Abstract
Objectives
To compare two methods of measuring fetal biparietal diameter (BPD) – outer‐to‐inner (BPDoi) vs. outer‐to‐outer (BPDoo) calliper placement – and to compare the differences in EFW calculated using the Hadlock 4 formula and other common EFW formulae.
Methods
A total of 543 fetuses underwent a single ultrasound prospectively performed by 40 sonographers between 14 and 40 weeks of gestation, taking into account the intra‐ and inter‐observer variability. The measurements for each fetus consisted of BPDoi and BPDoo, and EFW is calculated from HC, AC and FL measurements. The difference between BPDoo and BPDoi was estimated, and this difference was compared with gestational age using linear regression. Translational equations that allow interconversion of the two parameters were derived. EFW calculated from four different formulae using various combinations of biometric measurements was compared.
Results
The difference between BPDoi and BPDoo increases with gestational age, although this difference was small. For BPDoo, the regression equation is BPDoo = 0.555934 + 1.027318 × BPDoi. Similarly, for BPDoi, the regression equation is BPDoi = −0.403458 + 0.9714153 × BPDoo. There is a minimal difference in the EFW calculated from the four formulae, except for gestations prior to 27–28 weeks. EFW derived from INTERGROWTH‐21st formulae plot is higher than that from Hadlock 3 or Hadlock 4 before 27–28 weeks.
Conclusions
Although the absolute difference between BPDoo and BPDoi increased across gestational age, this difference was small. The method of BPD measurement should follow that as prescribed in the EFW equation used in the local context. Estimation of fetal weight using Hadlock 3, Hadlock 4 and INTERGROWTH‐21st is similar, with slight differences at gestations less than 27–28 weeks.
Keywords: biometry, biparietal diameter, fetal head, measurement, ultrasound
Introduction
Measurement of fetal biometry is a mainstay of modern obstetric care. Fetal head measurements are important for estimating gestational age and a component of assessing fetal growth. Various regression or volumetric formulae have incorporated different parameters of fetal biometry in the estimation of fetal weight. These invariably use a combination of biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC) and femur length (FL) and have varying degrees of accuracy. Correct identification of fetal growth disorders will help in the diagnosis of fetal growth restriction and macrosomia, prompting timely intervention and reducing perinatal morbidity and mortality.1, 2 Conversely, inadvertent failure to recognise these disorders will preclude appropriate antenatal monitoring and surveillance.
Two methods have been described for the measurement of BPD: the outer‐to‐inner (BPDoi) method, where callipers are placed from the leading edge of the near‐field parietal bone to the leading edge of the far‐sided parietal bone, and the outer‐to‐outer (BPDoo) method, which involves measuring from the leading edge of the near‐field parietal bone to the far edge of the far‐sided parietal bone.3 Variation in the assessment of the BPD differs among studies, and there is no consensus on the ‘gold standard’ of which method to use.4, 5, 6 Recent publications of new international growth charts have adopted the BPDoo methodology.7 This is chiefly to facilitate direct comparison between antenatal and post‐natal measurements of head size and growth.8, 9 However, there have been no studies comparing the differences between these two methods of BPD measurement, although a recent study by Napilitano et al. demonstrated that both measurements are equally reproducible.10 Another study measured both the BPDoi and BPDoo in the same cohort; however, the primary aim of this study was to create biometry charts and validate inter‐observer and intra‐observer variability.11 It is also uncertain how measurement of BPD using the BPDoo method might impact on institutions which have used the standard BPDoi technique.
Furthermore, various estimated fetal weight (EFW) regression formulae or volumetric calculations have been proposed using either a single or combination of measurements. While some of these methods have minor systematic error, it is not uncommon for studies to report random errors (as measured by the standard deviation of errors) of more than 7%.12 A systematic review comparing the performance of various methods for ultrasound estimation of fetal weight has demonstrated that those of Hadlock et al. provide generally more consistent mean (systematic) errors across the selected studies, with comparable random errors.12, 13, 14 Commonly known as the Hadlock 3 and 4 formulae, respectively, Hadlock 3 uses HC, AC and FL, while Hadlock 4 incorporates all four parameters.13, 14 Given the potential problems of head moulding, a review suggested preference of the Hadlock 3 over the Hadlock 4 formula.15 In addition, the INTERGROWTH‐21st project proposed an international EFW chart to complement the fetal measurement standards.7, 16 This regression formula only incorporates two biometric measurements: HC and AC. However, no prospective validation of these data has been performed.
Hence, the objectives of this study were as follows: (i) to assess the difference between the two methods of measuring fetal BPD measurements – BPDoi vs. BPDoo, taking into account the intra‐observer and inter‐observer variability and (ii) to compare the differences in EFW calculated using the Hadlock 3 and 4 formulae, using measured BPDoi and BPDoi derived from BPDoo, and INTERGROWTH‐21st EFW formulae.
Methods
The study was conducted in a university teaching hospital in Melbourne, Victoria, during the period November 2015 to October 2016. The total number of antenatal ultrasounds performed during the 12‐month period of the study was 12183. To assess the variability throughout pregnancy with an equal degree of accuracy, a minimum of 20 fetuses were recruited for every 5‐week gestational age window. The patients consist of both high‐ and low‐risk cases from a diverse ethnic background. Subjects were recruited based on a convenience sample. The inclusion criteria were as follows: (i) established gestational age from a quality first‐trimester ultrasound and (ii) gestation from 14 to 40 weeks. The exclusion criteria were as follows: (i) known fetal anomaly and (ii) fetal death in utero. Women were only included in the study once throughout the pregnancy. The study protocol was approved by the institutional review board as a low‐risk study.
Ultrasound scans
All ultrasound examinations were performed using two types of commercially available ultrasound machines (Philips iu22, Philips Ultrasound, Bothell, WA, USA or Samsung RS80A, Samsung Medison, Gangnam‐Gu, Seoul, Korea) with curvilinear abdominal transducers. The unit consists of 40 Australian accredited medical sonographers across four sites who were trained on the image requirements at the beginning of the study. At each examination, BPD images were acquired in the transthalamic plane, where the landmarks consist of the cavum septum pellucidum, thalami and absence of the cerebellum. The BPD images were acquired in duplicate. Calliper placements of the BPDoi were made with the intersection of the callipers placed from the outer edge of the proximal fetal calvarium to the inner edge of the distal fetal calvarium, at the widest part of the fetal skull (Figure 1a). Calliper placements of the BPDoo were made with the intersection of the callipers placed from the outer edge of the proximal fetal calvarium to the outer edge of the distal fetal calvarium (Figure 1b).
Figure 1.
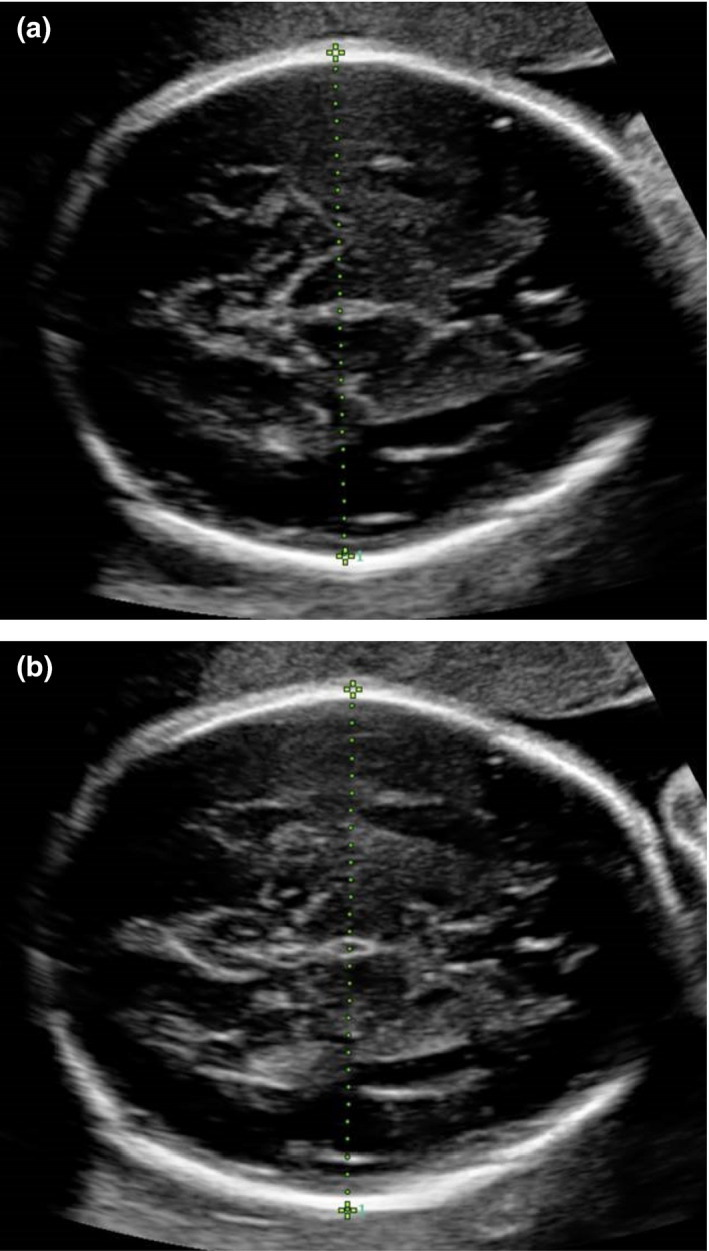
(a) Ultrasound Image of Fetal Biparietal Diameter, Measured Using Outer‐to‐Outer Calliper Placement (BPDoi) in the Transthalamic Plane. (b) Ultrasound Image of Biparietal Diameter, Measured Using Outer‐to‐outer Calliper Placement (BPDoo).
Intra‐ and inter‐observer variations were accounted for the two operators. During each scan, the first sonographer (Observer 1) obtained real‐time measurements of BPDoo and BPDoi on the first of two duplicate BPD images. The still ultrasound images were stored and the measurements recorded. Using the stored ultrasound images on the machine after the original calliper placements had been removed from the images, Observer 2 measured the BPDoi and BPDoo measurements on each of the two still images, blinded to Observer 1's measurements. Only one observer was present in the room at any one time. The intra‐observer variability for both methods was calculated for the measurements of Observer 2. Inter‐observer variability was calculated by comparing the measurements of Observer 1 with the second measurement of Observer 2.
The rest of the biometric measurements – the head circumference (HC), abdominal circumference (AC) and femur length (FL) – were measured in previously described methods.6, 17, 18 The estimated fetal weight (EFW) was calculated using (i) the established Hadlock 4 method using the measured BPDoi13; (ii) Hadlock 4 after applying the reverse coefficient to BPDoo to derive BPDoi; (iii) Hadlock 314; and (iv) INTERGROWTH‐21st formula.16 The body mass index (BMI) of the patient was obtained from the patient's history.
Statistical analysis
All analyses were performed using Stata software version 14 (StataCorp, College Station, Texas, USA).
The intra‐observer variability and inter‐observer variability of the BPDoo and BPDoi measurements were determined using the Bland–Altman method. Differences between measurements were plotted against the average values for visual inspection of variability in these measurements. Separate plots were drawn to assess inter‐ and intra‐observer agreement. Linear regression was used to assess the relationship between differences in BPDoo and BPDoi measurements and gestational age. To ascertain whether maternal BMI contributed to measurement variability, BMI was included as a covariate in the regression model. Translational equations to estimate BPDoo using BPDoi and BPDoi using BPDoo were also derived.
Fetal weight was estimated using four different methods:
(i) Hadlock 4 (using BPDoi, HC, AC and FL); (ii) Hadlock 4 after applying the reverse coefficient to BPDoo to derive BPDoi; (iii) Hadlock 3 (using HC, AC and FL) and (iv) INTERGROWTH‐21st formula (using HC and AC).13, 14, 16 The 3rd, 10th, 50th, 90th and 97th percentiles of estimated fetal weight were determined and plotted against gestational age.
Results
A total of 539 pregnancies of 543 fetuses were included. Four were twin pregnancies. All fetuses were scanned once only. Mean maternal BMI was 25.5 (range, 15–47.9). All measurements for BPD, HC, AC and FL were complete for all 543 fetuses. No BMI data were available in 57 (10.5%) measurements.
There was a good agreement between or within sonographers in the measurements performed on the same fetus. Figure 2 depicts Bland–Altman plots for the intra‐ and inter‐observer variability for BPDoi for differences in measurement units (mm) (Figure 2a and b) and for BPDoo (Figure 2c and d). The limits of agreement were within 3 mm for intra‐observers and 2 mm for inter‐observers. For BPOoi, the mean difference in intra‐observer measurements is 0.07 mm (CI −0.06 to 0.19) and the mean difference in inter‐observer measurements is −0.04 mm (CI −0.12 to 0.04). For BPDoo, the mean difference in intra‐observer measurements is 0.08 mm (CI −0.05 to 0.21) and the mean difference in inter‐observer measurements is −0.11 mm (CI −0.20 to −0.02).
Figure 2.
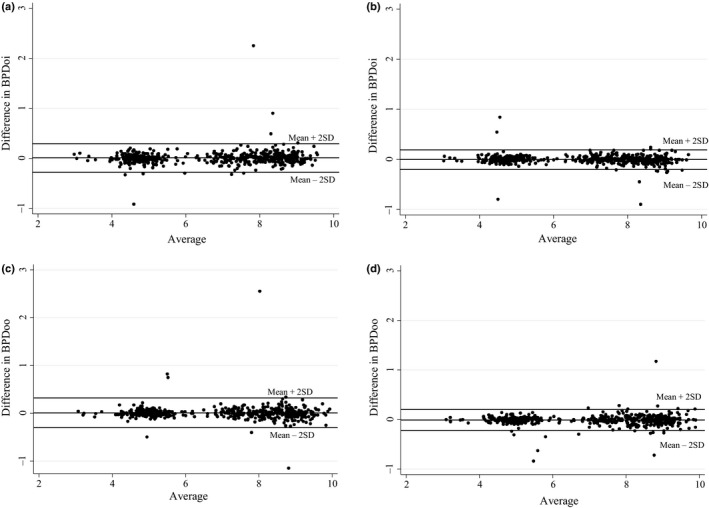
Intra‐observer and Inter‐observer Variability in BPDoi and BPDoo Measurements. (a) Intra‐observer Agreement Bland–Altman Plot for BPDoi. (b) Inter‐observer Agreement Bland–Altman Plot for BPDoi. (c) Intra‐observer Agreement Bland–Altman Plot for BPDoo. (d) Inter‐observer Agreement Bland–Altman Plot for BPDoo.
Difference in BPDoi and BPDoo with gestational age
The analysis showed that there was a moderate correlation between difference in outer‐to‐outer and outer‐to‐inner measurements and gestational age (r = 0.39). Gestational age was independently associated with a difference in outer‐to‐outer and outer‐to‐inner measurements after adjusting for BMI. On average, there was an estimated increase of 0.06 mm in difference between outer‐to‐outer and outer‐to‐inner measurements for every one‐week increase in gestational age. BMI was not associated with the difference between measurements (P = 0.22).
Figure 3 depicts the relationship between the difference in BPDoi and BPDoo (i.e. BPDoo–BPDoi) and gestational age. The translational equations to derive BPDoo using BPDoi and vice versa are as follows:
Figure 3.
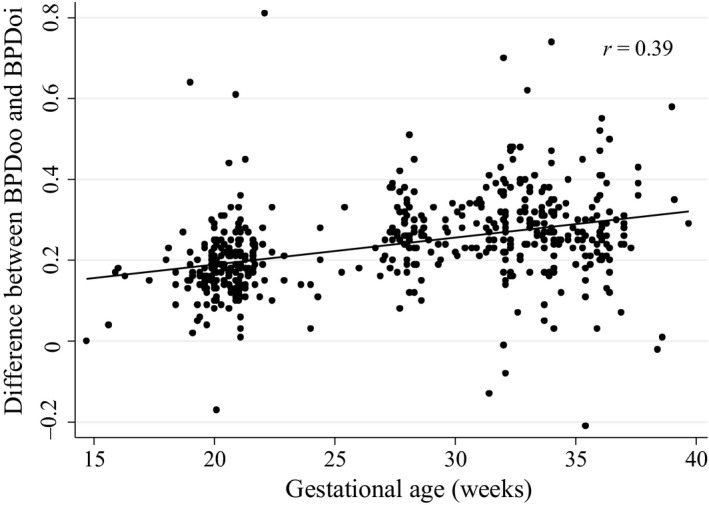
Relationship between Difference in Outer‐to‐outer and Outer‐to‐inner Measurements and Gestational Age.
For BPDoo, the regression equation is as follows:
Similarly for BPDoi, the regression equation is as follows:
Difference in EFW between Hadlock 4 and other EFW formula
Figure 4 shows the comparison of EFW derived from various formulae:
Figure 4.
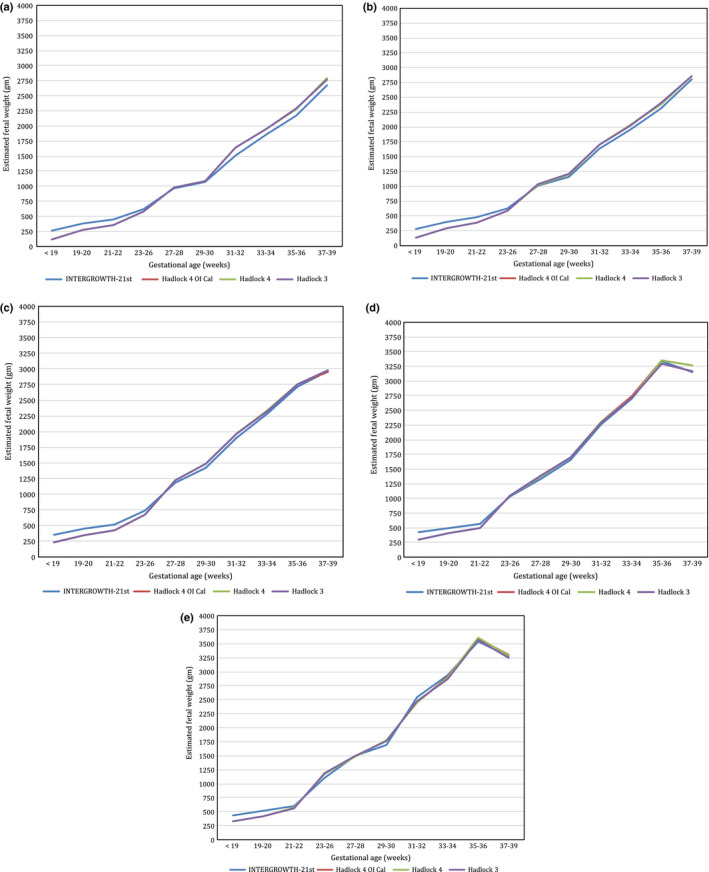
Estimated Fetal Weight Calculations Using Four Formulae: (1) Hadlock 4 Using Measured BPDoi; (2) Hadlock 4 Using BPDoi Derived from BPDoo; (3) Hadlock 3; (4) INTERGROWTH‐21st. Figures Shown are 3rd (Figure 4a), 10th (Figure 4b), 50th (Figure 4c), 90th (Figure 4d), and 97th Percentiles (Figure 4e). (a) 3rd Percentile of Estimated Fetal Weight Using Different Formulas According to the Gestational Age. (b) 10the Percentile of Estimated Fetal Weight Using Different Formulas According to the Gestational Age. (c) 50th Percentile of Estimated Fetal Weight Using Different Formulas According to the Gestational Age. (d) 90th Percentile of Estimated Fetal Weight Using Different Formulas According to the Gestational Age. (e) 97th Percentile of Estimated Fetal Weight Using Different Formulas According to the Gestational Age.
The EFW derived from Hadlock 4 using the measured BPDoi is virtually mirrored by the EFW derived using Hadlock 4 with BPDoi derived from the reverse coefficient of BPDoi. In addition, this is similar to Hadlock 3. For INTERGROWTH‐21st formula, the estimation is slightly dissimilar at smaller gestations, with INTERGROWTH‐21st showing higher EFW values than the other formulae below 27–28 weeks, converging around 27–28 weeks and diverging again with the other formulae with increasing gestation. This difference was statistically significant (P < 0.001). There was a strong correlation between difference in Hadlock 4‐ and INTERGROWTH‐21st‐estimated fetal weight and gestational age (r = 0.72; P < 0.001). On average, the difference in Hadlock 4‐ and INTERGROWTH‐21stt‐estimated foetal weight increases by 9.6 g for every one‐week increase in gestational age.
Discussion
Among the four common fetal biometric measurements, the description of HC, AC and FL is fairly standardised with well‐described and internationally agreed methodology.3 However, in the case of BPD, two equally reproducible methods of measurement have been in clinical use: BPDoi vs. BPDoo.10 The aim of this study was to determine the difference in BPD measurements between the two techniques – BPDoo and BPDoi, and how this difference varied across gestational age. We also aimed to propose a regression formula that will allow derivation of BPDoi from BPDoo and vice versa. Finally, both the measured BPDoi and derived BPDoi were incorporated into the calculation of EFW by Hadlock 3 and 4 formulae, and this was compared with a recently proposed INTERGROWTH‐21st EFW formula that did not include BPD. Our study showed that although the absolute difference between BPDoo and BPDoi increased across gestational age, this difference was small. To our knowledge, this is the first study that has assessed the difference between the two techniques of measuring BPD and proposed a regression formula that allows derivation of one from the other.
Various EFW regression formulae have been proposed that uses either BPDoi or BPDoo measurements. The original method for measuring BPD was the BPDoi technique as the inner margin of the fetal calvarium in the far field was more well defined when using static B scanners.19, 20, 21 Other charts of BPD measurement have employed the outer‐to‐outer method.7 The authors found that using modern ultrasound equipment, the measurement of BPD using either the BPDoo or BPDoi technique is equally reproducible.10 Consequently, as BPDoo can be used for both BPD and HC measurements and is also the method for measuring occipitofrontal diameter (OFD), using BPDoo would be the most efficient method as it can be adapted for all fetal head measurements. In addition, the BPDoo, as part of the HC (calculated), provides a useful tool for monitoring growth as it facilitates the tracking of head size and growth between the antenatal and post‐natal periods.7
However, in many institutions where the HC is measured directly rather than calculated, this is perhaps less relevant as the continuity of fetal head size and growth measurement will remain. Furthermore, the BPDoi technique is the established method of choice in many geographical regions. Thus, operators in these countries will need retraining to accurately determine calliper placement if BPDoo measurement is to be adopted. Besides monitoring fetal head size, the BPD is also used in the calculation of EFW using the Hadlock 4 formula, one of the most commonly used formulae globally. Should the BPDoo technique be adopted, this will necessitate the utility of a different EFW formula.
However, for institutions where BPDoo is the preferred technique, we propose a regression formula that allows the interconversion of BPDoi and BPDoo. Additionally, the EFW calculated from the two methods did not demonstrate a clinically significant difference between the Hadlock 4 (using measured BPDoi) and Hadlock 4 (using BPDoi calculated from BPDoo) (see Figure 4). However, we found that there is a slight difference between the EFW obtained via the Hadlock 4 and INTERGROWTH‐21st formulae at the lower end of gestation, before converging around 27–28 weeks and diverging again from 30 weeks’ gestation onwards. Assessment of the accuracy of the formula will require comparison against a ‘gold standard’, that is the birthweight (BW) with a preferred scan‐to‐delivery interval of less than 7 days. Unfortunately, this information was not available in our study. Previous studies have validated the use of Hadlock 3 and 4 formulae, while the INTERGROWTH‐21st regression formula has yet to be prospectively validated against birthweights outside of the INTERGROWTH‐21st study. Furthermore, the proposed EFW formula lowered the starting gestational age to 22 weeks, that is ‘2 weeks below the customary cut‐off of 24 weeks’ gestation for viability’. While the authors acknowledge that ‘this will facilitate the early recognition of fetal growth restriction around the recommended time of the second trimester anatomy scan and to anticipate the possible extension of the limit of viability’, prediction of birthweight at the extreme range of weights has been known to be most challenging and least accurate, as only a small proportion of births occurs at both extremes of the normal population curve.22 Hence, until prospective validation is performed, caution should be exercised when applying any EFW formula for the diagnosis of fetal growth restriction at the extreme lower end of gestation.
Traditionally, the BPD measurement was useful for dating a pregnancy, particularly around the 11‐ to 16‐week mark, and also for estimating fetal weights in weight equations. However, its role in dating pregnancies has become superseded by the increasing use of crown–rump length (CRL) in the first trimester, the most accurate way of determining gestational age. The role of BPD in EFW regression equations may also become less relevant as increasingly more weight is put on HC and AC, and less so on FL, as its measurement is associated with the highest inter‐ and intra‐observer variability compared to HC and AC.23 In clinical practice, the value of BPD is limited as it can be affected by a variety of other factors such as the fetal head shape and fetal presentation. In spite of this, until a new, alternative EFW formula is proposed that is widely accepted and validated, BPD measurements remain necessary for input into the many existing EFW formulae, such as Hadlock 3 or 4, which are two of the most widely accepted formulae for fetal weight estimation.
Our study also aimed to assess whether BMI affected BPDoo–BPDoi measurement. Our study found no association between BMI and the difference in BPDoo and BPDoi. Previous studies have found no association between BMI and variability of ultrasound measurements, although some have documented an association with maternal body fat distribution and, in particular, abdominal wall thickness. However, given that this study was examining the difference between two measurements, the effect of BMI is likely to be less pronounced as it will affect the two measurements in the same way and hence be negated in the subtraction.
Strength and limitations
A key strength of our study was taking into account the inter‐ and intra‐observer variability. This is particularly important as the absolute difference between the BPDoo and BPDoi measurements is small (fraction of millimetres), accuracy and reproducibility in the assessment of individual BPD values are essential in calculating the difference between the two values. Care was taken to ensure that the observers are blinded to the measurements of the other sonographer. The sonographers were not blinded to their own measurement, which could result in the sonographers making measurements to be artificially similar or biased in line with what would be expected at any given gestation. However, effort was taken to minimize this by only showing the measured numerical value but not the correlated gestational age of the measurement.
The study involved the entire team of 40 sonographers across the unit. It is recognised that to accurately assess the difference between BPDoo and BPDoi requires capturing an appropriate image and accurate calliper placement. Thus, a large team of 40 sonographers has the potential to introduce greater variability as multiple sonographers may have marginally different practices in image plane acquisition and/or calliper placement. However, we felt that the involvement of multiple sonographers would more accurately reflect clinical practice and increase generalisability of this study, although we acknowledge that there will be varying experience among the team. Attempts were made to reduce this variability as all had undergone training prior to commencement of the study. Another limitation of this study is the convenience nature of the sample. This may introduce bias as the sonographers and patients were not involved in a random manner. However, effort was made to obtain an equal distribution of patients across gestational age and sonographer practice variability minimised by having strict study protocols.
In conclusion, although the absolute difference between BPDoo and BPDoi increased across gestational age, this difference was small. In the light of recently published biometry charts, practices of BPD measurement may need to be changed, should ultrasound practice begin widespread adoption of BPD measurement technique different from their current local practice. However, until the questions regarding fetal weight estimation have been answered, it is unlikely that BPDoo will become the preferred method of measurement. Furthermore, given its limited value in clinical practice, it may remain the least preferred measurement of fetal biometry or may one day become an obsolete measurement altogether if a new alternative EFW formula is proposed that omits the BPD altogether. Until then, BPDoi and HC remain the current best practice for fetal head size measurements and are a key measurement in estimated fetal weight calculations.
Disclosure statement
We declare no financial support or relationships that may pose any conflict of interest.
Authorship declaration
We acknowledge that the authorship listing confirms with the journal's authorship policy and that all authors are in agreement with the content of the submitted manuscript.
Acknowledgements
We acknowledge the sonographers of Monash Health for participating in the study.
References
- 1.Gardosi J, Giddings S, Buller S, Southam M, Williams M. Preventing stillbirths through improved antenatal recognition of pregnancies at risk due to fetal growth restriction. Public Health 2014; 128: 698–702. [DOI] [PubMed] [Google Scholar]
- 2.Boulvain M, Irion O, Dowswell T, Thornton JG. Induction of labour at or near term for suspected fetal macrosomia. Cochrane Database Syst Rev 2016:CD000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salomon LJ, Alfirevic Z, Berghella V, Bilardo C, Hernandez‐Andrade E, Johnsen SL, et al. Practice guidelines for performance of the routine mid‐trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2011; 37: 116–26. [DOI] [PubMed] [Google Scholar]
- 4.International Society of Ultrasound in Obstetrics & Gynecology Education Committee . Sonographic examination of the fetal central nervous system: Guidelines for performing the ‘basic examination’ and the ‘fetal neurosonogram’. Ultrasound Obstet Gynecol 2007; 29: 109–16. [DOI] [PubMed] [Google Scholar]
- 5.Papageorghiou AT, Sarris I, Ioannou C, Todros T, Carvalho M, Pilu G, et al. Ultrasound methodology used to construct the fetal growth standards in the intergrowth‐21st project. BJOG 2013;120(Suppl 2):27–32. [DOI] [PubMed] [Google Scholar]
- 6.Chitty LS, Altman DG, Henderson A, Campbell S. Charts of fetal size: 2. Head measurements. Br J Obstet Gynaecol 1994; 101: 35–43. [DOI] [PubMed] [Google Scholar]
- 7.Papageorghiou AT, Ohuma EO, Altman DG, Todros T, Cheikh Ismail L, Lambert A, et al. International standards for fetal growth based on serial ultrasound measurements: the fetal growth longitudinal study of the intergrowth‐21st project. Lancet 2014; 384: 869–79. [DOI] [PubMed] [Google Scholar]
- 8.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross‐sectional study of the intergrowth‐21st project. Lancet 2014; 384: 857–68. [DOI] [PubMed] [Google Scholar]
- 9.Cheikh Ismail L, Knight HE, Ohuma EO, Hoch L, Chumlea WC. Anthropometric standardisation and quality control protocols for the construction of new, international, fetal and newborn growth standards: the intergrowth‐21st project. BJOG 2013;120(Suppl 2):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Napolitano R, Donadono V, Ohuma EO, Knight CL, Wanyonyi SZ, Kemp B, et al. Scientific basis for standardization of fetal head measurements by ultrasound: a reproducibility study. Ultrasound Obstet Gynecol 2016; 48: 80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang MW, Leung TN, Sahota DS, Lau TK, Chang AM. Customizing fetal biometric charts. Ultrasound Obstet Gynecol 2003; 22: 271–6. [DOI] [PubMed] [Google Scholar]
- 12.Dudley NJ. A systematic review of the ultrasound estimation of fetal weight. Ultrasound Obstet Gynecol 2005; 25: 80–9. [DOI] [PubMed] [Google Scholar]
- 13.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements–a prospective study. Am J Obstet Gynecol 1985; 151: 333–7. [DOI] [PubMed] [Google Scholar]
- 14.Hadlock FP, Harrist RB, Carpenter RJ, Deter RL, Park SK. Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology 1984; 150: 535–40. [DOI] [PubMed] [Google Scholar]
- 15.Westerway SC. Estimating fetal weight for best clinical outcome. Austr J Ultrasound Med 2012; 15: 13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stirnemann J, Villar J, Salomon LJ, Ohuma E, Ruyan P, Altman DG, et al. International estimated fetal weight standards of the intergrowth‐21st project. Ultrasound Obstet Gynecol 2017; 49: 478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chitty LS, Altman DG, Henderson A, Campbell S. Charts of fetal size: 4. Femur length. Br J Obstet Gynaecol 1994; 101: 132–5. [DOI] [PubMed] [Google Scholar]
- 18.Chitty LS, Altman DG, Henderson A, Campbell S. Charts of fetal size: 3. Abdominal measurements. Br J Obstet Gynaecol 1994; 101: 125–31. [DOI] [PubMed] [Google Scholar]
- 19.Chan LW, Fung TY, Leung TY, Sahota DS, Lau TK. Volumetric (3d) imaging reduces inter‐ and intraobserver variation of fetal biometry measurements. Ultrasound Obstet Gynecol 2009; 33: 447–52. [DOI] [PubMed] [Google Scholar]
- 20.Gull I, Fait G, Har‐Toov J, Kupferminc MJ, Lessing JB, Jaffa AJ, et al. Prediction of fetal weight by ultrasound: the contribution of additional examiners. Ultrasound Obstet Gynecol 2002; 20: 57–60. [DOI] [PubMed] [Google Scholar]
- 21.Hadlock FP, Deter RL, Harrist RB, Park SK. Fetal biparietal diameter: a critical re‐evaluation of the relation to menstrual age by means of real‐time ultrasound. J Ultrasound Med 1982; 1: 97–104. [DOI] [PubMed] [Google Scholar]
- 22.Kacem Y, Cannie MM, Kadji C, Dobrescu O, Lo Zito L, Ziane S, et al. Fetal weight estimation: comparison of two‐dimensional US and MR imaging assessments. Radiology 2013; 267: 902–10. [DOI] [PubMed] [Google Scholar]
- 23.Sarris I, Ioannou C, Chamberlain P, Ohuma E, Roseman F, Hoch L, et al. Intra‐ and interobserver variability in fetal ultrasound measurements. Ultrasound Obstet Gynecol 2012; 39: 266–73. [DOI] [PubMed] [Google Scholar]


