Abstract
Obstructive sleep apnea (OSA) and chronic obstructive pulmonary disease (COPD) are common chronic diseases. These two noncommunicable diseases (NCDs) are prevalent among approximately 10% of the general population. Approximately 1% of the population is affected by the co‐existence of both conditions, known as the overlap syndrome (OS). OS patients suffer from greater degrees of nocturnal oxygen desaturation and cardiovascular consequences than those with either condition in isolation. Besides OS, patients with COPD may suffer from a spectrum of sleep‐related breathing disorders, including hypoventilation and central sleep apnea. The article provides an overview of the pathogenesis, associated risk factors, prevalence, and management of sleep‐related breathing disorders in COPD. It examines respiratory changes during sleep caused by COPD and OSA. It elaborates upon the factors that link the two conditions together to lead to OS. It also discusses the clinical evaluation and diagnosis of these patients. Subsequently, it reviews the pathophysiological basis and the current evidence for three potential therapies: positive airway pressure therapy [including continuous positive airway pressure (CPAP) and bilevel positive airway pressure], oxygen therapy, and pharmacological therapy. It also proposes a phenotypic approach toward the diagnosis and treatment of OS and the entire spectrum of sleep‐related breathing disorders in COPD. It concludes with the current evidence gaps and future areas of research in the management of OS.
Keywords: COPD, OSA, overlap syndrome, PAP therapy, phenotype, sleep‐disordered breathing
1. INTRODUCTION
Human beings spend one‐third of their lifetimes in sleep, which is essential for restoration of physical and mental functions of the body.1 However, an estimated 45% of the world's population experiences issues with sleep that affect their health and quality of life.2 Obstructive sleep apnea (OSA) and chronic obstructive pulmonary disease (COPD) and are common chronic diseases, both affected by and affecting sleep. These two noncommunicable diseases (NCDs) are prevalent among approximately 10% of the general population each and are gradually increasing in prevalence worldwide.3, 4 Approximately 1% of the population is affected by the co‐existence of both conditions, known as the overlap syndrome (OS).3
In this manuscript, we will review the respiratory changes during sleep in normal individuals as well as the pathophysiological changes during sleep in OSA and COPD. We will examine the factors that link the two conditions together to lead to OS. We will discuss the prevalence, costs, pathogenesis, risk factors, and clinical evaluation of OS and other sleep‐related breathing disorders (SRBD) in COPD. Subsequently, we will review the pathophysiological basis and the current evidence for three potential therapies: positive airway pressure (PAP) therapy, oxygen therapy, and pharmacological therapy. We will also propose a phenotype‐guided to approach to the management of these patients. Finally, we will identify evidence gaps and future areas of research in the management of OS.
2. SLEEP AND BREATHING
Sleep and the respiratory system are intimately linked. A myriad of changes effect respiration during various phases of normal human sleep. These respiratory changes during sleep bear no adverse consequences for a healthy individual, however they can be detrimental for patients with disease states including obesity, chronic respiratory, and cardiac disease. The physiological changes during sleep affect the neural and chemical control of respiration, the respiratory muscles, and the upper airways.
First, there is diminished neural input to the brainstem respiratory centers from the higher centers during sleep which lessens the respiratory drive.5 This is associated with a fall in the ventilation and consequential blood gas changes: a slight fall in the arterial partial pressure of oxygen (PaO2) and a slight rise in the arterial partial pressure of carbon dioxide (PaCO2).6 This leads to an enhanced dependency on the chemoreceptors for chemical control of respiration in response to blood gas changes.7 Secondly, the activity of the non‐diaphragm respiratory muscles (i.e., the accessory muscles) is diminished during sleep and may be completely absent during rapid eye movement (REM) sleep.8 Finally, the muscle tone of the pharyngeal dilator muscles which maintain the upper airway patency is reduced during sleep, especially during REM sleep.9
3. OBSTRUCTIVE SLEEP APNEA (OSA)
OSA is the most common SRBD. OSA causes episodic collapse in the upper airway while sleeping resulting in momentary cessation or attenuation of breathing.10 OSA affects an estimated 936 million adults aged 30–69 years old globally, of whom an estimated 425 million have moderate to severe disease.11 Obesity is a major risk factor for OSA.12 OSA is also a major risk factor for other NCDs, notably cardiovascular disease (CVD), metabolic disorders, hypertension, depression, and stroke.10, 12, 13, 14, 15, 16
The economic burden of OSA is high.15, 17 In 2015, the estimated annual cost of OSA for USA was US $12.4 billion.11 A study of the Danish National Patient Registry found that OSA resulted in annual health care costs 2.8 times higher than the average annual health cost for up to 12 years before OSA diagnosis, compared with control subjects of comparable age‐, sex‐ and socioeconomic indicators.17, 18 OSA which is untreated or diagnosed late can double medical expenses due to comorbidity with CVD.17 An estimated 80% of individuals with OSA are diagnosed late, even if they have access to health care.16 In a US study, the upper third with most severe cases of OSA and comorbidities consumed 65–82% of all OSA patients’ costs.17
3.1. Respiratory changes during sleep in OSA
The number of obstructive events (apneas or hypopneas) occurring per hour of sleep is referred to as the apnea‐hypopnea index (AHI), with a value greater than 5 events/hour usually denoting a diagnosis of OSA. The most important risk factor for OSA is obesity which leads to fat deposition in the neck structures with narrowing of the upper airway. Hence, when the pharyngeal dilator muscles become hypotonic during sleep, there is an increased tendency for the upper airway to collapse.9 These episodic obstructive events lead to transient oxygen desaturations or “intermittent hypoxemia.”19
Patients with more severe obesity have heavier loads imposed upon the respiratory muscles on account of the thicker chest wall. Simultaneously, the diaphragm is compressed by the abdominal fat leading to pulmonary restriction.20 These factors may lead to profound hypoventilation during sleep, when there is reduced respiratory drive and respiratory muscle activity. Furthermore, obesity is associated with resistance to leptin. Resistance to this hormone has been implicated in reduced neural drive leading to central hypoventilation.21 Hence, patients with severe obesity and SRBD may develop persistent hypercapnia termed as the obesity hypoventilation syndrome (OHS).
4. CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD)
COPD refers to respiratory disease due to noxious stimuli which is characterized by irreversible or incompletely reversible airflow obstruction. The primary cause of COPD is first‐ or second‐hand tobacco smoke. Additional environmental risk factors include exposure to indoor and outdoor pollution, dust, and hazardous fumes.22 According to the Global Disease Burden Study of 2017, an estimated 272 million people worldwide are afflicted with COPD.23 In 2017, COPD led to 3.2 million deaths worldwide.23 Over 90% of deaths from COPD are in low‐ and middle‐income countries.22 In 2017, COPD was seventh leading cause of years of life lost. By 2040, it is expected to be the fourth leading cause.23 Patients with COPD may suffer from a spectrum of SRBD, including OSA, sleep‐related hypoventilation, and central sleep apnea (CSA).
4.1. Respiratory changes during sleep in COPD
In patients with COPD, the respiratory changes during sleep underlie a spectrum of SRBD. COPD is not a homogenous disease entity and it may manifest with varying degrees of chronic bronchitis and emphysema.4 Among patients with predominant emphysema, there is pre‐existing downward displacement of the diaphragm which is worsened during sleep. This puts the diaphragm at a mechanical disadvantage for breathing which is compensated using the accessory muscles during the awake state. However, during sleep (especially REM stage) there is diminished function of the accessory muscles, which may worsen hypoventilation in these patients.24 This results in frequent awakenings, reduced sleep efficiency and quality, and reduced REM sleep in COPD patients.25 Thus, patients with COPD have sustained hypoxemia during sleep due to a combination of ventilation‐perfusion mismatch and hypoventilation.26
5. OVERLAP SYNDROME (OS)
OS refers to the co‐existence of the OSA and COPD in the same patient.27 The co‐existence of the two conditions due to chance alone can yield a population prevalence of 1%.4 Bednarek et al showed the prevalence of OS to be 1% in a population study and concluded that neither OSA predisposes for COPD, nor vice versa.3 Other epidemiological studies have produced high variable results, possibly due to confounding risk factors in the studied samples. For instance, the prevalence of OSA in COPD patients has varied from 11% to 45%.28, 29, 30, 31 On the other hand, the prevalence of COPD in OSA patients has ranged from 9% to 41% in different studies.3, 31, 32, 33 It is also emphasized that definitions of OSA and COPD have varied between studies. Different apnea hypopnea index (AHI) cutoffs have been used to diagnose OSA in these studies.
Patients with COPD with predominant emphysema and severe airflow obstruction may be protected against developing OSA. This is because the hyperinflated emphysematous lungs reduce the collapsibility of the upper airways by a caudal traction effect.34 Patients with severe COPD and emphysema also have lower body mass indices (BMIs), which is also protective against OSA.35 In contrast, patients with relatively mild COPD who have a higher BMI tend to develop OSA, leading to the OS at a younger age.28, 34, 36 These patients may be heavy smokers with higher cumulation of pack‐years, which contributes to upper airway inflammation and OSA.30, 37 Furthermore, they often develop right heart failure at an earlier age. These patients experience rostral fluid shifts at night which worsens obstructive events due to edema of neck structures.38
Hence, the complex interaction between sleep, body‐mass index, and COPD may either prevent or promote development of OS. Further, OS is only one manifestation of an entire spectrum of potential SRBD in COPD patients. Specifically, depending on the relative severity of obesity and emphysema, the patients may develop hypercapnia due to obesity hypoventilation syndrome (OHS) or sleep‐related hypoventilation, respectively. Finally, patients with COPD may be at increased risk of left ventricular failure which is a known precipitant of central sleep apnea (CSA).39 There is a paucity of literature which has examined the prevalence of CSA in COPD or OS patients.
5.1. Clinical consequences of overlap syndrome
Both COPD and OSA can lead to respiratory and blood gas disturbances. OSA and COPD have each been individually linked with metabolic syndrome and cardiovascular morbidity.40, 41 In comparison with either OSA or COPD alone, OS patients have been found to have a worse quality of life,42 greater nocturnal hypoxemia,3 and a higher risk of pulmonary hypertension,43, 44 cardiovascular events,45 and mortality.46, 47 OS patients also have higher burdens of systemic hypertension, diabetes mellitus, and obesity compared to COPD alone. OS patients have higher levels of pro‐inflammatory mediators and more metabolic risk factors, and consequentially an increased susceptibility for cardiovascular disease.48, 49 In one large cohort, Kendzerska et al observed that COPD patients with nocturnal hypoxemia (who spent at least 10% of their total sleep time at an SpO2 below 90%) were found to have increased cardiac events (myocardial infarction, stroke, congestive heart failure, revascularization, or death) compared to patients with neither COPD nor OSA.45 In this cohort, untreated patients with COPD and severe OSA had the highest hazard; they were twice as likely to experience a cardiac event compared with patients with neither illness.
6. CLINICAL EVALUATION FOR OVERLAP SYNDROME
OS is a treatable condition, which makes prompt diagnosis and initiation of therapy essential. Common symptoms of OSA include snoring, daytime sleepiness, nocturnal choking, and unrestful sleep. Nonetheless, the finding of daytime sleepiness is not universal, and patients may instead present with daytime fatigue.50 Hence, questionnaires which assess sleepiness, such as the Epworth Sleepiness Scale (ESS) and the Berlin Questionnaire (BQ), may be inaccurate in predicting OSA in COPD patients.51 Furthermore, it has been found that OSA worsens quality of life in patients with COPD even in the absence of daytime sleepiness.42 The important physical examination findings which may suggest the possibility of OSA include obesity, increased neck circumference, and hypertension.
In a recent guideline, the American Thoracic Society (ATS) has promulgated the use of the STOPBANG (snoring, tiredness, observed apnea, high blood pressure, high BMI, age, neck circumference, and male gender) questionnaire to screen for OSA in COPD patients with chronic hypercapnic respiratory failure.52 Although direct evidence for the performance of the STOPBANG questionnaire in COPD is absent, it has been found to be the most sensitive screening tool for OSA overall with a pooled sensitivity of 0.93.53 The utility of the STOPBANG tool has been examined in a post‐hoc analysis of the Long‐Term Oxygen Treatment Trial (LOTT). The LOTT failed to demonstrate benefit of long‐term oxygen in COPD patients with borderline (89 – 93%), exercise‐related or nocturnal hypoxemia.54 The post‐hoc analysis has revealed that in this cohort, patients with intermediate‐to‐high risk for OSA (STOPBANG score ≥3) had an increased risk for mortality, hospitalizations, and acute exacerbations.55 This emphasizes the need for further studying the role of OSA screening and subsequent positive airway pressure (PAP) therapy in this patient population.56
In OSA patients, suspicion of OS should be considered in patients with significant smoking history and prominent respiratory symptoms. Patients with OS have greater nocturnal hypoxemia compared to those with OSA alone.3 Hence, OS should be suspected in patients who require either unanticipated oxygen during PAP titration or a bilevel PAP prescription.57 Pulmonary function testing using spirometry is required to confirm the diagnosis of COPD.
7. DIAGNOSIS OF OVERLAP SYNDROME
There is no formal guidance for the indications of performing a sleep study in patients with COPD. Extrapolating from the ATS guidelines for hypercapnic COPD patients, it is reasonable to consider diagnostic testing in all COPD patients with an intermediate‐to‐high risk for OSA using the STOPBANG questionnaire.52 Furthermore, COPD patients with pulmonary hypertension and borderline or nocturnal hypoxemia can be considered for sleep study. In‐lab attended polysomnography with PAP titration is the gold standard for the diagnosis and treatment of OS. It is desirable to non‐invasively record the PaCO2 to capture hypoventilation events and guide PAP titration. Transcutaneous CO2 monitoring is the preferred device for non‐invasive PaCO2 monitoring during sleep and unlike end‐tidal CO2 monitors, it does not impede PAP titration using a mask interface.58
Portable and home sleep apnea testing (HSAT) may have adequate sensitivity to diagnose OSA in COPD.59, 60 However, current guidelines recommend against use of HSAT in patients with significant chronic respiratory disease due to the following shortcomings.61 First, HSAT is unable to identify hypoventilation as a cause for hypoxemia in these patients, which may lead to inappropriate oxygen therapy or incorrect PAP prescription. Secondly, HSAT is often combined with auto‐PAP therapy. This may be followed by an inappropriate increase in expiratory positive airway pressure (EPAP) level which can worsen hyperinflation and lung function in COPD (see below).62, 63 The use of newer algorithms for auto‐titrating PAP therapy in OS remains an area of active research.64
8. PHYSIOLOGICAL BASIS OF POSITIVE AIRWAY PRESSURE (PAP) THERAPY
PAP therapy delivered via a mask interface is a non‐invasive externally applied pressure on the patient's airway. The two common modes of PAP used in OS are CPAP and bilevel positive airway pressure (BPAP). In CPAP, a constant pressure is applied which mechanically splints open the patient's upper airway and thereby prevents its collapse. It is the most common mode of PAP used for the therapy of OSA.65 BPAP employs two different levels of PAP during the breathing cycle: a higher level during inspiration, that is, the inspiratory PAP (IPAP); and a lower level during expiration, i.e., the expiratory PAP (EPAP). The difference between the IPAP and the EPAP supports the patient's inspiration by augmenting the tidal volume and providing rest to the respiratory muscles.65 Hence, BPAP is a form of non‐invasive ventilation (NIV). It is particularly useful in patients with hypoventilation due to overloaded respiratory muscles. The choice between CPAP and BPAP therapy can be illustrated with the example of OHS. Herein, randomized controlled trial (RCT) data has shown that patients with coexistent severe OSA (AHI >30 events/hour) derive similar benefit from CPAP and BPAP because the upper airway obstruction is the dominant pathophysiological abnormality and is corrected with CPAP.66 Contrarily, in OHS patients without severe OSA, hypoventilation is the dominant abnormality, and hence BPAP has been employed successfully to improve blood gases, sleepiness, and quality of life.67 On occasion, CPAP failure in OHS with severe OSA may also require use of BPAP therapy.
The use of PAP therapy in patients with COPD requires two important considerations. First, patients with emphysema phenotype suffer from expiratory airflow limitation, air trapping, and hyperinflation. This results in an inward chest recoil pressure at the end of expiration. This is known as the intrinsic or auto‐positive end expiratory pressure (auto‐PEEP).68 Consequently, the patient needs to overcome the auto‐PEEP prior to each inspiration. In the awake state, patients adapt using pursed lip breathing and prolonged expiration to minimize auto‐PEEP.69 However, during sleep the expiratory time is shortened leading to dynamic hyperinflation. The use of modest amounts of applied PEEP in the form of CPAP or EPAP may negate the auto‐PEEP, support ventilation and reduce muscle fatigue. CPAP of 8 cm H2O has been shown to reduce hyperinflation in stable COPD.70 However, if the applied PEEP is more than the auto‐PEEP, it may impose an additional expiratory load, thereby deterring ventilation. CPAP greater than 10 cm H2O may worsen hyperinflation.62, 63 In a study of OS patients, use of mean CPAP pressure of 11 cm H2O resulted in worsening of lung function.31
Secondly, patients with emphysema‐predominant COPD may have a downward displaced diaphragm which is mechanically disadvantaged. When combined with accessory muscle paralysis in REM sleep, this may result in sleep‐related hypoventilation.71 Such patients may have poor sleep quality with daytime fatigue and chronic hypercapnia. COPD patients with chronic hypercapnic respiratory failure are at increased risk of mortality. Often, these patients do not have OSA as defined by the AHI cut off because of the protective influence of emphysema on upper airway collapsibility.34 Hence, CPAP may not be useful in this phenotype. Rather, the use of BPAP, which supports ventilation in these patients, may reduce risk of mortality and hospitalizations with improved dyspnea and quality of life.52 Recently, trials have used high IPAP settings in these patients with a back‐up respiratory rate to achieve greater minute ventilation with an aim to normalize the PaCO2. This approach, termed as high‐intensity NIV has been shown to improve blood gases in physiological studies and is under further investigation.72, 73, 74
9. EVIDENCE FOR THE USE OF PAP THERAPY IN OVERLAP SYNDROME
To date, only observational studies of PAP therapy in OS patients have been conducted. PAP therapy in OS patients has been found to reduce pro‐inflammatory markers implicated in cardiovascular disease, including C‐reactive protein (CRP) and tumor necrosis factor‐α.75, 76 has been linked with physiological benefits in OS including improved arterial blood gases (reduced PaCO2 and increased PaO2),28, 32, 77 6‐minute walk distance,78 forced expiratory volume in 1 s (FEV1),32, 79 respiratory muscle strength,80 skeletal muscle strength,80 exercise capacity,80 and mean pulmonary artery pressure.81
Patients who are adherent to PAP therapy have been found to have reduced COPD exacerbations,82 COPD‐related hospitalizations,46, 83, 84 cardiovascular events,45 and mortality.46, 82, 85, 86 Most of these studies employed CPAP. Patients with emphysema have been found to have lower CPAP adherence compared with obese patients with daytime sleepiness.87
OS is amenable to phenotyping. Figure 1 shows that patients with COPD may suffer from a spectrum of SRBD, the exact nature of which depends on the relative severity of the emphysema and obesity. Patients with emphysema have higher sleep‐related hypoventilation due to the mechanically disadvantaged downwardly displaced diaphragm, but they are protected against OSA because of low BMI and caudal traction on the upper airways. In contrast, patients with obesity tend to have predominant OSA or OHS. Not shown in the figure, patient may also have co‐existent CSA, especially if there is co‐existent heart failure or opioid use. This figure is not to exact scale as the epidemiological studies of relative frequencies of different phenotypes have not been performed.
FIGURE 1.
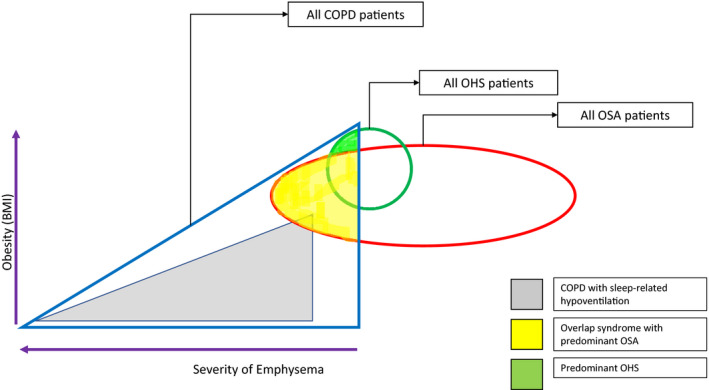
Proposed phenotypic classification of sleep‐related breathing disorders in Chronic Obstructive Pulmonary Disease. BMI, body mass index, COPD, chronic obstructive pulmonary disease, OSA, obstructive sleep apnea, OHS, obesity hypoventilation syndrome.
Currently, there is no formal guidance for the use of PAP therapy in patients with COPD and SRBD. In the absence of well‐designed RCTs, our understanding is limited to large observational studies and extrapolations of evidence from related conditions such as OSA, COPD with chronic hypercapnia and OHS. We have presented a decision‐tree of a proposed phenotype‐based management algorithm of SRBD in COPD (Figure 2).
FIGURE 2.
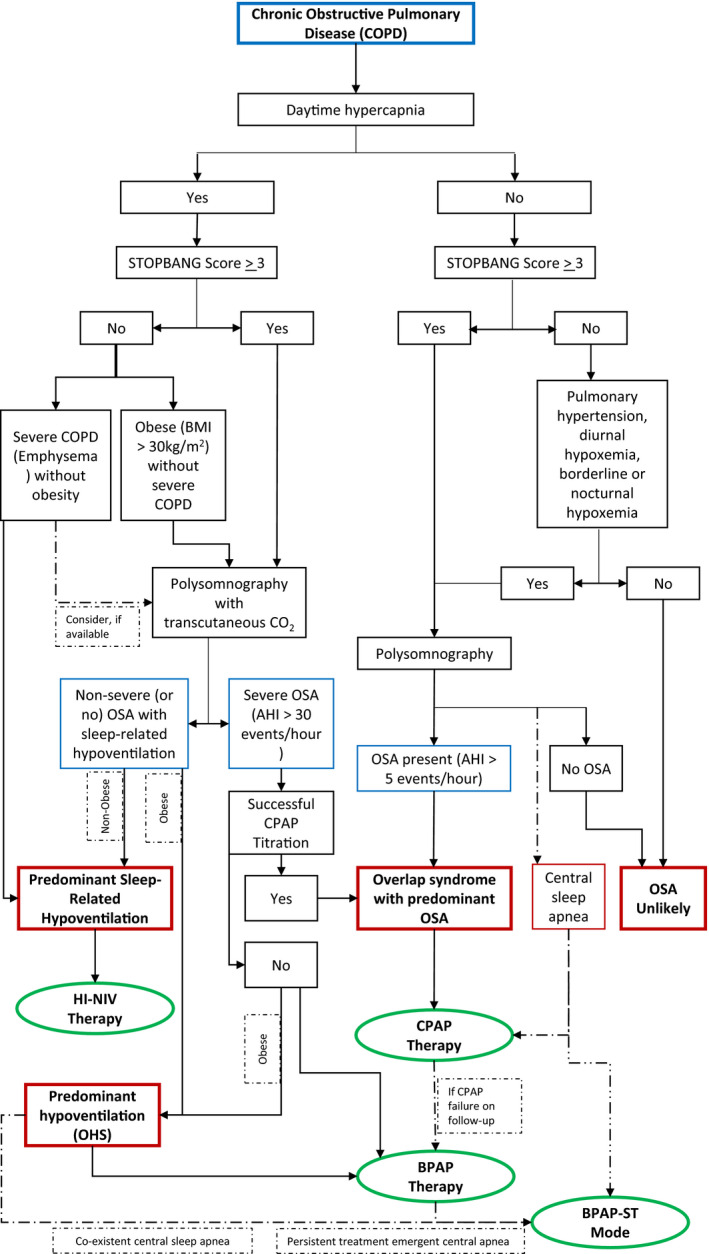
A phenotype‐guided approach toward the diagnosis and positive airway pressure therapy of sleep‐related breathing disorders with Chronic Obstructive Pulmonary Disease. BMI, body mass index; BPAP, bilevel positive airway pressure; COPD, chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure; high blood pressure, high BMI, age, neck circumference and male gender; NIV, non‐invasive ventilation; OHS, obesity hypoventilation syndrome; OSA, obstructive sleep apnea; STOPBANG, snoring, tiredness, observed apnea.
Figure 2 shows that, in patients of COPD with suspected SRBD, the decision for performing an in‐lab polysomnography depends on the presence of diurnal hypercapnia, risk for OSA (e.g., STOPBANG questionnaire), and pulmonary hypertension. In addition, patients with borderline or nocturnal hypoxemia may be potential candidates for diagnostic testing. Polysomnography can help in identifying the presence and the phenotype of SRBD in COPD patients. The choice between CPAP, BPAP or high‐intensity NIV depends on whether the predominant phenotype is OSA, OHS, or sleep‐related hypoventilation due to COPD, respectively. Additionally, the patient may have co‐existent CSA, especially if they have comorbid heart failure or opioid use. The use of CPAP or BPAP with ST mode may be considered for CSA.
10. OTHER THERAPIES FOR OVERLAP SYNDROME
10.1. Oxygen therapy
PAP therapy in OS has been shown to increase daytime oxygen saturation and may obviate need for oxygen in patients with borderline hypoxemia.32, 77, 79 As a corollary, inappropriate long‐term oxygen therapy in patients with borderline hypoxemia and OS may increase mortality, hospitalizations, and acute exacerbations.55 Supplementary oxygen therapy (with target SpO2 of 88 – 92%) should be considered in patients with OS who continue to have hypoxemia despite PAP therapy. However, in this situation, presence of hypoventilation during titration study may aid in choosing BPAP therapy over supplementary oxygen. However, head‐to‐head comparisons of BPAP versus CPAP with supplementary oxygen have not been made.88
10.2. Pharmacological therapy
The use of PAP therapy in OS has not been shown to reduce the need for bronchodilators in COPD.89 Long‐acting beta‐2 agonists (LABA) and long‐acting muscarinic antagonists (LAMA) should be continued as per COPD management guidelines. Both LABA and LAMA have been shown to increase nocturnal oxygen saturation without improving sleep quality.90, 91 The impact of inhaled corticosteroids (ICS) is more contentious. One study has shown that ICS may improve AHI, nocturnal hypoxemia, daytime PaCO2, and lung function via airway anti‐inflammatory effects.92 However, others have suggested that ICS may predispose to myopathy which may worsen upper airway collapsibility leading to OSA.88 Further, ICS is neither used as monotherapy nor as a first‐line therapy in COPD.84 Hence, the effects of ICS in OS need to be further studied before any conclusion may be drawn. The use of sedatives and opioids should be avoided in COPD patients due to the risk of worsening central hypoventilation or CSA.93 There are no studies examining the use of respiratory stimulants such as acetazolamide in OS. Their use has been shown to improve oxygenation without benefit in clinical outcomes in COPD. Further, acetazolamide may potentially cause harm (e.g., worsening respiratory acidosis in severe COPD).94 Hence, respiratory stimulants are not currently recommended in OS.
11. RECENT TRENDS AND FUTURE DIRECTIONS
The current definition of OS is restrictive and fails to acknowledge the entire spectrum of sleep‐disordered breathing which may be encountered in patients with COPD. No studies to date have examined the occurrence of CSA in COPD patients despite plausible pathophysiological links.95 Hence, it is imperative to conduct studies to characterize the prevalence of OSA, CSA, OHS, and pure hypoventilation related to COPD in this population. This will enable us to design trials for phenotype‐directed therapeutic modes of PAP therapy. Furthermore, the role of in‐lab polysomnography for NIV titration in COPD patients with chronic hypercapnic respiratory failure needs to be further examined.52
Although PAP therapy is currently the standard therapy for OS, there are no clinical trials which have examined its effects on clinical outcomes in this population. An ongoing trial is examining the effect of non‐invasive ventilation in overlap syndrome (NCT03184714). A randomized trial is studying the impact of early diagnosis and treatment of OSA in COPD patients requiring hospitalizations in preventing readmission (NCT03647462). Another randomized trial is comparing the effect of CPAP and BPAP therapy in correcting blood gases in hypercapnic OS patients (NCT03766542). A randomized trial is studying the non‐inferiority of home‐initiation of NIV versus hospitalized titration in OS (NCT02363413). Once the results of these trials are available, they will shed light on important aspects of PAP therapy for OS.
In the recent INOX trial, the application of nocturnal oxygen failed to reduce mortality or progression to long‐term oxygen therapy in COPD patients with nocturnal hypoxemia.96 However, the presence of sleep‐related hypoventilation in such patients cannot be excluded. Hence, the role of diagnosis and therapy of SRBD in COPD patients with borderline and nocturnal hypoxemia is another potential research area.
Finally, newer modes of non‐invasive ventilation need to be studied in OS. Volume‐assured pressure support (VAPS) with fixed or auto‐EPAP has been found to be effective in chronic hypoventilation in COPD patients in small studies.97 It has potential to assure adequate minute ventilation despite varying ventilatory drive and patient effort in different stages and postures of sleep. A novel non‐invasive mode specifically tailored for OS is the auto‐trilevel PAP. This mode employs a lower EPAP at beginning of expiration to counteract auto‐PEEP without causing dynamic hyperinflation. It uses a higher EPAP at end expiration, when upper airway collapsibility is most likely. In a pilot physiological study, it was superior to BPAP in improving AHI, nocturnal hypoxemia, sleep efficiency and daytime sleepiness.98
12. CONCLUSION
Sleep leads to changes in the respiratory function which bear no adverse impact on a healthy person but may be detrimental to patients with underlying respiratory diseases such as COPD. OSA and COPD are on the rise globally, and their co‐existence as OS can impair quality of life, worsen respiratory failure, and increase risk of pulmonary hypertension, cardiovascular events, or death compared to either disease alone. Further, COPD may be associated with a spectrum of SRBD including sleep‐related hypoventilation and CSA. Diagnosis requires high index of suspicion and an in‐lab polysomnography. A phenotype‐based approach of selecting PAP therapy which is tailored to correct the pathophysiology of SRBD demonstrates potential to improve clinical outcomes. To strengthen the evidence base, additional research is needed in the form of well‐designed clinical trials which use the phenotypic approach to the management of OS and SRBD in COPD.
CONFLICTS OF INTEREST
There are no conflicts of interest.
AUTHOR CONTRIBUTIONS
TMS and JCS designed the review, wrote, and edited the manuscript.
Suri TM, Suri JC. A review of therapies for the overlap syndrome of obstructive sleep apnea and chronic obstructive pulmonary disease. FASEB BioAdvances. 2021;3:683–693. 10.1096/fba.2021-00024
This article is part of the Global Voices for Prevention of Noncommunicable Diseases Special Collection.
REFERENCES
- 1.Eugene AR, Masiak J. The neuroprotective aspects of sleep. MEDtube Sci. 2015;3(1):35‐40. [PMC free article] [PubMed] [Google Scholar]
- 2.Wade A, Zisapel N, Lemoine P. Prolonged‐release melatonin for the treatment of insomnia: targeting quality of sleep and morning alertness. Aging Health. 2008;4(1):11‐21. 10.2217/1745509X.4.1.11. [DOI] [Google Scholar]
- 3.Bednarek M, Plywaczewski R, Jonczak L, Zielinski J. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population study. Respir Int Rev Thorac Dis. 2005;72(2):142‐149. 10.1159/000084044. [DOI] [PubMed] [Google Scholar]
- 4.Celli BR, Wedzicha JA. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med. 2019;381(13):1257‐1266. 10.1056/NEJMra1900500. [DOI] [PubMed] [Google Scholar]
- 5.Phillipson EA. Control of breathing during sleep. Am Rev Respir Dis. 1978;118(5):909‐939. 10.1164/arrd.1978.118.5.909. [DOI] [PubMed] [Google Scholar]
- 6.Stradling JR, Chadwick GA, Frew AJ. Changes in ventilation and its components in normal subjects during sleep. Thorax. 1985;40(5):364‐370. 10.1136/thx.40.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dempsey JA, Smith CA, Blain GM, Xie A, Gong Y, Teodorescu M. Role of central/peripheral chemoreceptors and their interdependence in the pathophysiology of sleep apnea. Adv Exp Med Biol. 2012;758:343‐349. 10.1007/978-94-007-4584-1_46. [DOI] [PubMed] [Google Scholar]
- 8.Tabachnik E, Muller NL, Bryan AC, Levison H. Changes in ventilation and chest wall mechanics during sleep in normal adolescents. J Appl Physiol. 1981;51(3):557‐564. 10.1152/jappl.1981.51.3.557. [DOI] [PubMed] [Google Scholar]
- 9.Jordan AS, White DP. Pharyngeal motor control and the pathogenesis of obstructive sleep apnea. Respir Physiol Neurobiol. 2008;160(1):1‐7. 10.1016/j.resp.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorasamy P. Obstructive sleep apnea and cardiovascular risk. Ther Clin Risk Manag. 2007;3(6):1105‐1111. [PMC free article] [PubMed] [Google Scholar]
- 11.Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature‐based analysis. Lancet Respir Med. 2019;7(8):687‐698. 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osman AM, Carter SG, Carberry JC, Eckert DJ. Obstructive sleep apnea: current perspectives. Nat Sci Sleep. 2018;10:21‐34. 10.2147/NSS.S124657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lebkuchen A, Freitas LS, Cardozo KHM, Drager LF. Advances and challenges in pursuing biomarkers for obstructive sleep apnea: implications for the cardiovascular risk. Trends Cardiovascular Med. 2021;31(4):242‐249 [DOI] [PubMed] [Google Scholar]
- 14.Salman LA, Shulman R, Cohen JB. Obstructive sleep apnea, hypertension, and cardiovascular risk: epidemiology, pathophysiology, and management. Curr Cardiol Rep. 2020;22(2):6. 10.1007/s11886-020-1257-y. [DOI] [PubMed] [Google Scholar]
- 15.Knauert M, Naik S, Gillespie MB, Kryger M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J Otorhinolaryngol ‐ Head Neck Surg. 2015;1(1):17‐27. 10.1016/j.wjorl.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding Q, Kryger M. Greater health care utilization and cost associated with untreated sleep apnea. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2020;16(1):5‐6. 10.5664/jcsm.8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarasiuk A, Reuveni H. The economic impact of obstructive sleep apnea. Curr Opin Pulm Med. 2013;19(6):639‐644. 10.1097/MCP.0b013e3283659e1e. [DOI] [PubMed] [Google Scholar]
- 18.Jennum P, Ibsen R, Kjellberg J. Social consequences of sleep disordered breathing on patients and their partners: a controlled national study. Eur Respir J. 2014;43(1):134‐144. 10.1183/09031936.00169212. [DOI] [PubMed] [Google Scholar]
- 19.Garvey JF, Taylor CT, McNicholas WT. Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur Respir J. 2009;33(5):1195‐1205. 10.1183/09031936.00111208. [DOI] [PubMed] [Google Scholar]
- 20.Mafort TT, Rufino R, Costa CH, Lopes AJ. Obesity: systemic and pulmonary complications, biochemical abnormalities, and impairment of lung function. Multidiscip Respir Med. 2016;11:28. 10.1186/s40248-016-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phipps PR, Starritt E, Caterson I, Grunstein RR. Association of serum leptin with hypoventilation in human obesity. Thorax. 2002;57(1):75‐76. 10.1136/thorax.57.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chronic obstructive pulmonary disease (COPD). 2021. https://www.who.int/news‐room/fact‐sheets/detail/chronic‐obstructive‐pulmonary‐disease‐(copd). Accessed February 13.
- 23.Viegi G, Maio S, Fasola S, Baldacci S. Global burden of chronic respiratory diseases. J Aerosol Med Pulm Drug Deliv. 2020;33(4):171‐177. 10.1089/jamp.2019.1576. [DOI] [PubMed] [Google Scholar]
- 24.Johnson MW, Remmers JE. Accessory muscle activity during sleep in chronic obstructive pulmonary disease. J Appl Physiol. 1984;57(4):1011‐1017. 10.1152/jappl.1984.57.4.1011. [DOI] [PubMed] [Google Scholar]
- 25.McSharry DG, Ryan S, Calverley P, Edwards JC, McNicholas WT. Sleep quality in chronic obstructive pulmonary disease. Respirol Carlton Vic. 2012;17(7):1119‐1124. 10.1111/j.1440-1843.2012.02217.x. [DOI] [PubMed] [Google Scholar]
- 26.Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis. 2011;6:199‐208. 10.2147/COPD.S10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med. 1985;6(4):651‐661. [PubMed] [Google Scholar]
- 28.Schreiber A, Cemmi F, Ambrosino N, Ceriana P, Lastoria C, Carlucci A. Prevalence and predictors of obstructive sleep apnea in patients with chronic obstructive pulmonary disease undergoing inpatient pulmonary rehabilitation. COPD. 2018;15(3):265‐270. 10.1080/15412555.2018.1500533. [DOI] [PubMed] [Google Scholar]
- 29.Macrea M, Campbell S, Martin T, Oursler KA. The peripheral neutrophils in subjects with COPD‐OSA overlap syndrome and severe comorbidities: a feasible inflammatory biomarker? Adv Clin Exp Med Off Organ Wroclaw Med Univ. 2018;27(12):1677‐1682. 10.17219/acem/75904. [DOI] [PubMed] [Google Scholar]
- 30.Steveling EH, Clarenbach CF, Miedinger D, et al. Predictors of the overlap syndrome and its association with comorbidities in patients with chronic obstructive pulmonary disease. Respir Int Rev Thorac Dis. 2014;88(6):451‐457. 10.1159/000368615. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien A, Whitman K. Lack of benefit of continuous positive airway pressure on lung function in patients with overlap syndrome. Lung. 2005;183(6):389‐404. 10.1007/s00408-005-2551-6. [DOI] [PubMed] [Google Scholar]
- 32.de Miguel J, Cabello J, Sánchez‐Alarcos JMF, Alvarez‐Sala R, Espinós D, Alvarez‐Sala JL. Long‐term effects of treatment with nasal continuous positive airway pressure on lung function in patients with overlap syndrome. Sleep Breath Schlaf Atm. 2002;6(1):3‐10. 10.1007/s11325-002-0003-6. [DOI] [PubMed] [Google Scholar]
- 33.López‐Acevedo MN, Torres‐Palacios A, Elena Ocasio‐Tascón M, Campos‐Santiago Z, Rodríguez‐Cintrón W. Overlap syndrome: an indication for sleep studies?: a pilot study. Sleep Breath Schlaf Atm. 2009;13(4):409‐413. 10.1007/s11325-009-0263-5. [DOI] [PubMed] [Google Scholar]
- 34.Krachman SL, Tiwari R, Vega ME, et al. Effect of emphysema severity on the apnea‐hypopnea index in smokers with obstructive sleep apnea. Ann Am Thorac Soc. 2016;13(7):1129‐1135. 10.1513/AnnalsATS.201511-765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Z, Yang D, Ge Z, Yan M, Wu N, Liu Y. Body mass index of patients with chronic obstructive pulmonary disease is associated with pulmonary function and exacerbations: a retrospective real world research. J Thorac Dis. 2018;10(8):5086‐5099. 10.21037/jtd.2018.08.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong MQ, Hu WH, Hu K, et al. Analysis of risk factors and consequences for concurrent obstructive sleep apnea in chronic obstructive pulmonary disease patients. Zhonghua Jie He He Hu Xi Za Zhi Zhonghua Jiehe He Huxi Zazhi Chin J Tuberc Respir Dis. 2019;42(11):832‐837. 10.3760/cma.j.issn.1001-0939.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Lin Y‐N, Li Q‐Y, Zhang X‐J. Interaction between smoking and obstructive sleep apnea: not just participants. Chin Med J (Engl). 2012;125(17):3150‐3156. [PubMed] [Google Scholar]
- 38.White LH, Bradley TD. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J Physiol. 2013;591(5):1179‐1193. 10.1113/jphysiol.2012.245159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Miguel DJ, Chancafe Morgan J, Jiménez GR. The association between COPD and heart failure risk: a review. Int J Chron Obstruct Pulmon Dis. 2013;8:305‐312. 10.2147/COPD.S31236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin H‐L, Yin S‐Q, Lin Q‐Y, Xu Y, Xu H‐W, Liu T. Prevalence of comorbidities in chronic obstructive pulmonary disease patients: a meta‐analysis. Medicine (Baltimore). 2017;96(19):e6836. 10.1097/MD.0000000000006836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong J‐Y, Zhang Y‐H, Qin L‐Q. Obstructive sleep apnea and cardiovascular risk: meta‐analysis of prospective cohort studies. Atherosclerosis. 2013;229(2):489‐495. 10.1016/j.atherosclerosis.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 42.Mermigkis C, Kopanakis A, Foldvary‐Schaefer N, et al. Health‐related quality of life in patients with obstructive sleep apnoea and chronic obstructive pulmonary disease (overlap syndrome). Int J Clin Pract. 2007;61(2):207‐211. 10.1111/j.1742-1241.2006.01213.x. [DOI] [PubMed] [Google Scholar]
- 43.Sun W‐L, Wang J‐L, Jia G‐H, et al. Impact of obstructive sleep apnea on pulmonary hypertension in patients with chronic obstructive pulmonary disease. Chin Med J (Engl). 2019;132(11):1272‐1282. 10.1097/CM9.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawryłkiewicz I, Pałasiewicz G, Pływaczewski R, Sliwiński P, Zieliński J. Effects of nocturnal desaturation on pulmonary hemodynamics in patients with overlap syndrome (chronic obstructive pulmonary disease and obstructive sleep apnea). Pneumonol Alergol Pol. 2000;68(1–2):37‐43. [PubMed] [Google Scholar]
- 45.Kendzerska T, Leung RS, Aaron SD, Ayas N, Sandoz JS, Gershon AS. Cardiovascular outcomes and all‐cause mortality in patients with obstructive sleep apnea and chronic obstructive pulmonary disease (overlap syndrome). Ann Am Thorac Soc. 2019;16(1):71‐81. 10.1513/AnnalsATS.201802-136OC. [DOI] [PubMed] [Google Scholar]
- 46.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325‐331. 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 47.Du W, Liu J, Zhou J, Ye D, OuYang Y, Deng Q. Obstructive sleep apnea, COPD, the overlap syndrome, and mortality: results from the 2005–2008 National Health and Nutrition Examination Survey. Int J Chron Obstruct Pulmon Dis. 2018;13:665‐674. 10.2147/COPD.S148735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Archontogeorgis K, Voulgaris A, Papanas N, et al. Metabolic syndrome in patients with coexistent obstructive sleep apnea syndrome and chronic obstructive pulmonary disease (overlap syndrome). Metab Syndr Relat Disord. 2020;18(6):296‐301. 10.1089/met.2019.0126. [DOI] [PubMed] [Google Scholar]
- 49.Zhou W, Li C‐L, Cao J, Feng J. Metabolic syndrome prevalence in patients with obstructive sleep apnea syndrome and chronic obstructive pulmonary disease: Relationship with systemic inflammation. Clin Resp J. 2020;14(12):1159‐1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Budhiraja R, Siddiqi TA, Quan SF. Sleep disorders in chronic obstructive pulmonary disease: etiology, impact, and management. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2015;11(3):259‐270. 10.5664/jcsm.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faria AC, da Costa CH, Rufino R. Sleep apnea clinical score, berlin questionnaire, or epworth sleepiness scale: which is the best obstructive sleep apnea predictor in patients with COPD? Int J Gen Med. 2015;8:275‐281. 10.2147/IJGM.S86479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macrea M, Oczkowski S, Rochwerg B, et al. Long‐term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease. an official american thoracic society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(4):e74‐e87. 10.1164/rccm.202006-2382ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiu H‐Y, Chen P‐Y, Chuang L‐P, et al. Diagnostic accuracy of the Berlin questionnaire, STOP‐BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta‐analysis. Sleep Med Rev. 2017;36:57‐70. 10.1016/j.smrv.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Long‐Term Oxygen Treatment Trial Research Group , Albert RK, Au DH, et al. A randomized trial of long‐term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375(17):1617‐1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donovan LM, Feemster LC, Udris EM, et al. Poor outcomes among patients with chronic obstructive pulmonary disease with higher risk for undiagnosed obstructive sleep apnea in the LOTT cohort. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2019;15(1):71‐77. 10.5664/jcsm.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis CA, Fergusson W, Eaton T, Zeng I, Kolbe J. Isolated nocturnal desaturation in COPD: prevalence and impact on quality of life and sleep. Thorax. 2009;64(2):133‐138. 10.1136/thx.2007.088930. [DOI] [PubMed] [Google Scholar]
- 57.Shetty S, Fernandes A, Patel S, Combs D, Grandner MA, Parthasarathy S. Unanticipated nocturnal oxygen requirement during positive pressure therapy for sleep apnea and medical comorbidities. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2017;13(1):73‐79. 10.5664/jcsm.6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Storre JH, Magnet FS, Dreher M, Windisch W. Transcutaneous monitoring as a replacement for arterial PCO(2) monitoring during nocturnal non‐invasive ventilation. Respir Med. 2011;105(1):143‐150. 10.1016/j.rmed.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Jen R, Orr JE, Li Y, et al. Accuracy of WatchPAT for the diagnosis of obstructive sleep apnea in patients with chronic obstructive pulmonary disease. COPD. 2020;17(1):34‐39. 10.1080/15412555.2019.1707789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang Y, Xu L, Han F, et al. Validation of the Nox‐T3 portable monitor for diagnosis of obstructive sleep apnea in patients with chronic obstructive pulmonary disease. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2019;15(4):587‐596. 10.5664/jcsm.7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep Apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2017;13(3):479‐504. 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lim TK. Effect of nasal‐CPAP on patients with chronic obstructive pulmonary disease. Singapore Med J. 1990;31(3):233‐237. [PubMed] [Google Scholar]
- 63.Holanda MA, Fortaleza SCB, Alves‐de‐Almeida M, et al. Continuous positive airway pressure effects on regional lung aeration in patients with COPD: a high‐resolution CT scan study. Chest. 2010;138(2):305‐314. 10.1378/chest.09-2850. [DOI] [PubMed] [Google Scholar]
- 64.Murphy PB, Arbane G, Ramsay M, et al. Safety and efficacy of auto‐titrating noninvasive ventilation in COPD and obstructive sleep apnoea overlap syndrome. Eur Respir J. 2015;46(2):548‐551. 10.1183/09031936.00205714. [DOI] [PubMed] [Google Scholar]
- 65.Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an american academy of sleep medicine systematic review, meta‐analysis, and GRADE assessment. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2019;15(2):301‐334. 10.5664/jcsm.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Masa JF, Mokhlesi B, Benítez I, et al. Long‐term clinical effectiveness of continuous positive airway pressure therapy versus non‐invasive ventilation therapy in patients with obesity hypoventilation syndrome: a multicentre, open‐label, randomised controlled trial. The Lancet. 2019;393(10182):1721‐1732. 10.1016/S0140-6736(18)32978-7. [DOI] [PubMed] [Google Scholar]
- 67.Masa JF, Corral J, Caballero C, et al. Non‐invasive ventilation in obesity hypoventilation syndrome without severe obstructive sleep apnoea. Thorax. 2016;71(10):899‐906. 10.1136/thoraxjnl-2016-208501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marini JJ. Dynamic hyperinflation and auto‐positive end‐expiratory pressure. Am J Respir Crit Care Med. 2011;184(7):756‐762. 10.1164/rccm.201102-0226PP. [DOI] [PubMed] [Google Scholar]
- 69.Ku S‐C. It’s time to reappraise the impact of Auto‐PEEP. Respir Care. 2016;61(2):258‐259. 10.4187/respcare.04658. [DOI] [PubMed] [Google Scholar]
- 70.Lopes AJ, Nery FP, Sousa FC, et al. CPAP decreases lung hyperinflation in patients with stable COPD. Respir Care. 2011;56(8):1164‐1169. 10.4187/respcare.01092. [DOI] [PubMed] [Google Scholar]
- 71.White JE, Drinnan MJ, Smithson AJ, Griffiths CJ, Gibson GJ. Respiratory muscle activity during rapid eye movement (REM) sleep in patients with chronic obstructive pulmonary disease. Thorax. 1995;50(4):376‐382. 10.1136/thx.50.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dreher M, Storre JH, Schmoor C, Windisch W. High‐intensity versus low‐intensity non‐invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax. 2010;65(4):303‐308. 10.1136/thx.2009.124263. [DOI] [PubMed] [Google Scholar]
- 73.Windisch W, Haenel M, Storre JH, Dreher M. High‐intensity non‐invasive positive pressure ventilation for stable hypercapnic COPD. Int J Med Sci. 2009;6(2):72‐76. 10.7150/ijms.6.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Köhnlein T, Windisch W, Köhler D, et al. Non‐invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2(9):698‐705. 10.1016/S2213-2600(14)70153-5. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Su M, Zhang X. Effects of continuous positive airway pressure treatment of inflammatory factors in patients with overlap syndrome. Zhonghua Yi Xue Za Zhi. 2014;94(6):416–419. 10.3760/CMA.J.ISSN.0376-2491.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 76.Nural S, Günay E, Halici B, Celik S, Ünlü M. Inflammatory processes and effects of continuous positive airway pressure (CPAP) in overlap syndrome. Inflammation. 2013;36(1):66‐74. 10.1007/s10753-012-9520-z. [DOI] [PubMed] [Google Scholar]
- 77.Lacedonia D, Carpagnano GE, Aliani M, et al. Daytime PaO2 in OSAS, COPD and the combination of the two (overlap syndrome). Respir Med. 2013;107(2):310‐316. 10.1016/j.rmed.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 78.Wang T‐Y, Lo Y‐L, Lee K‐Y, et al. Nocturnal CPAP improves walking capacity in COPD patients with obstructive sleep apnoea. Respir Res. 2013;14:66. 10.1186/1465-9921-14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mansfield D, Naughton MT. Effects of continuous positive airway pressure on lung function in patients with chronic obstructive pulmonary disease and sleep disordered breathing. Respirol Carlton Vic. 1999;4(4):365‐370. 10.1046/j.1440-1843.1999.00206.x. [DOI] [PubMed] [Google Scholar]
- 80.Nowiński A, Bieleń P, Jonczak L, Sliwiński P. Influence of treatment with continuous positive airway pressure on respiratory muscle function and physical fitness in patients with obstructive sleep apnoea and overlap syndrome. Pneumonol Alergol Pol. 2007;75(1):46‐56. [PubMed] [Google Scholar]
- 81.Toraldo DM, De Nuccio F, Nicolardi G. Fixed‐pressure nCPAP in patients with obstructive sleep apnea (OSA) syndrome and chronic obstructive pulmonary disease (COPD): a 24‐month follow‐up study. Sleep Breath Schlaf Atm. 2010;14(2):115‐123. 10.1007/s11325-009-0291-1. [DOI] [PubMed] [Google Scholar]
- 82.Jaoude P, El‐Solh AA. Predictive factors for COPD exacerbations and mortality in patients with overlap syndrome. Clin Respir J. 2019;13(10):643‐651. 10.1111/crj.13079. [DOI] [PubMed] [Google Scholar]
- 83.Konikkara J, Tavella R, Willes L, Kavuru M, Sharma S. Early recognition of obstructive sleep apnea in patients hospitalized with COPD exacerbation is associated with reduced readmission. Hosp Pract. 2016;44(1):41‐47. 10.1080/21548331.2016.1134268. [DOI] [PubMed] [Google Scholar]
- 84.Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Resp J. 2019;53(5):1900164. [DOI] [PubMed] [Google Scholar]
- 85.Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W, Malhotra A. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2013;9(8):767‐772. 10.5664/jcsm.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jaoude P, Kufel T, El‐Solh AA. Survival benefit of CPAP favors hypercapnic patients with the overlap syndrome. Lung. 2014;192(2):251‐258. 10.1007/s00408-014-9555-z. [DOI] [PubMed] [Google Scholar]
- 87.Theerakittikul T, Hatipoğlu U, Aboussouan LS. Hyperinflation on chest radiograph as a marker of low adherence to positive airway pressure therapy in the overlap syndrome. Respir Care. 2014;59(8):1267‐1274. 10.4187/respcare.03011. [DOI] [PubMed] [Google Scholar]
- 88.Malhotra A, Schwartz AR, Schneider H, et al. Research priorities in pathophysiology for sleep‐disordered breathing in patients with chronic obstructive pulmonary disease. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med. 2018;197(3):289‐299. 10.1164/rccm.201712-2510ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aro M, Saaresranta T, Vahlberg T, Anttalainen U. Medication of comorbidities in females with sleep‐disordered breathing during long‐term CPAP therapy. Respir Med. 2020;169:106014. 10.1016/j.rmed.2020.106014. [DOI] [PubMed] [Google Scholar]
- 90.Ryan S, Doherty LS, Rock C, Nolan GM, McNicholas WT. Effects of salmeterol on sleeping oxygen saturation in chronic obstructive pulmonary disease. Respir Int Rev Thorac Dis. 2010;79(6):475‐481. 10.1159/000235619. [DOI] [PubMed] [Google Scholar]
- 91.McNicholas WT, Calverley PMA, Lee A, Edwards JC. Tiotropium sleep study in COPD investigators. Long‐acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPD. Eur Respir J. 2004;23(6):825‐831. 10.1183/09031936.04.00085804. [DOI] [PubMed] [Google Scholar]
- 92.Rezaeetalab F, Rezaeitalab F, Dehestani V. Inhaled steroids reduce apnea‐hypopnea index in overlap syndrome. Pneumol Buchar Rom. 2013;62(4):212‐214. [PubMed] [Google Scholar]
- 93.Baillargeon J, Singh G, Kuo Y‐F, Raji MA, Westra J, Sharma G. Association of opioid and benzodiazepine use with adverse respiratory events in older adults with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2019;16(10):1245‐1251. 10.1513/AnnalsATS.201901-024OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adamson R, Swenson ER. Acetazolamide use in severe chronic obstructive pulmonary disease. pros and cons. Ann Am Thorac Soc. 2017;14(7):1086‐1093. 10.1513/AnnalsATS.201701-016FR. [DOI] [PubMed] [Google Scholar]
- 95.McNicholas WT, Hansson D, Schiza S, Grote L. Sleep in chronic respiratory disease: COPD and hypoventilation disorders. Eur Respir Rev Off J Eur Respir Soc. 2019;28(153): 10.1183/16000617.0064-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lacasse Y, Sériès F, Corbeil F, et al. Randomized trial of nocturnal oxygen in chronic obstructive pulmonary disease. N Engl J Med. 2020;383(12):1129‐1138. 10.1056/NEJMoa2013219. [DOI] [PubMed] [Google Scholar]
- 97.McArdle N, Rea C, King S, et al. Treating chronic hypoventilation with automatic adjustable versus fixed EPAP Intelligent Volume‐Assured Positive Airway Pressure Support (iVAPS): a randomized controlled trial. Sleep. 2017;40(10): 10.1093/sleep/zsx136. [DOI] [PubMed] [Google Scholar]
- 98.Su M, Huai DE, Cao J, et al. Auto‐trilevel versus bilevel positive airway pressure ventilation for hypercapnic overlap syndrome patients. Sleep Breath Schlaf Atm. 2018;22(1):65‐70. 10.1007/s11325-017-1529-y. [DOI] [PubMed] [Google Scholar]


