K, 2011. Occurrence of phthalate metabolites in human urine from several Asian countries. Environ. Sci. Technol. 45, 3138–3144. 10.1021/es103879m [DOI] [PubMed] [Google Scholar]
- Guyton KZ, Loomis D, Grosse Y, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H, Straif K, 2015. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 16, 490–491. 10.1016/S1470-2045(15)70134-8 [DOI] [PubMed] [Google Scholar]
- Hammel SC, Hoffman K, Lorenzo AM, Chen A, Phillips AL, Butt CM, Sosa JA, Webster TF, Stapleton HM, 2017. Associations between flame retardant applications in furniture foam, house dust levels, and residents’ serum levels. Environ. Int. 107, 181–189. 10.1016/j.envint.2017.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel SC, Hoffman K, Phillips A, Levasseur J, Lorenzo A, Webster TF, Stapleton HM, 2020. Comparing the use of silicone wristbands, hand wipes, and dust to evaluate children’s exposure to flame retardants and plasticizers. Environ. Sci. Technol. 10.1021/acs.est.9b07909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel SC, Hoffman K, Webster TF, Anderson KA, Stapleton HM, 2016. Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ. Sci. Technol. 50, 4483–4491. 10.1021/acs.est.6b00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel SC, Levasseur JL, Hoffman K, Phillips AL, Lorenzo AM, Calafat AM, Webster TF, Stapleton HM, 2019. Children’s exposure to phthalates and non-phthalate plasticizers in the home: The TESIE study. Environ. Int. 132, 105061. 10.1016/j.envint.2019.105061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel SC, Phillips AL, Hoffman K, Stapleton HM, 2018. Evaluating the Use of Silicone Wristbands To Measure Personal Exposure to Brominated Flame Retardants. Environ. Sci. Technol. 10.1021/acs.est.8b03755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handford CE, Elliott CT, Campbell K, 2015. A review of the global pesticide legislation and the scale of challenge in reaching the global harmonization of food safety standards. Integr. Environ. Assess. Manag. 11, 525–536. 10.1002/ieam.1635 [DOI] [PubMed] [Google Scholar]
- Harrad S, 2015. A meta-analysis of recent data on UK environmental levels of POP-BFRs in an international context: Temporal trends and an environmental budget. Emerg. Contam. 1, 39–53. 10.1016/j.emcon.2015.08.001 [DOI] [Google Scholar]
- Harrad S, Brommer S, Mueller JF, 2016. Concentrations of organophosphate flame retardants in dust from cars, homes, and offices: An international comparison. Emerg. Contam. 2, 66–72. 10.1016/j.emcon.2016.05.002 [DOI] [Google Scholar]
- Harrad S, Hazrati S, Ibarra C, 2006. Concentrations of Polychlorinated Biphenyls in Indoor Air and Polybrominated Diphenyl Ethers in Indoor Air and Dust in Birmingham, United Kingdom: Implications for Human Exposure. Environ. Sci. Technol. 40, 4633–4638. 10.1021/es0609147 [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM, 2006. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology 17, 682–691. 10.1097/01.ede.0000235996.89953.d7 [DOI] [PubMed] [Google Scholar]
- Hazrati S, Harrad S, 2006. Causes of Variability in Concentrations of Polychlorinated Biphenyls and Polybrominated Diphenyl Ethers in Indoor air. Environ. Sci. Technol. 40, 7584–7589. 10.1021/es0617082 [DOI] [PubMed] [Google Scholar]
- Heinzow B, Mohr S, Ostendorp G, Kerst M, Körner W, 2007. PCB and dioxin-like PCB in indoor air of public buildings contaminated with different PCB sources – deriving toxicity equivalent concentrations from standard PCB congeners. Chemosphere 67, 1746–1753. 10.1016/j.chemosphere.2006.05.120 [DOI] [PubMed] [Google Scholar]
- Herrick RF, McClean MD, Meeker JD, Baxter LK, Weymouth GA, 2004. An unrecognized source of PCB contamination in schools and other buildings. Environ. Health Perspect. 112, 1051–1053. 10.1289/ehp.6912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hites RA, 2018. Atmospheric Concentrations of PCB-11 Near the Great Lakes Have Not Decreased Since 2004. Environ. Sci. Technol. Lett. 5, 131–135. 10.1021/acs.estlett.8b00019 [DOI] [Google Scholar]
- Hong W-J, Jia H, Ma W-L, Sinha RK, Moon H-B, Nakata H, Minh NH, Chi KH, Li W-L, Kannan K, Sverko E, Li Y-F, 2016. Distribution, Fate, Inhalation Exposure and Lung Cancer Risk of Atmospheric Polycyclic Aromatic Hydrocarbons in Some Asian Countries. Environ. Sci. Technol. 50, 7163–7174. 10.1021/acs.est.6b01090 [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg. 5, 46–51. 10.1080/1047322X.1990.10389587 [DOI] [Google Scholar]
- Hu D, Hornbuckle KC, 2010. Inadvertent Polychlorinated Biphenyls in Commercial Paint Pigments. Environ. Sci. Technol. 44, 2822–2827. 10.1021/es902413k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Martinez A, Hornbuckle KC, 2008. Discovery of Non-Aroclor PCB (3,3′-Dichlorobiphenyl) in Chicago Air. Environ. Sci. Technol. 42, 7873–7877. 10.1021/es801823r [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd T, Stahlhut R, Meeker JD, Powell S-G, Hauser R, Huang T, Rich-Edwards J, 2012. Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ. Health Perspect. 120, 1307–1313. 10.1289/ehp.1104717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd TM, Huang T, Seely EW, Saxena AR, 2016. The association between phthalates and metabolic syndrome: The National Health and Nutrition Examination Survey 2001–2010. Environ. Heal. A Glob. Access Sci. Source 15, 1–12. 10.1186/s12940-016-0136-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jartun M, Ottesen RT, Steinnes E, Volden T, 2009. Painted surfaces – Important sources of polychlorinated biphenyls (PCBs) contamination to the urban and marine environment. Environ. Pollut. 157, 295–302. 10.1016/j.envpol.2008.06.036 [DOI] [PubMed] [Google Scholar]
- Jia C, Batterman S, 2010. A critical review of naphthalene sources and exposures relevant to indoor and outdoor air. Int. J. Environ. Res. Public Health 7, 2903–2939. 10.3390/ijerph7072903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinhui L, Yuan C, Wenjing X, 2017. Polybrominated diphenyl ethers in articles: a review of its applications and legislation. Environ. Sci. Pollut. Res. Int. 24, 4312–4321. 10.1007/s11356-015-4515-6 [DOI] [PubMed] [Google Scholar]
- Just AC, Adibi JJ, Rundle AG, Calafat AM, Camann DE, Hauser R, Silva MJ, Whyatt RM, 2010. Urinary and air phthalate concentrations and self-reported use of personal care products among minority pregnant women in New York city. J. Expo. Sci. Environ. Epidemiol. 20, 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmlein S, Hahn O, Jann O, 2003. Emissions of organophosphate and brominated flame retardants from selected consumer products and building materials. Atmos. Environ. 37, 5485–5493. 10.1016/j.atmosenv.2003.09.025 [DOI] [Google Scholar]
- Kile ML, Scott RP, O’Connell SG, Lipscomb S, MacDonald M, McClelland M, Anderson KA, 2016. Using silicone wristbands to evaluate preschool children’s exposure to flame retardants. Environ. Res. 147, 365–372. 10.1016/j.envres.2016.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-H, Jahan SA, Kabir E, Brown RJC, 2013. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 60, 71–80. 10.1016/j.envint.2013.07.019 [DOI] [PubMed] [Google Scholar]
- Kim K-H, Kabir E, Jahan SA, 2017. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 575, 525–535. 10.1016/j.scitotenv.2016.09.009 [DOI] [PubMed] [Google Scholar]
- Klocke C, Sethi S, Lein PJ, 2020. The developmental neurotoxicity of legacy vs. contemporary polychlorinated biphenyls (PCBs): similarities and differences. Environ. Sci. Pollut. Res. Int. 27, 8885–8896. 10.1007/s11356-019-06723-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen GA, Sanders JM, Birnbaum LS, 2016. Disposition of the Emerging Brominated Flame Retardant, 2-Ethylhexyl 2,3,4,5-Tetrabromobenzoate, in Female SD Rats and Male B6C3F1 Mice: Effects of Dose, Route, and Repeated Administration. Toxicol. Sci. 154, 392–402. 10.1093/toxsci/kfw176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Tremp J, Zennegg M, Seiler C, Minder-Kohler S, Beck M, Lienemann P, Wegmann L, Schmid P, 2005. Joint Sealants: An Overlooked Diffuse Source of Polychlorinated Biphenyls in Buildings. Environ. Sci. Technol. 39, 1967–1973. 10.1021/es048632z [DOI] [PubMed] [Google Scholar]
- La Guardia MJ, Hale RC, Harvey E, 2006. Detailed Polybrominated Diphenyl Ether (PBDE) Congener Composition of the Widely Used Penta-, Octa-, and Deca-PBDE Technical Flame-retardant Mixtures. Environ. Sci. Technol. 40, 6247–6254. 10.1021/es060630m [DOI] [PubMed] [Google Scholar]
- Lauby-Secretan B, Loomis D, Grosse Y, Ghissassi F. El, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K, 2013. Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 14, 287–288. 10.1016/S1470-2045(13)70104-9 [DOI] [PubMed] [Google Scholar]
- Levasseur JL, Hammel SC, Hoffman K, Phillips AL, Zhang S, Ye X, Calafat AM, Webster TF, Stapleton HM, 2021. Young children’s exposure to phenols in the home: Associations between house dust, hand wipes, silicone wristbands, and urinary biomarkers. Environ. Int. 147, 106317. 10.1016/j.envint.2020.106317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W-L, Ma W-L, Zhang Z-F, Liu L-Y, Song W-W, Jia H-L, Ding Y-S, Nakata H, Minh NH, Sinha RK, Moon H-B, Kannan K, Sverko E, Li Y-F, 2017. Occurrence and Source Effect of Novel Brominated Flame Retardants (NBFRs) in Soils from Five Asian Countries and Their Relationship with PBDEs. Environ. Sci. Technol. 51, 11126–11135. 10.1021/acs.est.7b03207 [DOI] [PubMed] [Google Scholar]
- Li W, Wang Y, Asimakopoulos AG, Covaci A, Gevao B, Johnson-Restrepo B, Kumosani TA, Malarvannan G, Moon H-B, Nakata H, Sinha RK, Tran TM, Kannan K, 2019. Organophosphate esters in indoor dust from 12 countries: Concentrations, composition profiles, and human exposure. Environ. Int. 133, 105178. 10.1016/j.envint.2019.105178 [DOI] [PubMed] [Google Scholar]
- Li Y, Chang Q, Duan H, Liu Y, Zhang J, Li J, 2019. Occurrence, levels and profiles of brominated flame retardants in daily-use consumer products on the Chinese market. Environ. Sci. Process. Impacts 21, 446–455. 10.1039/c8em00483h [DOI] [PubMed] [Google Scholar]
- Licina D, Morrison GC, Bekö G, Weschler CJ, Nazaroff WW, 2019. Clothing-Mediated Exposures to Chemicals and Particles. Environ. Sci. Technol. 53, 5559–5575. 10.1021/acs.est.9b00272 [DOI] [PubMed] [Google Scholar]
- Linares V, Belles M, Domingo JL, 2015. Human exposure to PBDE and critical evaluation of health hazards. Arch. Toxicol. 89, 335–356. 10.1007/s00204-015-1457-1 [DOI] [PubMed] [Google Scholar]
- Lu C, Adamkiewicz G, Attfield KR, Kapp M, Spengler JD, Tao L, Xie SH, 2013. Household Pesticide Contamination from Indoor Pest Control Applications in Urban Low-Income Public Housing Dwellings: A Community-Based Participatory Research. Environ. Sci. Technol. 47, 2018–2025. 10.1021/es303912n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucattini L, Poma G, Covaci A, de Boer J, Lamoree MH, Leonards PEG, 2018. A review of semi-volatile organic compounds (SVOCs) in the indoor environment: occurrence in consumer products, indoor air and dust. Chemosphere 201, 466–482. 10.1016/j.chemosphere.2018.02.161 [DOI] [PubMed] [Google Scholar]
- Ma Y, Harrad S, 2015. Spatiotemporal analysis and human exposure assessment on polycyclic aromatic hydrocarbons in indoor air, settled house dust, and diet: A review. Environ. Int. 84, 7–16. 10.1016/j.envint.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Ma Y, Venier M, Hites RA, 2012. 2-Ethylhexyl Tetrabromobenzoate and Bis(2-ethylhexyl) Tetrabromophthalate Flame Retardants in the Great Lakes Atmosphere. Environ. Sci. Technol. 46, 204–208. 10.1021/es203251f [DOI] [PubMed] [Google Scholar]
- Mariana M, Feiteiro J, Verde I, Cairrao E, 2016. The effects of phthalates in the cardiovascular and reproductive systems: A review. Environ. Int. 94, 758–776. 10.1016/j.envint.2016.07.004 [DOI] [PubMed] [Google Scholar]
- Marquart H, Brouwer DH, van Hemmen JJ, 2002. Removing Pesticides from the Hands with a Simple Washing Procedure Using Soap and Water. J. Occup. Environ. Med. 44. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Stapleton HM, 2010. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ. Health Perspect. 118, 318–323. 10.1289/ehp.0901332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Ford JB, Mínguez-Alarcón L, Keller M, Dadd R, James-Todd T, Hauser R, Nassan FL, Williams PL, Chavarro JE, Chiu Y-H, Gaskins AJ, Braun JM, Meeker JD, Souter I, Petrozza J, Toth TL, Calafat AM, 2018a. The Environment and Reproductive Health (EARTH) Study: a prospective preconception cohort. Hum. Reprod. Open 2018. 10.1093/hropen/hoy001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Williams PL, Minguez-Alarcon L, Carignan CC, Ford JB, Butt CM, Meeker JD, Stapleton HM, Souter I, Hauser R, 2018b. Organophosphate flame-retardant metabolite concentrations and pregnancy loss among women conceiving with assisted reproductive technology. Fertil. Steril. 110, 1137–1144.e1. 10.1016/j.fertnstert.2018.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL, Garfinkel R, Horton M, Camann D, Perera FP, Whyatt RM, Kinney PL, 2004. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest 126, 1071–1078. 10.1378/chest.126.4.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitro SD, Dodson RE, Singla V, Adamkiewicz G, Elmi AF, Tilly MK, Zota AR, 2016. Consumer Product Chemicals in Indoor Dust: A Quantitative Meta-analysis of U.S. Studies. Environ. Sci. Technol. 50, 10661–10672. 10.1021/acs.est.6b02023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison GC, Andersen HV, Gunnarsen L, Varol D, Uhde E, Kolarik B, 2018. Partitioning of PCBs from air to clothing materials in a Danish apartment. Indoor Air 28, 188–197. 10.1111/ina.12411 [DOI] [PubMed] [Google Scholar]
- Mostafalou S, Abdollahi M, 2013. Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 268, 157–177. 10.1016/j.taap.2013.01.025 [DOI] [PubMed] [Google Scholar]
- Mumford SL, Kim S, Chen Z, Gore-Langton RE, Boyd Barr D, Buck Louis GM, 2015. Persistent organic pollutants and semen quality: The LIFE Study. Chemosphere 135, 427–435. 10.1016/j.chemosphere.2014.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski A, Schmied-Tobies MIH, Rucic E, Schmidtkunz C, Küpper K, Leng G, Eckert E, Kuhlmann L, Göen T, Daniels A, Schwedler G, Kolossa-Gehring M, 2020. Metabolites of 4-methylbenzylidene camphor (4-MBC), butylated hydroxytoluene (BHT), and tris(2-ethylhexyl) trimellitate (TOTM) in urine of children and adolescents in Germany - human biomonitoring results of the German Environmental Survey GerES V (2014-. Environ. Res. 110345. 10.1016/j.envres.2020.110345 [DOI] [PubMed] [Google Scholar]
- N’Guessan R, Boko P, Odjo A, Chabi J, Akogbeto M, Rowland M, 2010. Control of pyrethroid and DDT-resistant Anopheles gambiae by application of indoor residual spraying or mosquito nets treated with a long-lasting organophosphate insecticide, chlorpyrifos-methyl. Malar. J. 9, 44. 10.1186/1475-2875-9-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassan F, Coull B, J. GA, A. ,WM, E. ,SN, B. ,FJ, Xiaoyun Y,M, C.A., M. ,BJ, Russ H, 2019. Personal Care Product Use in Men and Urinary Concentrations of Select Phthalate Metabolites and Parabens: Results from the Environment And Reproductive Health (EARTH) Study. Environ. Health Perspect. 125, 87012. 10.1289/EHP1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni K, Lu Y, Wang T, Shi Y, Kannan K, Xu L, Li Q, Liu S, 2013. Polybrominated diphenyl ethers (PBDEs) in China: Policies and recommendations for sound management of plastics from electronic wastes. J. Environ. Manage. 115, 114–123. 10.1016/j.jenvman.2012.09.031 [DOI] [PubMed] [Google Scholar]
- Nielsen JB, 2010. Efficacy of skin wash on dermal absorption: an in vitro study on four model compounds of varying solubility. Int. Arch. Occup. Environ. Health 83, 683–690. 10.1007/s00420-010-0546-y [DOI] [PubMed] [Google Scholar]
- O’Connell SG, Kincl LD, Anderson KA, 2014. Silicone wristbands as personal passive samplers. Environ. Sci. Technol. 48, 3327–3335. 10.1021/es405022f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula AM, Balmes JR, Eisen EA, Mann J, Noth EM, Lurmann FW, Pratt B, Tager IB, Nadeau K, Hammond SK, 2015. Ambient polycyclic aromatic hydrocarbons and pulmonary function in children. J. Expo. Sci. Environ. Epidemiol. 25, 295–302. 10.1038/jes.2014.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F, Herbstman J, 2011. Prenatal environmental exposures, epigenetics, and disease. Reprod. Toxicol. 31, 363–373. 10.1016/j.reprotox.2010.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreas M, Gill R, Takaku-Pugh S, Lytle E, Parry E, Wang M, Quinn J, Park J-S, 2016. Rapid methodology to screen flame retardants in upholstered furniture for compliance with new California labeling law (SB 1019). Chemosphere 152, 353–359. 10.1016/j.chemosphere.2016.02.102 [DOI] [PubMed] [Google Scholar]
- Phillips AL, Hammel SC, Konstantinov A, Stapleton HM, 2017. Characterization of Individual Isopropylated and tert-Butylated Triarylphosphate (ITP and TBPP) Isomers in Several Commercial Flame Retardant Mixtures and House Dust Standard Reference Material SRM 2585. Environ. Sci. Technol. 51, 13443–13449. 10.1021/acs.est.7b04179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston EV, McClean MD, Claus Henn B, Stapleton HM, Braverman LE, Pearce EN, Makey CM, Webster TF, 2017. Associations between urinary diphenyl phosphate and thyroid function. Environ. Int. 101, 158–164. 10.1016/j.envint.2017.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Li W-L, Liu L-Y, Zhang Z-F, Zhu N-Z, Song W-W, Ma W-L, Li Y-F, 2014. Levels, distribution and human exposure of new non-BDE brominated flame retardants in the indoor dust of China. Environ. Pollut. 195, 1–8. 10.1016/j.envpol.2014.08.008 [DOI] [PubMed] [Google Scholar]
- Qiu X, Zhu T, Li J, Pan H, Li Q, Miao G, Gong J, 2004. Organochlorine Pesticides in the Air around the Taihu Lake, China. Environ. Sci. Technol. 38, 1368–1374. 10.1021/es035052d [DOI] [PubMed] [Google Scholar]
- Ramesh A, Archibong, Hood D, Guo Z, Loganathan B, 2012. Global Environmental Distribution and Human Health Effects of Polycyclic Aromatic Hydrocarbons, in: Global Contamination Trends of Persistent Organic Chemicals. pp. 97–128. 10.1201/b11098-7 [DOI] [Google Scholar]
- Ravindra K, Sokhi R, Van Grieken R, 2008. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 42, 2895–2921. 10.1016/j.atmosenv.2007.12.010 [DOI] [Google Scholar]
- Reddam A, Tait G, Herkert N, Hammel SC, Stapleton HM, Volz DC, 2020. Longer commutes are associated with increased human exposure to tris(1,3-dichloro-2-propyl) phosphate. Environ. Int. 136, 105499. 10.1016/j.envint.2020.105499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenburg LA, Guo J, Du S, Cavallo GJ, 2010. Evidence for Unique and Ubiquitous Environmental Sources of 3,3′-Dichlorobiphenyl (PCB 11). Environ. Sci. Technol. 44, 2816–2821. 10.1021/es901155h [DOI] [PubMed] [Google Scholar]
- Rota M, Bosetti C, Boccia S, Boffetta P, La Vecchia C, 2014. Occupational exposures to polycyclic aromatic hydrocarbons and respiratory and urinary tract cancers: an updated systematic review and a meta-analysis to 2014. Arch. Toxicol. 88, 1479–1490. 10.1007/s00204-014-1296-5 [DOI] [PubMed] [Google Scholar]
- Sackmann K, Reemtsma T, Rahmberg M, Bunke D, 2018. Impact of European chemicals regulation on the industrial use of plasticizers and patterns of substitution in Scandinavia. Environ. Int. 119, 346–352. 10.1016/j.envint.2018.06.037 [DOI] [PubMed] [Google Scholar]
- Saillenfait A-M, Ndaw S, Robert A, Sabate J-P, 2018. Recent biomonitoring reports on phosphate ester flame retardants: a short review. Arch. Toxicol. 92, 2749–2778. 10.1007/s00204-018-2275-z [DOI] [PubMed] [Google Scholar]
- Shang H, Li Y, Wang T, Wang P, Zhang H, Zhang Q, Jiang G, 2014. The presence of polychlorinated biphenyls in yellow pigment products in China with emphasis on 3,3′-dichlorobiphenyl (PCB 11). Chemosphere 98, 44–50. 10.1016/j.chemosphere.2013.09.075 [DOI] [PubMed] [Google Scholar]
- Shen H, Huang Y, Wang R, Zhu D, Li W, Shen G, Wang B, Zhang Y, Chen Y, Lu Y, Chen H, Li T, Sun K, Li B, Liu W, Liu J, Tao S, 2013. Global Atmospheric Emissions of Polycyclic Aromatic Hydrocarbons from 1960 to 2008 and Future Predictions. Environ. Sci. Technol. 47, 6415–6424. 10.1021/es400857z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, Parks CG, Goldner WS, Kamel F, Umbach DM, Ward MH, Lerro CC, Koutros S, Hofmann JN, Beane Freeman LE, Sandler DP, 2018. Pesticide Use and Incident Hypothyroidism in Pesticide Applicators in the Agricultural Health Study. Environ. Health Perspect. 126, 97008. 10.1289/EHP3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Kamal R, Ahamed I, Wagh M, Bihari V, Sathian B, Kesavachandran CN, 2018. PAH exposure-associated lung cancer: an updated meta-analysis. Occup. Med. (Lond). 68, 255–261. 10.1093/occmed/kqy049 [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF, 2008. Alternate and New Brominated Flame Retardants Detected in U.S. House Dust. Environ. Sci. Technol. 42, 6910–6916. 10.1021/es801070p [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF, 2009. Detection of Organophosphate Flame Retardants in Furniture Foam and U.S. House Dust. Environ. Sci. Technol. 43, 7490–7495. 10.1021/es9014019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A, 2012. Novel and High Volume Use Flame Retardants in US Couches Reflective of the 2005 PentaBDE Phase Out. Environ. Sci. Technol. 46, 13432–13439. 10.1021/es303471d [DOI] [PMC free article] [PubMed] [Google Scholar]
- State of California, 2004. An act to amend Sections 108921 and 108922 of the Health and Safety Code, relating to toxic substances. State of California. [Google Scholar]
- Tang W, Wang D, Wang J, Wu Z, Li L, Huang M, Xu S, Yan D, 2018. Pyrethroid pesticide residues in the global environment: An overview. Chemosphere 191, 990–1007. 10.1016/j.chemosphere.2017.10.115 [DOI] [PubMed] [Google Scholar]
- The People’s Republic of China, 2014. GB 6675–2014: Toy Safety. General Administration of Quality Supervision, Inpsection, and Quarantine; Standardization Administration. [Google Scholar]
- The People’s Republic of China, 2013. Announcement No. 2032 of the Ministry of Agriculture. [Google Scholar]
- The People’s Republic of China, 2007. National Implementation Plan for the Stockholm Convention on Persistent Organic Pollutants. [Google Scholar]
- Travis SC, Aga DS, Queirolo EI, Olson JR, Daleiro M, Kordas K, 2020. Catching flame retardants and pesticides in silicone wristbands: Evidence of exposure to current and legacy pollutants in Uruguayan children. Sci. Total Environ. 740, 140136. 10.1016/j.scitotenv.2020.140136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A, 2018. Black plastics: Linear and circular economies, hazardous additives and marine pollution. Environ. Int. 117, 308–318. 10.1016/j.envint.2018.04.036 [DOI] [PubMed] [Google Scholar]
- Turner A, Filella M, 2017. Bromine in plastic consumer products - Evidence for the widespread recycling of electronic waste. Sci. Total Environ. 601–602, 374–379. 10.1016/j.scitotenv.2017.05.173 [DOI] [PubMed] [Google Scholar]
- U.S. Bureau of Labor Statistics, 2013. American Time Use Survey - 2012 Results. [Google Scholar]
- UK Statutory Instruments, 1988. The Furniture and Furnishings (Fire) (Safety) Regulations 1988. [Google Scholar]
- United Nations, 2015. Progress in pesticide risk assessment and phasing-out of highly hazardous pesticides in Asia. [Google Scholar]
- US Consumer Product Safety Commission, 2008. Consumer Product Safety Improvement Act of 2008. [Google Scholar]
- US Environmental Protection Agency, 1979. EPA Bans PCB Manufacture; Phases Out Uses. [Google Scholar]
- US EPA, 2020. Chlorpyrifos [WWW Document]. URL https://www.epa.gov/ingredients-used-pesticide-products/chlorpyrifos
- US EPA, n.d.Chlordane: Hazard Summary [WWW Document]. URL https://www.epa.gov/sites/production/files/2016-09/documents/chlordane.pdf
- US FDA, 2018. Cosmetics: phthalates [WWW Document]. URL https://www.fda.gov/Cosmetics/ProductsIngredients/Ingredients/ucm128250.htm (accessed 2.25.18).
- van den Berg H, Manuweera G, Konradsen F, 2017. Global trends in the production and use of DDT for control of malaria and other vector-borne diseases. Malar. J. 16, 401. 10.1186/s12936-017-2050-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg M, Kypke K, Kotz A, Tritscher A, Lee SY, Magulova K, Fiedler H, Malisch R, 2017. WHO/UNEP global surveys of PCDDs, PCDFs, PCBs and DDTs in human milk and benefit-risk evaluation of breastfeeding. Arch. Toxicol. 91, 83–96. 10.1007/s00204-016-1802-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen I, de Boer J, 2012. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88, 1119–1153. 10.1016/j.chemosphere.2012.03.067 [DOI] [PubMed] [Google Scholar]
- Vorkamp K, 2016. An overlooked environmental issue? A review of the inadvertent formation of PCB-11 and other PCB congeners and their occurrence in consumer products and in the environment. Sci. Total Environ. 541, 1463–1476. 10.1016/j.scitotenv.2015.10.019 [DOI] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Dietrich KN, Braun JM, Lanphear BP, Chen A, 2018. Exposure to polybrominated diphenyl ethers (PBDEs) and child behavior: Current findings and future directions. Horm. Behav. 101, 94–104. 10.1016/j.yhbeh.2017.11.008 [DOI] [PubMed] [Google Scholar]
- Wang S, Romanak KA, Hendryx M, Salamova A, Venier M, 2019a. Association between Thyroid Function and Exposures to Brominated and Organophosphate Flame Retardants in Rural Central Appalachia. Environ. Sci. Technol. 10.1021/acs.est.9b04892 [DOI] [PubMed] [Google Scholar]
- Wang S, Romanak KA, Stubbings WA, Arrandale VH, Hendryx M, Diamond ML, Salamova A, Venier M, 2019b. Silicone wristbands integrate dermal and inhalation exposures to semi-volatile organic compounds (SVOCs). Environ. Int. 132, 105104. 10.1016/j.envint.2019.105104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Romanak KA, Tarallo S, Francavilla A, Viviani M, Vineis P, Rothwell JA, Mancini FR, Cordero F, Naccarati A, Severi G, Venier M, 2020. The use of silicone wristbands to evaluate personal exposure to semi-volatile organic chemicals (SVOCs) in France and Italy. Environ. Pollut. 267, 115490. 10.1016/j.envpol.2020.115490 [DOI] [PubMed] [Google Scholar]
- Wang Y, Hou M, Zhang Q, Wu X, Zhao H, Xie Q, Chen J, 2017. Organophosphorus Flame Retardants and Plasticizers in Building and Decoration Materials and Their Potential Burdens in Newly Decorated Houses in China. Environ. Sci. Technol. 51, 10991–10999. 10.1021/acs.est.7b03367 [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhu H, Kannan K, 2019. A Review of Biomonitoring of Phthalate Exposures. Toxics 7. 10.3390/toxics7020021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Sjodin A, Webster TF, 2011. Exposure to PBDEs in the office environment: evaluating the relationships between dust, handwipes, and serum. Environ. Health Perspect. 119, 1247–1252. 10.1289/ehp.1003271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Webster TF, 2013. Associations between PBDEs in office air, dust, and surface wipes. Environ. Int. 59, 124–132. 10.1016/j.envint.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler CJ, Nazaroff WW, 2008. Semivolatile organic compounds in indoor environments. Atmos. Environ. 42, 9018–9040. 10.1016/j.atmosenv.2008.09.052 [DOI] [Google Scholar]
- Wise CF, Hammel SC, Herkert N, Ma J, Motsinger-Reif A, Stapleton HM, Breen M, 2020. Comparative Exposure Assessment Using Silicone Passive Samplers Indicates That Domestic Dogs Are Sentinels To Support Human Health Research. Environ. Sci. Technol. 54, 7409–7419. 10.1021/acs.est.9b06605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittassek M, Koch HM, Angerer J, Bruning T, 2011. Assessing exposure to phthalates - the human biomonitoring approach. Mol. Nutr. Food Res. 55, 7–31. 10.1002/mnfr.201000121 [DOI] [PubMed] [Google Scholar]
- Wong MH, Leung AOW, Chan JKY, Choi MPK, 2005. A review on the usage of POP pesticides in China, with emphasis on DDT loadings in human milk. Chemosphere 60, 740–752. 10.1016/j.chemosphere.2005.04.028 [DOI] [PubMed] [Google Scholar]
- Xiong P, Yan X, Zhu Q, Qu G, Shi J, Liao C, Jiang G, 2019. A Review of Environmental Occurrence, Fate, and Toxicity of Novel Brominated Flame Retardants. Environ. Sci. Technol. 53, 13551–13569. 10.1021/acs.est.9b03159 [DOI] [PubMed] [Google Scholar]
- Young AS, Hauser R, James-Todd TM, Coull BA, Zhu H, Kannan K, Specht AJ, Bliss MS, Allen JG, 2020. Impact of “healthier” materials interventions on dust concentrations of per- and polyfluoroalkyl substances, polybrominated diphenyl ethers, and organophosphate esters. Environ. Int. 106151. 10.1016/j.envint.2020.106151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Diamond ML, Robson M, Harrad S, 2011. Sources, Emissions, and Fate of Polybrominated Diphenyl Ethers and Polychlorinated Biphenyls Indoors in Toronto, Canada. Environ. Sci. Technol. 45, 3268–3274. 10.1021/es102767g [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tao S, 2009. Global atmospheric emission inventory of polycyclic aromatic hydrocarbons (PAHs) for 2004. Atmos. Environ. 43, 812–819. 10.1016/j.atmosenv.2008.10.050 [DOI] [Google Scholar]
- Zhu L, Lu H, Chen S, Amagai T, 2009. Pollution level, phase distribution and source analysis of polycyclic aromatic hydrocarbons in residential air in Hangzhou, China. J. Hazard. Mater. 162, 1165–1170. 10.1016/j.jhazmat.2008.05.150 [DOI] [PubMed] [Google Scholar]
- Zimmerman JB, Anastas PT, 2015. Toward substitution with no regrets. Science (80-. ). 347, 1198–1199. 10.1126/science.aaa0812 [DOI] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ, 2014. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ. Health Perspect. 122, 235–241. 10.1289/ehp.1306681 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.