ABSTRACT
Radiation-induced optic neuropathy is a rare complication of radiation therapy that often results in profound, irreversible vision loss. We present a unique case of a patient in whom optical coherence tomography detected an early underlying optic neuropathy despite being visually asymptomatic in the affected eye.
KEYWORDS: Optic neuropathy, magnetic resonance imaging, optical coherence tomography, radiation therapy
Case report
In February 2015 a previously healthy 74-year-old woman presented to the Neuro-ophthalmology service with painless, progressive vision loss in the right eye that had begun in 2011. Despite cataract surgery and pars plana vitrectomy for an epiretinal membrane the vision did not improve.
Her visual acuity was 20/400 in her right eye (OD) and 20/20 in her left eye (OS). She could detect 0/14 of the Ishihara colour plates OD and 14/14 OS. Automated perimetry showed significant temporal and inferior depression with a relatively preserved superior nasal quadrant OD and a mild nasal defect OS (Figure 1a). There was a relative afferent pupillary defect (RAPD) OD and 3 mm of relative proptosis OD. Optical coherence tomography (OCT) demonstrated a normal peripapillary retinal nerve fibre layer (pRNFL) thickness in both eyes (OU) but it was slightly thinner OD compared with OS with ganglion cell-inner plexiform layer (GCIPL) thinning OD (Figure 1b). Funduscopy revealed optic disc pallor OD and a normal optic disc OS. Cranial magnetic resonance imaging (MRI) with contrast demonstrated a homogeneous enhancing mass of the right skull base with invasion into the right orbital apex causing compressive optic neuropathy (Figure 1c). A subtotal resection of the mass was performed. Histopathological analysis was consistent with a grade 1 meningioma. The residual tumour was treated with fractionated radiation over 28 days for a total of 54 Gy, that was completed in August 2017.
Figure 1.

Paraclinical findings at time of presentation of the intracranial meningioma. (a) Automated perimetry showing temporal and inferior visual deficits with a relatively preserved superior nasal quadrant in the right eye and normal left eye. (b) Optical coherence tomography demonstrating asymmetric peripapillary retinal nerve fibre layer thickness (slightly thinner in the right eye compared with the left eye) and ganglion cell-inner plexiform layer thinning in the right eye, consistent with a right optic neuropathy. (c) Axial T1 post-contrast magnetic resonance imaging showing an enhancing lesion centred around the right cavernous sinus (arrow)
In May 2019, during a routine surveillance examination, she reported improved vision in the right eye and she felt her vision in the left eye was normal. Her visual acuity was 20/50 OD and 20/20 OS. Automated perimetry revealed a central scotoma OD and a mild nasal defect OS (Figure 2a). There was an RAPD OD. However, OCT showed significant pRNFL and GCIPL thinning OU (Figure 2b). Funduscopy revealed optic disc pallor OD and a normal optic disc OS (Figure 2c). Cranial MRI demonstrated enhancement of the left pre-chiasmal optic nerve (Figure 3). The combination of the MRI enhancement of the left optic nerve, mild visual field defect, and pRNFL and GCIPL thinning on OCT was consistent with radiation induced optic neuropathy (RION). Therefore, no further evaluation was felt to be needed. A 4-week course of oral prednisone (60 mg/day) and 1200 mg pentoxifylline was prescribed.
Figure 2.

Paraclinical findings 22 months after subtotal resection and fractionated radiation therapy. (a) Automated perimetry showing a central scotoma in the right eye and a small nasal defect in the left eye. (b) Optical coherence tomography demonstrating significant peripapillary retinal nerve fibre layer and ganglion cell-inner plexiform layer thinning in both eyes. (c) Fundus photograph demonstrating optic disc pallor in the right eye and a normal optic disc in the left eye
Figure 3.
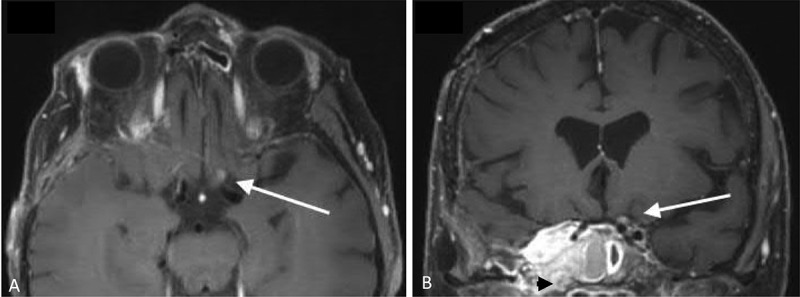
Brain magnetic resonance imaging 22 months after completion of fractionated radiation therapy. (a) T1-weighted post-contrast axial image showing mild focal expansion and enhancement of the pre-chiasmatic left optic nerve (arrow). (b) T1-weighted post-contrast coronal image showing mild focal expansion and enhancement of the pre-chiasmatic left optic nerve (arrow) and an unchanged, right sphenocavernous meningioma (black arrowhead)
At last follow-up, in August 2020, there was no subjective change in vision in either eye. Her visual acuity was 20/70 OD and 20/30 OS. Automated perimetry revealed a central and superonasal defect OD and a nasal defect OS. OCT continued to show superior and inferior pRNFL and GCIPL thinning OU. Cranial MRI demonstrated resolution of the left optic nerve enhancement.
Discussion
We present a unique case of an asymptomatic patient presenting with OCT and MRI findings consistent with RION. The OCT showed significant pRNFL and GCIPL thinning and there was pre-chiasmal optic nerve enhancement on MRI. The absence of optic disc pallor was consistent with prior observations of an increased sensitivity of OCT in detecting optic atrophy compared with funduscopy.1
RION is a rare complication of radiation therapy that often results in profound, irreversible visual loss.2 On average, RION occurs 18 months after the completion of radiation therapy at a single dose of >5 Gy for external beam radiation and >8 Gy for stereotactactic radiation therapy2,3 or a total cumulative radiation dose over 50 Gy.2–4 Most patients with RION present with painless subacute vision loss, with clinical evidence of an optic neuropathy and optic nerve enhancement on MRI.3 Although multiple treatments such as hyperbaric oxygen (HBO) therapy, corticosteroids, bevacizumab, pentoxifylline, and anticoagulation have been attempted, no proven effective treatment exists to date. Lessell and others have suggested that early detection of RION may optimise the efficacy of HBO and other interventions.2
MRI has been shown to be sensitive for the diagnosis of RION. Guy et al. reported on a series of 6 patients with RION and concluded that MRI is a reliable modality to distinguish RION from intrinsic optic nerve abnormalities.5 Furthermore, Hudgins et al.6 and Zimmerman et al.7 reviewed their patients with RION and also emphasised MRI for early detection. Recently, Archer et al. presented a series of 12 patients (15 eyes) with RION and noted optic nerve enhancement on MRI in 14 of 15 eyes that typically preceded vision loss but they did not describe OCT findings in these patients.4 Therefore, while there have been reported cases of optic nerve enhancement on MRI prior to RION-induced visual loss,2,4 we are not aware of a documented case of OCT changes prior to visual loss. Yousef and Finger described the OCT findings in 10 eyes in 10 patients with RION following plaque brachytherapy for choroidal melanoma.8 All patients had optic disc oedema with corresponding increase in pRNFL thickness and a 50% decrease in GCIPL thickness. The authors did not comment on how many patients were asymptomatic.
Lessell has suggested monitoring patients for RION with routine MRI for 10–20 months after the conclusion of external beam radiation therapy.2 Our case highlights that structural changes of the optic nerve can be seen prior to a patient reporting symptomatic visual changes. Therefore, OCT could be used as an adjunct screening tool in patients during surveillance after exposure of the anterior visual pathways to radiation therapy.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of interest statementDisclosure statement
The authors have no relevant conflicts of interest to declare
References
- 1.Pula JH, Kattah JC, Wang H, Marshall J, Eggenberger ER.. Ability of a neuro-ophthalmologist to estimate retinal nerve fiber layer thickness. Clin Ophthalmol. 2012;6:1477–1481. doi: 10.2147/OPTH.S34573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lessell S. Friendly fire: neurogenic visual loss from radiation therapy. J Neuroophthalmol. 2004;24(3):243–250. doi: 10.1097/00041327-200409000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Lee MS, Borruat FX. Should patients with radiation-induced optic neuropathy receive any treatment? J Neuroophthalmol. 2011;31:83–88. doi: 10.1097/WNO.0b013e31820d5361. [DOI] [PubMed] [Google Scholar]
- 4.Archer EL, Liao EA, Trobe JD. Radiation-induced optic neuropathy: clinical and imaging profile of twelve patients. J Neuroophthalmol. 2019;39(2):170–180. doi: 10.1097/WNO.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 5.Guy J, Mancuso A, Beck R, et al. Radiation-induced optic neuropathy: a magnetic resonance imaging study. J Neurosurg. 2009;74(3):426–432. doi: 10.3171/jns.1991.74.3.0426. [DOI] [PubMed] [Google Scholar]
- 6.Hudgins PA, Newman NJ, Dillon WP, Hoffman JC. Radiation-induced optic neuropathy: characteristic appearances on gadolinium-enhanced MR. Am J Neuroradiol. 1992;13:235–238. [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmerman CF, Schatz NJ, Glaser JS. Magnetic resonance imaging of radiation optic neuropathy. Am J Ophthalmol. 1990;110(4):389–394. doi: 10.1016/S0002-9394(14)77019-9. [DOI] [PubMed] [Google Scholar]
- 8.Yousef YA, Finger PT. Optical coherence tomography of radiation optic neuropathy. Ophthalmic Surg Lasers Imaging. 2012;43(1):6–12. doi: 10.3928/15428877-20111129-09. [DOI] [PubMed] [Google Scholar]


