Abstract
Aims/Introduction
The convergence of tuberculosis (TB) and diabetes mellitus (DM) is a new challenge in Asia as a result of the rising prevalence of diabetes mellitus with higher TB infection rates, and also because diabetes mellitus itself enhances TB disease activity and consequently the spread of TB. We aimed to address the risk presented by diabetes mellitus for TB infection.
Materials and Methods
Patients with diabetes mellitus were retrospectively recruited. The baseline assessments included age, sex, body mass index, fasting blood glucose, glycated hemoglobin, urine albumin‐to‐creatinine ratio and estimated glomerular filtration rate. TB was determined by meeting the international classification of disease, for TB diagnosis and receiving anti‐TB treatment for at least 2 months.
Results
In total, 9,750 individuals with diabetes mellitus were recruited. The event rate of TB was 47 (0.48%). Younger age, lower proportion of men, higher fasting blood glucose and glycated hemoglobin values, and better renal function (estimated glomerular filtration rate and urine albumin‐to‐creatinine ratio) were observed in the metformin‐exposed groups. Old age and male sex were associated with higher TB infection risk on multivariate analysis. Metformin users had a significantly lower risk for TB infection, whereas insulin users had a higher risk for TB infection. However, glycemic status had no effect on TB infection risk.
Conclusions
This study provides clinical evidence from a survey of TB in individuals with diabetes mellitus. Old age, male sex and insulin use were risk factors for TB infection. Metformin remains the first choice of treatment for diabetes mellitus and has a potential protective effect against TB infection.
Keywords: Diabetes mellitus, Metformin, Tuberculosis
Introduction
Mycobacterium tuberculosis (TB) infection remains a leading infectious disease in the modern world, particularly among Asian countries, such as Taiwan1. In fact, given that TB was the most commonly reported communicable disease in Taiwan for more than four decades, the Taiwan Centers for Disease Control launched the ‘Mobilization Plan to Reduce TB by half in Ten Years’ program in 2006. Accordingly, over a period of 10 years, the number of annual TB cases decreased from 16,472 in 2006 to 10,526 in 2015, and the annual incidence rate decreased from 72.5 person per 100,000 individuals to 45.6 persons per 100,000 individuals over this period1.
The convergence of TB and diabetes mellitus has become a new challenge in Asia, not only because of the rising prevalence of type 2 diabetes mellitus in populations with higher TB infection rates, but also because type 2 diabetes mellitus patients tend to have a higher TB disease severity due to the formation of pulmonary cavities that facilitate the spread of TB2, 3.
Bidirectional screening for TB and diabetes has been proposed by the World Health Organization (WHO) in 2011, and well‐established guidelines have been published to examine diabetes mellitus in patients with TB4. However, plans to assess TB in diabetes mellitus patients remain incomplete, primarily due to concerns regarding the cost‐effectiveness of such surveys. Therefore, a risk assessment of TB in patients with diabetes mellitus is required before deciding on the regular screening of TB in patients with diabetes mellitus. Recent reports have shown that medications for diabetes control, particularly metformin, affect the immune responses against TB. Therefore, we aimed to use the longitudinal medical records to assess the effects of medications for diabetes control, particularly metformin, on the development of TB in patients with diabetes mellitus, and sought to establish a model that incorporates clinical risk factors and medications, and consequently predicts TB development in patients with diabetes mellitus.
Materials and Methods
Study participants
We retrospectively recruited patients who enrolled in the diabetes pay‐for‐performance program of Taichung Veteran General Hospital, Taichung, Taiwan, between June 2007 and January 2014. The pay‐for‐performance program, also named the Diabetes Shared Care Program (DSCP), was initiated in 2001 by Taiwan’s Ministry of Health and Welfare to improve diabetes management, glycemic control, and to reduce the medical costs of diabetes. The percentage of diabetes mellitus patients treated under DSCP increased from 23.52% to 44.60% in 2005 and mid 2106, respectively5. The diabetes mellitus patients can opt in to the DSCP or not, and the physicians can decide if the patients enroll in the DSCP or not. Their electronic medical records were reviewed based on their initial medication, and their clinical parameters were recorded at the time of enrollment in the pay‐for‐performance program. Metformin users were defined as those using metformin within 3 months before enrollment in the pay‐for‐performance program. The use of antihypertensive medications and lipid‐lowering medications were also reviewed. The baseline variables assessed included age, sex, fasting blood glucose, glycated hemoglobin (HbA1c), micro‐albuminuria status, estimated glomerular filtration rate (eGFR), total cholesterol (TC), systolic blood pressure (SBP), and diastolic blood pressure (DBP). The status of glycemic control was defined based on the baseline fasting blood glucose/HbA1c. The Modification of Diet in Renal Disease equation was used for calculating eGFR6: 186 × plasma creatinine−1.154 × age−0.203. Individuals with a baseline eGFR >180 mL/min/1.73 m2 were excluded. The clinical end‐point was followed until 31 December 2014. The Ethics Committee of Taichung Veterans General Hospital approved this study and waived the requirement for informed consent with all data fully anonymized. All methods were carried out in accordance with the relevant guidelines and regulations.
Main outcome measures
New diagnosis of TB infection was considered as the primary end‐point. Previous or concurrent TB infection was excluded. Follow‐up duration of metformin and non‐metformin users in patients with diabetes mellitus was 2.8 ± 1.8 and 2.6 ± 1.8 years (P < 0.001). In patients, TB was determined by meeting the international classification of disease, ninth revision for TB diagnosis and receiving anti‐TB treatment (consisting of isoniazid, rifampicin, ethambutol and pyrazinamide) for at least 2 months.
Statistical analysis
Descriptive statistics for continuous variables were expressed as the mean ± standard deviation or number and percentage. The differences in clinical variables between groups were tested for statistical significance using the independent t‐test for continuous variables, and the χ2‐test for categorical variables. Kaplan–Meier curves for cumulative probability of tuberculosis infection were plotted for metformin users versus non‐metformin users and insulin users versus non‐insulin users. The Cox proportional hazards model was used to assess the impact of antidiabetic medications and the status of glycemic control on the risk of new TB infection. All of the statistical analyses were carried out using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA). All reported P‐values <0.05 were considered to show statistical significance.
Results
In total, 9,750 patients with type 2 diabetes mellitus were enrolled from Taichung Veterans General Hospital, Taichung, Taiwan. The event rate of TB was 0.48% (n = 47). Younger age, lower proportion of men, higher fasting blood glucose and HbA1c values, and better renal function (eGFR and urine albumin‐to‐creatinine ratio) were observed in the metformin‐exposed group. A greater proportion of sulfonylurea, dipeptidyl peptidase‐4 inhibitor and thiazolidinedione use, but lower proportion of insulin use was noted in the metformin‐exposed group. People with diabetes in the non‐metformin‐exposed group tended to smoke cigarettes heavily. More people received TB vaccination in the metformin‐exposed group (Table 1). Age >65 years and male sex were associated with a greater risk of TB infection on univariate and multivariate analysis. Metformin users had a significantly lower risk of TB infection, as compared with those not using metformin (P = 0.0020; Fig. 1); in contrast, insulin users had a significantly higher risk of TB infection compared with those without insulin use (P = 0.0071; Fig. 2). Multivariate analysis (Table 2) showed that metformin users had a 46% lower risk of TB infection compared with non‐metformin users (hazard ratio 0.54, 95% confidence interval 0.3–0.99, P = 0.0475). In contrast, the hazard ratio was 1.94 (95% confidence interval 1.04–3.61, P = 0.0365) in insulin users versus non‐insulin users. Glycemic status had no effect on TB infection.
Table 1.
Baseline characteristics of metformin and non‐metformin users among patients with diabetes mellitus
| Variables | Overall | Metformin (–) | Metformin (+) | P‐value |
|---|---|---|---|---|
| n (%) | 9,750 | 2,946 (30.22) | 6,804 (69.78) | |
| Age (years) | 65.1 ± 15.2 | 66.5 ± 18.5 | 64.5 ± 13.4 | <0.0001 |
| Male | 5,430 (55.7) | 1,744 (59.2) | 3,686 (54.2) | <0.0001 |
| BMI (kg/m2) | 25.7 ± 29.7 | 24.9 ± 18.9 | 26 ± 32.9 | 0.0800 |
| Systolic BP (mmHg) | 130.4 ± 13.1 | 129.8 ± 14 | 130.7 ± 12.7 | 0.0245 |
| Diastolic BP (mmHg) | 78.4 ± 8 | 77.2 ± 8.3 | 78.9 ± 7.8 | <0.0001 |
| HbA1c (%) | 7.6 ± 1.6 | 7.3 ± 1.5 | 7.7 ± 1.6 | <0.0001 |
| eGFR (mL/min/1.73 m2) | 80.3 ± 38.5 | 67.7 ± 50.4 | 85.8 ± 30.2 | <0.0001 |
| FBS (mg/dL) | 147.3 ± 48.5 | 141.6 ± 51.9 | 149.8 ± 46.7 | <0.0001 |
| TC (mg/dL) | 174.4 ± 38.8 | 173.6 ± 40.7 | 174.7 ± 37.9 | 0.1902 |
| LDL‐C (mg/dL) | 107.5 ± 34.2 | 106.6 ± 35.5 | 107.9 ± 33.6 | 0.0806 |
| HDL‐C (mg/dL) | 50.4 ± 14.2 | 51.2 ± 15.8 | 50.1 ± 13.3 | 0.0007 |
| UACR (mg/g) | 244.9 ± 769.8 | 444 ± 1099.5 | 162.3 ± 559.9 | <0.0001 |
| SU, n (%) | 4,290 (44) | 735 (25) | 3,555 (52.3) | <0.0001 |
| DPP4i, n (%) | 3,127 (32.1) | 631 (21.4) | 2,496 (36.7) | <0.0001 |
| TZD, n (%) | 1,096 (11.2) | 170 (5.8) | 926 (13.6) | <0.0001 |
| Insulin, n (%) | 3,330 (34.2) | 1,351 (45.9) | 1,979 (29.1) | <0.0001 |
| Statin, n (%) | 4,524 (46.4) | 1,189 (40.4) | 3,335 (49) | <0.0001 |
| ACEI, n (%) | 1,245 (12.8) | 324 (11) | 921 (13.5) | 0.0006 |
| ARB, n (%) | 3,489 (35.8) | 1,092 (37.1) | 2,397 (35.2) | 0.0821 |
| DM duration (years) | 7.3 ± 7.3 | 8.2 ± 8 | 6.9 ± 6.9 | <0.0001 |
| Smoke, n (%) | 3,305 (33.9) | 1,058 (35.91) | 2,247 (33.02) | 0.0057 |
| TB vaccine | 7,271 (74.57) | 1,918 (65.11) | 5,353 (78.67) | <0.0001 |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; DPP4i, dipeptidyl peptidase‐4 inhibitor; eGFR, estimated glomerular filtration rate; FBS, fasting blood glucose; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein‐cholesterol; LDL‐C, low‐density lipoprotein‐cholesterol; SU, sulfonylurea; TC, total cholesterol; TZD, thiazolidinedione; UACR, urine albumin‐to‐creatinine ratio.
Figure 1.
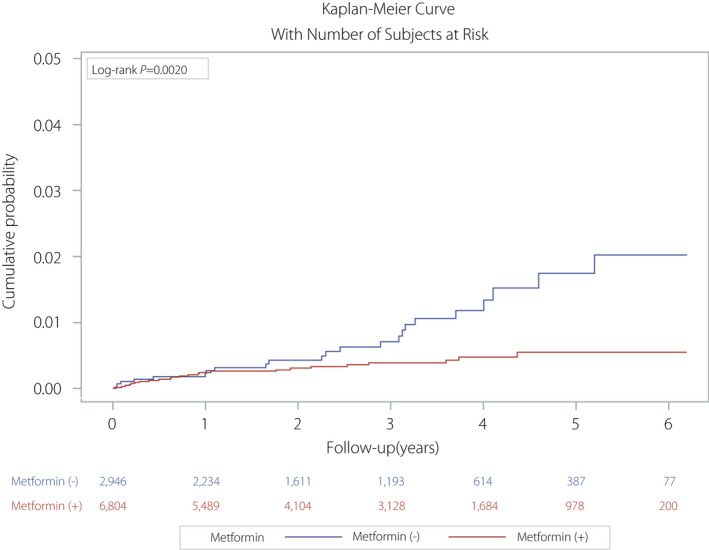
Kaplan–Meier curve for the cumulative probability of tuberculosis infection between metformin and non‐metformin users.
Figure 2.
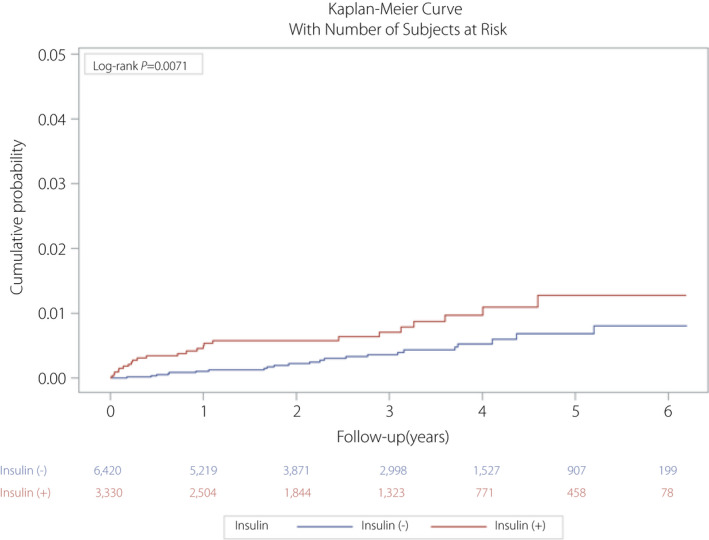
Kaplan–Meier curve for the cumulative probability of tuberculosis infection between insulin and non‐insulin users.
Table 2.
Predictors and comparison of hazard ratios for tuberculosis infection
| Variable | Univariate, HR (95% CI) | P‐value | Multivariate, HR (95% CI) | P‐value | P‐value |
|---|---|---|---|---|---|
| Age (>65 years vs ≤65 years) | 5.71 (2.42–13.44) | <0.0001 | 6.36 (2.66–15.23) | <.0001 | |
| Sex (male vs female) | 3.41 (1.65–7.06) | 0.0009 | 3.35 (1.59–7.05) | 0.0015 | |
| FBG (mg/dL) | |||||
| <100 | 0.62 (0.19–2.00) | 0.4202 | 0.54 (0.16–1.85) | 0.3289 | 0.1694* |
| 100–125 | 0.61 (0.29–1.26) | 0.1823 | 0.6 (0.27–1.32) | 0.2049 | |
| >125 | Reference | Reference | |||
| HbA1c (%) | |||||
| <7 | 0.53 (0.25–1.13) | 0.0986 | 0.64 (0.27–1.50) | 0.3013 | 0.3182* |
| 7–9 | 0.65 (0.31–1.33) | 0.2384 | 0.72 (0.34–1.51) | 0.3814 | |
| >9 | Reference | Reference | |||
| LDL‐C (mg/dL) | 1 (0.99–1.01) | 0.7100 | 1.01 (0.98–1.03) | 0.539 | |
| HDL‐C (mg/dL) | 0.98 (0.96–1) | 0.0874 | 1 (0.97–1.02) | 0.7183 | |
| TC (mg/dL) | 1 (0.99–1) | 0.2516 | 0.99 (0.97–1.02) | 0.5308 | |
| UACR (mg/g) | |||||
| <30 | Reference | Reference | 0.9106* | ||
| 30–300 | 1.46 (0.75–2.81) | 0.2643 | 1.01 (0.52–1.96) | 0.9882 | |
| >300 | 1.88 (0.87–4.04) | 0.1072 | 1.05 (0.47–2.34) | 0.9007 | |
| Diabetes treatment vs no use | |||||
| Metformin | 0.42 (0.24–0.74) | 0.0027 | 0.54 (0.3–0.99) | 0.0475 | |
| Any Insulin | 2.15 (1.22–3.82) | 0.0086 | 1.94 (1.04–3.61) | 0.0365 | |
FBS, fasting blood glucose; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein‐cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TC, total cholesterol; UACR, urine albumin‐to‐creatinine ratio.
Trend test.
Discussion
Our primary salient findings showed that metformin had a protective effect against TB infection in patients with diabetes mellitus. Furthermore, insulin use increased the risk of TB infection, whereas glycemic status did not affect the incidence of new TB infection.
Approximately 9.6 million individuals were estimated to have TB worldwide in 2014, and among the Asian countries, China and India primarily accounted for 58% of new TB cases7. The current goal of the WHO is to reduce the number of new TB cases by 90% in 2035, whereas the Taiwan Centers for Disease Control also aims to achieve an annual reduction in the number of new TB cases and to gradually eliminate TB in Taiwan by 2035. However, in Taiwan, a combination of a dense and aging population, increasing prevalence of diabetes mellitus, frequent travel to high‐burden countries, and foreign spouses and workers from Asian countries with high TB prevalence had made TB control more challenging7.
Diabetes mellitus affects people worldwide and markedly threatens public health due to its increasing prevalence globally, and particularly in Asian countries, as a result of an increased prevalence of obesity, changing patterns of diet and physical activity, and aging populations8, 9. Studies have found that patients with diabetes mellitus tend to have a higher incidence, higher severity and poor treatment outcome of TB compared with those without diabetes mellitus10, 11, 12. Thus, the convergence of epidemics of TB and diabetes mellitus has become a great concern, particularly in Asian countries, which have the highest TB burden and have experienced a marked increase in diabetes mellitus prevalence in recent years13. Dynamic TB transmission models have been used to analyze the potential effect of diabetes mellitus on TB epidemiology, and have shown that the cumulative reduction in TB incidence would be just 8.8% in 2035 under the current diabetes mellitus prevalence trends14. Therefore, diabetes mellitus is currently considered as a major obstacle to achieve the WHO goal of reducing the number of TB cases by 90% by 2035. Although the WHO has proposed the need for bidirectional screening for TB and diabetes mellitus, additional studies are required to elucidate the mechanisms underlying diabetes mellitus in patients with TB, which is essential to initiate appropriate measures for a bidirectional survey of diabetes mellitus and TB. In fact, comprehensive studies investigating diabetes mellitus in patients with TB have established practical guidelines for surveying and treating diabetes mellitus in TB patients15; however, plans to assess TB in diabetes mellitus patients remain incomplete due to the limited evidence in diabetes mellitus patients with complex antidiabetic treatments16, 17. A recently published study in India that used a WHO‐recommended symptom screen among patients visiting the clinic failed to determine any active TB cases; furthermore, the cost‐effectiveness of this approach was a problem due to the low sensitivity18. Nevertheless, the screening of TB in diabetes mellitus patients is vital, and additional studies are required to clarify the risk factors, such as age, duration of diabetes mellitus and glycemic control status, for TB infection in patients with diabetes mellitus through a regular assessment by endocrinologists. Thus, the resources for TB screening might be focused on high‐risk subjects. Our study showed that old age, male gender and insulin use, but not glycemic status, were risk factors for TB infection in diabetes mellitus patients. In a recent Taiwanese nationwide population‐based study, old age and male sex were found to be risk factors for death in diabetes mellitus patients with TB infection19. The researchers suggested that diabetes mellitus patients should undergo screening for TB infection, particularly elderly men who use insulin, as they were more susceptible to TB infection and had higher mortality after TB infection.
Metformin has been used for >40 years to enhance insulin sensitivity through adenosine monophosphate‐activated protein kinase signaling and has had unique roles in diseases other than diabetes mellitus, such as anticancer activities20. Recently, Vashisht et al.21 clearly showed that metformin reduced the growth of M. tuberculosis through the adenosine monophosphate‐activated protein kinase and reactive oxygen species pathway in an aerosol‐infected mouse TB infection model. Furthermore, metformin promotes the formation of anti‐inflammatory M2 macrophages and regulatory T and CD8 memory T cells, and leads to inflammation reduction22, 23, 24. Metformin use was found to reduce the risk of TB infection in a dose–response pattern in a cohort analysis of the Taiwan’s National Health Insurance Research Database25, 26. The present study was more powerful than that study. First, we included data on blood glucose and HbA1c values, which reflects the quality of glycemic control in patients. Second, we recorded body mass index (BMI) values, as they are vital in the selection of antidiabetic drugs27. Furthermore, a lower BMI is a risk factor of TB infection28, 29, 30. However, the BMI did not significantly differ between metformin users and non‐users in the present study, and hence, we believe that BMI does not affect the TB incidence between the groups. In contrast to metformin, insulin resistance has been found to be a risk factor for TB31. Insulin has recently been found to induce the production of regulatory T cells32, which can inhibit the immune response against M. tuberculosis. Thus, antidiabetic medications could affect TB development, and should be incorporated in the risk assessment of TB in patients with diabetes mellitus, consistent with that noted in the present study that metformin has a protective effect against TB, whereas insulin use is a risk factor.
Good blood glucose control of diabetes mellitus reduces the risk of developing TB33, whereas poor glycemic control is associated with a higher TB incidence34, 35. The present result showed that the glycemic status did not affect the incidence of TB, whereas the use of metformin and insulin did. Insulin use is a reliable indicator of more severe diabetes mellitus. Metformin users showed slightly poorer glycemic control than non‐metformin users. In the present study, we found that poor glycemic status might further increase the incidence of TB, although the effect was not significant. In contrast, the effects of metformin and insulin remained similar, both in terms of the cumulative incidence or relative risk estimation. These findings suggest that the effects of antidiabetic medications on TB infection might surpass the effects of the glycemic control in terms of magnitude.
The present retrospective study had several major limitations. First, the temporal relationship was difficult to assess, as no TB tests were carried out before study enrollment, and some baseline characteristics might result from undiagnosed TB infections. Second, other risk factors, such as smoking, heavy alcohol use or nutritional status, were not recorded. Nevertheless, the sex‐related difference might be attributed to the variation in cigarette smoking between Taiwanese men and women (prevalence of 34% and 4.8% in 2013, respectively)36. Third, we did not use the duration of diabetes, as it is not reliable and might vary due to recall memory errors. Fourth, we did not record the cumulative dose of metformin use; however, metformin is the first‐line therapy for diabetes mellitus in Taiwan, and the non‐metformin users were considered not to use metformin in the subsequent treatment, except few patients with diabetes mellitus were treated primarily with diet alone in the diabetes pay‐for‐performance program. Finally, we did not determine which individuals had type 1 or type 2 diabetes, but it is likely most had type 2 diabetes.
The results of the present study provide clinical evidence for the assessment of TB in patients with diabetes mellitus. Old age, male sex and insulin use were found to be risk factors for TB infection. Metformin remains the first choice of treatment for diabetes mellitus in individuals without any contraindication, and could have a potential protective effect against TB infection.
Disclosure
The authors disclose no conflict of interest.
Acknowledgments
This work was supported by grants from Taichung Veterans General Hospital, Taichung, Taiwan (TCVGH‐1067301B).
J Diabetes Investig. 2021; 12: 1603–1609
References
- 1.Taiwan National Infectious Disease Statistics System. 2016. Available online at https://nidss.cdc.gov.tw/en/SingleDisease.aspx?dc=1&dt=3&disease=010 (Chinese).
- 2.Tatar D, Senol G, Alptekin S, et al. Tuberculosis in diabetics: features in an endemic area. Jpn J Infect Dis 2009; 62: 423–427. [PubMed] [Google Scholar]
- 3.Thanh NP, Khue PM, Sy DN, et al. [Diabetes among new cases of pulmonary tuberculosis in Hanoi, Vietnam] Bull Soc Pathol Exot 2015; 108: 337–341. [DOI] [PubMed] [Google Scholar]
- 4.WHO . International Union Against Tuberculosis and Lung Disease. Collaborative Framework for Care and Control of Tuberculosis and Diabetes. WHO/HTM/TB/2011.15. Geneva: World Health Organization, 2011. [PubMed] [Google Scholar]
- 5.National Health Insurance Administration, Ministry of Health and Welfare . 2016–2017 National Health Insurance Annual Report. Taiwan: National Health Insurance Administration, Ministry of Health and Welfare; 2018. [Google Scholar]
- 6.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 7.Center for Disease Control, Department of Health, executive yuan, Taiwan. Tuberculosis annual report, 2017. Availlable online at https://www.cdc.gov.tw/uploads/files/201712/2bf81c5a‐4812‐4977‐9c02‐388aadba77a7.pdf (Chinese).
- 8.Mozaffarian D, Kamineni A, Carnethon M, et al. Lifestyle risk factors and new‐onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med 2009; 169: 798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popkin BM. Will China's nutrition transition overwhelm its health care system and slow economic growth? Health Aff (Millwood) 2008; 27: 1064–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008; 5: e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leegaard A, Riis A, Kornum JB, et al. Diabetes, glycemic control, and risk of tuberculosis: a population‐based case‐control study. Diabetes Care 2011; 34: 2530–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang CY, Bai KJ, Lin HH, et al. The influence of diabetes, glycemic control, and diabetes‐related comorbidities on pulmonary tuberculosis. PLoS One 2015; 10: e0121698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis 2009; 9: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan SC, Ku CC, Kao D, et al. Effect of diabetes on tuberculosis control in 13 countries with high tuberculosis: a modelling study. Lancet Diabet Endocrinol 2015; 3: 323–330. [DOI] [PubMed] [Google Scholar]
- 15.Harries AD, Kumar AM, Satyanarayana S, et al. Diabetes mellitus and tuberculosis: programmatic management issues. Int J Tubercul Lung Dis 2015; 19: 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Lin Y, Mi F, et al. Screening of patients with tuberculosis for diabetes mellitus in China. Trop Med Int Health 2012; 17: 1294–1301. [DOI] [PubMed] [Google Scholar]
- 17.India Diabetes Mellitus–Tuberculosis Study G . Screening of patients with diabetes mellitus for tuberculosis in India. Trop Med Int Health 2013; 18: 646‐654. [DOI] [PubMed] [Google Scholar]
- 18.Mave V, Nimkar S, Prasad H, et al. Tuberculosis screening among persons with diabetes mellitus in Pune, India. BMC Infect Dis 2017; 17: 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko PY, Lin SD, Hsieh MC, et al. Diabetes mellitus increased all‐cause mortality rate among newly‐diagnosed tuberculosis patients in an Asian population: a nationwide population‐based study. Diabetes Res Clin Pract 2017; 133: 115–123. [DOI] [PubMed] [Google Scholar]
- 20.Safe S, Nair V, Karki K. Metformin‐induced anticancer activities: recent insights. Biol Chem 2018; 399: 321–335. [DOI] [PubMed] [Google Scholar]
- 21.Vashisht R, Brahmachari SK. Metformin as a potential combination therapy with existing front‐line antibiotics for Tuberculosis. J Transl Med 2015; 13: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang SC, Everts B, Ivanova Y, et al. Cell‐intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol 2014; 15: 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin Y, Choi SC, Xu Z, et al. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med 2015; 7: 274ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearce EL, Walsh MC, Cejas PJ, et al. Enhancing CD8 T‐cell memory by modulating fatty acid metabolism. Nature 2009; 460: 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng CH. Metformin decreases risk of tuberculosis infection in type 2 diabetes patients. J Clin Med 2018; 7: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee MC, Chiang CY, Lee CH, et al. Metformin use is associated with a low risk of tuberculosis among newly diagnosed diabetes mellitus patients with normal renal function: a nationwide cohort study with validated diagnostic criteria. PLoS One 2018; 13: e0205807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American DA. 1. Promoting health and reducing disparities in populations. Diabetes Care 2017; 40: S6–S10. [DOI] [PubMed] [Google Scholar]
- 28.Edwards LB, Livesay VT, Acquaviva FA, et al. Height, weight, tuberculous infection, and tuberculous disease. Arch Environ Health 1971; 22: 106–112. [DOI] [PubMed] [Google Scholar]
- 29.Patra J, Jha P, Rehm J, et al. Tobacco smoking, alcohol drinking, diabetes, low body mass index and the risk of self‐reported symptoms of active tuberculosis: individual participant data (IPD) meta‐analyses of 72,684 individuals in 14 high tuberculosis burden countries. PLoS One 2014; 9: e96433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cegielski JP, Arab L, Cornoni‐Huntley J. Nutritional risk factors for tuberculosis among adults in the United States, 1971–1992. Am J Epidemiol 2012; 176: 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao F, Chen T, Zhao Y, et al. Insulin resistance: a potential marker and risk factor for active tuberculosis? Med Hypotheses 2011; 77: 66–68. [DOI] [PubMed] [Google Scholar]
- 32.Akbarpour M, Goudy KS, Cantore A, et al. Insulin B chain 9‐23 gene transfer to hepatocytes protects from type 1 diabetes by inducing Ag‐specific FoxP3+ Tregs. Sci Trans Med 2015; 7: 289ra81. [DOI] [PubMed] [Google Scholar]
- 33.Lo HY, Yang SL, Lin HH, et al. Does enhanced diabetes management reduce the risk and improve the outcome of tuberculosis? Int J Tuber Lung Dis 2016; 20: 376–382. [DOI] [PubMed] [Google Scholar]
- 34.Qiu H, Shi Y, Li Y, et al. Incident rate and risk factors for tuberculosis among patients with type 2 diabetes: retrospective cohort study in Shanghai, China. Trop Med Int Health 2017; 22: 830–838. [DOI] [PubMed] [Google Scholar]
- 35.Marupuru S, Senapati P, Pathadka S, et al. Protective effect of metformin against tuberculosis infections in diabetic patients: an observational study of south Indian tertiary healthcare facility. Brazilian J Infect Dis 2017; 21: 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiang CY, Chang HY. A population study on the time trend of cigarette smoking, cessation, and exposure to secondhand smoking from 2001 to 2013 in Taiwan. Popul Health Metr 2016; 14: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]


