The Australasian Society for Ultrasound in Medicine (ASUM) is the leading multidisciplinary medical ultrasound society advancing the clinical practice of diagnostic medical ultrasound for the highest standards of patient care in Australia and New Zealand. The Australasian College for Infection Prevention and Control (ACIPC) is the peak body for Infection Prevention and Control professionals in the Australasian region focused on promoting education and evidence based practice outcomes for a healthy community. This document was developed collaboratively by ASUM and ACIPC to establish nationally accepted guidelines for reprocessing ultrasound transducers. The requirements in these guidelines have been based on the standards of AS/NZS4187:2014 and AS/NZS4185:2006.1 These guidelines must be used as the minimum standard of practice for reprocessing ultrasound transducers and considered to be best practice at the time which they were issued.
1. Introduction
In Australia, ultrasound is increasingly utilised as an imaging modality in a diversity of care environments. Each ultrasound procedure involves contact between an ultrasound transducer and the patient's skin, mucous membranes, or sterile tissues. Failure to adhere to minimum infection control standards, including the proper cleaning and reprocessing of the equipment and transducers, increases the risk of pathogen transmission and subsequent infection. Lack of compliance with scientifically based guidelines for infection control has led to numerous outbreaks arising from ultrasound examinations,2, 3, 4, 5, 6, 7, 8, 9, 10 including cases of infection resulting from ultrasound‐guided procedures,4, 11, 12, 13 and ultrasound transducers that have not undergone appropriate disinfection (Medical Device Alert Ref: MDA/2012/037)14, 15 or have been damaged.16
1.1. Scope and target audience
The Guidelines for Reprocessing Ultrasound Transducers provides recommendations for the cleaning and disinfection of all medical ultrasound transducers and any additional equipment that may be utilised during the procedure, such as the keyboard and ultrasound gel. These guidelines are recommended for all individuals directly or indirectly involved with medical ultrasound.
Abbreviations
- ACIPC
Australasian College for Infection Prevention and Control
- ARTG
Australian Register of Therapeutic Goods
- AS/NZS 4815:2006
Australian/New Zealand Standard™ Office‐based health care facilities – Reprocessing of reusable medical and surgical instruments and equipment, and maintenance of the associated environment
- AS/NZS4187:2014
Australian/New Zealand Standard™ Reprocessing of reusable medical devices in health service organizations
- ASUM
Australasian Society for Ultrasound in Medicine
- FDA
Food and Drug Authority
- HAI
Healthcare‐Associated Infection
- HLD
High Level Disinfection
- IFU
Instructions for Use
- LLD
Low Level Disinfection
- MRC
Minimum Recommended Concentration
- NHMRC
National Health and Medical Research Council
- SDS
Safety Data Sheet: a form supplied with the product detailing the properties of the product
- TGO54
Therapeutic Goods Order No.54
- TGA
Therapeutic Goods Administration
2. Definition of terms (adapted from AS/NZS4187:2014)
2.1. Cleaning
The removal of contamination from an item to the extent necessary for further processing or for intended use.17
2.2. Disinfection
The destruction of many microorganisms (including human pathogens) using thermal or chemical means. Unlike sterilisation, disinfection is not effective against high numbers of bacterial endospores. Disinfectants are classified by grade as follows:
Low‐level instrument grade: disinfectant that kills vegetative bacteria, some fungi and some viruses.
Intermediate‐level instrument grade: disinfectant that kills vegetative bacteria, Mycobacteria, viruses and most fungi but not bacterial endospores.
High‐level instrument grade: disinfectant that kills all microorganisms with the exception of high numbers of bacterial endospores.
2.3. Sterilisation
Sterilisation destroys microorganisms on an object rendering it free from viable microorganisms.
3. Medical device classification
The Spaulding classification system states that medical devices are classified as non‐critical, semi‐critical or critical according to the risk of transmission of microorganisms associated with their use.
3.1. Non‐critical medical devices
Ultrasound transducers that come into contact with intact skin are considered non‐critical medical devices and as such are reprocessed by cleaning and may be followed by low‐level disinfection (LLD) method as described in Section 7.1 ‘Low‐level disinfection’.
3.2. Semi‐critical medical devices
Ultrasound transducers that come into contact with non‐intact skin and / or mucous membranes and transducers that have had likely contact with blood / body fluids are considered as semi‐critical medical devices due to the high risk of potential contamination. These transducers are reprocessed by cleaning followed by a high‐level disinfection (HLD) method as described in Section 7.2 ‘High‐level disinfection’.
3.3. Critical devices
Transducers are extremely delicate and heat sensitive and as such are reprocessed as a semi‐critical medical device by cleaning followed by a HLD method as described in Section ‘High‐level disinfection’. An appropriate sterile sheath or transducer cover is applied, allowing it to be used on the critical aseptic field (AS/NZS4187:2014 Clause 5.1.3 (e)).
4. Sterilisation and disinfection methods
While there are multiple methods of sterilisation in practice, sterilisation of ultrasound transducer is impractical, due to the heat sensitivity of transducers. High‐level disinfection of ultrasound transducers must kill all forms of bacteria (including mycobacteria), viruses, fungi and protozoa and achieve disinfection.
5. Mechanisms of infection
5.1. Endogenous infection
Endogenous infection occurs as a result of breakdown of a normal barrier, thereby allowing the patient's own flora to access a normally sterile site. This can occur during ultrasound‐assisted biopsy and other procedures where normally sterile sites are accessed. This mode of infection is an intrinsic risk in the collection of a biopsy from an ordinarily sterile site and is not related to the cleaning, disinfecting or sterilising of ultrasound equipment.
5.2. Exogenous infection
Exogenous infection results from an organism extrinsic to the patient's own microbiota. Disinfection and cleaning procedures are intended to prevent this type of infection. There are two kinds of exogenous infection: patient to patient and hospital environment to patient. The risk of exogenous infection is increased if ultrasound equipment / accessories: (i) have not been properly cleaned and disinfected (ii) have been damaged (iii) are poorly designed, and / or (iv) are contaminated by the ultrasound gel. Poor compliance with infection control guidelines increases the risk of infection.
Ultrasound procedures are used to collect biopsy samples for microbiological examination. Improper collection procedures can lead to the contamination of these samples and the erroneous diagnosis of infection.
6. Agents potentially transmitted by ultrasound procedures
Ultrasound procedures contaminated with pathogenic bacteria can cause disease and may lead to further spread of these organisms.7, 9, 10, 14, 15, 16, 18, 19, 20, 21 Of particular concern are the following:
Staphylococcus aureus (including Methicillin‐Resistant S. aureus (MRSA))
Vancomycin‐Resistant Enterococci (VRE)
Multi‐resistant gram‐negative organisms (MRGN)
Carbapenem‐resistant enterobacteraciae (CRE)
Mycobacterium tuberculosis complex (MBTC)
-
Non‐tuberculous ‘atypical’ mycobacteria
-
○
Mycobacteria are relatively resistant to most chemical disinfectants, including aldehydes. Transmission of mycobacteria has been associated with ultrasound procedures.2
-
○
-
Clostridium difficile
-
○
Spores of C. difficile are less resistant to some disinfectants than the spores of other bacterial species. High‐level disinfection procedures have been shown to inactivate C. difficile spores.
-
○
-
Neisseria gonorrhoeae, Chlamydia trachomatis, Treponema pallidum (syphilis), Mycoplasma genitallium
-
○
Transmission of these organisms is a specific risk when transoesophageal, transrectal or transvaginal ultrasounds are performed. These organisms may not be removed by low‐level disinfection wipes.22
-
○
Blood‐borne viruses (BBVs) such as the human immunodeficiency virus (HIV), Hepatitis B virus (HBV) and Hepatitis C virus (HCV) can be spread through contact with the blood or body fluid of infected persons. Many chemical disinfectants inactivate blood‐borne viruses; however, ineffective cleaning prior to disinfection limits the effectiveness of chemical disinfection and leads to the persistence of active virus after a disinfection procedure. Improperly cleaned and disinfected ultrasound equipment maybe capable of transmitting BBVs;18 however, ancillary equipment, particularly related to biopsy and the administration of intravenous medication, presents the highest risk of transmission.12 It is a vital and best surgical and anaesthetic practice that is adhered to in addition to appropriate ultrasound equipment cleaning and reprocessing to minimise the risk of transmission.
Human herpes virus 1 (HHV1) and human herpes virus 2 (HHV2) are common viruses that are relatively resistant to decontamination. Transducers are known to become contaminated with these viruses, even when a transducer cover is used.23 Transmission of herpes viruses during vaginal ultrasound in pregnancy may have consequences for a subsequent delivery and create a risk of neonatal herpes.
Human papilloma viruses (HPVs) are of particular concern when performing transvaginal ultrasound. Certain ‘high‐risk’ types (in particular 16 and 18) are agents of cervical cancer. This virus is known to be persistent in the environment and retain a high proportion of its infectivity after dehydration for 7 days. Several studies have examined the likelihood of transmission of this organism through transvaginal ultrasound using current infection control practices and found that transducer covers do not adequately prevent the contamination of transducers with HPVs24 and that low‐level disinfection may not remove HPV from ultrasound transducers.22, 24
Many other potentially infectious agents may be transmitted via improperly maintained, cleaned and disinfected ultrasound equipment, accessories and transducers, including protozoal pathogens such as Trichomonas vaginalis, intestinal parasites such as Entamoeba histolytica or fungal pathogens including dermatophytes and hyphomycetes.
7. Recommended cleaning and disinfection procedures
Cleaning is an essential prerequisite for all LLD and HLD processes. Organic residue may prevent the disinfectant from contacting all surfaces of the medical device being processed and may also bind and inactivate chemical disinfectants.25 If the transducer has grooves or crevices, then it must be cleaned with a soft brush prior to any LLD or HLD reprocessing.
Cleaning agents and instrument‐grade HLD methods must be intended for use on medical devices and entered the Australian Register of Therapeutic Goods (ARTG). All transducers must be cleaned according to the manufacturer's instructions.
Ultrasound transducers are heat‐sensitive items and as such will need to be disinfected using low‐temperature chemical sterilising / disinfecting agents or other approved automated systems. Any products used for cleaning or disinfection must be compatible with the ultrasound equipment as determined by the ultrasound equipment manufacturer. The instructions for use for any ultrasound equipment must be consulted to ensure compatibility prior to using any type of disinfectant on their transducers. Care should be taken to follow each disinfectant manufacturer's labelled conditions for the use of their specific products. Directions for use are not interchangeable between formulations from either the same or different manufacturers.
Some disinfectants may have associated toxicity issues, and personal protective equipment (PPE) need to be used, along with fume cabinets and / or any other safety instructions outlined on the Safety Data Sheet (SDS).
7.1. Low‐level disinfection
Manually remove all ultrasound gel prior to cleaning.
-
Clean transducer using a TGA‐approved disposable cleaning wipe or system intended for use on medical devices.
or
Clean transducer using freshly made up solution of cleaning agent at the correct concentration. Rinse thoroughly under running water to remove cleaning agent residues. Dry using a single‐use low linting cloth.
7.2. High‐level disinfection
High‐level disinfection, with an approved disinfectant method, is necessary for further statistical reduction in the number of microorganisms. The definitions given in TGO54 indicate that when used as recommended by the manufacturer, high‐level disinfection methods inactivate all microbial pathogens, except large numbers of bacterial endospores.
Following step 7.1, transducers must undergo high‐level disinfection (HLD) using a TGA‐approved instrument grade disinfection method following the manufacturer's instructions for use (IFU). Methods of high‐level disinfection include, but may not be limited to, the following:
-
Liquid high‐level instrument grade chemical disinfectants
or
-
Automated high‐level disinfection systems, for example chemical or light‐based
or
High‐level instrument grade disinfectant wipes.
7.2.1. Rinsing / neutralisation and drying
Rinsing / neutralisation is an important step to remove any disinfectant residue or by‐product post high‐level disinfection. All transducers must be rinsed with clean water post‐HLD to maintain the microbiological quality of the reprocessed transducer (AS/NZS4187:2014). Transducers should be dried using a single‐use low linting cloth.
Transducers used on critical aseptic fields require filtered or sterile water to be used for rinsing to comply with AS/NZS4187:2014 requirements. Immediately following HLD and rinsing, transducers should be dried using a sterile single‐use low linting cloth prior to insertion into the sterile sheath / sleeve or transducer cover.
7.3. Storage
After cleaning, all transducers must be stored in an appropriate environment to protect from environmental contamination (AS/NZS4187:2014, Table 5.1). It is recommended that a specific cabinet is used, but if this is not available the minimum standard recommended is a clean disposable cover applied to the transducer to mitigate risks from environmental contaminants.
8. Traceability
Records of HLD must be kept in accordance with the requirements specified in AS/NZS4187:2014 (Clause 2.4.3.2 (a)) to ensure a system of traceability is in place to enable recall procedures to be followed in case of decontamination failure.
AS/NZS4187:2014 requires documentation of the following:
Date of reprocessing;
Type of transducer and unique identification number, for example the serial number;
Person responsible for the cleaning and disinfection and release of the transducer for use;
Batch numbers and expiry dates of the disinfectant and any chemical indicator test strips used to check minimum recommended concentration (MRC);
-
For manual HLD processes:
Record of the immersion time in and out of the HLD, and where applicable the temperature of the solution; results of chemical indicator test strips used to check MRC and completion of the final rinse to remove HLD residues;
-
For automated HLD processes:
Record of the process cycle for automated cleaning and / or disinfection;
-
For HLD wipes:
Record of the batch number and expiry dates of the products used for cleaning, HLD and neutralisation.
9. Equipment cleaning
Any equipment that has been in contact with the patient or operator should be cleaned with a detergent / disinfectant wipe or solution between use, for example the leads to the transducer, the keyboard or the bed. This reduces the risk of potential cross‐contamination between the patients and the operator.22, 26, 27, 28, 29 Ensure that all cleaning agents used for the general environment have been approved by the equipment manufacturer.
10. Workflow
Workflows should promote best practice to reduce risk of contamination of clean areas with contaminated equipment. Workflows in the reprocessing area will be unidirectional to avoid the risks of cross‐contamination of clean and / or high‐level disinfected transducers. Reprocessing can be performed at the point of care (POC) or in a separate room. If conducted at POC, the products used must be safe to use in that setting. If reprocessing is performed in another room, a system for transducer transport must be implemented to ensure that dirty and clean transducers are not mixed. Separate transport containers for dirty and clean transducers are required.
11. Operator requirements and standard precautions
Every patient must be regarded as a potential source of harmful microorganisms, and appropriate precautions should be taken to prevent cross‐infection between patient and operator and from patient to patient. These ‘standard precautions’ are promoted throughout organisations. Hand hygiene both before and after direct patient contact, and before any procedure, is particularly important. Other precautions include the use of personal protective equipment (PPE), correct handling and disposal of waste and ensuring a clean working environment is maintained between patients.
All users of ultrasound equipment must be trained in reprocessing procedures. They should receive formal training in medical device cleaning and disinfection to ensure safe and effective reprocessing of the transducers. It is expected that minimum standard training will be completed by all ultrasound healthcare workers which includes hand hygiene and aseptic techniques. Annual updates are recommended.
12. Transducer / probe covers
All intracavity transducers should be covered with a single‐use high‐quality transducer cover. This may include some, but not all brands of condoms, specific transducer covers or surgical drapes. Prior to the application of a latex transducer cover, specific enquiry should be directed to the patient regarding latex sensitivity and, if appropriate, special non‐latex covers need to be utilised.
At the end of the procedure, using a gloved hand, the disposable cover should be removed and discarded, taking care not to contaminate the handle, cable or cord of the transducer.
Although the use of a disposable cover reduces the level of risk of contamination, covers can be perforated or contain small, unrecognised defects. Due to the reported breakage rate of transducer covers, for maximum safety, cleaning and high‐level disinfection of the transducer is recommended between each use.30 Covers used on transducers introduced into critical aseptic fields must be sterile and applied in a manner that prevents the contamination of the sterile barrier. It appears the risk of patient to patient transmission of infection post ultrasound guided procedures, where a sterile transducer cover is used, is low. To date, there are no studies evaluating the effectiveness of a variety of transducer covers used during an ultrasound guided procedure. However, in view of a recent investigation showing a majority of ultrasound machines in Emergency Departments were contaminated with blood,27 appropriate infection control measures should be implemented. This would include a sterile transducer cover for every real‐time ultrasound guided procedure, followed by HLD, unless the transducer is some distance away from the needle and contamination with blood or body fluid not possible.
13. Ultrasound gel recommendations
Ultrasound gels may be sterile or non‐sterile and have been the source of past outbreaks of infection. Ultrasound gels, that are defined as sterile, are unopened ultrasound gel packets or sachets that are specifically labelled as ‘sterile’. Ultrasound gel products that are labelled as non‐sterile or that are not labelled at all with respect to sterility are not sterile. The use of non‐sterile gel should be limited to low‐risk general examinations on intact skin. If a non‐sterile gel is being used on a patient who is on any transmission‐based precautions, a single‐use gel bottle or sachet should be used. Due to the risk of bacterial contamination and growth within a warm environment, heating of gel is not recommended.29 In circumstances where warm gel is necessary, the use of dry heat is the preferred method.31 After each use, lids should be closed on reusable gel bottles.
The basic minimum standards to minimise the infection transmission risk through the use of contaminated ultrasound gel are as follows:
Always follow the manufacturer's instructions for care and use;
Ensure reusable dispenser bottles are completely emptied, thoroughly washed and dried daily / weekly according to your facility's infection control practices;
Clean all reusable equipment according to the manufacturer's instructions;
For procedures that require the use of sterile gel, ensure that only unopened containers / sachets labelled ‘sterile’ are used;
Any unused portion of single‐use sterile gel packets must be discarded and not reused for another examination or patient.
Disclaimer
These joint guidelines are intended to provide best practice guidelines on the reprocessing of ultrasound transducers. The requirements in these guidelines have been based on the standards AS/NZS4187:2014 and AS/NZS4185:2006. The information within this document has been developed and reviewed by the Australasian College for Infection Prevention and Control (ACIPC) and the Australasian Society for Ultrasound in Medicine (ASUM) Working Committee and approved by ACIPC Board and ASUM Council. Although every effort has been made to ensure that these guidelines are accurate, neither ASUM or ACIPC, nor its employees or members accepts any liability for the consequences of any misleading statements or opinions.
These guidelines will be reviewed jointly by ASUM and ACIPC every 4 years unless evidence suggests an earlier review is required.
Guideline Authors
These joint guidelines were developed by a working committee representing ASUM and ACIPC. The document was produced by:
Dr Jocelyne M Basseal, Australasian Society for Ultrasound in Medicine (ASUM), Sydney NSW
Adj Assoc Prof Susan Campbell Westerway, Faculty of Dentistry & Health Sciences, Charles Sturt University NSW
Marija Juraja, Clinical Service Coordinator (CICP, GCNS) – Infection Prevention & Control Unit, Division of Acute Medicine, The Queen Elizabeth Hospital, Central Adelaide Local Health Network (CALHN), SA
Assoc Prof Thea F van de Mortel, School of Nursing and Midwifery, Griffith University, QLD
Terry E McAuley, Director STEAM Consulting Pty Ltd.
Assoc Prof James Rippey, Emergency Medicine Physician, Sir Charles Gairdner Hospital, WA
Simon Meyer‐Henry, CICP‐E, Infection Prevention & Management, Royal Perth Hospital, WA
Dr Samuel Maloney, Clinical Microbiologist, Gold Coast University Hospital, Visiting Research Fellow in the Institute for Glycomics, QLD
Amanda Ayers, Infection Control Consultant, Hand Hygiene Program Coordinator, Infection Prevention & Control, Bendigo Health, VIC
Susan Jain, MN, Centre for Healthcare Epidemiology and Staff Services, Prince of Wales Hospital, Sydney, NSW
Dr Karen Mizia, Consultant Obstetrician, Gynaecologist and Sonologist, Ultrasound Care, Randwick, Sydney, NSW
Denise Twentyman, Acting Safety Quality Infection Control Officer, Tennant Creek Hospital, Central Australia Health Service, NT.
Citation
These Guidelines should be cited as: Basseal JM, Westerway SC, Juraja M, van de Mortel T, McAuley TE, Rippey J, et al. Guidelines for reprocessing ultrasound transducers. Australas J Ultrasound Med 2017; 20: 30–40.
15. Appendices
Acknowledgements
The authors would like to acknowledge the assistance in reviewing the Guidelines from Dr Adrian Goudie (Fiona Stanley Hospital, Western Australia), Dr Fabricio Da Silva Costa (Monash Ultrasound for Women and Perinatal Services, Monash Medical Centre, Melbourne, Victoria), Prof Jon Hyett (Department of High Risk Obstetrics, Royal Prince Alfred Hospital, Sydney, NSW) and Ms Fiona Law (President Medical Imaging Nurses’ Association NSW & ACT, Prince of Wales/Sydney Children's Hospitals, NSW, Australia).
Appendix A. Reference Guide Tables for cleaning ultrasound transducers
A.1.
These tables were developed as a reference guide only to assist with ultrasound‐specific procedures undertaken by various ultrasound specialties.
Please note that all cleaning and disinfection products must be intended for use on medical devices, registered on the Australian Register of Therapeutic Goods (ARTG) and approved by the Therapeutic Goods Administration (TGA). All transducers must be cleaned according to the manufacturer's instructions.
Table 1.
Reference guide to cleaning ultrasound transducers in the Emergency Medicine Department
| Transducer | Procedure | Use of transducer cover |
Recommended cleaning method Low‐Level Disinfection (LLD) or High‐Level Disinfection (HLD) |
|---|---|---|---|
| External | Normal intact skin | No | LLD |
| Open wound, for example ulcers | Yes | HLD | |
| Intact infected skin | No | HLD | |
| Yes | HLDa | ||
|
US‐guided interventional procedure For example, joint aspiration; abscess drainage; foreign body removal; suprapubic bladder tap; nerve block |
Yes | HLDa | |
| Peripheral IV line insertion | Yes | HLDa | |
| CVC / PICC insertion | Yes | HLDa | |
| Pleural tap, ascites tap or drainage | Yes | HLD | |
| Intracavity | Transvaginal | Yes | HLD |
LLD can be performed if the transducer is classified as non‐critical. Non‐critical transducers do not contact non‐intact skin, blood or mucous membranes. If the transducer comes in direct contact with non‐intact skin, blood or mucous membranes transducers should be cleaned with HLD irrespective of the use of a transducer cover. If transducer cover is broken during a procedure, then HLD must be performed.
Table 2.
Reference guide to cleaning ultrasound transducers in the Radiology Department
| Transducer | Procedure | Use of transducer cover |
Recommended cleaning method Low‐Level Disinfection (LLD) or High‐Level Disinfection (HLD) |
|---|---|---|---|
| External | Intact skin | No | LLD |
| Open wound, for example ulcers | Yes | HLD | |
| Intact infected skin | No | HLD | |
| Yes | HLDa | ||
|
Externala Needle‐guided procedures |
MSK injection | Yes | HLDa |
| Joint aspiration; abscess drainage; foreign body removal; suprapubic bladder tap; nerve block | Yes | HLDa | |
| Peripheral IV line insertion | Yes | HLDa | |
| CVC / PICC insertion | Yes | HLDa | |
| Pleural tap, ascites tap or drainage | Yes | HLD | |
| Intracavity | Transvaginal | Yes | HLD |
| Transrectal / TRUSS | Yes | HLD | |
| Intraoperative | Yes | HLD | |
LLD can be performed if the transducer is classified as non‐critical. Non‐critical transducers do not contact non‐intact skin, blood or mucous membranes. If the transducer comes in direct contact with non‐intact skin, blood or mucous membranes transducers should be cleaned with HLD irrespective of the use of a transducer cover. If transducer cover is broken during a procedure, then HLD must be performed.
Table 3.
Reference guide to cleaning ultrasound transducers in the O&G Department
| Transducer | Procedure | Use of transducer cover | Recommended cleaning method Low‐Level Disinfection (LLD) or High‐Level Disinfection (HLD) |
|---|---|---|---|
| External | Intact skin | No | LLD |
| Non‐intact skin | Yes | HLD | |
|
Externala Needle‐guided procedure |
Amniocentesis CVS Suprapubic bladder tap for urine retention |
Yes | HLDa |
| Intracavity | Transvaginal | Yes | HLD |
| Intraoperative | Yes | HLD | |
LLD can be performed if the transducer is classified as non‐critical. Non‐critical transducers do not contact non‐intact skin, blood or mucous membranes. If the transducer comes in direct contact with non‐intact skin, blood or mucous membranes transducers should be cleaned and with HLD irrespective of the use of a transducer cover. If transducer cover is broken during a procedure, then HLD must be performed.
Table 4.
Reference guide to cleaning ultrasound transducers in the Vascular Department
| Transducer | Procedure | Use of transducer cover | Recommended cleaning method Low‐Level Disinfection (LLD) or High‐Level Disinfection (HLD) |
|---|---|---|---|
| External | Intact skin | No | LLD |
| Intact infected skin | No | HLD | |
| Yes | HLDa | ||
| Ulcerated skin | Yes | HLD | |
|
Internal Ultrasound‐guided surgical procedure |
IVUS probe | Not reusable – discard after use | |
| Intraoperative | Yes | HLD | |
LLD can be performed if the transducer is classified as non‐critical. Non‐critical transducers do not contact non‐intact skin, blood or mucous membranes. If the transducer comes in direct contact with non‐intact skin, blood or mucous membranes transducers should be cleaned with HLD irrespective of the use of a transducer cover. If transducer cover is broken during a procedure, then HLD must be performed.
Table 5.
Reference guide to cleaning ultrasound transducers in the Cardiac Department
| Transducer | Procedure | Use of transducer cover | Recommended cleaning method Low‐Level Disinfection (LLD) or High‐Level Disinfection (HLD) |
|---|---|---|---|
| External | Intact skin | No | LLD |
| Intact infected skin | No | HLD | |
| Yes | HLDa | ||
| Internal | TOE | Yes | HLD |
| No | HLD | ||
| Epicardial echo | Yes | HLD | |
| Intracardiac | Interventional | Not reusable – discard after use | |
| Intraoperative | Yes | HLD | |
LLD can be performed if the transducer is classified as non‐critical. Non‐critical transducers do not contact non‐intact skin, blood or mucous membranes. If the transducer comes in direct contact with non‐intact skin, blood or mucous membranes transducers should be cleaned with HLD irrespective of the use of a transducer cover. If transducer cover is broken during a procedure, then HLD must be performed.
Appendix B. Flow chart reference guide for reprocessing ultrasound transducers
B.1.
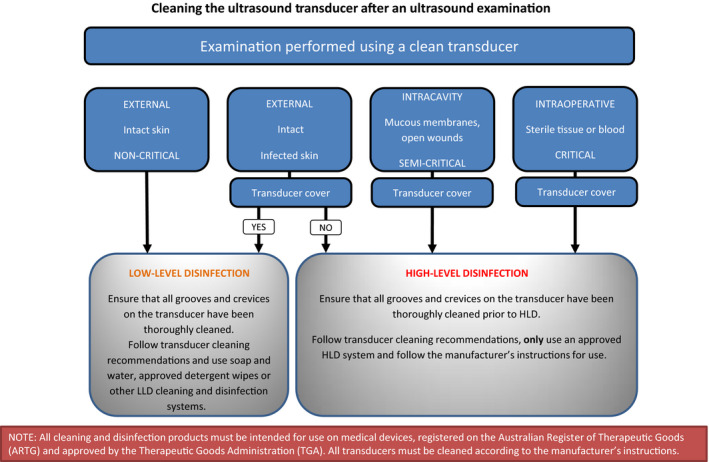
Flow chart 1:
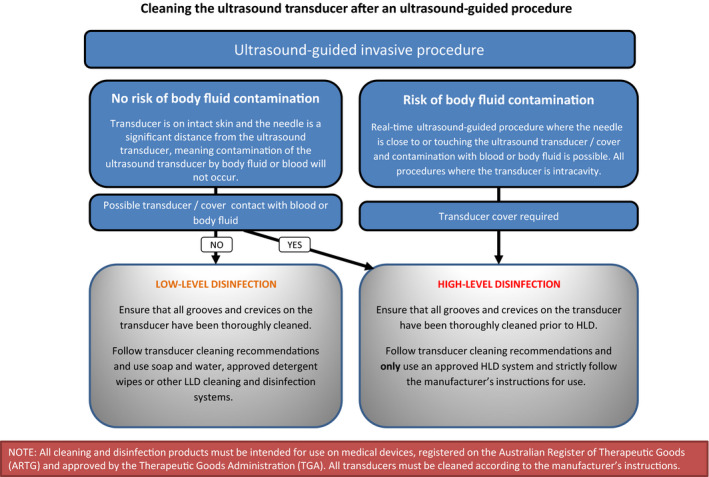
Flow chart 2:
National Safety & Quality Health Service Standards
 Governance for Safety and Quality in Health Care Governance for Safety and Quality in Health Care |
 Partnering with Consumers Partnering with Consumers |
 Preventing & Controlling Healthcare associated infections Preventing & Controlling Healthcare associated infections |
 Medication Safety Medication Safety |
 Patient Identification & Procedure Matching Patient Identification & Procedure Matching |
 Clinical Handover Clinical Handover |
 Blood and Blood Products Blood and Blood Products |
 Preventing & Managing Pressure Injuries Preventing & Managing Pressure Injuries |
 Recognising & Responding to Clinical Deterioration Recognising & Responding to Clinical Deterioration |
 Preventing Falls & Harm from Fall Preventing Falls & Harm from Fall |
| ✓ | ✓ | ✓ | ✓ |
Australasian Society for Ultrasound in Medicine (ASUM) , Basseal JM, Westerway SC, Juraja M, van de Mortel TF, McAuley TE, Rippey J, Meyer‐Henry S, Maloney S, Ayers A, Jain S, Mizia K, Twentyman D, Guidelines for Reprocessing Ultrasound Transducers. Australasian Journal of Ultrasound in Medicine. 2017;00:000–000. 10.1002/ajum.12042
Contributor Information
Australasian Society for Ultrasound in Medicine (ASUM):
Jocelyne M Basseal, Susan Campbell Westerway, Marija Juraja, Thea F van de Mortel, Terry E McAuley, James Rippey, Simon Meyer‐Henry, Samuel Maloney, Amanda Ayers, Susan Jain, Karen Mizia, and Denise Twentyman
14 References
- 1. Australian/New Zealand Standard AS/NZS 4815:2006 Office‐based health care facilities – Reprocessing of reusable medical and surgical instruments and equipment, and maintenance of the associated environment, Standards Australia and Standards New Zealand.
- 2. Centers for Disease Control and Prevention (CDC) . Pseudomonas aeruginosa Respiratory Tract Infections Associated with Contaminated Ultrasound Gel Used for Transesophageal Echocardiography — Michigan, December 2011–January 2012 (Morbidity and Mortality Weekly Report No. 61(15);262‐264); 2012. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6115a3.htm [PubMed]
- 3. Cheng A, Sheng W‐H, Huang Y‐C, Sun H‐Y, Tsai Y‐T, Chen M‐L, et al. Prolonged postprocedural outbreak of Mycobacterium massiliense infections associated with ultrasound transmission gel. Clin Microbiol Infect 2016; 22: 382.e1–11. [DOI] [PubMed] [Google Scholar]
- 4. Chittick P, Russo V, Sims M, Robinson‐Dunn B, Oleszkowicz S, Sawarynski K, et al. An outbreak of Pseudomonas aeruginosa respiratory tract infections associated with intrinsically contaminated ultrasound transmission gel. Infect Control Hosp Epidemiol 2013; 34: 850–3. [DOI] [PubMed] [Google Scholar]
- 5. Gaillot O, Maruéjouls C, Abachin É, Lecuru F, Arlet G, Simonet M, et al. Nosocomial outbreak of Klebsiella pneumoniae producing SHV‐5 extended‐spectrum β‐lactamase, originating from a contaminated ultrasonography coupling gel. J Clin Microbiol 1998; 36: 1357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacobson M, Wray R, Kovach D, Henry D, Speert D, Matlow A. Sustained endemicity of Burkholderia cepacia complex in a pediatric institution, associated with contaminated ultrasound gel. Infect Control Hosp Epidemiol 2006; 27: 362–6. [DOI] [PubMed] [Google Scholar]
- 7. Nannini EC, Ponessa A, Muratori R, Marchiaro P, Ballerini V, Flynn L, et al. Polyclonal outbreak of bacteremia caused by Burkholderia cepacia complex and the presumptive role of ultrasound gel. Braz J Infect Dis 2015; 19: 543–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olshtain‐Pops K, Block C, Temper V, Hidalgo‐Grass C, Gross I, Moses AE, et al. An outbreak of achromobacter xylosoxidans associated with ultrasound gel used during transrectal ultrasound guided prostate biopsy. J Urol 2011; 185: 144–7. [DOI] [PubMed] [Google Scholar]
- 9. Organ M, Grantmyre J, Hutchinson J. Burkholderia cepacia infection of the prostate caused by inoculation of contaminated ultrasound gel during transrectal biopsy of the prostate. Can Urol Assoc J 2010; 4: E58–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weist K, Wendt C, Petersen LR, Versmold H, Rüden H. An outbreak of pyodermas among neonates caused by ultrasound gel contaminated with methicillin‐susceptible Staphylococcus aureus . Infect Control Hosp Epidemiol 2000; 21: 761–4. [DOI] [PubMed] [Google Scholar]
- 11. Cervini P, Hesley GK, Thompson RL, Sampathkumar P, Knudsen JM. Incidence of infectious complications after an ultrasound‐guided intervention. Am J Roentgenol 2010; 195: 846–50. [DOI] [PubMed] [Google Scholar]
- 12. Ferhi K, Rouprêt TM, Mozer P, Ploussard G, Haertig A, De La Taille A. Hepatitis C transmission after prostate biopsy. Case Rep Urol, 2013; 2013: e797248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hiyama Y, Takahashi S, Uehara T, Ichihara K, Hashimoto J, Masumori N. A case of infective endocarditis and pyogenic spondylitis after transrectal ultrasound guided prostate biopsy. J Infect Chemother 2016; 22: 767–9. [DOI] [PubMed] [Google Scholar]
- 14. Bancroft EA, English L, Terashita D, Yasuda L. Outbreak of Escherichia coli infections associated with a contaminated transesophageal echocardiography probe. Infect Control Hosp Epidemiol 2013; 34: 1121–3. [DOI] [PubMed] [Google Scholar]
- 15. Paz A, Bauer H, Potasman I. Multiresistant Pseudomonas aeruginosa associated with contaminated transrectal ultrasound. J Hosp Infect 2001; 49: 148–9. [DOI] [PubMed] [Google Scholar]
- 16. Seki M, Machida N, Yamagishi Y, Yoshida H, Tomono K. Nosocomial outbreak of multidrug‐resistant Pseudomonas aeruginosa caused by damaged transesophageal echocardiogram probe used in cardiovascular surgical operations. J Infect Chemother 2013; 19: 677–81. [DOI] [PubMed] [Google Scholar]
- 17. Australian/New Zealand Standard: AS/NZS4187:2014 Reprocessing of Reusable Medical Devices in Health Service Organisation. Standards Australia and Standards New Zealand.
- 18. Gillespie JL, Arnold KE, Noble‐Wang J, Jensen B, Arduino M, Hageman J, et al. Outbreak of Pseudomonas aeruginosa infections after transrectal ultrasound‐guided prostate biopsy. Urology 2007; 69: 912–4. [DOI] [PubMed] [Google Scholar]
- 19. Medicines and Healthcare products Regulatory Agency . Reusable transoesophageal echocardiography, transvaginal and transrectal ultrasound probes (transducers) – failure to appropriately decontaminate Medical safety alert – GOV.UK (No. MDA/2012/038); 2012. Available from: https://www.gov.uk/drug-device-alerts/medical-device-alert-reusable-transoesophageal-echocardiography-transvaginal-and-transrectal-ultrasound-probes-transducers-failure-to-appropriately-decontaminate.
- 20. Ohara T, Itoh Y, Itoh K. Contaminated ultrasound probes: a possible source of nosocomial infections. J Hospital Infect 1999; 43: 73. [DOI] [PubMed] [Google Scholar]
- 21. Stigt JA, Wolfhagen MJ, Smulders P, Lammers V. The identification of Stenotrophomonas maltophilia contamination in ultrasound endoscopes and reproduction of decontamination failure by deliberate soiling tests. Respiration 2015; 89: 565–71. [DOI] [PubMed] [Google Scholar]
- 22. M'Zali F, Bounizra C, Leroy S, Mekki Y, Quentin‐Noury C, Kann M. Persistence of microbial contamination on transvaginal ultrasound probes despite low‐level disinfection procedure. PLoS ONE 2014; 9: e93368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amis S, Ruddy M, Kibbler CC, Economides DL, MacLean AB. Assessment of condoms as probe covers for transvaginal sonography. J Clin Ultrasound 2000; 28: 295–8. [DOI] [PubMed] [Google Scholar]
- 24. Casalegno J, Le Bail Carval K, Eibach D, Valdeyron M‐L, Lamblin G, Jacquemoud H, et al. High risk HPV contamination of endocavity vaginal ultrasound probes: an underestimated route of nosocomial infection? PLoS ONE 2012; 7: e48137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frazee BW. Emergency department ultrasonagraphic probe contamination and experimental mode of probe disinfection. Ann Emerg Med 2011; 58: 56–8. [DOI] [PubMed] [Google Scholar]
- 26. Alfa MJ. Intra‐cavity ultrasound probes: cleaning and high‐level disinfection are necessary for both the probe head and handle to reduce the risk of infection transmission. Infect Control Hosp Epidemiol 2015; 36: 585–6. [DOI] [PubMed] [Google Scholar]
- 27. Keys M, Sim B, Thom O, Tunbridge M, Barnett A, Fraser J. Efforts to Attenuate the Spread of Infection (EASI): a prospective, observational multicenter survey of ultrasound equipment in Australian emergency departments and intensive care units. Crit Care Resusc 2015; 17: 43–6. [PubMed] [Google Scholar]
- 28. Rutala WA, Weber D. Reprocessing semicritical items. Am J Infect Control 2016; 44: e53–62. [DOI] [PubMed] [Google Scholar]
- 29. Westerway S, Basseal JM, Brockway A, Hyett JA, Carter DA. Potential risks associated with an ultrasound examination – a bacterial perspective. J Ultrasound Med Biol 2016; 43: 421–6. [DOI] [PubMed] [Google Scholar]
- 30. Storment JM, Monga M, Blanco JD. Ineffectiveness of latex condoms in preventing contamination of the transvaginal ultrasound head. South Med J 1997; 90: 206–8. [DOI] [PubMed] [Google Scholar]
- 31. Oleszkowicz S, Chittick P, Russo V, Keller P, Sims M, Band J. Infections associated with use of ultrasound transmission gel: proposed guidelines to minimize risk. Infect Control Hosp Epidemiol 2012; 33: 1235. [DOI] [PubMed] [Google Scholar]


