Abstract
Structural and functional changes in the cerebral vasculature occur with advancing age, which may lead to impaired neurovascular coupling (NVC) and cognitive decline. Cyclooxygenase (COX) inhibition abolishes age-related differences in cerebrovascular reactivity, but it is unclear if COX inhibition impacts NVC. The purpose of this study was to examine the influence of aging on NVC before and after COX inhibition. Twenty-three young (age = 25 ± 4 yr) and 21 older (age = 64 ± 5 yr) adults completed two levels of difficulty of the Stroop and n-back tests before and after COX inhibition. Middle cerebral artery blood velocity (MCAv) was measured using transcranial Doppler ultrasound and mean arterial blood pressure (MAP) was measured using a finger cuff. Hemodynamic variables were measured at rest and in response to cognitive challenges. During the Stroop test, older adults demonstrated a greater increase in MCAv (young: 2.2 ± 6.8% vs. older: 5.9 ± 5.8%; P = 0.030) and MAP (young: 2.0 ± 4.9% vs. older: 4.8 ± 4.9%; P = 0.036) compared with young adults. There were no age-related differences during the n-back test. COX inhibition reduced MCAv by 30% in young and 26% in older adults (P < 0.001 for both). During COX inhibition, there were no age-related differences in the percent change in MCAv or MAP in response to the cognitive tests. Our results show that older adults require greater increases in MCAv and MAP during a test of executive function compared with young adults and that any age-related differences in NVC were abolished during COX inhibition. Collectively, this suggests that aging is associated with greater NVC necessary to accomplish a cognitive task.
Keywords: arterial stiffness, blood flow, blood pressure, executive function, working memory
INTRODUCTION
Cognitive aging manifests as a decrease in brain functional capacity in processing speed, memory, and executive function (1). Age-related structural changes in the brain, which contribute to cognitive aging, include decreases in white matter (2), gray matter (3), and total brain volume (4). Specifically, aging is associated with a decrease in gray matter volume of the prefrontal cortex, an area associated with executive function and cognitive performance (5, 6). Identifying risk factors associated with accelerated cognitive decline during midlife may help prevent or delay the development of clinical cognitive impairment and/or dementia later in life. It is speculated that vascular dysfunction precedes cognitive impairment (7), as hypoperfusion has been linked to brain atrophy and elevated white matter hyperintensities with age (8). Over the past decade, initiatives have been launched to investigate vascular contributions to cognitive impairment and dementia and establish measurable risk factors identifiable in middle-aged adults (9). Arterial stiffness is one such measurable risk factor that increases with advancing age (10, 11). Elevated arterial stiffness is associated with lower cerebral perfusion (12), higher white matter hyperintensity volume (13–15), and a decline in cognitive function (16, 17).
Neurovascular coupling (NVC) is defined as the relationship between neuronal activity and subsequent changes in blood flow, ensuring adequate delivery of oxygen to match metabolic demand (18). However, with advancing age and the development of pathologies such as hypertension or stroke, impairments in NVC may occur (18–20). Although reductions in NVC are multifaceted, structural and functional changes to the vasculature likely play a critical role in the development of cognitive decline (19, 21). Impaired NVC with advancing age suggests that changes in NVC may be an early marker of risk for cognitive decline. NVC can be assessed using acute cognitive challenges that induce a neuronal metabolic stimulus and require augmentation of blood flow. Previous studies evaluating hemodynamics during acute cognitive challenges have shown increases in middle cerebral artery blood velocity (MCAv) (22–25), with no change in cerebral pulsatility index (PI) (22). However, older adults exhibit greater cerebral PI during acute cognitive challenges compared with young adults, suggesting an augmented cerebral PI response with advancing age (25). Although measuring NVC in response to a cognitive challenge may represent the cerebrovascular response of day-to-day tasks, the effects of aging and acute reductions in cerebral blood velocity remain unclear.
Vasodilating prostaglandins have been established as a potential mechanism underlying the increase in cerebral blood velocity in response to hypercapnia (e.g., cerebrovascular reactivity) (26, 27), but the role of prostaglandins in NVC is unknown. Cyclooxygenase (COX) is the enzyme responsible for producing vasodilating prostaglandins such as prostacyclin (PGI2). Previous research has reported no effect of the common COX inhibitor aspirin on cognitive function (28) or cognitive test scores in older adults (29). However, indomethacin (INDO) is a COX inhibitor that lowers MCAv at rest in humans (30–32). Because aging is associated with a reduction in prostaglandin-mediated vasodilation (33, 34), the combination of older age and COX inhibition may alter NVC or the systemic hemodynamics. Our previous work has shown that COX inhibition with INDO increased central blood pressure and aortic augmentation index in older adults (35). However, the effects of COX inhibition on NVC and the systemic hemodynamic response to a cognitive challenge are unclear. Therefore, the impact of acute INDO administration could be important for understanding COX-mediated mechanisms, as well as the effect of COX inhibitors, which are frequently used to manage pain.
The purpose of this study was to evaluate age-related differences in NVC and the hemodynamic response to acute cognitive challenges before and after administration of a COX inhibitor in young and older adults. We hypothesized that older adults would exhibit an augmented NVC and hemodynamic response to acute cognitive challenges compared with young adults and hypothesized that this difference would be abolished during COX inhibition. Because of the increase in arterial stiffness with age (10, 11) and the association between increased arterial stiffness and a decline in cognitive function (16, 17), a secondary aim was to investigate associations between baseline arterial stiffness and NVC or hemodynamic responses to acute cognitive challenges.
METHODS
Participants
Twenty-three young (18–35 yr) and 21 older (55–75 yr) adults participated in this study. All participants had a body mass index (BMI) between 18 and 29 kg/m2. Participants were nonsmoking and excluded if they presented with a history or evidence of cardiovascular disease, stroke, hypertension, diabetes, hepatic disease, renal disease, hematological disease, neurovascular disease, peripheral vascular disease, or other chronic diseases as determined by a health history questionnaire. In addition, participants were excluded if they were currently taking any vasoactive medications or were prescribed nonsteroidal anti-inflammatory drugs (NSAIDs). Young women were studied during the early follicular phase of their menstrual cycle or the placebo pill phase of oral contraceptives (n = 8 young women). Older women were postmenopausal for >1 year and were not currently taking hormone replacement therapy. All study procedures were approved by the University of Wisconsin-Madison Institutional Review Board and performed according to the Declaration of Helsinki by obtaining written informed consent from each participant. This study was registered under ClinicalTrials.gov (NCT02653638).
Experimental Procedures
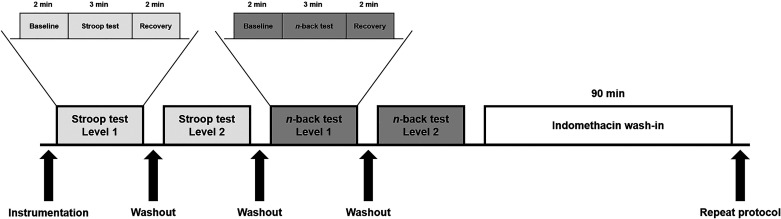
Participants arrived at the Bruno Balke Biodynamics Laboratory at the University of Wisconsin-Madison following an overnight fast having refrained from consuming caffeine or alcohol and performing strenuous exercise 24 h before the study. In addition, participants were asked to withhold over-the-counter medication on the day of the study and avoid the use of NSAIDs for 5 days leading up to the study visit. Upon arrival to the laboratory, height and weight were obtained using a standard scale and stadiometer. All study procedures were performed in a dim, temperature-controlled room kept between 22°C and 24°C. A timeline of the study protocol is shown in Fig. 1.
Figure 1.
Study day timeline. Mean arterial pressure, heart rate, and middle cerebral artery blood velocity were continuously measured throughout the protocol. Participants completed two levels of difficulty of the Stroop color and word test. Each level consisted of a 2-min baseline period, a 3-min cognitive test, and a 2-min recovery period followed by a brief washout period. Participants then completed two levels of difficulty of the n-back working memory test following the same protocol. Indomethacin was administered followed by a 90-min wash-in period. Then, the cognitive testing protocol was repeated.
Cardiovascular Measurements
Following 10 min of supine rest, arterial blood pressure was measured in triplicate using a brachial blood pressure cuff (CardioCap, Datex Ohmeda, GE Healthcare, Fairfield, CT), and the average of the three measurements was recorded. Heart rate (HR) was measured using a 3-lead electrocardiogram (CardioCap, Datex Ohmeda, GE Healthcare, Fairfield, CT). Beat-to-beat mean arterial blood pressure (MAP) was continuously measured using a noninvasive finger cuff (Finometer, Finapres Medical Systems, Amsterdam, The Netherlands). Central arterial stiffness (cfPWV) was assessed using applanation tonometry of the carotid and femoral sites (Sphygmocor, AtcorMedical, Sydney, NSW, Australia) as described previously (36). Briefly, participants rested for 15 min before three trials of cfPWV recorded by measuring the pulse at the carotid and the femoral artery. The total transit distance was calculated by adding the distance between the carotid artery and suprasternal notch to the distance between the suprasternal notch and femoral artery. cfPWV was calculated using the intersection tangent foot-to-foot algorithm. The average of three measurements of cfPWV is reported.
Cerebrovascular Measurements
For cerebrovascular assessments, blood velocity of the right middle cerebral artery (MCA) was measured using a 2-MHz transcranial Doppler ultrasound (TCD) (Spencer Technologies, Seattle, WA). Optimal MCAv signals were obtained according to techniques originally developed by Aaslid et al. (37) and using established guidelines (38). On an initial screening day, the MCA was imaged in each participant, and the depth, anatomical location, probe angle, and mean flow were recorded. On the experimental study day, the ultrasound probe was secured using an adjustable headband to maintain the position and angle of the probe throughout the protocol. Before each block of the cognitive challenges, optimal signal quality was confirmed. End-tidal CO2 (ETCO2) was measured at rest using a nasal cannula (CardioCap, Datex Ohmeda, GE Healthcare, Fairfield, CT).
Cognitive Challenges to Measure NVC
NVC was assessed by measuring the MCAv, MAP, and HR response to two cognitive challenges, the Stroop color and word test (Stroop), a test of executive function (39), and the n-back working memory test (n-back), a test of working memory (40). All participants completed two levels of difficulty of the Stroop test as described previously (41, 42). Following the Stroop test, participants completed two levels of difficulty of the n-back test. A laptop containing custom versions of the Stroop and n-back tests was positioned in front of the participant. The computer screen was adjusted to minimize head movement while performing the tests to minimize signal loss of the MCA. Prior to each cognitive test, the directions were read aloud to the participant by the researcher. All participants were familiarized with the cognitive challenges and allowed to practice a standardized set before measurement to ensure understanding. Baseline cardiovascular and cerebrovascular measurements were recorded for 2 min before the test. Next, each cognitive test was administered for 3 min followed by a 2-min recovery period with a brief washout period between tests. The protocol was repeated for each subsequent test (Fig. 1). Participants were asked to verbally provide answers and were instructed to complete the test to the best of their ability.
Cyclooxygenase Inhibition
Following the completion of the first block of cognitive testing, the COX inhibitor INDO was administered as previously described (32). Briefly, INDO was administered orally at 1.2 mg/kg of body weight with 10 mL of Maalox to reduce gastric irritation. After INDO was administered, participants remained in a semirecumbent position during a 90-min wash-in period. During the drug wash-in period, participants were permitted to read or quietly rest, and physiological variables were constantly monitored by the study team. Participants refrained from consuming anything other than small sips of water and did not engage in strenuous cognitive activity. After 10 min of rest in the supine position, baseline data were collected, and an identical block of cognitive tests was completed following the 90-min wash-in period.
Data Analysis and Statistics
All measurements were recorded using Windaq Data Acquisition Software at 250 Hz (DATAQ Instruments, Akron, OH) and stored offline for analysis. The measurements included: MCAv, cerebral PI, MAP, pulse pressure (PP), HR, and cerebrovascular conductance index (CVCi). Cerebral PI of the MCA was calculated as (systolic MCAv – diastolic MCAv)/mean MCAv. CVCi was calculated as MCAv/MAP. Baseline cardiovascular and cerebrovascular measurements were analyzed during the middle 60 s of the 2-min baseline period. The response to each cognitive test was analyzed using the average value obtained during the first 60 s of the 3-min cognitive test. Raw values for all cardiovascular measurements and the percent change from baseline are reported. The responses to each level of difficulty of both the Stroop and n-back tests were similar and highly correlated within participants (Stroop: average r = 0.67, n-back: average r = 0.67). For analysis, the response to each level of difficulty of the cognitive challenge was averaged such that NVC and hemodynamic responses to level 1 and level 2 of the Stroop or n-back tests are reported. Statistical analyses were completed using RStudio (The R Foundation, Vienna, Austria). Normality was assessed using Shapiro–Wilk tests. In the case of a non-normal distribution, Wilcoxon rank-sum tests and Wilcoxon signed-rank tests were used to evaluate age-related differences between or within groups for primary outcome variables. Participant demographics and baseline cardiovascular and cerebrovascular variables were compared between young and older adults using independent-samples t tests (two tailed). For our primary hypothesis, age-related differences in NVC and hemodynamic responses to cognitive challenges were compared using independent-samples t tests (one tailed). During INDO, age-related differences in NVC and hemodynamic responses to cognitive challenges were compared using independent-samples t tests (one tailed). Within-group differences in all baseline variables and NVC and hemodynamic responses to cognitive challenges before and during INDO were compared using dependent-samples t tests (two tailed). Effect sizes for all comparisons were calculated using Cohen’s d. As a secondary aim, associations between cfPWV obtained at baseline and NVC and hemodynamic responses to each cognitive test were analyzed using Pearson correlation coefficients. Statistical significance was set a priori at P < 0.05.
RESULTS
There were no age-related differences in BMI or HR (Table 1). At rest, older adults had greater diastolic blood pressure and MAP, but values were still in the normotensive range (Table 1). As expected, older adults had higher cfPWV at rest compared with young adults (Table 1). There were no age-related differences in ETCO2 values measured at rest during the control condition (young: 40 ± 5 mmHg vs. older: 41 ± 4 mmHg; P = 0.284, d = 0.33) and after INDO administration (young: 42 ± 2 mmHg vs. older: 41 ± 3 mmHg; P = 0.089, d = 0.53)
Table 1.
Participant characteristics and hemodynamic variables at rest
| Young Adults |
Older Adults |
P Value (Effect Size) | |
|---|---|---|---|
| n | 23 | 21 | |
| Sex, M/F | 10/13 | 11/10 | |
| Age, yr | 25 ± 4 | 64 ± 5 | <0.001 (8.61) |
| Weight, kg | 68 ± 10 | 69 ± 13 | 0.915 (0.03) |
| Height, cm | 171 ± 8 | 172 ± 8 | 0.960 (0.02) |
| Body mass index, kg/m2 | 23 ± 2 | 23 ± 3 | 0.854 (0.06) |
| Systolic blood pressure, mmHg | 119 ± 9 | 122 ± 9 | 0.345 (0.29) |
| Diastolic blood pressure, mmHg | 69 ± 7 | 75 ± 6 | 0.007 (0.87) |
| Mean arterial pressure, mmHg | 86 ± 7 | 91 ± 7 | 0.020 (0.73) |
| Heart rate, beats/min | 55 ± 8 | 55 ± 7 | 0.940 (0.02) |
| cfPWV, cm/s | 5.9 ± 0.9 | 7.8 ± 1.3 | <0.001 (1.63) |
Values are expressed as means ± SD. cfPWV, carotid-femoral pulse wave velocity. P value in bold indicates a significant difference between young and older adults (independent-samples t test).
Responses to the Stroop Color and Word Test
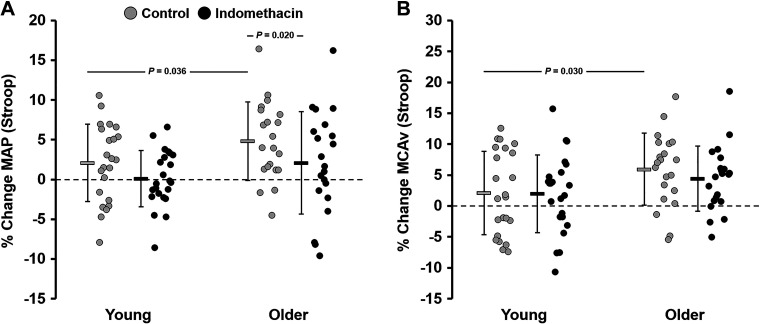
At baseline, before the start of the Stroop test, there were no differences in MAP, PP, HR, or cerebral PI between groups (Table 2). MCAv and CVCi were significantly lower in older adults compared with young adults (Table 2). In response to the Stroop test, there were no age-related differences in MAP, PP, HR, or cerebral PI values (Table 2). MCAv and CVCi values were significantly lower in older adults compared with young adults in response to the Stroop test (Table 2). Because there were age-related differences in some of the baseline values, when MAP and MCAv were expressed as a percent change from baseline, the changes in MAP (young: 2.0 ± 4.9% vs. older: 4.8 ± 4.9%; P = 0.036, d = 0.56) and MCAv (young: 2.2 ± 6.8% vs. older: 5.9 ± 5.8%; P = 0.030, d = 0.61) in response to the Stroop test were significantly greater in older adults compared with young adults (Fig. 2). However, there were no age-related differences in the percent change in PP (young: 3.5 ± 6.8% vs. older: 2.2 ± 7.2%; P = 0.280, d = 0.17), HR (young: 13 ± 10% vs. older: 14 ± 8.5%; P = 0.301, d = 0.16), CVCi (young: 0.3 ± 6.5% vs. older: 1.4 ± 5.8%; P = 0.276, d = 0.19), or cerebral PI (young: −0.3 ± 8.9% vs. older: −2.0 ± 5.2%; P = 0.215, d = 0.23).
Table 2.
Cerebrovascular and hemodynamic variables during the Stroop color and word test
| Stroop Color and Word Test | Young Adults | Older Adults | P Value (Effect Size) |
|---|---|---|---|
| MAP, mmHg | |||
| Control | |||
| Baseline | 90 ± 13 | 94 ± 11 | 0.152 (0.27) |
| Stroop | 92 ± 12 | 98 ± 13 | 0.051 (0.50) |
| Indomethacin | |||
| Baseline | 91 ± 12 | 96 ± 13 | 0.121 (0.36) |
| Stroop | 92 ± 13 | 98 ± 15 | 0.093 (0.41) |
| PP, mmHg | |||
| Control | |||
| Baseline | 74 ± 16 | 73 ± 12 | 0.445 (0.04) |
| Stroop | 76 ± 18 | 75 ± 11 | 0.415 (0.06) |
| Indomethacin | |||
| Baseline | 73 ± 14 | 76 ± 11 | 0.208 (0.25) |
| Stroop | 74 ± 14 | 76 ± 13 | 0.379 (0.09) |
| HR, beats/min | |||
| Control | |||
| Baseline | 60 ± 7 | 59 ± 8 | 0.231 (0.22) |
| Stroop | 68 ± 10 | 67 ± 10 | 0.387 (0.09) |
| Indomethacin | |||
| Baseline | 55 ± 8 | 55 ± 8 | 0.468 (0.02) |
| Stroop | 60 ± 9 | 62 ± 9 | 0.269 (0.19) |
| MCAv, cm/s | |||
| Control | |||
| Baseline | 60 ± 13 | 50 ± 16 | 0.017 (0.66) |
| Stroop | 63 ± 14 | 53 ± 17 | 0.027 (0.60) |
| Indomethacin | |||
| Baseline | 42 ± 8* | 37 ± 9* | 0.016 (0.67) |
| Stroop | 44 ± 9* | 38 ± 9* | 0.027 (0.60) |
| CVCi, cm/s/mmHg | |||
| Control | |||
| Baseline | 0.68 ± 0.17 | 0.54 ± 0.18 | 0.007 (0.78) |
| Stroop | 0.70 ± 0.19 | 0.55 ± 0.18 | 0.007 (0.77) |
| Indomethacin | |||
| Baseline | 0.47 ± 0.11* | 0.39 ± 0.12* | 0.012 (0.70) |
| Stroop | 0.48 ± 0.12* | 0.40 ± 0.12* | 0.015 (0.68) |
| Cerebral PI, AU | |||
| Control | |||
| Baseline | 0.71 ± 0.12 | 0.76 ± 0.10 | 0.054 (0.50) |
| Stroop | 0.70 ± 0.12 | 0.74 ± 0.11 | 0.126 (0.35) |
| Indomethacin | |||
| Baseline | 0.86 ± 0.16* | 0.89 ± 0.13* | 0.223 (0.23) |
| Stroop | 0.87 ± 0.17* | 0.89 ± 0.14* | 0.416 (0.08) |
Values are expressed as means ± SD. Cerebral PI, cerebral pulsatility index; CVCi, cerebrovascular conductance index; HR, heart rate; MAP, mean arterial blood pressure; MCAv, middle cerebral artery blood velocity; PP, pulse pressure. P value in bold indicates a significant difference between young and older adults at baseline or in response to the Stroop color and word test (independent-samples t test or Wilcoxon rank-sum test). *P < 0.05 significantly different vs. control at baseline or in response to the Stroop color and word test (dependent-samples t test or Wilcoxon signed-rank test).
Figure 2.
Percent change (% change) in cardiovascular and cerebrovascular variables in response to the Stroop color and word test before (control) and after (indomethacin) administration of indomethacin. Values are expressed as means % change ± SD, and individual data points are provided. A: percent change in mean arterial blood pressure (MAP). B: percent change in middle cerebral artery blood velocity (MCAv). P value indicates a significant difference between young (n = 23; 10 M, 13 F) and older adults (n = 21; 11 M, 10 F) (independent-samples t test or Wilcoxon rank-sum test) or within groups across conditions (dependent-samples t test or Wilcoxon signed-rank test) in response to the Stroop color and word test.
Responses to the n-Back Working Memory Test
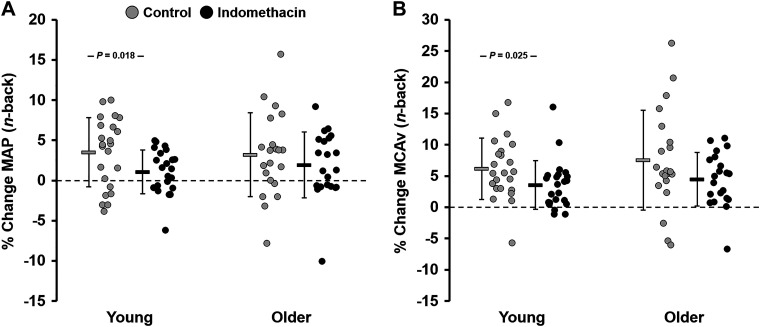
At baseline, before the start of the n-back test, there were no age-related differences in MAP, PP, or HR (Table 3). MCAv and CVCi were significantly lower, whereas cerebral PI was significantly higher in older adults compared with young adults (Table 3). In response to the n-back test, there were no age-related differences in PP or HR values (Table 3); however, MAP and cerebral PI values were significantly greater and both MCAv and CVCi values were significantly lower in older adults compared with young adults (Table 3). When these values were expressed as a percent change from baseline, the change in HR (young: 14 ± 8.7% vs. older: 9.0 ± 8.1%; P = 0.019, d = 0.65) was greater in young adults compared with older adults in response to the n-back test. In response to the n-back test, there were no age-related differences in the percent change in MAP (young: 3.5 ± 4.4% vs. older: 3.2 ± 5.2%; P = 0.435, d = 0.06), PP (young: 3.8 ± 5.6% vs. older: 2.2 ± 9.5%; P = 0.255, d = 0.20), MCAv (young: 6.2 ± 5.0% vs. older: 7.4 ± 8.0%; P = 0.272, d = 0.21), CVCi (young: 2.9 ± 5.8% vs. older: 4.5 ± 7.1%; P = 0.208, d = 0.24), or cerebral PI (young: −4.0 ± 5.9% vs. older: −4.3 ± 6.1%; P = 0.437, d = 0.06) (Fig. 3).
Table 3.
Cerebrovascular and hemodynamic variables during the n-back working memory test
| n-Back Working Memory Test | Young Adults | Older Adults | P Value (Effect Size) |
|---|---|---|---|
| MAP, mmHg | |||
| Control | |||
| Baseline | 91 ± 13 | 97 ± 13 | 0.054 (0.49) |
| n-Back | 94 ± 12 | 100 ± 12 | 0.046 (0.52) |
| Indomethacin | |||
| Baseline | 92 ± 13 | 98 ± 14 | 0.066 (0.46) |
| n-Back | 93 ± 13 | 100 ± 15 | 0.044 (0.52) |
| PP, mmHg | |||
| Control | |||
| Baseline | 74 ± 16 | 77 ± 14 | 0.289 (0.17) |
| n-Back | 77 ± 16 | 78 ± 11 | 0.431 (0.05) |
| Indomethacin | |||
| Baseline | 74 ± 14 | 76 ± 13 | 0.304 (0.16) |
| n-Back | 75 ± 14 | 77 ± 13 | 0.284 (0.17) |
| HR, beats/min | |||
| Control | |||
| Baseline | 59 ± 7 | 60 ± 8 | 0.292 (0.17) |
| n-Back | 67 ± 10 | 66 ± 11 | 0.282 (0.18) |
| Indomethacin | |||
| Baseline | 56 ± 8 | 56 ± 8 | 0.494 (0.01) |
| n-Back | 59 ± 8 | 60 ± 8 | 0.453 (0.04) |
| MCAv, cm/s | |||
| Control | |||
| Baseline | 60 ± 14 | 51 ± 16 | 0.022 (0.63) |
| n-Back | 64 ± 14 | 54 ± 16 | 0.022 (0.63) |
| Indomethacin | |||
| Baseline | 44 ± 9* | 38 ± 8* | 0.013 (0.70) |
| n-Back | 46 ± 9* | 40 ± 9* | 0.018 (0.65) |
| CVCi, cm/s/mmHg | |||
| Control | |||
| Baseline | 0.67 ± 0.19 | 0.52 ± 0.17 | 0.004 (0.85) |
| n-Back | 0.69 ± 0.19 | 0.55 ± 0.17 | 0.006 (0.80) |
| Indomethacin | |||
| Baseline | 0.49 ± 0.12* | 0.40 ± 0.12* | 0.006 (0.79) |
| n-Back | 0.50 ± 0.12* | 0.41 ± 0.12* | 0.007 (0.78) |
| Cerebral PI, AU | |||
| Control | |||
| Baseline | 0.71 ± 0.12 | 0.78 ± 0.12 | 0.030 (0.58) |
| n-Back | 0.67 ± 0.10 | 0.74 ± 0.10 | 0.016 (0.67) |
| Indomethacin | |||
| Baseline | 0.85 ± 0.15* | 0.90 ± 0.13* | 0.147 (0.32) |
| n-Back | 0.84 ± 0.16* | 0.88 ± 0.13* | 0.202 (0.26) |
Values are expressed as means ± SD. Cerebral PI, cerebral pulsatility index; CVCi, cerebrovascular conductance index; HR, heart rate; MAP, mean arterial blood pressure; MCAv, middle cerebral artery blood velocity; PP, pulse pressure. P value in bold indicates a significant difference between young and older adults at baseline or in response to the n-back working memory test (independent-samples t test or Wilcoxon rank-sum test). *P < 0.05 significantly different vs. control at baseline or in response to the n-back working memory test (dependent-samples t test or Wilcoxon signed-rank test).
Figure 3.
Percent change (% change) in cardiovascular and cerebrovascular variables in response to the n-back working memory test before (control) and after (indomethacin) administration of indomethacin. Values are expressed as means % change ± SD, and individual data points are provided. A: percent change in mean arterial blood pressure (MAP). B: percent change in middle cerebral artery blood velocity (MCAv). P value indicates a significant difference between young (n = 23; 10 M, 13 F) and older adults (n = 21; 11 M, 10 F) (independent-samples t test or Wilcoxon rank-sum test) or within groups across conditions (dependent-samples t test or Wilcoxon signed-rank test) in response to the n-back working memory test.
Effects of Indomethacin on Baseline Hemodynamics
The effects of INDO administration are shown in Table 2. In young adults, administration of INDO had no effect on MAP (P = 0.682, d = 0.08) or PP (P = 0.533, d = 0.09) but resulted in a decrease in HR (P < 0.001, d = 0.63), MCAv (P < 0.001, d = 1.6), and CVCi (P < 0.001, d = 1.4) compared with control (Table 2). In addition, INDO resulted in an increase in cerebral PI in young adults compared with control (P < 0.001, d = 1.1; Table 2). Similarly, in older adults, administration of INDO had no effect on MAP (P = 0.326, d = 0.17) or PP (P = 0.332, d = 0.20) but resulted in a decrease in HR (P = 0.021, d = 0.43), MCAv (P < 0.001, d = 1.1), and CVCi (P < 0.001, d = 1.0) compared with control (Table 2). INDO resulted in an increase in cerebral PI in older adults compared with control (P < 0.001, d = 1.1; Table 2).
Effects of Indomethacin on the Response to Cognitive Testing
During INDO, before the start of the Stroop test, there were no age-related differences in baseline MAP, PP, HR, or cerebral PI (Table 2); however, MCAv and CVCi were significantly lower in older adults compared with young adults (Table 2). In response to the Stroop test during INDO, there were no age-related differences in MAP, PP, HR, or cerebral PI values (Table 2); however, MCAv and CVCi values were significantly lower in older adults compared with young adults (Table 2). There were no age-related differences in the percent change in MAP (young: 0.3 ± 3.6% vs. older: 2.1 ± 6.4%; P = 0.124, d = 0.38), HR (young: 9.4 ± 11% vs. older: 12 ± 13%; P = 0.207, d = 0.25), MCAv (young: 2.0 ± 6.4% vs. older: 4.4 ± 5.4%; P = 0.095, d = 0.42), CVCi (young: 2.1 ± 6.6% vs. older: 2.8 ± 5.9%; P = 0.371, d = 0.12), or cerebral PI (young: 2.1 ± 1.3% vs. older: −0.6 ± 1.0%; P = 0.068, d = 0.44) in response to the Stroop test (Fig. 2). During INDO, young adults had a greater percent change in PP (young: 4.0 ± 6.4% vs. older: −0.1 ± 7.2%; P = 0.027, d = 0.60) in response to the Stroop test compared with older adults. During INDO, young adults had a similar percent change in MAP (control: 2.0 ± 4.9% vs. INDO: 0.3 ± 3.6%; P = 0.069, d = 0.46) and MCAv (control: 2.2 ± 6.8% vs. INDO: 2.0 ± 6.41.3%; P = 0.465, d = 0.02) in response to the Stoop test compared with control (Fig. 2). Older adults had a significantly lower percent change in MAP (control: 4.8 ± 4.9% vs. INDO: 2.1 ± 6.4%; P = 0.020, d = 0.48) and a similar percent change in MCAv (control: 5.9 ± 5.8% vs. INDO: 4.4 ± 5.4%; P = 0.173, d = 0.28) in response to the Stroop test compared with control (Fig. 2).
During INDO, before the start of the n-back test, there were no age-related differences in baseline MAP, PP, HR, or cerebral PI (Table 3); however, MCAv and CVCi were significantly lower in older adults compared with young adults (Table 3). In response to the n-back test during INDO, there were no age-related differences in PP, HR, or cerebral PI values (Table 3); however, MAP was significantly greater, whereas both MCAv and CVCi values remained significantly lower in older adults compared with young adults (Table 3). There were no age-related differences in the percent change in MAP (young: 1.0 ± 2.8% vs. older: 1.7 ± 4.1%; P = 0.263, d = 0.25), PP (young: 1.6 ± 4.7% vs. older: 1.8 ± 6.2%; P = 0.463, d = 0.04), HR (young: 7.2 ± 7.9% vs. older: 7.7 ± 9.0%; P = 0.416, d = 0.06), MCAv (young: 3.6 ± 3.8% vs. older: 4.5 ± 4.4%; P = 0.231, d = 0.22), CVCi (young: 2.6 ± 4.6% vs. older: 2.8 ± 4.2%; P = 0.440, d = 0.02), or cerebral PI (young: −0.7 ± 4.3% vs. older: −1.8 ± 3.6%; P = 0.191, d = 0.28) (Fig. 3). During INDO, young adults had a significantly lower percent change in MAP (control: 3.5 ± 4.4% vs. INDO: 1.0 ± 2.8%; P = 0.009, d = 0.67) and MCAv (control: 6.2 ± 5.0% vs. INDO: 3.6 ± 3.8%; P = 0.013, d = 0.59) in response to the n-back test compared with control (Fig. 3). There were no differences in the percent change in MAP (control: 3.2 ± 5.2% vs. INDO: 1.7 ± 4.1%; P = 0.101, d = 0.27) or MCAv (control: 7.4 ± 8.0% vs. INDO: 4.5 ± 4.4%; P = 0.070, d = 0.48) in response to the n-back test during INDO in older adults compared with control (Fig. 3).
Associations between Arterial Stiffness and NVC or Hemodynamic Responses to Cognitive Testing
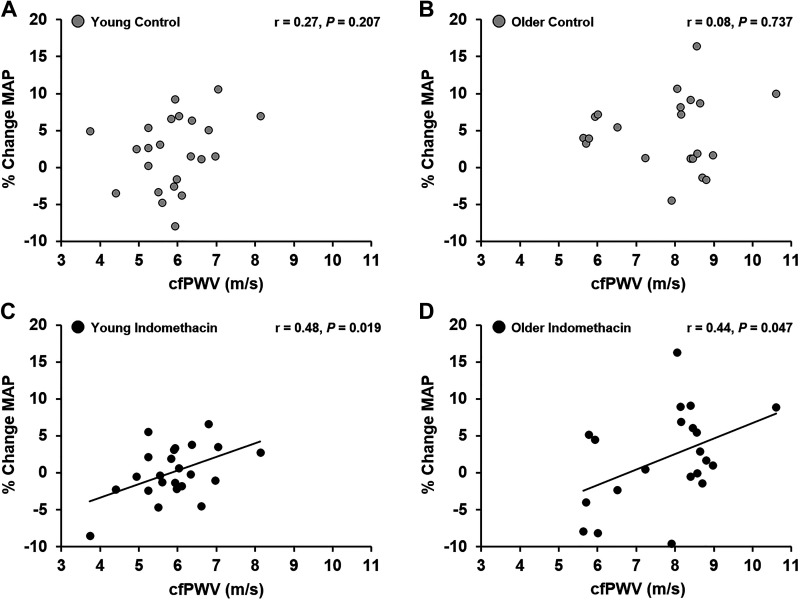
There was no association between cfPWV and the percent change in MAP in response to the Stroop test in young (Fig. 4A) or older adults (Fig. 4B). In addition, there was no association between cfPWV and the percent change in MAP in response to the n-back test in young (r = 0.33, P = 0.120) or older (r = 0.04, P = 0.856) adults. There were no associations between cfPWV and the percent change in HR or MCAv in response to the Stroop or n-back test in young or older adults. During INDO, there was a significant association between baseline cfPWV (which was measured during control condition) and the percent change in MAP in response to the Stroop test in young (Fig. 4C) and older adults (Fig. 4D). During INDO, there was no association between cfPWV and the percent change in MAP in response to the n-back test in young (r = 0.17, P = 0.451) or older (r = 0.06, P = 0.806) adults. There were no associations between cfPWV and the percent change in HR or MCAv in response to the Stroop or n-back test in young or older adults during INDO.
Figure 4.
Associations between percent change in mean arterial blood pressure (% change MAP) in response to the Stroop color and word test and carotid-femoral pulse wave velocity (cfPWV) at rest. A: young adults (n = 23; 10 M, 13 F) (control); B: older adults (n = 21; 11 M, 10 F) (control); C: young adults (n = 23; 10 M, 13 F) (indomethacin); D: older adults (n = 21; 11 M, 10 F) (indomethacin). P value indicates a significant association between cfPWV and the MAP response to the Stroop color and word test (Pearson correlation).
DISCUSSION
The primary aim of this study was to evaluate age-related differences in NVC and the hemodynamic response to acute cognitive challenges before and after administration of a COX inhibitor. The main findings of the present study are that older adults have an augmented NVC and hemodynamic response to the Stroop test compared with young adults; however, no age-related differences were observed in response to the n-back test. These results indicate that older adults have an augmented NVC and hemodynamic response to a test of executive function compared with young adults. COX inhibition reduced cerebral blood velocity by 30% in young and 26% in older adults; however, there were no age-related differences in NVC and hemodynamic responses to the Stroop or n-back tests during INDO. Taken together, the results of the present study suggest that older adults require a greater increase in MAP and MCAv to accomplish a cognitive test assessing executive function.
Previous studies have reported NVC and hemodynamic changes in response to cognitive challenges in young and older adults (22, 24, 25). The results of the present study build upon existing literature in this area by investigating, for the first time to our knowledge, age-related differences in NVC and hemodynamics before and after COX inhibition. In contrast to work conducted by Heffernan et al. (25), we observed age-related differences in MCAv values at rest and in response to both cognitive challenges, which may be attributed to the young adults being 10 yr younger (25 ± 4 yr in our study vs. 35 ± 10 yr in the study by Heffernan et al.). Furthermore, we report no age-related differences in cerebral PI in response to the Stroop test, whereas Heffernan et al. (25) reported greater cerebral PI in response to the Stroop test in older adults compared with young adults. However, in the present study, older adults demonstrated a greater cerebral PI response to the n-back test compared with young adults, suggesting that the modality of the cognitive challenge may impact the results. Specifically, older adults demonstrated a larger increase in MAP and MCAv only in response to the Stroop test compared with young adults during the control condition.
With advancing age, differential activation patterns of brain regions have been observed during the Stroop (43), n-back (44, 45), and other tests of working memory (46), possibly due to a change in strategy to accomplish the task (47). Furthermore, cognitive performance on such tasks declines with age (44, 45, 48, 49). Together, these age-related differences suggest that older adults activate different areas of the brain, and possibly to a greater degree, to accomplish the same cognitive challenge compared with young adults. Indeed, older adults demonstrate a greater percent change in blood flow in response to the Stroop test measured using functional magnetic resonance imaging (fMRI) than young adults (43). In addition to NVC and hemodynamic differences observed in this study, the type of cognitive activity presented by each test stimulates different regions of the brain as the Stroop test is a measure of executive function, whereas the n-back test is a measure of working memory. Previous works evaluating changes in regional cerebral blood flow during the Stroop test using positron emission tomography have shown activation of anterior regions of the brain (50, 51), such as the anterior cingulate cortex (52). In addition, compared with young adults, older adults activate different areas of the brain during the Stroop test as measured by fMRI, suggesting a shift in strategy to accomplish the task (43). Completion of the n-back test results in activation of several areas of the brain including the premotor cortices, prefrontal cortices, and posterior parietal cortices (40). Similarly, age-related differences in brain activation patterns have been observed during the n-back and other tests of working memory (44–46, 53–55). Specifically, older adults demonstrate an inability to augment prefrontal cortex activity as the difficulty of the task increases when compared with young adults (44, 54). These differences may be explained by diminished functional coupling between regions of the brain (53) and problems with sustained attention during progressive increases in difficulty of the n-back test (56). Taken together, the shift in processing strategy and differential activation patterns of areas of the brain with advancing age may explain the differences in MAP and MCAv responses observed between cognitive challenges in this study.
The use of NSAIDs and COX inhibitors has increased over time (57), and the existing literature on the relationship between COX inhibition and cardiovascular risk is conflicting (58). Previous studies suggest that some COX inhibitors increase cardiovascular risk (59), whereas others suggest no elevated cardiovascular risk (60, 61). Aspirin use is not associated with changes in cognitive function (28, 29); however, given that a previous study has shown that INDO administration resulted in an impairment in cognitive performance (62), it is important to consider the acute effects on NVC. INDO inhibits the COX enzyme, which blocks the production of PGI2. PGI2 is a powerful vasodilator that interacts with circulating platelets throughout the vasculature; however, the vasodilatory effects of PGI2 are attenuated in normal aging (34). Previous work from our laboratory has shown that age-related differences in MCAv and cerebrovascular reactivity to hypercapnia were abolished with INDO, suggesting that the role of vasodilating prostaglandins to augment cerebral blood flow decreases with age (32). The present study extends the existing literature on the effects of COX inhibition by investigating age-related differences in the response to cognitive challenges. During the control condition, we observed age-related differences in NVC and the hemodynamic response to the Stroop test, but no differences in response to the n-back test. Consistent with our previous work, COX inhibition decreased resting MCAv and CVCi in both young and older adults by 26%–30% and 28%–31%, respectively (32). Here, we show that COX inhibition abolishes age-related differences in NVC and the hemodynamic response to the Stroop test. When comparing young and older adults, COX inhibition did not significantly impact NVC or the hemodynamic response to the n-back test. Yet, in young adults, the percent change in MAP and MCAv in response to the n-back test was lower during INDO compared with the control condition. This suggests that young adults may rely more on vasodilating prostaglandins compared with older adults to complete a test of working memory. Taken together, COX inhibition abolished age-related differences in NVC and the hemodynamic response to a test of executive function. These findings add to our previous research demonstrating that age-related differences in cerebrovascular reactivity are abolished following COX inhibition (32).
We also sought to investigate associations between arterial stiffness and NVC or hemodynamic responses to cognitive challenges before and during COX inhibition. There were no significant associations between cfPWV and NVC or hemodynamic responses to either cognitive challenge during the control condition. Yet, there was a positive association between baseline cfPWV and the MAP response to the Stroop test during COX inhibition in both young and older adults. This positive association was not apparent in response to the n-back test in either group during COX inhibition. These data could suggest that individuals with higher arterial stiffness may rely more on increases in MAP to augment cerebral blood velocity during the Stroop test. It is important to note that these associations between arterial stiffness and the MAP response to the Stroop test were only observed during COX inhibition.
Although we did not observe age-related differences in resting MAP during COX inhibition, we have previously shown that COX inhibition augmented central blood pressure and increased aortic augmentation index in older adults (35). The associations between cfPWV and the MAP response to the Stroop test during COX inhibition could be a compensatory response to the effect of INDO on MCAv. Increases in MAP may be necessary to elevate cerebral blood flow during neuronal activation. However, the ability to augment cerebral blood flow via vasodilation of the microvessels may be influenced by arterial stiffness and other vasodilators affecting the endothelium including nitric oxide (NO) and prostaglandins (63). During COX inhibition, MCAv is lower, yet the percent increase in MCAv in response to the Stroop test was similar to the control condition, suggesting a different mechanism to ensure adequate NVC during INDO. The finding that age-related differences in the MAP response to the Stroop test were abolished following COX inhibition may partially explain the positive correlation between cfPWV and the MAP response to the Stroop test during INDO. This observation suggests that adults with higher arterial stiffness augment MAP to a greater degree to ensure adequate NVC during a test of executive function when prostaglandin production is inhibited. Furthermore, as arterial stiffness increases with age, greater reliance on blood pressure to maintain homeostatic cerebral blood flow may be one possible mechanism explaining the progressive increase in blood pressure with age (64).
Methodological Limitations
There were several strengths of the study. First, evaluating NVC and changes in hemodynamics using the average response of two levels of difficulty of two distinct cognitive tests. Although the two different cognitive challenges evoke differential activation patterns in the brain, the average NVC and hemodynamic response were highly correlated, which indicates that these responses are not driven by outliers. Second, beat-by-beat measurement of both MCAv and MAP to evaluate changes during cognitive challenges may provide a more accurate measurement compared with measurements taken periodically during the test (e.g., every 30 s, 1 min, or sporadically during the cognitive challenge), which previous studies have used. Third, to our knowledge, this is the first study to evaluate the effects of COX inhibition on NVC and hemodynamics in response to acute cognitive tests, which has implications for aging humans.
There were methodological limitations of the study, which include the use of TCD to measure MCAv, as this assumes that the diameter of the MCA is relatively constant as long as the insonation angle and depth were consistent. Previous studies evaluating changes in MCA diameter using MRI have revealed conflicting evidence suggesting the MCA diameter may change during a vasoactive stimulus (65–68). To our knowledge, there have been no studies evaluating changes in MCA diameter during an acute cognitive challenge. Furthermore, previous studies evaluating the hemodynamic response to cognitive challenges have used TCD to measure changes in MCAv (22, 24, 25, 69, 70). COX inhibition may affect MCA diameter, as INDO has been shown to decrease cerebral blood flow and relax pial and cerebral arteries in animal models (71, 72). In humans, INDO administration decreases MCAv and increases cerebral PI (30), which may suggest a vasoconstrictor response. Another limitation is that we did not score the cognitive challenges, although participants were not informed of this until after the completion of the study. Participants were encouraged to complete the cognitive challenge to the best of their ability. As the purpose of this study was to evaluate changes in hemodynamics in response to acute cognitive challenges, evaluating cognitive performance was not included because of the inability to accurately quantify cognitive function and the validity of these test scores while participants are fully instrumented lying in the supine position. Moreover, participants were instructed to provide answers to the cognitive challenges verbally and quietly to minimize jaw displacement. In addition, the quality of the signal was closely monitored, and the Doppler probe was secured with a headband to minimize changes in depth, angle, and position of the probe. Due to the verbal responses, accurate ETCO2 could not be measured during cognitive tests; therefore, only ETCO2 at rest is included.
Perspectives and Significance
Older adults demonstrated an augmented NVC and hemodynamic response to a test of executive function compared with young adults. COX inhibition decreased resting MCAv and CVCi in both young and older adults. However, during INDO, there were no age-related differences in NVC and hemodynamic responses to a test of executive function or working memory. There was a positive association between baseline cfPWV and the magnitude of the pressor response to the Stroop test in young and older adults during INDO. Collectively, these results suggest that older adults may need to augment MCAv and MAP to a greater degree when engaging in a test of executive function. In addition, individuals with higher central arterial stiffness, regardless of age, may rely more on augmenting blood pressure to ensure adequate cerebral perfusion during COX inhibition, which reduces cerebral blood velocity by ∼26%–30%. Understanding the effects of acute cognitive challenges on the vasculature and subsequent changes in NVC and hemodynamics may be important contributors to cognitive decline with advancing age. Further investigation into age-related differences in NVC and cerebral blood flow regulation and the role of COX during physiological stressors is warranted to understand the potential long-term effects on cerebrovascular health.
GRANTS
This study was funded by the National Institutes of Health Grants HL118154 (to J.N.B.) and HL007936 (to K.B.M.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.N.B. conceived and designed research; A.G.P., K.B.M., A.T.C., N.A.E., A.J.H., A.E.C., M.W.E., and J.N.B. performed experiments; A.G.P. analyzed data; A.G.P. and J.N.B. interpreted results of experiments; A.G.P. prepared figures; A.G.P. and J.N.B. drafted manuscript; A.G.P., K.B.M., A.T.C., N.A.E., A.J.H., A.E.C., M.W.E., and J.N.B. edited and revised manuscript; A.G.P., K.B.M., A.T.C., N.A.E., A.J.H., A.E.C., M.W.E., and J.N.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ronée Harvey and Samuel Gallo for help with data collection.
REFERENCES
- 1.Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med 29: 737–752, 2013. doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farokhian F, Yang C, Beheshti I, Matsuda H, Wu S. Age-related gray and white matter changes in normal adult brains. Aging Dis 8: 899–909, 2017. doi: 10.14336/AD.2017.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramanoël S, Hoyau E, Kauffmann L, Renard F, Pichat C, Boudiaf N, Krainik A, Jaillard A, Baciu M. Gray matter volume and cognitive performance during normal aging. A voxel-based morphometry study. Front Aging Neurosci 10: 235, 2018. doi: 10.3389/fnagi.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol 60: 989–994, 2003. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- 5.Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex 14: 721–730, 2004. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 6.Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MPJ, Evans AC, Jolles J, Uylings HBM. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage 17: 657–669, 2002. doi: 10.1006/nimg.2002.1173. [DOI] [PubMed] [Google Scholar]
- 7.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 5: 347–360, 2004. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 8.Vernooij MW, Van Der Lugt A, Ikram MA, Wielopolski PA, Vrooman HA, Hofman A, Krestin GP, Breteler MMB. Total cerebral blood flow and total brain perfusion in the general population: The Rotterdam Scan Study. J Cereb Blood Flow Metab 28: 412–419, 2008. doi: 10.1038/sj.jcbfm.9600526. [DOI] [PubMed] [Google Scholar]
- 9.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: 2672–2713, 2011. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000. doi: 10.1161/01.CIR.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 11.Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88: 1456–1462, 1993. doi: 10.1161/01.CIR.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 12.Tarumi T, Shah F, Tanaka H, Haley AP. Association between central elastic artery stiffness and cerebral perfusion in deep subcortical gray and white matter. Am J Hypertens 24: 1108–1113, 2011. doi: 10.1038/ajh.2011.101. [DOI] [PubMed] [Google Scholar]
- 13.Tsao CW, Seshadri S, Beiser AS, Westwood AJ, DeCarli C, Au R, Himali JJ, Hamburg NM, Vita JA, Levy D, Larson MG, Benjamin EJ, Wolf PA, Vasan RS, Mitchell GF. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology 81: 984–991, 2013. doi: 10.1212/WNL.0b013e3182a43e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell GF, Van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: The Age, Gene/Environment Susceptibility-Reykjavik Study. Brain 134: 3398–3407, 2011. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes JN, Harvey RE, Zuk SM, Lundt ES, Lesnick TG, Gunter JL, Senjem ML, Shuster LT, Miller VM, Jack CR, Joyner MJ, Kantarci K. Aortic hemodynamics and white matter hyperintensities in normotensive postmenopausal women. J Neurol 264: 938–945, 2017. doi: 10.1007/s00415-017-8476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazzouri AA, Newman AB, Simonsick E, Sink KM, Tyrrell KS, Watson N, Satterfield S, Harris T, Yaffe K; Health ABC Study. Pulse wave velocity and cognitive decline in elders: The health, aging, and body composition study. Stroke 44: 388–393, 2013. doi: 10.1161/STROKEAHA.112.673533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scuteri A, Tesauro M, Appolloni S, Preziosi F, Brancati AM, Volpe M. Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J Hypertens 25: 1035–1040, 2007. doi: 10.1097/HJH.0b013e3280895b55. [DOI] [PubMed] [Google Scholar]
- 18.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 100: 328–335, 2006. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 19.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol 312: H1–H20, 2017. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci 18: 419–434, 2017. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Roos A, Van Der Grond J, Mitchell G, Westenberg J. Magnetic resonance imaging of cardiovascular function and the brain: is dementia a cardiovascular-driven disease? Circulation 135: 2178–2195, 2017. doi: 10.1161/CIRCULATIONAHA.116.021978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heffernan KS, Spartano NL, Augustine JA, Lefferts WK, Hughes WE, Mitchell GF, Jorgensen RS, Gump BB. Carotid artery stiffness and hemodynamic pulsatility during cognitive engagement in healthy adults: a pilot investigation. Am J Hypertens 28: 615–622, 2015. doi: 10.1093/ajh/hpu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoemaker LN, Wilson LC, Lucas SJE, Machado L, Cotter JD. Cerebrovascular regulation is not blunted during mental stress. Exp Physiol 104: 1678–1687, 2019. doi: 10.1113/EP087832. [DOI] [PubMed] [Google Scholar]
- 24.Lefferts WK, DeBlois JP, Barreira TV, Heffernan KS. Neurovascular coupling during cognitive activity in adults with controlled hypertension. J Appl Physiol (1985) 125: 1906–1916, 2018. doi: 10.1152/japplphysiol.00100.2018. [DOI] [PubMed] [Google Scholar]
- 25.Heffernan KS, Augustine JA, Lefferts WK, Spartano NL, Hughes WE, Jorgensen RS, Gump BB. Arterial stiffness and cerebral hemodynamic pulsatility during cognitive engagement in younger and older adults. Exp Gerontol 101: 54–62, 2018. doi: 10.1016/j.exger.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Wagerle LC, Mishra OP. Mechanism of CO2 response in cerebral arteries of the newborn pig: role of phospholipase, cyclooxygenase, and lipoxygenase pathways. Circ Res 62: 1019–1026, 1988. doi: 10.1161/01.res.62.5.1019. [DOI] [PubMed] [Google Scholar]
- 27.Wagerle LC, Degiulio PA. Indomethacin-sensitive CO2 reactivity of cerebral arterioles is restored by vasodilator prostaglandin. Am J Physiol Heart Circ Physiol 266: H1332–H1338, 1994. doi: 10.1152/ajpheart.1994.266.4.h1332. [DOI] [PubMed] [Google Scholar]
- 28.Price JF, Stewart MC, Deary IJ, Murray GD, Sandercock P, Butcher I, Fowkes FGR; AAA Trialists. Low dose aspirin and cognitive function in middle aged to elderly adults: randomised controlled trial. BMJ 337: a1198, 2008. doi: 10.1136/bmj.a1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veronese N, Stubbs B, Maggi S, Thompson T, Schofield P, Muller C, Tseng PT, Lin PY, Carvalho AF, Solmi M. Low-dose aspirin use and cognitive function in older age: a systematic review and meta-analysis. J Am Geriatr Soc 65: 1763–1768, 2017. doi: 10.1111/jgs.14883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markus HS, Vallance P, Brown MM. Differential effect of three cyclooxygenase inhibitors on human cerebral blood flow velocity and carbon dioxide reactivity. Stroke 25: 1760–1764, 1994. doi: 10.1161/01.STR.25.9.1760. [DOI] [PubMed] [Google Scholar]
- 31.Xie A, Skatrud JB, Morgan B, Chenuel B, Khayat R, Reichmuth K, Lin J, Dempsey JA. Influence of cerebrovascular function on the hypercapnic ventilatory response in healthy humans. J Physiol 577: 319–329, 2006. doi: 10.1113/jphysiol.2006.110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnes JN, Schmidt JE, Nicholson WT, Joyner MJ. Cyclooxygenase inhibition abolishes age-related differences in cerebral vasodilator responses to hypercapnia. J Appl Physiol (1985) 112: 1884–1890, 2012. doi: 10.1152/japplphysiol.01270.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh N, Prasad S, Singer DRJ, Macallister RJ. Ageing is associated with impairment of nitric oxide and prostanoid dilator pathways in the human forearm. Clin Sci (Lond) 102: 595–600, 2002. doi: 10.1042/CS20010262. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson WT, Vaa B, Hesse C, Eisenach JH, Joyner MJ. Aging is associated with reduced prostacyclin-mediated dilation in the human forearm. Hypertension 53: 973–978, 2009. doi: 10.1161/HYPERTENSIONAHA.108.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes JN, Casey DP, Hines CN, Nicholson WT, Joyner MJ. Cyclooxygenase inhibition augments central blood pressure and aortic wave reflection in aging humans. Am J Physiol Heart Circ Physiol 302: H2629–H2634, 2012. doi: 10.1152/ajpheart.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harvey RE, Barnes JN, Hart ECJ, Nicholson WT, Joyner MJ, Casey DP. Influence of sympathetic nerve activity on aortic hemodynamics and pulse wave velocity in women. Am J Physiol Heart Circ Physiol 312: H340–H346, 2017. doi: 10.1152/ajpheart.00447.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 57: 769–774, 1982. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 38.Dewitt LD, Wechsler LR. Current concepts of cerebrovascular disease transcranial Doppler. Stroke 19: 915–921, 1988. doi: 10.1161/01.str.19.7.915. [DOI] [PubMed] [Google Scholar]
- 39.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol 18: 643–662, 1935. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- 40.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 25: 46–59, 2005. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol 480: 361–368, 1994. doi: 10.1113/jphysiol.1994.sp020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z, Barnes SA, Sokolnicki LA, Snyder EM, Johnson BD, Turner ST, Joyner MJ, Eisenach JH. Beta-2 adrenergic receptor polymorphisms and the forearm blood flow response to mental stress. Clin Auton Res 16: 105–112, 2006. doi: 10.1007/s10286-006-0329-4. [DOI] [PubMed] [Google Scholar]
- 43.Zysset S, Schroeter ML, Neumann J, von Cramon DY. Stroop interference, hemodynamic response and aging: an event-related fMRI study. Neurobiol Aging 28: 937–946, 2007. doi: 10.1016/j.neurobiolaging.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 44.Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett 392: 32–37, 2006. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 45.Qin S, Basak C. Age-related differences in brain activation during working memory updating: an fMRI study. Neuropsychologia 138: 107335, 2020. doi: 10.1016/j.neuropsychologia.2020.107335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith EE, Geva A, Jonides J, Miller A, Reuter-Lorenz P, Koeppe RA. The neural basis of task-switching in working memory: effects of performance and aging. Proc Natl Acad Sci USA 98: 2095–2100, 2001. doi: 10.1073/pnas.98.4.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gajewski PD, Hanisch E, Falkenstein M, Thönes S, Wascher E. What does the n-Back task measure as we get older? Relations between working-memory measures and other cognitive functions across the lifespan. Front Psychol 9: 2208, 2018. doi: 10.3389/fpsyg.2018.02208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Der Elst W, Van Boxtel MPJ, Van Breukelen GJP, Jolles J. The Stroop color-word test influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment 13: 62–79, 2006. doi: 10.1177/1073191105283427. [DOI] [PubMed] [Google Scholar]
- 49.Bopp KL, Verhaeghen P. Aging and n-back performance: a meta-analysis. J Gerontol B Psychol Sci Soc Sci 75: 229–240, 2020. doi: 10.1093/geronb/gby024. [DOI] [PubMed] [Google Scholar]
- 50.Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RSJ, Dolan RJ. Investigations of the functional anatomy of attention using the stroop test. Neuropsychologia 31: 907–922, 1993. doi: 10.1016/0028-3932(93)90147-R. [DOI] [PubMed] [Google Scholar]
- 51.Larrue V, Celsis P, Bès A, Marc-Vergnes JP. The functional anatomy of attention in humans: cerebral blood flow changes induced by reading, naming, and the stroop effect. J Cereb Blood Flow Metab 14: 958–962, 1994. doi: 10.1038/jcbfm.1994.128. [DOI] [PubMed] [Google Scholar]
- 52.Derbyshire SWG, Vogt BA, Jones AKP. Pain and Stroop interference tasks activate separate processing modules in anterior cingulate cortex. Exp Brain Res 118: 52–60, 1998. doi: 10.1007/s002210050254. [DOI] [PubMed] [Google Scholar]
- 53.Podell JE, Sambataro F, Murty VP, Emery MR, Tong Y, Das S, Goldberg TE, Weinberger DR, Mattay VS. Neurophysiological correlates of age-related changes in working memory updating. Neuroimage 62: 2151–2160, 2012. doi: 10.1016/j.neuroimage.2012.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prakash RS, Heo S, Voss MW, Patterson B, Kramer AF. Age-related differences in cortical recruitment and suppression: implications for cognitive performance. Behav Brain Res 230: 192–200, 2012. doi: 10.1016/j.bbr.2012.01.058. [DOI] [PubMed] [Google Scholar]
- 55.Kennedy KM, Boylan MA, Rieck JR, Foster CM, Rodrigue KM. Dynamic range in BOLD modulation: lifespan aging trajectories and association with performance. Neurobiol Aging 60: 153–163, 2017. doi: 10.1016/j.neurobiolaging.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daffner KR, Chong H, Sun X, Tarbi EC, Riis JL, Mcginnis SM, Holcomb PJ. Mechanisms underlying age- and performance-related differences in working memory. J Cogn Neurosci 23: 1298–1314, 2011. doi: 10.1162/jocn.2010.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davis JS, Lee HY, Kim J, Advani SM, Peng HL, Banfield E, Hawk ET, Chang S, Frazier-Wood AC. Use of non-steroidal anti-inflammatory drugs in US adults: changes over time and by demographic. Open Heart 4: e000550, 2017. doi: 10.1136/openhrt-2016-000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Antman EM, DeMets D, Loscalzo J. Cyclooxygenase inhibition and cardiovascular risk. Circulation 112: 759–770, 2005. doi: 10.1161/CIRCULATIONAHA.105.568451. [DOI] [PubMed] [Google Scholar]
- 59.Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 332: 1302–1305, 2006. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Konstam MA, Weir MR, Reicin A, Shapiro D, Sperling RS, Barr E, Gertz BJ. Cardiovascular thrombotic events in controlled, clinical trials of rofecoxib. Circulation 104: 2280–2288, 2001. doi: 10.1161/hc4401.100078. [DOI] [PubMed] [Google Scholar]
- 61.Shaya FT, Blume SW, Blanchette CM, Weir MR, Mullins CD. Selective cyclooxygenase-2 inhibition and cardiovascular effects: an observational study of a medicaid population. Arch Intern Med 165: 181–186, 2005. doi: 10.1001/archinte.165.2.181. [DOI] [PubMed] [Google Scholar]
- 62.Shoemaker LN, Wilson LC, Lucas SJE, Machado L, Walker RJ, Cotter JD. Indomethacin markedly blunts cerebral perfusion and reactivity, with little cognitive consequence in healthy young and older adults. J Physiol 599: 1097–1113, 2020. doi: 10.1113/JP280118. [DOI] [PubMed] [Google Scholar]
- 63.Hoiland RL, Caldwell HG, Howe CA, Nowak‐Flück D, Stacey BS, Bailey DM, Paton JFR, Green DJ, Sekhon MS, Macleod DB, Ainslie PN. Nitric oxide is fundamental to neurovascular coupling in humans. J Physiol 598: 4927–4939, 2020. doi: 10.1113/JP280162. [DOI] [PubMed] [Google Scholar]
- 64.Warnert EAH, Rodrigues JCL, Burchell AE, Neumann S, Ratcliffe LEK, Manghat NE, Harris AD, Adams Z, Nightingale AK, Wise RG, Paton JFR, Hart EC. Is high blood pressure self-protection for the brain? Circ Res 119: e140–e151, 2016. doi: 10.1161/CIRCRESAHA.116.309493. [DOI] [PubMed] [Google Scholar]
- 65.Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol (1985) 117: 1090–1096, 2014. doi: 10.1152/japplphysiol.00285.2014. [DOI] [PubMed] [Google Scholar]
- 66.Miller KB, Howery AJ, Rivera-Rivera LA, Johnson SC, Rowley HA, Wieben O, Barnes JN. Age-related reductions in cerebrovascular reactivity using 4D flow MRI. Front Aging Neurosci 11: 281, 2019. doi: 10.3389/fnagi.2019.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000. doi: 10.1161/01.STR.31.7.1672. [DOI] [PubMed] [Google Scholar]
- 68.Verbree J, Bronzwaer ASGT, Ghariq E, Versluis MJ, Daemen MJAP, Van Buchem MA, Dahan A, Van Lieshout JJ, Van Osch MJP. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol (1985) 117: 1084–1089, 2014. doi: 10.1152/japplphysiol.00651.2014. [DOI] [PubMed] [Google Scholar]
- 69.Kelley RE, Chang JY, Scheinman NJ, Levin BE, Duncan RC, Lee SC. Transcranial Doppler assessment of cerebral flow velocity during cognitive tasks. Stroke 23: 9–14, 1992. doi: 10.1161/01.STR.23.1.9. [DOI] [PubMed] [Google Scholar]
- 70.Vingerhoets G, Stroobant N. Lateralization of cerebral blood flow velocity changes during cognitive tasks. Stroke 30: 2152–2158, 1999. doi: 10.1161/01.str.30.10.2152. [DOI] [PubMed] [Google Scholar]
- 71.Pickard JD. Role of prostaglandins and arachidonic acid derivatives in the coupling of cerebral blood flow to cerebral metabolism. J Cereb Blood Flow Metab 1: 361–384, 1981. doi: 10.1038/jcbfm.1981.41. [DOI] [PubMed] [Google Scholar]
- 72.McCulloch J, Kelly PAT, Grome JJ, Pickard JD. Local cerebral circulatory and metabolic effects of indomethacin. Am J Physiol Heart Circ Physiol 243: H416–H423, 1982. doi: 10.1152/ajpheart.1982.243.3.h416. [DOI] [PubMed] [Google Scholar]