Abstract
Aims/Introduction
To examine the performance and identify the optimal threshold of vibration perception threshold (VPT) for diagnosing diabetic polyneuropathy (DPN) in a Chinese population according to multiple definitions of DPN as gold standards.
Materials and Methods
VPT was determined in 421 Chinese individuals with type 2 diabetes, who simultaneously completed a questionnaire of neuropathic symptoms, and underwent the assessment of signs of peripheral neuropathy and electromyography tests. Three definitions of DPN (i.e., clinician‐diagnosed DPN, abnormal nerve conduction and confirmed DPN) were taken as reference gold standards.
Results
Vibration perception threshold was a specific measure for all three groups of DPN outcomes, with the highest specificity noted for clinician‐diagnosed DPN (85.1%). The specificity for abnormal nerve conduction and confirmed DPN was 77.0 and 76.6%, respectively. The sensitivity of VPT was 67.0% for clinician‐diagnosed DPN, 66.5% for abnormal nerve conduction and 67.2% for confirmed DPN. The optimal cut‐off threshold for abnormal nerve conduction, as well as confirmed DPN, was VPT >14.9 V. The specificity and sensitivity of VPT >14.9 V as the cut‐off value for clinician‐diagnosed DPN were 85.6 and 66.2%, respectively. When taking clinician‐diagnosed DPN as the gold standard, the performance of VPT for diagnosing DPN was best with an area under the curve value of 0.804.
Conclusions
VPT measured using the neurothesiometer had relatively high specificity and best performance for diagnosing DPN when clinician‐diagnosed DPN rather than abnormal nerve conduction was taken as the gold standard in a Chinese population. A VPT value of ≥15 V might be equally applicable for diagnosing DPN in a Chinese population.
Keywords: Diabetic polyneuropathy, Type 2 diabetes mellitus, Vibration perception threshold
Vibration perception threshold measured using the neurothesiometer had relatively high specificity and best performance for diagnosing diabetic polyneuropathy when clinician‐diagnosed diabetic polyneuropathy rather than abnormal nerve conduction was taken as the gold standard in a Chinese population. A vibration perception threshold value of ≥15 V might be equally applicable for diagnosing diabetic polyneuropathy in a Chinese population.
Introduction
Diabetic polyneuropathy (DPN), which has a lifetime estimated prevalence up to 50–70%1, 2, 3, is one of the most common microvascular complications in patients with diabetes. It is highly correlated with the occurrence of diabetic foot ulceration, that results in great morbidity, mortality and significant economic burden4, 5. Thus, early diagnosis of DPN is the key factor for a better prognosis and preventing diabetic foot ulceration.
Nowadays, a set of neurological tests, including temperature sensation, pinprick sensation, vibration perception, pressure sensation and ankle reflexes, have been recommended as screening tests for DPN in clinical practice guidelines6, 7. However, carrying out the set of tests is time‐consuming, which hampers their widespread use in outpatient clinics or primary medical care units. Some studies found that the abnormal results on vibratory perception and pressure sensation are the most helpful signs8. The combination of testing vibration perception and pressure sensation had higher sensitivity and accuracy for identifying patients at risk of having DPN9, and was most commonly recommended in the primary care setting10.
Vibration perception thresholds (VPT) measures could potentially offer a quick and accurate screening instrument to evaluate DPN, and have been frequently used in clinical practice11, 12. Compared with various tuning fork applications, the neurothesiometer was the most reliable method of assessing VPT13, 14. However, the optimal cut‐off values for diagnosing DPN remain inconsistent. Most studies used VPT ≥25 V as one of the diagnostic criteria for DPN in people with diabetes15, 16, 17. In other studies, DPN was diagnosed based on a VPT ≥15 V18, 19. These cut‐off values of VPT were obtained through using different definitions of DPN as reference gold standards (e.g., nerve conduction function, neuropathy disability score or Michigan Neuropathy Screening Instrument). For Chinese individuals, however, which cut‐off value is more applicable remains unknown. Therefore, the current study aimed to evaluate the performance and identify the optimal threshold of VPT for DPN diagnosis in a Chinese population according to multiple definitions of DPN as gold standards.
Materials and Methods
Study population
A total of 421 individuals with type 2 diabetes mellitus were recruited from the clinic or were inpatients of the Department of Endocrinology and Metabolism of West China Hospital, Sichuan University, Chengdu, China, between July 2017 and January 2019. Diabetes was diagnosed according to the 1999 World Health Organization criteria20. We collected information on demographic features, lifestyle risk factors, history and treatment of comorbidities, and measured biochemical indices (e.g., glycosylated hemoglobin, liver and renal function, glucose and lipid) in the fasting state. The exclusion criteria were as follows: type 1 diabetes or specific types of diabetes, acute complications of diabetes, non‐diabetes‐related peripheral neuropathy, unstable cardiopulmonary conditions, severe liver disease, renal failure, malignant tumor, rheumatic disease and pregnancy or breast‐feeding.
The present study adhered to the tenets of the Declaration of Helsinki, and was approved by the Institutional Ethic Committee of West China Hospital, Sichuan University. Written informed consent was obtained from all participants.
Measurement of vibration perception threshold
Vibration perception was measured by a trained and experienced podiatric technician using a neurothesiometer (Beijing Laxons Technology Co., Ltd, Beijing, China) on the distal pulp of participants’ left great toe. The intensity of the stimulus was gradually increased from null to a voltage at which vibration was first detected by participants21. Three separate tests were carried out with participants’ eyes closed. The average of the three VPTs was used for analysis. A ‘null stimulus’ trial was added randomly to ensure participants’ adherence and understanding of the test requirements.
Definitions of DPN
Three definitions of DPN (i.e., clinician‐diagnosed DPN, confirmed DPN and abnormal nerve conduction) were used in the present study, and taken as reference gold standards to evaluate the performance of VPT for diagnosing DPN.
Clinician‐diagnosed DPN was defined by physicians by the presence of a combination of symptoms and signs of neuropathy according to the recommendations of various guidelines or expert panels7, 22. The symptoms of neuropathy included decreased sensation, numbness, prickling or stabbing and burning or aching, predominantly in the toes, feet or legs. The signs of neuropathy consisted of the abnormalities of pressure sensation, vibration perception, pinprick sensation, temperature sensation and ankle reflexes. The 10‐g monofilament, 128‐Hz tuning, pin, Tip‐Therm and percussion hammer were used to evaluate neuropathy signs. The five clinical tests were carried out in accordance with the methods described in a report from the Task Force of the Foot Care Interest Group of the American Diabetes Association22. After non‐diabetes‐related neuropathy was excluded, clinician‐diagnosing DPN was defined if patients had a combination of neuropathic symptoms and one or more positive signs7, 22.
Additionally, all patients were given electromyography tests with a Counterpoint electromyography instrument (Keypoint 4ch, Medtronic, Denmark) at the laboratory of the EMG Neurology Department. Abnormal nerve conduction was defined as the presence of one or more abnormal nerve conduction results (amplitude, conduction velocity or minimal F‐wave latency) in peroneal nerve parameter and sural nerve parameter23. Confirmed clinical neuropathy was defined as the presence of neuropathic symptoms or positive signs and abnormal nerve conduction7, 22.
Statistical analysis
Statistical analysis was carried out using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Quantitative variables expressed as the mean ± standard deviation or median with interquartile range, and categorical data were recorded as numbers and percentages. Between‐group differences of continuous variables were tested for significance using Student’s t‐test or the Wilcoxon rank test. A χ2‐test was used for categorical data.
Receiver operating characteristic curves were made to find the optimal cut‐off value of vibration perception threshold to prompt DPN under three different gold standards. The sensitivity, specificity, positive predictive value and negative predictive value (NPV) of cut‐off value were also calculated. Areas under the receiver operating characteristic curves (AUC) were used to show the performance of VPT in predicting clinician‐diagnosed DPN, confirmed DPN or abnormal nerve conduction. Generally, an AUC value of 0.5 indicates no better than chance, values between 0.70 and 0.79 indicate fair performance, values between 0.80 and 0.89 indicate good performance, and values ≥0.9 indicate excellent test performance24. Additionally, as VPT >25 V was used as the diagnostic criteria for DPN in many previous studies15, 16, 17, the sensitivity, specificity, positive predictive value and negative predictive value of VPT >25 V were also calculated in the present study to compare the performance of the optimal cut‐off value and VPT >25 V on DPN diagnosis.
Statistical significance was accepted as a two‐sided test with an alpha level of 0.05.
Results
Among the 421 participants included in the present study, DPN prevalence was highest when defined by clinician‐diagnosed neuropathy (53.9%). DPN defined by abnormal nerve conduction and confirmed DPN were present in 43.2 and 42.0% of all participants, respectively.
Characteristics of participants based on the status (yes/no) of clinician‐diagnosed DPN are shown in Table 1. Participants with clinician‐diagnosed DPN were more likely to be older, men, smokers and alcohol drinkers, and to have higher BMI, longer diabetes duration and a higher risk of having hypertension. The median level of VPT was higher among individuals with DPN versus without DPN (all P < 0.001), no matter which definition of DPN was taken as a gold standard (Figure 1).
Table 1.
Demographic and biochemical characteristics according to clinical diagnosed diabetic polyneuropathy status
| Characteristics | Clinician‐diagnosed diabetic polyneuropathy | ||
|---|---|---|---|
| No | Yes | P‐value | |
| n (%) | 194 (46.1) | 227 (53.9) | |
| Age (years) | 54.3 ± 15.8 | 62.1 ± 13.4 | <0.001 |
| Sex, male (%) | 103 (53.0) | 149 (65.6) | 0.009 |
| Race (%) | |||
| Han Chinese | 187 (96.4) | 219 (96.5) | 0.96 |
| Non‐Han Chinese | 7 (3.6) | 8 (3.5) | |
| Smoking (%) | 67 (34.5) | 103 (45.4) | 0.02 |
| Alcohol consumption (%) | 64 (33.0) | 109 (48.0) | 0.002 |
| Diabetes duration, year | 6.0 (1.0–11.0) | 10.0 (5.3–17.0) | <0.001 |
| Hypertension (%) | 91 (46.9) | 140 (61.7) | 0.002 |
| BMI (kg/m2) | 25.0 ± 4.0 | 24.3 ± 3.4 | 0.049 |
| SBP (mmHg) | 127.5 ± 18.3 | 132.5 ± 21.0 | 0.010 |
| DBP (mmHg) | 81.3 ± 12.0 | 80.3 ± 12.1 | 0.40 |
| FPG (mmol/L) | 7.4 (5.8–9.4) | 7.6 (6.2–10.1) | 0.15 |
| HbA1c (%) | 8.6 ± 2.4 | 9.0 ± 2.3 | 0.11 |
| TC (mmol/L) | 4.3 ± 1.1 | 4.2 ± 1.1 | 0.08 |
| TG (mmol/L) | 1.5 (0.9–2.2) | 1.3 (0.9–2.0) | 0.25 |
| LDL‐C (mmol/L) | 2.5 ± 0.9 | 2.4 ± 0.9 | 0.25 |
| HDL‐C (mmol/L) | 1.2 ± 0.4 | 1.2 ± 0.4 | 0.82 |
| Abnormal nerve conduction (%) | 5 (2.6) | 177 (78.0) | <0.001 |
BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
Figure 1.
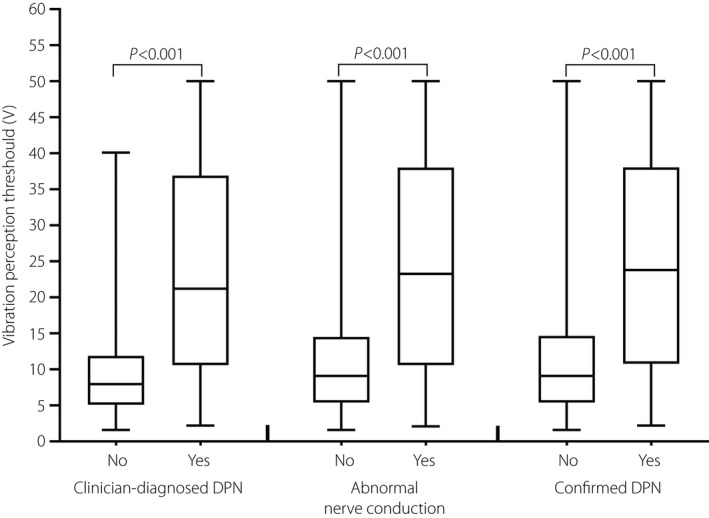
Vibration perception threshold levels by clinician‐diagnosed diabetic polyneuropathy (DPN), abnormal nerve conduction and confirmed diabetic polyneuropathy status.
VPT was a relatively specific predictor for all three groups of DPN outcomes (Table 2), with the highest specificity noted for clinician‐diagnosed DPN (85.1%). The specificity of VPT to predict abnormal nerve conduction and confirmed DPN was 77.0 and 76.6%, respectively (Table 2). For all the three groups, the sensitivity of VPT was similar and reached approximately 70% (67.0% for clinician‐diagnosed DPN, 66.5% for abnormal nerve conduction, 67.2% for confirmed DPN; Table 2). The optimal cut‐off threshold for clinician‐diagnosed DPN, abnormal nerve conduction and confirmed DPN was VPT >14.5 V, VPT >14.9 V and VPT >14.9 V, respectively (Table 2). If VPT >14.9 V was considered as the cut‐off value for clinician‐diagnosed DPN, the specificity would increase to 85.6% despite a slight decrease of the sensitivity (66.2%).
Table 2.
Performance of vibration perception threshold testing on the great toe
| Case/n | Associated criterion | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Kappa | |
|---|---|---|---|---|---|---|---|
| Clinician‐diagnosed diabetic polyneuropathy | 228/421 | >14.5 V† | 67.0 (60.4–73.0) | 85.1 (79.2–89.8) | 84.0 | 68.7 | 0.52 |
| Clinician‐diagnosed diabetic polyneuropathy | 228/421 | >14.9 V | 66.2 (59.5–72.2) | 85.6 (79.8–90.2) | 84.1 | 67.8 | 0.52 |
| Clinician‐diagnosed diabetic polyneuropathy | 228/421 | >25 V | 43.6 (37.1–50.3) | 96.4 (92.7–98.5) | 93.4 | 59.4 | 0.52 |
| Abnormal nerve conduction | 181/421 | >14.9 V† | 66.5 (59.1–73.3) | 77.0 (71.1–82.2) | 68.7 | 75.1 | 0.43 |
| Abnormal nerve conduction | 181/421 | >25 V | 48.4 (40.9–55.9) | 92.5 (88.4–95.5) | 83.0 | 70.2 | 0.43 |
| Confirmed diabetic polyneuropathy | 177/421 | >14.9 V† | 67.2 (59.8–74.1) | 76.6 (70.8–81.8) | 67.6 | 76.3 | 0.44 |
| Confirmed diabetic polyneuropathy | 177/421 | >25 V | 49.2 (41.6–56.8) | 92.2 (88.1–95.2) | 82.1 | 71.4 | 0.44 |
The optimal cut‐off threshold. NPV, negative predictive value; PPV, positive predictive value; VPT, vibration perception threshold.
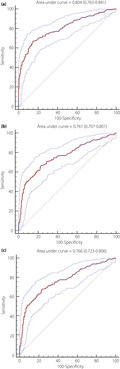
Receiver operating characteristic curves were used to show the performance of VPT against all three groups of DPN outcomes. Figure 2 shows the relationship between the true positive rate (sensitivity) and the false positive rate (1‐specificity) for various VPT values to predict clinician‐diagnosed DPN (Figure 2a), abnormal nerve conduction (Figure 2b) and confirmed DPN (Figure 2c). On a basis of the different definitions of DPN, the AUC of VPT ranged from 0.761 to 0.804 – which indicated fair‐to‐good performance – were lowest for abnormal nerve conduction and were highest for clinician‐diagnosed DPN.
Figure 2.
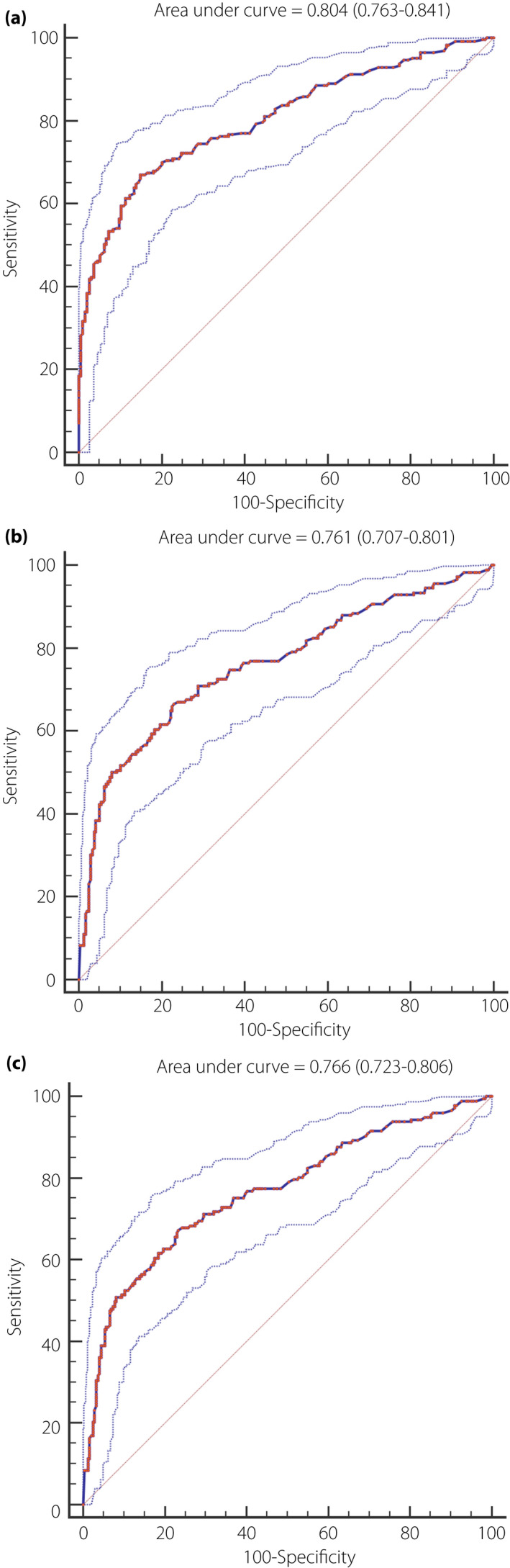
Receiver operating characteristic curve for the accuracy of vibration perception threshold testing at the great toe for (a) clinician‐diagnosed diabetic polyneuropathy, (b) abnormal nerve conduction and (c) confirmed diabetic polyneuropathy.
Discussion
In the current study, we found that VPT obtained with the neurothesiometer had relatively high specificity of diagnosing DPN, even if different definitions of DPN were taken as reference gold standards. Thus, it was also a reliable measure of DPN in the Chinese population as in the Western population14. When taking the clinician‐diagnosed DPN as the gold standard, the performance of VPT for diagnosing DPN was best with values of AUC >0.8. Although different gold standards were applied in the study, VPT >14.9 showed high sensitivity and specificity under all these gold standards.
Vibration perception threshold as a traditional method can be used to easily and accurately identify DPN in clinic or primary care11. It could reflect impairment of large nerve fibers and provide evidence for diagnosis of DPN25. Previously, some studies reported that VPT testing was a sensitive measure in the diagnosis of DPN25, 26. For example, among patients with type 1 diabetes, the results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study showed that the sensitivity of VPT to predict clinician‐diagnosed neuropathy, abnormal nerve conduction and confirmed DPN was 80%, 75 and 87%, respectively25. In the present study, however, the specificity of VPT was more notable than the sensitivity for diagnosing DPN, particularly clinician‐diagnosed DPN. Likewise, a pilot study in Indian patients with type 2 diabetes mellitus showed that the specificity of VPT for neuropathy detection got to 89.7–92.6%, but the sensitivity only was 62.5–50%, irrespective of the VPT cut point for diagnosing DPN27. Previous studies showed that there was a physiological increase in VPT with age, which could result in a higher cut point of VPT for diagnosing polyneuropathy in an older population28. In the present study, we further assessed optimal cut points and performance of VPT according to two age classes: <65 and ≥65 years. As expected, compared with overall research participants, the elderly (aged ≥65 years) had higher cut points of VPT (23.0 V or 25.9 V) for diagnosing DPN, whereas the young‐middle (<65 years) had lower cut points of VPT (12.7 V or 12.8 V) for diagnosing DPN, irrespective of different reference gold standards (Table S1). However, the sensitivity of VPT for neuropathy detection was reduced in the elderly, even if the change of the sensitivity of VPT in the young‐middle was unremarkable, compared with overall research participants (Table S1). Therefore, the sensitivity of VPT for diagnosing DPN might decrease with increasing age. In addition, DPN severity showed a negative association with the sensitivity of VPT in the previous literature29, 30. The VPT obtained with the neurothesiometer was more sensitive for the diagnosis of DPN in individuals with mild neuropathy30. However, we did not stratify the participants according to DPN severity in the present study. Thus, it remains unknown whether the relatively low sensitivity of VPT was associated with relatively severe DPN in our study, which needs to be further studied.
Interestingly, the present study found that the performance of VPT for diagnosing DPN was best when considering the presence of a combination of neuropathic symptoms and any abnormality of five signs of neuropathy, namely clinician‐diagnosed DPN, as the gold standard. Whereas taking abnormal nerve conduction as the gold standard, the lowest AUC value (0.761) of VPT was observed in the study. In contrast, Martin et al.25 reported that VPT testing for predicting confirmed DPN had the best performance with an AUC value of 0.800, whereas an AUC value of VPT for predicting clinician‐diagnosed DPN was lowest (0.745). These discrepancies might be attributed to different types of diabetes in study participants and different devices used to assess VPT25, 31. Despite these inconsistent results, as a traditional method for diagnosing DPN, the cut‐off value of VPT testing, which was recommended and frequently used14, 32, 33, was obtained based on abnormal nerve conduction as the gold standard. However, many patients initially present with symptoms and signs of small‐fiber neuropathy, but without abnormal nerve conduction2. Abnormal nerve conduction usually reflects the DPN in the later stage34. Therefore, use of abnormal nerve conduction as the gold standard might produce a high cut‐off value of VPT, which would be inclined to miss part of DPN at the early stage and reduce the performance of VPT. Among patients with type 2 diabetes mellitus, whether clinician‐diagnosed DPN rather than other definitions of DPN is a more appropriate gold standard to assess the performance of VPT remains controversial and requires further large‐sized observational studies to provide more evidence.
In previous studies, the VPT cut point of 25 V was generally for diagnosing DPN and predicting foot ulceration14, 32, 33, 35, 36, 37, 38. The criterion was established based on the risk of foot ulceration in patients with diabetes, but without lower limb ischemia35. Among these patients, as DPN was the major pathogenic mechanism of the development of food ulceration, VPT ≥25 V was also used as the diagnostic measure for DPN in subsequent studies32, 36, 38. Given that foot ulceration is usually a consequence of peripheral neuropathy with a long‐time course39, however, DPN at the early stage might be easily missed according to the diagnostic measure of VPT ≥25 V.
The results from the present study also showed that the sensitivity of VPT ≥25 V for diagnosing DPN was relatively low (43.6–49.2%). In the current study, the optimal cut‐off threshold for clinician‐diagnosed DPN, abnormal nerve conduction and confirmed DPN was VPT >14.5 V, VPT >14.9 V and VPT >14.9 V, respectively. Even if a VPT value of >14.9 V was taken as the cut‐off value for clinician‐diagnosed DPN, only slight changes in sensitivity and specificity were noted compared with the optimal cut‐off threshold. All these cut‐off values approach a VPT ≥15 V, which was defined as impaired vibration perception in other ethnic groups18, 19, 40. Therefore, a VPT value of ≥15 V should be equally applicable for diagnosing DPN in a Chinese population.
Generally, DPN was present in approximately 50% of individuals with diabetes3, 41. Similarly, the prevalence of DPN was 53.9% in the present study. Furthermore, compared with those without DPN, participants with DPN were older, had longer diabetes duration, and there were higher proportions of smokers and alcohol drinkers, and were likely to have hypertension, which are well‐established risk factors of DPN development, as reported in previous studies42, 43. These suggested that the study population enrolled in the present study was at low risk of selection bias.
Several limitations of the present study deserve mention. First, it was a cross‐sectional study, failing to observe the long‐term outcome of DPN in patients with high VPT. Thus, further prospective cohort studies are warranted to assess if the optimal cut‐off threshold of VPT is appropriate for predicting the long‐term outcome of DPN. Second, the participants in the present study reside mainly in southwestern China, possibly affecting the generalizability of the results. Further studies are required to replicate the present findings in a nationally representative Chinese population. In addition, these analyses in the study did not address the association between VPT testing and DPN severity. It might merit exploration in future study.
In conclusion, we found that VPT measured using the neurothesiometer had relatively high specificity and best performance for diagnosing DPN when the clinician‐diagnosed DPN rather than abnormal nerve conduction was taken as the gold standard in a Chinese population. Consistent with previous reports18, 19, 40, a VPT value of ≥15 V might be equally applicable for diagnosing DPN in a Chinese population. In clinical application, however, the influence of the sensory attenuation on the sensitivity of VPT ≥15 V for diagnosing DPN requires attention in the elderly population.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 | Optimal cut points and performance of vibration perception threshold testing according to two age classes: <65 and ≥65 years.
Acknowledgments
This study was supported by the National Science and Technology Major Project (Grant No. 2017 ZX09304023), National Natural Science Foundation of China Youth Science Fund Project (Grant No. 81700087), Science and Technology Bureau of Chengdu City (Grant No. 2017‐CY02‐00028‐GX), Health Medical Big Data Application and Innovation Project in Sichuan (Grant No. 2018gfgw001), 1.3.5 Project for disciplines of excellence, West China Hospital, Sichuan University (Grant No. ZYGD18025), and National Key R&D Program of China (Grant No. 2017YFC1309605).
J Diabetes Investig. 2021; 12: 1663–1670
References
- 1.Dyck PJ, Kratz KM, Karnes JL, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population‐based cohort: the Rochester Diabetic Neuropathy Study. Neurology 1993; 43: 817–824. [DOI] [PubMed] [Google Scholar]
- 2.Løseth S, Stålberg EV, Lindal S, et al. Small and large fiber neuropathy in those with type 1 and type 2 diabetes: a 5‐year follow‐up study. J Peripher Nerv Syst 2016; 21: 15–21. [DOI] [PubMed] [Google Scholar]
- 3.Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005; 28: 956–962. [DOI] [PubMed] [Google Scholar]
- 4.Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. N Engl J Med 2004; 351: 48–55. [DOI] [PubMed] [Google Scholar]
- 5.Xu Z, Ran X. Diabetic foot care in China: challenges and strategy. Lancet Diabetes Endocrinol 2016; 4: 297–298. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association . 10. Microvascular complications and foot care: standards of medical care in diabetes‐2018. Diabetes Care 2018; 41(Suppl 1): S105–S118. [DOI] [PubMed] [Google Scholar]
- 7.Jia W, Weng J, Zhu D, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev 2019; 35: e3158. [DOI] [PubMed] [Google Scholar]
- 8.Kanji JN, Anglin RE, Hunt DL, et al. Does this patient with diabetes have large‐fiber peripheral neuropathy? JAMA 2010; 303: 1526–1532. [DOI] [PubMed] [Google Scholar]
- 9.Skopljak A, Sukalo A, Batic‐Mujanovic O, et al. Assessment of diabetic polyneuropathy and plantar pressure in patients with diabetes mellitus in prevention of diabetic foot. Med Arch 2014; 68: 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chicharro‐Luna E, Pomares‐Gómez FJ, Ortega‐Ávila AB, et al. Variability in the clinical diagnosis of diabetic peripheral neuropathy. Prim Care Diabetes 2020; 14: 53–60. [DOI] [PubMed] [Google Scholar]
- 11.Garrow AP, Boulton AJ. Vibration perception threshold–a valuable assessment of neural dysfunction in people with diabetes. Diabetes Metab Res Rev 2006; 22: 411–419. [DOI] [PubMed] [Google Scholar]
- 12.van Deursen RW, Sanchez MM, Derr JA, et al.Vibration perception threshold testing in patients with diabetic neuropathy: ceiling effects and reliability. Diabet Med 2001; 18: 469–475. [DOI] [PubMed] [Google Scholar]
- 13.Baker N. An alternative to a 10‐g monofilament or tuning fork? Two new, simple, easy‐to‐use screening tests for determining foot ulcer risk in people with diabetes. Diabet Med 2012; 29: 1477–1479. [DOI] [PubMed] [Google Scholar]
- 14.Lanting SM, Spink MJ, Tehan PE, et al. Non‐invasive assessment of vibration perception and protective sensation in people with diabetes mellitus: inter‐ and intra‐rater reliability. J Foot Ankle Res 2020; 13: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 16.Kärvestedt L, Mårtensson E, Grill V, et al. The prevalence of peripheral neuropathy in a population‐based study of patients with type 2 diabetes in Sweden. J Diabetes Complications 2011; 25: 97–106. [DOI] [PubMed] [Google Scholar]
- 17.Adams OP, Herbert JR, Howitt C, et al. The prevalence of peripheral neuropathy severe enough to cause a loss of protective sensation in a population‐based sample of people with known and newly detected diabetes in Barbados: a cross‐sectional study. Diabet Med 2019; 36: 1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiles PG, Pearce SM, Rice PJ, et al. Vibration perception threshold: influence of age, height, sex, and smoking, and calculation of accurate centile values. Diabet Med 1991; 8: 157–161. [DOI] [PubMed] [Google Scholar]
- 19.Ponirakis G, Elhadd T, Chinnaiyan S, et al. Prevalence and management of diabetic neuropathy in secondary care in Qatar. Diabetes Metab Res Rev 2020; 36: e3286. [DOI] [PubMed] [Google Scholar]
- 20.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553. [DOI] [PubMed] [Google Scholar]
- 21.Claus D, Mustafa C, Vogel W, et al. Assessment of diabetic neuropathy: definition of norm and discrimination of abnormal nerve function. Muscle Nerve 1993; 16: 757–768. [DOI] [PubMed] [Google Scholar]
- 22.Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010; 33: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.England JD, Gronseth GS, Franklin G, et al. Distal symmetrical polyneuropathy: definition for clinical research. Muscle Nerve 2005; 31: 113–123. [DOI] [PubMed] [Google Scholar]
- 24.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983; 148: 839–843. [DOI] [PubMed] [Google Scholar]
- 25.Martin CL, Waberski BH, Pop‐Busui R, et al. Vibration perception threshold as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the DCCT/EDIC study. Diabetes Care 2010; 33: 2635–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong DG, Lavery LA, Vela SA, et al. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med 1998; 158: 289–292. [DOI] [PubMed] [Google Scholar]
- 27.Ghosal S, Stephens J, Mukherjee A. Quantitative vibration perception threshold in assessing diabetic neuropathy: is the cut‐off value lower for Indian subjects? [Q‐VADIS Study]. Diabetes Metab Syndr 2012; 6: 85–89. [DOI] [PubMed] [Google Scholar]
- 28.Maffei L, Premrou V, Roldan P, et al. Vibration perception threshold in the screening of sensorimotor distal symmetric polyneuropathy: the need of more accurate age‐specific reference values. J Diabetes Sci Technol 2014; 8: 621–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos TRM, Melo JV, Leite NC, et al. Usefulness of the vibration perception thresholds measurement as a diagnostic method for diabetic peripheral neuropathy: results from the Rio de Janeiro type 2 diabetes cohort study. J Diabetes Complications 2018; 32: 770–776. [DOI] [PubMed] [Google Scholar]
- 30.Bril V, Perkins BA. Comparison of vibration perception thresholds obtained with the Neurothesiometer and the CASE IV and relationship to nerve conduction studies. Diabet Med 2002; 19: 661–666. [DOI] [PubMed] [Google Scholar]
- 31.Borire AA, Issar T, Kwai NC, et al. Correlation between markers of peripheral nerve function and structure in type 1 diabetes. Diabetes Metab Res Rev 2018; 34: e3028. [DOI] [PubMed] [Google Scholar]
- 32.Feldman EL, Stevens MJ, Thomas PK, et al. A practical two‐step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994; 17: 1281–1289. [DOI] [PubMed] [Google Scholar]
- 33.Boulton AJ, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care 2008; 31: 1679–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breiner A, Lovblom LE, Perkins BA, et al. Does the prevailing hypothesis that small‐fiber dysfunction precedes large‐fiber dysfunction apply to type 1 diabetic patients? Diabetes Care 2014; 37: 1418–1424. [DOI] [PubMed] [Google Scholar]
- 35.Young MJ, Breddy JL, Veves A, et al. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds: a prospective study. Diabetes Care 1994; 17: 557–560. [DOI] [PubMed] [Google Scholar]
- 36.Abbott CA, Vileikyte L, Williamson S, et al. Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care 1998; 21: 1071–1075. [DOI] [PubMed] [Google Scholar]
- 37.Lu B, Yang Z, Wang M, et al. High prevalence of diabetic neuropathy in population‐based patients diagnosed with type 2 diabetes in the Shanghai downtown. Diabetes Res Clin Pract 2010; 88: 289–294. [DOI] [PubMed] [Google Scholar]
- 38.Azzopardi K, Gatt A, Chockalingam N, et al. Hidden dangers revealed by misdiagnosed diabetic neuropathy: a comparison of simple clinical tests for the screening of vibration perception threshold at primary care level. Prim Care Diabetes 2018; 12: 111–115. [DOI] [PubMed] [Google Scholar]
- 39.Vas PRJ, Edmonds ME. Early recognition of diabetic peripheral neuropathy and the need for one‐stop microvascular assessment. Lancet Diabetes Endocrinol 2016; 4: 723–725. [DOI] [PubMed] [Google Scholar]
- 40.Goel A, Shivaprasad C, Kolly A, et al. Comparison of electrochemical skin conductance and vibration perception threshold measurement in the detection of early diabetic neuropathy. PLoS One 2017; 12: e0183973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev 2012; 28(Suppl 1): 8–14. [DOI] [PubMed] [Google Scholar]
- 42.Tesfaye S, Stevens LK, Stephenson JM, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia 1996; 39: 1377–1384. [DOI] [PubMed] [Google Scholar]
- 43.Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med 2005; 352: 341–350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Optimal cut points and performance of vibration perception threshold testing according to two age classes: <65 and ≥65 years.


