Abstract
Aims/Introduction
Discontinuation of diabetes care has been studied mostly in patients with prevalent diabetes and not in patients with newly diagnosed diabetes, whose dropout risk is highest. Because enrolling patients in a prospective study will influence adherence, we retrospectively examined whether guideline‐recommended practices, defined as nutritional guidance or ophthalmological examination, can prevent patient discontinuation of diabetes care after its initiation.
Materials and Methods
We retrospectively identified adults with newly screened diabetes during checkups using a large Japanese administrative claims database (JMDC, Tokyo, Japan) that contains laboratory data and lifestyle questionnaires. We defined discontinuation of physician visits as a follow‐up interval exceeding 6 months. We divided the patients into those who received guideline‐recommended practices (nutritional guidance or ophthalmology consultation) within the same month as the first visit and those who did not. We calculated propensity scores and carried out inverse probability of treatment weighting analyses to compare discontinuation between the two groups.
Results
We identified 6,508 patients with at least one physician consultation for diabetes care within 3 months after their checkup, including 4,574 patients without and 1,934 with guideline‐recommended practices. After inverse probability of treatment weighting, patients with guideline‐recommended practices had a significantly lower proportion of discontinuation than those without (17.2% vs 21.8%; relative risk 0.79, 95% confidence interval 0.69–0.91).
Conclusions
This study is the first to show that after adjustment for both patient and healthcare provider factors, guideline‐recommended practices within the first month of physician consultation for diabetes care can decrease subsequent discontinuation of physician visits in patients with newly diagnosed diabetes.
Keywords: Adherence, Clinical epidemiology, Nutrition guidance
Nutritional or ophthalmological care is recommended in diabetes guidelines. Newly‐screened diabetes patients receiving such care are less likely to discontinue care. Nutritional guidance is especially important among guideline‐recommended practices.

Introduction
Diabetes mellitus is increasing in prevalence, and has become a worldwide problem with associated health and economic burdens1. To prevent exacerbation and complications of diabetes, continuation of diabetes care is crucial2. Evidence suggests that continuation of pharmacotherapy for diabetes can improve all‐cause mortality and prevent hospitalization3. Although continuation of diabetes care is important, patients are likely to discontinue the care by themselves soon after its initiation4, 5, 6. A similar trend in which the highest discontinuation rate was observed during the initial stage of treatment intervention was also seen in patients with obesity7. Therefore, prevention of dropout during the initial stage of diabetes care is considered particularly important.
Several studies have examined factors associated with discontinuation of diabetes care5, 6, 8, 9, 10, 11, 12, 13, 14. A meta‐analysis of such studies showed that poor knowledge of diabetes was a common positive predictor for discontinuation15. A recent study investigating patients with prevalent diabetes showed that nutritional guidance and ophthalmological examination, which are recommended in guidelines worldwide16, 17, as well as in the Japanese guideline18, 19, were associated with a lower risk of discontinuation of diabetes care8. Therefore, guideline‐recommended practices, which we define as practices including nutritional guidance or ophthalmological examination, might have the potential to improve treatment adherence among patients with diabetes at the most important timing for treatment intervention, during the initial stage of diabetes care.
However, evidence is lacking on effective methods for decreasing discontinuation of diabetes follow up in newly diagnosed patients during the initial stage of care. Furthermore, the patients in previous studies appeared well‐motivated and less likely to drop out, because they were prevalent patients who were already receiving treatment for diabetes5, 6, 8, 9, 10, 11, 12, 13, 14 or volunteered for a health promotion program8. Thus, it remains unknown what kind of medical care would be effective for reducing discontinuation during the prime time for dropout, just after receiving the diagnosis of diabetes. It also remains to be clarified whether guideline‐recommended practices are effective for preventing discontinuation of diabetes care among patients with newly diagnosed diabetes. Considering that enrolling patients in a prospective study in which examination of the adherence to the continuation of diabetes care would affect subsequent discontinuation rates, a retrospective study where indication bias is removed as much as possible is warranted.
In the present study, we aimed to clarify whether guideline‐recommended practices, including nutritional guidance and ophthalmological examination, can reduce discontinuation of diabetes care in patients with newly diagnosed diabetes using a nationwide database. This is the first study to examine a way in which to reduce the dropout rate after adjustment for patient factors, such as a history of physician visits for other purposes, severity of diabetes or attitude toward lifestyle modifications, and healthcare provider factors, such as prescription of antihyperglycemics or performance of urinary albumin/protein quantitative examination.
Materials and Methods
Data source
We used the Japanese administrative claims database (JMDC) in Tokyo, Japan. The details of the database were described previously20. Briefly, the JMDC database contains claims data submitted to health insurers by clinics, hospitals and pharmacies since January 2005. Most of the insured individuals in the database are employees of Japanese companies and their family members. Therefore, data for individuals aged >65 years are scarce in the registered population21. For the claims data, we used recorded diagnoses based on International Classification of Diseases 10th Revision codes and drug specifications based on World Health Organization Anatomical Therapeutic Chemical Classification System (WHO‐ATC) codes.
The database also includes information on annual checkups for lifestyle‐related diseases21 for approximately 40% of the whole population in the JMDC database. The checkups include blood pressure recordings, clinical laboratory tests (e.g., complete blood count, blood glucose, biochemistry and urine dipstick), questionnaires on lifestyle factors (e.g., smoking and alcohol habits), medical history of stroke or ischemic heart disease, health behavior changes based on a transtheoretical model, and willingness to receive health instructions from public health nurses. Individuals suspected of having diabetes at the time of a checkup are urged to see a doctor21. The thresholds for this recommendation are a fasting blood glucose ≥126 mg/dL or glycated hemoglobin (HbA1c) ≥6.5% (≥48 mmol/mol).
Individuals who satisfied all of the following criteria were included in the study: (i) age ≥ 20 years; (ii) fasting blood glucose ≥126 mg/dL; (iii) HbA1c ≥6.5% (≥48 mmol/mol) at the baseline checkup; and (iv) actual visit to a physician for diabetes care within 3 months after the checkup. For each patient, the baseline period was the period between 1 year before the checkup and the month when the person first saw a physician for diabetes care (Figure 1). During the 1 year before the baseline checkup, there must have been no claims for a disease name registered as diabetes, measurements of diabetes markers, such as HbA1c or glycated albumin, or prescription of antidiabetic medications (WHO‐ATC codes starting with A10). Therefore, all included individuals were patients with newly diagnosed diabetes. We defined physician visits for diabetes care as having diabetes mellitus‐related disease names, HbA1c or glycated albumin tests, or medications for diabetes. We excluded individuals without health insurance coverage throughout the observation period (Figure 1).
Figure 1.
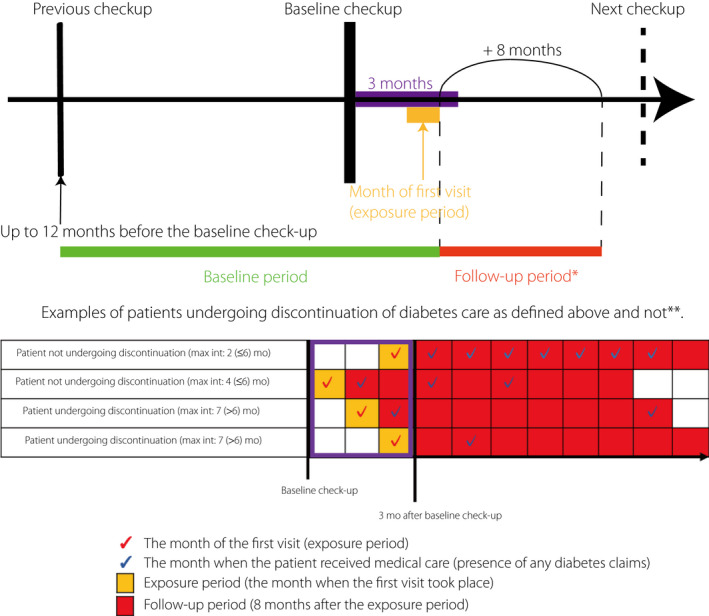
Study design and time course for the included patients. *We placed censoring weights for all included patients, considering those censored during the follow‐up period. **Each box represents 1 month. Max int, maximal interval; mo, months.
The institutional review board of the Graduate School of Medicine of The University of Tokyo approved the study protocol. Owing to the anonymous nature of the data, the requirement for informed consent was waived.
Study outcomes and variables
We obtained the following information from the checkup data: sex; age; body mass index; blood pressure; waist circumference; laboratory data for HbA1c, fasting blood glucose, triglyceride, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, serum creatinine and urinary protein dipstick; smoking and alcohol habits; health behavior changes based on a transtheoretical model; and willingness to receive health instructions from public health nurses. We also collected data on HbA1c and fasting blood glucose from the previous checkup, at 1 year before the baseline checkup. Additionally, we obtained medication history for dyslipidemia (WHO‐ATC codes starting with C10) and hypertension (WHO‐ATC codes starting with C02, C03, C04, C07, C08 or C09) from the claims data in the 12 months before the checkup. We determined a binary variable on whether the registered person was an insured employee themselves, and the frequency of physician visits evaluated as the number of months among the previous 12 months (0–12). We considered the following variables related to procedures and clinical or demographic characteristics in the first month of diabetes care: urinary quantitative albumin or protein examination, ophthalmological examination, nutritional guidance, total medical cost in the first month, visited facility (hospital or clinic), prescription for hypoglycemia and number of visited medical facilities for diabetes or other diseases. Nutritional guidance is provided by registered nutritionists based on physicians’ requests and is reimbursed by insurers in the Japanese medical insurance system.
Blood pressure was categorized according to the definition of the Japanese Society of Hypertension: normal (systolic blood pressure <140 mmHg and diastolic blood pressure <90 mmHg), grade 1 hypertension (systolic blood pressure 140–159 mmHg or diastolic blood pressure 90–99 mmHg), grade 2 hypertension (systolic blood pressure of 160–179 mmHg or diastolic blood pressure 100–109 mmHg) and grade 3 hypertension (systolic blood pressure ≥180 mmHg or diastolic blood pressure ≥110 mmHg)22.
For the first month of diabetes care, we gained information on the total medical cost in the same month. We included this variable because in some populations, cost during hospitalization was associated with subsequent discontinuation of care23, and therefore cost in the first month might affect discontinuation of diabetes care. All costs were converted into $US assuming that ¥100 was equivalent to $US1.
We defined guideline‐recommended practices as nutritional guidance and ophthalmology examination, because current clinical guidelines for management of patients with diabetes recommend not only pharmacotherapy, but also nutritional intervention and/or ophthalmology consultation16, 17, 19. We divided the patients into those with and without exposure to guideline‐recommended practices in the month when their first visit for diabetes took place.
The outcome was discontinuation of physician visits, defined as absence of re‐visit within 6 months during the observation period of 8 months after the first physician consultation for diabetes care (Figure 1). We set the observation period to 8 months, because adoption of a longer follow‐up period would provide an opportunity to undergo the next annual checkup, which might bias the results.
Multiple imputation
We applied a multiple imputation method, because there were missing values for alcohol or smoking habits and questionnaires for lifestyle factors. We prepared 20 imputed datasets by way of sequential imputation using chained equations for missing data and the ‘mi impute chained’ syntax in Stata24.
Inverse probability of treatment weighting
We used propensity scores to minimize confounding by indications for guideline‐recommended practices25. When estimating the propensity scores for receiving guideline‐recommended practices (practices including nutritional guidance or ophthalmological examination), we used a generalized linear model within each multiple‐imputed dataset26. The dependent variable was receipt of guideline‐recommended practices, and the independent variables were age, sex, body mass index, waist circumference, fasting blood glucose, HbA1c, blood pressure, triglycerides, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, urinary protein, prescription histories of antidyslipidemia or antihypertensive drugs determined by claims in the past 12 months, insured person, frequency of physician visits defined by number of visits within 12 months before the first visit, alcohol intake frequency, smoking history, stage in change model for lifestyle modifications, willingness to receive health instruction from public health nurses, urinary quantitative albumin or protein examination carried out within the first month of the visit, total medical cost in the first month, facility type for initial diabetes management (hospital or clinic), prescription of antidiabetic medication in the first month and HbA1c or blood glucose levels in the previous year. We used the estimated propensity scores for inverse probability of treatment weighting (IPTW). Each patient was weighted by the inverse probability of being in the observed group: patients who received guideline‐recommended practices were weighted by the reciprocal of the propensity score, and those who did not receive guideline‐recommended practices were weighted by the reciprocal of 1 minus the propensity score; the weights were then stabilized by the proportion of patients in each group27, 28. To account for censoring during patient follow up, mainly because of alterations in medical insurers, we calculated propensity scores for censoring with all of the variables used to calculate the propensity scores for receipt of guideline‐recommended practices plus whether the person received guideline‐recommended practices. To take censoring into consideration, we calculated the final weights by using the stabilized weights with the propensity scores for receipt of guideline‐recommended practices multiplied by the stabilized weights with the propensity scores for censoring as previously reported28. We calculated standardized differences to compare patient characteristics between those with guideline‐recommended practices and those without. We defined absolute standardized differences of >10% as being out of balance29.
Statistical analysis
First, we summarized the background characteristics of the eligible population before carrying out multiple imputation. Next, we summarized the weighted and unweighted background characteristics of the patients with and without guideline‐recommended practices.
We compared the proportions of discontinuation of medical care in the propensity‐score IPTW groups using a χ2‐test. We then calculated the discontinuation rates and relative risks (RRs) by RR regression using a log‐link binomial generalized linear model, with discontinuation of physician visits as the dependent variable and receipt of guideline‐recommended practices as the independent variable without and with IPTW in each dataset. We unified the estimates gained from the 20 imputed datasets, and obtained combined estimates and standard errors based on Rubin’s rules, using the ‘mi estimate: binreg’ syntax in Stata30.
As a secondary analysis to determine which of the two interventions (nutritional guidance or ophthalmological examination) was more effective, we examined the individual effects of the two interventions on discontinuation of physician visits. Specifically, we evaluated the effects of nutritional guidance or ophthalmological examination with and without IPTW in each dataset after calculating propensity scores for receipt of each procedure.
All hypothetical tests had a two‐sided significance level of 0.05, and all statistical analyses were carried out using Stata version 16 software (StataCorp, College Station, TX, USA).
Results
Study population
Among the population with checkup data in the database, we identified 6,836 adults who were screened as diabetes mellitus at their annual checkup, but had no history of treatment or diagnosis in the previous year. We subsequently excluded 328 patients who were aged <20 years or censored during the first 3 months of diabetes care. The background characteristics of the included patients divided by the discontinuation status are summarized in Table 1.
Table 1.
Characteristics of eligible patients with and without discontinuation of follow‐up visits for diabetes
| Variable | Category | No discontinuation | Discontinuation | P‐value |
|---|---|---|---|---|
| 5,194 | 1,314 | |||
| Age (years) | 20–39 | 261 (5.0%) | 87 (6.6%) | 0.006 |
| 40–49 | 1,848 (35.6%) | 412 (31.4%) | ||
| 50–59 | 2,103 (40.5%) | 569 (43.3%) | ||
| ≥60 | 982 (18.9%) | 246 (18.7%) | ||
| Sex | Male | 4,046 (77.9%) | 1,117 (85.0%) | <0.001 |
| Body mass index (kg/m2) | ≤18.49 | 69 (1.3%) | 10 (0.8%) | 0.410 |
| 18.50–24.99 | 1,830 (35.2%) | 470 (35.8%) | ||
| 25.00–29.99 | 2,129 (41.0%) | 538 (40.9%) | ||
| ≥30.00 | 1,165 (22.4%) | 292 (22.2%) | ||
| Missing | 1 (<0.1%) | 4 (0.3%) | ||
| Waist circumference (cm) | M: <85 cm, F: <90 cm | 1,544 (29.7%) | 366 (27.9%) | 0.200 |
| M: ≥85 cm, F: ≥90 cm | 3,497 (67.3%) | 905 (68.9%) | ||
| Missing | 153 (2.9%) | 43 (3.3%) | ||
| Fasting blood glucose (mg/dL) | 126–199 | 3,771 (72.6%) | 1,118 (85.1%) | <0.001 |
| ≥200 | 1,423 (27.4%) | 196 (14.9%) | ||
| HbA1c, % (mmol/mol) | 6.5–6.9 (48–52) | 1,214 (23.4%) | 557 (42.4%) | <0.001 |
| 7.0–7.9 (53–63) | 1,557 (30.0%) | 419 (31.9%) | ||
| 8.0–8.9 (64–74) | 710 (13.7%) | 119 (9.1%) | ||
| ≥9.0 (≥75) | 1,713 (33.0%) | 219 (16.7%) | ||
| Blood pressure | Normal | 3,349 (64.5%) | 867 (66.0%) | 0.080 |
| Grade 1 hypertension | 1,228 (23.6%) | 319 (24.3%) | ||
| Grade 2 hypertension | 451 (8.7%) | 97 (7.4%) | ||
| Grade 3 hypertension | 162 (3.1%) | 27 (2.1%) | ||
| Missing | 4 (0.1%) | 4 (0.3%) | ||
| Triglycerides (mg/dL) | <150 | 2,587 (49.8%) | 688 (52.4%) | 0.110 |
| 150–299 | 1,855 (35.7%) | 458 (34.9%) | ||
| ≥300 | 736 (14.2%) | 161 (12.3%) | ||
| Missing | 16 (0.3%) | 7 (0.5%) | ||
| LDL cholesterol (mg/dL) | <120 | 1,428 (27.5%) | 374 (28.5%) | 0.470 |
| 120–139 | 1,158 (22.3%) | 306 (23.3%) | ||
| ≥140 | 2,531 (48.7%) | 617 (47.0%) | ||
| Missing | 77 (1.5%) | 17 (1.3%) | ||
| HDL cholesterol (mg/dL) | <40 | 819 (15.8%) | 194 (14.8%) | 0.370 |
| ≥40 | 4,371 (84.2%) | 1,119 (85.2%) | ||
| Missing | 4 (0.1%) | 1 (0.1%) | ||
| Urinary protein dipstick test | − or ± | 4,388 (84.5%) | 1,151 (87.6%) | <0.001 |
| ≥+ | 713 (13.7%) | 132 (10.0%) | ||
| Missing | 93 (1.8%) | 31 (2.4%) | ||
| Antidyslipidemia prescription | + | 486 (9.4%) | 81 (6.2%) | <0.001 |
| Antihypertensive prescription | + | 975 (18.8%) | 159 (12.1%) | <0.001 |
| Insured person | Identical person | 4,034 (77.7%) | 1,116 (84.9%) | <0.001 |
| Dependent | 1,160 (22.3%) | 198 (15.1%) | ||
| Frequency of physician visits in previous year (months/year) | 0 | 1,074 (20.7%) | 304 (23.1%) | <0.001 |
| 1–4 | 1,825 (35.1%) | 522 (39.7%) | ||
| 5–12 | 2,295 (44.2%) | 488 (37.1%) | ||
| Frequency of alcohol intake | Rarely | 1,807 (34.8%) | 402 (30.6%) | <0.001 |
| Occasionally | 1,504 (29.0%) | 379 (28.8%) | ||
| Regularly | 1,096 (21.1%) | 344 (26.2%) | ||
| Missing | 787 (15.2%) | 189 (14.4%) | ||
| Smoking | – | 3,230 (62.2%) | 757 (57.6%) | 0.003 |
| + | 1,615 (31.1%) | 461 (35.1%) | ||
| Missing | 349 (6.7%) | 96 (7.3%) | ||
| Stage in change model for lifestyle modifications | Precontemplation | 620 (11.9%) | 183 (13.9%) | 0.050 |
| Contemplation | 1,488 (28.6%) | 378 (28.8%) | ||
| Determination | 865 (16.7%) | 244 (18.6%) | ||
| Action | 459 (8.8%) | 99 (7.5%) | ||
| Maintenance | 754 (14.5%) | 169 (12.9%) | ||
| Missing | 1,008 (19.4%) | 241 (18.3%) | ||
| Willingness to receive health instruction from public health nurses | – | 2,386 (45.9%) | 648 (49.3%) | 0.480 |
| + | 1,374 (26.5%) | 354 (26.9%) | ||
| Missing | 1,434 (27.6%) | 312 (23.7%) | ||
| Urinary protein/albumin quantitative test performed in first month | + | 802 (15.4%) | 115 (8.8%) | <0.001 |
| Ophthalmological examination performed in first month | + | 944 (18.2%) | 143 (10.9%) | <0.001 |
| Nutritional guidance performed in first month | + | 1,083 (20.9%) | 180 (13.7%) | <0.001 |
| Total cost in first month ($US) | ≤149 | 1,327 (25.5%) | 549 (41.8%) | <0.001 |
| 150–299 | 1,930 (37.2%) | 415 (31.6%) | ||
| ≥300 | 1,937 (37.3%) | 350 (26.6%) | ||
| Main visited medical facility (clinic or hospital) | Clinic | 3,382 (65.1%) | 866 (65.9%) | 0.590 |
| Hospital | 1,812 (34.9%) | 448 (34.1%) | ||
| Antidiabetic prescription in first month | + | 2,657 (51.2%) | 308 (23.4%) | <0.001 |
| HbA1c in previous year, % (mmol/L) | ≤6.4 (≤47) | 765 (14.7%) | 264 (20.1%) | 0.001 |
| ≥6.5 (≥48) | 1,136 (21.9%) | 288 (21.9%) | ||
| Missing | 3,293 (63.4%) | 762 (58.0%) | ||
| Fasting blood glucose in previous year (mg/dL) | ≤125 | 1,072 (20.6%) | 340 (25.9%) | 0.002 |
| ≥126 | 840 (16.2%) | 195 (14.8%) | ||
| Missing | 3,282 (63.2%) | 779 (59.3%) |
F, female; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; M, male.
We classified the 6,508 eligible patients who consulted a doctor after their checkup into those who received guideline‐recommended practices (n = 1,934) and those who did not (n = 4,574; Figure 2). Those who received guideline‐recommended practices were more likely to be women, have a smaller waist circumference, have higher fasting blood glucose and HbA1c at the checkup, undergo urinary protein/albumin quantitative examination, utilize hospital care rather than clinic care, be prescribed hypoglycemic agents, accrue more medical costs in the first month of physician visits, and have higher HbA1c at the previous checkup; in contrast, they were less likely to have antidyslipidemia or antihypertensive drugs prescribed during the previous 12 months.
Figure 2.
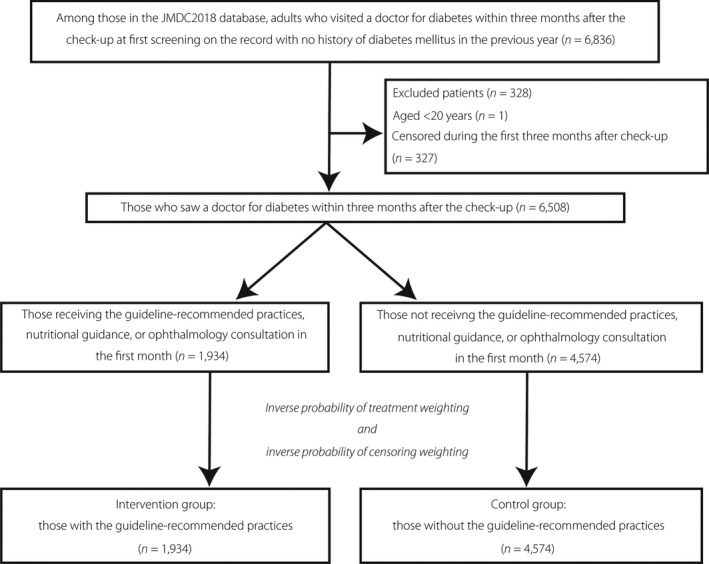
Flow chart of patient selection. JMDC, Japanese administrative claims database.
The factors for receipt of guideline‐recommended practices during the initial period of diabetes care in patients with newly diagnosed diabetes were determined by multivariable logistic regression analysis. We examined the propensity score distributions between those with and without receipt of guideline‐recommended practices without and with IPTW (Figure 3). After IPTW, the distribution of the patient characteristics was more balanced (Table 2, Figure 3).
Figure 3.
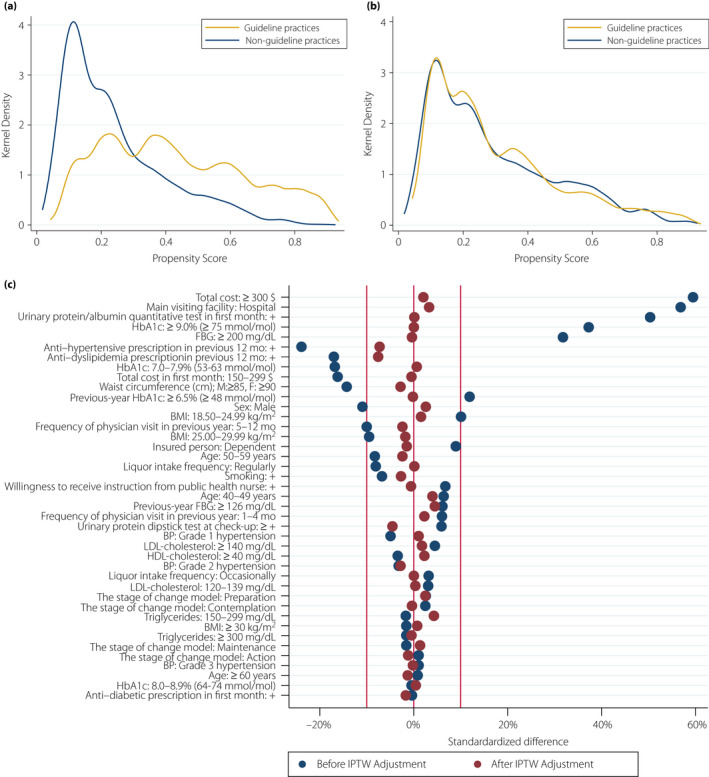
Kernel density plots showing the distributions of propensity scores in patients who received guideline‐recommended practices and those who did not (a) before and (b) after inverse probability of treatment weighting. (c) The standardized differences were smaller after inverse probability of treatment weighting. (a,b) Guideline practices, those who received guideline‐recommended practices; non‐guideline practices, those who did not receive guideline‐recommended practices. BMI, body mass index; BP, blood pressure; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; IPTW, inverse probability of treatment weighting; mo, months.
Table 2.
Characteristics of eligible patients before and after inverse probability of treatment weighting
| Variable | Category | Before IPTW | After IPTW | ||||
|---|---|---|---|---|---|---|---|
| Not receiving practices | Receiving practices | Standardized difference | Not receiving practices | Receiving practices | Standardized difference | ||
| Age (years) | 20–39 | 5.1 | 6.0 | 4.4 | 5.4 | 5.5 | 0.2 |
| 40–49 | 34.1 | 36.3 | 4.7 | 34.5 | 35.5 | 2.0 | |
| 50–59 | 42.3 | 38.2 | −8.4 | 40.8 | 39.6 | −2.4 | |
| ≥60 | 18.6 | 19.5 | 2.3 | 19.2 | 19.4 | 0.5 | |
| Sex | Male | 80.6 | 76.4 | −10.1 | 79.4 | 80.5 | 2.6 |
| Body mass index (kg/m2) | ≤18.49 | 1.1 | 1.6 | 4.2 | 1.1 | 1.1 | −0.1 |
| 18.50–24.99 | 34.1 | 38.4 | 9.1 | 35.2 | 35.1 | −0.3 | |
| 25.00–29.99 | 42.3 | 37.9 | −9.1 | 41.4 | 41.9 | 1.1 | |
| ≥30.00 | 22.5 | 22.1 | −0.9 | 22.3 | 21.9 | −0.9 | |
| Waist circumference (cm) | M: <85, F: <90 | 28.7 | 33.9 | 11.1 | 30.2 | 29.6 | −1.2 |
| M: ≥85, F: ≥90 | 71.3 | 66.1 | −11.1 | 69.8 | 70.4 | 1.2 | |
| Fasting blood glucose (mg/dL) | 126–199 | 79.4 | 65.1 | −32.3 | 75.9 | 76.0 | 0.3 |
| ≥200 | 20.6 | 34.9 | 32.3 | 24.1 | 24.0 | −0.3 | |
| HbA1c, % (mmol/mol) | 6.5–6.9 (48–52) | 29.8 | 21.1 | −20.1 | 27.5 | 28.0 | 1.2 |
| 7.0–7.9 (53–63) | 32.7 | 24.8 | −17.5 | 30.8 | 30.5 | −0.7 | |
| 8.0–8.9 (64–74) | 12.8 | 12.6 | −0.7 | 12.8 | 12.8 | 0.0 | |
| ≥9.0 (≥75) | 24.7 | 41.5 | 36.4 | 29.0 | 28.8 | −0.5 | |
| Blood pressure | Normal | 64.1 | 66.6 | 5.3 | 64.8 | 65.2 | 0.8 |
| Grade 1 hypertension | 24.5 | 22.2 | −5.6 | 24.1 | 24.0 | −0.2 | |
| Grade 2 hypertension | 8.5 | 8.3 | −0.5 | 8.3 | 8.3 | −0.1 | |
| Grade 3 hypertension | 2.9 | 2.9 | −0.1 | 2.8 | 2.6 | −1.4 | |
| Triglycerides (mg/dL) | <150 | 49.8 | 51.7 | 3.8 | 50.2 | 49.6 | −1.3 |
| 150–299 | 36.0 | 34.8 | −2.4 | 35.7 | 36.4 | 1.4 | |
| ≥300 | 14.2 | 13.4 | −2.2 | 14.1 | 14.0 | −0.2 | |
| LDL cholesterol (mg/dL) | <120 | 28.5 | 27.0 | −3.4 | 28.0 | 28.6 | 1.2 |
| 120–139 | 22.5 | 23.4 | 2.0 | 22.8 | 22.5 | −0.8 | |
| ≥140 | 48.9 | 49.6 | 1.3 | 49.1 | 48.9 | −0.4 | |
| HDL cholesterol (mg/dL) | <40 | 15.1 | 16.7 | 4.3 | 15.6 | 15.1 | −1.3 |
| ≥40 | 84.9 | 83.3 | −4.3 | 84.4 | 84.9 | 1.3 | |
| Urinary protein dipstick test | − or ± | 87.6 | 84.9 | −7.7 | 87.0 | 87.5 | 1.4 |
| ≥+ | 12.4 | 15.1 | 7.7 | 13.0 | 12.5 | −1.4 | |
| Anti‐dyslipidemia prescription | + | 9.9 | 5.9 | −14.6 | 8.7 | 8.3 | −1.4 |
| Anti‐hypertensive prescription | + | 19.8 | 11.8 | −22.1 | 17.3 | 16.1 | −3.1 |
| Insured person | Identical person | 80.1 | 76.8 | −8.1 | 79.4 | 80.2 | 1.9 |
| Dependent | 19.9 | 23.2 | 8.1 | 20.6 | 19.8 | −1.9 | |
| Frequency of physician visits in previous year (months/year) | 0 | 20.6 | 22.5 | 4.6 | 21.3 | 20.5 | −1.9 |
| 1–4 | 35.2 | 38.1 | 6.0 | 36.0 | 36.5 | 1.1 | |
| 5–12 | 44.2 | 39.4 | −9.7 | 42.7 | 43.0 | 0.6 | |
| Frequency of alcohol intake | Rarely | 38.8 | 41.8 | 6.0 | 39.6 | 40.0 | 0.7 |
| Occasionally | 34.0 | 34.5 | 1.2 | 34.2 | 33.6 | −1.3 | |
| Regularly | 27.2 | 23.7 | −8.1 | 26.2 | 26.4 | 0.6 | |
| Smoking | + | 34.9 | 33.1 | −3.9 | 34.4 | 35.0 | 1.3 |
| Stage in change model for lifestyle modifications | Precontemplation | 15.7 | 14.5 | −3.3 | 15.3 | 15.3 | 0.0 |
| Contemplation | 35.0 | 36.6 | 3.3 | 35.5 | 36.0 | 1.0 | |
| Preparation | 21.2 | 20.8 | −1.0 | 21.0 | 20.7 | −0.7 | |
| Action | 10.4 | 11.0 | 1.9 | 10.6 | 10.1 | −1.5 | |
| Maintenance | 17.7 | 17.1 | −1.5 | 17.6 | 17.9 | 0.7 | |
| Willingness to receive health instruction from public health nurses | + | 35.3 | 38.5 | 6.7 | 36.1 | 35.9 | −0.5 |
| Urinary protein/albumin quantitative test performed in first month | + | 8.5 | 27.4 | 50.8 | 13.4 | 14.2 | 2.3 |
| Total cost in first month (US$) | ≤149 | 35.0 | 14.1 | −50.1 | 29.0 | 27.6 | −2.9 |
| 150–299 | 38.3 | 30.7 | −16.0 | 36.4 | 35.9 | −1.1 | |
| ≥300 | 26.7 | 55.2 | 60.6 | 34.6 | 36.5 | 3.9 | |
| Main visited medical facility (clinic or hospital) | Clinic | 73.2 | 46.5 | −56.5 | 65.4 | 63.0 | −4.9 |
| Hospital | 26.8 | 53.5 | 56.5 | 34.6 | 37.0 | 4.9 | |
| Anti‐diabetic prescription in first month | + | 45.8 | 44.9 | −1.8 | 45.4 | 44.3 | −2.3 |
| HbA1c in previous year, % (mmol/mol) | ≤6.4 (≤47) | 40.9 | 35.3 | −11.6 | 39.4 | 38.4 | 1.91.9 |
| ≥6.5 (≥48) | 59.1 | 64.7 | 11.6 | 60.6 | 61.6 | 1.9 | |
| Fasting blood glucose in previous year (mg/dL) | ≤125 | 56.2 | 54.3 | −4.0 | 55.7 | 55.3 | −0.9 |
| ≥126 | 43.8 | 45.7 | 4.0 | 44.3 | 44.7 | 0.9 | |
Data are shown as percentages. F, female; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; IPTW, inverse probability of treatment weighting; M, male.
Outcomes
Table 3 shows the results of regression analyses for the primary and secondary outcomes in the main analysis. Those who received guideline‐recommended practices had a significantly lower proportion of discontinuation than those who did not (unweighted analysis 14.7 vs 22.5%; weighted analysis 17.2 vs 21.8%; Table 3). The analysis without IPTW yielded an RR of 0.65 (95% confidence interval [CI] 0.58–0.74), whereas the analysis with IPTW yielded an RR of 0.79 (95% CI 0.69–0.91).
Table 3.
Regression analysis of relative risks for interventions and discontinuation before and after inverse probability of treatment weighting
| Intervention | Model | Without intervention | With intervention | Relative risk | 95% CI | P‐value |
|---|---|---|---|---|---|---|
| Practices including nutritional guidance and ophthalmological examination | Before IPTW | 22.5% | 14.7% | 0.65 | 0.58–0.74 | <0.001 |
| After IPTW | 21.8% | 17.2% | 0.79 | 0.69–0.91 | 0.001 | |
| Nutritional guidance | Before IPTW | 21.6% | 14.3% | 0.66 | 0.57–0.76 | <0.001 |
| After IPTW | 21.1% | 15.9% | 0.75 | 0.62–0.90 | 0.002 | |
| Ophthalmological examination | Before IPTW | 21.6% | 13.2% | 0.61 | 0.52–0.72 | <0.001 |
| After IPTW | 20.7% | 19.3% | 0.93 | 0.74–1.17 | 0.551 |
CI, confidence interval; IPTW, inverse probability of treatment weighting.
Regarding the secondary outcomes, nutritional guidance alone was associated with a lower proportion of discontinuation (RR 0.75, 95% CI 0.62–0.90), whereas ophthalmological examination alone was not (RR 0.93, 95% CI 0.74–1.17).
Discussion
In the present observational study using real‐world data, we showed that guideline‐recommended practices within the first month of physician consultation were inversely associated with discontinuation of medical visits for diabetes care. This is the first study to examine a method to prevent discontinuation of diabetes care after adjustment for a number of important patient and healthcare provider factors.
Although there are a few articles on the association between guideline‐recommended practices and discontinuation of diabetes care, the included patients were limited to patients with prevalent diabetes or those registered in a health promotion program. A recent study targeting patients with prevalent diabetes showed that seeking nutritional guidance and ophthalmological examination was associated with a lower proportion of discontinuation8. The present study has distinct characteristics from the previous study in that we examined the effects of guideline‐recommended practices on the total included population where there was no longer an association between the confounders and the receipt of guideline‐recommended practices using IPTW31, and we used real‐world data rather than a registered cohort for a health promotion program. Most importantly, we focused on patients just after the initiation of diabetes care, which is the time associated with the highest risk of dropout.
Although the exact mechanism remains unclear in the present study, there is a possible explanation that guideline‐recommended practices, particularly nutritional guidance, work to reduce discontinuation. In nutritional guidance, registered nutritionists provide information on healthy eating patterns based on personal and cultural preferences, healthy behaviors, and barriers for behavioral changes32. Through this guidance, patients can become motivated and recognize the necessity of visiting nutritionists or physicians again. Therefore, we assume that the decrease in discontinuation was brought about by behavior modification of patients, improved patient motivation or consciousness toward diabetes care through these facilitations.
The present results suggest that patients who are diagnosed with diabetes and begin visiting physicians should receive nutritional guidance. Although a previous report showed that patients who see doctors specializing in diabetes care are more likely to receive nutritional guidance than those who do not see such specialists33, it is still desirable for patients who see non‐specialists to have an opportunity to receive nutritional guidance. This is extremely important, because most patients with diabetes are treated by primary care physicians34. To resolve this gap in the opportunity to receive nutritional guidance, primary care physicians and diabetologists should collaborate against the discontinuation of diabetes care.
Although nutritional guidance was associated with a lower proportion of discontinuation in the present study, ophthalmological examination was not. This difference can be explained by several reasons. As one reason, the reported proportion of patients who adhered to interval recommendations for follow‐up eye appointments was relatively low (approximately 30%), even in a registered prospective cohort, and its discontinuation was determined by patients based on their knowledge of diabetes control35. Thus, ophthalmological examination might have had a weaker effect on preventing discontinuation than nutritional guidance. Another reason for the lack of association between ophthalmological examination and discontinuation might be that most of those who underwent screening for diabetic eye complications proved to have no or mild lesions and were less likely to have another ophthalmological follow up36. Furthermore, no or mild eye lesions arising from diabetes necessitate one annual follow up36, and thus ophthalmological examination might not have effectively urged patients to complete a follow‐up visit within 6 months.
We found a higher proportion of overall discontinuation than a German database study (approximately 20 vs 5.5%)8, although both countries examined have adopted a national insurance system. This large difference probably arose for several reasons. As one reason, the previous study investigated participants in a national health promotion program for high‐quality diabetes care8, and thus there might have been some motivation to continue diabetes care among both physicians and patients. Another reason might be that the previous study investigated patients with prevalent diabetes who were already receiving treatment for diabetes. Meanwhile, we observed an unbiased rate of discontinuation in a country where a national insurance system has been adopted, and the present findings will be useful for future practice in diabetology.
The strengths of the present study lie in the observation of patients with newly diagnosed diabetes, use of real‐world data, use of methodological approaches to support causal inference and a large sample size. As aforementioned, newly diagnosed patients have a high risk of dropout from diabetes care4, 5, 7, which revisits the importance of intervention timing to prevent such discontinuation of diabetes care. Second, the use of real‐world data might have removed the selection bias, which could be related to inherent motivation of patients and caregivers, as seen in previous registry cohort studies5, 6, 8, 9, 11, 12. Third, the use of multiple imputation and IPTW with censoring weights might have reduced possible biases37, 38. Importantly, the IPTW method enabled our comparative study to use the whole population of patients with newly diagnosed diabetes27. Fourth, we adjusted for not only background characteristics of both medical practitioners and patients, including severity of diabetes, but also patients’ attitude toward health promotion, such as the stage in the change model for lifestyle modifications or willingness to receive health instruction from public health nurses. Finally, we obtained the sample size of 6,508 newly diagnosed patients at the initial stage of diabetes care. These epidemiological and statistical processes were made possible using big data incorporated with laboratory data and detailed medical procedures and information available, as were used in previous publications21, 39.
The present study also had several limitations. It was a retrospective observational study without randomization, and the guideline‐recommended medical care was determined at the discretion of the treating physicians. However, to minimize confounding by indications, such as severity of diabetes, we carried out IPTW analyses using propensity scores based on many characteristics of the patients, healthcare providers and medical facilities. Although the use of randomized controlled trials would resolve some other confounding factors, obtaining consent to participate in the trial would affect patient motivation and sampling, and lead to biases in the effects of guideline‐recommended practices. In this sense, the present observational study using real‐world data adjusted for important confounders is justified. Another limitation might be the short follow‐up period and exposure period. We observed only 8 months after the first physician visit, because use of longer follow‐up periods would expose the population to the next annual checkup, which might influence the care‐seeking behavior of the patients. In this respect, we observed ‘early’ discontinuation in patients with newly diagnosed diabetes. We set the exposure period as only 1 month, because a longer exposure period would exclude patients who discontinued diabetes care within 1 month, which would result in failure to capture discontinuation of diabetes care within 1 month. As another limitation, we must acknowledge that although we determined patients with newly diagnosed diabetes by excluding patients who had received any diabetes‐related care in the previous 12 months, some of the included patients might have undergone discontinuation of diabetes care before that time period. In addition, the study population was mainly aged in their 20s to 50s; thus, the present results might not be applicable to advanced‐age patients with diabetes. However, considering that the risk of discontinuation of diabetes care is higher in younger than older generations15, our target might be justifiable. Finally, although we adjusted for 21 measured confounders, including those related to patients’ motivation toward treatment and those related to the extent to which physicians carried out detailed investigations, unmeasured confounders might have remained in the multivariable regression analyses for the propensity score calculation. For example, we did not adjust for education level, occupation and socioeconomic status, because these factors were not recorded in the database.
In conclusion, the present retrospective cohort study using a large‐scale nationwide claims database showed that guideline‐recommended practices, especially nutritional guidance, in patients with diabetes might reduce the subsequent discontinuation of physician visits for diabetes care. These findings can shed light on diabetes practice and reinforce the importance of implementing guideline‐recommended practices from the early stage of diabetes care.
Disclosure
AO, SY, KIK and TK are members of the Department of Prevention of Diabetes and Lifestyle‐related Diseases, which is a cooperative program between The University of Tokyo and Asahi Mutual Life Insurance Company, and KIK is employed by Asahi Mutual Life Insurance Company. SO is a member of the Department of Eat‐loss Medicine, which is a cooperative program between The University of Tokyo and ITO EN Ltd. H Yamana and NM are members of the Department of Health Services Research, which is a cooperative program between The University of Tokyo and Tsumura & Co.
Acknowledgments
This work was supported by grants from the Ministry of Health, Labor and Welfare, Japan (19AA2007) and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20K18957, 20H03907 and 17H05077). This work was also supported by a junior scientist development grant from the Japan Diabetes Society to A.O.
J Diabetes Investig. 2021; 12: 1619–1631
References
- 1.Gakido E, Afshin A, Abajobir AA, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1345–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun P, Lian J. Treatment adherence in newly diagnosed type 2 diabetes: patient characteristics and long‐term impact of adherence on inpatient care utilization. Postgrad Med 2016; 128: 338–345. [DOI] [PubMed] [Google Scholar]
- 3.Khunti K, Seidu S, Kunutsor S, et al. Association between adherence to pharmacotherapy and outcomes in type 2 diabetes: a meta‐analysis. Diabetes Care 2017; 40: 1588–1596. [DOI] [PubMed] [Google Scholar]
- 4.Catalan VS, Couture JA, LeLorier J. Predictors of persistence of use of the novel antidiabetic agent acarbose. Arch Intern Med 2001; 161: 1106–1112. [DOI] [PubMed] [Google Scholar]
- 5.Graber AL, Davidson P, Brown AW, et al. Dropout and relapse during diabetes care. Diabetes Care 1992; 15: 1477–1483. [DOI] [PubMed] [Google Scholar]
- 6.Tino S, Wekesa C, Kamacooko O, et al. Predictors of loss to follow up among patients with type 2 diabetes mellitus attending a private not for profit urban diabetes clinic in Uganda ‐ a descriptive retrospective study. BMC Health Serv Res 2019; 19: 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inelmen EM, Toffanello ED, Enzi G, et al. Predictors of drop‐out in overweight and obese outpatients. Int J Obes (Lond) 2005; 29: 122–128. [DOI] [PubMed] [Google Scholar]
- 8.Fullerton B, Erler A, Pohlmann B, et al. Predictors of dropout in the German disease management program for type 2 diabetes. BMC Health Serv Res 2012; 12: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chew BH, Lee PY, Shariff‐Ghazali S, et al. Predictive factors of follow‐up non‐attendance and mortality among adults with type 2 diabetes mellitus‐ an analysis of the Malaysian diabetes registry 2009. Curr Diabetes Rev 2015; 11: 122–131. [DOI] [PubMed] [Google Scholar]
- 10.Wiwanitkit V. Loss of follow‐up of diabetic patients: what are the reasons? Indian J Endocrinol Metab 2011; 15: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masuda Y, Kubo A, Kokaze A, et al. Personal features and dropout from diabetic care. Environ Health Prev Med 2006; 11: 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benoit SR, Ji M, Fleming R, et al. Predictors of dropouts from a San Diego diabetes program: a case control study. Prev Chronic Dis 2004; 1: A10. [PMC free article] [PubMed] [Google Scholar]
- 13.Simmons D, Fleming C. Prevalence and characteristics of diabetic patients with no ongoing care in South Auckland. Diabetes Care 2000; 23: 1791–1793. [DOI] [PubMed] [Google Scholar]
- 14.Kawahara R, Amemiya T, Yoshino M, et al. Dropout of young non‐insulin‐dependent diabetics from diabetic care. Diabetes Res Clin Pract 1994; 24: 181–185. [DOI] [PubMed] [Google Scholar]
- 15.Lee RRS, Samsudin MI, Thirumoorthy T, et al. Factors affecting follow‐up non‐attendance in patients with Type 2 diabetes mellitus and hypertension: a systematic review. Singapore Med J 2019; 60: 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association. 4 . Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes‐2019. Diabetes Care 2019; 42(Suppl. 1): S34–S45. [DOI] [PubMed] [Google Scholar]
- 17.Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre‐diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 2013; 34: 3035–3087. [DOI] [PubMed] [Google Scholar]
- 18.Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int 2020; 11: 165–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haneda M, Noda M, Origasa H, et al. Japanese clinical practice guideline for diabetes 2016. J Diabetes Investig 2018; 9: 657–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ono S, Ono Y, Matsui H, et al. Factors associated with hospitalization for seasonal influenza in a Japanese nonelderly cohort. BMC Public Health 2016; 16: 922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono S, Ono Y, Matsui H, et al. Impact of clinic follow‐up visits on body weight control in people with prediabetes or diabetes mellitus: Japanese nonelderly cohort study. Fam Pract 2017; 34: 552–557. [DOI] [PubMed] [Google Scholar]
- 22.The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Chapter 2. Measurement and clinical evaluation of blood pressure. Hypertension Res 2014; 37: 266–278. [Google Scholar]
- 23.Nwabuo CC, Dy SM, Weeks K, et al. Factors associated with appointment non‐adherence among African‐Americans with severe, poorly controlled hypertension. PLoS One 2014; 9: e103090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aloisio KM, Micali N, Swanson SA, et al. Analysis of partially observed clustered data using generalized estimating equations and multiple imputation. Stata J 2014; 14: 863–883. [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc 1984; 79: 516–524. [Google Scholar]
- 26.Leyrat C, Seaman SR, White IR, et al. Propensity score analysis with partially observed covariates: how should multiple imputation be used? Stat Methods Med Res 2019; 28: 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015; 34: 3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000; 11: 550–560. [DOI] [PubMed] [Google Scholar]
- 29.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 2009; 38: 1228–1234. [Google Scholar]
- 30.Aloisio KM, Swanson SA, Micali N, et al. Analysis of partially observed clustered data using generalized estimating equations and multiple imputation. Stata J 2014; 14: 863–883. [PMC free article] [PubMed] [Google Scholar]
- 31.Brookhart MA, Wyss R, Layton JB, et al. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes 2013; 6: 604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 2019; 42: 731–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yabe D, Higashiyama H, Kadowaki T, et al. Real‐world Observational Study on Patient Outcomes in Diabetes (RESPOND): study design and baseline characteristics of patients with type 2 diabetes newly initiating oral antidiabetic drug monotherapy in Japan. BMJ Open Diabetes Res Care 2020; 8: e001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugiyama T, Imai K, Ihana‐Sugiyama N, et al. Variation in process quality measures of diabetes care by region and institution in Japan during 2015–2016: an observational study of nationwide claims data. Diabetes Res Clin Pract 2019; 155: 107750. [DOI] [PubMed] [Google Scholar]
- 35.Keenum Z, McGwin G Jr, Witherspoon CD, et al. adherence to recommended follow‐up eye care after diabetic retinopathy screening in a publicly funded county clinic and factors associated with follow‐up eye care use. JAMA Ophthalmol 2016; 134: 1221–1228. [DOI] [PubMed] [Google Scholar]
- 36.Murchison AP, Hark L, Pizzi LT, et al. Non‐adherence to eye care in people with diabetes. BMJ Open Diabetes Res Care 2017; 5: e000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howe CJ, Cole SR, Lau B, et al. Selection bias due to loss to follow up in cohort studies. Epidemiology (Cambridge, Mass) 2016; 27: 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009; 338: b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takano A, Ono S, Yamana H, et al. Factors associated with long‐term prescription of benzodiazepine: a retrospective cohort study using a health insurance database in Japan. BMJ Open 2019; 9: e029641. [DOI] [PMC free article] [PubMed] [Google Scholar]


