Abstract
It is clear that excessive mucosal immune activation and intestinal barrier dysfunction both contribute to inflammatory bowel disease (IBD) pathogenesis. T cell protein tyrosine phosphatase (TCPTP), which extinguishes signaling in immune cells, is linked to IBD and other immune-mediated diseases. In this issue of the JCI, Marchelletta and Krishnan et al. demonstrate that, in intestinal epithelial cells, TCPTP regulates tight junction permeability in vivo. Intestinal epithelial TCPTP loss potentiated cytokine-induced barrier loss, and this synergized with effects of TCPTP loss in immune cells. This work implicates a single mutation as the cause of distinct functional aberrations in diverse cell types and demonstrates how one genetic defect can drive multihit disease pathogenesis.
TCPTP expression may regulate immune cell activation
Inflammatory bowel disease (IBD) has been associated with over 240 genetic polymorphisms. In most cases, these gene variants are insufficient to cause disease, and their relative contributions to pathogenesis are difficult to assess. Large genome-wide association studies have linked many SNPs and associated genes to multiple immune-mediated diseases. The protein tyrosine phosphatase non-receptor type 2 (PTPN2) gene has been linked to Crohn’s disease, ulcerative colitis, type I diabetes, and rheumatoid arthritis (1). In adults, PTPN2 polymorphisms increase the risk of IBD only modestly (odds ratio, approximately 1.35), but a recent report implicating a PTPN2 mutation in a patient with very early onset IBD suggests that the effects may be much greater in some contexts (2).
PTPN2 encodes T cell protein tyrosine phosphatase (TCPTP), which dampens JAK/STAT pathway activation (3). Because JAK/STAT pathways intersect with many other signaling events, TCPTP regulates diverse processes in many cell types. Consequences of TCPTP loss include unrestrained T cell receptor signaling, exaggerated cytokine responses, increased MAPK signaling, excessive growth factor receptor activation, and defective autophagosome formation (4, 5). The observation that TCPTP expression is upregulated in response to IFN-γ but also attenuates IFN-γ signaling (3, 6) suggests that increased TCPTP expression in inflammatory diseases, including IBD, may be a critical mechanism of regulating immune cell activation. Consistently, TCPTP deletion in T cells causes severe, systemic autoimmune disease in mice (5).
TCPTP in intestinal barrier regulation
In this issue of the JCI, Marchelletta and Krishnan et al. focus on the role of TCPTP in intestinal barrier regulation (7). Disease-associated human PTPN2 polymorphisms result in loss of TCPTP function, making Ptpn2 WT, knockout, and heterozygous mice a useful system. Both heterozygous and knockout mice showed markedly increased permeability to 4 kDa dextran, relative to WT mice. In contrast, the barrier to 70 kDa dextran was maintained (7). These results implicate increased flux across the low-capacity paracellular leak pathway, accommodating macromolecules up to approximately 125 Å in diameter, and exclude epithelial damage as the source of barrier loss (8). The leak pathway is regulated by myosin light chain kinase, which phosphorylates myosin II regulatory light chain to trigger endocytosis of the tight junction protein occludin (9, 10). Consistent with this process, Marchelletta and Krishnan et al. found that myosin II regulatory light chain phosphorylation was increased and occludin was internalized in epithelial cells of Ptpn2-knockout mice (also referred to as Tcptp-deficient mice). Although not explored here, it is likely that myosin light chain kinase expression was also increased (11, 12).
Marchelletta and Krishnan et al. also demonstrated increased expression of the pore-forming tight junction protein claudin-2 in Ptpn2-knockout mice. Claudin-2 may contribute to the increased transmucosal conductance, i.e., reduced resistance, measured in the distal ileum and cecum of Ptpn2-knockout mice, but cannot explain increased 4 kDa dextran flux, as claudin-2 channels exclude molecules larger than water and monovalent cations. The observed claudin-2 upregulation may be secondary to increased mucosal IL-13 and IL-22, each of which promotes claudin-2 transcription, were increased within intestinal mucosae of Ptpn2-knockout mice. In addition to these cytokine-mediated effects, Marchelletta and Krishnan et al. considered the possibility that TCPTP loss in epithelial cells could directly amplify STAT signaling and claudin-2 expression. To pursue this possibility, the authors developed intestinal epithelial-specific Ptpn2-knockout mice (also called TCPTP-deficient mice). In the absence of exogenous insults, intestinal epithelial STAT signaling and claudin-2 expression were upregulated in these mice, supporting the conclusion that epithelial TCPTP loss contributes to disease (7).
The complex in vivo milieu makes it difficult to definitively demonstrate epithelium-intrinsic effects of TCPTP mutation. Marchelletta and Krishnan et al. turned to a stably expressed dominant-negative TCPTPC126S mutant in human intestinal epithelial cells. This mutant was sufficient to increase both claudin-2 expression and pore pathway permeability. siRNA blockade of claudin-2 expression partially corrected this transepithelial electrical resistance (TER) loss (7). TCPTP dysfunction is therefore sufficient to activate claudin-2 expression and increase permeability of the high-capacity, charge- and size-selective pore pathway via an epithelium-intrinsic process (8).
Analyses of tissues from intestinal epithelial-specific Ptpn2-knockout mice confirmed increased claudin-2 expression and reduced TER, but 4 kDa dextran permeability was unchanged. However, in vitro studies showed that both STAT1 activation and 4 kDa dextran permeability increases induced by IFN-γ were potentiated in TCPTPC126S-expressing epithelial cells, consistent with the role of TCPTP in extinguishing cytokine signaling (7). Hyperactivation of STAT and MAPK pathways in TCPTP-deficient epithelial cells is therefore likely to explain increased pore and leak pathway permeabilities by promoting transcription of claudin-2 and myosin light chain kinase, respectively (13, 14).
A vicious cycle that establishes disease
Why is permeability of the leak pathway increased in universal, but not intestinal epithelial-specific, Ptpn2-knockout mice? One possibility is that epithelial TCPTP dysfunction leads to increased claudin-2 expression and pore pathway permeability that, secondarily, triggers mucosal immune cell activation (Figure 1). In TCPTP-deficient immune cells, cytokine release is excessive (15, 16). Cytokine signaling is exaggerated in TCPTP-deficient epithelial cells and leads to excessive leak pathway permeability increases that ultimately feed back onto a hyperactive mucosal immune system, cause further cytokine signaling and barrier loss, and create a vicious cycle that establishes disease. This model is supported by the enhanced mucosal immune activation observed in mice with intestinal epithelial claudin-2 overexpression and increased pore pathway permeability as well as the modest, microbiota-driven mucosal immune activation that occurs in mice with intestinal epithelial myosin light chain kinase hyperactivation and increased leak pathway permeability (16–18).
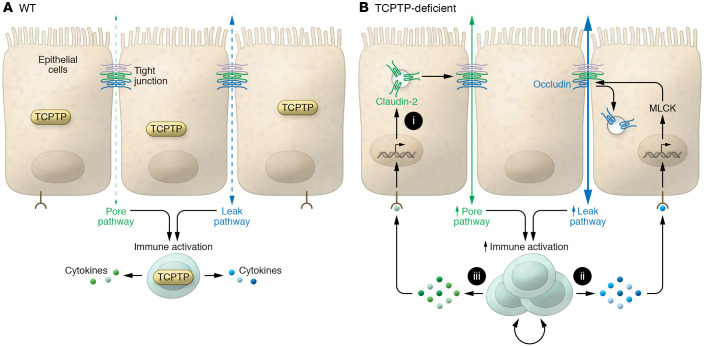
Figure 1. A cycle of barrier loss and mucosal immune hyperactivation.
(A) WT cells that express TCPTP are able to effectively dampen immune responses to limit changes in flux across the pore pathway (hatched green arrow), permeable to sodium and water, and the leak pathway (hatched blue arrow), permeable to larger molecules, including proteins and polysaccharides. (B) TCPTP loss in intestinal epithelial cells leads to epithelium-intrinsic signaling that upregulates claudin-2 expression (i). The resulting claudin-2–related increases in pore pathway (solid green arrow) permeability trigger excessive cytokine secretion in TCPTP-deficient immune cells. This cytokine release activates myosin light chain kinase (MLCK) to cause occludin internalization and leak pathway (solid blue arrow) permeability increases (ii). The exaggerated immune responses may also cause further increases in claudin-2 expression (iii). These cytokine-induced changes in permeabilities of and flux across pore and leak pathways trigger further immune activation, thereby creating a vicious cycle that leads to disease. In this manner, dysfunction of a single protein, TCPTP, results in excessive signaling in both epithelial and immune cells in development of a dysregulated cycle that leads to disease.
Neither pore nor leak pathway permeability increases alone are sufficient to induce disease (15–18). We can therefore infer that disease in universal Ptpn2-knockout mice and humans with germline PTPN2 mutations requires events beyond those caused by epithelial TCPTP dysfunction. As described in the proposed model (Figure 1), these events likely include defective immunoregulation. Marchelletta and Krishnan et al. therefore provide insight into how modification of a single gene can trigger events in separate, but functionally synergistic, tissue types to generate phenotypes that could not be predicted by examining either tissue in isolation (7). This concept can be expanded to include synergistic effects of multiple mutations on the same or separate cell types, consistent with the polygenic nature of most immune-mediated disease.
Questions and insights
The work by Marchelletta and Krishnan et al. (7) is a substantial advance, but, as with any good study, the results also raise new questions. For example, if the deletion of Ptpn2 acts primarily through the hyperactivation of the JAK/STAT signaling pathways, do JAK/STAT inhibitors reverse this phenotype? Do pore and leak pathway permeability increases make distinct or overlapping contributions to immune activation? This work also underscores the perennial question that arises when considering immune activation and mucosal barrier loss, which is the chicken and which is the egg?
Overall, Marchelletta and Krishnan et al. have deftly separated pore and leak pathways and discovered that, via epithelium-intrinsic processes, claudin-2–mediated pore pathway permeability is increased when epithelial TCPTP function is perturbed in vivo (7). Leak pathway permeability increases require extrinsic stimuli, e.g., cytokines, whose effects are augmented in the absence of intact epithelial TCPTP. These findings shed light on the multiple mechanisms by which PTPN2 polymorphisms contribute to disease pathogenesis (7). The data may also provide insight into some of the relationships between intestinal barrier loss and IBD risk (19, 20).
Acknowledgments
Studies in the authors’ laboratory are supported by US Department of Defense (PR181271) and NIH grants (R01DK61931 and R01DK68271) to JRT and the Harvard Digestive Disease Center (NIH grant P30DK034854).
Version 1. 09/01/2021
Electronic publication
Footnotes
Conflict of interest: JRT is a cofounder of, and shareholder in, Thelium Therapeutics and has consulted for Entrinsic Bioscience.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(17):e151414. https://doi.org/10.1172/JCI151414.
Contributor Information
Yan Y. Sweat, Email: ysweat@bwh.harvard.edu.
Jerrold R. Turner, Email: jrturner@bwh.harvard.edu.
References
- 1.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parlato M, et al. Loss-of-function mutation in PTPN2 causes aberrant activation of JAK signaling Via STAT and very early onset intestinal inflammation. Gastroenterology. 2020;159(5):1968–1971. doi: 10.1053/j.gastro.2020.07.040. [DOI] [PubMed] [Google Scholar]
- 3.Simoncic PD, et al. The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr Biol. 2002;12(6):446–453. doi: 10.1016/S0960-9822(02)00697-8. [DOI] [PubMed] [Google Scholar]
- 4.van Vliet C, et al. Selective regulation of tumor necrosis factor-induced Erk signaling by Src family kinases and the T cell protein tyrosine phosphatase. Nat Immunol. 2005;6(3):253–260. doi: 10.1038/ni1169. [DOI] [PubMed] [Google Scholar]
- 5.Wiede F, et al. T cell protein tyrosine phosphatase attenuates T cell signaling to maintain tolerance in mice. J Clin Invest. 2011;121(12):4758–4774. doi: 10.1172/JCI59492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scharl M, et al. Protection of epithelial barrier function by the Crohn’s disease associated gene protein tyrosine phosphatase n2. Gastroenterology. 2009;137(6):2030–2040. doi: 10.1053/j.gastro.2009.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchelletta RR, et al. T cell protein tyrosine phosphatase protects intestinal barrier function by restricting epithelial tight junction remodeling. J Clin Invest. 2021;131(17):138230. doi: 10.1172/JCI138230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 9.Zolotarevsky Y, et al. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123(1):163–172. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 10.Marchiando AM, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189(1):111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blair SA, et al. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006;86(2):191–201. doi: 10.1038/labinvest.3700373. [DOI] [PubMed] [Google Scholar]
- 12.Wang F, et al. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology. 2006;131(4):1153–1163. doi: 10.1053/j.gastro.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber CR, et al. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285(16):12037–12046. doi: 10.1074/jbc.M109.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham WV, et al. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J Biol Chem. 2006;281(36):26205–26215. doi: 10.1074/jbc.M602164200. [DOI] [PubMed] [Google Scholar]
- 15.Tsai PY, et al. IL-22 upregulates epithelial Claudin-2 to drive diarrhea and enteric pathogen clearance. Cell Host Microbe. 2017;21(6):671–681.e4. doi: 10.1016/j.chom.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raju P, et al. Inactivation of paracellular cation-selective claudin-2 channels attenuates immune-mediated experimental colitis in mice. J Clin Invest. 2020;130(10):5197–5208. doi: 10.1172/JCI138697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su L, et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136(2):551–563. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edelblum KL, et al. The microbiome activates CD4 T-cell-mediated immunity to compensate for increased intestinal permeability. Cell Mol Gastroenterol Hepatol. 2017;4(2):285–297. doi: 10.1016/j.jcmgh.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turpin W, et al. Increased intestinal permeability is associated with later development of Crohn’s disease. Gastroenterology. 2020;159(6):2092–2100. doi: 10.1053/j.gastro.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Wyatt J, et al. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993;341(8858):1437–1439. doi: 10.1016/0140-6736(93)90882-H. [DOI] [PubMed] [Google Scholar]