Abstract
Aims/Introduction
This study aimed to investigate the risk of diabetic ketoacidosis (DKA) in insulin‐treated type 1 diabetes patients administered sodium–glucose cotransporter 2 (SGLT2) inhibitors in real‐world clinical practice.
Materials and Methods
We carried out a real‐world, retrospective, observational cohort study using Japanese Medical Data Vision, a diagnosis procedure combination database. We identified insulin‐treated adult type 1 diabetes patients enrolled from December 2018 to October 2019. We assessed the incidence and risk of DKA in type 1 diabetes patients using SGLT2 inhibitors in an ‘on‐label’ manner. Cox multivariate regression analyses were carried out to determine clinical factors linked to SGLT2 inhibitor‐associated DKA.
Results
Of 11,475 type 1 diabetes patients, 1,898 (16.5%) were prescribed SGLT2 inhibitors. DKA occurred in 139 (7.3%) of these patients, with 20.2 incidences per 100 person‐years. These patients also showed significantly higher DKA rates than did those not receiving SGLT2 inhibitors (hazard ratio 1.66, 95% confidence interval 1.33–2.06; P < 0.001). The mean time until DKA onset in SGLT2 inhibitor‐treated type 1 diabetes patients was 30.6 ± 30.1 days. The risk of SGLT2 inhibitor‐associated DKA increased in type 1 diabetes patients irrespective of sex, age or body mass index. However, the risk did not increase in type 1 diabetes patients receiving continuous subcutaneous insulin infusion, which warrants further investigation because of the small number of type 1 diabetes patients receiving continuous subcutaneous insulin infusion.
Conclusions
‘On‐label’ SGLT2 inhibitor use might increase DKA risk among insulin‐treated type 1 diabetes patients irrespective of sex, age or body mass index. Both type 1 diabetes patients and healthcare providers should be wary of DKA, especially during the first month of initiating SGLT2 inhibitors.
Keywords: Diabetic ketoacidosis, Sodium–glucose cotransporter 2 inhibitor, Type 1 diabetes
This study investigated the risk of diabetic ketoacidosis (DKA) in type 1 diabetes patients using sodium–glucose cotransporter 2 (SGLT2) inhibitors in an ‘on‐label’ manner. We found that SGLT2 inhibitors might increase the DKA risk among insulin‐treated type 1 diabetes patients irrespective of sex, age and body mass index, and that the mean time until DKA onset in SGLT2 inhibitor‐treated type 1 diabetes patients was 30.6 days. Physicians should fully consider the appropriate use of SGLT2 inhibitors in type 1 diabetes patients to prevent DKA.

Introduction
Type 1 diabetes is an autoimmune disease in which pancreatic β‐cell destruction causes insulin deficiency, leading to hyperglycemia and ketoacidosis1. To prevent diabetic complications, type 1 diabetes patients must strictly control blood glucose levels using lifelong insulin therapy. The USA‐based type 1 diabetes Exchange Registry showed that despite the widespread use of insulin analogs, insulin pumps and continuous glucose monitoring systems, just 21% of USA adult type 1 diabetes patients achieve target glycated hemoglobin A1c of <7% (53 mmol/mol)2. Thus, non‐insulin adjunctive therapies are required to improve long‐term glycemic control in type 1 diabetes patients.
Presently, sodium–glucose cotransporter 2 (SGLT2) inhibitors are widely used for the treatment of type 2 diabetes. SGLT2 inhibitors reduce hyperglycemia by inhibiting proximal tubular reabsorption of glucose in the kidney, making the glucose‐lowering effect independent of insulin secretion3. Thus, SGLT2 inhibitors are considered effective glucose‐lowering agents, and are prescribed as insulin therapy adjuncts for type 1 diabetes patients. Representative large‐scale randomized controlled trials (RCTs) using SGLT2 inhibitors, such as dapagliflozin (Dapagliflozin Evaluation in Patients with Inadequately Controlled Type 1 Diabetes [DEPICT] studies)4, 5, 6, empagliflozin (Empagliflozin as Adjunctive to Insulin Therapy [EASE] studies)7 and canagliflozin8, in addition to insulin therapy in adults with type 1 diabetes have shown moderate reductions in glycated hemoglobin A1c, glycemic variability, total daily insulin dose, blood pressure and bodyweight and increased ‘time in range’ without an increase in hypoglycemia. Similar findings were observed in adult type 1 diabetes patients in a multicenter, retrospective, observational study reported in Japan9.
However, these RCTs also showed substantial increases in the risk of SGLT2 inhibitor‐associated diabetic ketoacidosis (DKA)4, 5, 6, 7, 8. The underlying mechanism for this type of DKA is reportedly associated with a metabolic imbalance between glucose‐lowering and anticatabolic effects of SGLT2 inhibitors, which are intensified by an inadequate reduction in insulin dose10. A recent meta‐analysis showed a combined risk ratio of 2.66 for DKA (95% confidence interval [CI] 1.32–5.35) in RCTs comparing canagliflozin, dapagliflozin or empagliflozin with a placebo11. Similarly, another meta‐analysis of clinical trials found a high possibility of DKA induction with SGLT2 inhibitors (relative risk [RR] 4.49, 95% CI 2.88–6.99)12. Nevertheless, because controlled trial environments closely supervise patients and use sophisticated study protocols to reduce DKA risk, it remains unclear whether the observed DKA rates accurately reflect SGLT2 inhibitor use by type 1 diabetes patients in real‐world settings. A recent study showed that ‘off‐label’ use of SGLT2 inhibitors induced real‐world DKA rates of 4.5–7.3 per 100 person‐years in type 1 diabetes patients13. They also observed more DKA events than expected with sotagliflozin (a dual SGLT1/2 inhibitor) use in clinical trials13. These findings suggest a serious concern that ‘on‐label’ use of SGLT2 inhibitors by type 1 diabetes patients might increase DKA risk in real‐world clinical practice.
Since December 2018, two SGLT2 inhibitors, ipragliflozin and dapagliflozin, have been approved and indicated for use in Japan with insulin in adults with type 1 diabetes. Therefore, we investigated the true DKA risk in type 1 diabetes patients using SGLT2 inhibitors as add‐on therapy to insulin, and the association between this risk and patient clinical characteristics during real‐world ‘on‐label’ use of SGLT2 inhibitors.
Materials and methods
Research design
This was a real‐world, retrospective, observational cohort study using data from the Medical Data Vision (MDV) administrative claims database (Medical Data Vision Co., Ltd, Tokyo, Japan). MDV is a nationwide hospital‐based claims database covering almost 31 million cumulative patients since April 2008 who, as of October 2019, had been treated as inpatients or outpatients at any of the approximately 360 hospitals in Japan (21% of all hospitals) that participate in the diagnosis procedure combination/per diem payment system. We extracted administrative claims made between 1 December 2018 and 31 October 2019 (referred to as the target selection period). For reference, ipragliflozin and dapagliflozin were approved and indicated for use in Japan with insulin in adults with type 1 diabetes in December 2018 and March 2019, respectively. We censored patients at the time of discontinuing SGLT2 inhibitors, developing DKA, losing insurance coverage or death. For patients prescribed SGLT2 inhibitors, the observation start date was set as the date of the first prescription. For patients treated without SGLT2 inhibitors, the observation start date was defined as the date of the first visit to a hospital after 1 December 2018.
Study population
We extracted data on 3,129,105 patients diagnosed with diabetes mellitus (International Classification of Diseases, 10th revision [ICD‐10] code: E11–E14) registered between 1 April 2008 and 31 October 2019, in the MDV database. From the extracted data, we selected data on patients diagnosed with type 1 diabetes (ICD‐10 code: E10) based on exclusion criteria described in Figure S1. Thereafter, 11,475 adult type 1 diabetes patients registered in the MDV database during the target selection period were identified and divided into two groups based on the presence or absence of SGLT2 inhibitor prescription.
Outcome
The major outcome was increased DKA risk in type 1 diabetes patients following SGLT2 inhibitor use. Clinical factors linked to increased DKA risk in type 1 diabetes patients using SGLT2 inhibitors were determined. DKA was defined using an inpatient or emergency department diagnosis with an ICD‐9‐CM code 250.1x or ICD‐10 code E1x.1x at any position14.
Patient characteristics
Age, sex and body mass index (BMI) were included as baseline characteristics and identified using claim data in the same month as the observation start date. Obesity was defined as having BMI ≥25 kg/m2 15.
Statistical analysis
Patient data following a normal distribution (age and BMI) were expressed as the mean ± standard deviation. Continuous variables were analyzed using the unpaired t‐test. Categorical variables were analyzed using the χ2‐test and expressed as absolute numbers or percentages. Hazard ratios (HRs) for risk of DKA occurrence were analyzed using the Cox proportional hazards model adjusted for SGLT2 inhibitor prescription (yes/no), sex, age (as categories with 10‐year increments or as <25, 25–44, 45–64, ≥65 years), BMI (cut‐off value 25 kg/m2), and insulin regimens of multiple daily injection (MDI) and continuous subcutaneous insulin infusion (CSII). Kaplan–Meier survival curves and log‐rank tests were used for SGLT2 inhibitor prescription (with/without). Significance was set at P < 0.05. The cut‐off value for number of days since the observation start date to date of DKA onset was calculated using receiver operating characteristic analysis. All statistical analyses were carried out using Statistical Package for Social Sciences (SPSS) version 25 (SPSS Inc., Chicago, IL, USA; IBM Version).
Ethical considerations
The present study was carried out in accordance with ethical guidelines for medical and health research involving human subjects. The ethics board of Kitasato University approved the study (Control number: B19‐285), and provided permission to review patient records and use the corresponding data. All patient data were anonymized and contained no personal data; therefore, informed consent was not required.
Results
Clinical characteristics of type 1 diabetes patients
We identified 11,475 type 1 diabetes patients, of which 1,898 (16.5%) were treated with SGLT2 inhibitors (SGLT2i[+]) and 9,577 (83.5%) were treated without SGLT2 inhibitors (SGLT2i[−]; Table 1, Figure S1). The mean age was 53.6 ± 19.3 years and was significantly higher in SGLT2i(+) patients than in SGLT2i(−) patients (P = 0.003; Table 1). The proportion of patients aged <25 or ≥65 years was significantly lower in the SGLT2i(+) group, whereas the proportion of patients aged 45–64 years was significantly higher in the SGLT2i(+) group (P < 0.001). The proportion of obese patients with BMI ≥25 kg/m2 was significantly higher in the SGLT2i(+) than in the SGLT2i(−) group (42.4% and 20.0%, respectively; P < 0.001). This suggested that healthcare providers tend to prescribe SGLT2 inhibitors to type 1 diabetes patients with a higher BMI. There was no significant difference in the proportion of CSII users between the two groups.
Table 1.
Baseline clinical characteristics of all type 1 diabetes patients
| Characteristics | Overall (n = 11,475) | SGLT2i(+) (n = 1,898) | SGLT2i(−) (n = 9,577) | P‐value [SGLT2i(+) vs SGLT2i(−)] |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 5,469 (47.7) | 900 (47.4) | 4,569 (47.7) | 0.976 |
| Female | 6,006 (52.3) | 998 (52.6) | 5,008 (52.3) | |
| Age | ||||
| Mean (years) | 53.6 ± 19.3 | 55.8 ± 15.0 | 53.3 ± 2.0 | 0.003 |
| Distribution, n (%) | ||||
| <25 | 1,009 (8.8) | 54 (2.8) | 955 (10.0) | <0.001 |
| 25–44 | 2,480 (21.6) | 439 (23.1) | 2,041 (21.3) | |
| 45–64 | 4,144 (36.1) | 848 (44.7) | 3,296 (34.4) | |
| ≥65 | 3,842 (33.5) | 557 (29.3) | 3,285 (34.3) | |
| BMI | ||||
| Mean (kg/m2) | 22.3 ± 5.0 | 24.5 ± 4.9 | 21.8 ± 4.8 | 0.211 |
| Distribution, n (%) | ||||
| <25 | 8,750 (76.3) | 1,093 (57.6) | 7,657 (80.0) | <0.001 |
| ≥25 | 2725 (23.7) | 805 (42.4) | 1,920 (20.0) | |
| Insulin regimen, n (%) | ||||
| MDI | 10,600 (92.4) | 1,767 (93.1) | 8,833 (92.2) | 0.194 |
| CSII | 875 (7.6) | 131 (6.9) | 744 (7.8) | |
Data shown as mean ± standard deviation. Body mass index (BMI) is weight in kilograms divided by the square of height in meters.
CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injection; SGLT2i(+), patients treated with sodium–glucose cotransporter 2 inhibitors; SGLT2i(−), patients treated without sodium–glucose cotransporter 2 inhibitors.
Clinical characteristics of type 1 diabetes patients developing DKA
Diabetic ketoacidosis was observed in 823 (7.2%) of 11,475 type 1 diabetes patients; that is, 139 (7.3%) of 1,898 SGLT2i(+) patients and 684 (7.1%) of 9,577 SGLT2i(−) patients (Table 2). The mean age of DKA patients was 45.6 ± 20.7 years and was significantly higher in SGLT2i(+) patients (P = 0.002). The proportion of patients aged <25 years was significantly lower, and that of patients aged 25–44 years was significantly higher in SGLT2i(+) patients. The mean BMI of DKA patients was 21.5 ± 4.6 kg/m2 and was significantly higher in SGLT2i(+) patients. The proportion of patients with BMI ≥25 kg/m2 was significantly higher in SGLT2i(+) than in SGLT2i(−) patients (38.1% and 15.4%, respectively; P < 0.001). No significant difference in the proportion of CSII users was observed between the two groups.
Table 2.
Baseline clinical characteristics of type 1 diabetes patients presenting with diabetic ketoacidosis
| Characteristics | Overall (n = 823) | SGLT2i(+) (n = 139) | SGLT2i(−) (n = 684) | P‐value (SGLT2i[+] vs SGLT2i[−]) |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 380 (46.2) | 58 (41.7) | 322 (47.1) | 0.249 |
| Female | 443 (53.8) | 81 (58.3) | 362 (52.9) | |
| Age | ||||
| Mean (years) | 45.6 ± 20.7 | 50.3 ± 15.5 | 44.7 ± 21.5 | 0.002 |
| Distribution, n (%) | ||||
| <25 | 147 (17.9) | 8 (5.8) | 139 (20.3) | <0.001 |
| 25–44 | 232 (28.2) | 49 (35.3) | 183 (26.8) | |
| 45–64 | 271 (32.9) | 54 (38.8) | 217 (31.7) | |
| ≥65 | 173 (21.0) | 28 (20.1) | 145 (21.2) | |
| BMI | ||||
| Mean (kg/m2) | 21.5 ± 4.6 | 24.5 ± 4.9 | 21.0 ± 4.5 | <0.001 |
| Distribution, n (%) | ||||
| <25 | 665 (80.8) | 86 (61.9) | 579 (84.6) | <0.001 |
| ≥25 | 158 (19.2) | 53 (38.1) | 105 (15.4) | |
| Insulin regimen, n (%) | ||||
| MDI | 706 (85.8) | 124 (89.2) | 582 (85.1) | 0.205 |
| CSII | 117 (14.2) | 15 (10.8) | 102 (14.9) | |
Data shown as mean ± standard deviation. Body mass index (BMI) is weight in kilograms divided by the square of height in meters.
CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injection; SGLT2i(+), patients treated with sodium–glucose cotransporter 2 inhibitors; SGLT2i(−), patients treated without sodium–glucose cotransporter 2 inhibitors.
Risk of DKA in type 1 diabetes patients
DKA incidence was 9.68 (95% CI 9.04–10.4) per 100 person‐years in type 1 diabetes patients (Table 3). Notably, the incidence was higher in SGLT2i(+) patients than in SGLT2i(−) patients (20.2, 95% CI 17.1–23.9 and 8.70, 95% CI 8.12–9.43, respectively, per 100 person‐years). Kaplan–Meier curves showed that during the 334‐day observation period, DKA incidence was significantly higher in SGLT2i(+) patients (P < 0.001; Figure S2). DKA incidence decreased continuously as patient age increased (Table 3). As expected, DKA incidence was higher in CSII users than in MDI users (19.7, 95% CI 16.4–23.6 and 8.92, 95% CI 8.29–9.61, respectively, per 100 person‐years). SGLT2i(+) patients showed a higher rate of DKA (HR 1.66, 95% CI 1.33–2.06, P < 0.001; Figure 1a). HR did not differ between sexes, but decreased with increasing age, and was significantly higher (1.33‐fold) in patients with BMI <25 kg/m2 than in those with BMI ≥25 kg/m2 (P = 0.005). Intriguingly, HR was comparable between CSII and MDI patients.
Table 3.
Rates of diabetic ketoacidosis in all type 1 diabetes patients as per baseline clinical characteristics
| Characteristics | n | DKA events | DKA/100 person‐years |
|---|---|---|---|
| Overall patients | 11,475 | 823 | 9.68 (9.04–10.4) |
| SGLT2 inhibitors | |||
| Yes | 1,898 | 139 | 20.2 (17.1–23.9) |
| No | 9,577 | 684 | 8.70 (8.12–9.43) |
| Sex | |||
| Male | 5,469 | 380 | 9.35 (8.46–10.3) |
| Female | 6,006 | 443 | 10.0 (9.09–10.9) |
| Age (years) | |||
| <25 | 1,009 | 147 | 21.0 (17.8–24.6) |
| 25–44 | 2,480 | 232 | 12.9 (11.3–14.6) |
| 45–64 | 4,144 | 271 | 9.0 (7.99–10.1) |
| ≥65 | 3,842 | 173 | 5.78 (4.98–6.71) |
| BMI (kg/m2) | |||
| <25 | 8,750 | 665 | 10.1 (9.36–10.9) |
| ≥25 | 2,725 | 158 | 8.23 (7.04–9.62) |
| Insulin regimen | |||
| MDI | 10,600 | 706 | 8.92 (8.29–9.61) |
| CSII | 875 | 117 | 19.7 (16.4–23.6) |
Body mass index (BMI) is weight in kilograms divided by the square of height in meters.
CSII, continuous subcutaneous insulin infusion; DKA, diabetic ketoacidosis; MDI, multiple daily injection; SGLT2, sodium–glucose cotransporter 2.
Figure 1.
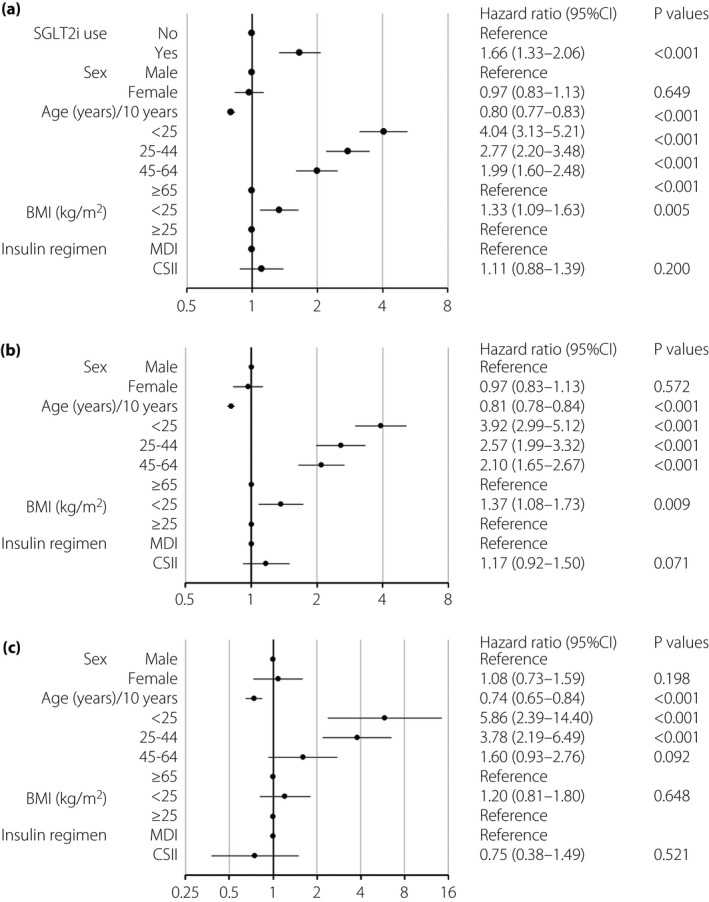
Hazard ratio of diabetic ketoacidosis (DKA) in type 1 diabetes patients. Forest plots show the hazard ratios (HRs) for DKA in type 1 diabetes patients as per baseline clinical characteristics. (a) HR for DKA among all type 1 diabetes patients. (b) HR for DKA among type 1 diabetes patients who did not receive sodium–glucose cotransporter 2 inhibitors (SGLT2is). (c) HR for DKA among type 1 diabetes patients using SGLT2is. Circles represent the HR, and horizontal bars extend from the lower limit to the upper limit of the 95% confidence interval (CI) of the estimated HR. BMI, body mass index; CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injection.
Risk of DKA in type 1 diabetes patients treated with and without SGLT2 inhibitors
The incidence of DKA (per 100 person‐years) with respect to all clinical parameters shown in Table S1 were higher in SGLT2i(+) patients than in SGLT2i(−) patients. DKA incidence decreased with increasing patient age, irrespective of treatment with SGLT2 inhibitors. In both SGLT2i(+) and SGLT2i(−) patients, DKA incidence was higher in patients with BMI <25 kg/m2 than in those with BMI ≥25 kg/m2. DKA incidence was also higher in patients receiving CSII than in those receiving MDI, irrespective of SGLT2 inhibitor use. For reference, SGLT2i(+) CSII patients showed a higher incidence of 31.0 person‐years than did SGLT2i(−) CSII patients and MDI patients treated with or without SGLT2 inhibitors. However, the 95% CI had a broad range (18.7–51.4), suggesting insufficient sample size for precise analysis. Therefore, the value might not accurately represent DKA incidence.
Regarding SGLT2i(−) patients (Figure 1b), sex differences were not associated with HR of DKA. HR decreased with increasing patient age and was significantly higher (1.37‐fold) in patients with BMI <25 kg/m2 than in those with BMI ≥25 kg/m2 (P = 0.009). Furthermore, patients receiving CSII showed a 1.17‐fold higher risk of DKA than did patients receiving MDI, although this difference was not statistically significant. For SGLT2i(+) patients (Figure 1c), sex difference was not associated with HR of DKA; HR decreased with increasing age, and other findings were similar to those noted for SGLT2i(−) patients. Obesity was not associated with DKA risk. HR was comparable between SGLT2i(+) patients receiving CSII and MDI.
Clinical factors linked to increased DKA risk in type 1 diabetes patients receiving SGLT2 inhibitors
We investigated DKA risk with respect to the baseline clinical characteristics of SGLT2i(+) versus SGLT2i(−) patients. The HR of SGLT2 inhibitor‐associated DKA was significantly increased, irrespective of sex and BMI (Figure 2). The HR was also significantly higher in patients aged 25–44, 45–64 and ≥65 years (HR 1.90, 1.44 and 2.09, respectively), and showed an increasing, although not significant, trend in patients aged <25‐years (HR 1.65), presumably owing to the small sample number. The HR significantly increased in patients receiving MDI (P < 0.001), but not in those receiving CSII. These findings suggest that SGLT2 inhibitor‐associated DKA risk might increase in type 1 diabetes patients irrespective of sex, age or BMI, and that this risk might increase in those receiving MDI but not CSII.
Figure 2.
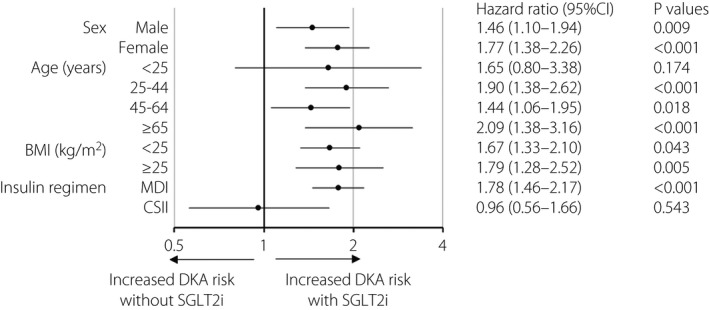
Forest plot of the association between the onset of diabetic ketoacidosis (DKA) and use of sodium–glucose cotransporter 2 inhibitors (SGLT2is) in type 1 diabetes patients. Forest plot shows the hazard ratios (HRs) for DKA as per baseline clinical characteristics of type 1 diabetes patients receiving SGLT2is versus those not receiving SGLT2is. Circles represent the HR, and horizontal bars extend from the lower limit to the upper limit of the 95% confidence interval (CI) of the estimated HRs. BMI, body mass index; CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injection.
Time until DKA onset in SGLT2 inhibitor‐treated type 1 diabetes patients
The mean time until DKA onset in SGLT2i(+) patients was 30.6 ± 30.1 days. The cut‐off value as determined by the receiver operating characteristic analysis was 38 days (Figure S3).
Discussion
The present study is the first to show that SGLT2i(+) patients have a 1.66‐fold higher HR of DKA than SGLT2i(−) patients under ‘on‐label’ use of SGLT2 inhibitors, showing that DKA risk is lower in clinical practice than noted in previous RCTs11. Although we could not identify triggers for DKA, previous DEPICT studies showed that most cases of SGLT2 inhibitor‐associated DKA can be attributed to insulin pump failure, reduced/missed (inadequate) insulin doses, infection or excessive alcohol intake4, 5, 6. After SGLT2 inhibitors were approved for adult patients with type 1 diabetes, the Japan Diabetes Society issued a recommendation for minimizing DKA risk in SGLT2 inhibitor‐treated type 1 diabetes patients, including repeatedly educating patients and healthcare providers about the early signs and symptoms of DKA, self‐managing sick days, risk factors for a DKA event, and limiting the reduction of total insulin dose to <20%16. Such notifications could lead to lower risks of DKA in SGLT2 inhibitor‐treated type 1 diabetes patients in the present study than in previous RCTs.
DKA incidence (7.2%) in SGLT2i(+)type 1 diabetes patients was higher in the present study than in previous phase III trials targeting Japanese type 1 diabetes patients receiving insulin therapy (ipragliflozin, 0% for 24 weeks; dapagliflozin, 2.0% for 52 weeks)17, 18. Furthermore, the DKA rate was 20.2 per 100 person‐years in the present study, higher than that in a recent study in which, under real‐world ‘off‐label’ use of SGLT2 inhibitors, the DKA rate ranged from 4.5 to 7.3 per 100 person‐years in type 1 diabetes patients13. The increased DKA rate in the present study might be associated with the increased DKA rate observed even in the SGLT2i(−) patients – 8.70 (95% CI 8.12–9.43) per 100 person‐years. This was higher than that shown by a previous study in the UK19 – 3.6 per 100 person‐years in type 1 diabetes patients from 1998 to 2013 – during which, SGLT2 inhibitors were unavailable in clinical practice. Thus, the baseline increase in DKA risk in SGLT2i(−) patients might contribute to the higher DKA rate in SGLT2i(+) patients in the present study. Behind this background, capillary blood ketone monitoring by patients themselves is not approved in Japan, possibly leading to a delayed or missed diagnosis of DKA. For reference, we compared DKA incidence within 1 month after initiating SGLT2 inhibitors between the first half (December 2018 to April 2019) and the latter half (May 2019 to October 2019) of the observation period. As a result, DKA incidences in the first half and latter half of the periods were 5.9% (59/937) and 4.1% (37/902), respectively. The DKA incidence tended to be higher in the former groups than in the latter groups, although not significantly (P = 0.071, using the χ2‐test). These findings suggest that both patients and healthcare providers might have been unfamiliar with the medication in the first half of the period.
Although there was no significant difference in baseline BMI between SGLT2i(+) and SGLT2i(−) patients, the distribution of obesity was significantly higher in SGLT2i(+) patients than in SGLT2i(−) patients. Therefore, we investigated whether the presence or absence of obesity is associated with the risk of SGLT2 inhibitor‐associated DKA. As a result, although the DKA risk was higher in SGLT2i(−) patients with BMI <25 kg/m2 than in patients with BMI ≥25 kg/m2, the risk was comparable between SGLT2i(+) patients with BMI <25 kg/m2 and those with BMI ≥25 kg/m2. Regarding the relationship with BMI, DEPICT studies showed a possible increased risk of DKA in type 1 diabetes patients with lower BMI treated with dapagliflozin (5 mg/day)20. In response to these findings, the Japan Diabetes Society has warned healthcare providers that DKA might develop in type 1 diabetes patients prescribed SGLT2 inhibitors, especially in those with low BMI16. This might help decrease the DKA risk in SGLT2i(+) patients with BMI <25 kg/m2 in the present study. However, it is notable that SGLT2 inhibitor‐associated DKA risk increased equally in type 1 diabetes patients with BMI <25 kg/m2 and those with BMI ≥25 kg/m2 (Figure 2), suggesting that healthcare providers have to be aware of possible DKA occurrence in insulin‐treated type 1 diabetes patients initiating SGLT2 inhibitors, irrespective of BMI.
In the present study, a slightly higher, but not significant, DKA risk was observed in CSII users than in MDI users among overall and SGLT2i(−) patients. These findings are largely concordant with those from previous meta‐analyses21, 22. In fact, a previous meta‐analysis showed that CSII is associated with increased DKA risk mainly due to insulin pump malfunction and/or catheter occlusion22; thus, both of which might also be associated with the increasing trend in DKA risk in CSII users. Furthermore, previous clinical studies23, 24, 25 suggest that SGLT2 inhibitor‐associated DKA might occur more frequently in type 1 diabetes patients who receive CSII than in those who receive MDI. However, the DKA risk was comparable between the SGLT2i(+) patients who used either CSII or MDI in the present study. In addition, type 1 diabetes patients who used MDI, but not CSII, had an increased risk of SGLT2 inhibitor‐associated DKA. Although the small number of patients with CSII did limit the statistical power, our findings might be sufficient to confidently recommend methods to mitigate DKA risk in CSII users initiating SGLT2 inhibitors; for example, self‐monitoring blood glucose levels more frequently, bringing patient attention to insulin pump failures, providing STICH protocols for sick days26 or selecting patients with the ability to comprehend the treatment.
The present study showed that the mean duration of the SGLT2 inhibitor treatment until the onset of DKA was 30.6 days, and that DKA risk significantly increased within 38 days of initiating SGLT2 inhibitors. The most common causal factor for DKA was reportedly inadequate insulin delivery through missed doses or insulin pump failure20. Therefore, both type 1 diabetes patients and healthcare providers should be wary of DKA caused by inadequate insulin therapy, especially during the first month of initiating SGLT2 inhibitors.
Several limitations arise from the use of diagnostic codes and prescription claims to classify type 1 diabetes patients and ascertain DKA events. In addition, information on possible confounding factors for DKA, such as glycated hemoglobin A1c, insulin secretion capacity (C‐peptide) and accurate dose of SGLT2 inhibitors, was only available for a limited number of patients. Thus, our analyses could not be adjusted for such confounders. Furthermore, we could not clarify the triggers for DKA development. Finally, as described above, the risk of SGLT2 inhibitor‐associated DKA for CSII users in the present study might not be accurate, because CSII users were comparatively few in number. Thus, we emphasize that this result might not necessarily be true for other clinical practices.
In conclusion, the present study revealed that insulin‐treated type 1 diabetes patients treated with ‘on‐label’ use of SGLT2 inhibitors showed a 1.66‐fold higher DKA rate than did those treated without SGLT2 inhibitors in real‐world clinical practice. Furthermore, type 1 diabetes patients using SGLT2 inhibitors showed a high incidence of DKA; that is, 20.2 per 100 person‐years. The risk of SGLT2 inhibitor‐associated DKA was high in type 1 diabetes patients irrespective of sex, age or BMI. The risk was also higher in those who received MDI, but not in those who received CSII, which warrants further investigation because of the small number of type 1 diabetes patients with CSII. In addition, type 1 diabetes patients developed DKA within the first month of SGLT2 inhibitor initiation. Following these findings, we emphasize anew the importance of proper ‘on‐label’ use of SGLT2 inhibitors in insulin‐treated type 1 diabetes patients to minimize DKA risk.
Acknowledgment
No specific funding or grant was received for this work.
Disclosure
AS received lecture fees from Astellas Pharma Inc., Eli Lilly Japan K.K., Novo Nordisk Pharma Inc. and Sanofi K.K. The other authors declare no conflict of interest.
Supporting information
Figure S1 | Patient disposition.
Figure S2 | Kaplan–Meier analysis of the cumulative incidence of diabetic ketoacidosis in patients with type 1 diabetes treated with and without sodium–glucose cotransporter 2 inhibitors.
Figure S3 | Cutoff value for the duration of treatment with sodium–glucose cotransporter 2 inhibitors until the onset of diabetic ketoacidosis in patients with type 1 diabetes.
Table S1 | Incidences of diabetic ketoacidosis in patients with type 1 diabetes treated with and without sodium–glucose cotransporter 2 inhibitors as per baseline clinical characteristics.
J Diabetes Investig. 2021; 12: 1586–1593
References
- 1.American DA. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37(Suppl 1): S81–S90. [DOI] [PubMed] [Google Scholar]
- 2.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther 2019; 21: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Bommel EJ, Muskiet MH, Tonneijck L, et al. SGLT2 inhibition in the diabetic kidney‐from mechanisms to clinical outcome. Clin J Am Soc Nephrol 2017; 12: 700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT‐1): 24 week results from a multicentre, double‐blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5: 864–876. [DOI] [PubMed] [Google Scholar]
- 5.Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT‐1 52‐week study. Diabetes Care 2018; 41: 2552–2559. [DOI] [PubMed] [Google Scholar]
- 6.Mathieu C, Dandona P, Gillard P, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT‐2 study): 24‐week results from a randomized controlled trial. Diabetes Care 2018; 41: 1938–1946. [DOI] [PubMed] [Google Scholar]
- 7.Rosenstock J, Marquard J, Laffel LM, et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care 2018; 41: 2560–2569. [DOI] [PubMed] [Google Scholar]
- 8.Henry RR, Thakkar P, Tong C, et al. Efficacy and safety of canagliflozin, a sodium‐glucose cotransporter 2 inhibitor, as add‐on to insulin in patients with type 1 diabetes. Diabetes Care 2015; 38: 2258–2265. [DOI] [PubMed] [Google Scholar]
- 9.Chiba K, Nomoto H, Nakamura A, et al. Sodium‐glucose cotransporter 2 inhibitors reduce day‐to‐day glucose variability in patients with type 1 diabetes. J Diabetes Investig 2021; 12: 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa W, Hirota Y. Sodium‐glucose cotransporter 2 inhibitor‐associated diabetic ketoacidosis in patients with type 1 diabetes: metabolic imbalance as an underlying mechanism. J Diabetes Investig 2019; 10: 879–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu J, Tang L, Meng H, et al. Effects of sodium‐glucose cotransporter (SGLT) inhibitors in addition to insulin therapy on glucose control and safety outcomes in adults with type 1 diabetes: a meta‐analysis of randomized controlled trials. Diabetes Metab Res Rev 2019; 35: e3169. [DOI] [PubMed] [Google Scholar]
- 12.Li K, Xu G. Safety and efficacy of sodium glucose co‐transporter 2 inhibitors combined with insulin in adults with type 1 diabetes: a meta‐analysis of randomized controlled trials. J Diabetes 2019; 11: 645–655. [DOI] [PubMed] [Google Scholar]
- 13.Hampp C, Swain RS, Horgan C, et al. Use of sodium‐glucose cotransporter 2 inhibitors in patients with type 1 diabetes and rates of diabetic ketoacidosis. Diabetes Care 2020; 43: 90–97. [DOI] [PubMed] [Google Scholar]
- 14.Bobo WV, Cooper WO, Epstein RA Jr, et al. Positive predictive value of automated database records for diabetic ketoacidosis (DKA) in children and youth exposed to antipsychotic drugs or control medications: a Tennessee Medicaid study. BMC Med Res Methodol 2011; 11: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Examination Committee of Criter . New criteria for ‘obesity disease’ in Japan. Circ J 2002; 66: 987–992. [DOI] [PubMed] [Google Scholar]
- 16.Committee on the Proper Use of SI . Recommendations on the proper use of SGLT2 inhibitors. J Diabetes Investig 2020; 11: 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaku K, Isaka H, Sakatani T, et al. Efficacy and safety of ipragliflozin add‐on therapy to insulin in Japanese patients with type 1 diabetes mellitus: a randomized, double‐blind, phase 3 trial. Diabetes Obes Metab 2019; 21: 2284–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Araki E, Watada H, Uchigata Y, et al. Efficacy and safety of dapagliflozin in Japanese patients with inadequately controlled type 1 diabetes (DEPICT‐5): 52‐week results from a randomized, open‐label, phase III clinical trial. Diabetes Obes Metab 2020; 22: 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong VW, Juhaeri J, Mayer‐Davis EJ. Trends in hospital admission for diabetic ketoacidosis in adults with type 1 and type 2 diabetes in England, 1998–2013: a retrospective cohort study. Diabetes Care 2018; 41: 1870–1877. [DOI] [PubMed] [Google Scholar]
- 20.Paik J, Blair HA. Dapagliflozin: a review in type 1 diabetes. Drugs 2019; 79: 1877–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pala L, Dicembrini I, Mannucci E. Continuous subcutaneous insulin infusion vs modern multiple injection regimens in type 1 diabetes: an updated meta‐analysis of randomized clinical trials. Acta Diabetol 2019; 56: 973–980. [DOI] [PubMed] [Google Scholar]
- 22.Fazeli Farsani S, Brodovicz K, Soleymanlou N, et al. Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): a systematic literature review. BMJ Open 2017; 7: e016587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garg SK, Henry RR, Banks P, et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med 2017; 377: 2337–2348. [DOI] [PubMed] [Google Scholar]
- 24.Danne T, Cariou B, Banks P, et al. HbA1c and hypoglycemia reductions at 24 and 52 weeks with sotagliflozin in combination with insulin in adults with type 1 diabetes: the European inTandem2 study. Diabetes Care 2018; 41: 1981–1990. [DOI] [PubMed] [Google Scholar]
- 25.Buse JB, Garg SK, Rosenstock J, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American inTandem1 study. Diabetes Care 2018; 41: 1970–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garg SK, Peters AL, Buse JB, et al. Strategy for mitigating DKA risk in patients with type 1 diabetes on adjunctive treatment with SGLT inhibitors: a STICH protocol. Diabetes Technol Ther 2018; 20: 571–575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Patient disposition.
Figure S2 | Kaplan–Meier analysis of the cumulative incidence of diabetic ketoacidosis in patients with type 1 diabetes treated with and without sodium–glucose cotransporter 2 inhibitors.
Figure S3 | Cutoff value for the duration of treatment with sodium–glucose cotransporter 2 inhibitors until the onset of diabetic ketoacidosis in patients with type 1 diabetes.
Table S1 | Incidences of diabetic ketoacidosis in patients with type 1 diabetes treated with and without sodium–glucose cotransporter 2 inhibitors as per baseline clinical characteristics.


