Abstract
Cardiogenic shock is frequently seen following acute myocardial infarction complicated by the rupture of interventricular septum and formation of functional ventricular septal defect. Despite significant advances in medical, interventional and surgical management, the mortality in this group of patients remains very high. We present a case of refractory cardiogenic shock following an exclusion bovine pericardial patch repair of post infarction ventricular septal defect, where the residual functional left ventricular cavity size was insufficient to maintain end organ function. This case illustrates the concept of “Normal ejection fraction low cardiac output cardiogenic shock”, where reporting left ventricular ejection fraction number in isolation can be misleading.
Keywords: cardiogenic shock; infarct; post infarction ventricular septal defect; ejection fraction, cardiac output; transthoracic echocardiography and transoesophageal echocardiography
Case report
A 68‐year‐old female was admitted to a district hospital with symptoms of pre‐syncope on a background of neck pain radiating into the chest and left shoulder. She had preceding diarrhoea and vomiting for 5 days. Her background medical history included hypertension, hyperlipidemia, long‐standing breathlessness with poor exercise tolerance. Clinical examination on presentation revealed a regular pulse of 90/min with a blood pressure of 100/56 mmHg with a harsh systolic murmur at the apex of the heart. A 12 lead electrocardiogram showed anterior ST elevation myocardial infarction (Figure 1) and blood tests revealed a significantly elevated serum troponin. Patient received thrombolysis and antiplatelet agents and was transferred to our hospital.
Figure 1.
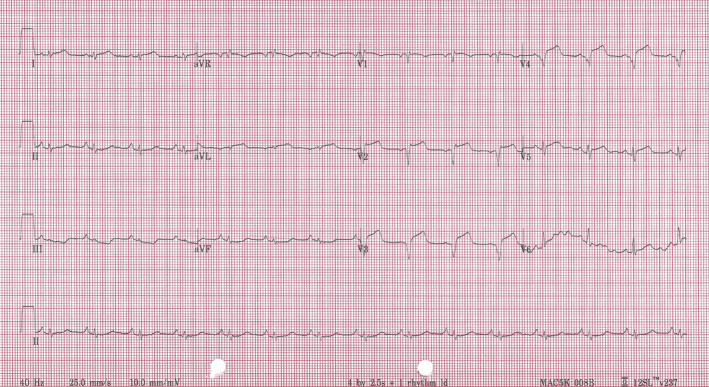
ECG showing ST elevation anterior myocardial infarction.
Patient underwent urgent coronary angiogram which revealed multivessel coronary artery disease, and an occluded left anterior descending artery unsuitable for intervention (Figure 2).
Figure 2.
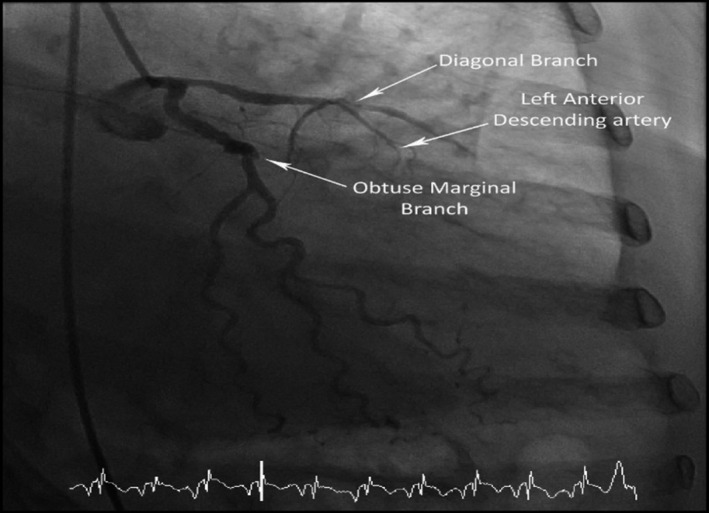
A coronary angiogram demonstrating an occluded LAD.
A transthoracic echocardiogram demonstrated large anterior and anteroseptal area of severe hypokinesis and ruptured interventricular septum with moderately severe left to right shunt via an apical ventricular septal defect (VSD; Figure 3).
Figure 3.
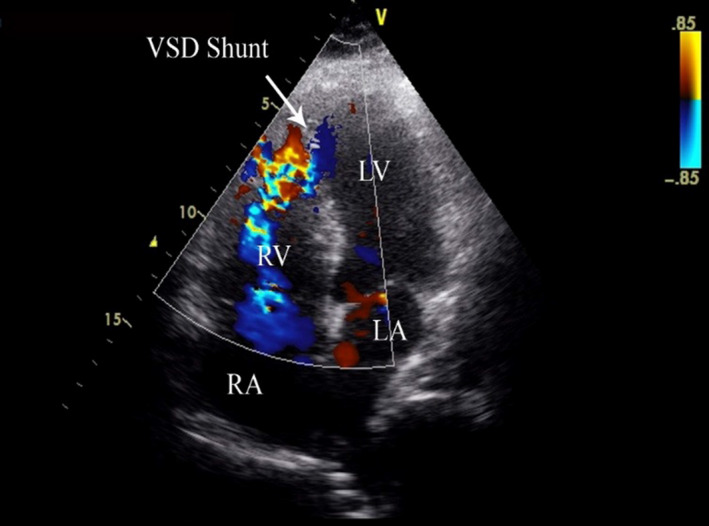
TTE showing an apical ventricular septal defect with left to right shunt.
A rapid deterioration in clinical condition with cardiogenic shock and acute kidney injury occurred requiring insertion of an intraaortic balloon pump (IABP) for haemodynamic support. The patient was urgently transferred to the operating theatre for rescue VSD repair. A large apico–anterior left ventricular infarct was found on sternotomy. The septum was approached by a linear transinfarct left ventriculostomy. The anterior two‐thirds of interventicular septum was found to be necrotic with a double orifice apical septal tear located a third of the way up from the apex. An infarct exclusion technique with a bovine pericardial patch was used to repair the VSD. The patient was transferred to the intensive care unit with IABP and inotrope support.
A progressive deterioration with oliguric renal failure and ischaemic hepatitis occurred following the surgical repair. A repeat transthoracic echocardiography revealed biventricular systolic impairment with stroke volume of 28 mL measured in left ventricular outflow tract (LVOT) along with a residual distal VSD with left to right shunt at the site of pericardial repair. A decision for re‐do VSD repair was made in view of ongoing multiorgan failure. The intraoperative findings revealed a segment of the pericardial patch to have partially dehisced posteriorly away from the infarcted tissue. Another patch was sutured over the infarcted septal area and then sandwiched into the left ventriculostomy incision suture line. At the end of the procedure an intraoperative transoesophageal echocardiography confirmed no residual VSD.
The patient continued to deteriorate in spite of aggressive organ support in intensive care. A transoesophageal echocardiography at this time revealed a residual post‐exclusion left ventricular cavity with a very small end‐diastolic volume <30 mL as measured by Simpson's technique. A normal ejection fraction of 70% was reported, however the forward stroke volume was noted to be only 21 mL on Doppler measurements in LVOT. The excluded LV cavity was akinetic and was partially filled with clot (Figure 4).
Figure 4.
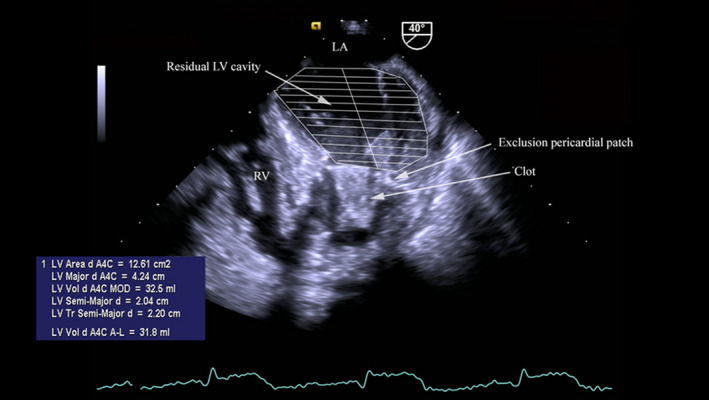
TOE showing the excluded and functional LV cavities and LVEDV.
A small dehiscence of the pericardial patch adjacent to the lateral LV wall was noted resulting in bidirectional shunt between two LV cavities. A small residual shunt was also seen between the LV exclusion cavity and RV (Figure 5).
Figure 5.
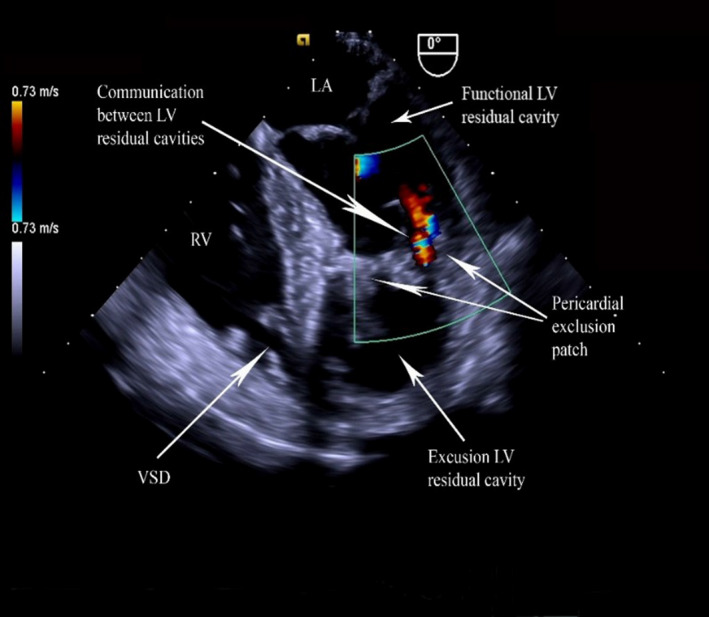
TOE showing bidirectional flow between the cavities.
A significant portion of the SV was being diverted into the residual excluded LV cavity (calculated SV index 9 mL/m2). Patient continued to deteriorate with severe global hypoperfusion and irreversible shock leading to death.
Discussion
Ventricular septal defect is an uncommon but well recognised complication of acute myocardial infarction and is associated with poor prognosis.1 Medical and interventional management alone often is inadequate to achieve haemodynamic stability leading to a surgical rescue intervention. The development of shock is the most important predictor of poor survival.2, 3
Surgical repair is difficult and can be complicated by recurrent VSD, reported to be between 10% and 40% due to perioperative failure of repair.4 While early surgical intervention is associated with a higher risk of mortality,5, 6 it remains the best therapeutic option in a clinical setting of cardiogenic shock. Several surgical procedures are used in an attempt to correct acute septal rupture, which includes infarctectomy, reconstruction of septum and ventricular walls with Dacron fabric or bovine patches and left ventricular exclusion techniques.
The LV exclusion technique utilises bovine pericardial patch sutured to the intact peri‐infarct epicardium, separating the infarcted part of the ventricular cavity from the circulation. A large myocardial infarction leaves little intact endocardial surface for optimal suturing of the exclusion patch, subjecting it to a very high risk of dehiscence.
It is important to remember that ejection fraction is derived as a factor of stroke volume from end‐diastolic volume. In a large proportion of non‐critically ill patients, it represents a reasonable surrogate measurement for adequacy of circulation but not in patients with shock. Hence in severe shock states, cardiac output measured as a calculation of stroke volume times the heart rate is a more reliable physiological quantitative parameter, a true reflection of circulatory status and global end organ perfusion.
This case illustrates how reported high left ventricular ejection fraction can be misleading when used in a context of an anatomically small left ventricular cavity, a unique phenomenon of ‘low cardiac output normal ejection fraction cardiogenic shock’. Hence it is important to use indexed SV parameters to reflect haemodynamic status.
References
- 1.Poulsen SH, Praestholm M, Munk K, Wierup P, Egeblad H, Nielsen‐Kudsk JE. Ventricular septal rupture complicating acute myocardial infarction: clinical characteristics and contemporary outcome. Ann Thorac Surg 2008; 85: 1591–6. [DOI] [PubMed] [Google Scholar]
- 2.Bolooki H. Emergency cardiac procedures in patients in cardiogenic shock due to complications of coronary artery disease. Circulation 1989; 79(6 Pt 2): I137–48. [PubMed] [Google Scholar]
- 3.Labrousse L, Choukroun E, Chevalier JM, Madonna F, Robertie F, Merlico F, et al. Surgery for post infarction ventricular septal defect (VSD): risk factors for hospital death and long term results. Eur J Cardiothorac Surg 2002; 21: 725–32. [DOI] [PubMed] [Google Scholar]
- 4.Deja MA, Szostek J, Widenka K, Szafron B, Spyt TJ, Hickey MS, et al. Post infarction ventricular septal defect – can we do better? Eur J Cardiothorac Surg 2000; 18: 194–201. [DOI] [PubMed] [Google Scholar]
- 5.Jones MT, Schofield PM, Dark JF, Moussalli H, Deiraniya AK, Lawson RA, et al. Surgical repair of acquired ventricular septal defect. Determinants of early and late outcome. J Thorac Cardiovasc Surg 1987; 93: 680–6, 11. [PubMed] [Google Scholar]
- 6.Blanche C, Khan SS, Matloff JM, Chaux A, DeRobertis MA, Czer LS, et al. Results of early repair of ventricular septal defect after an acute myocardial infarction. J Thorac Cardiovasc Surg 1992; 104: 961–5. [PubMed] [Google Scholar]


