Abstract
Importance:
The US Preventive Services Task Force (USPSTF) is updating its 2016 colorectal cancer screening recommendations.
Objective:
To provide updated model-based estimates of the benefits, burden, and harms of colorectal cancer screening strategies and to identify strategies that may provide an efficient balance of life-years gained (LYG) from screening and colonoscopy burden to inform the USPSTF.
Design, Setting, and Participants:
Comparative modeling study using 3 microsimulation models of colorectal cancer screening in a hypothetical cohort of 40-year-old US individuals at average risk of colorectal cancer.
Exposures:
Screening from ages 45, 50, or 55 years to ages 70, 75, 80, or 85 years with fecal immunochemical testing (FIT), multitarget stool DNA testing, flexible sigmoidoscopy alone or with FIT, computed tomography colonography, or colonoscopy. All persons with an abnormal noncolonoscopy screening test result were assumed to undergo follow-up colonoscopy. Screening intervals varied by test. Full adherence with all procedures was assumed.
Main Outcome and Measures:
Estimated LYG relative to no screening (benefit), lifetime number of colonoscopies (burden), number of complications from screening (harms), and balance of incremental burden and benefit (efficiency ratios). Efficient strategies were those estimated to require fewer additional colonoscopies per additional LYG relative to other strategies.
Results:
Estimated LYG from screening strategies ranged from 171 to 381 per 1000 40-year-olds. Lifetime colonoscopy burden ranged from 624 to 6817 per 1000 individuals, and screening complications ranged from 5 to 22 per 1000 individuals. Among the 49 strategies that were efficient options with all 3 models, 41 specified screening beginning at age 45. No single age to end screening was predominant among the efficient strategies, although the additional LYG from continuing screening after age 75 were generally small. With the exception of a 5-year interval for computed tomography colonography, no screening interval predominated among the efficient strategies for each modality. Among the strategies highlighted in the 2016 USPSTF recommendation, lowering the age to begin screening from 50 to 45 years was estimated to result in 22 to 27 additional LYG, 161 to 784 additional colonoscopies, and 0.1 to 2 additional complications per 1000 persons (ranges are across screening strategies, based on mean estimates across models). Assuming full adherence, screening outcomes and efficient strategies were similar by sex and race and across 3 scenarios for population risk of colorectal cancer.
Conclusions and Relevance:
This microsimulation modeling analysis suggests that screening for colorectal cancer with stool tests, endoscopic tests, or computed tomography colonography starting at age 45 years provides an efficient balance of colonoscopy burden and life-years gained.
Colorectal cancer remains the second leading cause of US cancer deaths.1 While randomized trials have shown that screening reduces colorectal cancer incidence and colorectal cancer mortality,2 there are many ways to screen, and trial-based evaluation of the long-term outcomes of the full spectrum of screening strategies is not feasible. In 2016, the US Preventive Services Task Force (USPSTF) recommended colorectal cancer screening starting at age 50 years and continuing until age 75 years (A recommendation), offering a number of screening modalities.3 To help inform an update of the colorectal cancer screening recommendations,3, 4 the USPSTF requested an updated modeling study to estimate long-term outcomes of screening strategies, along with an updated evidence review.2
METHODS
This analysis updated a 2016 modeling analysis estimating how the benefits, burden, and harms of screening average-risk, asymptomatic adults for colorectal cancer vary by modality, interval for repeated testing, and ages to begin and end screening.4 It incorporated recent evidence reporting increasing colorectal cancer incidence rates among recent birth cohorts5 and evaluated whether modeled benefits, burden, and harms of screening vary by race and sex. More information about the models and findings from additional analyses are included in the full modeling report.6
Model Descriptions
This study used 3 independently-developed microsimulation models: Simulation Model of CRC (SimCRC), CRC Simulated Population Model for Incidence and Natural History (CRC-SPIN), and Microsimulation Screening Analysis (MISCAN) for colorectal cancer. All were used in the 2016 analysis,4 although they were updated to incorporate observed trends in the population risk of colorectal cancer.5 CRC-SPIN was also updated7 following a validation study.8 Each model has a natural history and a screening component, summarized below. SimCRC was programmed in C++, CRC-SPIN in R, and MISCAN in Delphi.
Natural History Component
All models describe the natural history of colorectal cancer in an average-risk unscreened population, assuming that all colorectal cancer arises from an adenomatous polyp.9 Alternative ways in which colorectal cancer can arise, such as from serrated polyps,10 were not modeled. Simulated persons were assumed to be free of adenomas and colorectal cancer at birth (Figure 1). As they age, they are at risk of developing 1 or more adenomas, each of which may grow in size. Adenomas may progress to preclinical colorectal cancer, after which they may become symptomatic, leading to colorectal cancer diagnosis. Persons may die of other causes at any time11; persons with clinically detected colorectal cancer are also at risk of dying of colorectal cancer.12
Figure 1. Natural History of Colorectal Cancer and the Effects of Screening as Simulated by SimCRC, CRC-SPIN, and MISCAN.
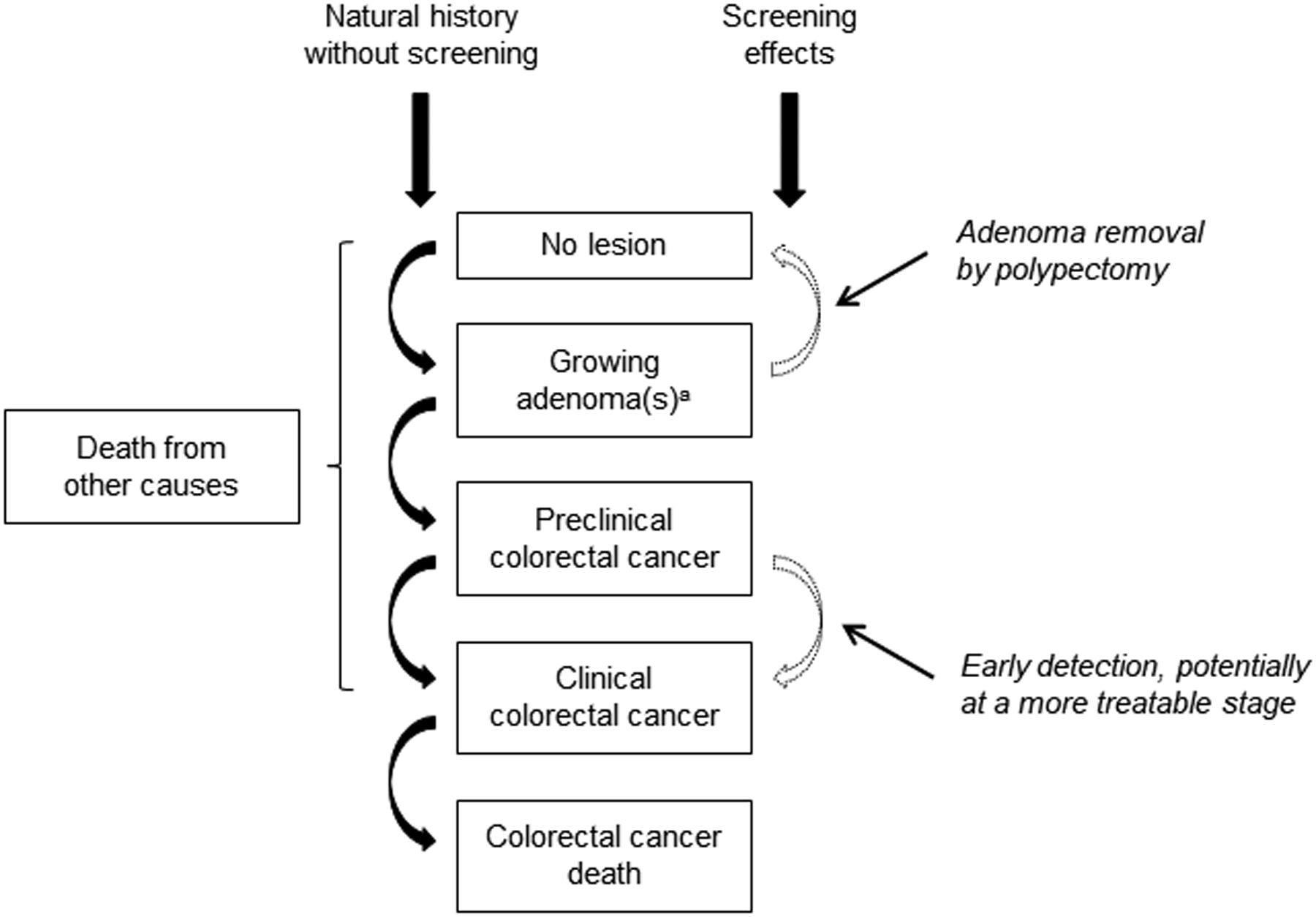
The opportunity to intervene in the natural history through screening (adenoma detection and removal, and early detection) is noted by the dotted lines. Screening can either remove a precancerous lesion (ie, adenoma), thus moving a person to the “no lesion” state, or diagnose a preclinical cancer, which, if detected at an earlier stage, may be more amenable to treatment. Each person’s life history is simulated in the absence of screening and in the presence of screening, such that the effect of a given screening strategy on each simulated person’s outcomes are known.
a Simulation Model of CRC (SimCRC) and Microsimulation Screening Analysis (MISCAN) simulate categorical adenoma size (1 to <6 mm; 6 to <10 mm, ≥10 mm), whereas CRC Simulated Population Model for Incidence and Natural History (CRC-SPIN) simulates continuous adenoma size. SimCRC and CRC-SPIN assume that all adenomas have the potential to progress to colorectal cancer, whereas MISCAN assumes that some adenomas are nonprogressive (ie, they do not grow or progress to cancer after reaching a certain size category) and that the likelihood that an adenoma is progressive increases with age. None of the models simulate adenoma histology.
Each model’s natural history component was initially calibrated to adenoma prevalence data and colorectal cancer incidence prior to the dissemination of screening in the US.13 The models do not simulate family history of colorectal cancer, although people with a family history are included in the data used to calibrate the models. For this analysis, the natural history components were adjusted to reflect increasing colorectal cancer incidence among birth cohorts since the 1950s.5 The magnitude of the increase was estimated by the ratio of colorectal cancer incidence (excluding tumors that are not the target of colorectal cancer screening) among 20- to 44-year-olds in 2012–2016 (the 5 most recent years of Surveillance, Epidemiology, and End Results [SEER] data available) relative to 1975–1979 (the years of SEER data used for initial model calibration).14 Base-case analyses assumed this incidence rate ratio (IRR) to be 1.19. Scenario analyses were also simulated assuming IRRs of 1.00 (no change in incidence from the prescreening era) and 1.52 (the approximate upper bound of the 95% CI for the IRR estimated from age-period-cohort modeling of SEER incidence rates; see the Supplement for details on estimation of IRRs). In all scenarios, the increase in incidence was assumed to arise from an increase in adenoma risk that is carried forward with age.
Screening Component
The models’ screening components allow for interruption of the adenoma-carcinoma sequence through detection and removal of adenomas and for the detection of preclinical cancer, potentially at an earlier stage (Figure 1). The ability of a test to detect lesions is a function of its sensitivity and, for endoscopic tests, whether the lesion is within reach of the scope (Table 1). All persons with an abnormal noncolonoscopy screening test result were assumed to undergo follow-up colonoscopy. Patients with a history of adenoma(s) were assumed to undergo surveillance with colonoscopy, with surveillance intervals based on published guidelines.19 The models incorporate the risk of fatal20 and nonfatal21 complications from colonoscopy and have been externally validated.22
Table 1.
Screening Test Characteristics Used in the Analysis
| Screening test/test characteristic | Value | Source |
|---|---|---|
| FIT (per person) | Lin et al,2 2021 | |
| Specificity | 0.97 | |
| 0.07a | ||
| Sensitivity for adenomas ≥10 mm | 0.22b | |
| Sensitivity for colorectal cancer | 0.74 | |
| sDNA-FIT (per person) | Lin et al,2 2021 | |
| Specificity | 0.91 | |
| 0.15a | ||
| Sensitivity for adenomas ≥10 mm | 0.42b | |
| Sensitivity for colorectal cancer | 0.94 | |
| Colonoscopy (within reach, per lesion)c | ||
| Specificity | 0.86d | Schroy et al,15 2013 |
| Sensitivity for adenomas 1 to <6 mm | 0.75 | van Rijn et al,16 2006 |
| Sensitivity for adenomas 6 to <10 mm | 0.85 | van Rijn et al,16 2006 |
| Sensitivity for adenomas ≥10 mm | 0.95 | van Rijn et al,16 2006 |
| Sensitivity for colorectal cancer | 0.95 | By assumptione |
| SIG (within reach, per lesion) | ||
| Specificity | 0.87d | Weissfeld et al,17 2005 |
| Sensitivity for adenomas 1 to <6 mm | 0.75 | By assumptionf |
| Sensitivity for adenomas 6 to <10 mm | 0.85 | By assumptionf |
| Sensitivity for adenomas ≥10 mm | 0.95 | By assumptionf |
| Sensitivity for colorectal cancer | 0.95 | By assumptionf |
| CTC (per lesion) | Johnson et al,18 2008 | |
| Specificity | 0.88g | |
| Sensitivity for adenomas 1 to <6 mm | NR | |
| Sensitivity for adenomas 6 to <10 mm | 0.57 | |
| Sensitivity for adenomas ≥10 mm | 0.84 | |
| Sensitivity for colorectal cancer | 0.84e | |
Abbreviations: CTC, computed tomography colonography; FIT, fecal immunochemical test (with positivity cutoff of 20 μg of hemoglobin per gram of feces); NR, not reported (adenoma size < 6 mm [the threshold size for referral to colonoscopy]); sDNA-FIT, multitarget stool DNA test with a fecal immunochemical assay; SIG, flexible sigmoidoscopy.
For persons with nonadvanced adenomas. For persons with adenomas 1 mm to <6 mm, sensitivity was assumed to equal the positivity rate in persons without adenomas. Sensitivity for persons with adenomas 6 mm to <10 mm was chosen such that the weighted mean sensitivity for persons with adenomas 1 mm to <6 mm and 6 mm to <10 mm was equal to the sensitivity for nonadvanced adenomas.
For persons with advanced adenomas (ie, adenomas ≥10 mm, adenomas with advanced histology, or both); the studies in the meta-analysis in Lin et al2 did not provide sensitivity for adenomas ≥10 mm separately from advanced adenomas.
The same test characteristics were assumed to apply to all colonoscopies, regardless of indication. No correlation in findings at CTC or SIG and follow-up colonoscopy was assumed.
The lack of specificity with endoscopy reflects detection of nonadenomatous polyps, which, in the case of sigmoidoscopy, may lead to unnecessary follow-up colonoscopy, and in the case of colonoscopy, leads to unnecessary polypectomy, which is associated with an increased risk of complications.
Sensitivity for cancer was assumed to be the same as sensitivity for adenomas ≥10 mm because of the small number of cancers detected in screening studies.
Sensitivity for flexible sigmoidoscopy was assumed to equal that of colonoscopy within reach of the sigmoidoscope and 0 for lesions beyond reach of the scope.
The lack of specificity with CTC reflects detection of nonadenomatous lesions ≥6 mm, artifacts, stool, and adenomas smaller than the 6-mm threshold for colonoscopy referral that are measured as ≥6 mm.
Screening Strategies
The following screening modalities were evaluated: a fecal immunochemical test (FIT) representative of the OC-Sensor family of tests (Polymedco) with a cutoff of 20 μg of hemoglobin per gram of feces; a stool DNA test with a FIT assay (sDNA-FIT), marketed as Cologuard (Exact Sciences); flexible sigmoidoscopy, alone or with FIT; colonoscopy; and computed tomography colonography. Multiple ages to begin screening (45, 50, 55 years) and end screening (70, 75, 80, 85 years) and screening intervals were evaluated for each modality, resulting in 163 unique strategies (Table 2). No screening was performed after the stopping age, but surveillance continued through at least age 85. Perfect adherence with all screening, follow-up, and surveillance procedures was assumed, reflecting outcomes among persons with full willingness to participate.
Table 2.
Screening Strategies Evaluated by the Modelsa
| Modality | Screening interval, y | Age to begin screening, y | Age to end screening, yb | No. of (unique) strategiesc |
|---|---|---|---|---|
| No screening | 1 (1) | |||
| COL | 5, 10, 15 | 45, 50, 55 | 70, 75, 80, 85 | 36 (26) |
| CTC | 5, 10 | 45, 50, 55 | 70, 75, 80, 85 | 24 (20) |
| SIG | 5, 10 | 45, 50, 55 | 70, 75, 80, 85 | 24 (20) |
| FIT | 1, 2, 3 | 45, 50, 55 | 70, 75, 80, 85 | 36 (36) |
| sDNA-FIT | 1, 2, 3 | 45, 50, 55 | 70, 75, 80, 85 | 36 (36) |
| SIG + FITd | 10_1, 10_2 | 45, 50, 55 | 70, 75, 80, 85 | 24 (24) |
| Total | 181 (163) | |||
Abbreviations COL, colonoscopy; CTC, computed tomography colonography; FIT, fecal immunochemical test (with positivity cutoff of 20 μg of hemoglobin per gram of feces); sDNA-FIT, multitarget stool DNA test with a fecal immunochemical assay; SIG, flexible sigmoidoscopy without biopsy.
See the full report6 for a complete list of screening strategies evaluated by the models.
Age to end screening is the last age at which screening happens; screening tests could be performed at but not after this age.
The number of unique strategies excludes those with different ages to end screening that result in screens at the same ages (eg, COL every 10 years from ages 50–70 years and from ages 50–75 years both result in screening at ages 50, 60, and 70 years).
The first interval is for SIG and the second interval is for FIT. If SIG and FIT were due the same year, it was assumed that the FIT was performed first. Persons with a positive FIT result did not have a SIG. Instead they had a follow-up colonoscopy. Those with a negative FIT result had a SIG, and those with SIG findings subsequently had a follow-up colonoscopy.
Outcomes
Outcomes were simulated for a hypothetical cohort of average risk US adults who were unscreened and free of diagnosed colorectal cancer at age 40 years and were tallied through death. The primary benefit of screening was expressed as life-years gained (LYG) from the prevention or delay of colorectal cancer death, accounting for the loss of years from fatal complications. The burden of screening was measured by the lifetime number of required colonoscopies. Numbers of noncolonoscopy tests were also reported. Harms were expressed by the number of colonoscopy complications (serious gastrointestinal events [perforations, bleeding, transfusion], other gastrointestinal events [paralytic ileus, nausea and vomiting, dehydration, abdominal pain], and cardiovascular events [myocardial infarction, angina, arrhythmia, congestive heart failure, cardiac or respiratory arrest, syncope, hypotension, shock]).21 Other outcomes included the number of colorectal cancer cases and colorectal cancer deaths.
Analysis
Model output was analyzed in R version 3.6.1 (R Foundation for Statistical Computing). The relative efficiency of colorectal cancer screening across strategies was assessed by evaluating the tradeoff in burden (colonoscopies) vs benefits (LYG). Because of large differences in the number of noncolonoscopy tests across modalities, efficiency analyses were performed by class of screening modality. FIT and sDNA-FIT were grouped together as exclusively stool-based modalities with comparable burden. The remaining modalities each comprised a unique class because of differences in bowel preparation, invasiveness, need for sedation, and number and type of noncolonoscopy tests.
Within each class, the subset of strategies that provide an efficient balance of colonoscopies and LYG were identified. A strategy is efficient if no other strategy, or combination of strategies, within the class is estimated to provide more LYG and require fewer colonoscopies. Strategies were compared by plotting their estimated LYG (relative to no screening) on the horizontal axis and their estimated number of lifetime colonoscopies on the vertical axis. The “efficient frontier” is the line connecting the efficient strategies.23 Strategies on the efficient frontier were compared using an efficiency ratio, which represents the estimated number of additional colonoscopies required for each additional LYG. Because an inefficient strategy providing outcomes similar to an efficient strategy may be a reasonable option on other grounds23 (eg, for consistency of screening ages across modalities), efficiency ratios were also calculated for “near-efficient” strategies, defined a priori as being within 3 days of life gained per person of the efficient frontier. For the strategies highlighted by the USPSTF in 2016, this absolute distance of 3 days is generally consistent with the relative measure used in the 2016 decision analysis (ie, LYG within 2% of the efficient frontier).4 Hereafter, “efficient” refers to both efficient and near-efficient strategies.
Analyses by Race and Sex
In consultation with the USPSTF, analyses were conducted by race and sex for the 2 most commonly-used screening modalities in the US: colonoscopy and FIT.24 Prior to performing these analyses, we conducted a comprehensive review of the literature on race and colorectal cancer.25 We concluded that the primary driver of differences in colorectal cancer incidence by race is access to screening and subsequent care, rather than biological differences in natural history. This review found that Black-White differences in colorectal cancer incidence began only after the dissemination of screening, that there is strong evidence that Black adults have had lower rates of colorectal cancer screening than White adults, and that there is limited evidence for Black-White differences in findings at screening, including detection of adenomas, advanced adenomas, and colorectal cancer.25 Accordingly, the modeling analyses assumed no differences in the natural history of colorectal cancer by race (ie, development of clinically detected colorectal cancer in the absence of screening), incorporating only Black-White differences in all-cause mortality11 and in stage-specific relative survival after diagnosis.12
RESULTS
In the absence of screening, the models simulated identical life expectancy among 40-year-olds with no prior diagnosis of colorectal cancer, 40.2 years, and estimated that 77 to 85 per 1000 40-year-olds would be diagnosed with colorectal cancer in their lifetimes and 32 to 34 would die of colorectal cancer (eTable 1 in the Supplement). Compared with no screening, all colorectal cancer screening strategies were estimated to yield substantial increases in life expectancy (171–381 LYG per 1000) and reductions in the lifetime number of colorectal cancer cases and colorectal cancer deaths (16–74 and 15–32 cases and deaths averted per 1000, respectively). In the models, lifetime colonoscopies ranged from 624 to 6817 per 1000, and the lifetime number of colonoscopy complications ranged from 5 to 22 per 1000 (ranges are across strategies and models; see the full report6 for detailed outcomes).
Efficiency Analysis
Figure 2, Figure 3, and eFigures 1 through 3 in the Supplement show the estimated number of lifetime number of colonoscopies and LYG per 1000 for each screening strategy within a class. Although absolute estimation differed by model, general patterns were similar. Within each class, estimated LYG from screening increased when screening was initiated in the models at an earlier age, although the increase was smaller for MISCAN compared with SimCRC and CRC-SPIN.
Figure 2. Lifetime Number of Colonoscopies and Life-Years Gained for a Cohort of 40-year-olds for Colonoscopy Screening Strategiesa.
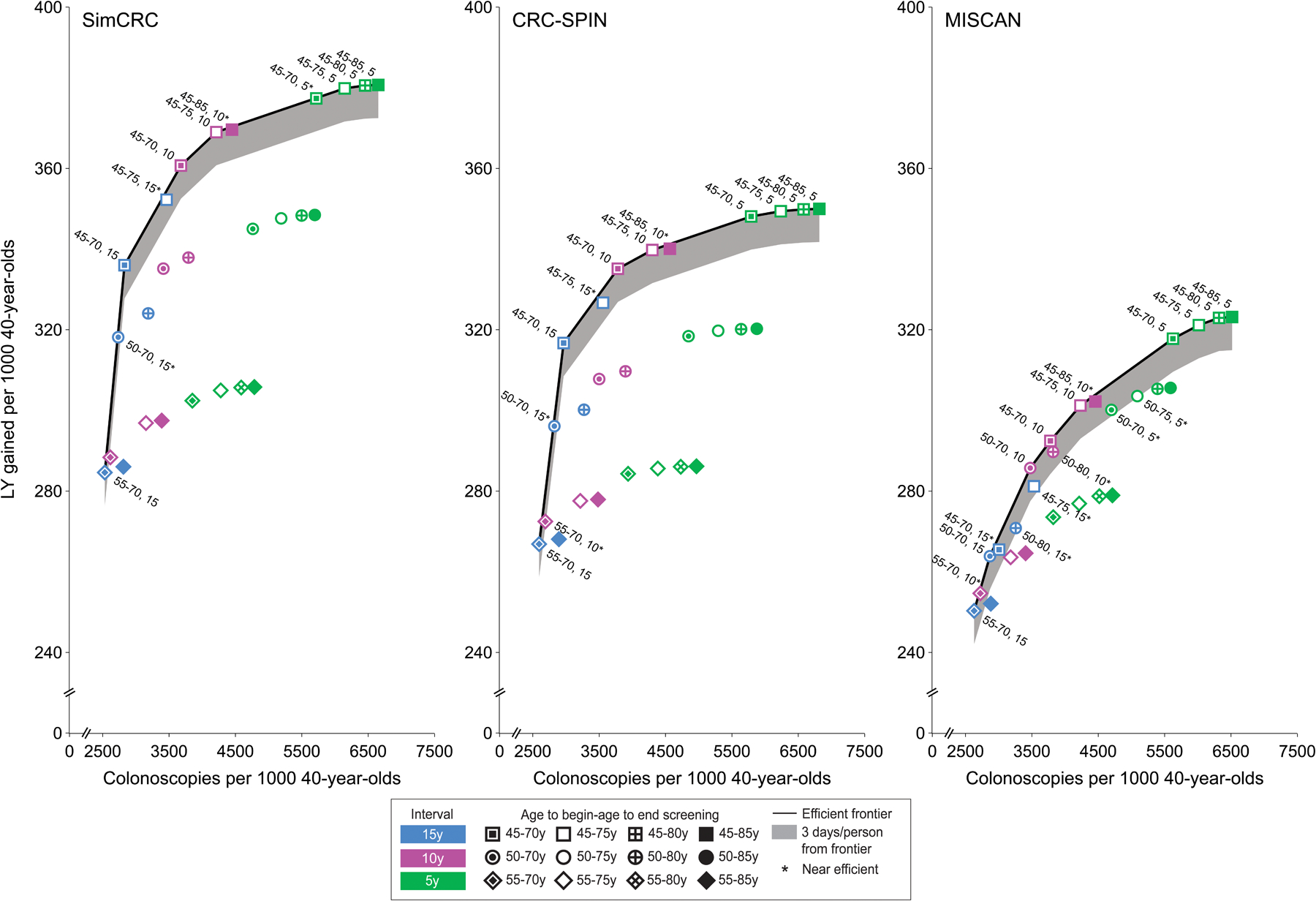
CRC-SPIN indicates CRC Simulated Population Model for Incidence and Natural History; MISCAN, Microsimulation Screening Analysis; SimCRC, Simulation Model of CRC.
a Analyses assume an increased population incidence of colorectal cancer based on an incidence rate ratio comparing incidence among current 20- to 44-year-olds (ie, in 2012–2016) vs 20- to 44-year-olds in the time period used for initial model calibration (ie, 1975–1979) of 1.19. An interactive version of this figure is available at https://resources.cisnet.cancer.gov/projects/#crcr/uspstf2021
Figure 3. Lifetime Number of Colonoscopies and Life-Years Gained for a Cohort of 40-year-olds for Stool-Based Screening Strategiesa.
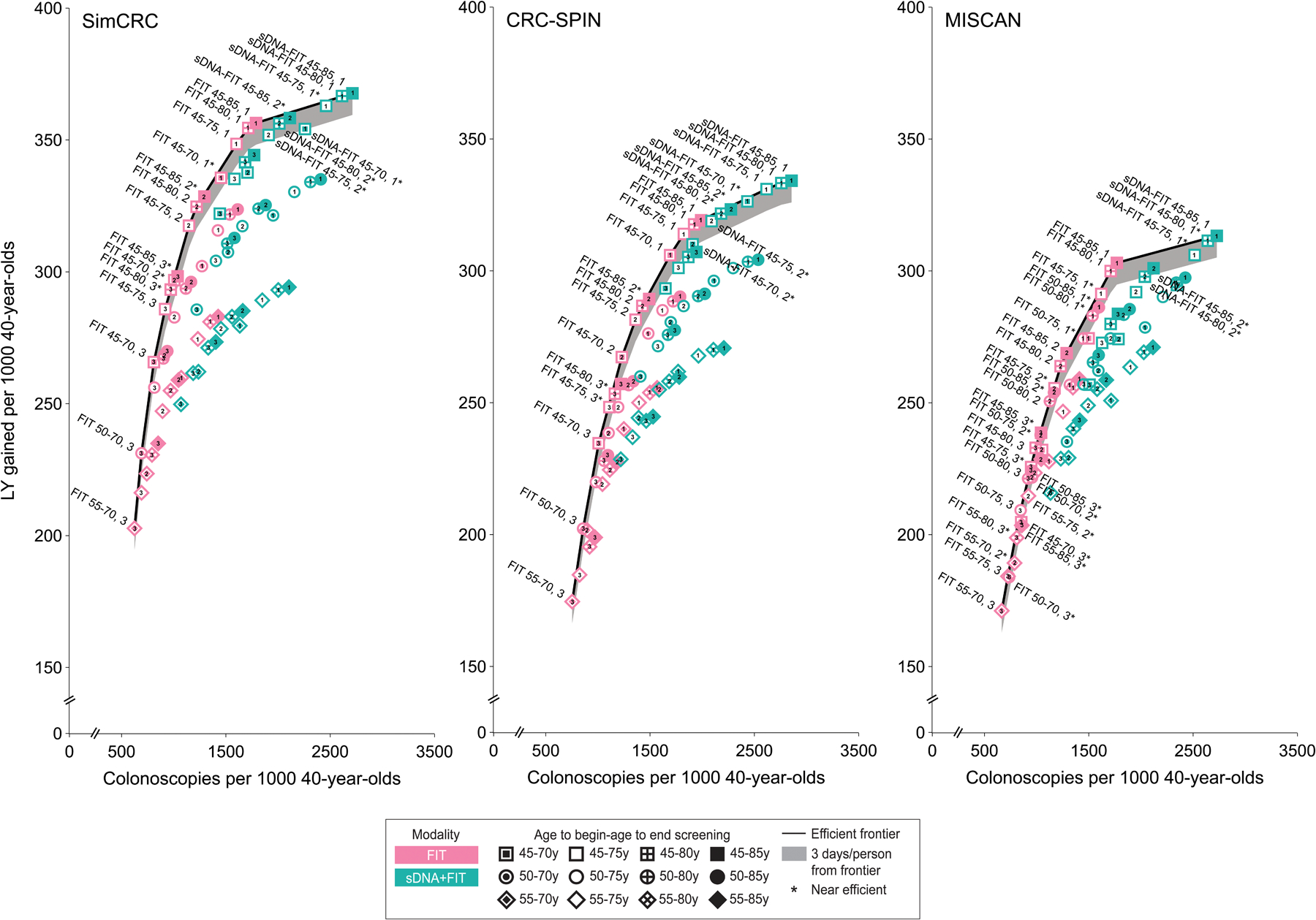
See Figure 2 legend for expanded abbreviations.
a Analyses assume an increased population incidence of colorectal cancer based on an incidence rate ratio comparing incidence among current 20- to 44-year-olds (ie, in 2012–2016) vs 20- to 44-year-olds in the time period used for initial model calibration (ie, 1975–1979) of 1.19. An interactive version of this figure is available at https://resources.cisnet.cancer.gov/projects/#crcr/uspstf2021
Forty-nine unique screening strategies were efficient for all 3 models (Table 3). The majority (41/49) were those with screening starting at age 45 years. None of the strategies highlighted in the 2016 USPSTF colorectal cancer screening recommendations,3 which specified screening from ages 50 to 75, were efficient in all 3 models.
Table 3.
Outcomes for Efficient and Near-Efficient Strategies with SimCRC, CRC-SPIN, and MISCANa
| Unique strategies simulated, No. | Efficient or near-efficient strategies with all modelsb | Range of outcomes per 1000 across models | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CRC | Efficiency ratio (Δ COL / Δ LYG)e |
||||||||
| No. | Strategyc | COLs | Non-COL testsd | Complications | Cases | Deaths | LYG | ||
| Colonoscopy | |||||||||
| 26 | 11 | COL 55–70, 15 | 2532–2630 | 0 | 13–14 | 21–40 | 7–11 | 250–285 | Defaultf |
| COL 50–70, 15 | 2734–2868 | 0 | 11–13 | 18–40 | 6–11 | 264–318 | 6g-18 | ||
| COL 45–70, 15 | 2829–3006 | 0 | 10–12 | 18–41 | 6–12 | 265–336 | 6–85g | ||
| COL 45–75, 15 | 3463–3558 | 0 | 15–16 | 14–37 | 4–10 | 281–352 | 38g-59g | ||
| COL 45–70, 10 | 3679–3782 | 0 | 12–14 | 13–37 | 4–10 | 292–361 | 34–45 | ||
| COL 45–75, 10 | 4212–4300 | 0 | 15–17 | 12–34 | 3–8 | 301–369 | 52–112 | ||
| COL 45–85, 10 | 4449–4566 | 0 | 17–19 | 11–34 | 3–8 | 302–370 | 227g-828g | ||
| COL 45–70, 5 | 5626–5789 | 0 | 15–17 | 10–32 | 3–8 | 318–377 | 84–180g | ||
| COL 45–75, 5 | 6016–6235 | 0 | 17–19 | 10–31 | 3–7 | 321–380 | 116–344 | ||
| COL 45–80, 5 | 6320–6581 | 0 | 19–20 | 9–30 | 2–7 | 323–381 | 169–736 | ||
| COL 45–85, 5 | 6516–6817 | 0 | 20–22 | 9–30 | 2–7 | 323–381 | 926–2190 | ||
| Strategy highlighted in 2016 | COL 50–75, 10 | 3414–3500 | 0 | 13–15 | 15–36 | 5–9 | 286–335 | D, D, 28 | |
| Stool tests (FIT or sDNA-FIT) | |||||||||
| 72 | 16 | FIT 55–70, 3 | 624–754 | 4637–4710 | 5–7 | 47–65 | 17–20 | 171–203 | Defaultf |
| FIT 50–70, 3 | 691–858 | 5663–5757 | 5–7 | 43–64 | 15–20 | 184–231 | 2–6g | ||
| FIT 45–70, 3 | 810–1007 | 7299–7435 | 6–8 | 38–62 | 13–18 | 205–266 | 3–6g | ||
| FIT 45–75, 3 | 917–1110 | 8300–8475 | 7–9 | 36–60 | 11–16 | 226–286 | 5–7g | ||
| FIT 45–80, 3 | 971–1163 | 8866–9043 | 8–10 | 35–60 | 10–14 | 233–293 | 6–8g | ||
| FIT 45–75, 2 | 1147–1361 | 11420–11731 | 8–11 | 29–55 | 9–13 | 256–318 | 7–9 | ||
| FIT 45–80, 2 | 1220–1426 | 12249–12576 | 9–12 | 28–54 | 7–12 | 264–325 | 8–12 | ||
| FIT 45–85, 2 | 1288–1492 | 13160–13487 | 10–13 | 27–54 | 6–11 | 269–329 | 12–25g | ||
| FIT 45–75, 1 | 1602–1824 | 18950–19680 | 10–13 | 20–46 | 6–10 | 291–348 | 15g-16 | ||
| FIT 45–80, 1 | 1710–1923 | 20622–21368 | 11–14 | 19–45 | 5–9 | 300–355 | 14–27 | ||
| FIT 45–85, 1 | 1769–1990 | 21850–22567 | 12–15 | 19–44 | 4–8 | 303–356 | 19–43 | ||
| sDNA-FIT 45–80, 2 | 2012–2181 | 9928–10167 | 11–14 | 18–43 | 5–9 | 298–356 | 26g-176g | ||
| sDNA-FIT 45–85, 2 | 2114–2275 | 10620–10828 | 13–15 | 17–42 | 4–8 | 301–358 | 69g-375g | ||
| sDNA-FIT 45–75, 1 | 2462–2617 | 13494–13888 | 12–14 | 15–38 | 4–9 | 306–363 | 53–251g | ||
| sDNA-FIT 45–80, 1 | 2614–2758 | 14608–14966 | 13–15 | 14–37 | 4–8 | 311–367 | 62–104g | ||
| sDNA-FIT 45–85, 1 | 2713–2856 | 15424–15721 | 14–16 | 14–36 | 3–8 | 313–368 | 94–111 | ||
| Strategies highlighted in 2016 | sDNA-FIT 50–75, 3 | 1405–1576 | 5939–6074 | 9–12 | 26–50 | 8–12 | 257–304 | D, D, D | |
| FIT 50–75, 1 | 1423–1619 | 15562–16160 | 9–12 | 23–47 | 7–11 | 274–316 | D, D, 29g | ||
| sDNA-FIT 50–75, 1 | 2156–2295 | 11132–11463 | 11–14 | 18–39 | 6–9 | 290–330 | D, D, D | ||
| Flexible sigmoidoscopy | |||||||||
| 20 | 7 | SIG 55–70, 10 | 907–1340 | 1623–1708 | 7–10 | 35–48 | 13–16 | 204–210 | Defaultf |
| SIG 45–70, 10 | 1155–1635 | 2480–2622 | 7–10 | 29–45 | 11–14 | 234–262 | 4–73g | ||
| SIG 45–75, 10 | 1360–1800 | 2946–3173 | 9–12 | 26–43 | 9–12 | 245–278 | 13g-18 | ||
| SIG 45–70, 5 | 1586–2020 | 4013–4446 | 9–11 | 25–40 | 9–12 | 263–302 | 11–20 | ||
| SIG 45–75, 5 | 1680–2119 | 4389–4935 | 10–12 | 24–39 | 8–11 | 269–309 | 19–27 | ||
| SIG 45–80, 5 | 1749–2196 | 4681–5326 | 11–13 | 23–38 | 7–10 | 271–311 | 29–49 | ||
| SIG 45–85, 5 | 1793–2235 | 4877–5602 | 12–13 | 23–38 | 7–10 | 272–312 | 78–98 | ||
| Strategy highlighted in 2016 | SIG 50–75, 5 | 1510–1927 | 3646–4134 | 10–12 | 26–40 | 9–11 | 256–279 | D, D, 19g | |
| Flexible sigmoidoscopy + FIT | |||||||||
| 24 | 10 | SIG+FIT 55–70, 10_2 | 1230–1547 | 6084–6685 | 8–11 | 27–45 | 9–13 | 241–266 | Defaultf |
| SIG+FIT 50–70, 10_2 | 1512–1835 | 8519–9277 | 9–12 | 22–42 | 7–11 | 274–314 | 6g-9 | ||
| SIG+FIT 45–70, 10_2 | 1617–1947 | 10174–11005 | 9–12 | 20–42 | 7–11 | 280–340 | 5–24g | ||
| SIG+FIT 45–75, 10_2 | 1835–2130 | 11710–12693 | 11–13 | 18–39 | 5–9 | 294–354 | 15–22 | ||
| SIG+FIT 45–80, 10_2 | 1889–2154 | 12266–13375 | 11–14 | 17–39 | 4–9 | 296–357 | 15–25 | ||
| SIG+FIT 45–70, 10_1 | 1903–2148 | 15867–17141 | 10–13 | 17–40 | 6–10 | 292–353 | 19g-88g | ||
| SIG+FIT 45–85, 10_2 | 1988–2235 | 13131–14286 | 13–15 | 17–39 | 4–8 | 298–358 | 38g-78g | ||
| SIG+FIT 45–75, 10_1 | 2102–2331 | 17858–19217 | 11–14 | 15–37 | 4–9 | 304–363 | 22g-34 | ||
| SIG+FIT 45–80, 10_1 | 2203–2379 | 19076–20649 | 12–15 | 15–37 | 4–8 | 307–366 | 21–53 | ||
| SIG+FIT 45–85, 10_1 | 2293–2463 | 20204–21763 | 14–16 | 14–37 | 3–8 | 309–367 | 46–81 | ||
| Strategy highlighted in 2016 | SIG+FIT 50–75, 10_1 | 1840–2048 | 14257–15636 | 11–13 | 18–39 | 6–10 | 287–330 | D, D, 18g | |
| Computed tomography colonography | |||||||||
| 20 | 5 | CTC 55–70, 10 | 939–1029 | 1695–1705 | 7–9 | 32–57 | 12–19 | 181–245 | Defaultf |
| CTC 45–70, 5 | 1569–1677 | 4372–4436 | 9–11 | 20–45 | 6–12 | 271–348 | 11–21g | ||
| CTC 45–75, 5 | 1672–1791 | 4804–4893 | 10–13 | 18–42 | 5–11 | 283–355 | 11–21 | ||
| CTC 45–80, 5 | 1744–1882 | 5131–5254 | 11–14 | 17–40 | 4–9 | 288–358 | 13–38 | ||
| CTC 45–85, 5 | 1790–1939 | 5348–5504 | 12–15 | 17–40 | 4–9 | 290–359 | 32–104 | ||
| Strategy highlighted in 2016 | CTC 50–75, 5 | 1519–1626 | 4006–4088 | 10–12 | 20–43 | 6–11 | 268–325 | D, D, 9 | |
Abbreviations: COL, colonoscopy; CRC, colorectal cancer; CTC, computed tomography colonography; D, dominated; FIT, fecal immunochemical test (with positivity cutoff for of 20 μg of hemoglobin per gram of feces); LYG, life-years gained compared with no screening; sDNA-FIT, multitarget stool DNA test with a fecal immunochemical assay; SIG, flexible sigmoidoscopy.
Analyses assume an increased population risk of colorectal cancer based on an incidence rate ratio comparing colorectal cancer risk among 20- to 44-year-olds in 2012–2016 vs 1975–1979 of 1.19. See the full report6 for details.
For comparison purposes, strategies highlighted by the US Preventive Services Task Force in 20163 are included even though they were not estimated to be efficient or near-efficient in all 3 models in this analysis.
Strategies are denoted by the screening modality, age to begin screening-age to end screening, interval. For example, CTC 50–75, 5 indicates CTC every 5 years from age 50 years to age 75 years and SIG+FIT 50–75, 10_1 indicates sigmoidoscopy every 10 years and annual FIT from age 50 years to age 75 years.
For SIG+FIT, the number reported is the total number of tests. Numbers of each test can be found in the full report.6
None of the strategies highlighted by the US Preventive Services Task Force in 20163 were estimated to be efficient or near efficient in all 3 models. For these strategies, the efficiency ratio column indicates whether the 2016 highlighted strategy was dominated and not near efficient (D) or, if efficient, the efficiency ratio in SimCRC, CRC-SPIN, and MISCAN, respectively. For all other strategies, this column indicates the range of efficiency ratios across models.
Strategy requiring the fewest colonoscopies and providing the fewest LYG.
Near efficient (ie, within 3 days of life gained per person of the efficient frontier).
Unlike the age to begin screening, no single age to end screening was predominant among efficient strategies. However, for most modalities, the estimated increase in LYG from extending screening beyond age 75 years was small in comparison with the increase in colonoscopies (Table 3). For example, with 10-yearly colonoscopy starting at age 45, extending the age to end screening from 75 to 85 was estimated to increase lifetime colonoscopies by 5% to 6% (from 4212–4300 to 4449–4566 per 1000; ranges are across models), whereas LYG increased by less than 1% (from 301–369 to 302–370 per 1000). With annual FIT from age 45, extending the age to end screening from age 75 to age 80 years was estimated to increase lifetime colonoscopies by 5% to 7% (from 1602–1824 to 1710–1923 per 1000) and LYG by 1% to 3% (from 291–348 to 300–355 per 1000). Extending the age to end FIT screening from age 80 to age 85 was estimated to increase lifetime colonoscopies by 3% to 4% (to 1769–1990 per 1000) and LYG by less than 1% to 1% (to 303–356 per 1000).
With the exception of computed tomography colonography (5-year interval), efficient strategies within each class included multiple screening intervals; no interval was predominant (Table 3). The number of additional colonoscopies required for each additional LYG increased with shorter screening intervals. For colonoscopy from age 45 to 75 years, shortening the interval from 10 to 5 years was estimated to increase the lifetime colonoscopies by 42% to 46% across models (from 4212–4300 to 6016–6235 per 1000), while LYG was estimated to increase by 3% to 7% (from 301–369 to 321–380 per 1000) (Table 3).
Of the 16 efficient stool-based strategies, 11 were those with FIT (Table 3, Figure 3), including triennial, biennial, and annual FIT screening starting at age 45 years. Annual and biennial sDNA-FIT strategies starting at age 45 were also efficient, albeit with a relatively high colonoscopy burden relative to the LYG. For example, compared with annual FIT from ages 45 to 85, screening with biennial sDNA-FIT from ages 45 to 80 was estimated to increase the lifetime number of colonoscopies by 10% to 15% (from 1769–1990 to 2012–2181 per 1000; range is across models), while LYG were estimated to be similar to the FIT strategy (+1% in CRC-SPIN [from 319 to 322 per 1000], unchanged in SimCRC [356 per 1000], and −2% in MISCAN [from 303 to 298 per 1000]). None of the models found the triennial sDNA-FIT strategies to be efficient relative to other stool-based options.
In general, estimated LYG with 5-yearly sigmoidoscopy were comparable to LYG with biennial FIT (Table 3). Sigmoidoscopy with interval FIT was estimated to provide LYG and colorectal cancer deaths averted similar to those estimated for colonoscopy screening over the same age range.
Figure 4 and Figure 5 summarize the model-estimated changes in outcomes if screening were to begin at age 45 years instead of age 50 for strategies highlighted by the USPSTF in 2016.3 Models that lowered the age to begin screening showed 22 to 27 additional LYG (8%−9% increase), 2 to 3 fewer colorectal cancer cases (4%−6% reduction), and 0.9 to 1 fewer colorectal cancer deaths (3%−5% reduction) but also showed 0.1 to 2 additional complications (1%−14% increase) and required 161 to 784 additional colonoscopies (10%−23% increase) and 0 (with colonoscopy) to 3553 additional noncolonoscopy tests (no change to a 24% increase) over the lifetimes of 1000 persons (numbers are mean estimates across models).
Figure 4. Benefits of Colorectal Cancer Screening Strategies Highlighted by the US Preventive Services Task Force in 20163 and the Change in Outcomes When Screening is Started at Age 45 Years Instead of Age 50.
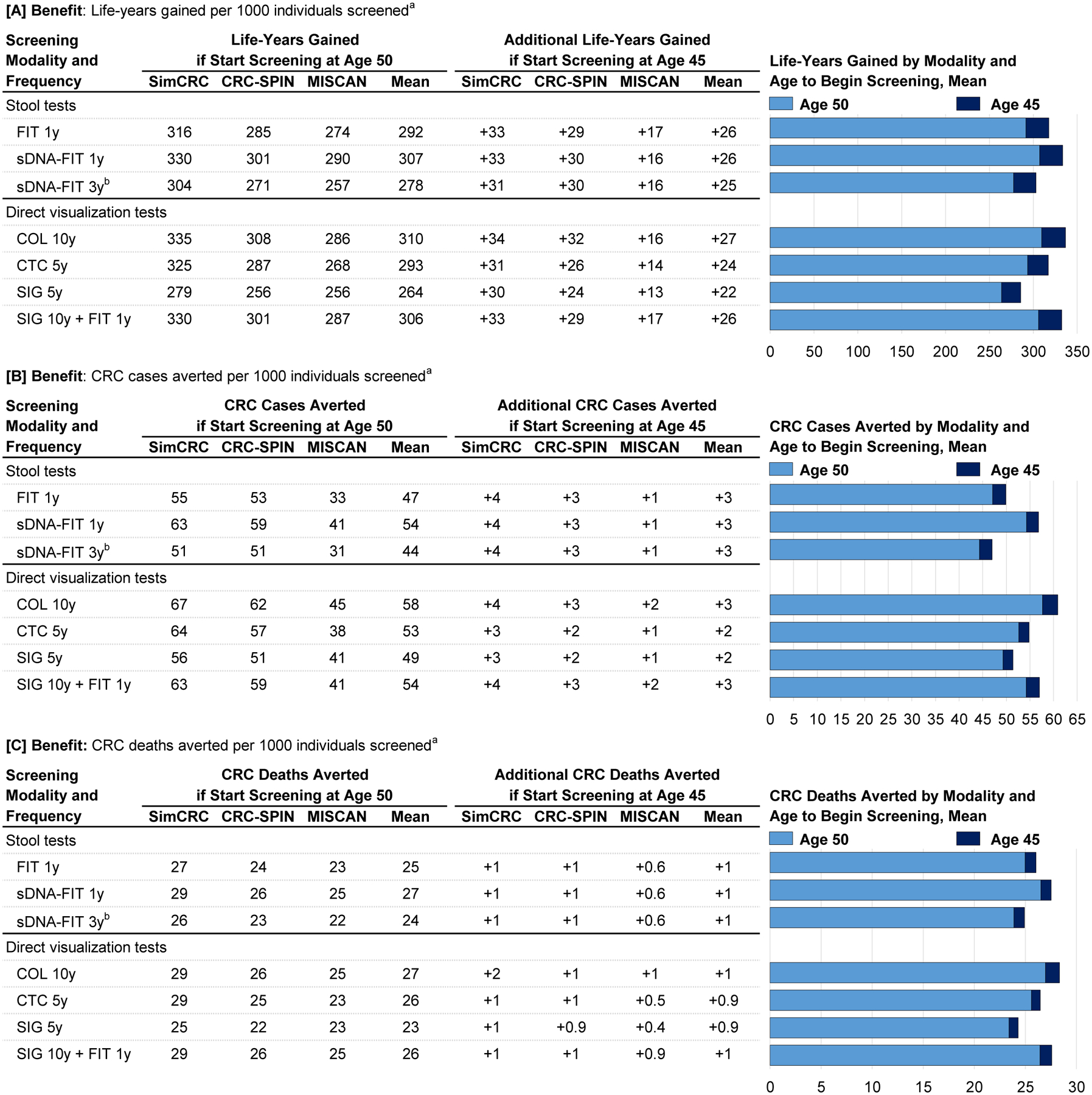
COL indicates colonoscopy; CRC colorectal cancer; CRC-SPIN, CRC Simulated Population Model for Incidence and Natural History; CTC, computed tomography colonography; FIT indicates fecal immunochemical test (with positivity cutoff for of 20 μg of hemoglobin per gram of feces); sDNA-FIT, multitarget stool DNA test with a fecal immunochemical assay; SIG, flexible sigmoidoscopy; SimCRC, Simulation Model of CRC.
a Outcomes are expressed over the lifetimes of 1000 40-year-olds who start screening at age 45 or at age 50 and are screened to age 75, assuming an increased population incidence of colorectal cancer based on an incidence rate ratio comparing incidence among current 20- to 44-year-olds (ie, in 2012–2016) vs 20- to 44-year-olds in the time period used for initial model calibration (ie, 1975–1979) of 1.19.
b Compared with other options for stool-based screening, this strategy was not estimated to provide an efficient balance of the burden (ie, lifetime number of colonoscopies) and the benefit (life-years gained) of screening.
Figure 5. Complications, Colonoscopy Burden, and Non-Colonoscopy Burden of Colorectal Cancer Screening Strategies Highlighted by the USPSTF in 20163 and the Change in Outcomes When Screening is Started at Age 45 Years Instead of Age 50.
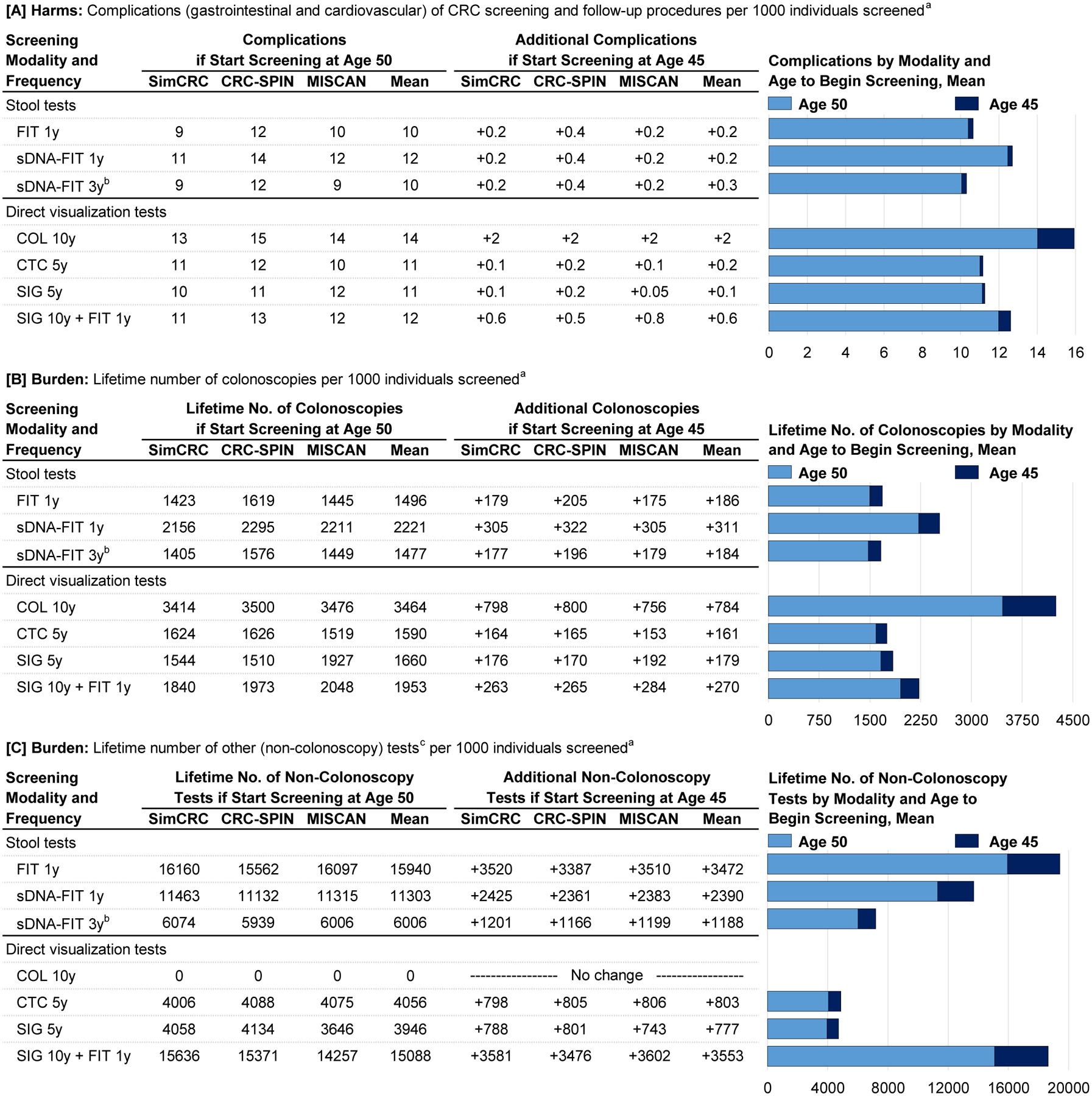
See Figure 4 legend for expanded abbreviations.
a Outcomes are expressed over the lifetimes of 1000 40-year-olds who start screening at age 45 or at age 50 and are screened to age 75, assuming an increased population incidence of colorectal cancer based on an incidence rate ratio comparing incidence among current 20- to 44-year-olds (ie, in 2012–2016) vs 20- to 44-year-olds in the time period used for initial model calibration (ie, 1975–1979) of 1.19.
b Compared with other options for stool-based screening, this strategy was not estimated to provide an efficient balance of the burden (ie, lifetime number of colonoscopies) and the benefit (life-years gained) of screening.
c Other (noncolonoscopy) tests include FIT, sDNA-FIT, CTC, SIG.
Findings by Race and Sex
Compared with the total population, model-estimated life expectancy among unscreened 40-year-olds with no prior colorectal cancer diagnosis was 2 years higher for White women (42.2 vs 40.2 years), similar for Black women (40.1 years), 2 years lower for White men (38.4 years), and 5 years lower for Black men (35.2 years). In the absence of screening, the model-estimated lifetime number of diagnosed colorectal cancer cases was higher for White men compared with the total population (86 vs 81 cases per 1000, respectively; estimates are the mean across models) and lower than the total population for the other groups (77 cases per 1000 White women, 73 cases per 1000 Black men, and 70 cases per 1000 Black women) (eTable 1 in the Supplement).
Among men, the models estimated that the lower lifetime risk of colorectal cancer diagnosis also led to a lower lifetime risk of colorectal cancer death in Black vs White adults in the absence of screening (eTable 1 in the Supplement) despite the worse Black vs White relative survival after colorectal cancer diagnosis.12 The pattern differed for women by race: Black women were estimated to have lower lifetime colorectal cancer incidence than White women in the absence of screening but higher lifetime risk of colorectal cancer death. This implies that the difference in Black vs White lifetime colorectal cancer incidence was offset by the worse Black vs White relative survival after colorectal cancer diagnosis.12
Because of these differences in the estimated lifetime risk of colorectal cancer death among Black vs White adults by sex in the absence of screening, estimated LYG from both colonoscopy and FIT were generally lower for Black vs White men and generally similar for Black vs White women (eFigures 4–5 in the Supplement). However, efficient strategies for each race-sex group were generally the same, and efficiency ratios were similar by race among each sex (eTables 2–3 in the Supplement), suggesting that with equal access to quality care, the relative balance of the colonoscopy burden and the LYG from screening differs slightly by sex but not by race. For example, across models, efficiency ratios for 10-yearly colonoscopy from age 45 to 75 years ranged from 48 to 143 for White men, 46 to 142 for Black men, 57 to 100 for White women, and 51 to 93 for Black women. Among all 4 race-sex groups, efficient screening options for all 3 models were predominantly those beginning at age 45 (eFigures 4–5 and eTables 2–3 in the Supplement).
Scenario Analyses
For each modality, efficient strategies were similar for the 3 colorectal cancer population risk scenarios (eTables 4–8, eFigures 6–7 in the Supplement); efficient strategies in all 3 models were primarily those with screening beginning at age 45 years regardless of risk scenario.
Potential Implications of Adherence
Because this analysis was intended to inform population guidelines, perfect adherence to screening strategies was assumed to estimate achievable benefit for people who adhere to recommendations. However, perfect adherence does not occur in daily life.2 The potential implications of nonadherence can be inferred by comparing model-estimated outcomes with different start ages and screening intervals. These comparisons are described in the Supplement and are highlighted in eTables 9 through 12 in the Supplement. For example, lack of adherence with annual FIT from ages 45 to 75 years, resulting in a 3-year screening interval, was estimated to reduce LYG by 18% to 23% (eTable 12 in the Supplement). Similarly, lack of adherence with 10-yearly colonoscopy, resulting in a 15-year interval, was estimated to reduce LYG by 4% to 7%; if adherent with only the first colonoscopy, LYG would be estimated to decrease by 25% to 44%.
DISCUSSION
This comparative modeling study yielded largely consistent findings across models: assuming perfect adherence, routine screening for colorectal cancer was estimated to reduce the lifetime risk of being diagnosed with and of dying of colorectal cancer, with possibly substantial LYG. Most of the efficient strategies were those with screening beginning at age 45 years. No consistent pattern emerged for the age to end screening, although the estimated LYG from continued screening after age 75 were small. For most modalities, no screening interval was predominant. Efficient strategies were robust to assumptions about population risk of colorectal cancer.
The findings from this analysis are consistent with those of the 2016 decision analysis of colorectal cancer screening for the USPSTF.4 However, in 2016, there was limited evidence to support screening before age 50 years. While no trials have reported on the effect of screening among asymptomatic adults aged 45 to 49 and data on the findings at screening at these ages remain sparse,26–28 there is clearer evidence that colorectal cancer incidence in the US is increasing before age 50.5,29
The SimCRC and CRC-SPIN models estimated that most (and often, nearly all) efficient strategies begin at age 45 years. The estimated LYG and colonoscopy burden of screening were lowest for MISCAN, and MISCAN found strategies with screening beginning at 45 identified by SimCRC and CRC-SPIN. Prior work examining differences in model estimation found that these differences are primarily attributable to assumptions about the nature of adenomas.13 With SimCRC and CRC-SPIN, all adenomas have the potential to progress to colorectal cancer. With MISCAN, some adenomas are assumed to be nonprogressive, and the probability that an adenoma is nonprogressive decreases with age at onset. Because of this age dependency, which is the result of model structure and calibration, MISCAN estimates a smaller benefit from removing adenomas present at age 45 compared with SimCRC or CRC-SPIN.
A key change from the 2016 analysis is inclusion of analyses examining the benefits, harms, and colonoscopy burden of colorectal cancer screening strategies by race and sex. Based on a recent review of the literature on race and colorectal cancer,25 these analyses assumed that the risk of developing adenomas and the progression of adenomas to colorectal cancer did not differ by Black vs White race. However, the analyses incorporated race- and sex-specific all-cause mortality rates11 and relative survival probabilities after colorectal cancer diagnosis.12 Because Black adults have a shorter life expectancy than White adults,11 the model-estimated lifetime incidence of colorectal cancer diagnosis in the absence of screening was lower in Black vs White adults by sex (eTable 1 in the Supplement). While this lower lifetime incidence in the absence of screening may appear to conflict with current SEER data demonstrating higher colorectal cancer incidence among Black adults,14 SEER incidence rates are observed in the presence of screening. Racial disparities in screening have been well documented. Black adults are less likely than White adults to be up to date with screening recommendations, to receive colonoscopy after an abnormal noncolonoscopy test result, and to be screened by endoscopists with higher adenoma detection rates.25
Unlike the current analysis, MISCAN and SimCRC analyses to inform the 2018 American Cancer Society (ACS) colorectal cancer screening guidelines30 assumed differences in natural history by Black vs White race. These analyses were carried out before publication of recent reviews demonstrating limited evidence of Black vs White differences in findings at colorectal cancer screening.25,31 Accordingly, differences in outcomes by race were larger in analyses for the ACS than in this analysis. However, both analyses found that starting screening at age 45 years provided an efficient balance of colonoscopies and LYG for the asymptomatic average-risk population as a whole and by race.
Findings from this analysis for the total population are also largely consistent with MISCAN analyses for the ACS for the total population,32 despite use of different estimates of the magnitude of the observed increase in colorectal cancer incidence among recent birth cohorts. Analyses for the ACS were based on (larger) published estimates5 of the increase in incidence, which included tumors that are not the target of colorectal cancer screening (eg, carcinoid tumors). These tumors were excluded from analysis of SEER data used to inform risk scenarios for the USPSTF. Because the incidence of adenocarcinoma is increasing more slowly than that of carcinoid tumors of the colon or rectum,29 the estimated increase in population risk of colorectal cancer is lower when these tumors are excluded (see the Supplement for details). However, despite the different assumptions about colorectal cancer incidence rates in the absence of screening, analyses for the USPSTF described here and analyses for the ACS both suggest that starting colorectal cancer screening at age 45 years provides an efficient balance of the colonoscopy burden and the LYG from screening.
With many recommended options for colorectal cancer screening, deciding which strategy to adopt may be overwhelming for patients and their clinicians. In addition to helping patients decide whether to begin screening at age 45 years or at age 50, the estimated long-term outcomes from the models may help inform the trade-offs of screening with one approach over another. For example, if choosing among the screening strategies recommended by the USPSTF in 2016, a patient who wishes to maximize LYG and minimize the lifetime risk of colorectal cancer diagnosis may opt for 10-yearly colonoscopy screening, while a patient who wishes to minimize the lifetime number of colonoscopies required may opt for screening with either 3-yearly sDNA-FIT or annual FIT. For whichever option is chosen, model findings may also help illustrate to patients the potential implications of failure to adhere with recommended screening ages and intervals.
A strength of this study is that it used 3 distinct simulation models to estimate the LYG and colonoscopy burden of screening. Each model is based on different assumptions about the natural history of colorectal cancer, although all are calibrated to similar end points.13 Their differences—in dwell times, location of adenomas, progressive vs nonprogressive adenomas, among others—provide a range of outcomes that reflect a sensitivity analysis on underlying model assumptions. The similar relative estimations across classes of screening modalities and similar rankings of strategies within classes of modalities across the 3 models demonstrate the robustness of the findings.
Limitations
This study has several limitations. First, although the modeling results provide a lifetime framework for evaluating benefits and burdens from a screening program, empirical data on test sensitivity and specificity are based on a single screen. Outcomes at repeated rounds of stool testing have been reported in 2 small studies33,34; findings suggest that test results are correlated. While all 3 models assumed independence of repeated tests, an analysis using MISCAN showed that incorporation of correlated results across repeated screening tests would have only a modest effect on model-estimated outcomes.33
Second, the models did not simulate the serrated polyp pathway to colorectal cancer10 because of insufficient evidence on the prevalence of serrated polyps by age, their malignant potential, and the ability of screening tests to detect them. A modeling study by Greuter et al35 estimated the effect of incorporating the serrated pathway on screening effectiveness and found little difference in model results when assuming that 0% vs 30% of cancers arise from this pathway. Exploratory analyses with MISCAN also showed that inclusion of the serrated pathway had limited effect on optimal screening strategies.36
Third, the models assumed that the increased population risk of colorectal cancer arises from an increase in adenoma risk. Whether the observed increase in colorectal cancer incidence among younger adults is caused (instead or in addition) by faster progression to malignancy is unknown. In prior analyses with MISCAN32 the effects of each of these assumptions were evaluated; findings with respect to screening initiation at age 45 years were robust.
Fourth, models can only approximate reality. While the models described here have been extensively calibrated and validated, they are limited by uncertainty in the data used to inform inputs and assumptions. Nevertheless, model-based estimates are important because they provide patients and their clinicians with information they can use to make decisions about when and how to screen for colorectal cancer. Such decisions would otherwise be left to individual judgement, as that information cannot feasibly be obtained from clinical studies. Modeling studies are not a substitute for empirical evidence. Instead they synthesize, build from, and extend empirical evidence to provide insights into questions about screening practices.
Conclusions
This microsimulation modeling analysis suggests that screening for colorectal cancer with stool tests, endoscopic tests, or computed tomography colonography starting at age 45 years provides an efficient balance of colonoscopy burden and life-years gained.
Supplementary Material
Funding/Support
This research was funded under contract HHSA-290-2015-00007-I-EPC5, Task Order 8 from the Agency for Healthcare Research and Quality (AHRQ), US Department of Health and Human Services, via the Kaiser Permanente Evidence-based Practice Center (EPC), under a contract to support the US Preventive Services Task Force (USPSTF). Drs. Knudsen, Rutter, Peterse, Meester, Zauber, Kuntz, and Lansdorp-Vogelaar and Ms. Lietz and Ms. Seguin were also supported in part by grants U01CA199335 and U01CA253913 from the National Cancer Institute.
Role of the Funder/Sponsor:
Investigators worked with USPSTF members, AHRQ staff, and the EPC review team to define the scope of the project and key questions to be addressed. AHRQ staff provided project oversight, reviewed the report to ensure that the analysis met methodological standards, and distributed the draft for peer review. Otherwise, AHRQ had no role in the conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript findings. The opinions expressed in this document are those of the authors and do not reflect the official position of AHRQ, the US Department of Health and Human Services, or the National Cancer Institute.
Footnotes
Conflict of Interest Disclosures
Ms. Siegel is Scientific Director of Surveillance Research at the American Cancer Society. Her contributions to the report are solely the responsibility of the author and do not represent the official view of the American Cancer Society. No other authors reported disclosures.
Additional Information:
A draft version of this modeling report underwent external peer review from 3 content experts (Douglas Corley, MD, PhD, MPH [Kaiser Permanente], Jennifer Croswell, MD, MPH [National Cancer Institute], and Jason A. Dominitz, MD, MHS [Veterans Affairs Puget Sound Health Care System and University of Washington]). Comments from reviewers were presented to the USPSTF during its deliberation of the evidence and were considered in preparing the final report.
Publisher's Disclaimer: Editorial Disclaimer:
This modeling study is presented as a document in support of the accompanying USPSTF Recommendation Statement. It did not undergo additional peer review after submission to JAMA.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2.Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the U.S. Preventive Services Task Force. Evidence Synthesis No. 202. Agency for Healthcare Research and Quality; 2021. AHRQ publication 20–05271-EF-1. [PubMed] [Google Scholar]
- 3.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. ; US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(23):2564–2575. doi: 10.1001/jama.2016.5989 [DOI] [PubMed] [Google Scholar]
- 4.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315(23):2595–2609. doi: 10.1001/jama.2016.6828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst. 2017;109 (8):djw322. doi: 10.1093/jnci/djw322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knudsen AB, Rutter CM, Peterse EF, et al. Colorectal Cancer Screening: An Updated Decision Analysis for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality; 2021. AHRQ publication 20–05271-EF-2. [PubMed] [Google Scholar]
- 7.Rutter CM, Ozik J, DeYoreo M, Collier N. Microsimulation model calibration using incremental mixture approximate Bayesian computation. Ann Appl Stat. 2019;13(4):2189–2212. doi: 10.1214/19-AOAS1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutter CM, Knudsen AB, Marsh TL, et al. Validation of models used to inform colorectal cancer screening guidelines: accuracy and implications. Med Decis Making. 2016;36(5):604–614. doi: 10.1177/0272989X15622642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morson B President’s address: the polyp-cancer sequence in the large bowel. Proc R Soc Med. 1974;67(6, pt 1):451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107(9):1315–1329. doi: 10.1038/ajg.2012.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arias E, Xu JQ. United States life tables, 2017. Natl Vital Stat Rep. 2019;68(7):1–66. [PubMed] [Google Scholar]
- 12.Rutter CM, Johnson EA, Feuer EJ, Knudsen AB, Kuntz KM, Schrag D. Secular trends in colon and rectal cancer relative survival. J Natl Cancer Inst. 2013;105(23):1806–1813. doi: 10.1093/jnci/djt299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuntz KM, Lansdorp-Vogelaar I, Rutter CM, et al. A systematic comparison of microsimulation models of colorectal cancer: the role of assumptions about adenoma progression. Med Decis Making. 2011;31(4):530–539. doi: 10.1177/0272989X11408730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SEER*Stat Database: Incidence—SEER 9 Regs Research Data with Delay-Adjustment, Malignant Only, Nov 2018 Sub (1975–2016) <Katrina/Rita Population Adjustment>—Linked To County Attributes—Total US, 1969–2017 Counties. National Cancer Institute. Released April 2019. AccessedApril 13, 2021. https://www.seer.cancer.gov
- 15.Schroy PC III, Coe A, Chen CA, O’Brien MJ, Heeren TC. Prevalence of advanced colorectal neoplasia in white and black patients undergoing screening colonoscopy in a safety-net hospital. Ann Intern Med. 2013;159(1):13–20. doi: 10.7326/0003-4819-159-1-201307020-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101(2):343–350. doi: 10.1111/j.1572-0241.2006.00390.x [DOI] [PubMed] [Google Scholar]
- 17.Weissfeld JL, Schoen RE, Pinsky PF, et al. ; PLCO Project Team. Flexible sigmoidoscopy in the PLCO cancer screening trial: results from the baseline screening examination of a randomized trial. J Natl Cancer Inst. 2005;97(13):989–997. doi: 10.1093/jnci/dji175 [DOI] [PubMed] [Google Scholar]
- 18.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359(12):1207–1217. doi: 10.1056/NEJMoa0800996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2020;115(3):415–434. doi: 10.14309/ajg.0000000000000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95(3):230–236. doi: 10.1093/jnci/95.3.230 [DOI] [PubMed] [Google Scholar]
- 21.van Hees F, Zauber AG, Klabunde CN, Goede SL, Lansdorp-Vogelaar I, van Ballegooijen M. The appropriateness of more intensive colonoscopy screening than recommended in Medicare beneficiaries: a modeling study. JAMA Intern Med. 2014;174(10):1568–1576. doi: 10.1001/jamainternmed.2014.3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeYoreo M, Lansdorp-Vogelaar I, Knudsen AB, Kuntz KM, Zauber AG, Rutter CM. Validation of colorectal cancer models on long-term outcomes from a randomized controlled trial. Med Decis Making. 2020;40(8):1034–1040. doi: 10.1177/0272989X20961095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mark DH. Visualizing cost-effectiveness analysis. JAMA. 2002;287(18):2428–2429. doi: 10.1001/jama.287.18.2428 [DOI] [PubMed] [Google Scholar]
- 24.Cancer Trends Progress Report. National Cancer Institute. Published February2019. AccessedApril 8, 2021. https://progressreport.cancer.gov
- 25.Rutter CM, Knudsen AB, Lin JS, Bouskill KE. Black and white differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30(1):3–12. doi: 10.1158/1055-9965.EPI-19-1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med. 2002;346(23):1781–1785. doi: 10.1056/NEJM200206063462304 [DOI] [PubMed] [Google Scholar]
- 27.Rundle AG, Lebwohl B, Vogel R, Levine S, Neugut AI. Colonoscopic screening in average-risk individuals ages 40 to 49 vs 50 to 59 years. Gastroenterology. 2008;134(5):1311–1315. doi: 10.1053/j.gastro.2008.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butterly LF, Siegel RL, Fedewa S, Robinson CM, Jemal A, Anderson JC. Colonoscopy outcomes in average-risk screening equivalent young adults: data from the New Hampshire Colonoscopy Registry. Am J Gastroenterol. 2021;116(1):171–179. doi: 10.14309/ajg.0000000000000820 [DOI] [PubMed] [Google Scholar]
- 29.Montminy EM, Zhou M, Maniscalco L, et al. Contributions of adenocarcinoma and carcinoid tumors to early-onset colorectal cancer incidence rates in the United States. Ann Intern Med. 2021;174(2):157–166. doi: 10.7326/M20-0068 [DOI] [PubMed] [Google Scholar]
- 30.Meester RGS, Peterse EFP, Knudsen AB, et al. Optimizing colorectal cancer screening by race and sex: microsimulation analysis II to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018;124(14):2974–2985. doi: 10.1002/cncr.31542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imperiale TF, Abhyankar PR, Stump TE, Emmett TW. Prevalence of advanced, precancerous colorectal neoplasms in black and white populations: a systematic review and meta-analysis. Gastroenterology. 2018;155(6):1776–1786. doi: 10.1053/j.gastro.2018.08.020 [DOI] [PubMed] [Google Scholar]
- 32.Peterse EFP, Meester RGS, Siegel RL, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018;124(14):2964–2973. doi: 10.1002/cncr.31543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Meulen MP, Lansdorp-Vogelaar I, van Heijningen EM, Kuipers EJ, van Ballegooijen M. Nonbleeding adenomas: evidence of systematic false-negative fecal immunochemical test results and their implications for screening effectiveness—a modeling study. Cancer. 2016;122(11):1680–1688. doi: 10.1002/cncr.29952 [DOI] [PubMed] [Google Scholar]
- 34.Hubbard RA, Johnson E, Hsia R, Rutter CM. The cumulative risk of false-positive fecal occult blood test after 10 years of colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2013;22(9):1612–1619. doi: 10.1158/1055-9965.EPI-13-0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greuter MJ, Demirel E, Lew JB, et al. Long-term impact of the Dutch Colorectal Cancer Screening Program on cancer incidence and mortality—model-based exploration of the serrated pathway. Cancer Epidemiol Biomarkers Prev. 2016;25(1):135–144. doi: 10.1158/1055-9965.EPI-15-0592 [DOI] [PubMed] [Google Scholar]
- 36.Naber SK, Kuntz KM, Henrikson NB, et al. Cost-effectiveness of age-specific screening intervals for people with family histories of colorectal cancer. Gastroenterology. 2018;154(1):105–116. doi: 10.1053/j.gastro.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


