Abstract
Background:
Lentigo maligna/lentigo maligna melanoma (LM/LMM) poses a treatment and surgical challenge given unpredictable subclinical extension resulting in incomplete excision.
Objectives:
To describe the demographic, clinical, and pathologic characteristics of incompletely excised LM/LMM. To evaluate the potential role of reflectance confocal microscopy (RCM).
Patients and Methods:
A retrospective review of a melanoma database at a tertiary cancer center for patients referred with ‘incompletely excised LM/LMM’ or ‘incompletely excised melanoma’ between October 2006 and July 2017. We recorded clinical and pathological data and surgical margins needed to clear the residual LM/LMM. The second part consisted of a prospective cohort of patients in which RCM was performed when presenting with incompletely excised LM/LMM.
Results:
We included a total of 67 patients (retrospective + prospective cohort); mean age was 64.9 (SD 11.3) years and 52.2% were males. For the retrospective cohort (n=53), the mean scar size was 3.4 cm. The average initial margins excised prior to presentation were 4.8 mm (range 3 – 7 mm). The average additional margin needed to clear the residual, incompletely excised LM/LMM was 7.8 mm. For the prospective cohort (n=14) there were no differences in age, gender, or size when compared to the retrospective cohort. RCM had a diagnostic accuracy of 78.6%, a sensitivity of 90.9%, a specificity of 33.3% and a positive predictive value of 83.3% for the detection of incompletely excised LM/LMM.
Conclusions:
Incompletely excised LM/LMM is a poorly characterized clinical-pathological scenario that may require considerable extra margins for microscopic clearance. RCM may emerge as a valuable tool for the evaluation of patients with incompletely excised LM/LMM.
Keywords: reflectance confocal microscopy, biopsy, melanoma, incompletely excised, residual diagnosis, dermatologic surgery, staged excision, Mohs micrographic surgery
Introduction:
Lentigo maligna/lentigo maligna melanoma (LM/LMM) can pose diagnostic and surgical challenges due to subclinical extension that is difficult to detect with the naked eye. This can result in incomplete excision of the LM/LMM;1,2 which, in turn, can lead to subsequent additional surgery or potential increase in local recurrence.1–3 Furthermore, the head and neck location of LM/LMM is associated with functional and cosmetic implications, complicating the overall management of incompletely excised melanomas.
Incompletely excised melanoma is defined as the presence of involved pathologic margins after surgical excision. Studies have shown that after wide local excision (WLE), margins are microscopically involved in 2.7% - 9% of melanomas,4–7 with differences based on melanoma subtype and treating specialty (i.e. plastic surgeon, surgical oncologist or dermatologist). When focusing on LM/LMM, studies have shown microscopic margins are very frequently involved beyond the standard recommended surgical margins.8,9 Furthermore, incomplete excision is more likely to be detected when comprehensive circumferential excision techniques such as staged excision or Mohs micrographic surgery are used for LM/LMM.3,5,10–12 The assessment of incompletely excised LM/LMM is complex due to lack of clinically evident residual pigmentation. Additionally, many patients have already undergone complex reconstructions with tissue rearrangement at time of presentation. These factors collectively make evaluation of patients with incompletely excised LM/LMM challenging.13,14
Studies have shown that the presence of desmoplasia on histology and location on the head and neck are associated with positive margins of melanoma after WLE.1,5 However, current data is limited, derived from single-center retrospective studies, predominantly from the plastic surgery literature.5–7 Strategies for the evaluation of these patients are needed to guide management. Reflectance confocal microscopy (RCM) is a non-invasive imaging tool that uses a near-infrared laser and provides with quasi-histological views in vivo. RCM has been used as a diagnostic tool for both primary15 and recurrent melanomas,16 as well as to map melanomas of the LM/LMM type prior to surgical excision17–19 and to monitor non-surgical treatment modalities.20,21 Studies have not specifically evaluated this clinical-pathological scenario and the role of RCM in the evaluation of incompletely excised LM/LMM. In the present study, we sought to describe the demographic and clinical characteristics of incompletely excised LM/LMM in a cohort of patients and to evaluate the role of RCM.
Patients and methods:
This was an IRB approved study, performed at a tertiary cancer center between October 2006 and June 2019. All patients were seen for a consultation in the Dermatologic surgery unit by an expert dermatologic surgeon. This study consisted of 2 parts, a retrospective and a prospective cohort.
Retrospective LM/LMM database review:
In the first part, a retrospective review of a LM/LMM database including all patients undergoing surgery in our unit. Database was screened for patients referred for ‘incompletely excised LM/LMM’ or ‘incompletely excised melanoma’ or ‘positive margins’ between October 2006 and July 2017.
We included consecutive adult (≥18 years old) patients with a primary LM/LMM that underwent WLE and a histopathology report that showed ‘positive margins’ at any surgical margin and had no clinical or dermoscopic evidence of residual melanoma on retrospective chart and image review. We excluded patients with recurrent LM/LMM and patients that did not undergo surgery after initial consultation due to the lack of histopathological margin assessment. Therefore, this cohort corresponded to patients that presented to our institution with an initial WLE that had no clinical/dermoscopic residual lesion and presented with microscopic positive margins.
Demographics and clinical data:
Patients had an initial consultation including clinical and Wood’s lamp examination. Demographic characteristics (age, sex) were recorded as well as LM/LMM location and scar maximum diameter (cm). We also recorded the initial surgical excision margins as per the clinical notes. All excision specimens were evaluated by an expert dermatopathologist to confirm the diagnosis, Breslow tumor thickness (mm), and status of positive margins. Clinical photos were taken with a digital camera (VEOS DS3, Canfield Inc., NJ, USA).
Prospective cohort and reflectance confocal microscopy evaluation:
In the second part, a prospective cohort of consecutive patients with incompletely excised LM/LMM were evaluated in our unit between August 2017 and June 2019. Similar to the first part (retrospective cohort), the same clinical and demographic characteristics were recorded (age, sex, tumor location, maximum scar diameter, and Breslow thickness). In addition, each patient was evaluated with RCM.
Reflectance confocal microscopy analysis:
RCM examination was performed during the initial consultation visit, with a commercially-available, handheld RCM device (Vivascope 3000, Caliber I.D., Rochester, NY, USA). All patients signed informed consent prior to RCM imaging. RCM examination was obtained with the aid of adhesive paper tape, as previously described.22 The distance from the paper tape to the scar was 3 – 5 mm, according to the surgeon’s assessment. Single images, stacks, and videos were taken. RCM LM/LMM criteria used to evaluate the center of the scar were those previously described by Guitera et al.15 Margins were considered ‘RCM-positive’ if there were ≥1 large round cell (>20 μm) or ≥3 dendritic or round cells regardless of size, as described by Pellacani et al.18
The scar tissue was first evaluated with the intent to assess the entire surface with RCM. Then, margins were imaged by positioning the probe next to the paper tape.22 RCM results were recorded as ‘RCM-positive’ or ‘RCM-negative’. All evaluations were performed by the same 2 investigators (C.N-D. and M.C.) for consensus. A third investigator (S.A. or A.M.R.) helped to solve disagreements. The findings of RCM evaluation did not alter the predetermined treatment course, as deemed by the surgeon.
Histopathological analysis and correlation:
For both the retrospective and prospective cohorts, excisions were performed in a staged manner as described by Hazan et al.3 Excised tissue was sent to pathology for formalin fixed, paraffin embedded and histopathologic evaluation. Results were recorded in a database as ‘pathology-positive’ (presence of any residual melanoma) or ‘pathology-negative’ (absence of residual melanoma features). Additional surgical margins were calculated based on the clinical margins (mm) required for histopathologic clearance in the stage excision: Final margins were calculated by adding the initial surgical margins performed (mm) plus the subsequent staged excision margins (mm).
Statistical analysis:
Data was analyzed using SPSS 23.0 (SPSS, Armonk, NY, USA). All values are expressed as mean and standard deviation (SD). Fisher’s square test was used for categorical variables. Odds ratios (ORs) were calculated. Independent-samples student’s t-test was performed for comparisons of continuous variables. A 2-tailed P<0.05 was considered to indicate statistical significance. Diagnostic accuracy, area under the curve (AUC), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the RCM findings and final histopathological diagnosis was calculated.
Results:
A total of 67 patients had incompletely excised LM/LMM in the study period. Patient demographics and clinical characteristics are outlined in Table 1. No demographic or clinical differences were found between both cohorts.
Table 1:
Demographic and clinical characteristics of incompletely excised lentigo maligna/lentigo maligna melanoma patients.
| Variable | Total (n = 67) | Retrospective group (n = 53) | Prospective group (n = 14) | P-value* |
|---|---|---|---|---|
| Age, y, mean (SD) | 64.9 (11.3) | 64.0 (11.1) | 68.4 (11.7) | 0.2 |
| Gender, male, % (n) | 52.2% (35) | 54.7% (29) | 42.9% (6) | 0.4 |
| Scar maximum diameter, cm (SD) | 3.4 (2.0) | 3.4 (2.1) | 3.4 (1.9) | 0.8 |
| Breslow, mm, mean (SD) | 0.2 (0.6) | 0.2 (0.7) | 0.18 (0.3) | 0.8 |
|
| ||||
| Location | ||||
| Cheek | 38.8% (26) | 37.7% (20) | 12.8% (6) | -- |
| Nose | 4.5% (3) | 5.7% (3) | -- | -- |
| Periorbital | 13.4% (9) | 15.1% (8) | 7.1% (1) | -- |
| Temple | 4.5% (3) | 3.8% (2) | 7.1% (1) | -- |
| Ear | 1.5% (1) | -- | 7.1% (1) | -- |
| Lips | 4.5% (3) | 1.9% (1) | 14.2% (2) | -- |
| Forehead | 8.9% (6) | 9.4% (5) | 7.1% (1) | -- |
| Jawline | 2.9% (2) | 3.8% (2) | -- | -- |
| Scalp | 1.5% (1) | -- | 7.1% (1) | -- |
| Neck | 1.5% (1) | 1.9% (1) | -- | -- |
| Trunk | 1.5% (1) | 1.9% (1) | -- | -- |
| Extremity | 16.4% (11) | 18.9% (10) | 7.1% (1) | -- |
p-value calculated between retrospective and prospective groups. SD = standard deviation.
Retrospective LM/LMM database review:
Fifty-three patients fulfilled the inclusion criteria. Mean age was 64.0 years (SD 11.1; 34 – 83 years). Twenty-nine (54.7%) were males. Twenty-eight (51.9%) patients were Skin type II. Eye color was blue in 21 (39.6%) patients and brown in 20 (37.7%) patients. Thirty-seven (69.8%) patients had brown hair color. Twenty-six (49.1%) patients had history of keratinocyte carcinoma, 11 (20.8%) had personal history of melanoma, and 10 (18.9%) had family history of melanoma.
LM/LMM characteristics:
Forty-two (79.2%) patients had melanoma on the head and neck and 11 were located on trunk and extremities (20.7%); 29 (54.7%) were on the left side, 22 (41.5%) on the right side, and 2 (3.8%) on midline. There were no differences between laterality and gender (p=0.3).
Mean scar diameter was 3.4 cm (SD 2.1; 0.5 – 8.5 cm). Mean Breslow thickness was 0.2 mm (SD 0.7; 0 – 4.5 mm). The initial margins excised prior to presentation were on average 4.8 mm (SD 1.1; 3.0 – 7.0 mm). The total average (additional) margin needed to clear the incompletely excised LM/LMM was 7.8 mm (SD 3.7; 3.0 – 20.0 mm). Additional margin needed to clear incompletely excised LM/LMM was 7.4 mm (SD 3.1 mm) for in situ and 8.7 mm (SD 4.9 mm) for invasive melanomas (p=0.4). After definitive staged excision, 40% (n=16) of patients required a complex reconstruction (i.e. flap or graft); 10.4% (n=7) of the surgical defects were repaired in the operating room under general anesthesia.
Prospective cohort and reflectance confocal microscopy evaluation:
Fourteen patients with incompletely excised LM/LMM were evaluated with RCM between August 2017 and July 2019. Mean age was 68.4 years (SD 11.7); there were no differences between the two cohorts regarding age (p=0.2) and gender (p=0.4). Mean maximum scar diameter was 3.4 cm (SD 1.9).
Reflectance confocal microscopy:
RCM was associated with a diagnostic accuracy of 78.6% (95% CI 49.2 – 95.3%) and an area under the curve (AUC) of 0.62 when compared to histopathological evaluation. The sensitivity of RCM compared to the gold standard histopathology was 90.9% (95% CI 58.7 – 99.8%), specificity 33.3% (95% CI 0.8 – 90.6%), PPV 83.3% (95% CI 68.7 – 91.9%), and NPV 50.0% (95% CI 7.9 – 92.1%). RCM details can be found in Table 2, and Figures 1 – 3. Mean time from initial WLE to RCM examination was 3.0 months (SD 1.8; range 1.0 – 8.0 months).
Table 2:
Reflectance confocal microscopy features seen in incompletely excised LM/LMM (n=14). DEJ = dermoepidermal junction.
| Variable | % (n) |
|---|---|
|
| |
| Suprabasal epidermis features: | |
| Atypical honeycomb | 28.6% (4) |
| Pagetoid cells | 14.3% (2) |
| Basal layer and DEJ features: | |
| Dendritic cells DEJ | 85.7% (12) |
| Folliculotropism | 57.1% (8) |
| Cobblestone pattern | 21.4% (3) |
| DEJ disarray | 14.3% (2) |
| Superficial dermis features: | |
| Dense nests | 7.1% (1) |
| Inflammation | 78.6% (11) |
| Plump cells | 14.3% (2) |
| Reticular collagen | 28.6% (4) |
| Amorphous collagen | 28.6% (4) |
| Solar elastosis | 28.6% (4) |
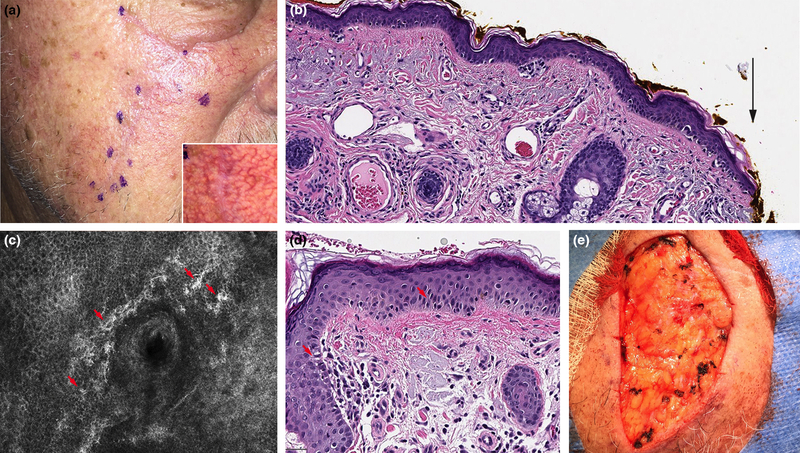
Figure 1:
Incompletely excised lentigo maligna (LM) detected with reflectance confocal microscopy (RCM). A. Right cheek LM excision scar (rotation flap) with no residual clinical pigmentation. B. Residual LM extending to side margins following initial wide local excision (black arrow) (H&E 10X). C. RCM image with bright, large, nucleated dendritic cells with folliculotropism (red arrows) (750 × 750 μm). D. Residual LM confirmed in final excision (H&E 20X). E. Final surgical defect.
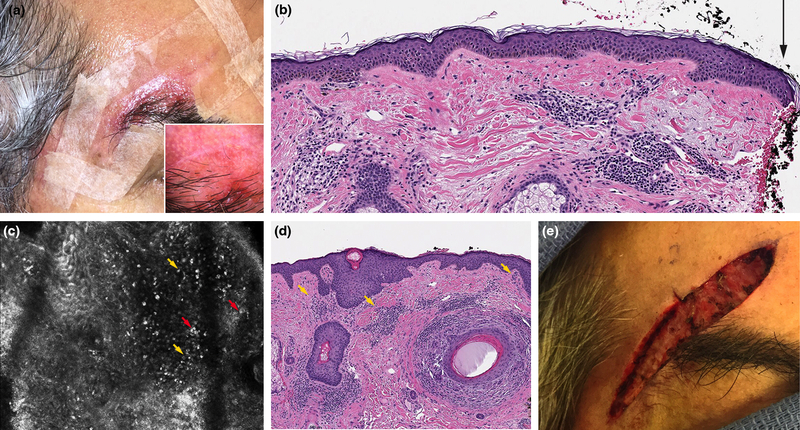
Figure 3:
Incompletely excised lentigo maligna (LM) inflammation affected reflectance confocal microscopy (RCM) interpretation. A. Right temple LM excision scar (inflamed) with no residual clinical pigmentation. B. Residual LM extending to side margins following initial wide local excision (black arrow) (H&E 10X). C. RCM with a dense inflammatory infiltrate (yellow arrows); interpreted as round nucleated cells suspicious for residual LM but with low confidence. D. No residual LM/ on final excision. Aggregates of inflammation were seen (yellow arrows) (H&E 10X). E. Final surgical defect.
Only one case of LM/LMM outside the head and neck was evaluated (lower extremity); it was an incompletely excised LMM Breslow thickness 0.4 mm. In this case, RCM evaluation correctly detected the residual LM component.
Discussion:
In this study of incompletely excised LM/LMM patients seen in a tertiary cancer center over a period of 13 years, we found that the additional average margin needed to histopathologically clear residual LM/LMM was 7.8 mm. Moreover, this additional margin occurred despite lack of clinically evident pigmentation. These findings are clinically relevant since the 7.8 mm ‘extra’ surgical margin was in addition to a prior WLE (average of 4.8 mm) with an average total margin of 12.6 mm for clearing these LM/LMM cases. We found no difference between in situ (LM) and invasive cases (LMM) in terms of extra margins needed for clearance. Our results confirm the relevance of utilizing circumferential margin-controlled surgery for LM/LMM to ensure clear margins prior to reconstruction/tissue rearrangement given unpredictable subclinical extension.1,2,5,12,23–26
Recognizing incompletely excised LM/LMM is important since if left untreated, it may lead to subsequent recurrent melanoma. Despite this, the true biological consequences of microscopically positive margins are not widely understood and the true incidence of recurrence in this clinical-pathological scenario is unkwnown.12,25 Management of incompletely excised melanoma includes re-excision5 and/or adjuvant topical imiquimod.27 Observation may be indicated in selected cases; however, since the true biological relevance and behavior is not clear, this recommendation must be given with caution and close monitoring is needed.12
Strategies are needed to address LM/LMM excision challenges since subclinical extension is unpredictable and variable. LM/LMM incomplete excision can be avoided utilizing surgical techniques that provide complete margin assessment (e.g. staged excision procedures or Mohs micrographic surgery).3,10,23,26 Non-randomized studies suggest lower recurrence rates of LM/LMM when treated with circumferential margin assessment, possibly explained by comprehensive evaluation of margins.26,28 RCM emerges as a novel, non-invasive tool to aid in the presurgical mapping of LM/LMM detecting subclinical extension.17–19
In addition to mapping margins of primary LM/LMM,17–19 RCM may be used for the evaluation of patients with incompletely excised LM/LMM. RCM can provide with in vivo, real-time, non-invasive assessment of the complete scar area. Most of our patients had large scars that involved the entire facial cosmetic units making treatment planning very challenging. In theory, treatment could be focused to the specific areas of residual LM/LMM. However, given difficulty in defining residual LM/LMM areas clinically, treatment is performed to the whole scar area. In patients with complex reconstructions, ‘blind’ excision of the entire scar tissue might be associated with considerable morbidity: RCM could potentially guide management of incompletely excised LM/LMM by directing surgery to targeted areas or guiding non-invasive topical treatment (e.g. imiquimod).
We found that RCM was associated with a diagnostic accuracy of 78.6%, a sensitivity of 90.9%, and a specificity of 33.3% for the detection of residual LM/LMM, when compared to histopathology. With a sensitivity of >90%, RCM has the potential to accurately locate the area of residual LM/LMM.16,29 Nevertheless, specificity in our study was relatively low (<50%). This could be attributed to the presence of inflammation after recent surgery. Post-surgical inflammation can be detected on RCM up to 6 months after surgery;30 the median time of presentation in our cohort, post initial excision was 3 months (range 1 – 8 months). This limitation of RCM examination has been previously reported in basal cell carcinomas evaluated after a recent biopsy; with reported RCM specificities as low as 23%.31,32
False positives and false negatives:
In the present study, two false positives were identified – this might be explained due to post-surgical inflammation. RCM examination was performed 1 month after surgery for the 1st case which still showed clinical erythema (Figure 3). The RCM examination of the 2nd false positive case was performed 2.5 months post-surgery. Ideally, RCM evaluation should be performed when scars are no longer erythematous and without crusts/ulcerations. We also found 1 false negative case in a patient with a cheek scar. After re-reviewing the images no RCM evidence of residual LM/LMM was found which could be explained by the presence of focal residual LM/LMM on final pathology. The present study also complements the current literature on the feasibility of RCM evaluation of previously biopsied/surgically treated tissue.16,31–33
Limitations:
This was a single-center study conducted in a tertiary cancer center; therefore, generalizability of this study might be limited. Regarding RCM evaluation, inflammation from recent surgery may complicate RCM assessment of scars and produce false positive cases: histopathology-confirmed negative cases were only 3 in the prospective cohort and therefore, specificity was disproportionally affected. As Guitera et al. highlighted, hypomelanotic/amelanotic LM/LMM can be more challenging to evaluate with RCM;34 however, given that most of the patients included were referred from other institutions, the pigmentation status of the primary LM/LMM at the time of initial surgery was unknown. Furthermore, we evaluated with RCM only one case located outside the head and neck; thus, we were unable to perform stratify results according to location. Additional studies should look into the role of RCM in assessing residual LM/LMM outside the face.35,36 Nevertheless, head and neck location is most commonly complicated by positive margins.1,12 Finally, we did not systematically record dermoscopic images in our analysis. This was given part of the inclusion criteria was the lack of clinical and dermoscopic evidence of residual LM/LMM.
Conclusion:
Incompletely excised LM/LMM is a poorly characterized clinical-pathological scenario which may require considerable additional margins to achieve microscopic clearance. RCM is emerging as a valuable tool for the evaluation of patients presenting with incompletely excised LM/LMM. Ultimately, most of these complex patients benefit for multidisciplinary tumor board discussions.37 Larger, multicentric studies are needed to confirm our results.
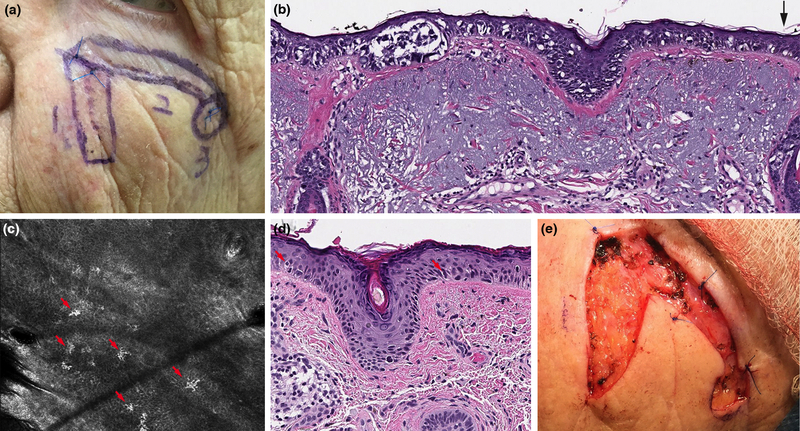
Figure 2:
Incompletely excised lentigo maligna (LM) detected with reflectance confocal microscopy (RCM). A. Left cheek LM excision scar (rotation flap) with no residual clinical pigmentation. B. Residual LM extending to side margins following initial wide local excision (black arrow) (H&E 10X). C. RCM image with bright, large, nucleated dendritic cells at the dermoepidermal junction (red arrows) (750 × 750 μm). D. Residual LM confirmed in final excision (H&E 20X). E. Final surgical defect.
Funding:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Conflicts of interest:
Ashfaq A. Marghoob: received honorarium from 3GEN for dermoscopy lectures, received royalties from publishing companies for books and book chapters, received dermoscopy equipment for testing and feedback, received payment from the American Dermoscopy Meeting for organizing and lecturing at the annual meeting.
Chih-Shan Jason Chen: received research funding from Apollo Medical Optics, Inc.
Anthony Rossi: Dr. Rossi has no relevant conflicts of interest related to this manuscript but has received grant funding from The Skin Cancer Foundation and the A.Ward Ford Memorial Grant for research related to this work. He also served on advisory board, as a consultant, or given educational presentations: for Allergan, Inc; Galderma Inc; Evolus Inc; Elekta; Biofrontera, Quantia; Merz Inc; Dynamed; Skinuvia, Perf-Action, and LAM therapeutics.
References:
- 1.Rzepecki AK, Hwang CD, Etzkorn JR, et al. The “Rule of 10s” versus the “Rule of 2s”: High complication rates after conventional excision with postoperative margin assessment of specialty site versus trunk and proximal extremity melanomas. J Am Acad Dermatol. 2018. [DOI] [PubMed] [Google Scholar]
- 2.Fosko SW, Navarrete-Dechent CP, Nehal KS. Lentigo Maligna-Challenges, Observations, Imiquimod, Confocal Microscopy, and Personalized Treatment. JAMA Dermatol. 2018;154(8):879–881. [DOI] [PubMed] [Google Scholar]
- 3.Hazan C, Dusza SW, Delgado R, Busam KJ, Halpern AC, Nehal KS. Staged excision for lentigo maligna and lentigo maligna melanoma: A retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58(1):142–148. [DOI] [PubMed] [Google Scholar]
- 4.Fallowfield ME, Cook MG. Re-excisions of scar in primary cutaneous melanoma: a histopathological study. Br J Dermatol. 1992;126(1):47–51. [DOI] [PubMed] [Google Scholar]
- 5.Karanetz I, Stanley S, Knobel D, et al. Melanoma Extirpation with Immediate Reconstruction: The Oncologic Safety and Cost Savings of Single-Stage Treatment. Plast Reconstr Surg. 2016;138(1):256–261. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan SR, Liu DZ, Mathes DW, Isik FF. Head and neck malignant melanoma: local recurrence rate following wide local excision and immediate reconstruction. Ann Plast Surg. 2012;68(1):33–36. [DOI] [PubMed] [Google Scholar]
- 7.Osborne JE, Hutchinson PE. A follow-up study to investigate the efficacy of initial treatment of lentigo maligna with surgical excision. Br J Plast Surg. 2002;55(8):611–615. [DOI] [PubMed] [Google Scholar]
- 8.Kunishige JH, Doan L, Brodland DG, Zitelli JA. 9-mm Surgical margin required for both LM and MIS as diagnosed in real-world community practice. J Am Acad Dermatol. 2019;81(4):e117–e118. [DOI] [PubMed] [Google Scholar]
- 9.Kunishige JH, Doan L, Brodland DG, Zitelli JA. Comparison of surgical margins for lentigo maligna versus melanoma in situ. J Am Acad Dermatol. 2019;81(1):204–212. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. Melanoma (Version 2.2018). https://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. Accessed April 4, 2018.
- 11.Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47(5):743–748. [DOI] [PubMed] [Google Scholar]
- 12.Navarrete-Dechent C, Aleissa S, Ariyan C, Busam KJ, Nehal KS. Melanoma in situ margins: 9 mm for ALL cases may not be appropriate. J Am Acad Dermatol. 2019. [DOI] [PubMed] [Google Scholar]
- 13.Rigual NR, Popat SR, Jayaprakash V, Jaggernauth W, Wong M. Cutaneous head and neck melanoma: the old and the new. Expert Rev Anticancer Ther. 2008;8(3):403–412. [DOI] [PubMed] [Google Scholar]
- 14.Hoersch B, Leiter U, Garbe C. Is head and neck melanoma a distinct entity? A clinical registry-based comparative study in 5702 patients with melanoma. Br J Dermatol. 2006;155(4):771–777. [DOI] [PubMed] [Google Scholar]
- 15.Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130(8):2080–2091. [DOI] [PubMed] [Google Scholar]
- 16.Navarrete-Dechent C, Cordova M, Liopyris K, et al. Reflectance confocal microscopy and dermoscopy aid in evaluating repigmentation within or adjacent to lentigo maligna melanoma surgical scars. Journal of the European Academy of Dermatology and Venereology. 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yelamos O, Cordova M, Blank N, et al. Correlation of Handheld Reflectance Confocal Microscopy With Radial Video Mosaicing for Margin Mapping of Lentigo Maligna and Lentigo Maligna Melanoma. JAMA Dermatol. 2017;153(12):1278–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellacani G, De Carvalho N, Ciardo S, et al. The smart approach: feasibility of lentigo maligna superficial margin assessment with hand-held reflectance confocal microscopy technology. J Eur Acad Dermatol Venereol. 2018. [DOI] [PubMed] [Google Scholar]
- 19.Navarrete-Dechent C, Cordova M, Aleissa S, et al. Lentigo maligna melanoma mapping using reflectance confocal microscopy correlates with staged excision: A prospective study. J Am Acad Dermatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarrete-Dechent C, Cordova M, Postow MA, et al. Evaluation of the Response of Unresectable Primary Cutaneous Melanoma to Immunotherapy Visualized With Reflectance Confocal Microscopy: A Report of 2 Cases. JAMA Dermatol. 2019;155(3):347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alarcon I, Carrera C, Alos L, Palou J, Malvehy J, Puig S. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71(1):49–55. [DOI] [PubMed] [Google Scholar]
- 22.Navarrete-Dechent C, Cordova M, Aleissa S, et al. Use of paper tape to guide reflectance confocal microscopy navigation of large skin lesions. J Am Acad Dermatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunishige JH, Doan L, Brodland DG, Zitelli JA. Comparison of surgical margins for lentigo maligna versus melanoma in situ. Journal of the American Academy of Dermatology. 2019;81(1):204–212. [DOI] [PubMed] [Google Scholar]
- 24.Kunishige JH, Brodland DG, Zitelli JA. Surgical margins for melanoma in situ. J Am Acad Dermatol. 2012;66(3):438–444. [DOI] [PubMed] [Google Scholar]
- 25.Weyers W “Personalized Excision” of Malignant Melanoma-Need for a Paradigm Shift in the Beginning Era of Personalized Medicine. Am J Dermatopathol. 2019. [DOI] [PubMed] [Google Scholar]
- 26.Hou JL, Reed KB, Knudson RM, et al. Five-year outcomes of wide excision and Mohs micrographic surgery for primary lentigo maligna in an academic practice cohort. Dermatol Surg. 2015;41(2):211–218. [DOI] [PubMed] [Google Scholar]
- 27.Swetter SM, Chen FW, Kim DD, Egbert BM. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72(6):1047–1053. [DOI] [PubMed] [Google Scholar]
- 28.Moyer JS, Rudy S, Boonstra PS, et al. Efficacy of Staged Excision With Permanent Section Margin Control for Cutaneous Head and Neck Melanoma. JAMA Dermatol. 2017;153(3):282–288. [DOI] [PubMed] [Google Scholar]
- 29.Mataca E, Migaldi M, Cesinaro AM. Impact of Dermoscopy and Reflectance Confocal Microscopy on the Histopathologic Diagnosis of Lentigo Maligna/Lentigo Maligna Melanoma. Am J Dermatopathol. 2018. [DOI] [PubMed] [Google Scholar]
- 30.Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2(2):202a210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahu A, Yelamos O, Iftimia N, et al. Evaluation of a Combined Reflectance Confocal Microscopy-Optical Coherence Tomography Device for Detection and Depth Assessment of Basal Cell Carcinoma. JAMA Dermatol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navarrete-Dechent C, Cordova M, Aleissa S, et al. Reflectance confocal microscopy confirms residual basal cell carcinoma on clinically negative biopsy sites before Mohs micrographic surgery: A prospective study. J Am Acad Dermatol. 2019;81(2):417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webber SA, Wurm EM, Douglas NC, et al. Effectiveness and limitations of reflectance confocal microscopy in detecting persistence of basal cell carcinomas: a preliminary study. Australas J Dermatol. 2011;52(3):179–185. [DOI] [PubMed] [Google Scholar]
- 34.Guitera P, Menzies SW, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions. Br J Dermatol. 2016;175(6):1311–1319. [DOI] [PubMed] [Google Scholar]
- 35.Persechino F, De Carvalho N, Ciardo S, et al. Folliculotropism in pigmented facial macules: Differential diagnosis with reflectance confocal microscopy. Exp Dermatol. 2018;27(3):227–232. [DOI] [PubMed] [Google Scholar]
- 36.Borsari S, Pampena R, Benati E, et al. In vivo dermoscopic and confocal microscopy multistep algorithm to detect in situ melanomas. Br J Dermatol. 2018;179(1):163–172. [DOI] [PubMed] [Google Scholar]
- 37.Mori S, Navarrete-Dechent C, Petukhova TA, et al. Tumor Board Conferences for Multidisciplinary Skin Cancer Management: A Survey of US Cancer Centers. J Natl Compr Canc Netw. 2018;16(10):1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]