Abstract
Background
Nutcracker phenomenon (NCP) describes the compression of the left renal vein between the aorta and the superior mesenteric artery. Nutcracker Syndrome (NCS) refers to the clinical manifestations of NCP.
Aims
This paper aims to provide education and ultrasound protocol for Clinicians and Sonographers who encounter patients with the symptoms of NCS during their course of practice.
Methods
The following report examines two case studies where a diagnosis of NCP was made from clinical history and ultrasound findings. Based on these case studies, we would like to propose an appropriate ultrasound scanning protocol for patients presenting with symptoms of gonadal vein incompetence.
Results
The above case studies highlight the need for further assessment with ultrasound to help diagnose cases of NCP.
Conclusion
The proposed ultrasound techniques are a valid protocol extension to the ultrasound examination to help diagnose NCP.
Keywords: compression syndrome, gonadal vein incompetence, nutcracker phenomenon, nutcracker syndrome, varicocele
The term nutcracker phenomenon (NCP) refers to compression of the left renal vein (LRV) between the aorta and the superior mesenteric artery (SMA). Nutcracker syndrome (NCS) refers to the clinical manifestations of NCP.1
Symptoms of NCS include haematuria, thought to be caused by extravasation of blood due to increased venous pressure from the stenosis of the LRV.2 Proteinuria, predominantly seen in paediatric cases of NCS, is thought to be associated with change in renal haemodynamics. This change is due to an increase in norepinephrine and angiotensin II.3 Left‐sided flank pain is a symptom linked to pelvic venous congestion. This is caused by venous collaterals from high venous pressures.4 It is also associated with renal colic from the passage of blood clots through the left ureter.4 Varicocele, usually of the left testis, is due to gonadal vein incompetence.1 Varicose veins are occasionally associated with gonadal vein incompetence.5 These extend from the pelvic region and into the lower limbs. Urinary frequency is caused by pelvic venous congestion extending to the veins in the bladder wall (Table 1).4
Table 1.
Symptoms of NCS
|
All these symptoms have been described as sequelae of NCP.
The demographics of NCP is not known. However, the literature suggests that there is a higher incidence amongst the female population with peak presentation between the ages of 30–40 years.1, 6, 7 It has been reported that there is a link between low body habitus, increased height and NCP.1, 8
The finding of NCP is usually coincidental during to the investigation of one or more of the described symptoms.
The following report examines two case studies where a diagnosis of NCP was made from clinical history and ultrasound findings. Based on these case studies, we would like to propose an appropriate ultrasound scanning protocol for patients presenting with symptoms of gonadal vein incompetence.
Case study 1
A 25‐year‐old male presented for investigation of lower abdominal pain and urinary frequency. This was exacerbated whilst sitting. He had a history of left testicular varicocele, surgically ligated in China 10 years previously.
Physical examination revealed a soft recurrent left‐sided varicocele. Varicosities extended up to the inguinal canal from the scrotum.
He was referred for an ultrasound examination. Examination of the abdomen showed a reduced aorta‐SMA angle of 19 degrees at the origin of the SMA. The LRV was stenosed between the aorta and the SMA. Measurement of the stenosis revealed a LRV diameter change from 8.5 mm to 1 mm. Velocity measurements in the LRV proximal to the defect were 37 cm/s. At the stenotic portion, the velocity measurement was 250 cm/s. The velocity ratio was 7:1. His kidneys were otherwise unremarkable. The ultrasound revealed dilated veins in the left inguinal canal and there was a left‐sided varicocele. Measurement of the veins in the inguinal canal gave a diameter 3.5 mm. These veins drained into the pelvic veins directly.
Subsequent phlebogram confirmed the diagnosis of NCP. The left testicular vein was disconnected by previous surgery. There was no filling of the varicocele from the left testicular vein (Figure 1 and 2).
Figure 1.
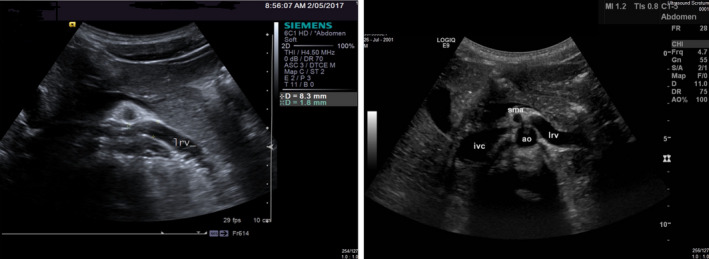
Two Images of Compression of the Proximal LRV between the Aorta and SMA on Ultrasound Examination.
Figure 2.
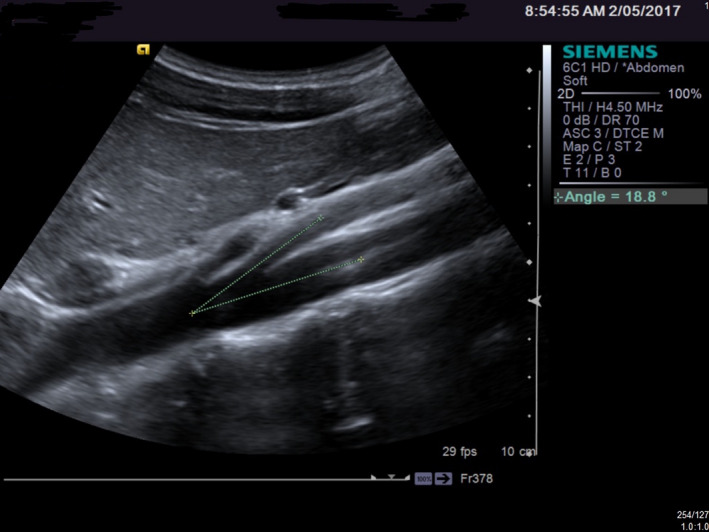
An Example of a Reduced Aortic/SMA Angle.
Case study 2
A 13‐year‐old boy presented to our ultrasound department with a varicocele and abdominal pain for a scrotal and renal ultrasound. On examination, a large left‐sided varicocele was seen to extend from the left hemiscrotum up into the inguinal canal. The kidneys were assessed and were seen to be normal.
The LRV was seen to be dilated proximally, towards the renal hilum. This measured up to 8.8 mm. The part of the renal vein to pass between the aorta and the SMA was seen to be compressed down to a diameter of approximately 1 mm.
The examination was extended to include measurements of the aorta/SMA angle and the velocity of flow in the LRV proximal to, and within, the compressed portion of vein.
The aorta/SMA angle was measured at 15. The velocity of the LRV flow proximal to the narrowing was 23 cm/sec with an increase to 350 cm/s at the narrowing. This produces a velocity ratio up to 16:1. A diagnosis of ‘Nutcracker Syndrome’ was made (Figures 3 and 4).
Figure 3.

Flow Velocities in the LRV.
Figure 4.
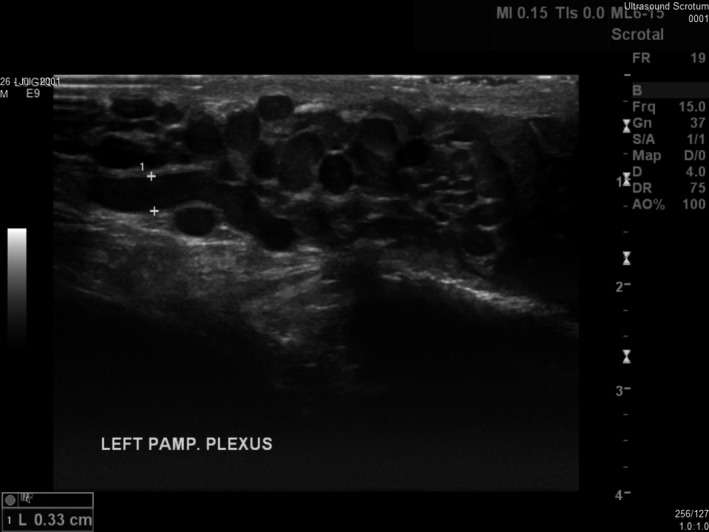
An Example of a Left‐sided Varicocele.
Recommended protocol in instances of suspected nutcracker phenomenon:
Begin the scan by selecting a low‐frequency curved array transducer on an abdominal venous setting. The patient should be fasted but well hydrated. This eliminates bowel gas whilst maintaining venous filling.
The sonographer should survey the aorta at the level of the SMA in both a transverse and longitudinal plane. This assessment is to rule out any space‐occupying lesions that could be compressing the LRV.
Measure the angle of the aorta and SMA origin in a longitudinal plane.
The LRV should then be assessed looking for entrapment. If positive, measure the AP diameter of the lumen at the proximal aspect to the compression point, at the defect and distal to it.
Measure the velocity of flow in the LRV proximally and at the compressed portion. The velocity ratio should be greater than 5:1.10
Assess the surrounding structures for any other causes of possible compression of the LRV.
Assess and measure both kidneys looking for any pathology.
Assess the vasculature within the inguinal region looking for incompetence. If suspected, measure veins with an AP diameter over 3 mm.
Assess the scrotum for varicocele in the case of male patients and ovarian vein incompetence in female patients. This can be achieved by scanning the region using colour Doppler and getting the patient to cough. The vasculature should flash with colour during the strain of the cough. If there is incompetence, a second flash of reversed colour straight after will display refluxed flow (Table 2).
Table 2.
Ultrasound features consistent with NCS
Limitations of this protocol include obtaining the correct angle in Pulse‐wave Doppler mode to get an accurate velocity measurement of the LRV. Pitfall effecting the ultrasound results include patients body habitus, as a greater body mass may make access to the region more difficult. Bowel gas is another pitfall that needs to be considered as it can affect image quality. Patient dehydration is another consideration as it can affect the filling of the patients’ veins. Lastly, patient positioning can affect the examination as venous flow is posturally affected.
This paper aims to provide education and ultrasound protocol for clinicians and sonographers who come across patients with the symptoms of NCS during their course of practice. The above case studies highlight the need for further assessment with ultrasound to help diagnose cases of NCP.
References
- 1.Kurklinsky AK, Rooke TW. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc 2010; 85(6): 552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin JI, Park JM, Lee JS, Kim MJ. Effect of renal Doppler ultrasound on the detection of nutcracker syndrome in children with hematuria. Eur J Pediatr 2007; 166(5): 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gulleroglu K, Gulleroglu B, Baskin E. Nutcracker syndrome. World J Nephrol 2014; 3(4): 277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scultetus AH, Villavicencio JL, Gillespie DL. The nutcracker syndrome: its role in the pelvic venous disorders. J Vasc Surg 2001; 34(5): 812–9. [DOI] [PubMed] [Google Scholar]
- 5.Takemura T, Iwasa H, Yamamoto S, Hino S, Fukushima K, Isokawa S, et al. Clinical and radiological features in four adolescents with nutcracker syndrome. Pediatr Nephrol 2000; 14: 1002. [DOI] [PubMed] [Google Scholar]
- 6.Barnes RW, Fleisher HL, Redman JF, Smith JW, Harshfield DL, Ferris EJ. Mesoaortic compression of the left renal vein (the so‐called nutcracker syndrome): repair by a new stenting procedure. J Vasc Surg 1988; 8(4): 415–21. [DOI] [PubMed] [Google Scholar]
- 7.Shin JI, Lee JS, Kim MJ. The prevalence, physical characteristics and diagnosis of nutcracker syndrome. Eur J Vasc Endovasc Surg 2006; 32(3): 335–6. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed K, Sampath R, Khan MS. Current trends in the diagnosis and management of renal nutcracker syndrome: a review. Eur J Vasc Endovasc Surg 2006; 31(4): 410–6. [DOI] [PubMed] [Google Scholar]
- 9.Weerakkody Y, De Souza D, et al. Nutcracker syndrome | Radiology Reference Article | Radiopaedia.org. https://radiopaedia.org/articles/nutcracker-syndrome. Accessed 21st May 2017
- 10.Cheon JE, Kim WS, Kim IO, Kim SH, Yeon KM, Ha IS, et al. Nutcracker syndrome in children with gross haematuria: Doppler sonographic evaluation of the left renal vein. Pediatr Radiol 2006; 36(7): 682–6. [DOI] [PubMed] [Google Scholar]


