Abstract
Background:
Hormonal therapy for menopause has been found to be the most efficacious treatment, but it may be associated with adverse effects in some of the women. Rheum rhaponticum root extract (”ERr 731”), which is available worldwide, is a natural, reliable, effective, and well-tolerated remedy for women in perimenopausal women with menopausal symptoms (MSs), but there is no Indian study demonstrating its efficacy, safety, and tolerability till date.
Objective:
This study aimed to evaluate the efficacy and safety of ERr 731 in alleviating MSs in perimenopausal Indian women.
Patients and Methods:
In this open-labeled prospective study, 129 perimenopausal women were treated with tablet containing 4 mg of Rr dried root extract once daily for 12 weeks. The Menopause Rating Scale (MRS) II score, endometrial thickness (ET), blood pressure, glycemic status, lipid profile, and high-sensitivity C-reactive protein (hs-CRP) level were periodically assessed and compared.
Results:
A significant reduction (67% by 12th week) in the mean MRS II score was observed from baseline till the end of 12 weeks (18.1; 95% confidence interval [CI]: 17.0–19.2; P < 0.001). A monotonic reduction in the mean total MRS II score over time was found (1.51 units/week; 95% CI: 1.42–1.60 units/week; P < 0.001) noticeable. There was a reduction in the mean ET from baseline till the end of 12 weeks, although the change was not significant. There were significant reductions in the mean fasting (6.3 mg/dl; 95% CI: 1.7–11.0 mg/dl; P = 0.008) and postprandial (6.3 mg/dl; 95% CI: 1.0–11.7; P = 0.021) blood glucose levels and glycated hemoglobin level (0.30%; 95% CI: 0.085–0.520; P = 0.007) at 12 weeks. No significant changes were noted in terms of blood pressure, lipid profile, and hs-CRP level. The drug was found to be safe.
Conclusion:
ERr 731 was well tolerated and was found to be efficacious and safe in alleviating MSs in Indian perimenopausal women.
Keywords: Estrogen, menopausal symptoms, perimenopausal, R. rhaponticum, VMS
INTRODUCTION
Menopause hormone therapy is the most efficient treatment for symptoms of acute climacteric syndrome and for efficient prevention of long-term estrogen deficiency.[1] The transition from perimenopausal state to menopause is often troublesome mainly because of menopausal symptoms (MSs) such as palpitations, restlessness or fidgety, fatigability, difficulty in concentration, forgetfulness, mood swings, headaches or migraines, or insomnia.[2] Further, these women also experience vasomotor symptoms (VMS) encompassing hot flushes and night sweats during this period.[3] MS and VMS are experienced by 63%–70% of western women[4] and nearly 60% of Indian women.[5]
MSs are related to a reduced estrogen level in the blood. Estrogen exerts its effects principally through two different receptors, estrogen receptor (ER)-α (ER-α) and-β (ER-β).[6] Hormone replacement therapy (HRT) is effective in the management of MS.[7] However, HRT carries several inherent risks, which may be associated with breast cancer, endometrial cancer, and venous thromboembolism in selected high-risk women who have family history of cancer and had a history of cancer.[7,8,9] Hence, alternative therapies devoid of such risks are looked for. Thus, it was of our interest to study the safety and efficacy of one such plant Rheum rhaponticum (Rr) which has shown beneficial effects for the treatment of MSs across the globe but has never been studied in India.
The plant Rr, commonly known as Siberian Rhubarb, originates from Central Asia. A special phytoisolate (”Rr”) obtained from this plant has been very well researched and introduced in clinical practice since 1950 in Germany for the treatment of MS.[10] A special root extract from this plant, referred to as “ERr 731,” has been used since 1993 to alleviate MS. The extract consists mainly of rhaponticin and desoxyrhaponticin, as well as small quantities of the aglycones trans-rhapontigenin and desoxyrhapontigenin.[2] The extract does not contain any estrogens, and therefore, its actions are independent of estrogen.[11]
ERr 731 (Estroease, Lupin Pharmaceuticals Ltd., India) is approved as an food for special medical purpose by the Food Safety and Standards Authority of India. Preclinical studies have demonstrated the safety of ERr 731, in terms of endometrial proliferation.[12] The clinical efficacy of ERr 731 has been shown in the western population.[2,11,13] However, this extract has not been studied yet in the Indian population. Hence, this study was performed to evaluate the efficacy and safety of ERr 731 in alleviating MS in perimenopausal Indian women. We herein report the results of interim analysis.
PATIENTS AND METHODS
Ethics
This study was performed with centralized approval from the ethics committee. Informed consent was obtained from all participants before enrollment. This study has adhered to the principles of the declaration of Helsinki.
Study design
This was a multicentric, single-arm, open-labeled, prospective interventional study conducted at 15 gynecology outpatient departments in various institutes in India from June 2020 to January 2021. The inclusion criteria were women of age 45–55 years having MS and VMS, those with a Menopause Rating Scale (MRS) II score[14,15] of >18 at the time of enrollment, and those with a history of irregular menstrual cycles in the preceding 12 months implying progression toward menopause. The exclusion criteria were participants on drugs interfering with menstruation, history of salpingo-oophorectomy or hysterectomy performed within the last 6 months, comorbid conditions (diabetes mellitus, cardiovascular disease(s), cancers, and other major comorbid conditions), history of regular menstrual cycles during the last 3 months before enrollment, history of treatment with HRT in the last 6 months, history of treatment of VM in the last 1 month, ongoing treatment with any selective ER modulator, presence of any organic cause of amenorrhea (dysgenesis, cervical stenosis, Asherman syndrome, etc.), pregnant or lactating women, women who were likely to be nonadherent to treatment, those who were unwilling to provide informed consent to participate in this study, and those who were otherwise deemed unfit by the investigator(s). All recruited women were administered ERr 731 enteric-coated tablets containing 4 mg of Rr dried root extract (Estroease, Lupin Ltd., India) once daily for 12 weeks.[16]
Assessment of outcome parameters
General examination (including blood pressure measurement), ultrasound examination of the endometrium for measuring the endometrial thickness (ET) performed on day 4–7 of menstrual cycle in women with a history of irregular menses, and laboratory investigations (serum lipid profile, fasting and postprandial (PP) blood glucose levels, glycated hemoglobin (HbA1c) level, and high-sensitivity C-reactive protein [hs-CRP] level) were performed in all recruited women at baseline and end of treatment (12 weeks). The MRS II scores were evaluated at baseline and weeks 4, 8, and 12; and mammography was performed only at baseline. All participants were monitored for adverse effects from the investigational drug.
Statistical analyses
For efficacy analysis, participants having complete results at every time point were included. For safety analysis, participants receiving at least one dose of the investigational drug were included. The continuous variables were compared by paired t-test or analysis of variance. All analyses were performed by SPSS version 22 (IBM Corp., Armonk, NY). P < 0.05 was considered statistically significant.
RESULTS
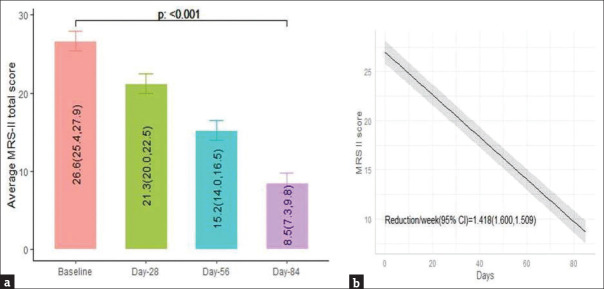
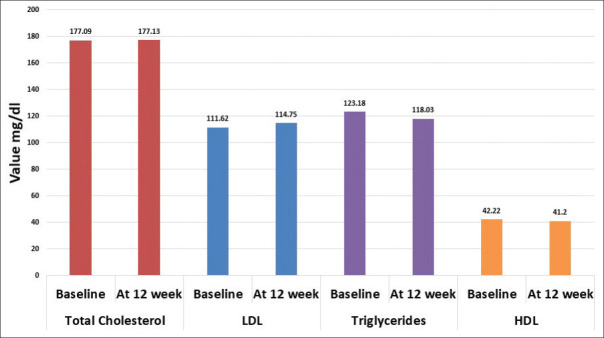
Across the centers, 129 women were identified, 108 were enrolled, 104 were active participants (4 were drop-outs), and 70 completed the full treatment [Figure 1]. A significant reduction in the mean MRS II score was observed from baseline till the end of 12 weeks (18.1; 95% confidence interval [CI]: 17.0–19.2; P < 0.001). The percentage reduction was 67.99%. A monotonic reduction in the mean total MRS II score over time following intervention was found (1.51 units/week; 95% CI: 1.42–1.60 units/week; P < 0.001) [Figure 2]. Significant changes in each item of the MRS II score over time were noted [Table 1 and Figure 3]. There was a reduction in the mean ET from baseline till the end of 12 weeks, although the change was not statistically significant. The results of mammography at baseline were normal in 63/70 participants. The remaining seven women did not provide their consent to undergo radiological assessment. The mean total cholesterol level was slightly increased, whereas the mean high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglyceride levels were decreased after 12 weeks of treatment than those values at baseline; however, the changes were not statistically significant [Table 2].
Figure 1.
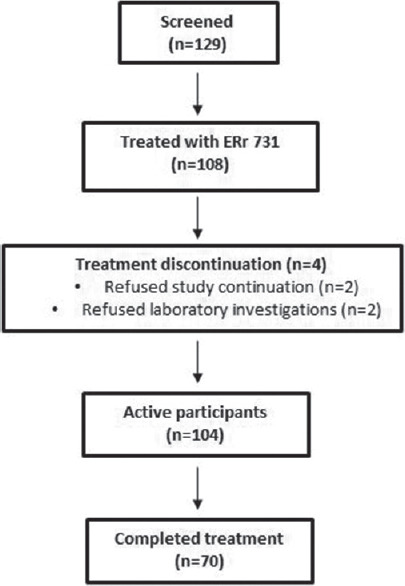
Study flowchart
Figure 2.
Mean changes in MRS II score (a) and monotonic reduction of the total MRS II score (b) from baseline till the end of 12 weeks. The numbers in the bars represent the mean (lower control limit, upper control limit). The shaded area represents 95% CI
Table 1.
Changes in each item of Menopause Rating Scale II score over time (n=70)
| MRS-II parameters | Baseline | Week 4 | Week 8 | Week 12 | Percentage difference baseline to 12th week | P* |
|---|---|---|---|---|---|---|
| Somatic subscale | ||||||
| Joint muscular discomfort | 2.5 (2.2-2.7) | 1.8 (1.6-2.1) | 1.3 (1.1-1.5) | 0.8 (0.5-1.0) | 68.00 | <0.001 |
| Sleep problems | 2.7 (2.5-2.9) | 2.3 (2.0-2.5) | 1.7 (1.5-1.9) | 0.8 (0.6-1.0) | 70.37 | <0.001 |
| Heart discomfort | 2.8 (2.6-3.1) | 2.3 (2.0-2.5) | 1.6 (1.3-1.8) | 0.8 (0.5-1.0) | 71.43 | <0.001 |
| Hot flushes | 2.8 (2.6-3.0) | 2.2 (2.0-2.4) | 1.5 (1.3-1.7) | 0.7 (0.5-0.9) | 75.00 | <0.001 |
| Urogenital subscale | ||||||
| Dryness of vagina | 1.9 (1.7-2.1) | 1.6 (1.3-1.8) | 1.2 (1.0-1.5) | 0.8 (0.5-1.0) | 57.89 | <0.001 |
| Bladder problems | 2.1 (1.9-2.4) | 1.8 (1.5-2.0) | 1.3 (1.1-1.5) | 0.9 (0.6-1.1) | 57.14 | <0.001 |
| Sexual problems | 1.9 (1.6-2.1) | 1.6 (1.4-1.8) | 1.2 (1.0-1.4) | 0.7 (0.5-0.9) | 63.16 | <0.001 |
| Psychological subscale | ||||||
| Physical and mental exhaustion | 2.4 (2.2-2.6) | 1.9 (1.7-2.1) | 1.4 (1.2-1.6) | 0.8 (0.6-1.0) | 66.67 | <0.001 |
| Anxiety | 2.4 (2.1-2.6) | 1.9 (1.6-2.1) | 1.3 (1.0-1.5) | 0.8 (0.6-1.0) | 66.67 | <0.001 |
| Irritability | 2.6 (2.4-2.9) | 1.9 (1.7-2.1) | 1.4 (1.2-1.6) | 0.9 (0.7-1.1) | 65.38 | <0.001 |
| Depressive mood | 2.6 (2.4-2.8) | 2.1 (1.9-2.3) | 1.4 (1.2-1.6) | 0.7 (0.5-0.9) | 73.08 | <0.001 |
*Comparison between results at baseline and 12 weeks. The data are represented by mean (95% CI). ANOVA was performed. MRS: Menopause Rating Scale, CI: Confidence interval, ANOVA: Analysis of variance
Figure 3.
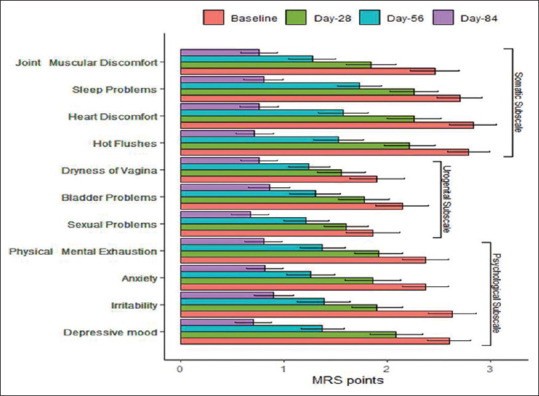
Mean changes in the individual items of Menopause Rating Scale II score from baseline till the end of 12 weeks. The error bars represent standard deviations. MRS: Menopause Rating Scale
Table 2.
Changes in endometrial thickness and lipid profile from baseline to week 12 (n=70)
| Parameters | Group | Values, mean (SD) | Mean difference (95% CI) | P |
|---|---|---|---|---|
| Endometrial thickness (mm) | Baseline | 4.42 (2.29) | 0.014 (−0.377-0.406) | 0.942 |
| Week 12 | 4.41 (2.01) | |||
| Total cholesterol (mg/dl) | Baseline | 177.09 (33.99) | −0.035 (−5.177-5.106) | 0.989 |
| Week 12 | 177.13 (37.37) | |||
| HDL cholesterol (mg/dl) | Baseline | 42.22 (9.09) | 1.016 (−0.967-2.999) | 0.310 |
| Week 12 | 41.20 (8.32) | |||
| LDL cholesterol (mg/dl) | Baseline | 111.62 (33.81) | 3.128 (−9.056-2.800) | 0.296 |
| Week 12 | 114.75 (37.73) | |||
| Triglycerides (mg/dl) | Baseline | 123.18 (45.87) | 5.149 (−3.788-14.086) | 0.254 |
| Week 12 | 118.03 (43.24) |
Paired t-test was performed. HDL: High-density lipoprotein, LDL: Low-density lipoprotein, CI: Confidence interval, SD: Standard deviation
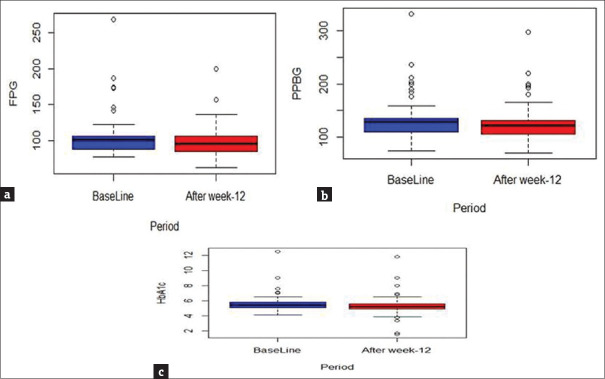
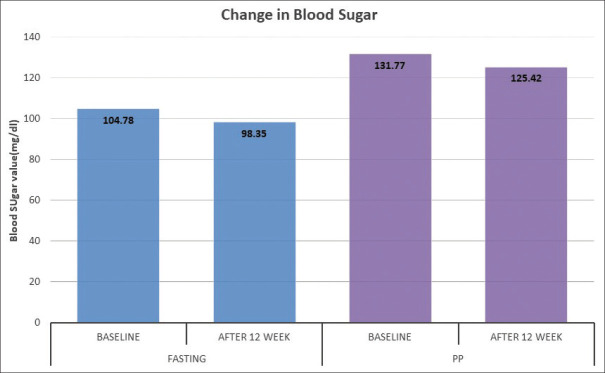
Both mean fasting (6.3 mg/dl; 95% CI: 1.7–11.0 mg/dl; P = 0.008) and PP (6.3 mg/dl; 95% CI: 1.0–11.7; P = 0.021) blood glucose levels showed significant reduction from baseline till the end of 12 weeks. A small but significant reduction in the mean HbA1c level (0.30%; 95% CI: 0.085–0.520; P = 0.007) was noted at the end of 12 weeks from that at baseline [Figures 4-6]. The change in the mean systolic (−0.386 mm Hg; 95% CI: −2.47–1.69 mm Hg) and diastolic (0.943 mm Hg; 95% CI: −0.63–2.51) blood pressure from baseline till the end of 12 weeks was not significant. The changes in the lipid profile from baseline till the end of 12 weeks are shown in Figure 7. No significant change was observed in the hs-CRP level from baseline to that at the end of 12 weeks [Figure 8].
Figure 4.
Box plots showing changes in fasting blood glucose level (a), postprandial blood glucose level (b), and glycated hemoglobin level (HbA1c) level (c) from baseline till the end of 12 weeks
Figure 6.
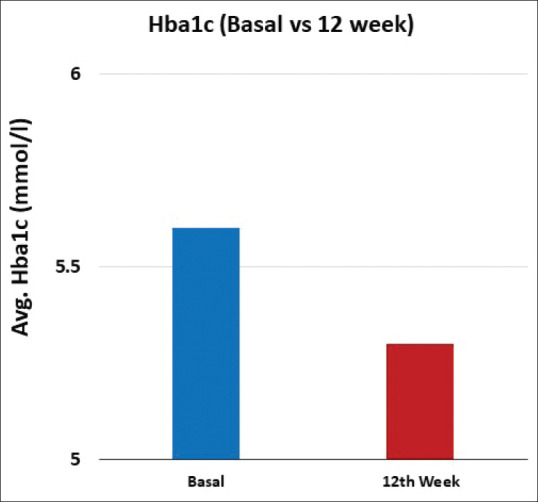
Bar graphs representing Hba1c level, from baseline and at the end of 12 weeks
Figure 7.
Bar graphs representing Lipid profile, from baseline and at the end of 12 weeks
Figure 8.
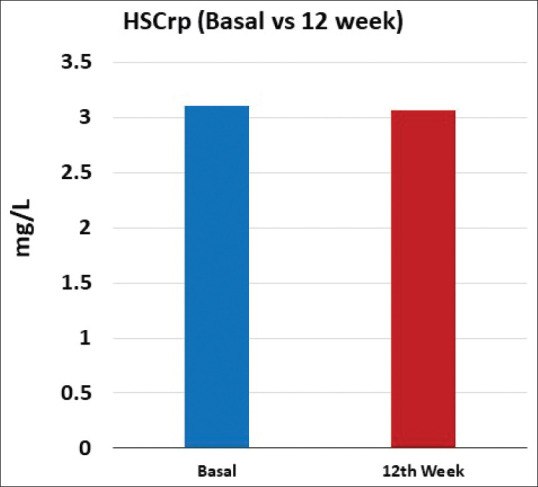
Bar graphs representing Hs-CRP from baseline and at the end of 12 weeks
Figure 5.
Bar graphs representing fasting and postprandial (PP) blood glucose level, from baseline and at the end of 12 weeks
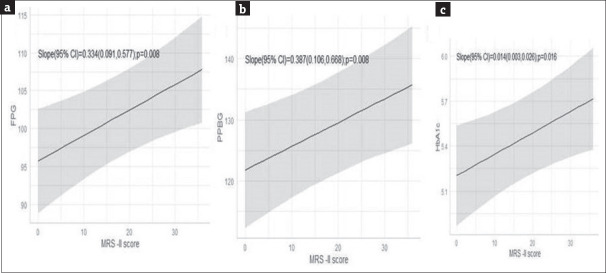
An interesting direct relationship was observed between the MRS II scores and glycemic parameters. There was a reduction by − 0.33 units (95% CI: 0.09–0.58 units; P = 0.008) of fasting blood glucose level, −0.39 units (95% CI: 0.11–0.67 units; P = 0.008) of PP blood glucose level, and − 0.014 units (95% CI: 0.003–0.026 units; P = 0.016) of HbA1c following per unit reduction in the MRS II score [Figure 9]. The drug was well tolerated with only five participants reporting symptoms (withdrawal bleeding in one, nausea in two, and weakness and dizziness in two participants).
Figure 9.
Correlation of MRS II with fasting blood glucose level (a), post-prandial blood glucose level (b), and glycated hemoglobin (HbA1c) level (c). The shaded areas represent 95% CIs
DISCUSSION
To the best of our knowledge, this is the first study evaluating the efficacy and safety of ERr 731 in alleviating MS in Indian perimenopausal women. The changes in the MRS II score were evident from week 4. The changes persisted implying a sustained alleviation of MRS in 12 weeks. The results are similar to other published studies.[2,13,17] Each of the MRS II score items showed significant improvements, as reported earlier.[11] An open-labeled multicentric study in Germany confirmed the efficacy and safety of ERr 731 in improving MS in 252 peri- and postmenopausal women. The maximum benefit was obtained within the first 3 months of treatment. Moreover, women having severe MS (MRS score of >18) showed the highest improvements.[16] Treatment with ERr 731 for 12 weeks was also found to alleviate anxiety, reduce somatic pains and hot flushes, and improve general well-being more than placebo.[13] Another multicentric placebo-controlled randomized control trial confirmed the efficacy and safety of ERr 731 in alleviating MS in 112 perimenopausal women.[17]
In this study, ERr 731 treatment led to a reduction in fasting blood glucose, PP blood glucose, and HbA1c levels. Although none of the participants had diabetes on enrollment, ERr 731 may be considered safe in not deranging the glycemic status. There were no significant changes in the lipid profile with treatment, implying that ERr 731 can be safely used in perimenopausal women who often have dyslipidemia.[18,19] This is the first study evaluating the effects of ERr 731 treatment on lipid profile. Furthermore, there were no significant changes in ET, hs-CRP level, and blood pressure. Thus, ERr 731 does not increase cardiovascular risks and can be used in perimenopausal women who often have hypertension as well.[20]
The mechanism of action of ERr 731 has not been fully elucidated. After ingestion, large amounts of the aglycones might be released due to deglycosylation of rhaponticin and desoxyrhaponticin by intestinal bacteria.[21] ERr 731 may exert its biological effects via selective binding to, and activation of, ER-β without acting on ER-α. This may explain the anxiolytic effects of ERr 731.[22,23] In tissues where both ER-α and ER-β are expressed, ER-β may function as a negative regulator of ER-α, offering relief of MS, as well as protection against inflammation and proliferation.[23]
Estrogen-based HRT increases ET implying secretory or proliferative endometrial activity. The most frequent symptoms with HRT are bleeding, spotting, nausea, and malaise, resulting in treatment discontinuation by 80% of the women within the first 3 months.[24,25] Unopposed estrogen-based HRT carries several inherent risks, such as the risks of breast cancer, endometrial cancer, and venous thromboembolism, irrespective of other risk factors.[7,8,9] Since these effects are primarily mediated by ER-α,[26,27] and ERr 731 has no ER-α agonistic activity,[22,23] it is devoid of these adverse effects. A postmarketing study performed in Germany had clarified that the incidence of adverse effects following ERr 731 usage is very low.[10]
It is pertinent to discuss the limitations of this study. First, this was an open-labeled single-arm study without a comparator arm, inviting some inherent biases in the study design. However, the open-labeled design allowed us to evaluate the long-term effects of ERr 731 in the intended patient population. Single-arm design is employed when the objective of the trial is to obtain preliminary evidence of treatment efficacy and collect additional safety data, like in this case.[28] Furthermore, being a single-arm study, the patients could serve as their own control. Second, the duration of treatment was short. The final analysis will be performed at the end of 24th week of therapy.
CONCLUSION
Treatment with the extract of the root of Rr (ERr 731) for 12 weeks was found to be efficacious in alleviating MS in Indian perimenopausal women. The euglycemic status was maintained throughout 12 weeks' duration. There were no significant changes in ET, blood pressure, lipid profile, and hs-CRP level. ERr 731 demonstrated short-term safety during these 12 weeks' follow-up. Further large-scale randomized control trials are required to validate our findings.
Financial support and sponsorship
This study was funded by Lupin Ltd., India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to acknowledge Insignia Communications for assisting in the data analysis and 3MD Healthcare Communication Consultants for assisting in drafting the manuscript.
REFERENCES
- 1.Fait T. Menopause hormone therapy: Latest developments and clinical practice. Drugs Context. 2019;8:212551. doi: 10.7573/dic.212551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasper I, Ventskovskiy BM, Rettenberger R, Heger PW, Riley DS, Kaszkin-Bettag M. Long-term efficacy and safety of the special extract ERr 731 of Rheum rhaponticum in perimenopausal women with menopausal symptoms. Menopause. 2009;16:117–31. doi: 10.1097/GME.0b013e3181806446. [DOI] [PubMed] [Google Scholar]
- 3.Nudy M, Jiang X, Aragaki AK, Manson JE, Shadyab AH, Foy AJ, et al. The severity of vasomotor symptoms and number of menopausal symptoms in postmenopausal women and select clinical health outcomes in the Women's Health Initiative Calcium and Vitamin D randomized clinical trial. Vol. 27. Menopause: The Journal of The North American Menopause Society; 2020. pp. 1265–73. [DOI] [PubMed] [Google Scholar]
- 4.Greenblum CA, Rowe MA, Neff DF, Greenblum JS. Midlife women: Symptoms associated with menopausal transition and early postmenopause and quality of life. Menopause. 2013;20:22–7. doi: 10.1097/gme.0b013e31825a2a91. [DOI] [PubMed] [Google Scholar]
- 5.Jadhav A, Bavaskar Y. An epidemiological study of the perimenopausal and menopausal health problems in women living in an urban area of Mumbai, Maharashtra. Int J Community Med Public Health. 2017;4:3088–93. [Google Scholar]
- 6.Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol. 2019;116:135–70. doi: 10.1016/bs.apcsb.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palacios S, Stevenson JC, Schaudig K, Lukasiewicz M, Graziottin A. Hormone therapy for first-line management of menopausal symptoms: Practical recommendations. Women's Health. 2019;15:1–8. doi: 10.1177/1745506519864009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphries KH, Gill S. Risks and benefits of hormone replacement therapy: The evidence speaks. CMAJ. 2003;168:1001–10. [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: Scientific review. JAMA. 2002;288:872–81. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- 10.Chang JL, Montalto MB, Heger PW, Thiemann E, Rettenberger R, Wacker J. Rheum rhaponticum Extract (ERr 731): Postmarketing Data on Safety Surveillance and Consumer Complaints. Integr Med (Encinitas) 2016;15:34–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Heger M, Ventskovskiy BM, Borzenko I, Kneis KC, Rettenberger R, Kaszkin-Bettag M, et al. Efficacy and safety of a special extract of Rheum rhaponticum (ERr 731) in perimenopausal women with climacteric complaints: A 12-week randomized, double-blind, placebo-controlled trial. Menopause. 2006;13:744–59. doi: 10.1097/01.gme.0000240632.08182.e4. [DOI] [PubMed] [Google Scholar]
- 12.Papke A, Kretzschmar G, Zierau O, Kaszkin-Bettag M, Vollmer G. Effects of the special extract ERr 731 from Rheum rhaponticum on estrogen-regulated targets in the uterotrophy model of ovariectomized rats. J Steroid Biochem Mol Biol. 2009;117:176–84. doi: 10.1016/j.jsbmb.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Kaszkin-Bettag M, Ventskovskiy BM, Kravchenko A, Rettenberger R, Richardson A, Heger PW, et al. The special extract ERr 731 of the roots of Rheum rhaponticum decreases anxiety and improves health state and general well-being in perimenopausal women. Menopause. 2007;14:270–83. doi: 10.1097/01.gme.0000251932.48426.35. [DOI] [PubMed] [Google Scholar]
- 14.Lange Lordseeker Production J. MRS-Menopause Rating Scale. [[Last accessed on 2021 Jul 06]]. Available from: https://zeg-berlin.de/expertise/diagnostics-tools/menopause-rating-scale/mrs-outcome-measure/
- 15.Heinemann K, Ruebig A, Potthoff P, Schneider HP, Strelow F, Heinemann LA, et al. The Menopause Rating Scale (MRS) scale: A methodological review. Health Qual Life Outcomes. 2004;2:45. doi: 10.1186/1477-7525-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaszkin-Bettag M, Beck S, Richardson A, Heger PW, Beer AM. Efficacy of the special extract ERr 731 from rhapontic rhubarb for menopausal complaints: A 6-month open observational study. Altern Ther Health Med. 2008;14:32–8. [PubMed] [Google Scholar]
- 17.Kaszkin-Bettag M, Ventskovskiy BM, Solskyy S, Beck S, Hasper I, Kravchenko A, et al. Confirmation of the efficacy of ERr 731 in perimenopausal women with menopausal symptoms. Altern Ther Health Med. 2009;15:24–34. [PubMed] [Google Scholar]
- 18.Wu ZY, Wu XK, Zhang YW. Relationship of menopausal status and sex hormones to serum lipids and blood pressure. Int J Epidemiol. 1990;19:297–302. doi: 10.1093/ije/19.2.297. [DOI] [PubMed] [Google Scholar]
- 19.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321:641–6. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- 20.Maas AH, Franke HR. Women's health in menopause with a focus on hypertension. Neth Heart J. 2009;17:68–72. doi: 10.1007/BF03086220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park EK, Choo MK, Yoon HK, Kim DH. Antithrombotic and antiallergic activities of rhaponticin from Rhei Rhizoma are activated by human intestinal bacteria. Arch Pharm Res. 2002;25:528–33. doi: 10.1007/BF02976613. [DOI] [PubMed] [Google Scholar]
- 22.Vollmer G, Papke A, Zierau O. Treatment of menopausal symptoms by an extract from the roots of rhapontic rhubarb: The role of estrogen receptors. Chin Med. 2010;5:7. doi: 10.1186/1749-8546-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Möller F, Zierau O, Jandausch A, Rettenberger R, Kaszkin-Bettag M, Vollmer G. Subtype-specific activation of estrogen receptors by a special extract of Rheum rhaponticum (ERr 731), its aglycones and structurally related compounds in U2OS human osteosarcoma cells. Phytomedicine. 2007;14:716–26. doi: 10.1016/j.phymed.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Mattsson LA, Skouby S, Rees M, Heikkinen J, Kudela M, Stadnicki-Kolendo A, et al. Efficacy and tolerability of continuous combined hormone replacement therapy in early postmenopausal women. Menopause Int. 2007;13:124–31. doi: 10.1258/175404507781605596. [DOI] [PubMed] [Google Scholar]
- 25.Cagnacci A, Venier M. The Controversial History of Hormone Replacement Therapy. Medicina (Kaunas) 2019;55:602. doi: 10.3390/medicina55090602. 1:11. doi: 10.3390/medicina55090602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marquardt RM, Kim TH, Shin JH, Jeong JW. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis?? Int J Mol Sci. 2019;20:3822. doi: 10.3390/ijms20153822. 1-28. doi: 10.3390/ijms20153822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han SJ, Begum K, Foulds CE, Hamilton RA, Bailey S, Malovannaya A, et al. The Dual Estrogen Receptor α Inhibitory Effects of the Tissue-Selective Estrogen Complex for Endometrial and Breast Safety. Mol Pharmacol? 2016;89:14–26. doi: 10.1124/mol.115.100925. doi: 10.1124/mol.115.100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans SR. Clinical trial structures. J Exp Stroke Transl Med. 2010;3:8–18. doi: 10.6030/1939-067x-3.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]