Abstract
Aims/Introduction
To assess efficacy and safety of insulin degludec/liraglutide (IDegLira) in Japanese participants with type 2 diabetes across different baseline characteristics.
Materials and Methods
Data from two randomized controlled trials were used: DUAL I Japan (n = 819 insulin‐naïve participants) and DUAL II Japan (n = 210 insulin‐experienced participants). Outcomes were assessed according to baseline glycated hemoglobin ( HbA1c; <8.0%, ≥8.0–<9.0%, ≥9.0%), body mass index (<25, ≥25–<30, ≥30 kg/m2) and age (<65, ≥65 years).
Results
In DUAL I Japan, reductions in HbA1c with IDegLira versus degludec and liraglutide were observed across all subgroups (treatment differences: −0.48% to −0.72% vs degludec, −0.29% to −0.73% vs liraglutide). Results were similar with IDegLira versus degludec in DUAL II Japan (treatment differences: −0.82% to −1.61%). Treatment‐by‐subgroup interactions were significant for IDegLira versus liraglutide for baseline HbA1c and age in DUAL I Japan, and for IDegLira versus degludec for baseline HbA1c in DUAL II Japan. In DUAL I Japan, IDegLira was associated with less weight gain than degludec in most subgroups. In DUAL II Japan, IDegLira was associated with a small mean weight loss (except for baseline HbA1c ≥9.0%) versus a small gain for degludec (except for age ≥65 years subgroup); treatment‐by‐subgroup interactions were not significant. Total daily insulin dose was lower with IDegLira versus degludec across all categories, except for age >65 years in DUAL II Japan.
Conclusions
IDegLira reduced HbA1c in Japanese participants with type 2 diabetes across baseline HbA1c, body mass index and age categories, without unexpected safety issues.
Keywords: Insulin degludec/liraglutide, Japan, Type 2 diabetes mellitus
The aim of this subgroup analysis was to assess efficacy and safety of insulin degludec/liraglutide in Japanese participants with type 2 diabetes across different baseline characteristics. Overall, insulin degludec/liraglutide reduced glycated hemoglobin in Japanese participants with type 2 diabetes across baseline glycated hemoglobin, body mass index and age categories, without unexpected safety issues.

Introduction
Insulin degludec/liraglutide (IDegLira) is a soluble fixed‐ratio combination of insulin degludec (degludec) and the glucagon‐like peptide‐1 receptor agonist, liraglutide1. The global DUAL clinical trial program investigated the efficacy and safety of IDegLira across a range of patient populations with type 2 diabetes2, 3, 4, 5, 6, 7, 8, 9. Following this trial program, two randomized trials, DUAL I Japan and DUAL II Japan, investigated the use of IDegLira in Japanese participants with type 2 diabetes uncontrolled on previous diabetes treatment with an oral antidiabetic drug or basal or pre‐mix insulin, respectively10, 11.
In DUAL I Japan, IDegLira was associated with significantly greater glycated hemoglobin (HbA1c) reductions versus liraglutide 1.8 mg and degludec after 52 weeks (both P < 0.0001)10. Bodyweight increased to a lesser extent with IDegLira versus degludec, but, as expected, there was a significant difference in favor of liraglutide versus IDegLira. The rate of severe or blood glucose (BG)‐confirmed hypoglycemia (<3.1 mmol/L [<56 mg/dL]) for IDegLira was lower compared with degludec, but higher compared with liraglutide10. Gastrointestinal adverse events (AEs) were reported by 34.9%, 22.9% and 41.8% of participants in the IDegLira, degludec and liraglutide groups, respectively10. In DUAL II Japan, IDegLira provided superior reduction from baseline in HbA1c after 26 weeks versus degludec (P < 0.0001)11. IDegLira was associated with bodyweight loss compared with weight gain for degludec, and the rates of severe or BG‐confirmed hypoglycemia were comparable between treatment groups11.
Disease progression and clinical characteristics, such as HbA1c, body mass index (BMI) and age, are often considered when individualizing diabetes treatment options. Elderly patients represent a unique group due to increased risk of hypoglycemia and frailty, and therefore careful consideration is required when setting glycemic targets and choosing treatments12. The Japan Diabetes Society/Japan Geriatrics Society recommend specific glycemic targets in elderly patients depending on various characteristics13. Hence, it is important to investigate the results of DUAL I Japan and DUAL II Japan regarding these characteristics to inform physicians which patients might benefit most from IDegLira treatment.
We report subgroup analyses of DUAL I Japan and DUAL II Japan investigating the efficacy and safety of IDegLira in patients grouped according to trial baseline characteristics (HbA1c, BMI and age).
Materials and Methods
For the present subgroup analyses of DUAL I Japan and DUAL II Japan (further details in Supporting Information)10, 11, patients were grouped according to baseline HbA1c (<8.0%, ≥8.0–<9.0%, ≥9.0%), BMI (<25, ≥25–<30, ≥30 kg/m2) and age (non‐elderly [<65 years] and elderly [≥65 years]). Baseline HbA1c cut‐offs were chosen to ensure a similar number of patients in each HbA1c subgroup. The obesity and age cut‐offs aligned with the BMI/age categories, as defined by the Japan Society for the Study of Obesity and the Japan Diabetes Society/Japan Geriatrics Society, respectively13, 14.
Statistical analysis
In the subgroup analyses, changes in HbA1c and bodyweight, end‐of‐trial (EOT) total daily insulin dose, and confirmed hypoglycemia rates were assessed for each study across baseline HbA1c, BMI and age categories using the cut‐offs described above. Analyses of all end‐points assessed across baseline HbA1c categories were post‐hoc, as were the analyses of change in bodyweight and total insulin dose by baseline BMI, and total insulin dose by age.
Changes from baseline in HbA1c and bodyweight, and EOT total daily insulin dose were analyzed using an analysis of covariance (ancova) model, which included treatment, pre‐trial diabetes treatment, subgroup (baseline HbA1c, BMI or age), and interaction between treatment and subgroup as fixed factors. For analysis of change in HbA1c and bodyweight, corresponding baseline value was included as a covariate. For analysis of total daily insulin dose, baseline HbA1c (both trials) and baseline insulin dose (DUAL II Japan only) were included as covariate(s). In each case, missing data were imputed using last observation carried forward.
From the model, treatment differences with 95% confidence intervals were estimated for each subgroup, and the treatment‐by‐subgroup interaction was tested to assess if the treatment effect was affected by the subgroups. The number of severe or BG‐confirmed hypoglycemia and AEs (including gastrointestinal AEs) are presented descriptively by treatment and subgroup.
Results
All 819 participants from DUAL I Japan were included in the analysis. From DUAL II Japan, all 210 participants were included in the analysis.
Participant distribution across baseline HbA1c, baseline BMI and age categories is shown in Table 1 and discussed in the Supporting Information.
Table 1.
Number of participants in each baseline characteristic category
| IDegLira | Degludec | Liraglutide 1.8 mg | |
|---|---|---|---|
| DUAL I Japan (total) | 275 | 271 | 273 |
| Baseline HbA1c, n (%) | |||
| <8.0% | 107 (39) | 105 (39) | 128 (47) |
| ≥8.0 to < 9.0% | 80 (29) | 70 (26) | 71 (26) |
| ≥9.0% | 88 (32) | 96 (35) | 74 (27) |
| Baseline BMI, n (%) | |||
| <25 kg/m2 | 122 (44) | 117 (43) | 118 (43) |
| ≥25 to <30 kg/m2 | 112 (41) | 107 (39) | 109 (40) |
| ≥30 kg/m2 | 41 (15) | 47 (17) | 46 (17) |
| Age, n (%) | |||
| <65 years | 197 (72) | 183 (68) | 202 (74) |
| ≥65 years | 78 (28) | 88 (32) | 71 (26) |
| DUAL II Japan (total) | 105 | 105 | |
| Baseline HbA1c, n (%) | |||
| <8.0% | 29 (28) | 27 (26) | |
| ≥8.0 to < 9.0% | 40 (38) | 46 (44) | |
| ≥9.0% | 36 (34) | 32 (30) | |
| Baseline BMI, n (%) | |||
| <25 kg/m2 | 26 (25) | 28 (27) | |
| ≥25 to <30 kg/m2 | 64 (61) | 53 (50) | |
| ≥30 kg/m2 | 15 (14) | 24 (23) | |
| Age, n (%) | |||
| <65 years | 75 (71) | 85 (81) | |
| ≥65 years | 30 (29) | 20 (19) | |
BMI, body mass index; HbA1c, glycated hemoglobin; IDegLira, insulin degludec/liraglutide; n, number of participants.
The overall results for the subgroup analyses on change in HbA1c, change in bodyweight and EOT insulin dose are summarized in Table S1 and presented in more detail below.
Change in HbA1c
Estimated treatment differences
Based on the 95% confidence intervals of the estimated treatment differences (ETDs), IDegLira was associated with greater improvements in HbA1c versus both degludec and liraglutide across all participant subgroups (baseline HbA1c, baseline BMI and age) in both DUAL I Japan and DUAL II Japan (Figure 1). In DUAL I Japan, the ETD ranged from –0.48% to −0.72% for IDegLira versus degludec, and from –0.29% to –0.73% for IDegLira versus liraglutide. In DUAL II Japan, the ETDs were similar with IDegLira versus degludec, ranging from −0.82% to −1.61%.
Figure 1.
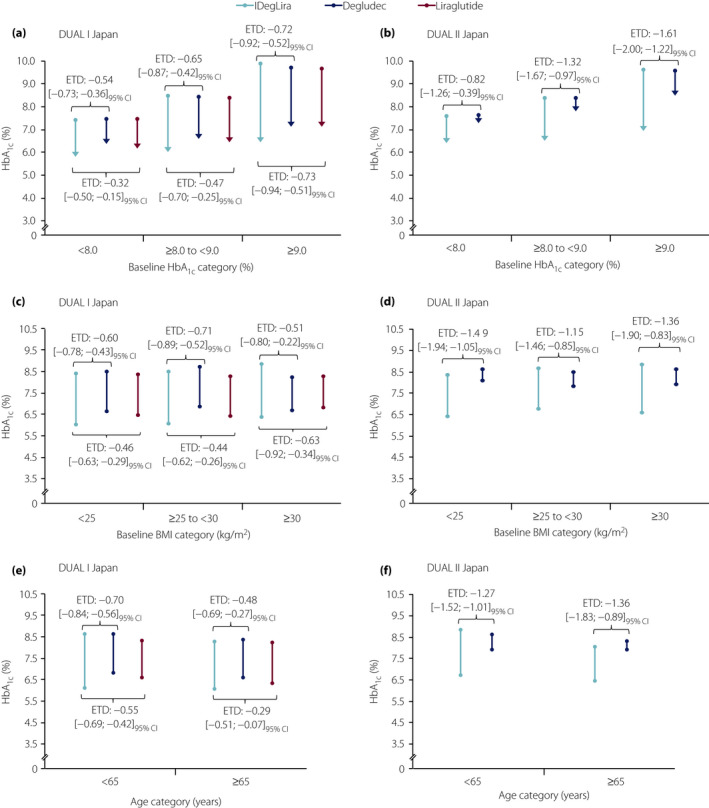
Change in glycated hemoglobin (HbA1c) with insulin degludec/liraglutide (IDegLira), degludec and liraglutide according to (a,b) baseline glycated hemoglobin (HbA1c), (c,d) baseline body mass index (BMI) and (e,f) age in DUAL I Japan and DUAL II Japan. Points represent the observed mean HbA1c at baseline and the arrows represent the observed mean HbA1c at end‐of‐trial. Changes from baseline in HbA1c were analyzed using an analysis of covariance (ancova) model, which included treatment, pre‐trial diabetes treatment, subgroup (baseline HbA1c, BMI or age), and interaction between treatment and subgroup as fixed factors. For analysis of change in HbA1c, baseline HbA1c was included as a covariate. Missing data were imputed using last observation carried forward. From the model, treatment differences with 95% confidence intervals (95% CI) were estimated for each subgroup, and the treatment‐by‐subgroup interaction was tested to assess if the treatment effect was affected by the subgroups. ETD, estimated treatment difference.
Treatment interaction
In DUAL I Japan, although the magnitude of the ETD in HbA1c between IDegLira and degludec increased with increasing baseline HbA1c, the treatment‐by‐subgroup interaction was not significant (P = 0.4432). In contrast, the interaction for IDegLira versus liraglutide was significant (P = 0.0194), showing that IDegLira was more effective than liraglutide for change in HbA1c with increasing baseline HbA1c.
In DUAL II Japan, the treatment‐by‐subgroup interaction according to baseline HbA1c was statistically significant for IDegLira versus degludec (P = 0.0312), again indicating that IDegLira was more effective than degludec for change in HbA1c with increasing baseline HbA1c.
The treatment‐by‐subgroup interaction for change in HbA1c according to baseline BMI was not significant (P > 0.05; Table S1 a and b) for IDegLira versus liraglutide (DUAL I Japan) or IDegLira versus degludec (DUAL I Japan and DUAL II Japan).
The treatment‐by‐subgroup interaction for change in HbA1c according to age was significant (P = 0.0481) for IDegLira versus liraglutide (DUAL I Japan), indicating that IDegLira was more effective than liraglutide in participants aged <65 years. It was not significant for IDegLira versus degludec in either trial.
Change in bodyweight
Estimated treatment differences
In DUAL I Japan, IDegLira was associated with less weight gain than degludec regardless of baseline HbA1c, baseline BMI or age (Figure 2). The ETD ranged from–0.24 kg to –1.47 kg for IDegLira versus degludec, and from +3.34 kg to +4.83 kg for IDegLira versus liraglutide. In liraglutide‐treated patients, there was an overall decrease in bodyweight in all subgroups, compared with an increase in those treated with IDegLira; ETDs favored liraglutide across all subgroups (Figure 2).
Figure 2.

Change in bodyweight with insulin degludec/liraglutide (IDegLira), degludec and liraglutide according to (a,b) baseline glycated hemoglobin (HbA1c), (c,d) baseline body mass index (BMI) and (e,f) age in DUAL I Japan and DUAL II Japan. Bars represent the observed mean change from baseline to end‐of‐trial (EOT) in bodyweight. Changes from baseline in bodyweight were analyzed using an analysis of covariance (ancova) model, which included treatment, pre‐trial diabetes treatment, subgroup (baseline HbA1c, BMI or age), and interaction between treatment and subgroup as fixed factors. For analysis of change in bodyweight, baseline bodyweight was included as a covariate. Missing data were imputed using last observation carried forward. From the model, treatment differences with 95% confidence intervals (95% CI) were estimated for each subgroup, and the treatment‐by‐subgroup interaction was tested to assess if the treatment effect was affected by the subgroups. ETD, estimated treatment difference.
In DUAL II Japan, IDegLira treatment was associated with bodyweight loss in participants with baseline HbA1c <8.0%, baseline HbA1c ≥8.0 to <9.0%, baseline BMI ≥25 to <30 kg/m2 and age <65 years, versus weight gain for degludec‐treated participants; in all of these subgroups, there was an overall decrease in bodyweight in IDegLira‐treated patients, compared with an overall increase in those treated with degludec (Figure 2). There was a greater weight loss associated with IDegLira treatment in the participants aged ≥65 years versus those aged <65 years (Figure 2). The magnitude of the ETDs ranged from –0.06 to –2.29 kg for IDegLira versus degludec.
Treatment interaction
The treatment‐by‐subgroup interaction for change in bodyweight was not statistically significant (P > 0.05; Table S1) for any of the analyses in DUAL I Japan or DUAL II Japan.
EOT insulin dose
Estimated treatment differences
In DUAL I Japan, the EOT daily total insulin dose was numerically lower for IDegLira versus degludec across all participant subgroups (baseline HbA1c, baseline BMI and age; Figure 3). The magnitude of the ETDs ranged from − 4.39 U to −17.62 U.
Figure 3.
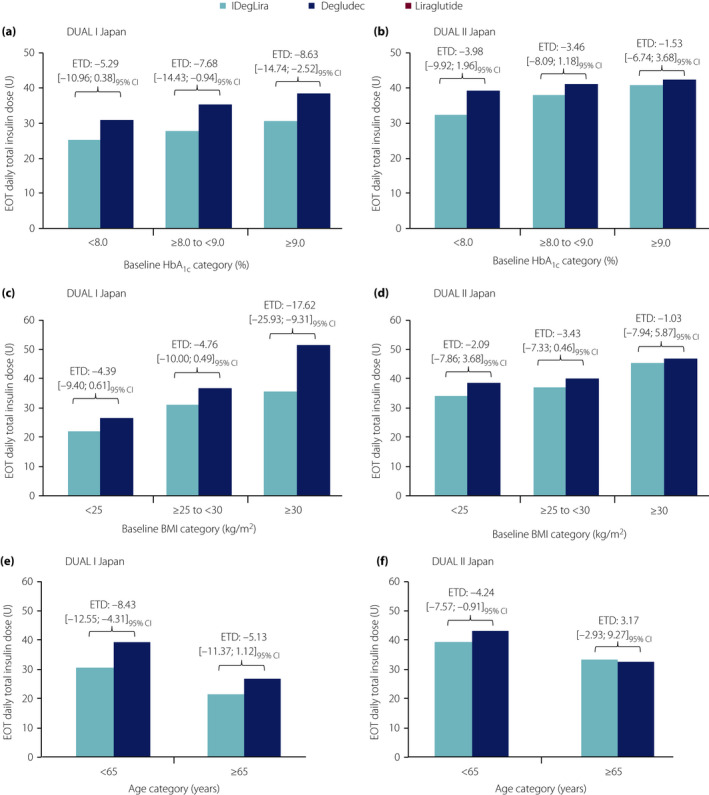
End‐of‐trial (EOT) daily total insulin dose in insulin degludec/liraglutide (IDegLira)‐ and degludec‐treated participants according to (a,b) baseline glycated hemoglobin (HbA1c), (c,d) baseline body mass index (BMI) and (e,f) age in DUAL I Japan and DUAL II Japan. Bars represent the observed mean EOT daily total insulin dose. EOT total daily insulin dose was analyzed using an analysis of covariance (ancova) model, which included treatment, pre‐trial diabetes treatment, subgroup (baseline HbA1c, BMI or age), and interaction between treatment and subgroup as fixed factors. Baseline HbA1c (both trials) and baseline insulin dose (DUAL II Japan only) were included as covariate(s). Missing data were imputed using last observation carried forward. From the model, treatment differences with 95% confidence intervals (95% CI) were estimated for each subgroup, and the treatment‐by‐subgroup interaction was tested to assess if the treatment effect was affected by the subgroups. ETD, estimated treatment difference.
In DUAL II Japan, the EOT daily total insulin dose was lower for IDegLira versus degludec regardless of baseline HbA1c or baseline BMI; however, it was lower in participants aged <65 years and greater in those aged ≥65 years (Figure 3). The magnitude of the ETDs across participant subgroups (baseline HbA1c, baseline BMI and age) ranged from −4.24 U to +3.17 U.
Treatment interaction
In DUAL I Japan, the treatment‐by‐subgroup interaction for insulin dose was not statistically significant for baseline HbA1c or age, but was statistically significant for baseline BMI (IDegLira vs degludec; P = 0.0190; Figure 3c; Table S1e). In DUAL II Japan, the interaction was not statistically significant for baseline HbA1c or baseline BMI, but was statistically significant for age (P = 0.037; Figure 3f; Table S1f).
Hypoglycemia
In DUAL I Japan, the rates of severe or BG‐confirmed hypoglycemia were lower with IDegLira versus degludec across all subgroups (Table S2a). In DUAL II Japan, the rate was lower with IDegLira versus degludec for the baseline HbA1c <8.0% and ≥8.0 to <9.0% subgroups, but higher than IDegLira versus degludec for the baseline HbA1c ≥9.0% subgroup (Table S2b). In DUAL II Japan, the rate was lower with IDegLira versus degludec for the baseline BMI ≥25 to <30 kg/m2 subgroup, but higher with IDegLira versus degludec in the baseline BMI <25 kg/m2 subgroup (Table S2b). Compared with degludec, hypoglycemia rates for IDegLira were higher in non‐elderly participants and lower in elderly participants.
As expected, rates of severe or BG‐confirmed hypoglycemia were higher with IDegLira and degludec versus liraglutide across all subgroups (DUAL I Japan; Table S2a).
AEs
There was no consistent pattern in overall AE rates for IDegLira versus degludec across the subgroups in DUAL I Japan or DUAL II Japan (Table S3). In contrast, the rate of gastrointestinal AEs was consistently higher with IDegLira than degludec across all subgroups in DUAL I Japan and DUAL II Japan (Table S4). Rates of gastrointestinal AEs in DUAL I Japan were lower with IDegLira versus liraglutide for those with baseline HbA1c ≥8.0% (Table S4a).
Conclusions
The aim of the current subgroup analyses of DUAL I Japan and DUAL II Japan was to understand the treatment response with IDegLira for patients in clinical practice by evaluating the efficacy and safety across patient subgroups split by baseline HbA1c, baseline BMI and age.
Based on the ETDs, IDegLira was associated with improvements in HbA1c versus degludec monotherapy and liraglutide monotherapy in all participant subgroups; that is, irrespective of their baseline HbA1c, baseline BMI or age. The magnitude of the ETD in HbA1c between IDegLira and comparators increased with increasing baseline HbA1c in both DUAL I Japan and DUAL II Japan, suggesting, as one might expect, that combination treatment is more effective in those with higher baseline HbA1c. A significant treatment‐by‐baseline HbA1c interaction was observed for IDegLira versus liraglutide in insulin‐naïve participants (DUAL I Japan). For IDegLira versus degludec, this trend was more pronounced in DUAL II Japan (significant interaction) than DUAL I Japan (no significant interaction). This might be because the degludec dose was capped in the DUAL II Japan trial (maximum dose 50 U), but not in DUAL I Japan. There was also a significant treatment interaction with age for IDegLira versus liraglutide in DUAL I Japan, indicating that although IDegLira provides effective glycemic control in both younger (aged <65 years) and older participants (aged ≥65 years), it is more effective than liraglutide in the former.
As previously reported, the effects of a glucagon‐like peptide‐1 receptor agonist are expected to be affected by remaining β‐cell function15. For example, Usui et al. reported the relationship between β‐cell function and the glucose‐lowering effect of liraglutide in combination with basal insulin15. Their results suggested that, for individuals with depleted β‐cell function (C‐peptide index <1.103 ng/mL), liraglutide + basal inulin requires additional oral antidiabetic drug therapy or bolus insulin to achieve glycemic control (HbA1c <7.0% 1 year after treatment initiation). IDegLira might be similarly affected. However, as C‐peptide or other β‐cell function‐related parameters were not measured in DUAL II Japan, we did not carry out subgroup analyses by β‐cell function this time. Further consideration is required on this point.
The EOT insulin dose was numerically lower with IDegLira than degludec across all subgroups, except older age, in DUAL II Japan. However, the ETD favored IDegLira in only some groups (baseline HbA1c ≥8.0 to 9.0% and ≥9.0% in DUAL I Japan, baseline BMI ≥30 kg/m2 in DUAL I Japan and age <65 years in both studies). The treatment interaction was significant for baseline BMI in DUAL I Japan and age in DUAL II Japan, suggesting that IDegLira has a greater insulin‐sparing effect in those with insulin‐naïve type 2 diabetes with a baseline BMI ≥30 kg/m2 and in younger insulin‐experienced patients. Therefore, IDegLira could be particularly effective in insulin‐naïve patients with obesity and insulin‐experienced patients aged <65 years.
In insulin‐naïve participants (DUAL I Japan), both IDegLira and degludec were associated with mean weight gain; however, the magnitude of the increase was lower with IDegLira in all subgroups, except those with a baseline BMI ≥30 kg/m2. Liraglutide was associated with mean weight loss, and the ETD versus IDegLira favored liraglutide in all subgroups. In insulin‐experienced participants (DUAL II Japan), IDegLira was associated with a small mean weight loss in most subgroups, whereas degludec was associated with a small mean weight gain; ETDs favored IDegLira for some, but not all, subgroups. However, there was no significant treatment interaction for weight change for any of the subgroups. Although uncertain, the weight gain observed with IDegLira in the HbA1c ≥9.0% subgroup may have resulted from the greater insulin dose required for these participants compared with participants in the other HbA1c subgroups. Furthermore, participants with baseline HbA1c ≥9.0% might have had glycosuria, which likely improved as they received the diabetes treatment, resulting in weight gain versus baseline.
The present results are broadly consistent with those of the post‐hoc analyses of the global DUAL I and DUAL II studies16, in which Rodbard et al. showed that HbA1c reductions were significantly greater with IDegLira versus degludec or liraglutide alone across four categories of baseline HbA1c (≤7.5–9.0%) in DUAL I. In DUAL II, HbA1c reductions were significantly greater with IDegLira than with degludec in all but the lowest HbA1c category. Although categories of baseline BMI were not explored in that report, they have been investigated by Lingvay et al. in the post‐hoc analyses of DUAL V, which included participants with type 2 diabetes uncontrolled on basal insulin17. Lingvay et al.17 reported reductions in mean HbA1c across all baseline HbA1c, fasting plasma glucose and BMI categories, with significantly greater reductions versus insulin glargine 100 units/mL. Contrary to our findings in participants with type2 diabetes uncontrolled on basal insulin (DUAL II Japan), IDegLira was associated with weight loss across all baseline BMI categories; in DUAL II Japan, negligible weight change was associated with IDegLira treatment in participants with baseline BMI <25 kg/m2 and baseline BMI ≥30 kg/m2. The differing bodyweight results might be due to the difference in EOT total insulin dose associated with IDegLira treatment across baseline BMI categories <25, ≥25 to < 30 and ≥30 kg/m2, respectively: 37 U, 42 U and 45 U in DUAL V17, and 34.1 U, 37.2 U and 45.4 U in DUAL II Japan.
Hypoglycemia rates were lower in participants with baseline BMI ≥30 kg/m2 versus those with baseline BMI ≥25 to <30 kg/m2 or BMI <25 kg/m2, observed in both DUAL I Japan and DUAL II Japan, and consistent with the results of the post‐hoc analyses of DUAL V17. For participants of DUAL I Japan, who were insulin‐naïve, IDegLira treatment was associated with a numerically higher rate of hypoglycemia in elderly participants than non‐elderly participants, potentially due to varying food intake between participants in the two age subgroups. Notably, the rate of gastrointestinal AEs was numerically higher in elderly participants versus non‐elderly participants. Although IDegLira does not pose any particular concerns regarding severe hypoglycemia, attention should be paid to the occurrence of hypoglycemia in elderly patients, who are generally prone to hypoglycemia.
Interpretation of some of these analyses is limited by the post‐hoc nature and the relatively low number of participants in each subgroup. In Japan, elderly patients aged ≥75 years account for one‐third of the total population with type 2 diabetes18. Therefore, a possible limitation of the current analyses is that the number of elderly patients included, especially those aged ≥75 years, might not have been sufficient to support the conclusion of the treatment‐by‐subgroup interaction for the efficacy and safety profiles according to age. In addition, the limitations of DUAL II Japan apply to the subgroup analyses of these data reported here. That is, that the dose of degludec was capped at 50 U and that, consequently, it is unclear whether the same outcomes would have been observed in participants switching from basal or pre‐mix insulin of >50 U.
The strengths of these subgroup analyses include that the data used were from two randomized controlled trials. In addition, these analyses add to the body of evidence that consistently shows the clinical benefits of IDegLira3, 4, 5, 7, 8, 19, 20, 21.
In summary, the results of these subgroup analyses show that IDegLira is an effective treatment option for Japanese patients with type 2 diabetes across a broad range of baseline HbA1c, BMI and age categories.
Disclosure
MK has received research support from Nippon Boehringer Ingelheim Co. Ltd., Sanofi K.K., Ono Pharmaceutical Co., Ltd., MSD K.K., Takeda Pharmaceutical Company Ltd., Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc., Sumitomo Dainippon Pharma Co. Ltd., Teijin Pharma Ltd., Novo Nordisk Pharma Ltd., Kissei Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co. Ltd., Daiichi Sankyo Company Ltd., Ono Pharmaceutical Co., Eli Lilly Japan K., Taisho Toyama Pharmaceutical Co., Ltd., Novartis Pharma K.K. and Kowa Pharmaceutical Co. Ltd; and has participated in speakers' bureaus for Nippon Boehringer Ingelheim Co. Ltd., Sanofi K.K., Ono Pharmaceutical Co., Ltd., Msd K.K., Takeda Pharmaceutical Company Ltd., Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc., Sumitomo Dainippon Pharma Co., Ltd., Teijin Pharma Ltd., Novo Nordisk Pharma Ltd., Kissei Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co. Ltd., Terumo Corporation, Daiichi Sankyo Company Ltd., Eli Lilly Japan K., Taisho Toyama Pharmaceutical Co., Ltd., Novartis Pharma K.K., Bayer Yakuhin Ltd., Medtronic Japan Co., Ltd. and Kowa Pharmaceutical Co. Ltd. HW has received grants from Kowa, Sanofi, Yakult, Eli Lilly, Novartis, Sanwa Kagaku Kenkyusho, Abbott Japan, Astellas Pharma, Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo Pharma, Pfizer, Kissei Pharma, Kyowa Hakko Kirin, Mitsubishi Tanabe Pharma, Merck Sharp & Dohme, Novo Nordisk, Ono Pharmaceutical, Teijin, Taisho‐Toyama and Souiken; and has received personal fees from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Dainippon Sumitomo Pharma, Eli Lilly, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Novo Nordisk, Ono Pharmaceutical, Sanofi, Sanwa Kagaku Kenkyusho, Kyowa Hakko Kirin, Terumo Corporation, Fuji Film and Takeda. SK has received honoraria for lectures from Sumitomo Dainippon Pharma Co., Ltd., Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., AstraZeneca K.K. and Mitsubishi Tanabe Pharma Corporation. BRA and TN are employees and shareholders in Novo Nordisk. KK has received honoraria or consulting fees from Astellas Pharma, AstraZeneca, Kowa Pharmaceutical, Mitsubishi Tanabe Pharma, MSD, Nippon Boehringer Ingelheim, Novo Nordisk Pharma, Sanwa Kagaku Kenkyusho, Dainippon Sumitomo Pharma, Taisho Toyama Pharmaceutical and Takeda.
Supporting information
Table S1 | Overall results for subgroup analysis of change in (a,b) glycated hemoglobin, (c,d) bodyweight and (e,f) end‐of‐trial total daily insulin dose with insulin degludec/liraglutide, degludec and liraglutide in DUAL I Japan and DUAL II Japan.
Table S2 | Rates of severe or blood glucose‐confirmed hypoglycemia across participant subgroups in (a) DUAL I Japan and (b) DUAL II Japan.
Table S3 | Rates of adverse events across participant subgroups in (a) DUAL I Japan and (b) DUAL II Japan.
Table S4 | Rates of gastrointestinal adverse events across participant subgroups in (a) DUAL I Japan and (b) DUAL II Japan.
Acknowledgments
Medical writing and editorial support, under the guidance of the authors, was provided by Matthew Robinson DPhil and Beverly La Ferla MRes from Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc, funded by Novo Nordisk.
J Diabetes Investig. 2021; 12: 1610–1618
References
- 1.Kapitza C, Bode B, Ingwersen SH, et al. Preserved pharmacokinetic exposure and distinct glycemic effects of insulin degludec and liraglutide in IDegLira, a fixed‐ratio combination therapy. J Clin Pharmacol 2015; 55: 1369–1377. [DOI] [PubMed] [Google Scholar]
- 2.Gough SC, Bode BW, Woo VC, et al. One‐year efficacy and safety of a fixed combination of insulin degludec and liraglutide in patients with type 2 diabetes: results of a 26‐week extension to a 26‐week main trial. Diabetes Obes Metab 2015; 17: 965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buse JB, Vilsboll T, Thurman J, et al. Contribution of liraglutide in the fixed‐ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care 2014; 37: 2926–2933. [DOI] [PubMed] [Google Scholar]
- 4.Linjawi S, Bode BW, Chaykin LB, et al. The efficacy of IDegLira (Insulin Degludec/Liraglutide Combination) in adults with type 2 diabetes inadequately controlled with a GLP‐1 receptor agonist and oral therapy: DUAL III randomized clinical trial. Diabetes Ther 2017; 8: 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodbard HW, Bode BW, Harris SB, et al. Safety and efficacy of insulin degludec/liraglutide (IDegLira) added to sulphonylurea alone or to sulphonylurea and metformin in insulin‐naive people with Type 2 diabetes: the DUAL IV trial. Diabet Med 2017; 34: 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lingvay I, Perez Manghi F, Garcia‐Hernandez P, et al. Effect of insulin glargine up‐titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: The DUAL V randomized clinical trial. JAMA 2016; 315: 898–907. [DOI] [PubMed] [Google Scholar]
- 7.Harris SB, Kocsis G, Prager R, et al. Safety and efficacy of IDegLira titrated once weekly versus twice weekly in patients with type 2 diabetes uncontrolled on oral antidiabetic drugs: DUAL VI randomized clinical trial. Diabetes Obes Metab 2017; 19: 858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billings LK, Doshi A, Gouet D, et al. Efficacy and safety of IDegLira versus basal‐bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: The DUAL VII randomized clinical trial. Diabetes Care 2018; 41: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 9.Aroda VR, Gonzalez‐Galvez G, Gron R, et al. Durability of insulin degludec plus liraglutide versus insulin glargine U100 as initial injectable therapy in type 2 diabetes (DUAL VIII): a multicentre, open‐label, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol 2019; 7: 596–605. [DOI] [PubMed] [Google Scholar]
- 10.Kaku K, Araki E, Tanizawa Y, et al. Superior efficacy with a fixed‐ratio combination of insulin degludec and liraglutide (IDegLira) compared with insulin degludec and liraglutide in insulin‐naive Japanese patients with type 2 diabetes in a phase 3, open‐label, randomized trial. Diabetes Obes Metab 2019; 21: 2674–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watada H, Kaneko S, Komatsu M, et al. Superior HbA1c control with the fixed‐ratio combination of insulin degludec and liraglutide (IDegLira) compared with a maximum dose of 50 units of insulin degludec in Japanese individuals with type 2 diabetes in a phase 3, double‐blind, randomized trial. Diabetes Obes Metab 2019; 21: 2694–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelhafiz AH, Rodriguez‐Manas L, Morley JE, et al. Hypoglycemia in older people – a less well recognized risk factor for frailty. Aging Dis 2015; 6: 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Report C. Glycemic targets for elderly patients with diabetes: Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee on Improving Care for Elderly Patients with Diabetes. J Diabetes Investig 2017; 8: 126–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Japan Society for the Study of Obesity (JASSO) . Guidelines for the management of obesity disease 2016 [article online], Available from http://www.jasso.or.jp/data/magazine/pdf/chart_A.pdf. Accessed February 2020 (Japanese).
- 15.Usui R, Sakuramachi Y, Seino Y, et al. Retrospective analysis of liraglutide and basal insulin combination therapy in Japanese type 2 diabetes patients: The association between remaining beta‐cell function and the achievement of the glycated hemoglobin target 1 year after initiation. J Diabetes Investig 2018; 9: 822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodbard HW, Buse JB, Woo V, et al. Benefits of combination of insulin degludec and liraglutide are independent of baseline glycated haemoglobin level and duration of type 2 diabetes. Diabetes Obes Metab 2016; 18: 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lingvay I, Harris S, Jaeckel E, et al. Insulin degludec/liraglutide (IDegLira) was effective across a range of dysglycaemia and body mass index categories in the DUAL V randomized trial. Diabetes Obes Metab 2018; 20: 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of Health Labour and Welfare (Japan) . National Health and Nutrition Survey Report [article online], 2018, 2018. Available from https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/h30‐houkoku_00001.html Accessed October 2020 (Japanese). [Google Scholar]
- 19.Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed‐ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open‐label, randomised, 26‐week, treat‐to‐target trial in insulin‐naive patients with type 2 diabetes. Lancet Diabetes Endocrinol 2014; 2: 885–893. [DOI] [PubMed] [Google Scholar]
- 20.Lingvay I, Pérez Manghi F, Garcia‐Hernandez P, et al. Effect of insulin glargine up‐titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: The DUAL V randomized clinical trial. JAMA 2016; 315: 898–907. [DOI] [PubMed] [Google Scholar]
- 21.Philis‐Tsimikas A, Billings LK, Busch R, et al. Superior efficacy of insulin degludec/liraglutide versus insulin glargine U100 as add‐on to sodium‐glucose co‐transporter‐2 inhibitor therapy: a randomized clinical trial in people with uncontrolled type 2 diabetes. Diabetes Obes Metab 2019; 21: 1399–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Overall results for subgroup analysis of change in (a,b) glycated hemoglobin, (c,d) bodyweight and (e,f) end‐of‐trial total daily insulin dose with insulin degludec/liraglutide, degludec and liraglutide in DUAL I Japan and DUAL II Japan.
Table S2 | Rates of severe or blood glucose‐confirmed hypoglycemia across participant subgroups in (a) DUAL I Japan and (b) DUAL II Japan.
Table S3 | Rates of adverse events across participant subgroups in (a) DUAL I Japan and (b) DUAL II Japan.
Table S4 | Rates of gastrointestinal adverse events across participant subgroups in (a) DUAL I Japan and (b) DUAL II Japan.


