Abstract
Objective
It remains unknown how to stratify the risk of clinical relapse of chronic hepatitis B (CHB) patients after stopping nucleos(t)ide analogs (NAs) antiviral therapy.
Methods
The current post hoc analysis included 122 non-cirrhotic patients with chronic hepatitis B virus infection who were positive for hepatitis B envelope antigen (HBeAg) and discontinued long-term NA therapy after achieving HBeAg seroconversion for a median of 2.5 years. Post hoc analysis of end-of-treatment (EOT) hepatitis B core-related antigen (HBcrAg) levels was performed using a chemiluminescent enzyme immunoassay.
Results
A total of 78/122 (63.9%) patients experienced sustained response after NAs cessation, and 44/122 (36.1%) patients experienced clinical relapse. In multivariate analysis, EOT HBcrAg (hazard ratio [HR] = 2.105 95% CI: 1.440–3.077, p < 0.001), hepatitis B surface antigen (HBsAg) ≥100 IU/mL (HR = 4.406, 95% CI 1.567–12.389, p = 0.005) and age (HR = 1.051, 95% CI: 1.010–1.093, p = 0.049) were independently associated with clinical relapse. A cut-off value of 4.0 log10 U/mL of HBcrAg was defined by maximized Youden’s index. An EOT HBcrAg level of ≥4.0 log10 U/mL was associated with higher risks of clinical relapse (65.8% vs 23.2%, p<0.001) and HBeAg reversion (27.5% vs 1.6%, p < 0.001). In majority of patients (n = 91) who had a high EOT HBsAg level (≥100 IU/mL), serum HBcrAg level could further discriminate patients at low risk of clinical relapse. Patients with an HBcrAg level ≥4.0 log10 U/mL had significantly higher cumulative incidence rates of clinical relapse (78.1% vs 29.4%, p < 0.001) and HBeAg reversion (29.4% vs 0%, p < 0.001).
Conclusion
Serum EOT HBcrAg level can be a predictor of off-treatment relapse in patients with CHB. An HBcrAg level of 4.0 log10 U/mL may identify patients at high risk of clinical relapse after treatment cessation.
Keywords: chronic hepatitis B, discontinuation, hepatitis B core-related antigen, hepatitis B surface antigen
Introduction
Nucleos(t)ide analogs (NAs) can effectively suppress hepatitis B virus (HBV) replication and reduce the risk of complications such as cirrhosis and hepatocellular carcinoma. Although NAs are widely used in clinical practice,1–3 their capacity to achieve hepatitis B surface antigen (HBsAg) clearance, and thus a functional cure for HBV infection and cessation of treatment, is unsatisfactory.4,5 A systematic review reported a relapse rate of 18–79% after discontinuation of NA treatment based on recommendations in different guidelines.6 Notably however, several studies have described sustained response and higher incidence of HBsAg clearance in patients who have discontinued NA treatment.7,8 Thus, it remains uncertain whether NA discontinuation can be recommended before HBsAg loss, and which patients with chronic hepatitis B infection are likely to benefit from a finite NA treatment approach.
Studies have identified HBsAg as a potential end-of-treatment (EOT) biomarker to predict relapse after NA therapy cessation.9 Recently, few studies described an undetectable hepatitis B core-related antigen (HBcrAg) level predicts a low likelihood of relapse in hepatitis B envelope antigen (HBeAg)-negative patients,10,11 but contradictory results have also been reported. There are many discrepancies in HBcrAg levels between HBeAg-negative and HBeAg-positive patients.12 Whether HBcrAg status can predict clinical relapse in HBeAg-positive patients remains to be determined.
The aims of the current study were to investigate the capacity of HBcrAg levels to predict relapse after NA discontinuation in HBeAg-positive patients, and determine a reliable cut-off value. The results of the study may facilitate the identifications of subpopulations at low-risk relapse after NA cessation, enabling those patients to benefit from a limited treatment period by way of low economic burden, reduced side effects, and a higher quality of life.
Materials and Methods
Patient Population and Study Design
The current investigation was a prospective observational cohort study (clinical study no. ChiCTR-OOCO17013970). Patients were recruited at Nanfang Hospital, Southern Medical University, Guangzhou, China. Consecutive patients were enrolled if they met the international criteria for discontinuing NA treatment and agreed to discontinue effective NA treatment.
The inclusion criteria were consistent with APASL guidelines and have been reported elsewhere.13 Briefly, HBeAg-positive patients were required to achieve HBeAg seroconversion and undetectable HBV DNA followed by at least 12 months of consolidation therapy. At the time of discontinuation, all the patients were HBeAg-negative and had undetectable HBV DNA. Patients with combined HBV-hepatitis C virus or hepatitis D virus infection, biopsy-proven cirrhosis or liver stiffness >9 kPa as determined via fibroscan (Echosens, Paris, France), alcohol abuse, a liver transplantation history, or malignancy were excluded.
After cessation of NA therapy, all the patients were followed monthly for first 3 months, then every 3 months for 2 years, then every 6 months thereafter. The primary endpoint was clinical relapse defined as an HBV DNA level >2,000 IU/mL with an alanine aminotransferase (ALT) level greater than twice the upper limit of normal (ULN, 40 U/L). All patients with clinical relapse were retreated with either entecavir or tenofovir and discontinued from the study protocol. Secondary endpoints of interest were virologic relapse defined as HBV DNA >2,000 IU/mL, and HBsAg loss.
Biochemical and Virologic Testing
Local standardized automated techniques were used for biochemical testing. Serum HBV DNA levels were assessed via the Cobas TaqMan polymerase chain reaction HBV assay (Roche Diagnostics, Basel, Switzerland) with a lower limit of detection of 20 IU/mL. HBsAg, HBeAg, and anti-hepatitis B e antibody levels were determined using ARCHITECT i1000SR (Abbott Laboratories, Chicago, IL, USA). Serum HBcrAg levels were quantified using the fully automated LUMIPULSE® chemiluminescent enzyme immunoassay analyzer (Fujirebio Inc., Tokyo, Japan) as previously described.14 The lower limit of sensitivity was 2 log10 U/mL and the concentration estimation range was 3–7 log10 U/mL. An HBcrAg level >2 log10 U/mL was considered to be detectable Sera containing >7.0 log10 U/mL of HBcrAg were diluted 10-fold or 100-fold with normal human serum, then retested to obtain the exact titer concentration.
Statistical Analysis
Univariate analyses of normally distributed continuous variables were performed using Student’s t-test and expressed as means ± standard deviation (SD). Non-normally distributed continuous variables were analyzed using the Wilcoxon rank-sum test and expressed as medians and interquartile ranges (IQR). Pearson’s chi-squared test or Fisher’s exact test was used as appropriate for comparisons of categorical data expressed as numbers and percentages.
A Cox proportional regression model was used to test for factors significantly associated with clinical relapse. Factors with p < 0.20 were included in a multivariate Cox proportional hazards regression model. Time-dependent receiver operating characteristic (ROC) curves were generated using the “survivalROC” package via the “Kaplan-Meier” method in R 3.5.2 (Institute for Statistics and Mathematics, Vienna, Austria; http://www.r-project.org/). Kaplan–Meier analysis with the Log rank test was used to calculate the cumulative rate of clinical relapse. Graphs were plotted using GraphPad Prism version 7.0 (GraphPad Software, San Diego, CA, USA). All other analyses were performed using IBM SPSS version 23.0 (IBM, Armonk, NY, USA). All statistical tests were 2-sided, and p values <0.05 were considered statistically significant.
Results
Baseline Characteristics
A total of 122 patients who were HBsAg-positive and HBV DNA-negative at EOT were included in the statistical analysis. A total of 78 (63.9%) patients exhibited a sustained response after NA cessation, and 44 (36.1%) patients exhibited clinical relapse (Table 1). The mean serum HBsAg level at EOT was significantly lower in the sustained response group than in the clinical relapse group (2.35 ± 1.20 vs 2.82 ± 0.85 log10 IU/mL, p = 0.025). A similar trend in serum HBcrAg was observed in the sustained response group (3.59 ± 0.82 vs 4.15 ± 0.72 log10 U/mL, p < 0.001).
Table 1.
Clinical Characteristics of the Study Cohort
| Characteristics of EOT | Total (n=122) | Sustained Response (n=78, 63.9%) | Clinical Relapse (n=44, 36.1%) | p-value |
|---|---|---|---|---|
| Age (years) | 34 (29–40) | 33 (22–39) | 36.5 (31–41) | 0.109 |
| Male (%) | 95 (77.9%) | 60 (76.9%) | 35 (79.5%) | 0.738 |
| Antiviral regiment | 0.168 | |||
| ETV/TDF (%) | 71 (58.2%) | 49 (62.8%) | 22 (50%) | |
| Others (%) | 51 (41.8%) | 29 (37.2%) | 22 (50%) | |
| Treatment duration, (years) | 4.7 (3.2–6.2) | 4.3 (2.9–6.4) | 5.0 (3.7–6.0) | 0.398 |
| Consolidation treatment duration (years) | 2.5 (1.3–3.6) | 3.0 (1.5–3.9) | 2.0 (1.2–3.4) | 0.125 |
| ALT level (ULN) | 0.42 (0.34–0.54) | 0.50 (0.38–0.68) | 0.48 (0.43–0.59) | 0.324 |
| HBV DNA (log10 IU/mL) | UD | UD | UD | - |
| HBsAg (log10 IU/mL) | 2.52±1.11 | 2.35±1.20 | 2.82±0.85 | 0.025 |
| <100 IU/mL (%) | 31 (25.4%) | 27 (34.6%) | 4 (9.15%) | <0.001 |
| ≥100 IU/mL (%) | 91 (74.6%) | 51 (65.4%) | 40 (90.9%) | |
| HBcrAg (log10 U/mL) | 3.80±0.83 | 3.59±0.82 | 4.15±0.72 | <0.001 |
| Liver siffness value (kPa) | 5.3 (4.5–6.2) | 5.3 (4.6–6.0) | 5.8 (4.8–6.6) | 0.149 |
Notes: Variables are expressed as means ± SD, median (interquartile range), or n (%).
Abbreviations: ALT, alanine aminotransferase; EOT, end of treatment; ETV, entecavir; TDF, tenofovir; ULN, upper limit of normal; HBeAg, hepatitis B envelope antigen; HBsAg, hepatitis B surface antigen; HBcrAg, hepatitis B core-relative antigen; UD, undetectable (<20 IU/mL).
Factors Associated with Relapse
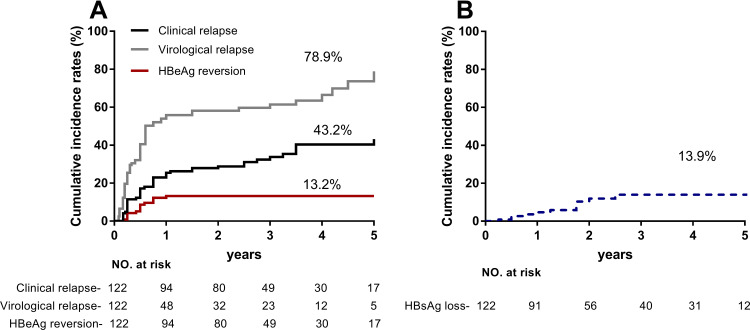
During a median 3.0 years (IQR: 2.1–5.0 years) of follow-up, clinical relapse, virological relapse, and HBeAg reversion were, respectively, observed in 44, 75, and 15 patients within 5 years of follow-up, with cumulative incidence of 43.2%, 79.3%, and 13.2%. Twelve patients were cleared HBsAg, resulting in a cumulative incidence of 13.9% at year 5 (Figure 1). No patients developed signs of decompensated liver disease. Univariate and multivariate analyses were conducted to explore risk factors associated with clinical relapse. High EOT HBcrAg (hazard ratio [HR] = 2.105, 95% confidence interval [CI] 1.440–3.077, p<0.001), HBsAg ≥100 IU/mL (HR = 4.406, 95% CI 1.567–12.389, p = 0.005), and age (HR = 1.051, 95% CI 1.010–1.093, p = 0.049) were independently associated with clinical relapse (Table 2). Higher EOT HBcrAg level was associated with an increased incidence of virological relapse (Supplementary Table 1).
Figure 1.
Cumulative rates of virologic relapse, clinical relapse, HBeAg reversion, and HBsAg loss after discontinuation of NA therapy. (A) Clinical relapse, virological relapse, and HBeAg reversion were respectively observed in 44, 75, and 15 patients within 5 years of follow-up, with the cumulative incidence of 43.2%, 79.3%, and 13.2%. (B) Twelve patients cleared HBsAg, resulting in a cumulative incidence of 13.9% at year 5.
Table 2.
Cox Proportional Hazard Model for Virological Relapse HBeAg-Positive Patients (n=122)
| Variable | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (years) | 1.032 | 0.994–1.032 | 0.098 | 1.051 | 1.010–1.093 | 0.013 |
| Male vs Female | 1.127 | 0.541–2.346 | 0.750 | |||
| First-line vs Second-line drugs† | 0.702 | 0.388–1.271 | 0.242 | |||
| Treatment duration, (years) | 1.077 | 0.961–1.207 | 0.201 | |||
| Consolidation treatment duration (years) | 0.886 | 0.744–1.056 | 0.176 | 0.961 | 0.796–1.159 | 0.676 |
| ALT (U/L) | 0.982 | 0.952–1.013 | 0.255 | |||
| HBsAg (log10 IU/mL) | 1.328 | 0.970–1.818 | 0.077 | |||
| ≥100 IU/mL | 3.842 | 1.374–100.743 | 0.010 | 4.406 | 1.567–12.389 | 0.005 |
| <100 IU/mL | Ref | |||||
| HBcrAg (log10 U/mL) | 2.116 | 1.451–3.086 | <0.001 | 2.105 | 1.440–3.077 | <0.001 |
| ≥4.0 log10 U/mL | 4.042 | 2.301–7.099 | <0.001 | |||
| <4.0 log10 U/mL | Ref | |||||
Notes: †First-line, entecavir, tenofovir; second-line, lamivudine, adefovir, telbivudine, and combination treatment.
Abbreviations: ALT, alanine aminotransferase; ULN, upper limit of normal; HBeAg, hepatitis B envelope antigen; HBsAg, hepatitis B surface antigen; HBcrAg, hepatitis B core-relative antigen; ns, no significance; HR, hazard ratio; 95% CI, 95% confidence interval.
Serum HBcrAg Level and the Prediction of Clinical Relapse After NA Discontinuation
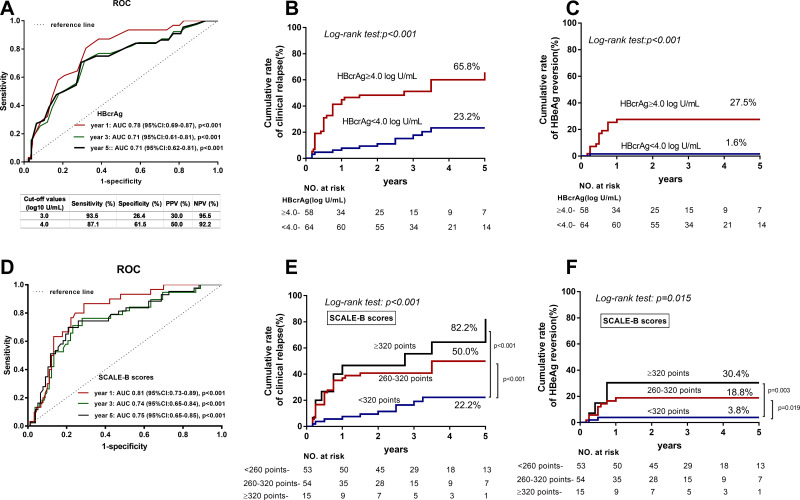
ROC analysis was performed to evaluate the capacity of HBcrAg levels at EOT to predict clinical relapse. The respective area under the ROC curves (AUCs) of HBcrAg for the prediction of clinical relapse after NA therapy discontinuation at year 1,3,5 were 0.78 (p < 0.001), 0.71 (p < 0.001) and 0.71 (p < 0.001), respectively (Figure 2A). Hence, a cut-off value of 4.0 log10 U/mL was defined by maximized Youden’s index on the 5-year ROC curve. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were 87.1%, 61.5%, 50% and 92.2%, respectively. In stratification analysis performed using Kaplan-Meier curves, an EOT HBcrAg level ≥4.0 log10 U/mL was associated with a higher cumulative incidence of clinical relapse (65.8% vs 23.2%, Log rank test: p < 0.001), as well as HBeAg reversion (27.5% vs 1.6%, Log rank test: p < 0.001), compared to level <4.0 log10 U/mL at year 5 (Figures 2B and C).
Figure 2.
ROC curves of HBcrAg and SCALE-B scores to predict clinical relapse after discontinuation of NA therapy. (A) Respective AUCs of HBcrAg for predicting of clinical relapse after NA discontinuation at 1, 3, and 5 years were 0.78 (p < 0.001), 0.71 (p < 0.001) and 0.71 (p<0.001). (B) In stratification analysis EOT HBcrAg level ≥ 4.0 log10 U/mL was associated with a higher risk of clinical relapse (65.8% vs 23.2%, p < 0.001). (C) EOT HBcrAg level ≥ 4.0 log10 U/mL was associated with a higher risk of HBeAg reversion (27.5% vs 1.6%, p < 0.001). (D) Respective AUCs of SCALE-scores for predicting of clinical relapse after NA discontinuation at 1, 3, and 5 years were 0.81 (p < 0.001), 0.74 (p < 0.001) and 0.75 (p<0.001). (E) SCALE-B score < 260 points (22.2%) was associated with a lower risk of clinical relapse, compared to 260–320 points (50%, p < 0.001) and ≥ 320 points (82.2%, p < 0.001). (F) SCALE-B score < 260 points (3.8%) was associated with a lower risk of HBeAg reversion, compared to 260–320 points (18.8%, p < 0.001) and ≥ 320 points (30.4%, p < 0.001).
To verify the published SCALB score, the discriminative performance of SCALB score was assessed by AUCs, and being 0.81, 0.74 and 0.75 at 1, 3, and 5 years, respectively (Figure 2D). The cumulative incidences of clinical relapse and HBeAg reversion in low- (<260 points), mediate- (260–320 points), and high (≥320 points) subgroups at 5 years were 22.2%, 50%, 82.2% (Log rank test: p < 0.001), and 3.8%, 18.8%, 30.4% (Log rank test: p = 0.015), respectively (Figures 2E and F).
HBcrAg and HBsAg Combined for the Prediction of 5-Year Relapse Risk After NA Discontinuation
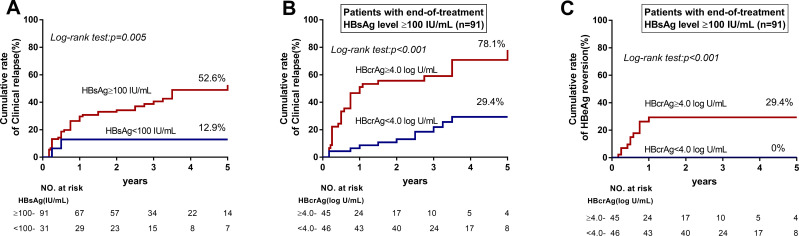
An EOT HBsAg level <100 IU/mL is associated with a lower risk of relapse after NA discontinuation (Figure 3A), but the majority of patients (n = 91) had a high EOT HBsAg level (≥100 IU/mL). Among those patients with a high EOT HBsAg level, serum HBcrAg level could further discriminate patients at low risk of clinical relapse. Patients with an HBcrAg level ≥4.0 log10 U/mL had significantly higher cumulative incidence rates of clinical relapse (78.1% vs 29.4%, p < 0.001) and HBeAg reversion (29.4% vs 0%, p < 0.001) (Figures 3B and C). In multivariable analysis, HBcrAg was associated with higher clinical relapse rate (HR = 2.102; 95% CI 1.422–3.107; p < 0.001) (Table 3).
Figure 3.
Cumulative rates of clinical relapse in patients with HBsAg levels <100 IU/mL and HBsAg levels ≥100 IU/mL. (A) An EOT HBsAg level < 100 IU/mL was associated with a lower risk of relapse after NA discontinuation. (B) Of patients with an EOT HBsAg level ≥100 IU/mL, those with an HBcrAg level ≥4.0 log10 U/mL had significantly higher cumulative incidence rates of clinical relapse (78.1% vs 29.4%, p < 0.001). (C) Of patients with an EOT HBsAg level ≥100 IU/mL, those with an HBcrAg level ≥4.0 log10 U/mL had significantly higher cumulative incidence rates of HBeAg reversion (29.4% vs 0%, p < 0.001).
Table 3.
Cox Proportional Hazard Model for Clinical Relapse in Patients with HBsAg Level ≥100 IU/mL (n=91)
| Variable | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (years) | 1.039 | 0.997–1.098 | 0.07 | 1.029 | 0.985–1.074 | 0.103 |
| Male vs Female | 1.107 | 0.527–2.326 | 0.789 | |||
| First-line vs Second-line drugs† | 0.911 | 0.487–1.704 | 0.711 | |||
| Treatment duration (years) | 1.108 | 0.975–1.259 | 0.117 | 1.134 | 0.986–1.258 | 0.102 |
| Consolidation treatment duration (years) | 1.092 | 0.981–1.215 | 0.106 | 0.754 | 0.586–0.971 | 0.029 |
| ALT (U/L) | 0.974 | 0.940–1.010 | 0.158 | 0.968 | 0.929–1.008 | 0.118 |
| HBsAg (log10 IU/mL) | 0.723 | 0.379–1.379 | 0.324 | |||
| HBcrAg (log10 U/mL) | 2.060 | 1.383–3.068 | <0.001 | 2.102 | 1.422–3.107 | <0.001 |
| ≥4.0 log10 U/mL | 4.202 | 2.082–8.482 | <0.001 | |||
| <4.0 log10 U/mL | Ref | |||||
Notes: †First-line, entecavir, tenofovir; second-line, lamivudine, adefovir, telbivudine, and combination treatment.
Abbreviations: ALT, alanine aminotransferase; ULN, upper limit of normal; HBeAg, hepatitis B envelope antigen; HBsAg, hepatitis B surface antigen; HBcrAg, hepatitis B core-relative antigen; ns, no significance; HR, hazard ratio; 95% CI, 95% confidence interval.
Prediction of HBsAg Loss
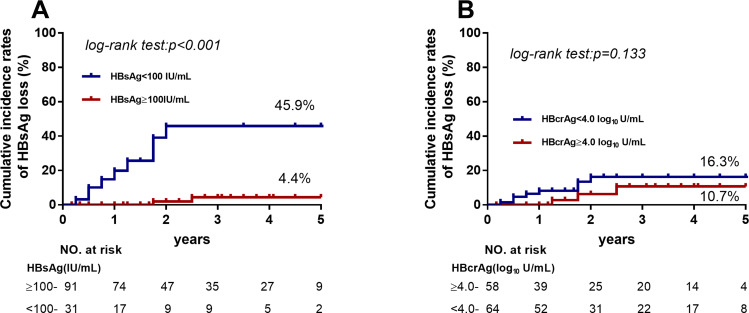
To further evaluate variables associated with HBsAg loss, EOT HBsAg levels and HBcrAg levels were compared in patients with HBsAg loss during follow-up. Patients with HBsAg levels <100 IU/mL were remarkably more likely to exhibit HBsAg loss (45.9% vs 4.4%, p < 0.001, Figure 4). Of the 12 patients who developed HBsAg loss, only 4 had HBcrAg levels <3.0 log10 IU/mL at discontinuation. The off-treatment serum HBsAg, HBV DNA, and ALT levels of these patients are present in Supplementary Figure 1. Interestingly, EOT HBcrAg was positively correlated with peak HBV DNA levels during the follow-up period (Supplementary Figure 2).
Figure 4.
Cumulative rates of HBsAg loss among patients with HBsAg levels <100 IU/mL and HBsAg levels ≥100 IU/mL. (A) Patients with HBsAg levels <100 IU/mL were significantly more likely to exhibit HBsAg loss (45.9% vs 4.4%, p < 0.001). (B) Patients with HBcrAg levels ≥4.0 log10 U/mL had higher cumulative incidence rates of HBsAg loss but not significantly.
Discussion
Collectively, the results of the current study highlight the potential of using serum HBcrAg level as a biomarker to identify candidates for safe NA treatment discontinuation. HBcrAg has been evaluated in a variety of settings in CHB patients and may serve as a new serum biomarker for HBV infection, treatment and prognosis.15 The results of the present study are concordant with those of other studies in which EOT HBcrAg levels were significantly associated with off-NA HBV relapse, yielding AUCs 0.61–0.77 for predicting clinical relapse.16–19 In contrast, in another two recent studies conducted in Taiwan EOT HBcrAg level was not an independent predictor of HBV relapse in HBeAg-negative patients after the cessation of entecavir or tenofovir.20,21 The discrepancy in the predictive value of EOT HBcrAg between studies may be partly due the heterogeneous characteristics of the patients included; ie, differences in ethnicity, immune status, and therapeutic regimens. Moreover, most previous studies have focused on HBeAg-negative patients or a mixture of HBeAg-positive and HBeAg-negative patients. Given this, the current study focused on HBeAg-positive patients constitutes a degree of complementary clinical validation of the use of HBcrAg in clinical practice.
Patients with HBcrAg levels ≥4.0 log10 U/mL were at significantly higher risk of clinical relapse (65.8%) and HBeAg reversion (27.5%). These results validated the cut-off values recommended in the Japan Society of Hepatology guidelines, which propose a dual threshold (HBcrAg levels of 3.0 and 4.0 log10 U/mL) to predict relapse risk after the cessation of NA treatment.22 The cut-off value of HBcrAg established in this study is comparable with that determined in other studies.16,17,23 Kaewdech et al showed that EOT HBcrAg level of 3 log10 U/mL yielded NPV of 90% in predicting off-treatment relapse.19 Notably however, the cut-off value of 3.0 log10 U/mL was not considered in the present study because specificity would only have been 26.4% if it had been adopted, suggesting that the predictive value of this cut-off value is not satisfactory. Nevertheless, studies have reported higher HBcrAg levels in HBeAg-positive patients than in HBeAg-negative patients.24 It is therefore reasonable to use a higher threshold in a group of HBeAg-positive patients than may be used in an HBeAg-negative group or a pooled group. Moreover, a higher and thus more lenient cut-off value would allow physicians to identify additional candidates for the discontinuation of NA treatment. Notably however, more extensive clinical validation is warranted before serum HBcrAg levels can be used in clinical practice.
Consistent with previous research,10,21 low levels of HBsAg (<100 IU/mL) in current study could identify patients at low risk of clinical relapse (12.9%). On the contrary, a combination of high EOT HBsAg level (≥100 IU/mL) and EOT HBcrAg level (≥4.0 log10 U/mL) alarmed high risk of clinical relapse (78.1%) and HBeAg reversion (29.4%), and should be considered as a marker against the cessation of NA therapy. Instead of combining HBsAg and HBcrAg to improve the predicting value of off-treatment relapse, Hsu et al have attempted to conduct a SCALE-B score formulated with EOT HBcrAg, HBsAg, age, ALT, and tenofovir use.18 The present study lent support to validity of the SCALE-B score, particularly its capability of using 320 points to identify patients at high risk of clinical relapse (82.2%). However, it may be limited to the clinical practice because only 15 of 122 patients were ≥320 points. A lower AUC value of SCALE-B score for predicting clinical relapse was shown in the present cohort (0.74) than the initial cohort (0.90), but better than studies by Sonneveld et al (0.626) and Papatheodoridi et al (no significance).10,25 Except for the ethnicity, the discrepancy is probably due to the cohorts having different HBeAg status and evaluations for clinical relapse.
The incidence of HBsAg loss was relatively low in the present study, and it was 13.9% (12/122) at 5-years of follow-up. Similar rates of HBsAg loss were reported in studies from Taiwan.26,27 Higher rate has been found in Caucasian patients,7 and this may be due to differences in races and possibly HBV genotypes. Irrespective differences between HBV-infected population, the current study verified that an EOT HBsAg level <100 IU/mL is important for achieving HBsAg loss after discontinuation, which reached 45.9%. Previous studies demonstrated that undetectable EOT HBcrAg level (<3.0 log10 U/mL) could predict HBsAg loss after discontinuation.7,18 In contrast, the present study showed only 4/12 patients who developed HBsAg loss had undetectable HBcrAg, suggesting that undetectable HBcrAg is not essential to achieving HBsAg loss. Because the clearance of HBsAg is probably affected by immune mechanisms, such as the reactivation of HBV-specific CD4 and CD8 T cells,28,29 as well as the expression of chemokines (CXCL13and IP10) to attract functional T cells.30 HBsAg exists in the form of small particles in the serum and carries a variety of B cell and T cell epitopes on its surface, which may induce the immune response, then reduces HBsAg and even clears HBsAg. The present study showed that anti-HBs levels were all negative (<2 IU/mL) except one (5.73 IU/mL), and were detectable in 8/12 patients (range 32.68 to 319.20 IU/mL) after HBsAg loss (data not shown), reflecting anti-HBs neutralization may play an important role in HBsAg loss. Studies have proved that HBcrAg acts as an indicator of cccDNA transcription activity.31,32 As such, HBcrAg is related to the rebound of virus after drug withdrawal. HBcrAg was only weakly correlated with HBsAg in the current study, and this may be due to the small range of HBsAg levels in the majority of the patients enrolled (113/122 patients had HBsAg levels ranging from −1 to 3.7 log10 IU/mL) (Supplementary Figure 3). Limited to the low incidence rates of HBsAg loss in CHB patients, more data are needed to define the predicting value of HBcrAg for HBsAg loss.
The current study had some limitations. A total of 51 cases treatment with drugs that are no longer used as first-line drugs in patients with chronic HBV. Nevertheless, there were no statistically significant differences between treatment drugs with respect to clinical relapse, which may be due to their similar mechanisms of HBV suppression. Another study limitation was that HBV genotype were only available for a minority of the patients (40/122; 32.8%), and the genotypes were all either B or C (data not shown). Consequently, the contribution of HBV genotype to clinical relapse or HBsAg loss could not be reliably evaluated. Lastly, the study was focused on EOT characteristics, and did not account for the kinetics of on-treatment and off-treatment HBcrAg levels.
Conclusion
In the present study, an EOT HBcrAg level <4.0 log10 U/mL was a reliable predictor of the safety stopping NA treatment in patients with chronic HBV infection.
Acknowledgments
The authors would like to thank all the patients included in this study, and the nurses who assisted in patient management and collection of serum samples.
Funding Statement
This study was supported by grants from the National Science Foundation of China (No. 81971949), National Science and Technology Key Project on “Major Infectious Diseases such as HIV/AIDS, Viral Hepatitis Prevention and Treatment” (Nos. 2017ZX09304016 and 2017ZX10302201004008) and Clinical Research Startup Program of Southern Medical University by High-level University Construction Funding of Guangdong Provincial Department of Education (No. LC2016PY003). The funding sources did not have any influence on the study design, data collection, analysis and interpretation of the data, writing of the manuscript, or decision to submit for publication.
Abbreviations
HBV, hepatitis B virus; CHB, chronic hepatitis B; ALT, alanine aminotransferase; NA, nucleos(t)ide analog; EOT, end-of-treatment; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; HBcrAg, hepatitis B core-related antigen; ULN, the upper limit of normal; LLOD, the lower limit of detection; CLEIA, chemiluminescent enzyme immunoassay; ROC, receiver operating characteristic; SD, standard deviation; HR, hazard ratio; CI, confidence interval.
Data Sharing Statement
Authors can confirm all relevant data are included in the article and materials are available on request from the authors.
Ethics Approval and Consent to Participate
The study was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Ethics Committee of Nanfang Hospital (study identifier NFEC-201209-K3). All patients provided written informed consent, agreed to follow the protocol and take specimens, and were willing to anonymously publish details of the medical record.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–283. doi: 10.1002/hep.28156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu LZ, Sun J, Hou J, Chan H. Improvements in the management of chronic hepatitis B virus infection. Expert Rev Gastroenterol Hepatol. 2018;12(11):1153–1166. doi: 10.1080/17474124.2018.1530986 [DOI] [PubMed] [Google Scholar]
- 4.Jeng WJ, Chen YC, Chien RN, Sheen IS, Liaw YF. Incidence and predictors of hepatitis B surface antigen seroclearance after cessation of nucleos(t)ide analogue therapy in hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2018;68(2):425–434. doi: 10.1002/hep.29640 [DOI] [PubMed] [Google Scholar]
- 5.Fung J, Seto WK, Wong DK, Lai CL, Yuen MF. Hepatitis B surface antigen levels after hepatitis B e-antigen seroclearance: a longitudinal follow-up study. Liver Int. 2015;35(3):854–859. doi: 10.1111/liv.12596 [DOI] [PubMed] [Google Scholar]
- 6.Chang ML, Liaw YF, Hadziyannis SJ. Systematic review: cessation of long-term nucleos(t)ide analogue therapy in patients with hepatitis B e antigen-negative chronic hepatitis B. Aliment Pharmacol Ther. 2015;42(3):243–257. doi: 10.1111/apt.13272 [DOI] [PubMed] [Google Scholar]
- 7.Papatheodoridis GV, Manolakopoulos S, Su TH, et al. Significance of definitions of relapse after discontinuation of oral antivirals in HBeAg-negative chronic hepatitis B. Hepatology. 2018;68(2):415–424. doi: 10.1002/hep.29497 [DOI] [PubMed] [Google Scholar]
- 8.Hadziyannis SJ, Sevastianos V, Rapti I, Vassilopoulos D, Hadziyannis E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology. 2012;143(3):629–636. doi: 10.1053/j.gastro.2012.05.039 [DOI] [PubMed] [Google Scholar]
- 9.van Bommel F, Berg T. Stopping long-term treatment with nucleos(t)ide analogues is a favourable option for selected patients with HBeAg-negative chronic hepatitis B. Liver Int. 2018;38(Suppl 1):90–96. doi: 10.1111/liv.13654 [DOI] [PubMed] [Google Scholar]
- 10.Papatheodoridi M, Hadziyannis E, Berby F, et al. Predictors of hepatitis B surface antigen loss, relapse and retreatment after discontinuation of effective oral antiviral therapy in noncirrhotic HBeAg-negative chronic hepatitis B. J Viral Hepat. 2020;27(2):118–126. doi: 10.1111/jvh.13211 [DOI] [PubMed] [Google Scholar]
- 11.Van Hees S, Chi H, Hansen B, et al. Sustained off-treatment viral control is associated with high hepatitis B surface antigen seroclearance rates in Caucasian patients with nucleos(t)ide analogue-induced HBeAg seroconversion. J Viral Hepat. 2019;26(6):766–769. doi: 10.1111/jvh.13084 [DOI] [PubMed] [Google Scholar]
- 12.Gou Y, Zhao Y, Rao C, et al. Predictive value of hepatitis B core-related antigen (HBcrAg) during the natural history of hepatitis B virus infection. Clin Lab. 2017;63(7):1063–1070. doi: 10.7754/Clin.Lab.2017.161034 [DOI] [PubMed] [Google Scholar]
- 13.Cao J, Chi H, Yu T, et al. Off-treatment hepatitis B virus (HBV) DNA levels and the prediction of relapse after discontinuation of nucleos(t)ide analogue therapy in patients with chronic hepatitis B: a prospective stop study. J Infect Dis. 2017;215(4):581–589. doi: 10.1093/infdis/jix025 [DOI] [PubMed] [Google Scholar]
- 14.Chuaypen N, Posuwan N, Chittmittraprap S, et al. Predictive role of serum HBsAg and HBcrAg kinetics in patients with HBeAg-negative chronic hepatitis B receiving pegylated interferon-based therapy. Clin Microbiol Infect. 2018;24(3):306–307. doi: 10.1016/j.cmi.2017.07.016 [DOI] [PubMed] [Google Scholar]
- 15.Mak LY, Wong DK, Cheung KS, Seto WK, Lai CL, Yuen MF. Review article: hepatitis B core-related antigen (HBcrAg): an emerging marker for chronic hepatitis B virus infection. Aliment Pharmacol Ther. 2018;47(1):43–54. doi: 10.1111/apt.14376 [DOI] [PubMed] [Google Scholar]
- 16.Fan R, Peng J, Xie Q, et al. Combining hepatitis B virus RNA and hepatitis B core-related antigen: guidance for safely stopping nucleos(t)ide analogues in hepatitis B e antigen-positive patients with chronic hepatitis B. J Infect Dis. 2020;222(4):611–618. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto A, Tanaka E, Minami M, et al. Low serum level of hepatitis B indicates unlikely reactivation core-related antigen of hepatitis after cessation of lamivudine therapy. Hepatol Res. 2007;37(8):661–666. [DOI] [PubMed] [Google Scholar]
- 18.Hsu YC, Nguyen MH, Mo LR, et al. Combining hepatitis B core-related and surface antigens at end of nucleos(t)ide analogue treatment to predict off-therapy relapse risk. Aliment Pharmacol Ther. 2019;49(1):107–115. [DOI] [PubMed] [Google Scholar]
- 19.Kaewdech A, Tangkijvanich P, Sripongpun P, et al. Hepatitis B surface antigen, core-related antigen and HBV RNA: predicting clinical relapse after NA therapy discontinuation. Liver Int. 2020;40(12):2961–2971. doi: 10.1111/liv.14606 [DOI] [PubMed] [Google Scholar]
- 20.Huang PY, Wang JH, Hung CH, Lu SN, Hu TH, Chen CH. The role of hepatitis B virus core-related antigen in predicting hepatitis B virus relapse after cessation of entecavir in hepatitis B e antigen-negative patients. J Viral Hepat. 2021. doi: 10.1111/jvh.13528 [DOI] [PubMed] [Google Scholar]
- 21.Kuo YH, Wang JH, Hung CH, Lu SN, Hu TH, Chen CH. Combining end-of-treatment HBsAg and baseline hepatitis B core-related antigen reduce HBV relapse rate after tenofovir cessation. Hepatol Int. 2021;15(2):301–309. doi: 10.1007/s12072-021-10159-w [DOI] [PubMed] [Google Scholar]
- 22.Drafting Committee for Hepatitis Management Guidelines and the Japan Society of Hepatology. JSH guidelines for the management of hepatitis b virus infection. Hepatol Res. 2014;44(Suppl S1):1–58. [DOI] [PubMed] [Google Scholar]
- 23.Shinkai N, Tanaka Y, Orito E, et al. Measurement of hepatitis B virus core-related antigen as predicting factor for relapse after cessation of lamivudine therapy for chronic hepatitis B virus infection. Hepatol Res. 2006;36(4):272–276. doi: 10.1016/j.hepres.2006.08.005 [DOI] [PubMed] [Google Scholar]
- 24.Zhang ZQ, Wang YB, Lu W, et al. Performance of hepatitis B core-related antigen versus hepatitis B surface antigen and hepatitis B virus DNA in predicting HBeAg-positive and HBeAg-negative chronic hepatitis. Ann Lab Med. 2019;39(1):67–75. doi: 10.3343/alm.2019.39.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonneveld MJ, Park JY, Kaewdech A, et al. Prediction of sustained response after nucleo(s)tide analogue cessation using HBsAg and HBcrAg levels: a multicenter study (CREATE). Clin Gastroenterol Hepatol. 2020. doi: 10.1016/j.cgh.2020.12.005 [DOI] [PubMed] [Google Scholar]
- 26.Chen CH, Hsu YC, Lu SN, et al. The incidence and predictors of HBV relapse after cessation of tenofovir therapy in chronic hepatitis B patients. J Viral Hepat. 2018;25(5):590–597. doi: 10.1111/jvh.12851 [DOI] [PubMed] [Google Scholar]
- 27.Yao CC, Hung CH, Hu TH, et al. Incidence and predictors of HBV relapse after cessation of nucleoside analogues in HBeAg-negative patients with HBsAg</= 200 IU/mL. Sci Rep. 2017;7(1):1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rinker F, Zimmer CL, Honer ZSC, et al. Hepatitis B virus-specific T cell responses after stopping nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B. J Hepatol. 2018;69(3):584–593. doi: 10.1016/j.jhep.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 29.Rivino L, Le Bert N, Gill US, et al. Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J Clin Invest. 2018;128(2):668–681. doi: 10.1172/JCI92812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia M, Liao G, Chen H, et al. Plasma CXCL13 is a predictive factor for HBsAg loss and clinical relapse after discontinuation of nucleos(t)ide analogue treatment. Clin Immunol. 2019;198:31–38. doi: 10.1016/j.clim.2018.11.016 [DOI] [PubMed] [Google Scholar]
- 31.Testoni B, Lebosse F, Scholtes C, et al. Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J Hepatol. 2019;70(4):615–625. doi: 10.1016/j.jhep.2018.11.030 [DOI] [PubMed] [Google Scholar]
- 32.Chen EQ, Wang ML, Tao YC, et al. Serum HBcrAg is better than HBV RNA and HBsAg in reflecting intrahepatic covalently closed circular DNA. J Viral Hepat. 2019;26(5):586–595. doi: 10.1111/jvh.13061 [DOI] [PubMed] [Google Scholar]