Abstract
Extracellular vesicles (EVs) are a subclass of biological nanoparticles secreted by most cell types. Once secreted, EVs can travel long distances to deliver their content to target cells thereby playing a key role in cell‐to‐cell communication and supporting both physiological and pathological processes. In recent years, the functional versatility of EVs has come to be more widely appreciated. Their heterogeneous structure encloses solubilized bioactive cargoes including proteins and nucleic acids. EVs mirror the secreting cell in composition therefore representing a novel source of diagnostic and prognostic biomarkers. Moreover, due to their unique structure, EVs constitute a promising class of biocompatible nanovehicles for drug delivery as well. Importantly, and of burgeoning interest, is the fact that EVs have the intrinsic ability to breach biological barriers including the complex blood–brain barrier (BBB), whose restrictive nature represents a significant therapeutic challenge. EVs have been shown to contribute to the progression of a variety of brain diseases including metastatic brain cancer, neurodegenerative diseases, and acute pathologies including infections and ischemia. In this review, the role of EVs in the maintenance and regulation of the BBB under normal physiological and pathologic conditions are discussed. Applications of EVs as therapeutic and diagnostic tools in the treatment of diseases that affect the central nervous system are presented as are limitations hindering their broad translation and potential solutions to resolve them.
Keywords: brain metastasis, brain tumor, exosome, homeostasis, microvesicle, neurodegenerative disease, tumor microenvironment
1. INTRODUCTION
Biological fluids are populated by a plethora of circulating biogenic nanoparticles that differ in size, density, and composition and share an important role in cell‐to‐cell communication.1, 2 Over the past four decades, one specific subclass of biological nanoparticles named extracellular vesicles (EVs) has gained much attention in several scientific fields due to their chemico‐physical and biological properties.3, 4, 5, 6 EVs are heterogeneous in size and density and may be classified as exosomes, microvesicles (also named ectosomes, microparticles, or if tumor‐derived, oncosomes7) and apoptotic bodies based on the originating pathway.1, 2 Exosomes originate from the multivesicular bodies and are released in the extracellular space upon the fusion of the endosomes with the plasma membrane. In contrast, microvesicles originate from the outward budding of the plasma membrane whereas apoptotic bodies are the product of cell death via apoptosis.2 Interestingly, EV heterogeneity could suggest specific biological properties unique to each EV‐subclass. However, the currently applied EV size‐ (e.g., size‐exclusion chromatography, tangential flow filtration) and density‐based (e.g., ultracentrifugation, density gradient) isolation methods do not allow efficient separation of the different EV subclasses therefore hampering the study of their respective properties.8 To date, the EV‐intracellular originating pathway remains the only valid criterion to distinguish between exosomes, microvesicles, and apoptotic bodies as no biomarkers (single and combined) or chemico‐physical characteristics have been validated for this purpose.8 The broad EV heterogeneity remains a complex topic that is currently the subject of intense investigation. For this reason, we will refer to the cell‐derived nanoparticles discussed in the present review with the broad term of EVs.
Extracellular vesicles are released by both eukaryotic and prokaryotic cells, are delimited by cellular membrane and encase small coding and noncoding genetic materials and proteins.6 Along with classic routes of intercellular communication (e.g., secretion of autocrine/paracrine signaling factors), EVs represent an additional strategy for cells to convey messages to other cells in their surrounding environment and in distant organs.9 Once released from the originating cells, EVs may interact with the surrounding microenvironment, intravasate, travel short or long distances, extravasate at the target site and deliver their bioactive content to a specific cell modulating its phenotype.10 EVs have the intrinsic ability to breach biological barriers even the more complex ones such as the blood–brain barrier (BBB).11, 12
The term BBB refers to the entire set of small vessels and capillaries that form a dense network that vascularizes the brain and that simultaneously protects it from exogenous (bacteria, viral particles, others) and endogenous (glutamate, glycine, norepinephrine, epinephrine, peptide hormones, antibodies, others) blood‐circulating neurotoxins.1, 13 On a micron scale, the BBB consists of a homogeneous layer of brain microvascular endothelial cells (BECs) connected by unique lateral occluding junctions that prevent the paracellular transport or passive diffusion of molecules and particles through the barrier, making it highly selective.1, 13 To complete the complex BBB superstructure, BECs are supported by pericytes on the parenchymal side. Pericytes are contractile immune‐like cells embedded in a thin layer of extracellular matrix (ECM) called the basal lamina and the astrocytic glia end‐feet that help tighten the barrier.13, 14, 15 Together, the BBB cells which are the BECs, pericytes and astrocytes, and the brain cells, namely neurons, microglia, and oligodendrocytes, form the neurovascular unit (NVU) which is essential for central nervous system (CNS) homeostasis.15
Cell communication is fundamental between both sides of the BBB and it is, at least in part, mediated via EVs.16 It is now widely accepted that systemic and BBB‐derived EVs initiate, promote and support physiological blood‐to‐brain transport as well as pathological chronic (e.g., neurodegenerative diseases, primary and secondary tumors)17 and acute (e.g., infections, ischemia)18, 19, 20, 21 processes.
This review discusses the role of EVs in the maintenance and regulation of the BBB under normal physiological conditions as well as on the EV–BBB interactions that favor and support various brain pathologies. In addition, the recent principal applications of EVs as therapeutic and diagnostic tools in the treatment of pathologies that affect the CNS are presented as well as the limitations hindering their broad translation.
2. PHYSIOLOGICAL REGULATION OF THE BBB
The BBB is unique as it is characterized by having a high metabolic rate, high electrical resistance and numerous tight and adherent intercellular junctions.13, 22 Together, these features limit the paracellular transport of molecules while favoring a selective transcellular transport mediated by membrane proteins, receptors, and adsorptive transcytosis (Figure 1A).23 Efflux pumps actively remove exogenous molecules that may be harmful to BBB integrity and for the brain itself.23 BBB transport undergoes physiological variations throughout the life of individuals becoming more permeable with age. It has been shown that the BBB of healthy elderly mice (20–24 months old) becomes leakier to various tracers including endogenous proteins such as components of the plasma secretome.24 Compared to the BECs of young (3 months old) mice, BECs of 2‐year‐old mice showed a decreased expression of membrane proteins (e.g., transferrin receptors, lipoproteins receptors, clathrin and clathrin adaptors) that mediate receptor‐mediated transcytosis in favor of an increased transcytosis of large proteins (e.g., albumin and antibodies) through nonspecific caveolar vesicles. This transport shift was accompanied by other signs of physiological ageing, namely decreased pericyte coverage, the presence of ectopic brain calcifications and an increased expression of alkaline phosphatase (ALPL) on BEC surfaces. Interestingly, the targeting of vascular ALPL by approved selective inhibitors rescued vascular expression of transferrin receptors and increased BEC receptor‐ mediated transcytosis, potentially representing a novel strategy to modulate the BBB transport in the elderly population.24
FIGURE 1.
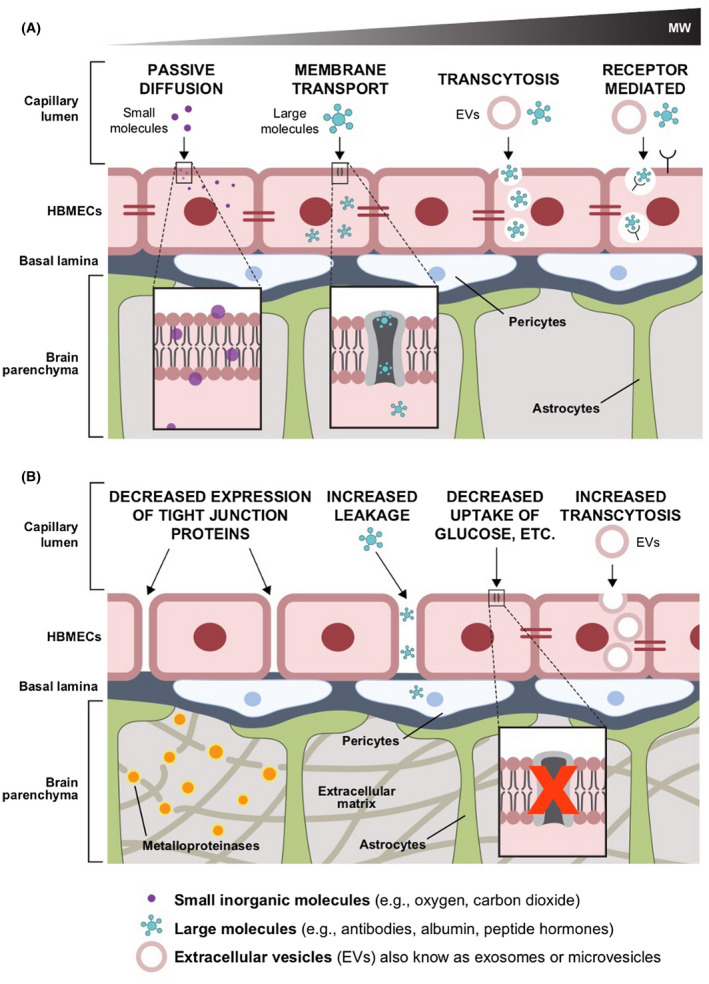
Examples of pathways and mechanisms of transport at the blood–brain barrier during (A) physiological conditions (e.g., passive diffusion, receptor‐mediated transcytosis) and (B) pathological processes, such as primary and metastatic brain cancer, and neurodegenerative diseases such as Parkinson's and Alzheimer's diseases (e.g., decreased expression of tight junction proteins, increased paracellular leakage, decreased expression of membrane protein for active transport of molecules, and increased cancer‐extracellular vesicle [EV] transcytosis)
Cells release biological nanoparticles such as lipoproteins, transferrin, and EVs2 that are fundamental for short and long distance cell‐to‐cell communication. Receptor‐based and adsorptive transcytosis are the two currently known routes through which complex circulating nanoparticles are transported through the BBB to the brain. Given the critical role that EV trafficking across the BBB plays during such significant pathological processes such as primary and metastatic brain tumors and some neurodegenerative diseases,11, 16, 17, 25 understanding the transcytosis of circulating EVs and their role in brain homeostasis remains an important research opportunity.
Taken together, it is clear that BBB structure and its role in brain homeostasis represent a double‐edged sword for the treatment of brain conditions as it regulates a selective transport mechanism while also hindering the delivery of the majority of therapeutic molecules.23 Elucidating the mechanisms through which EVs interact with the BBB under physiological and pathological conditions may advance the development of new vehicles3 for targeted brain delivery as well as the discovery and validation of diagnostic and prognostic biomarkers of brain pathologies.
3. EVs AND THE BBB IN BRAIN PATHOLOGIES
3.1. Primary and metastatic brain tumors
Cancer‐derived EVs have been unequivocally linked to the development and progression of primary and metastatic brain tumors26, 27, 28 providing further evidence in support of EV interactions with the BBB.1, 11, 25, 29
3.1.1. Primary brain tumors
Primary brain cancers, many of which are fatal, suffer from a lack of effective diagnostic or prognostic biomarkers, have limited treatment options and are often diagnosed at an advanced stage, leading to poor patient prognosis and survival.1, 11, 30
During their rapid growth in a spatially limited brain environment, brain tumors display ECM remodeling strategies and cytotoxic effects resulting in the loss or aberrant transformation of BBB cellular and extracellular components.31 As a result, the BBB in close proximity of a brain tumor, the blood–tumor barrier, is characterized by a compromised integrity and becomes permeable to large molecules and circulating leukocytes (Figure 1B).31 The blood–tumor barrier permits the transfer of blood‐borne neurotoxins into the brain parenchyma, thereby compromising neuronal viability and the function of preexisting blood vessels while favoring the formation of new leaky, abnormal capillaries.31 Similarly, EVs from primary brain cancers are found circulating in the blood and other systemic biological fluids supporting the finding that EVs can also be secreted by intracranial tumors and trafficked across both sides of the BBB (Figure 2A).
FIGURE 2.
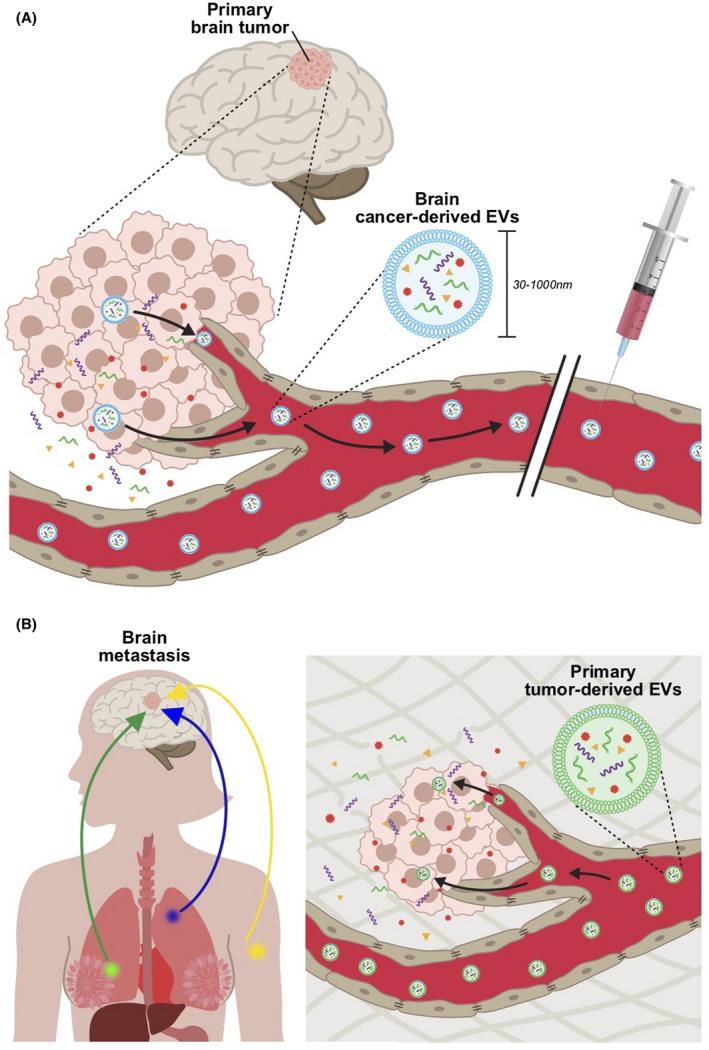
Tumor‐derived extracellular vesicle (EVs) in cancer pathogenesis and progression. (A) EVs secreted by primary tumors are released into blood circulation and other biological fluids (cerebrospinal fluid, urine, etc.) and may represent a useful source of tumor‐derived biomarkers for early diagnosis. (B) EVs secreted by brain seeking primary tumor cells travel long distances, are transcytosed through the intact blood–brain barrier and target and modulate the basal phenotype of healthy brain parenchyma cells
Gliomas are the most common category of primary brain tumors in adults, accounting for ~29% of all the adult brain cancers. In children, the most common form of gliomas is astrocytoma which accounts for 52% of all pediatric gliomas.32, 33 Gliomas originate from macroglial cells and are poorly responsive to current therapeutics.33 Glioblastoma multiforme (GBM) is the most common form of glioma in adults and is characterized by highly undifferentiated tumor cells, heterogeneity and defective angiogenesis.34 Patient‐derived stem‐like GBM cells cultured as neurospheres in neurobasal medium secrete large amounts of EVs (109 EVs/mL of conditioned media).35 These EVs carry selectively enriched genetic material, predominantly noncoding ribosomal RNA, along with microRNAs and low levels (below 10%) of coding messenger RNA able to impact the phenotype of BECs,35, 36 astrocytes, neurons, and microglia.35 Recently, it has been shown that a subpopulation of self‐renewing stem cell‐like GBM cells located in the NVU in close contact with the BBB secrete EVs enriched in pro‐angiogenic factors, in particular vascular endothelial growth factor‐A (VEGF‐A).34 Interestingly, GBM‐secreted EVs (GBM‐EVs) target BECs and induce the formation and sprouting of new vessels through a VEGF‐A‐dependent mechanism.34 Furthermore, VEGF‐A containing EVs isolated from GBM cells cultured under hypoxic conditions increase BBB permeability by decreasing the expression of tight junction proteins, namely claudin 5 and occludin, in BECs.37
Other than stimulating BECs through VEGF‐A‐dependent pathways, EVs derived from primary human GBM cells (GBM8) cultured as spheroids in neurobasal medium were shown to transfer a subset of eight micro‐RNAs (miRNAs) to BECs, which are responsible for modulating BECs gene transcription and protein expression toward a pro‐angiogenic phenotype.38 The mechanistic role of EVs in other rare intracranial brain cancers, in particular pediatric brain cancers, has also been studied but to a lesser extent compared to adult brain tumors.32 Further studies are required to determine the interaction of tumor‐derived and host EVs with the BBB within the context of pediatric brain tumors.
3.1.2. Metastatic brain tumors
Metastatic brain tumors, also known as secondary brain tumors, result from the dissemination of cancer cells from systemic primary tumors and their survival and proliferation in the brain microenvironment. Brain metastasis is the most common type of brain tumor in adults and occurs in a variety of cancer types, most commonly lung cancer, melanoma, and breast cancer.39 EVs have been shown to interact with the components of the BBB and contribute to different stages of brain metastasis. BECs have been identified as one of the recipients of tumor‐derived EVs, specifically, EVs derived from the brain‐seeking MDA‐MB‐231 breast cancer cell line. Transfer of miR‐105 and miR‐181c from EVs to BECs led to a decrease in the expression of zona occludens 1, a tight junction molecule and the reorganization of actin filaments, both leading to an increase in the permeability of the BBB (Figure 1B).40, 41 Furthermore, EVs derived from brain‐seeking breast cancer cells were shown to be enriched in the cell migration‐inducing and hyaluronan‐binding protein.42 When transferred to BECs, they diminished the vascular co‐option phenotype of tumor cells in the brain and further induced an inflammatory phenotype in both BECs and microglial cells.42
Importantly, tumor‐derived EVs can find their way to the brain parenchyma by crossing the brain endothelial barrier. Our group was the first to show that breast cancer‐derived EVs exhibit transcellular transport across the endothelial barrier and breach the BBB without compromising its integrity.29 We have further shown that, once on the abluminal side, breast cancer‐derived EVs can be taken up by astrocytes and transfer miR‐301a, which alters the matrix modulating function of the cells by decreasing the expression of tissue inhibitor of matrix metalloproteinase 225 as part of pre‐metastatic niche preparation. Breast cancer‐derived EVs have also been shown to transfer miR‐122 to astrocytes, which reduced glucose uptake of these cells and created a favorable metabolic environment in the brain for tumor growth.43 The reported EV interactions in brain metastasis are not limited to tumor‐derived EVs. Phosphatase and tensin homolog (PTEN) loss, which has been known as a common characteristic of metastatic brain tumors, was recently shown to be a nongenetic feature and a result of environmental cues transferred via EVs.44 Astrocyte EVs were shown to transfer miR‐19a to metastatic breast cancer cells in the brain, which led to PTEN downregulation and increased cell proliferation in these cells. PTEN loss could be reversed by blocking EV secretion in astrocytes.44
Overall, these and other studies demonstrate that EVs can drive bidirectional cross talk between tumor cells and BBB cells and can promote metastasis formation in the brain. It should be noted that our understanding of the role of EVs in brain metastasis has been limited, to a large extent, to breast cancer brain metastasis. With several brain metastatic cell lines now available for other cancers such as lung cancer and melanoma,45 future studies are warranted to evaluate the effect of EVs across different cancer types.
3.2. Neurodegenerative diseases
Neurodegenerative diseases include a broad group of pathologies, all of which progressively affect neuronal viability in the CNS leading to important neurological and psychiatric symptoms that heavily impact the patient's quality of life and survival.46, 47, 48, 49 The most common neurodegenerative diseases are Alzheimer's disease (AD), which represents the most common form of dementia in the elderly individual, Parkinson's disease (PD) and motor neuron diseases, which are progressive disorders affecting movement.49
The pathology of neurodegenerative diseases is based on the accumulation of misfolded aggregated proteins (e.g., α‐synuclein in PD and amyloid‐β, and tau protein in AD) that display cytotoxic effects and are transmitted to adjacent cells in a prion‐like fashion. EVs, as key effectors in intercellular communication, appear to be involved in the cross talk between healthy and affected cells and in the advancement of these pathologies.47
Chronic inflammation precedes the appearance of neurodegenerative clinical symptoms and promotes their progression. Astrocyte EVs promote AD progression directly by transferring neurotoxic amyloid‐β to surrounding neurons50 or indirectly by targeting neurons and delivering pro‐inflammatory molecules that stimulate amyloid‐β production and damage the target cells.51, 52 In both cases, EVs derived from activated astrocytes display neurotoxic effects on the surrounding brain cells favoring neuroinflammation and degeneration.
Similarly, astrocytes stimulated with lipopolysaccharide (LPS) secrete EVs enriched in miR‐34a that, once taken up by cultured dopaminergic neurons, decrease their level of antiapoptotic proteins thereby increasing their sensitivity towards neurotoxins.53 In vivo, rats that received a single intracerebral injection of astrocyte EVs showed an accelerated loss of dopaminergic neurons, further supporting the role of EVs released by activated astrocytes in PD pathogenesis.53
Recent findings suggest that other peripheral organs and tissues may contribute to the pathogenesis of the neurodegenerative disease, in particular PD.54 The abnormal accumulation of α‐synuclein in the brain of patients affected by PD is supported by active transport of peripheric α‐synuclein across the BBB to the brain.54 Erythrocytes contain a high concentration of α‐synuclein that is, at least in part, secreted via EVs. EVs isolated from erythrocytes of PD patients and healthy controls were administered to murine models with LPS‐induced systemic inflammation.54 Interestingly, after BBB permeability was increased by LPS, erythrocyte‐derived EVs were transferred, likely through absorption‐mediated transcytosis, across the BBB and colocalized with brain microglia. EVs derived from PD erythrocytes incubated with cultured microglia cells caused a significant increase (40%) in induced nitric oxide synthase expression supporting a pro‐inflammatory brain microenvironment that may contribute to PD pathogenesis and progression.54
Taken together, these and other studies support the involvement of EVs in the pathogenesis of neurodegenerative diseases. However, the specific role of EVs in the context of neurodegenerative diseases, for instance, whether EVs are involved in the transmission or degradation pathway of the aberrant proteins, is a complex topic that, to date, is still not well understood.49
3.3. Neuroimmune diseases
A growing body of literature demonstrates that EVs released from a variety of immune and nonimmune cells can induce pro‐ and anti‐inflammatory immune responses in their target cells. For instance, EVs can contribute to antigen presentation and modulation of T‐cell activity through their surface MHC molecules or programmed death‐ligand 1, respectively.55, 56, 57 The immunomodulatory function of EVs have also been described within the brain microenvironment, in relation to the components of the BBB and within the context of inflammatory, ischemic, and infectious brain diseases.18, 19, 20, 21 Importantly, EVs can regulate immune modulation in brain stems by mediating EV‐driven cross talk between the brain cells and peripheral immune and nonimmune cells. For instance, brain endothelial EVs containing inflammatory cargo were shown to have a strong correlation with the severity of acute ischemic stroke,21 with in vitro studies suggesting their role in the facilitation of monocyte extravasation across the BBB during inflammation.58 Monocyte EVs were also shown to change the expression profile of BECs toward an inflammatory phenotype.20 Microglia, the resident macrophages of the brain, also release EVs that alter the behavior of neurons and contribute to neuroinflammation59, 60; however, their role in the regulation of the BBB is not well understood. The choroid plexus‐derived EVs released during systemic inflammation were also shown to be taken up by astrocytes and microglia and upregulate the expression of inflammatory genes in the brain.19
Different pathogens have also been shown to exert their infectious effects on the brain through EVs. For instance, plasma EVs from mice with cerebral malaria were taken up by BECs when injected in healthy mice and were sufficient to induce cerebral malaria‐like symptoms.19 Furthermore, platelet‐derived EVs were shown to increase the adherence of malaria‐infected erythrocytes to the BECs thereby facilitating BBB alterations during cerebral malaria.19 HIV infection also increased the transfer of amyloid‐beta from BECs to astrocytes and pericytes through endothelial EVs, a process that was suggested to be involved in HIV‐associated neurocognitive disorder.61 It is worth noting that, similar to host cell‐derived EVs, microbial EVs derived from pathogenic and nonpathogenic bacteria have been recently introduced as major players in the modulation of immune responses.62, 63 The similarities between the microbial EVs and their eukaryotic counterparts in the mechanisms of internalization into recipient cells prompts the hypothesis that microbial EVs could be taken up by BECs or breach the BBB and, therefore, contribute to immune regulation in the brain. Recent studies have demonstrated that EVs derived from Aggregatibacter actinomycetemcomitans, a pathogen of periodontal diseases, could cross the BBB and were taken up by infiltrating macrophages and resident microglia, leading to inflammatory cytokine production.64, 65 This mechanism was suggested as a potential explanation for the association between periodontal diseases and AD.66 Future studies are needed to elucidate the role of microbial EVs derived from pathogens or nonpathogenic resident microbiota in the immune regulation of the BBB and the brain microenvironment.
4. PRECLINICAL AND CLINICAL INVESTIGATIONS OF EVs AS THERAPEUTIC AND DIAGNOSTIC STRATEGIES FOR BRAIN PATHOLOGIES
As a function of their promising therapeutic and diagnostic potential, EVs are under intense preclinical and clinical investigations in many diseases, including cancers and neurodegenerative, cardiovascular, and infectious diseases.67, 68, 69, 70 To date, according to the Clinicaltrials.gov database (https://www.clinicaltrials.gov/), there are over 200 ongoing and completed clinical trials using EVs as either therapeutic agents or diagnostic biomarkers. Among these trials, approximately 15 of them are related to brain pathologies such as PD, AD, stroke, and brain cancers which are discussed below.
Extracellular vesicles derived from the CNS are trafficked across the BBB, released into the bloodstream or in other fluids and may serve as a good source of diagnostic and prognostic biomarkers.48, 71, 72 EV components have shown great potential as brain cancer biomarkers.73 EGFR mRNA, in particular the GBM‐specific mutant splice variant EGFRvIII, was found to be enriched in EVs isolated from GBM patient serum samples.36 Similarly, miR‐21 was consistently found to be enriched in EVs isolated from blood samples of patients affected by GBM36, 74 as well as from the CSF of patients with leptomeningeal metastasis.75 Interestingly, miR‐21 was also found to be enriched in EVs isolated from brains of murine models of traumatic brain injury where the brain trauma was mimicked with a craniotomy followed by a controlled damage of the dura.76 GBM‐EVs have been shown to cross the intact BBB, be released into the bloodstream and carry a DNA signature specific to the originating malignant cells, in particular, isocitrate dehydrogenase 1 mutations.77 Notably, these GBM‐EVs were isolated from plasma samples of xenotransplanted orthotopic murine models and from serum samples from patients that underwent surgery for GBM (n = 20) or brain metastatic cancer (n = 1).77 In another study, a nuclear magnetic resonance system was adapted for the detection and analysis of the protein signatures of EVs derived from blood samples of GBM patients, which were used to analyze primary tumor mutations and predict the outcome of GBM therapy in vivo.78 Notably, there are ongoing clinical trials investigating EVs as predictive biomarkers for high‐grade GBM patients (NCT03576612; estimated study completion February 2022) or breast cancer leptomeningeal metastases (NCT03974204; estimated study completion date October 2023).
Neurodegenerative conditions are frequently diagnosed after the appearance of clinical manifestations, such as dementia and impaired movements, which are signs of advanced‐stage disease. Early symptoms of neurological changes are difficult to detect due to the lack of early diagnostic biomarkers.79 A systemic comparison of EV miRNAs isolated from blood samples and brain tissues of patients affected by AD have permitted the identification of potential biomarkers of AD and have also supported the hypothesis that EVs are trafficked across both sides of the BBB.79 Some candidate miRNAs have also been shown to be present in both blood and brain of patients affected by AD compared to healthy controls paving the way for the validation of novel blood‐based AD biomarkers.79 Similarly, EVs isolated from the blood and CSF of patients affected by amyotrophic lateral sclerosis were enriched in a subset of up and downregulated miRNAs with diagnostic potential.71 These candidate miRNAs were identified by next‐generation sequencing and validated by droplet digital polymerase chain reaction. Some of the identified miRNAs correlated with disability progression as well.71
Extracellular vesicles isolated from blood80 and saliva81 of patients affected by PD versus healthy controls have also been shown to carry specific molecules such as, but not limited to, α‐synuclein, that may represent potential prognostic markers for PD. Moreover, disease‐specific molecules carried by EVs can serve as therapeutic targets for PD‐targeted therapy. It has also been reported that EVs isolated from the urine and CSF of patients with PD exhibited elevated levels and autophosphorylation of leucine‐rich repeat kinase 2 (LRRK2), which is closely correlated with the LRRK2 gene mutation status in patients with PD.82, 83, 84 It has been known that the LRRK2 gene mutation can cause late‐onset PD.82, 83 These findings have led to three clinical trials (NCT01860118, NCT03775447, and NCT04603326) investigating the role of LRRK2 and other EV biomarkers for predicting disease susceptibility and progression as well as predicting the therapeutic efficacy of sunitinib, a potent LRRK2 kinase and angiogenesis inhibitor in PD patients.
Similarly, Tau, a microtubule‐associated protein whose pathological aggregation can cause neurodegenerative disorders, has been shown to be highly enriched in ectosomes, plasma membrane‐originated EVs, which are isolated from the interstitial fluid of a rat model of sporadic tauopathy.14 These findings revealed a new mechanism as to how intracellular Tau proteins are transferred into extracellular fluids, leading to pathological spread of Tau. A clinical trial (NCT03381482) is ongoing using ectosome‐associated Tau protein as a novel predictive biomarker for AD. Ectosomes were isolated from the CSF of patients with AD and their ectosomal Tau levels were quantified by ELISA and Nanosight measurements.
Extracellular vesicles are also being investigated for their potential in the treatment of brain diseases. EVs can be isolated from cells with intrinsic therapeutic potential (e.g., stem cells and immune cells)67 or can be loaded with therapeutic agents.68 Both strategies have been under clinical investigation.67, 68, 69, 70 EVs isolated from mesenchymal stem cells (MSC‐EVs) maintain the anti‐apoptotic, immunomodulatory, and regenerative functions of their parental cells.5, 70 In preclinical investigations, MSC‐EVs have shown protective effects in treating several pathologies, including AD.85, 86, 87, 88 The therapeutic benefits of MSC‐EVs as a promising regenerative medicine led to a number of clinical trials in brain diseases. MSC‐EVs administered via a nasal drip route have been investigated for treating patients with AD in an ongoing Phase I/II clinical trial (NCT04388982). In addition, intravenously administered MSC‐EVs have been investigated for treating patients with stroke (NCT03384433, ongoing trial). EVs have also been engineered to deliver various therapeutic payloads, including small molecules, peptides, proteins, and genetic therapeutics.5, 67, 68 EVs can be loaded via exogenous (e.g., co‐incubation, sonication, or electroporation) or endogenous methods (e.g., EVs are released by engineered donor cells).69 Notably, engineered EVs have shown promise as therapeutic in preclinical studies.5, 67, 68, 69 In September 2020, a Phase I clinical trial was launched to investigate the efficacy of modified EVs as an anticancer therapy. EVs were engineered via a fusion‐protein approach to display pro‐inflammatory interleukin‐12 (IL‐12) on their surface5, 67, 88 and then injected intratumorally for the treatment of advanced solid tumors, including GBM. The initial results of this clinical trial showed that local injection of IL‐12‐loaded EVs was well tolerated in healthy volunteers without treatment‐related adverse effects and systematic IL‐12 exposure; the multidose efficacy evaluation for cancer patients is ongoing.
Extracellular vesicles are an emerging class of biological medicine providing promising therapeutic and diagnostic opportunities for many brain pathologies in urgent need of therapeutic intervention. To accelerate clinical translation of EV‐based medicines, several critical challenges must be addressed including increasing the yield and the consistency of EVs isolated from patient's body fluids,60 manufacturing EVs or EV mimetics4 in a scaled‐up and GMP manner,68 increasing EV loading efficiency69 and enhancing EV targeting and BBB breaching/crossing abilities.11, 25, 70
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors contributed equally to the design and writing of this review.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the support of NIH R21 CA253051‐01 (MAM), the Breast Cancer Research Foundation (MAM), the Michael B. Rukin Foundation (MAM), the Goodman Family and the Faculty Career Development Fellowship of Boston Children's Hospital (PG). The authors thank Kristin Johnson of the Vascular Biology Program at Boston Children's Hospital for assistance with the illustrations.
Busatto S, Morad G, Guo P, Moses MA. The role of extracellular vesicles in the physiological and pathological regulation of the blood–brain barrier. FASEB BioAdvances. 2021;3:665–675. 10.1096/fba.2021-00045
This article is part of the Extracellular Vesicles and Homeostasis Special Collection.
Sara Busatto and Golnaz Morad contributed equally.
REFERENCES
- 1.Morad G, Moses MA. Brainwashed by extracellular vesicles: the role of extracellular vesicles in primary and metastatic brain tumour microenvironment. J Extracell Vesicles. 2019;8:1627164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busatto S, Zendrini A, Radeghieri A, et al. The nanostructured secretome. Biomater Sci. 2019;8:39‐63. [DOI] [PubMed] [Google Scholar]
- 3.Guo P, Huang J, Moses MA. Cancer nanomedicines in an evolving oncology landscape. Trends Pharmacol Sci. 2020;41:730‐742. [DOI] [PubMed] [Google Scholar]
- 4.Guo P, Busatto S, Huang J, Morad G, Moses MA. A facile magnetic extrusion method for preparing endosome‐derived vesicles for cancer drug delivery. Adv Func Mater. 2021. 10.1002/adfm.202008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song Y, Kim Y, Ha S, et al. The emerging role of exosomes as novel therapeutics: biology, technologies, clinical applications, and the next. Am J Reprod Immunol. 2020;85(2):e13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EL Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347‐357. [DOI] [PubMed] [Google Scholar]
- 7.Di Vizio D, Morello M, Dudley AC, et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol. 2012;181:1573‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raposo G, Stahl PD. Extracellular vesicles: a new communication paradigm? Nat Rev Mol Cell Biol. 2019;20:509‐510. [DOI] [PubMed] [Google Scholar]
- 10.Kanada M, Bachmann MH, Hardy JW, et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci USA. 2015;112:E1433‐E1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morad G, Carman CV, Hagedorn EJ, et al. Tumor‐derived extracellular vesicles breach the intact blood‐brain barrier via transcytosis. ACS Nano. 2019;13:13853‐13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto J, Stewart T, Banks WA, Zhang J. The transport mechanism of extracellular vesicles at the blood‐brain barrier. Curr Pharm Des. 2017;23:6206‐6214. [DOI] [PubMed] [Google Scholar]
- 13.Smriti Gupta SD, Sandhir R. Anatomy and physiology of blood‐brain barrier. In: Gao H, Gao X, eds. Brain Targeted Drug Delivery System. Elsevier; 2019:7‐31. Imprint: Academic Press. [Google Scholar]
- 14.Abbott NJ. Astrocyte‐endothelial interactions and blood‐brain barrier permeability. J Anat. 2002;200:629‐638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbott NJ, Ronnback L, Hansson E. Astrocyte‐endothelial interactions at the blood‐brain barrier. Nat Rev Neurosci. 2006;7:41‐53. [DOI] [PubMed] [Google Scholar]
- 16.Pegtel DM, Peferoen L, Amor S. Extracellular vesicles as modulators of cell‐to‐cell communication in the healthy and diseased brain. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciregia F, Urbani A, Palmisano G. Extracellular vesicles in brain tumors and neurodegenerative diseases. Front Mol Neurosci. 2017;10:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andras IE, Toborek M. Extracellular vesicles of the blood‐brain barrier. Tissue Barriers. 2016;4:e1131804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balusu S, Van Wonterghem E, De Rycke R, et al. Identification of a novel mechanism of blood‐brain communication during peripheral inflammation via choroid plexus‐derived extracellular vesicles. EMBO Mol Med. 2016;8:1162‐1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalvi P, Sun B, Tang N, Pulliam L. Immune activated monocyte exosomes alter microRNAs in brain endothelial cells and initiate an inflammatory response through the TLR4/MyD88 pathway. Sci Rep. 2017;7:9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simak J, Gelderman MP, Yu H, Wright V, Baird AE. Circulating endothelial microparticles in acute ischemic stroke: a link to severity, lesion volume and outcome. J Thromb Haemost. 2006;4:1296‐1302. [DOI] [PubMed] [Google Scholar]
- 22.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood‐brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223‐236. [DOI] [PubMed] [Google Scholar]
- 23.Fu BM. Transport across the blood‐brain barrier. Adv Exp Med Biol. 2018;1097:235‐259. [DOI] [PubMed] [Google Scholar]
- 24.Yang AC, Stevens MY, Chen MB, et al. Physiological blood‐brain transport is impaired with age by a shift in transcytosis. Nature. 2020;583:425‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morad G, Daisy CC, Otu HH, Libermann TA, Dillon ST, Moses MA. Cdc42‐dependent transfer of mir301 from breast cancer‐derived extracellular vesicles regulates the matrix modulating ability of astrocytes at the blood‐brain barrier. Int J Mol Sci. 2020;21:3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoshino A, Costa‐Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peinado H, Aleckovic M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro‐metastatic phenotype through MET. Nat Med. 2012;18:883‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre‐metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011;21:139‐146. [DOI] [PubMed] [Google Scholar]
- 29.Morad G, Carman CV, Hagedorn EJ, et al. Tumor‐derived extracellular vesicles breach the intact blood‐brain barrier via transcytosis. ACS Nano. 2019;12:13853‐13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Legler JM, Ries LA, Smith MA, et al. Cancer surveillance series [corrected]: brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst. 1999;91:1382‐1390. [DOI] [PubMed] [Google Scholar]
- 31.Arvanitis CD, Ferraro GB, Jain RK. The blood‐brain barrier and blood‐tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20:26‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuzesi A, Kling T, Wenger A, et al. Pediatric brain tumor cells release exosomes with a miRNA repertoire that differs from exosomes secreted by normal cells. Oncotarget. 2017;8:90164‐90175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubois LG, Campanati L, Righy C, et al. Gliomas and the vascular fragility of the blood brain barrier. Front Cell Neurosci. 2014;8:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Treps L, Perret R, Edmond S, Ricard D, Gavard J. Glioblastoma stem‐like cells secrete the pro‐angiogenic VEGF‐A factor in extracellular vesicles. J Extracell Vesicles. 2017;6:1359479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei Z, Batagov AO, Schinelli S, et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat Commun. 2017;8:1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao C, Wang H, Xiong C, Liu Y. Hypoxic glioblastoma release exosomal VEGF‐A induce the permeability of blood‐brain barrier. Biochem Biophys Res Commun. 2018;502:324‐331. [DOI] [PubMed] [Google Scholar]
- 38.Lucero R, Zappulli V, Sammarco A, et al. Glioma‐derived miRNA‐containing extracellular vesicles induce angiogenesis by reprogramming brain endothelial cells. Cell Rep. 2020;30(7):2065–2074.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cagney DN, Martin AM, Catalano PJ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population‐based study. Neuro Oncol. 2017;19:1511‐1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tominaga N, Kosaka N, Ono M, et al. Brain metastatic cancer cells release microRNA‐181c‐containing extracellular vesicles capable of destructing blood‐brain barrier. Nat Commun. 2015;6:6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou W, Fong MY, Min Y, et al. Cancer‐secreted miR‐105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrigues G, Hoshino A, Kenific CM, et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat Cell Biol. 2019;21:1403‐1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fong MY, Zhou W, Liu L, et al. Breast‐cancer‐secreted miR‐122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17:183‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, Zhang S, Yao J, et al. Microenvironment‐induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valiente M, Van Swearingen AED, Anders CK, et al. Brain metastasis cell lines panel: a public resource of organotropic cell lines. Cancer Res. 2020;80:4314‐4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haddad M, Perrotte M, Landri S, Lepage A, Fulop T, Ramassamy C. Circulating and extracellular vesicles levels of N‐(1‐carboxymethyl)‐L‐lysine (CML) differentiate early to moderate Alzheimer's disease. J Alzheimers Dis. 2019;69:751‐762. [DOI] [PubMed] [Google Scholar]
- 47.Thompson AG, Gray E, Heman‐Ackah SM, et al. Extracellular vesicles in neurodegenerative disease—pathogenesis to biomarkers. Nat Rev Neurol. 2016;12:346‐357. [DOI] [PubMed] [Google Scholar]
- 48.Shaimardanova AA, Solovyeva VV, Chulpanova DS, James V, Kitaeva KV, Rizvanov AA. Extracellular vesicles in the diagnosis and treatment of central nervous system diseases. Neural Regen Res. 2020;15:586‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeuchi T. Pathogenic and protective roles of extracellular vesicles in neurodegenerative diseases. J Biochem. 2020;169:181‐186. [DOI] [PubMed] [Google Scholar]
- 50.Sollvander S, Nikitidou E, Brolin R, et al. Accumulation of amyloid‐beta by astrocytes result in enlarged endosomes and microvesicle‐induced apoptosis of neurons. Mol Neurodegener. 2016;11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z, Moniruzzaman M, Dastgheyb RM, et al. Astrocytes deliver CK1 to neurons via extracellular vesicles in response to inflammation promoting the translation and amyloidogenic processing of APP. J Extracell Vesicles. 2020;10:e12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goetzl EJ, Schwartz JB, Abner EL, Jicha GA, Kapogiannis D. High complement levels in astrocyte‐derived exosomes of Alzheimer disease. Ann Neurol. 2018;83:544‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mao S, Sun Q, Xiao H, Zhang C, Li L. Secreted miR‐34a in astrocytic shedding vesicles enhanced the vulnerability of dopaminergic neurons to neurotoxins by targeting Bcl‐2. Protein Cell. 2015;6:529‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsumoto J, Stewart T, Sheng L, et al. Transmission of alpha‐synuclein‐containing erythrocyte‐derived extracellular vesicles across the blood‐brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson's disease? Acta Neuropathol Commun. 2017;5:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poggio M, Hu T, Pai CC, et al. Suppression of exosomal PD‐L1 induces systemic anti‐tumor immunity and memory. Cell. 2019;177(2):414‐427.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ricklefs FL, Alayo Q, Krenzlin H, et al. Immune evasion mediated by PD‐L1 on glioblastoma‐derived extracellular vesicles. Sci Adv. 2018;4:eaar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen‐presenting vesicles. J Exp Med. 1996;183:1161‐1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jy W, Minagar A, Jimenez JJ, et al. Endothelial microparticles (EMP) bind and activate monocytes: elevated EMP‐monocyte conjugates in multiple sclerosis. Front Biosci. 2004;9:3137‐3144. [DOI] [PubMed] [Google Scholar]
- 59.Kumar A, Stoica BA, Loane DJ, et al. Microglial‐derived microparticles mediate neuroinflammation after traumatic brain injury. J Neuroinflammation. 2017;14:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prada I, Gabrielli M, Turola E, et al. Glia‐to‐neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation‐induced synaptic alterations. Acta Neuropathol. 2018;135:529‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andras IE, Leda A, Contreras MG, et al. Extracellular vesicles of the blood‐brain barrier: role in the HIV‐1 associated amyloid beta pathology. Mol Cell Neurosci. 2017;79:12‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alpdundar Bulut E, Bayyurt Kocabas B, Yazar V, et al. Human gut commensal membrane vesicles modulate inflammation by generating M2‐like macrophages and myeloid‐derived suppressor cells. J Immunol. 2020;205:2707‐2718. [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Eagen WJ, Lee JC. Orchestration of human macrophage NLRP3 inflammasome activation by Staphylococcus aureus extracellular vesicles. Proc Natl Acad Sci USA. 2020;117:3174‐3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han EC, Choi SY, Lee Y, Park JW, Hong SH, Lee HJ. Extracellular RNAs in periodontopathogenic outer membrane vesicles promote TNF‐alpha production in human macrophages and cross the blood‐brain barrier in mice. FASEB J. 2019;33:13412‐13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ha JY, Choi SY, Lee JH, Hong SH, Lee HJ. Delivery of periodontopathogenic extracellular vesicles to brain monocytes and microglial IL‐6 promotion by RNA cargo. Front Mol Biosci. 2020;7:596366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dioguardi M, Crincoli V, Laino L, et al. The role of periodontitis and periodontal bacteria in the onset and progression of Alzheimer's disease: a systematic review. J Clin Med. 2020;9:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cully M. Exosome‐based candidates move into the clinic. Nat Rev Drug Discov. 2021;20:6‐7. [DOI] [PubMed] [Google Scholar]
- 68.Wiklander OPB, Brennan MA, Lotvall J, Breakefield XO, El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019;11(492):eaav8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klyachko NL, Arzt CJ, Li SM, Gololobova OA, Batrakova EV. Extracellular vesicle‐based therapeutics: preclinical and clinical investigations. Pharmaceutics. 2020;12:1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials—an ISEV position paper. J Extracell Vesicles. 2015;4:30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saucier D, Wajnberg G, Roy J, et al. Identification of a circulating miRNA signature in extracellular vesicles collected from amyotrophic lateral sclerosis patients. Brain Res. 2019;1708:100‐108. [DOI] [PubMed] [Google Scholar]
- 72.Gagliardi D, Bresolin N, Comi GP, Corti S. Extracellular vesicles and amyotrophic lateral sclerosis: from misfolded protein vehicles to promising clinical biomarkers. Cell Mol Life Sci. 2020;78:561‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hallal S, Ebrahimkhani S, Shivalingam B, Graeber MB, Kaufman KL, Buckland ME. The emerging clinical potential of circulating extracellular vesicles for non‐invasive glioma diagnosis and disease monitoring. Brain Tumor Pathol. 2019;36:29‐39. [DOI] [PubMed] [Google Scholar]
- 74.Akers JC, Ramakrishnan V, Kim R, et al. MiR‐21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): a platform for glioblastoma biomarker development. PLoS One. 2013;8:e78115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee KY, Im JH, Lin W, et al. Nanoparticles in 472 human cerebrospinal fluid: changes in extracellular vesicle concentration and miR‐21 expression as a biomarker for leptomeningeal metastasis. Cancers (Basel). 2020;12:2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harrison EB, Hochfelder CG, Lamberty BG, et al. Traumatic brain injury increases levels of miR‐21 in extracellular vesicles: implications for neuroinflammation. FEBS Open Bio. 2016;6:835‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia‐Romero N, Carrion‐Navarro J, Esteban‐Rubio S, et al. DNA sequences within glioma‐derived extracellular vesicles can cross the intact blood‐brain barrier and be detected in peripheral blood of patients. Oncotarget. 2017;8:1416‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shao H, Chung J, Balaj L, et al. Protein typing of circulating microvesicles allows real‐time monitoring of glioblastoma therapy. Nat Med. 2012;18:1835‐1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng L, Vella LJ, Barnham KJ, McLean C, Masters CL, Hill AF. Small RNA fingerprinting of Alzheimer's disease frontal cortex extracellular vesicles and their comparison with peripheral extracellular vesicles. J Extracell Vesicles. 2020;9:1766822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Picca A, Guerra F, Calvani R, et al. Mitochondrial signatures in circulating extracellular vesicles of older adults with Parkinson's disease: results from the EXosomes in PArkiNson's Disease (EXPAND) study. J Clin Med. 2020;9:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cao Z, Wu Y, Liu G, et al. alpha‐Synuclein in salivary extracellular vesicles as a potential biomarker of Parkinson's disease. Neurosci Lett. 2019;696:114‐120. [DOI] [PubMed] [Google Scholar]
- 82.Fraser KB, Moehle MS, Daher JP, et al. LRRK2 secretion in exosomes is regulated by 14‐3‐3. Hum Mol Genet. 2013;22:4988‐5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang S, Liu Z, Ye T, et al. Elevated LRRK2 autophosphorylation in brain‐derived and peripheral exosomes in LRRK2 mutation carriers. Acta Neuropathol Commun. 2017;5:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang S, Kelly K, Brotchie JM, Koprich JB, West AB. Exosome markers of LRRK2 kinase inhibition. NPJ Parkinsons Dis. 2020;6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214‐222. [DOI] [PubMed] [Google Scholar]
- 86.Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell‐derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kordelas L, Rebmann V, Ludwig AK, et al. MSC‐derived exosomes: a novel tool to treat therapy‐refractory graft‐versus‐host disease. Leukemia. 2014;28:970‐973. [DOI] [PubMed] [Google Scholar]
- 88.Reza‐Zaldivar EE, Hernandez‐Sapiens MA, Minjarez B, Gutierrez‐Mercado YK, Marquez‐Aguirre AL, Canales‐Aguirre AA. Potential effects of MSC‐derived exosomes in neuroplasticity in Alzheimer's disease. Front Cell Neurosci. 2018;12:317. [DOI] [PMC free article] [PubMed] [Google Scholar]


