Abstract
Aims/Introduction
Painful diabetic peripheral neuropathy (pDPN) is associated with small nerve fiber degeneration and regeneration. This study investigated whether the presence of pDPN might influence nerve regeneration in patients with type 2 diabetes undergoing intensive glycemic control.
Materials and Methods
This exploratory substudy of an open‐label randomized controlled trial undertook the Douleur Neuropathique en 4 questionnaire and assessment of electrochemical skin conductance, vibration perception threshold and corneal nerve morphology using corneal confocal microscopy in participants with and without pDPN treated with exenatide and pioglitazone or basal–bolus insulin at baseline and 1‐year follow up, and 18 controls at baseline only.
Results
Participants with type 2 diabetes, with (n = 13) and without (n = 28) pDPN had comparable corneal nerve fiber measures, electrochemical skin conductance and vibration perception threshold at baseline, and pDPN was not associated with the severity of DPN. There was a significant glycated hemoglobin reduction (P < 0.0001) and weight gain (P < 0.005), irrespective of therapy. Participants with pDPN showed a significant increase in corneal nerve fiber density (P < 0.05), length (P < 0.0001) and branch density (P < 0.005), and a decrease in the Douleur Neuropathique en 4 score (P < 0.01), but no change in electrochemical skin conductance or vibration perception threshold. Participants without pDPN showed a significant increase in corneal nerve branch density (P < 0.01) and no change in any other neuropathy measures. A change in the severity of painful symptoms was not associated with corneal nerve regeneration and medication for pain.
Conclusions
This study showed that intensive glycemic control is associated with greater corneal nerve regeneration and an improvement in the severity of pain in patients with painful diabetic neuropathy.
Keywords: Corneal confocal microscopy, Exenatide, Painful diabetic neuropathy
There has been a resurgence of interest in identifying new drug targets or, predictive biomarkers of disease‐modifying therapies in diabetic neuropathy. We show that the presence of painful diabetic neuropathy was associated with greater corneal nerve regeneration and an improvement in painful neuropathic symptoms in patients with type 2 diabetes undergoing intensive glycemic control.
INTRODUCTION
In patients with type 1 diabetes, 6.5 years of intensive glycemic control reduced the incidence of diabetic peripheral neuropathy (DPN) by 60%, prevented peroneal nerve conduction velocity slowing1 and continued to benefit patients 8 years after completion of the DCCT2. However, in patients with type 2 diabetes, the United Kingdom Prospective Diabetes Study (UKPDS)3 and Veterans Affairs Co‐operative Study in Type 2 Diabetes Mellitus (VA‐CSDM) trial4 reported no impact on the incidence of DPN, and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial showed no effect on vibration perception over a period of 6 years5. Furthermore, multiple phase III clinical trials failed to show an improvement in diabetic neuropathy, and there are currently no US Food and Drug Administration approved therapies for DPN6. It is unclear whether this failure is a consequence of inadequate translation of experimental therapies, inadequate end‐points or the enrolment of patients with a widely varying severity of DPN7.
The prevalence of painful diabetic peripheral neuropathy (pDPN) and DPN increases with age and duration of diabetes8. Hyperglycemia, hyperlipidemia and hypertension are associated with DPN9; whereas obesity, physical inactivity and smoking cigarettes are associated with pDPN8, 10. Neuropathic pain might be present at any stage of DPN11, and has been linked to a complex interplay between ongoing small nerve fiber degeneration and regeneration12, 13. Indeed, skin biopsy studies have shown comparable intra‐epidermal nerve fiber density in patients with and without painful neuropathy14 and painful diabetic neuropathy15, 16. However, more detailed immunohistological studies have shown an increase in regenerating intra‐epidermal17, 18 and dermal nerve fibers containing substance P and calcitonin gene‐related peptide in patients with painful compared with painless diabetic neuropathy19. Recently, Bönhof et al.20 showed comparable intra‐epidermal nerve fiber density, but increased growth‐associated protein‐43 staining indicative of regenerating dermal nerves in patients with painful diabetic neuropathy. We also utilized corneal confocal microscopy (CCM) to show significantly greater corneal sub‐basal nerve plexus degeneration in patients with painful compared with painless DPN15, 21, 22. These studies suggest that patients with painful diabetic neuropathy might have greater small fiber degeneration, but also an increased capacity for nerve regeneration.
CCM has been used to identify early small fiber regeneration in several clinical trials23. Indeed, early corneal nerve regeneration occurred 6 months24 after pancreas and kidney transplantation, and was followed by an improvement in nerve conduction and neuropathic symptoms after 24 months24, 25. We also recently reported that exenatide and pioglitazone or basal–bolus insulin effectively reduce glycated hemoglobin (HbA1c)26 and induce corneal nerve regeneration27.
The present substudy of the Qatar study26 assessed whether the presence of pDPN might influence nerve regeneration in patients with type 2 diabetes undergoing intensive glycemic control with exenatide and pioglitazone or insulin.
MATERIALS AND METHODS
This was an exploratory substudy of an open‐label, randomized controlled trial (clinicaltrials.gov ID: NCT02887625)26, that examined the efficacy of exenatide and pioglitazone versus basal–bolus insulin in patients with poorly controlled type 2 diabetes. This substudy has not been registered in a public clinical trial database. Participants with type 2 diabetes were enrolled from the National Diabetes Center in Hamad General Hospital, Doha, Qatar, and studied at baseline and 1‐year follow up, and control participants without diabetes were enrolled from Rumailah Hospital, Doha, Qatar, and studied at baseline only from October 2016 to November 2018.
The present study received ethical approval from the Hamad Medical Corporation IRB (IRB# 13‐00076), and all participants consented to participate in the study. The study followed the tenets of the declaration of Helsinki.
Study cohort
Individuals aged 18–75 years with HbA1c >7.5% (>58 mmol/mol) on near maximum dose of metformin (>1,500 mg/day) and sulfonylurea (>4 mg glimepiride or >60 mg gliclazide); with normal liver and kidney function, and electrocardiogram; and stable bodyweight (±1 kg) in the past year were recruited. The exclusion criteria are described in detail in our previous report27, but included any cause of neuropathy apart from diabetes, corneal dystrophies, corneal surgery or trauma in the past year, antidiabetic treatment other than metformin and sulfonylureas, diabetic proliferative retinopathy, and abnormally high albumin excretion.
Interventions
Participants were randomized to receive exenatide and pioglitazone or glargine and aspart insulin treatment to achieve and maintain an HbA1c <7.0% (<53 mmol/mol)27.
Demographic and metabolic measures
Age, sex, diabetes duration, bodyweight, body mass index (BMI), blood pressure, HbA1c, total cholesterol, triglyceride, high‐density lipoprotein (HDL) and low‐density lipoprotein (LDL) were recorded from the electronic health record.
DPN assessment
pDPN was defined on a Douleur Neuropathique en 4 (DN4) questionnaire score ≥4, as previously described28. The DN4 questionnaire has been validated for distinguishing neuropathic pain from non‐neuropathic pain29 in Arabic30 and for pDPN28. It consists of 10 questions relating to symptoms and signs, and each question is equally weighted. A score ≥4 has 80% sensitivity and 92% specificity for pDPN28. The questionnaire was administered by the investigator in English or Arabic. Medications for pDPN were recorded.
CCM was carried out using the HRT‐3‐RCM device (Heidelberg Engineering GmbH, Heidelberg, Germany), as described in our previously published protocol31. Corneal nerve fiber density (CNFD; fibers/mm2), length (CNFL) (mm/mm2) and branch density (CNBD) (branches/mm2) were quantified manually using CCMetrics32.
Sudomotor function was measured by electrochemical skin conductance (ESC) using Sudoscan (Impeto Medical SAS, Paris, France), as described previously33. Sudoscan evaluates sympathetic innervation based on sweat chloride concentrations generated by the sweat gland in response to the voltage applied33, and is reported as ESC in microSiemens (µS).
Vibration perception threshold (VPT) was measured using a Neurothesiometer (Horwell Scientific Laboratory Supplies, London, UK) on the pulp of the large toe on both feet, and the average value of three measurements was recorded as a VPT in Volts (V) ranging from 0 to 50 V.
Statistical analysis
Continuous variables between controls, participants with type 2 diabetes, with and without pDPN were compared using one‐way anova. Continuous variables were compared between the two study groups (with and without pDPN) using the unpaired t‐test, whereas categorical variables were compared using the χ2‐test. Within each group, changes between baseline and 1‐year follow up were compared for the continuous variables using the paired t‐test. Linear regression was used to analyze the association between the change in CCM measures with type of treatment, change in DN4 score, bodyweight, diastolic blood pressure, HbA1c, cholesterol, triglyceride and LDL. No adjustment for multiple testing was carried out, as the study was exploratory in nature. All analyses were carried out using IBM SPSS (version 23; Armonk, NY, USA). A two‐tailed P value of ≤0.05 was considered significant.
RESULTS
Difference between participants with and without pDPN and healthy controls
A total of 41 participants with type 2 diabetes, with (n = 13) and without (n = 28) pDPN, and 18 control participants were studied (Table 1). The proportion of those treated with basal–bolus insulin (P = 0.84) or a combination of exenatide and pioglitazone (P = 0.84) were comparable between the two groups. Three out of 13 participants with pDPN (23%) were taking medication to relieve pain. Participants with type 2 diabetes were age‐matched with control participants. Participants with pDPN were older (P < 0.01), had a higher DN4 score (P < 0.0001) and a lower percentage undertook physical activity (P < 0.05) compared with participants without pDPN. Sex (P = 0.94), duration of diabetes (P = 0.27), HbA1c (P = 0.66), total cholesterol (P = 1.00), triglyceride (P = 0.72), HDL (P = 0.28), LDL (P = 0.45), systolic blood pressure (P = 0.83), diastolic blood pressure (P = 0.91), bodyweight (P = 0.51) and BMI (P = 0.12) were comparable between participants with and without pDPN.
Table 1.
Comparison of baseline characteristics between patients with type 2 diabetes with and without painful diabetic peripheral neuropathy and healthy controls
|
Controls (n = 18) |
Patients without painful diabetic neuropathy (n = 28) |
Patients with painful diabetic neuropathy (n = 13) |
P‐value | |
|---|---|---|---|---|
| Age (years) | 53.0 ± 11.0 | 50.7 ± 9.4 | 57.6 ± 5.1 | <0.01 |
| Diabetes duration (years) | 12.0 ± 8.0 | 9.3 ± 6.3 | 0.27 | |
| Male, n (%) | 16/28 (69.6) | 7/12 (30.4) | 0.94 | |
| Basal‐bolus insulin, n (%) | 12/28(42.9) | 6/13(46.2) | 0.84 | |
| Exenatide plus pioglitazone, n (%) | 16/28(57.1) | 7/13(53.8) | ||
| Physical activity | 11/27 (49.7) | 1/12(8.3) | <0.05 | |
| HbA1c (mmol/mol) | 90.1 ± 21.1 | 87.0 ± 20.7 | 0.66 | |
| HbA1c (%) | 10.4 ± 1.9 | 10.1 ± 1.9 | ||
| Total cholesterol (mmol/L) | 4.9 ± 0.9 | 4.9 ± 1.2 | 1.00 | |
| Triglyceride (mmol/L) | 1.9 ± 1.2 | 2.1 ± 1.1 | 0.72 | |
| HDL (mmol/L) | 1.2 ± 0.5 | 1.1 ± 0.3 | 0.28 | |
| LDL (mmol/L) | 2.9 ± 0.9 | 2.6 ± 0.7 | 0.45 | |
| Systolic BP (mmHg) | 129.1 ± 15.9 | 127.5 ± 25.4 | 0.83 | |
| Diastolic BP (mmHg) | 78.1 ± 11.4 | 77.6 ± 14.2 | 0.91 | |
| Bodyweight (kg) | 85.0 ± 13.4 | 89.5 ± 22.2 | 0.51 | |
| BMI (kg/m2) | 29.9 ± 4.7 | 33.7 ± 7.6 | 0.12 | |
| DN4 score | 0 ± 0 | 1.1 ± 1.0†† | 5.5 ± 1.4††† | <0.0001 |
| CNFD (fibers/mm2) | 33.7 ± 5.7 | 27.4 ± 8.0‡ | 26.0 ± 8.7‡ | 0.64 |
| CNBD (branches/mm2) | 110.4 ± 45.0 | 67.3 ± 32.1†† | 54.0 ± 27.6††† | 0.20 |
| CNFL (mm/mm2) | 25.1 ± 4.3 | 18.8 ± 4.8††† | 17.4 ± 5.6††† | 0.46 |
| VPT (V) | 7.2 ± 4.1 | 7.8 ± 4.4† | 14.1 ± 8.0† | 0.07 |
| ESC feet (µS) | 66.9 ± 18.4 | 66.6 ± 17.4 | 64.2 ± 24.1 | 0.79 |
Numeric variables and frequency distribution for categorical variables are summarized as the mean ± standard deviation or n (%), and were compared between patients with and without painful diabetic peripheral neuropathy using the unpaired t‐test and χ2‐test, respectively. Variables between controls and patients with type 2 diabetes with and without painful diabetic peripheral neuropathy were compared using one‐way anova, and significant differences between them are denoted as ‡ P ≤ 0.05, † P ≤ 0.01, †† P ≤ 0.001, ††† P ≤ 0.0001. BMI, body mass index; BP, blood pressure; DN4, Douleur Neuropathique en 4; CNBD corneal nerve branch density; CNFD, corneal nerve fiber density; CNFL, corneal nerve fiber length; ESC, electrochemical skin conductance; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; VPT, vibration perception threshold. Bold values are indicates statistically significant.
Participants with type 2 diabetes had a significantly higher DN4 score (P ≤ 0.001) and VPT (P ≤ 0.01), and lower corneal nerve fiber measures (P ≤ 0.05), but comparable ESC compared with healthy controls. Corneal nerve fiber measures, ESC and VPT were comparable between participants with and without pDPN.
Difference in clinical characteristics and neuropathy measures between baseline and 1‐year follow up
Between baseline and 1‐year follow up, participants with pDPN showed a significant decrease in HbA1c (P < 0.0001), increase in bodyweight (P < 0.01), but no change in total cholesterol (P = 0.06), triglyceride (P = 0.26), HDL (P = 0.72), LDL (P = 0.19), systolic blood pressure (P = 0.16), diastolic blood pressure (P = 0.39) and BMI (P = 0.18; Table 2; Figures 1 and 2). There was a significant decrease in the DN4 score (P < 0.01), and an increase in CNFD (P < 0.05), CNBD (P < 0.01) and CNFL (P < 0.0001), but no change in ESC (P = 0.96) and VPT (P = 0.32).
Table 2.
Baseline and 1‐year follow‐up clinical and neuropathy measures of patients with type 2 diabetes with and without painful diabetic neuropathy
| Patients without painful diabetic neuropathy (n = 28) | P‐value |
Patients with painful diabetic neuropathy (n = 13) |
P‐value | |||
|---|---|---|---|---|---|---|
| Baseline | 1‐year follow up | Baseline | 1‐year follow up | |||
| HbA1c (mmol/mol) | 90.1 ± 21.1 | 53.5 ± 11.7↓ | <0.0001 | 87.0 ± 20.7 | 60.1 ± 20.2↓ | <0.0001 |
| HbA1c (%) | 10.4 ± 1.9 | 7.0 ± 1.1↓ | <0.0001 | 10.1 ± 1.9 | 7.7 ± 1.8↓ | <0.0001 |
| Total cholesterol (mmol/L) | 4.9 ± 0.9 | 4.3 ± 0.9↓ | <0.01 | 4.9 ± 1.2 | 4.2 ± 0.7 | 0.06 |
| Triglyceride (mmol/L) | 1.9 ± 1.2 | 1.5 ± 1.1↓ | <0.01 | 2.1 ± 1.1 | 1.8 ± 1.0 | 0.26 |
| HDL (mmol/L) | 1.2 ± 0.5 | 1.2 ± 0.3 | 0.30 | 1.1 ± 0.3 | 1.1 ± 0.2 | 0.72 |
| LDL (mmol/L) | 2.8 ± 0.9 | 2.5 ± 1.0↓ | <0.05 | 2.6 ± 0.7 | 2.3 ± 0.5 | 0.19 |
| Systolic BP (mmHg) | 129.1 ± 15.9 | 124.2 ± 16.4 | 0.12 | 127.5 ± 25.4 | 135.2 ± 15.3 | 0.16 |
| Diastolic BP (mmHg) | 78.1 ± 11.4 | 70.6 ± 9.8↓ | <0.0001 | 77.6 ± 14.2 | 74.4 ± 11.6 | 0.39 |
| Bodyweight (kg) | 85.0 ± 13.4 | 88.9 ± 15.4↑ | <0.0001 | 89.5 ± 22.2 | 95.6 ± 24.8↑ | <0.01 |
| BMI (kg/m2) | 29.9 ± 4.7 | 30.2 ± 4.7 | 0.19 | 33.7 ± 7.6 | 34.4 ± 8.5 | 0.18 |
| DN4 score | 1.1 ± 1.0 | 1.0 ± 1.1 | 0.66 | 5.5 ± 1.4 | 4.23 ± 1.9↓ | <0.01 |
| CNFD (fibers/mm2) | 27.4 ± 8.0 | 27.6 ± 6.5 | 0.91 | 26.0 ± 8.7 | 31.5 ± 8.2↑ | <0.05 |
| CNBD (branches/mm2) | 67.3 ± 32.1 | 81.8 ± 37.8↑ | <0.01 | 54.0 ± 27.6 | 102.2 ± 58.9↑ | <0.01 |
| CNFL (mm/mm2) | 18.8 ± 4.8 | 20.0 ± 4.7 | 0.13 | 17.4 ± 5.6 | 23.5 ± 5.8↑ | <0.0001 |
| VPT (V) | 7.8 ± 4.4 | 7.6 ± 3.9 | 0.77 | 14.1 ± 8.0 | 13.0 ± 7.1 | 0.32 |
| ESC feet (µS) | 66.6 ± 17.4 | 64.7 ± 16.4 | 0.54 | 64.2 ± 24.1 | 64.0 ± 23.3 | 0.96 |
Numeric variables are summarized as the mean ± standard deviation. Variables were compared using the paired t‐test. BMI, body mass index; BP, blood pressure; DN4, Douleur Neuropathique en 4; CNBD corneal nerve branch density; CNFD, corneal nerve fiber density; CNFL, corneal nerve fiber length; ESC, electrochemical skin conductance; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; VPT, vibration perception threshold. Bold values are indicates statistically significant.
Figure 1.
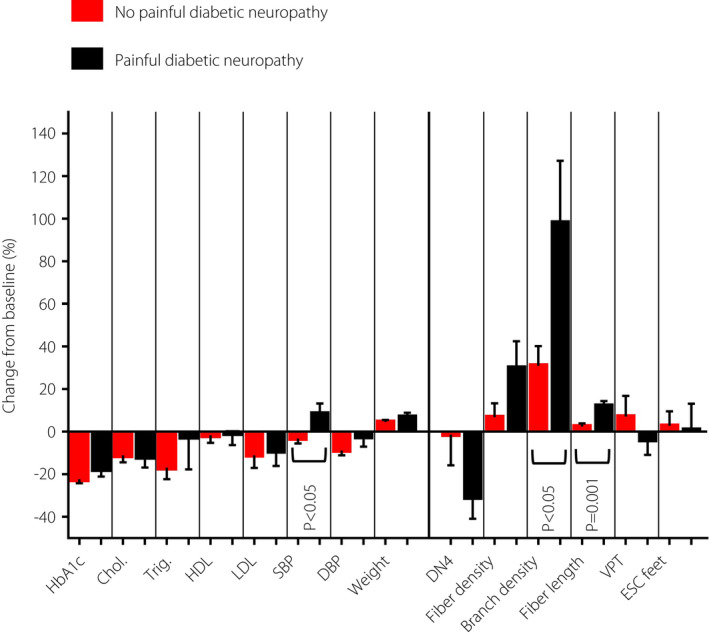
Comparison of percentage change in clinical and neuropathy measures over a 1‐year period between patients with and without painful diabetic neuropathy. Chol, total cholesterol; DBP, diastolic blood pressure; DN4, Douleur Neuropathique en 4; ESC, electrochemical skin conductance; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure; Trig, triglyceride; VPT, vibration perception threshold.
Figure 2.
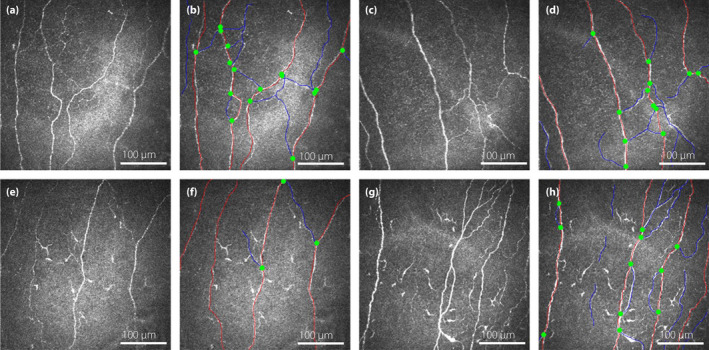
Corneal confocal microscopy images of the sub‐basal nerve plexus showing corneal nerve morphology in a patient with type 2 diabetes without painful diabetic peripheral neuropathy at (a) baseline and (c) 1‐year follow up with (b,d) the nerves traced, and a patient with type 2 diabetes and painful diabetic peripheral neuropathy (e) at baseline and (g) 1‐year follow up with (f,h) the nerves traced. The main nerve fibers are highlighted in red and the branches in blue. The origin of the branches is shown as green dots.
Participants without pDPN had a significant decrease in HbA1c (P < 0.0001), increase in bodyweight (P < 0.0001), decrease in total cholesterol (P < 0.01), triglyceride (P < 0.01), LDL (P < 0.05) and diastolic blood pressure (P < 0.0001), but no change in HDL (P = 0.30), systolic blood pressure (P = 0.12) and BMI (P = 0.19). There was a significant increase in CNBD (P < 0.01), but no change in CNFD (P = 0.91), CNFL (P = 0.13), DN4 score (P = 0.66), ESC (P = 0.54) and VPT (P = 0.77).
Difference in change in clinical and neuropathy measures between those with and without pDPN
Participants with pDPN showed a comparable change in HbA1c (P = 0.14), total cholesterol (P = 0.79), triglyceride (P = 0.72), HDL (P = 0.59), LDL (P = 0.87), diastolic blood pressure (P = 0.30), body weight (P = 0.28) and BMI (P = 0.24) compared with those without pDPN (Table 3; Figure 1). Systolic blood pressure increased in participants with pDPN, whereas it decreased in participants without pDPN (P < 0.05). Participants with pDPN had a significantly greater increase in CNBD (P < 0.05) and CNFL (P = 0.001) compared with those without pDPN. Both groups showed a comparable change in the DN4 score (P = 0.40), ESC (P = 0.77) and VPT (P = 0.53).
Table 3.
Comparison of changes in clinical and neuropathy measures over a 1‐year period between patients with and without painful diabetic neuropathy
| Patients without painful diabetic neuropathy (n = 28) | Patients with painful diabetic neuropathy (n = 13) | P‐value | |
|---|---|---|---|
| ΔHbA1c (mmol/mol) | −36.6 ± 16.2 | −26.8 ± 20.2 | 0.14 |
| ΔHbA1c (%) | −3.4 ± 1.5 | −2.5 ± 1.9 | 0.14 |
| ΔTotal cholesterol (mmol/L) | −0.6 ± 0.9 | −0.7 ± 1.2 | 0.79 |
| ΔTriglyceride (mmol/L) | −0.4 ± 0.8 | −0.3 ± 0.9 | 0.72 |
| ΔHDL (mmol/L) | −0.1 ± 0.4 | 0 ± 0.3 | 0.59 |
| ΔLDL (mmol/L) | −0.4 ± 0.8 | −0.3 ± 0.8 | 0.87 |
| ΔSystolic BP (mmHg) | −4.9 ± 16.1 | 7.7 ± 18.7 | <0.05 |
| ΔDiastolic BP (mmHg) | −7.6 ± 9.6 | −3.2 ± 13.0 | 0.30 |
| ΔBodyweight (kg) | 4.0 ± 4.7 | 6.1 ± 6.3 | 0.28 |
| ΔBMI (kg/m2) | 0 ± 1.7 | 0.8 ± 1.9 | 0.24 |
| ΔDN4 score | 0.1 ± 1.1 | −0.6 ± 2.7 | 0.40 |
| ΔCNFD (fibers/mm2) | 0.2 ± 8.0 | 5.5 ± 7.4 | 0.06 |
| ΔCNBD (branches/mm2) | 14.5 ± 25.4 | 48.2 ± 46.0 | <0.05 |
| ΔCNFL (mm/mm2) | 1.2 ± 3.9 | 6.1 ± 3.8 | 0.001 |
| ΔVPT (V) | −0.2 ± 3.8 | −1.1 ± 2.8 | 0.53 |
| ΔESC feet (µS) | −2.0 ± 14.1 | −0.2 ± 14.4 | 0.77 |
Numeric variables are summarized as the mean ± standard deviation. Variables were compared using unpaired t‐test. BMI, body mass index; BP, blood pressure; DN4, Douleur Neuropathique en 4; CNBD corneal nerve branch density; CNFD, corneal nerve fiber density; CNFL, corneal nerve fiber length; ESC, electrochemical skin conductance; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; VPT, vibration perception threshold. Bold values are indicates statistically significant.
Association of change in CCM measures and painful symptoms with clinical characteristics
The change in CCM measures was not associated with the type of treatment (P = 0.47) and decrease in HbA1c (P = 0.61). However, it was negatively associated with bodyweight gain, with every 2‐kg increase in bodyweight, CNFD decreased by 1 fiber/mm (95% confidence interval −1.0–0.0), but this association just missed statistical significance (P = 0.0501).
The change in DN4 score had no association with medications for neuropathic pain at baseline (P = 0.21), decrease in HbA1c (P = 0.81) or gain in bodyweight (P = 0.67).
There was no association between the change in DN4 score and change in CNFD (P = 0.93), CNBD (P = 0.25) or CNFL (P = 0.28).
DISCUSSION
The present study shows that treatment of patients with type 2 diabetes and poor glycemic control with exenatide and pioglitazone or basal–bolus insulin markedly improves glycemic control, and is associated with an improvement in painful diabetic neuropathy and corneal nerve regeneration.
Painful symptoms in DPN have been associated with active nerve degeneration and regeneration12. Indeed, although there are no differences in intra‐epidermal nerve fiber density between those with and without pDPN34, 35, there was a significantly lower CNFL in patients with pDPN compared with those with painless DPN15, 21; and in another study, CNFD was significantly lower in patients with pDPN22. Quantitative sensory testing has also shown increased thermal thresholds in patients with pDPN compared with those with painless DPN36, 37. More recently, we showed lower intra‐epidermal nerve fiber density and corneal nerve fibers in a large group of patients with painful compared with painless diabetic neuropathy38. In this study, CCM measures, sudomotor function and vibration perception threshold were comparable between patients with and without pDPN, although the number of patients with painful diabetic neuropathy was much smaller than in previous studies15, 21, 22, 38.
A large improvement in HbA1c (>2–3%) has been reported to be associated with treatment‐induced neuropathic pain and autonomic neuropathy39. However, the present study showed that despite a mean reduction in HbA1c of 3.4% among those without pDPN, and 2.5% among those with pDPN, there was no increase in the DN4 score, consistent with our previous findings27. Furthermore, of the 27 patients without pDPN, only one developed pDPN after 1 year of intensive glycemic control.
There is a need for better neuropathy phenotyping to enable trial enrichment of participants who are more likely to respond to therapies, whether to reduce the severity of pain with therapies targeting pain or nerve regeneration in clinical trials of disease‐modifying therapies for DPN. Thus, there has been a resurgence of interest in identifying biomarkers of specific pain mechanisms that might allow more effective targeted use of existing therapies35. Quantitative sensory testing has been used in a phenotype‐stratified randomized, double‐blind, placebo‐controlled study to show that oxcarbazepine had a significantly greater effect in patients with an irritable nociceptor phenotype40. Similarly, the conditioned pain modulation test has been used to identify altered descending spinal pathways to predict greater efficacy of duloxetine41. We also showed altered rate‐dependent depression of the H‐reflex, indicative of abnormal descending inhibitory pathways in patients with pDPN22. However, a deep phenotyping approach to identify outcomes of disease‐modifying therapies has not been undertaken to date. Indeed, despite multiple trials of disease‐modifying therapies, there are currently no US Food and Drug Administration‐approved therapies for DPN6. Several studies showed that subclinical small nerve fiber injury precedes large fiber damage in DPN42, 43. Furthermore, early small fiber repair has been shown in several small clinical intervention trials23, 44, and after pancreas and kidney transplantation, normalization of glycemia was associated with corneal nerve regeneration after 6 months, followed by an improvement in neuropathic symptoms and nerve conduction after 24 months24, 25. More recently, we showed that both exenatide and pioglitazone or basal–bolus insulin effectively reduce HbA1c and induce corneal nerve regeneration, independent of changes in HbA1c, bodyweight and lipids27. Preclinical studies have reported that glucagon‐like peptide‐1 receptor agonists have a neuroprotective effect45 and suppresses pain hypersensitivity in diabetes46, and although earlier clinical trials showed no benefit47, 48, we recently showed corneal nerve regeneration with exenatide27. Thiazolidinediones have been reported to alleviate neuropathic pain by attenuating proinflammatory cytokine expression49, and preclinical studies show a prevention of nerve conduction slowing50, 51. Indeed, the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial reported that rosiglitazone significantly reduced the 4‐year cumulative incidence of clinical DPN compared with insulin52. Insulin treatment has also been shown to have a neurotrophic effect and reduce tactile allodynia53, and intensive insulin treatment might prevent nerve conduction slowing54 and loss of ankle reflexes55.
We acknowledge this is a small exploratory study with potential confounders in relation to the small cohort size and effect of different treatments. Nevertheless, we showed that patients with pDPN have optimal nerve regeneration in response to improved glycemic control. We also showed that nerve regeneration might be limited due to weight gain, and, of course, recently we showed that weight loss with bariatric surgery is associated with corneal nerve regeneration56. Disease‐modifying treatments are also more likely to be of benefit in early or mild neuropathy where there is predominantly small fiber damage57, 58. These findings highlight the complex pathogenesis and risk factors determining outcomes in clinical trials of diabetic neuropathy and argues strongly for pre‐trial enrichment of participants.
We conclude that pDPN is associated with greater corneal nerve regeneration and improvement in painful neuropathic symptoms in patients with type 2 diabetes after intensive glycemic control. The underlying mechanism is not clear and merits further study.
DISCLOSURE
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
We thank all the participants for their efforts and commitment to be involved in the study. This work was funded by the Qatar National Research Fund (BMRP‐5726113101 & NPRP 5‐273‐3‐079). AstraZeneca provided exenatide for the Qatar Study.
J Diabetes Investig. 2021; 12: 1642–1650
Clinical Trial Registry
Exenatide Plus Pioglitazone Versus Insulin in Poorly Controlled T2DM
REFERENCES
- 1.The Diabetes Control and Complications Trial Research Group . The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann Intern Med 1995; 122: 561–568. [DOI] [PubMed] [Google Scholar]
- 2.Martin CL, Albers J, Herman WH, et al. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care 2006; 29: 340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 4.Azad N, Emanuele NV, Abraira C, et al. The effects of intensive glycemic control on neuropathy in the VA cooperative study on type II diabetes mellitus (VA CSDM). J Diabetes Complications 1999; 13(5–6): 307–313. [DOI] [PubMed] [Google Scholar]
- 5.Callaghan BC, Little AA, Feldman EL, et al. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev 2012: Cd007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik RA. Wherefore Art Thou, O treatment for diabetic neuropathy? Int Rev Neurobiol 2016; 127: 287–317. [DOI] [PubMed] [Google Scholar]
- 7.Malik RA. Why are there no good treatments for diabetic neuropathy? Lancet Diabetes Endocrinol 2014; 2: 607–609. [DOI] [PubMed] [Google Scholar]
- 8.Ponirakis G, Elhadd T, Chinnaiyan S, et al. Prevalence and risk factors for diabetic neuropathy and painful diabetic neuropathy in primary and secondary healthcare in Qatar. J Diabetes Investig 2021; 12: 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponirakis G, Elhadd T, Chinnaiyan S, et al. Prevalence and management of diabetic neuropathy in secondary care in Qatar. Diabetes Metab Res Rev 2020; 36: e3286. [DOI] [PubMed] [Google Scholar]
- 10.Ponirakis G, Elhadd T, Chinnaiyan S, et al. Prevalence and risk factors for painful diabetic neuropathy in secondary healthcare in Qatar. J Diabetes Investig 2019; 10: 1558–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veves A, Manes C, Murray HJ, et al. Painful neuropathy and foot ulceration in diabetic patients. Diabetes Care 1993; 16: 1187–1189. [DOI] [PubMed] [Google Scholar]
- 12.Yagihashi S, Yamagishi S, Wada R. Pathology and pathogenetic mechanisms of diabetic neuropathy: correlation with clinical signs and symptoms. Diabetes Res Clin Pract. 2007; 77: S184–S189. [DOI] [PubMed] [Google Scholar]
- 13.Spallone V, Greco C. Painful and painless diabetic neuropathy: one disease or two? Curr Diab Rep 2013; 13: 533–549. [DOI] [PubMed] [Google Scholar]
- 14.Truini A, Biasiotta A, Di Stefano G, et al. Does the epidermal nerve fibre density measured by skin biopsy in patients with peripheral neuropathies correlate with neuropathic pain? Pain 2014; 155: 828–832. [DOI] [PubMed] [Google Scholar]
- 15.Quattrini C, Tavakoli M, Jeziorska M, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes 2007; 56: 2148–2154. [DOI] [PubMed] [Google Scholar]
- 16.Cheung A, Podgorny P, Martinez JA, et al. Epidermal axonal swellings in painful and painless diabetic peripheral neuropathy. Muscle Nerve 2015; 51: 505–513. [DOI] [PubMed] [Google Scholar]
- 17.Cheng HT, Dauch JR, Porzio MT, et al. Increased axonal regeneration and swellings in intraepidermal nerve fibers characterize painful phenotypes of diabetic neuropathy. J Pain 2013; 14: 941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galosi E, La Cesa S, Di Stefano G, et al. A pain in the skin. Regenerating nerve sprouts are distinctly associated with ongoing burning pain in patients with diabetes. Eur J Pain 2018; 22: 1727–1734. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson P, Provitera V, Caporaso G, et al. Increased peptidergic fibers as a potential cutaneous marker of pain in diabetic small fiber neuropathy. Pain 2021; 162: 778–786. [DOI] [PubMed] [Google Scholar]
- 20.Bönhof GJ, Strom A, Püttgen S, et al. Patterns of cutaneous nerve fibre loss and regeneration in type 2 diabetes with painful and painless polyneuropathy. Diabetologia 2017; 60: 2495–2503. [DOI] [PubMed] [Google Scholar]
- 21.Kalteniece A, Ferdousi M, Petropoulos I, et al. Greater corneal nerve loss at the inferior whorl is related to the presence of diabetic neuropathy and painful diabetic neuropathy. Sci Rep 2018; 8: 3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall AG, Lee‐Kubli C, Azmi S, et al. Spinal disinhibition in experimental and clinical painful diabetic neuropathy. Diabetes 2017; 66: 1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brines M, Dunne AN, van Velzen M, et al. ARA 290, a nonerythropoietic peptide engineered from erythropoietin, improves metabolic control and neuropathic symptoms in patients with type 2 diabetes. Mol Med 2015; 20: 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tavakoli M, Mitu‐Pretorian M, Petropoulos IN, et al. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes 2013; 62: 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azmi S, Jeziorska M, Ferdousi M, et al. Early nerve fibre regeneration in individuals with type 1 diabetes after simultaneous pancreas and kidney transplantation. Diabetologia 2019; 62: 1478–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdul‐Ghani M, Migahid O, Megahed A, et al. Combination therapy with exenatide plus pioglitazone versus basal/bolus insulin in patients with poorly controlled type 2 diabetes on sulfonylurea plus metformin: the Qatar study. Diabetes Care 2017; 40: 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponirakis G, Abdul‐Ghani MA, Jayyousi A, et al. Effect of treatment with exenatide and pioglitazone or basal‐bolus insulin on diabetic neuropathy: a substudy of the Qatar Study. BMJ Open Diabetes Res Care. 2020; 8: e001420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spallone V, Morganti R, D'Amato C, et al. Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabet Med. 2012; 29: 578–585. [DOI] [PubMed] [Google Scholar]
- 29.Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005; 114: 29–36. [DOI] [PubMed] [Google Scholar]
- 30.Terkawi AS, Abolkhair A, Didier B, et al. Development and validation of Arabic version of the douleur neuropathique 4 questionnaire. Saudi J Anaesth 2017; 11: S31–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petropoulos IN, Alam U, Fadavi H, et al. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care 2013; 36: 3646–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dabbah MA, Graham J, Petropoulos IN, et al. Automatic analysis of diabetic peripheral neuropathy using multi‐scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Med Image Anal 2011; 15: 738–747. [DOI] [PubMed] [Google Scholar]
- 33.Casellini CM, Parson HK, Richardson MS, et al. Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol Ther 2013; 15: 948–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uceyler N, Vollert J, Broll B, et al. Sensory profiles and skin innervation of patients with painful and painless neuropathies. Pain 2018; 159: 1867–1876. [DOI] [PubMed] [Google Scholar]
- 35.Smith SM, Dworkin RH, Turk DC, et al. The potential role of sensory testing, skin biopsy, and functional brain imaging as biomarkers in chronic pain clinical trials: IMMPACT considerations. J Pain. 2017; 18: 757–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Themistocleous AC, Ramirez JD, Shillo PR, et al. The Pain in Neuropathy Study (PiNS): a cross‐sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain 2016; 157: 1132–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raputova J, Srotova I, Vlckova E, et al. Sensory phenotype and risk factors for painful diabetic neuropathy: a cross‐sectional observational study. Pain 2017; 158: 2340–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferdousi M, Azmi S, Kalteniece A, et al. Greater small nerve fibre damage in the skin and cornea of type 1 diabetic patients with painful compared to painless diabetic neuropathy. Eur J Neurol 2021. 10.1111/ene.14757 [DOI] [PubMed] [Google Scholar]
- 39.Gibbons CH, Freeman R. Treatment‐induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes. Brain 2015; 138: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demant DT, Lund K, Vollert J, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double‐blind, placebo‐controlled phenotype‐stratified study. Pain 2014; 155: 2263–2273. [DOI] [PubMed] [Google Scholar]
- 41.Yarnitsky D, Granot M, Nahman‐Averbuch H, et al. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain 2012; 153: 1193–1198. [DOI] [PubMed] [Google Scholar]
- 42.Malik RA, Veves A, Tesfaye S, et al. Small fibre neuropathy: role in the diagnosis of diabetic sensorimotor polyneuropathy. Diabetes Metab Res Rev. 2011; 27: 678–684. [DOI] [PubMed] [Google Scholar]
- 43.Breiner A, Lovblom LE, Perkins BA, et al. Does the prevailing hypothesis that small‐fiber dysfunction precedes large‐fiber dysfunction apply to type 1 diabetic patients? Diabetes Care 2014; 37: 1418–1424. [DOI] [PubMed] [Google Scholar]
- 44.Ishibashi F, Taniguchi M, Kosaka A, et al. Improvement in neuropathy outcomes with normalizing HbA1c in patients with type 2 diabetes. Diabetes Care 2019; 42: 110–118. [DOI] [PubMed] [Google Scholar]
- 45.Himeno T, Kamiya H, Naruse K, et al. Beneficial effects of exendin‐4 on experimental polyneuropathy in diabetic mice. Diabetes 2011; 60: 2397–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong N, Xiao Q, Zhu B, et al. Activation of spinal glucagon‐like peptide‐1 receptors specifically suppresses pain hypersensitivity. J Neurosci. 2014; 34: 5322–5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaiswal M, Martin CL, Brown MB, et al. Effects of exenatide on measures of diabetic neuropathy in subjects with type 2 diabetes: results from an 18‐month proof‐of‐concept open‐label randomized study. J Diabetes Complications 2015; 29: 1287–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brock C, Hansen CS, Karmisholt J, et al. Liraglutide treatment reduced interleukin‐6 in adults with type 1 diabetes but did not improve established autonomic or polyneuropathy. Br J Clin Pharmacol 2019; 85: 2512–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maeda T, Kiguchi N, Kobayashi Y, et al. Pioglitazone attenuates tactile allodynia and thermal hyperalgesia in mice subjected to peripheral nerve injury. J Pharmacol Sci 2008; 108: 341–347. [DOI] [PubMed] [Google Scholar]
- 50.Qiang X, Satoh J, Sagara M, et al. Inhibitory effect of troglitazone on diabetic neuropathy in streptozotocin‐induced diabetic rats. Diabetologia 1998; 41: 1321–1326. [DOI] [PubMed] [Google Scholar]
- 51.Yamagishi S, Ogasawara S, Mizukami H, et al. Correction of protein kinase C activity and macrophage migration in peripheral nerve by pioglitazone, peroxisome proliferator activated‐gamma‐ligand, in insulin‐deficient diabetic rats. J Neurochem 2008; 104: 491–499. [DOI] [PubMed] [Google Scholar]
- 52.Pop‐Busui R, Lu J, Brooks MM, et al. Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Cohort. Diabetes Care 2013; 36: 3208–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoybergs YM, Meert TF. The effect of low‐dose insulin on mechanical sensitivity and allodynia in type I diabetes neuropathy. Neurosci Lett 2007; 417: 149–154. [DOI] [PubMed] [Google Scholar]
- 54.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study. Diabetes Res Clin Pract 1995; 28: 103–117. [DOI] [PubMed] [Google Scholar]
- 55.Ismail‐Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010; 376: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adam S, Azmi S, Ho JH, et al. Improvements in diabetic neuropathy and nephropathy after bariatric surgery: a prospective cohort study. Obes Surg 2021; 31: 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azmi S, Ferdousi M, Petropoulos IN, et al. Corneal confocal microscopy identifies small‐fiber neuropathy in subjects with impaired glucose tolerance who develop type 2 diabetes. Diabetes Care 2015; 38: 1502–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Divisova S, Vlckova E, Hnojcikova M, et al. Prediabetes/early diabetes‐associated neuropathy predominantly involves sensory small fibres. J Peripher Nerv Syst. 2012; 17: 341–350. [DOI] [PubMed] [Google Scholar]


