Abstract
Peripheral artery disease (PAD) is characterized by the accumulation of atherosclerotic plaques in the lower extremity conduit arteries, which impairs blood flow and walking capacity. Dietary nitrate has been used to reduce blood pressure (BP) and improve walking capacity in PAD. However, a standardized dose for PAD has not been determined. Therefore, we sought to determine the effects of a body mass-normalized moderate dose of nitrate (0.11 mmol nitrate/kg) as beetroot juice on serum nitrate/nitrite, vascular function, walking capacity, and tissue oxygen utilization capacity in patients with PAD. A total of 11 patients with PAD received either nitrate supplement or placebo in a randomized crossover design. Total serum nitrate/nitrite, resting BP, brachial and popliteal artery endothelial function (flow-mediated dilation, FMD), arterial stiffness (pulse-wave velocity, PWV), augmentation index (AIx), maximal walking distance and time, claudication onset time, and skeletal muscle oxygen utilization were measured pre- and postnitrate and placebo intake. There were significant group × time interactions (P < 0.05) for serum nitrate/nitrite, FMD, BP, walking distance and time, and skeletal muscle oxygen utilization. The nitrate group showed significantly increased serum nitrate/nitrite (Δ1.32 μM), increased brachial and popliteal FMD (Δ1.3% and Δ1.7%, respectively), reduced peripheral and central systolic BP (Δ−4.7 mmHg and Δ−8.2 mmHg, respectively), increased maximal walking distance (Δ92.7 m) and time (Δ56.3 s), and reduced deoxygenated hemoglobin during walking. There were no changes in PWV, AIx, or claudication (P > 0.05). These results indicate that a body-mass normalized moderate dose of nitrate may be effective and safe for reducing BP, improving endothelial function, and improving walking capacity in patients with PAD.
Keywords: beetroot juice, exercise tolerance, nitrite, pulse-wave velocity, vascular function
INTRODUCTION
Peripheral artery disease (PAD) is a vascular disease in which atherosclerotic plaque accumulation in the conduit arteries attenuates blood flow and causes chronic ischemia, which is a lack of oxygen, to the lower extremities (1, 2). Early-stage PAD typically manifests as intermittent claudication (leg pain during walking); however, as the disease progresses, patients can experience ischemic leg pain at rest and develop plantar ulcers that may ultimately entail foot or limb amputation (1). These symptoms impair walking capacity and reduce overall quality of life (3). Determination of potential therapies for patients with PAD is a pressing matter, as alleviating symptoms and improving vascular function may subsequently improve quality of life and delay disease progression in this population.
It has been suggested that the endothelium plays critical roles in vascular homeostasis, and disturbances in endothelial function are thought to precede the manifestation of atherosclerotic diseases (4). Attenuated bioavailability of nitric oxide (NO), a potent vasodilator, is known to be associated with endothelial dysfunction (5, 6), and patients with PAD have commonly showed reduced NO bioavailability and endothelial dysfunction (7–9). Previous studies suggested that exogenous dietary nitrate (NO3−) intake can induce an increase in NO3− reserves in the body that can be reduced to bioactive NO (10–13), and recent studies have suggested that dietary NO3− intake increases NO bioavailability in patients with PAD (14, 15). Following dietary NO3− intake, patients with PAD have also experienced reduced blood pressure (BP) (14, 15), improved skeletal muscle oxygen utilization (15), vascular conductance of the lower limb (14), and exercise tolerance (14, 15) following NO3− ingestion. Despite these positive vascular and functional effects in patients with PAD, there have been no documented improvements in endothelial function following acute NO3− intake in this population (15). This absence of improved endothelial function in patients with PAD may be due to the fact that the previous study used the same dose for each participant, and there was a large variation in participant body mass (84.5 ± 16.5 kg) (15). To our knowledge, no studies have sought to establish a standardized dose of NO3− administration normalized per kg of body mass that can be used safely to efficiently improve endothelial function and walking capacity in patients with PAD. Therefore, the purpose of the present study was to examine the impacts of a consistent body mass-normalized acute moderate dose of NO3− in the form of beetroot juice on NO bioavailability, endothelial function, BP, arterial stiffness, walking capacity, and muscle oxygen utilization capacity in patients with PAD. Our hypothesis was threefold: 1) a moderate body-mass normalized dose of NO3− intake would increase serum NO bioavailability, which would in turn 2) improve endothelial function and reduce BP, which would contribute to 3) reduced arterial stiffness, improved walking capacity and exercise tolerance, and improved skeletal muscle oxygen utilization capacity in patients with PAD.
METHODS
Participants
Patients with PAD (n = 11, 5 males and 6 females) were recruited for study participation. Disease staging, claudication history, and ankle-brachial index of <0.90 were determined by both self-report and by physician diagnosis, and all participants were classified as Fontaine stage IIa and IIb (16). Participants were also required to have a stable BP, lipid, and/or diabetes regimen for ≥6 wk before participation, and all females were postmenopausal (no menses for ≥12 consecutive mo). Participants were excluded from the study if they had a history of pain at rest (Fontaine stage III) and/or tissue loss due to PAD (Fontaine stage IV), limited walking capacity due to conditions other than PAD, renal disease, already include a form of NO3− intake in their regimen, and/or had an allergy to beetroot juice. Participants were asked to withhold medications (at least 12 h before the visits) and were asked to refrain from mouthwash use for ≥ 24 h (17), as antibacterial mouthwash can negatively impact the oral reduction of NO3− to NO2− (18). Participants were also asked to track what they consumed the day before their first visit so they could consume the same foods before their second visit. In addition, participants were requested to not alter their dietary habits during the 2-wk washout period. All procedures were performed according with the protocols approved by the Institutional Review Board and performed in accordance with the Declaration of Helsinki. All participants provided written, informed consent before beginning the study. This study was registered with https://clinicaltrials.gov/ (NCT03506646).
Design
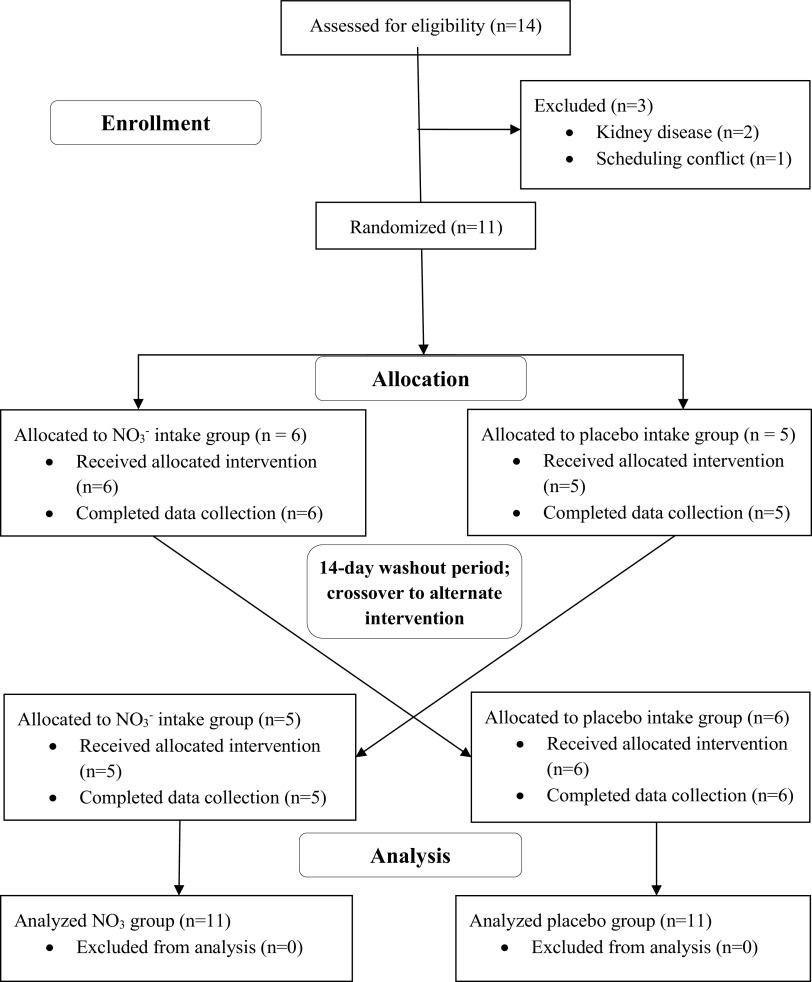
This study used a randomized, double-blinded, placebo-controlled, crossover design with a washout period of 14 days between the 2 study visits (Fig. 1). Participants were randomly assigned to receive either the body mass-normalized NO3− supplement (Beet It, James White Drinks, Ipswich, UK) or the nitrate-void placebo (PL). Our pilot data (unpublished) based on the low (∼0.05 ± 0.01 mmol NO3−/kg), moderate (∼0.11 ± 0.01 mmol NO3−/kg), and high doses (∼0.22 ± 0.03 mmol NO3−/kg) reported by Wylie et al. (19) showed that a low dose was not sufficient to produce a detectable improvement in endothelial function, and a high dose induced side effects such as nausea and gastrointestinal issues. We found that the moderate dose (∼0.11 mmol NO3−/kg) could elicit improvements in endothelial function without any adverse side effects. The dose per participant was calculated by multiplying body mass by 0.11 mmol, which was then used to calculate milliliters of supplement to be administered in a standard disposable drinking cup. The PL consisted of tapioca powder capsules that possessed no nitrate or antioxidant properties. Participants were informed that we were researching the effects of commercially available juice and pills on their vascular function and walking capacity; therefore, they were not aware that the capsules were PL. All assessments were performed after an overnight fast and at the same time of day (9:00 AM ± 1 h). Anthropometric measurements were obtained upon arrival at both visits. Following anthropometrics, baseline experimental measurements were conducted, after which the NO3− supplement or PL were consumed. All experimental measurements were repeated ∼1 h following ingestion, as NO3− intake in the form of beetroot juice has been reported to significantly increase concentrations of both NO3− and NO2− in the blood within 1 h after consumption (20, 21). All data analyses were performed in a blinded manner.
Figure 1.
Randomized placebo-controlled crossover design, participant allocation, and analysis. NO3−, nitrate.
Anthropometrics
Anthropometric measurements included height, body mass, and body composition. Height was measured using a stadiometer (nearest 0.5 cm). Body mass was measured using a standard scale (nearest 0.1 kg), and body mass index was calculated by taking the body mass divided by height squared (kg/m2). Body fat percentage was assessed using bioelectrical impedance analysis in duplicate to the nearest 0.1% (HBF – 306 C, Omron Healthcare, Lake Forest, IL), and the average of the 2 measures was recorded as the body fat percentage.
Resting Heart Rate and Blood Pressure
Participants sat for 15 min in a quiet and temperature-controlled room. Resting heart rate and BP were assessed in duplicate using an automated sphygmomanometer (HEM – FL 31, Omron Healthcare, Lake Forest, IL). The two measurements were taken with 5 min separating each measurement, and the average of the 2 was recorded as the resting heart rate and BP.
Blood Sampling
Blood was drawn (10 mL) from an antecubital vein with EDTA tubes before and after NO3− and PL intake. Samples were centrifuged at 3,500 rpm for 10 min at 4°C. Serum samples were stored in 2.0-mL microcentrifuge tubes and were stored at −80°C for later analysis of serum NO bioavailability. NO bioavailability [total NO3− and nitrite (NO2−)] was measured using a commercially available nitrate/nitrite colorimetric assay kit (Cat. No. 780001, Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions.
Endothelial Function
Endothelial function was assessed using brachial artery flow-mediated dilation (FMD) and popliteal artery FMD using a Doppler ultrasound (Terason uSmart 3300, Terason Division Teratech Corporation, Burlington, MA), rapid inflation cuff system (E20 Rapid Cuff and cuff model SC5, D.E. Hokanson, Bellevue, WA), and a 3-lead electrocardiogram system (7700 Series Trigger Monitor, IvyBiomedical Systems Inc., Branford, CT) as previously described (22). Briefly, for both brachial and popliteal assessments, the rapid inflation cuff was placed just distal to the antecubital fossa and just distal to the popliteal fossa, respectively (22). For both assessments, resting arterial diameter was recorded with the ultrasound probe ∼1–2 cm proximal to the rapid-inflation cuff for 5 min (22). The cuff was inflated to a pressure of 250 mmHg after baseline diameter was obtained and remained inflated for 5 min (22). The cuff was then deflated and the reactive hyperemic arterial response was recorded continuously on R-wave trigger for 5 min (22). Data were analyzed using an image capturing and automated edge-detection software (Vascular Imager, Vascular Research Tools 6, Medical Imaging Applications, Coralville, IA) as previously described (22). The relative artery diameter changes were calculated as previously described (22).
Arterial Stiffness and Pulse-Wave Analysis
Assessments of arterial stiffness, augmentation index (AIx), AIx adjusted to 75 beats/min (AIx@75), augmentation pressure, pulse pressure, and carotid-to-femoral pulse-wave velocity (PWV) were measured using applanation tonometry (SphygmoCor XCEL, AtCor Medical, Sydney, Australia) as previously described (22, 23). Briefly, AIx, AIx@75, augmentation pressure, and pulse pressure were assessed with a cuff placed on the upper arm between the elbow and shoulder to collect pressure waveforms which estimate central aortic pressure (22, 23). To assess carotid-to-femoral PWV, the carotid pulse was measured using applanation tonometry and femoral pulse waves were measured with a cuff placed on the upper leg between the hip and knee (22, 23).
Indices of peripheral arterial stiffness, carotid-to-radial PWV and carotid-to-ankle PWV, were assessed using applanation tonometry with participants in the supine position (Complior Analyze, Alam Medical, Saint-Quentin-Fallavier, France) as previously described (22, 23). Briefly, pulse waveforms for carotid-to-radial PWV and carotid-to-ankle PWV were recorded for 30–60 s (22, 23). Assessments of central systolic and diastolic BP, central pulse pressure, deceleration time, and maximum first derivative of pressure (dP/dtmax) were provided by the central pressure analysis (22, 23).
Walking Capacity and Skeletal Muscle Oxygen Utilization Capacity
Maximal walking capacity was assessed on a standard treadmill (Lode, B.V, Groningen, The Netherlands) using the modified Gardner protocol (22, 24). Participants were asked to notify the test administrators about their onset of claudication (the exact moment their leg pain starts) and to walk as long as they could tolerate even if the claudication persisted (maximal walking time and distance). The test was ended once patients could not tolerate their symptoms (i.e., severe claudication).
Gastrocnemius oxygen utilization capacity was measured with a portable near-infrared spectroscopy (NIRS) unit (Artinis PortaMon, Einsteinweg, The Netherlands) and Oxysoft software (v3.0.103.3, Einsteinweg, The Netherlands) during the exercise testing as previously described (22). Briefly, the NIRS device was adhered to the gastrocnemius, at the approximate location where the participant felt the most pain, using a commercially available double-sided adhesive and a commercially available black bandage to prevent extraneous light from reaching the device (15, 22). NIRS data were collected continuously at a frequency of 10 Hz and used to estimate the change in tissue saturation (StO2), deoxygenated hemoglobin concentration ([HHb]), and oxygenated hemoglobin concentration ([O2Hb]) at resting baseline and during the walking test. Upon walking test completion, the participant stood in a resting standing position and a thigh rapid inflation cuff (cuff model SC10, D.E. Hokanson, Bellevue, WA) was placed on the thigh ∼3–4 cm proximal to the knee on the limb with the NIRS device. The cuff was inflated to ∼250–260 mmHg for 5 min to deoxygenate the tissue with the same rapid inflation cuff system used for FMD (22, 25). The cuff pressure was released after 5 min, and the peak hyperemic response following cuff release was used to determine the 100% oxygenation level for each participant and was used to calculate normalized [HHb] (22, 25). The raw NIRS data were reduced to 1 Hz files and exported to Microsoft Excel files for later analysis.
Statistical Analysis
The Shapiro–Wilk test was used for all dependent variables to determine the normality of the data. Independent t tests were used to determine any differences between subject characteristics at the NO3− and PL visits. A two-way repeated measures analysis of variance (ANOVA) [group (NO3− and PL) × time (before and after supplement intake)] was used to compare the changes between NO3− and PL intake within and between groups on the dependent variables. When a significant main effect or interaction was found, paired t tests were used for post hoc comparisons for the normally distributed variables, and paired samples Wilcoxon tests were used for nonnormally distributed variables. Based on a power calculation from a previous study, a sample size of a minimum of total 14 (n = 7/group) would allow for 80% power to detect differences between FMD between NO3− versus PL (26). Associations between dependent variables were assessed using Pearson’s product-moment correlation coefficient. Effect size analyses were conducted using Cohen’s d and interpreted as 0.2 as a small effect size, 0.5 as a medium effect size, and 0.8 as a large effect size (22, 27). All analyses were performed using SPSS 26.0 (IBM, Armonk, NY). Descriptive characteristics are presented as means ± SD, and all other data are presented as means ± SE Statistical significance was set to P < 0.05.
RESULTS
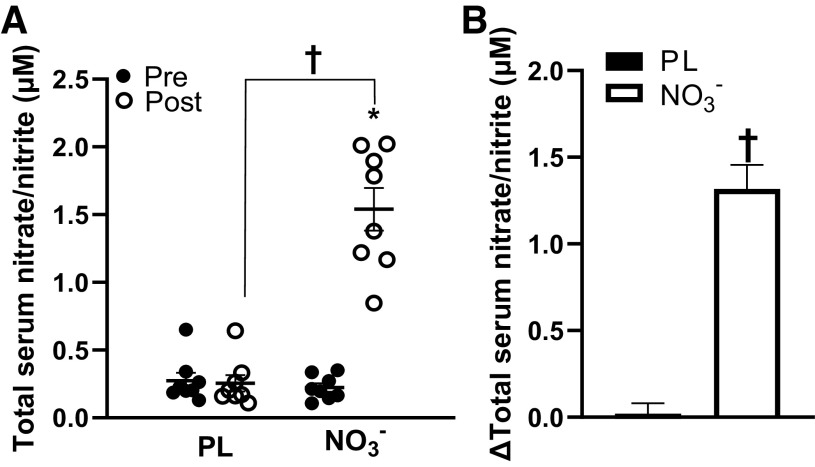
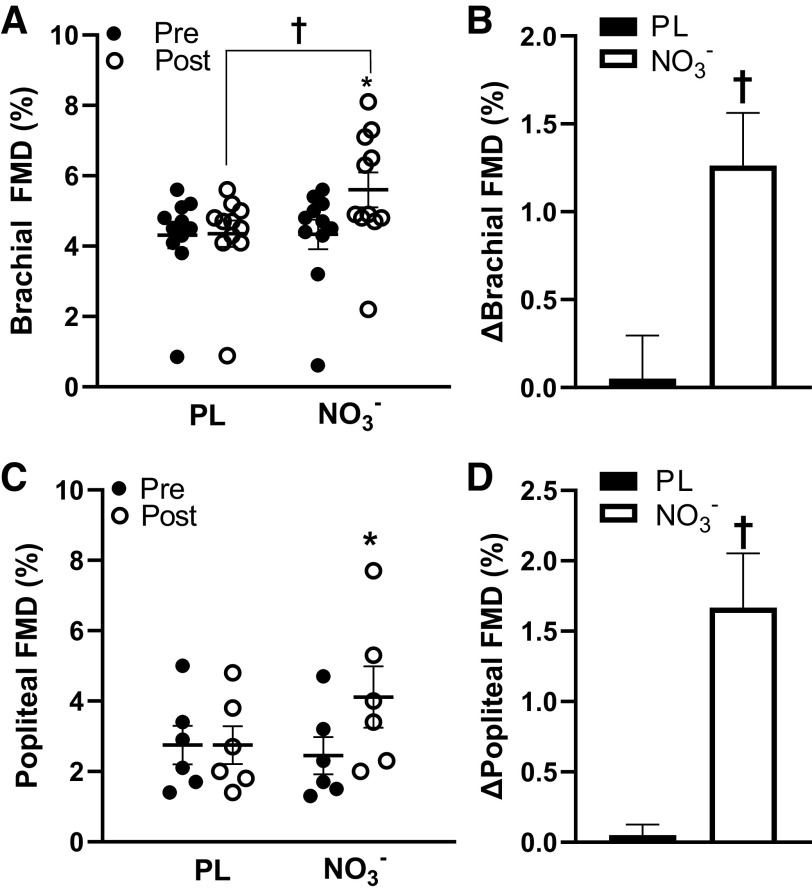
The moderate body mass-normalized dose of NO3− was tolerated well by all participants and no adverse side effects were reported (Table 1). The average dose of the supplement was ∼0.11 ± 0.01 mmol NO3−/kg, equating to ∼91.8 ± 20.0 mL of the beetroot juice supplement. Some participants reported beeturia (red-pink urine), which is consistent with previous studies (11, 19, 28). Participants presented with several other comorbidities, such as hypertension, prediabetes, dyslipidemia, and arthritis, and were taking medications for these comorbidities (Table 2). There were significant group × time interactions (P < 0.05) for serum total NO3− and NO2−, brachial and popliteal FMD, peripheral and central systolic BP, walking distance and time, and skeletal muscle oxygen utilization. Serum total NO3− and NO2− significantly increased (Δ1.32 ± 0.15 μM, P < 0.01) after NO3− intake compared with placebo (Fig. 2, A and B). Brachial artery FMD significantly increased (Δ1.3 ± 0.3%, P < 0.01), whereas central systolic BP was reduced (Δ−8.2 ± 3.1 mmHg, P = 0.014) after NO3− intake compared with placebo (Fig. 3, A and B, Table 3). Maximal walking distance also significantly increased (Δ92.7 ± 36.8 m, P = 0.029) after NO3− intake compared with placebo (Fig. 4, A and B). A time-effect was noted for reduced systolic BP (Δ−4.7 ± 1.4 mmHg, P = 0.021; Table 3) and increased popliteal FMD (Δ1.7 ± 0.4%, P < 0.01; Fig. 3, C and D). A group-effect was noted for maximal walking time (Δ56.3 ± 25.4 s, P = 0.015; Fig. 4, C and D). In addition, there were several significant changes between the NO3− intake and PL groups in oxygen utilization capacity. [HHb] was significantly lower following NO3− intake compared with PL at 60 s, 80 s, 100 s, 120 s, and 140 s during walking (Fig. 5A), but there were no significant differences in [StO2] or [HbO2] following NO3− intake compared with PL. There were no changes in resting heart rate, diastolic BP, central diastolic BP, deceleration time, dP/dtmax, peripheral or central arterial stiffness, AIx, AIx@75, augmentation pressure, pulse pressure, or time to onset of claudication (P > 0.05) following NO3− intake (Table 3, Fig. 4E).
Table 1.
Participant characteristics at the nitrate (NO3−) and placebo (PL) visits
| PL (n = 11) | NO3− (n = 11) | |
|---|---|---|
| Age, yr | 70.0 ± 7.0 | 70.0 ± 7.0 |
| Height, cm | 163.2 ± 14.0 | 163.2 ± 14.0 |
| Body mass, kg | 77.3 ± 16.5 | 77.0 ± 16.5 |
| BMI, kg/m2 | 29.1 ± 6.4 | 29.0 ± 6.0 |
| Body fat, % | 37.4 ± 9.4 | 37.6 ± 9.2 |
Values are presented as means ± SD. BMI, body mass index. Student’s t tests were used for comparison between the PL and NO3- groups.
Table 2.
Participant comorbidities, conditions, medications, and smoking history
| Comorbidity or Condition | n |
|---|---|
| Hypertension | 8 |
| Dyslipidemia | 10 |
| Prediabetes | 3 |
| Arthritis | 7 |
| Medications | |
| Angiotensin-converting enzyme inhibitors | 3 |
| Angiotensin II receptor blockers | 2 |
| Diabetic medication (metformin) | 3 |
| Beta blockers | 5 |
| Calcium channel blockers | 2 |
| Anticoagulants | 2 |
| Diuretics | 1 |
| Statins | 9 |
| Nonsteroidal antiinflammatory medication | 9 |
| Sleep aids | 3 |
| Smoking history | |
| Current | 1 |
| Former | 4 |
| Never | 6 |
Figure 2.
Total serum nitrate and nitrite levels (μM) pre- and post-placebo (PL) and nitrate (NO3−) intake. A: total serum nitrate/nitrite significantly increased post-NO3− and was significantly greater than post-PL (n = 8, d = 4.1) B: changes in total serum nitrate and nitrite levels in the PL and NO3− groups. Values are presented as means ± SE. Two-way repeated analysis of variance (ANOVA) [group (NO3− and PL) x time (before and after supplement intake)] with paired t tests for post hoc comparisons. Effect size analyses were conducted using Cohen’s d. *P < 0.01 vs. Pre †P < 0.01 vs. PL.
Figure 3.
Flow-mediated dilation (FMD, %) in the brachial and popliteal arteries pre- and post-placebo (PL) and nitrate (NO3−) intake. A: percent brachial artery FMD significantly increased post-NO3− and was significantly greater than post-PL (n = 11, d = 0.8). B: changes in brachial FMD in the PL and NO3− groups. C: percent popliteal artery FMD significantly increased post-NO3− (n = 6, d = 0.8). D: changes in popliteal FMD in the PL and NO3− groups. Values are presented as means ± SE Two-way repeated analysis of variance (ANOVA) [group (NO3− and PL) x time (before and after supplement intake)] with paired t tests for post hoc comparisons for normally distributed data and Wilcoxon tests for nonnormally distributed data. Effect size analyses were conducted using Cohen’s d. *P < 0.01 vs. Pre †P < 0.01 vs. PL.
Table 3.
Participant resting heart rate, blood pressures, and hemodynamic analyses pre- and postnitrate (NO3−) and placebo (PL) intake
| PL (n = 11) |
NO3− (n = 11) |
||||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Δ | Pre | Post | Δ | Cohen's d | |
| Resting heart rate, beats/min | 68.9 ± 3.8 | 69.0 ± 3.2 | 0.1 ± 0.8 | 70.0 ± 3.2 | 69.6 ± 3.4 | −0.4 ± 0.9 | 0.1 |
| Systolic BP, mmHg | 133.6 ± 4.7 | 134.8 ± 5.0 | 1.2 ± 1.9 | 133.2 ± 4.9 | 128.5 ± 4.9* | −4.7 ± 1.4 | 0.3 |
| Diastolic BP, mmHg | 81.2 ± 1.9 | 81.5 ± 2.0 | 0.3 ± 2.0 | 81.2 ± 1.8 | 79.2 ± 1.7 | −2.0 ± 1.3 | 0.4 |
| Central systolic BP, mmHg | 125.5 ± 4.6 | 126.5 ± 4.6 | 1.0 ± 1.4 | 127.7 ± 4.1 | 119.5 ± 5.2*† | −8.2 ± 3.1 | 0.5 |
| Central diastolic BP, mmHg | 80.0 ± 2.5 | 80.1 ± 2.3 | 0.1 ± 0.4 | 81.6 ± 1.7 | 79.0 ± 1.7 | −2.6 ± 1.3 | 0.4 |
| Carotid-to-radial PWV, m/s | 9.3 ± 0.3 | 9.4 ± 0.3 | 0.1 ± 0.2 | 9.4 ± 0.3 | 9.1 ± 0.3 | −0.3 ± 0.1 | 0.3 |
| Carotid-to-ankle PWV, m/s | 10.3 ± 0.3 | 10.3 ± 0.3 | 0.0 ± 0.2 | 10.4 ± 0.3 | 9.9 ± 0.6 | −0.5 ± 0.4 | 0.4 |
| Carotid-to-femoral PWV, m/s | 9.4 ± 0.8 | 9.6 ± 0.8 | 0.2 ± 0.1 | 9.3 ± 0.8 | 9.0 ± 0.5 | −0.3 ± 0.4 | 0.2 |
| Deceleration time, ms | 620.0 ± 60.7 | 618.7 ± 64.6 | −1.3 ± 11.1 | 614.9 ± 54.3 | 629.8 ± 50.8 | 14.9 ± 19.1 | 0.1 |
| dP/dtmax, mmHg/s | 729.8 ± 65.2 | 720.7 ± 72.0 | −9.1 ± 60.4 | 720.9 ± 47.6 | 755.5 ± 77.9 | 34.6 ± 76.2 | 0.2 |
| Central pulse pressure, mmHg | 45.5 ± 4.3 | 46.5 ± 4.4 | 1.0 ± 1.4 | 46.1 ± 3.5 | 41.4 ± 5.2 | −4.7 ± 3.9 | 0.3 |
| Peripheral pulse pressure, mmHg | 48.2 ± 6.4 | 53.0 ± 7.9 | 4.8 ± 1.9 | 51.8 ± 8.6 | 49.6 ± 8.9 | −2.2 ± 3.3 | 0.1 |
| Augmentation pressure, mmHg | 18.0 ± 3.1 | 20.6 ± 5.3 | 2.6 ± 2.4 | 21.6 ± 5.3 | 20.2 ± 4.6 | −1.4 ± 2.4 | 0.1 |
| AIx, mmHg | 39.4 ± 1.0 | 38.8 ± 2.7 | −0.6 ± 2.8 | 40.2 ± 3.7 | 39.8 ± 1.9 | −0.4 ± 3.6 | 0.2 |
| AIx@75 (%) | 30.6 ± 1.7 | 30.4 ± 4.0 | −0.2 ± 4.0 | 32.2 ± 4.5 | 32.4 ± 2.8 | 0.2 ± 3.2 | 0.1 |
Values are presented as means ± SE. AIx, augmentation index; AIx@75, augmentation index adjusted to 75 beats/min; BP, blood pressure; dP/dtmax, maximum first derivative of pressure; PWV, pulse-wave velocity. Two-way repeated analysis of variance (ANOVA) [group (NO3− and PL) × time (before and after supplement intake)] with paired t tests for post hoc comparisons. Effect size analyses were conducted using Cohen’s d. *P < 0.05 vs. Pre. †P < 0.05 vs. PL.
Figure 4.
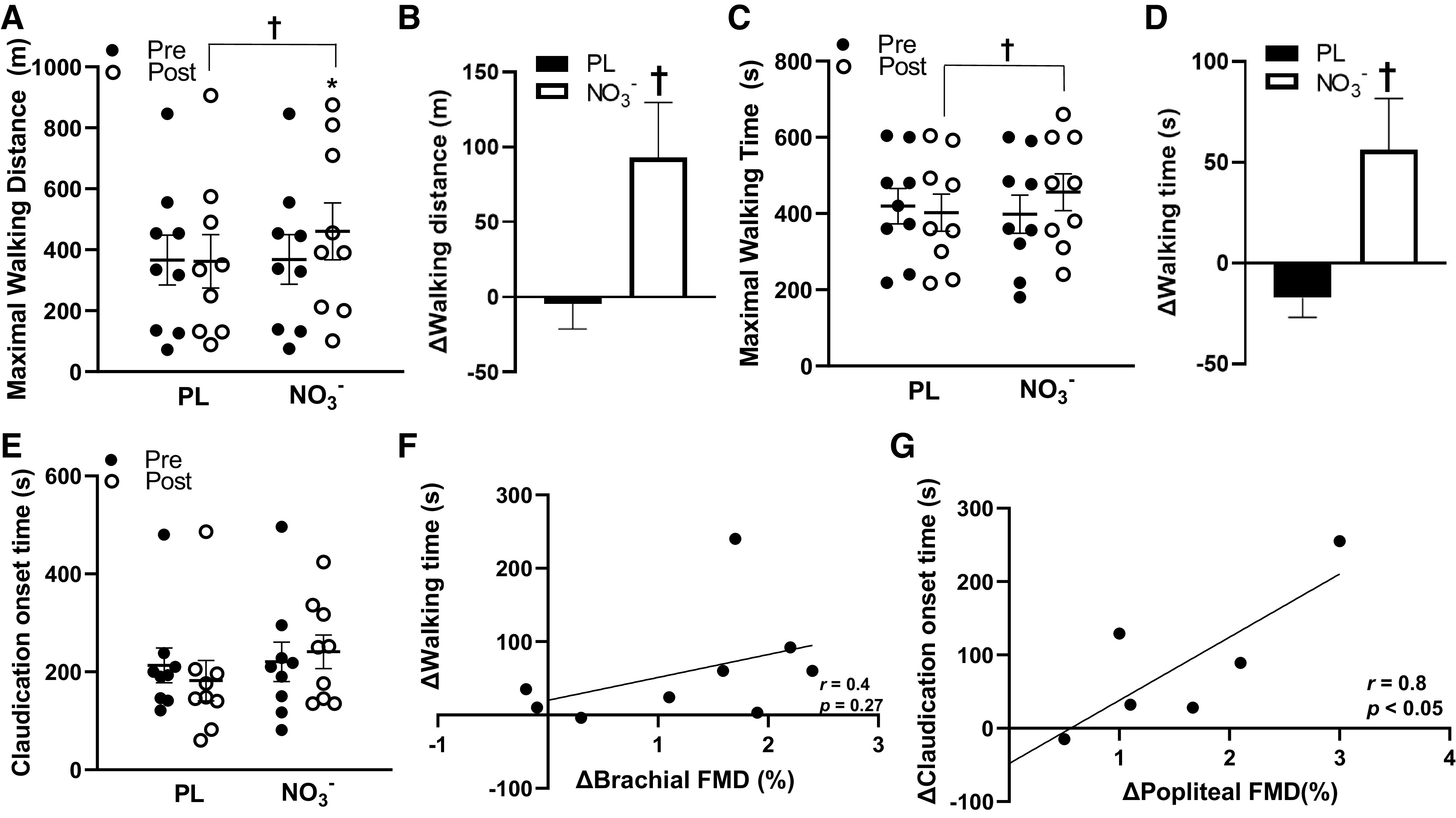
Maximal walking time (s), maximal walking distance (m), and time to claudication(s) pre- and post-placebo (PL) and nitrate (NO3−) intake and relationships between changes in flow-mediated dilation (FMD), maximal walking time, and time to onset of claudication in the NO3− group. A: maximal walking distance significantly increased post-NO3− and was significantly greater than post-PL (n = 9, d = 0.4). B: changes in maximal walking distance in the PL and NO3− groups. C: maximal walking time was significantly greater in post-NO3− compared with post-PL (n = 9, d = 0.4). D: changes in maximal walking time in the PL and NO3− groups. E: there were no significant changes in time to onset of claudication pre- or post-PL or NO3− intake (n = 9, d = 0.5). F: positive relationship between changes in walking time (s) and changes brachial FMD (%) in the NO3− group (n = 9, r = 0.4, P = 0.27). G: positive relationship between changes in time to onset of claudication(s) and changes popliteal FMD (%) in the NO3− group (n = 6, r = 0.8, P < 0.05). Values are presented as means ± SE. Two-way repeated analysis of variance (ANOVA) [group (NO3− and PL) x time (before and after supplement intake)] with paired t tests for post hoc comparisons. Pearson’s product-moment correlation coefficient was used for correlations. Effect size analyses were conducted using Cohen’s d. *P < 0.05 vs. Pre †P < 0.05 vs. PL.
Figure 5.
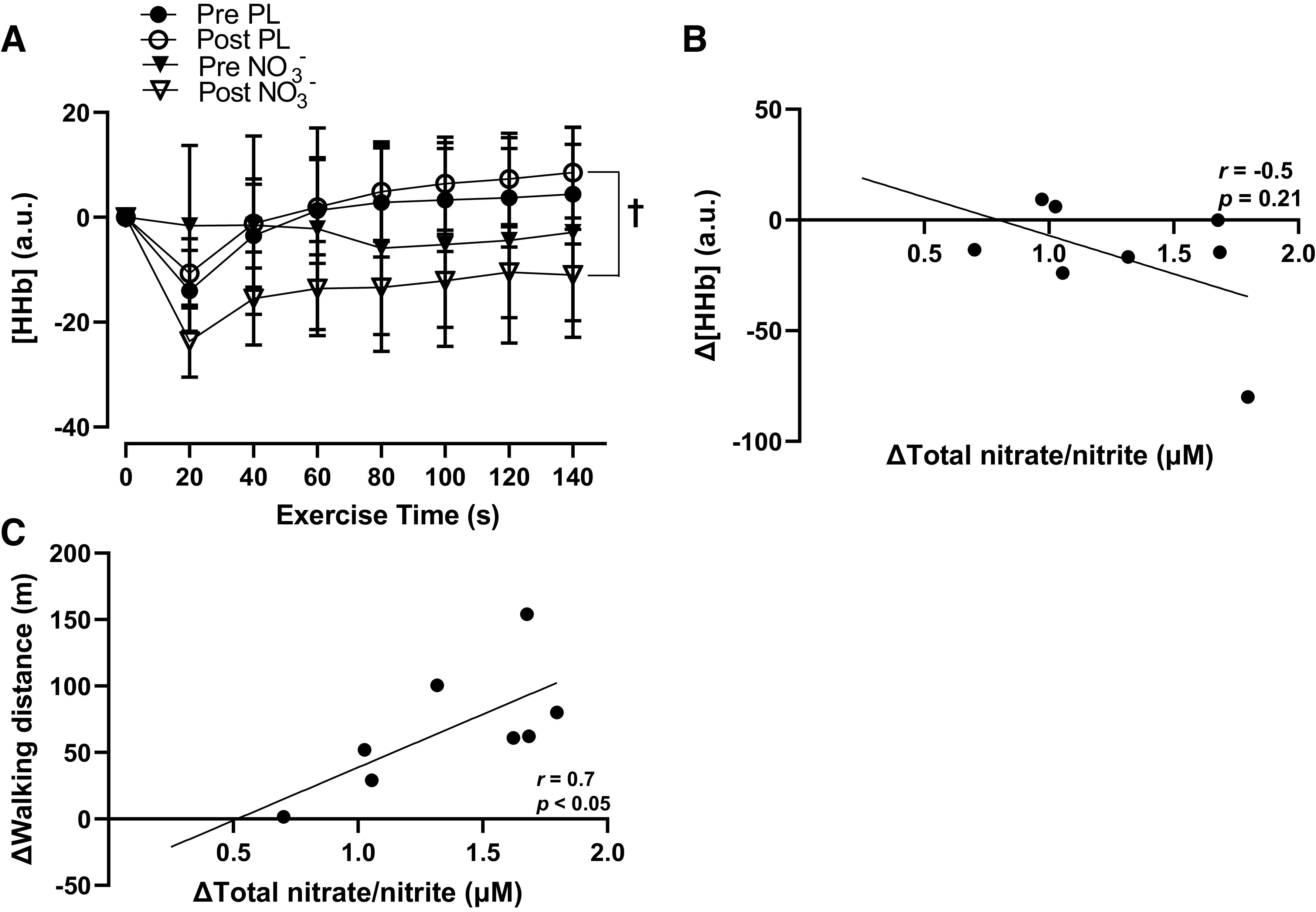
Group mean changes in deoxygenated hemoglobin ([HHb], a.u.) pre- and post-placebo (PL) and nitrate (NO3−) intake every 20 s during the maximal walking capacity test and relationships between changes in total nitrate/nitrite levels, [HHb], and maximal walking distance in the NO3− group. A: post-NO3− and post-PL [HHb] were significantly different (P < 0.05) only at 60, 80, 100, 120, and 140 s (n = 8, d = 1.2). B: negative relationship between changes in total nitrate/nitrite concentration (μM) and deoxyhemoglobin concentration ([HHb], a.u.) in the NO3− group (n = 8, r = −0.5, P = 0.21). C: positive relationship between changes in total nitrate/nitrite concentration (μM) and walking distance (m) in the NO3− group (n = 8, r = 0.7, P < 0.05). Values are presented as means ± SE. Two-way repeated analysis of variance (ANOVA) [group (NO3− and PL) x time (before and after supplement intake)] with paired t tests for post hoc comparisons. Pearson’s product-moment correlation coefficient was used for correlations. Effect size analyses were conducted using Cohen’s d. †Post-PL and post-NO3− significantly different (P < 0.05) only at 60, 80, 100, 120, and 140 s.
DISCUSSION
This study presents with several novel findings that may be relevant for patients with PAD. First, a moderate dose of NO3− intake normalized to body mass can be a safe and beneficial dietary strategy for improving NO bioavailability and reducing high BP in patients with PAD. Second, to our knowledge, we are the first group to demonstrate the impacts of a body mass-normalized moderate dose of NO3− intake on brachial and popliteal artery endothelial function in patients with PAD. Third, we found that a body mass-normalized moderate dose of NO3− significantly improves walking capacity and skeletal muscle oxygen utilization capacity during walking in patients with PAD.
NO Bioavailability and Endothelial Function
Endothelial dysfunction has been documented as a key contributor to the manifestation of atherosclerotic diseases (4). Numerous mechanisms have become increasingly implicated in endothelial dysfunction, whereas growing evidence suggests that dietary NO3− may support improvements in the redox environment and NO bioaction, which may support endothelial function in patients with PAD (29–31).
Previously, the impacts of dietary NO3− on vascular endothelial function have been explored in both healthy and patient populations. Several studies have supported the notion that both acute and chronic intake of dietary NO3− can improve conduit artery endothelial function in healthy older adults (13, 32), individuals with hypertension (33, 34), and individuals with hypercholesterolemia (12). However, there have been no noted improvements in endothelial function following NO3− intake in patients with PAD (15). Although Kenjale et al. (15) used a similar dose to our study, their participants were substantially heavier than our participants (84.5 ± 16.5 kg vs. 77.0 ± 16.5 kg), thus making their overall dose lower. Although their nonnormalized dose showed improvements in NO bioavailability, it may have not been sufficient to induce alterations of endothelial nitric oxide synthase (eNOS)-derived NO in response to reactive hyperemia (15). Our findings show that brachial FMD (Δ1.3%, d = 0.8; Fig. 3, A and B) and popliteal FMD (Δ1.7%, d = 0.9; Fig. 3, C and D) both improved after a body mass-normalized moderate dose of NO3− intake, and the mechanisms underlying these improvements may be largely explained by increased blood NO3−-mediated improvements in redox balance. We found that NO3− intake improves blood total NO3− and NO2− levels (Fig. 2, A and B), and this may have served to reduce reactive oxygen species (ROS) levels (13, 33). Attenuated ROS levels may have supported improvements in the redox environment and antioxidant defense system, and this may have contributed to improved NO-mediated vasodilatory function (13, 33). Walker and colleagues (2019) (13) suggested that improved FMD following acute NO3− intake was likely mediated by augmented antioxidant defense and reduced ROS that may collectively contribute to increased eNOS activity, which is a key enzyme for endothelium-mediated NO production. In addition, Velmurugan et al. (12) stated that an improvement in FMD after 4 wk of NO3− intake was likely due to reduced ROS-mediated NO scavenging, as oxidized low-density lipoprotein was reduced after NO3− intake. NO3−-mediated improvements in the redox environment may be a viable explanation for the increased FMD in the present study, as it is well-accepted that the FMD response is primarily eNOS-derived NO dependent (35, 36). Future research should entail assessments of plasma oxidant status in addition to measuring NO bioavailability in order to determine the impacts of NO3− intake on the redox environment.
In addition to NO3−, beetroot juice possesses several nonnitrate bioactive compounds in small amounts, including flavonoids, ascorbic acid, polyphenols, and several minerals (37, 38). We and others have previously demonstrated that several of these bioactive compounds alone at higher concentrations can induce improvements in endothelial function in healthy and disease populations, with these improvements likely mediated by an enhanced redox environment (39, 40). Accordingly, these bioactive compounds in beetroot juice may have had a slight additive effect on improving endothelial function in the present study. Furthermore, Tropea et al. (41) recently showed that pregnant eNOS−/− mice demonstrated significantly increased endothelium-dependent relaxation following dietary NO3− supplementation in the form of beetroot juice. The authors suggested that the endothelium-derived hyperpolarization (EDH) component of endothelial function may have been enhanced by NO3− supplementation in these eNOS−/− mice (41). Taken together, it may be hypothesized that both antioxidant property-mediated improvements in the redox environment (facilitated by both NO3− and other bioactive compounds) and EDH component-mediated effects of the beetroot juice may be potential contributors to the improvements in FMD in the present study. However, these hypotheses warrant further investigation in patients with PAD.
Furthermore, NO3− may have directly influenced vascular smooth muscle tone. NO3− is an NO-donor that can directly induce vascular smooth muscle relaxation. The nitrate-nitrite-NO pathway functions to reduce NO3− to bioactive NO in the blood and tissues (42). NO can diffuse into the vascular smooth muscle, which results in a signaling cascade that can induce relaxation (endothelium-independent vasodilation) of the vascular smooth muscle (43). In the present study, the nitrate-nitrite-NO pathway may have played a role in endothelium-independent vasodilation and a subsequent reduction in systemic vascular resistance as indicated by reduced systolic BP (Table 3), and this reduction in systemic vascular resistance can contribute to increased blood flow in accordance with Ohm’s Law (44). Despite the potential influence of NO3− on vascular smooth muscle relaxation, the magnitude of this contribution relative to other potential mechanisms requires further investigation.
Walking Capacity and Skeletal Muscle Oxygen Transfer and Utilization Capacity
Several research groups have focused on restoring NO levels as an avenue for improving physical capacity in patients with PAD. These studies primarily used NO-donor drugs, which are pharmacological agents that directly supply NO, whereas dietary NO3−, often in the form of beetroot juice, is a naturally occurring form of NO3−. Gresele et al. (45) reported that intake of a NO-donor (NCX-4016; 800 mg 2×/day) for 6 mo does not improve pain-free walking distance but can slow atherosclerotic progression in patients with PAD. The authors suggested that the lack of improvement may be due to the large variability of their walking data and the heterogeneity of PAD disease staging within their patient sample (45). Conversely, both acute (∼0.09–0.13 mmol/kg) and chronic (8.5 mmol, no body mass reported) dietary NO3- intake (nonbody mass-normalized doses) has been shown to improve assessments of walking capacity, including improvements in walking time, walking distance, and time to onset of claudication in patients with PAD (14, 15). Our findings are in agreement with these previous studies, as we found significant improvements in maximal walking time (Δ56.3 s, d = 0.4) and maximal walking distance (Δ92.7 m, d = 0.4) following a body mass-normalized moderate dose of NO3− intake (Fig. 4, A–D).
Although the mechanisms underlying the improvements in walking capacity are not entirely clear, it is well-accepted that reduced walking capacity in PAD is partially due to attenuated blood flow and perfusion that may cause hypoxic conditions within the lower extremities (46). The endothelium plays an essential role in regulating local skeletal muscle tissue blood flow and perfusion, particularly at the level of the microcirculation (47, 48). Of note, we recently reported that microcirculatory endothelial dysfunction is present in the leg skeletal muscles of patients with PAD, which may suggest there is an attenuated capacity to regulate flow and perfusion within the lower extremity (22). In the present study, the improvements in walking capacity may be partially explained by the improved endothelial function-mediated increase in blood flow and tissue perfusion, which may have facilitated enhanced transport of oxygen and nutrients locally within the skeletal muscle microcirculatory network, thus contributing to improved walking capacity (49). Previous studies have reported that dietary NO3− supplementation can improve blood flow and tissue perfusion in the leg muscle of murine models with chronic hindlimb ischemia (50, 51). These authors primarily attributed these improvements to enhanced endothelial function by the contribution of NO and the nitrate-nitrite-NO pathway (50, 51). Furthermore, NO3− supplementation (∼0.07 mmol NO3−/kg/day for 4 days) in humans was shown to delay muscular fatigue during leg exercise with experimentally-induced ischemia, and the authors said this was due to NO3−-mediated improvements in leg skeletal muscle perfusion (52). Their results suggest that NO3−-mediated improvements in tissue perfusion may serve to delay muscular fatigue, and this can be particularly relevant for patients with PAD. Interestingly, our study provides evidence that supports the improved endothelial function-mediated increase in skeletal muscle tissue perfusion. We found a positive moderate association between improvements in brachial FMD and improvements in maximal walking time (r = 0.4; Fig. 4F), and improvements in popliteal FMD were strongly associated with improvements in time to claudication onset (r = 0.8; Fig. 4G). These findings may suggest that NO3− supplement intake induced an improvement in endothelial function, which may serve to increase walking time and exercise tolerance in patients with PAD. More specifically, the strong relationship between popliteal endothelial function and walking tolerance may indicate that leg endothelial function plays a crucial role for improvements in tissue perfusion and delaying claudication. These results confirm the findings from our previous studies, as we reported that leg endothelial function and lower extremity blood flow are associated with exercise performance and functional capacity in PAD (22, 53, 54).
In addition, the nitrate-nitrite-NO pathway may have functioned as a compensatory mechanism to support vasodilation, blood flow, and tissue perfusion within the lower extremity in patients with PAD (42). Dietary NO3− is reduced to NO2− by the enterosalivary circulation and oral commensal bacteria, which generates a reservoir of NO2− that can be converted to NO in the blood and tissues (55, 56). Importantly, this nitrate-nitrite-NO pathway has been shown to facilitate vasodilation under hypoxic conditions (42), and HHb has specifically been shown to act as a key NO2− reducing agent (NO2− reductase) under these conditions of low oxygen tension (57, 58). During walking, the lower limb in PAD deoxygenates at a faster rate when compared with healthy controls, resulting in a greater concentration of HHb (59). This greater concentration of HHb may serve to facilitate vasodilation in the lower limbs in PAD, especially when NO2− is readily available. The NO2− and HHb reaction-mediated increase in vasodilation may produce significant increases in blood flow and tissue perfusion in the lower extremity during walking in PAD. We found that NO bioavailability was increased (Fig. 2, A and B) and [HHb] was reduced following NO3− intake compared with PL at 60 s, 80 s, 100 s, 120 s, and 140 s during walking (Fig. 5A). These results may imply that HHb functioned as a NO2− reducing agent to produce NO, which is well-aligned with previous findings in patients with PAD (15). Of note, we found a moderate negative association between reductions in [HHb] and increases in serum NO bioavailability (r = −0.5; Fig. 5B). This relationship may suggest that when NO3− and NO2− levels are higher, there may be more potential for HHb to act as a NO2− reducing agent to produce NO during walking in patients with PAD. In addition, we noted a strong positive relationship between increases in NO3− and NO2− levels and increases in walking distance (r = 0.7; Fig. 5C), which may further support the NO bioavailability-mediated improvements in tissue perfusion and walking capacity.
Arterial Stiffness and Augmentation Index
Arterial stiffening refers to alterations in mechanical and structural properties of the artery that perturb adequate arterial function and reduce distensibility of the vascular tree (60). Arterial stiffness is commonly assessed as pulse-wave velocity (PWV) (61, 62). Augmentation index (AIx) and AIx adjusted to 75 beats/min (AIx@75) are also often used as markers for central arterial stiffness and can be affected by both structural and hemodynamic influences (23, 63, 64).
Arterial stiffness has been reported to be associated with the formation of atherosclerotic lesions and affects individuals with PAD (65, 66). Previous interventions utilizing dietary NO3− for several populations have demonstrated mixed results regarding changes in arterial stiffness and AIx. Acute dietary NO3− intake (∼0.11–0.14 mmol NO3−/kg) was shown to reduce AIx@75 in healthy younger adults but showed no changes in healthy older adults (67). Interestingly, some positive effects of dietary NO3− were noted for clinical populations such as individuals with hypertension and hypercholesterolemia. In individuals with hypertension, NO3− intake (∼6.4 mmol NO3−/day for 4 wk, no body mass reported) induced reductions in PWV and AIx (34). Velmurugan et al. (12) reported that NO3− intake (∼6.0 mmol NO3−/day for 6 wk, no body mass reported) can reduce AIx and PWV in individuals with hypercholesterolemia. Despite these improvements noted in clinical populations, these studies are short- to midterm supplementation studies and did not report specific body mass normalized doses of NO3− for their subject populations (12, 34); therefore, it is hard to find standard doses of NO3− for clinical populations. To our knowledge, we are the first group to investigate the impacts of a body mass-normalized dose of dietary NO3− on arterial stiffness in patients with PAD. There were no significant changes in carotid-to-radial PWV (Δ−0.3 m/s, P = 0.13, d = 0.3), carotid-to-femoral PWV (Δ−0.34 m/s, P = 0.27, d = 0.2), carotid-to-ankle PWV (Δ−0.5 m/s, P = 0.38, d = 0.4), AIx (Δ−0.4%, P = 0.97, d = 0.1), or AIx@75 (Δ−0.2%, P = 0.93, d = 0.1) following NO3− intake (Table 3) in our study. The absence of improvements in arterial stiffness may be partially due to the fact that arterial stiffness is a result of structural changes within the vasculature, and an acute moderate dose of NO3− intake may not be sufficient to produce a detectable impact on arterial stiffness in PAD. Chronic body mass-normalized moderate doses of NO3- supplementation studies for patients with PAD should be considered to further assess the potential long-term impacts of NO3− on arterial stiffness and central vascular health.
Experimental Considerations
Our study has some experimental considerations that should be acknowledged. We have a couple of variables including popliteal FMD (bifurcations effect on measurement reliability/precision) and blood draw (needle apprehension) that have a lower sample size, which are limitations to some of our experiments and correlation analyses. It has been recently noted that technical settings for FMD and ultrasound scanning have been suggested, which included that the ultrasound probe should be placed at least 2–3 cm proximal to arterial bifurcations on a straight segment of the vessel to avoid confounding factors such as vortices and turbulent flow (68, 69). Unfortunately, some of our patients with PAD (n = 5) showed multiple bifurcations during artery scanning, and a straight arterial segment was not accessible, which prevents sufficient probe placement for the popliteal artery. Furthermore, we performed our measurements ∼ 1 h after NO3− ingestion. Previous studies using similar (∼0.09–0.13 mmol NO3−/kg) and higher (∼0.29–0.43 mmol NO3−/kg) doses and suggested that peak blood levels of NO3− and NO2− would be around 2–3 h post consumption (11, 15), and we found that blood levels of NO3− and NO2− significantly increase ∼1 h after ingestion, which is consistent with previous studies (11, 20, 21). Even though measurements were conducted sooner than the potential NO3− and NO2− peak in the blood, our results show that the vascular and functional benefits of dietary NO3− intake may occur in as little as 1 h in patients with PAD. However, more research is warranted to fully understand the relationship between the metabolism of a body mass-normalized moderate dose of dietary NO3− and how long these vascular and functional benefits may be maintained in patients with PAD.
In addition, we asked participants to repeat foods consumed before each visit and to not modify their dietary habits, and strongly suggested that they limit their nitrite-rich foods and other antioxidant property-rich food and supplements, but we did not require diet logs to be prepared and collected. Future studies, most particularly for supplementation studies, a diet-tracking component should be incorporated to monitor general macronutrient profile, caloric intake, and/or any additional higher-nitrate food consumed (e.g., spinach, other leafy green vegetables, beets, etc.). Furthermore, medications were withheld for ≥12 h before the study visits and use of medications was not ceased during the 2-wk washout period. Some blood pressure-lowering medications (e.g., β-blockers, ACE inhibitors, angiotensin II receptor blockers, etc.) have half-lives and residual activity that can extend past 12 h (70–73). The potential residual effects of blood pressure medications may have impacted the magnitude by which blood pressure was reduced by the acute dietary NO3−. Even though the mechanisms of action are different between these blood pressure medications (i.e., blocking of beta and angiotensin II receptors, and inhibition of angiotensin-converting enzyme) and dietary nitrate (NO donor), future studies should consider incorporating better control for these differences in medication half-lives and their possible lingering effects on blood pressure. Last, we did not directly investigate the impacts of additional comorbidities due to our relatively small sample size. Conditions such as prediabetes, arthritis, and smoking history may exacerbate systemic inflammation, ROS production, and skeletal muscle tissue perfusion that are independent of PAD (74–77). This may in turn attenuate the functional response to dietary NO3−, which may differentiate potential “responders” and “nonresponders” to dietary NO3− despite substantial increases in NO bioavailability (Supplemental materials; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.14713992). However, these concepts warrant further study with a larger sample size.
Perspectives and Significance
Our results show that blood NO bioavailability, endothelial function, BP, walking capacity, and tissue oxygen transfer and utilization capacity in the skeletal muscle were improved after an acute body mass-normalized moderate dose of dietary NO3−. In fact, these results may have several clinical implications for patients with PAD. We noted clinically relevant increases in both brachial FMD (Δ1.3%) and popliteal FMD (Δ1.7%) after a moderate dose of dietary NO3− intake, which may play a role in protecting or slowing the disease progression by promoting improved vascular homeostatic control (78, 79). In addition, these results may be advantageous for this population in terms of walking independence, as we noted significant improvements in walking capacity (both time and distance) after a moderate dose of dietary NO3− intake. Patients with PAD often lack the physical capacity and desire to participate in exercise due to their poor health-related quality of life (80), which may make nutritional and dietary interventions of greater interest as a therapy to support walking capacity. Although there were no statistically significant improvements in time to onset of claudication, there was a medium effect size (Δ20 s, P = 0.29, d = 0.5), which may indicate a clinically meaningful increase in pain-free walking time. Even though this delay in claudication onset time was not significant, our findings for increases in maximal walking time (∼14%) and maximal walking distance (∼25%) may support the notion that patients had improved claudication tolerance or a reduction in perceived claudication severity considering that they were able to walk for a longer distance and period of time. Overall, acute intake of a moderate body mass-normalized dose of dietary NO3− may induce beneficial effects on vasculature function and walking capacity for patients with PAD while inducing no known side effects. Thus, moderate doses of dietary NO3− (0.11 mmol NO3−/kg) would be an efficient nutritional therapy for delaying disease progression and also improving quality of life in this disease population.
GRANTS
This work was funded, in part, by the University of Nebraska at Omaha Graduate Research and Creative Activity (GRACA) grant (to E.J.P.), NASA Nebraska Space Grant Fellowship (to E.J.P.), the NASA Nebraska Space Grant NNX15AI09H (to S.-Y.P.), and the Center for Research in Human Movement Variability National Institutes of Health Grant P20GM109090 (to S.-Y.P).
DISCLOSURES
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
E.J.P. and S.-Y.P. conceived and designed research; E.J.P., T.K.W., S.K.Y., and S.-Y.P. performed experiments; E.J.P., T.K.W., and S.-Y.P. analyzed data; E.J.P. and S.-Y.P. interpreted results of experiments; E.J.P. and T.K.W. prepared figures; E.J.P. and S.-Y.P. drafted manuscript; E.J.P., T.K.W., S.K.Y., and S.-Y.P. edited and revised manuscript; E.J.P., T.K.W., S.K.Y., and S.-Y.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the participants.
REFERENCES
- 1.Shu J, Santulli G. Update on peripheral artery disease: epidemiology and evidence-based facts. Atherosclerosis 275: 379–381, 2018. doi: 10.1016/j.atherosclerosis.2018.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belch JJ, Topol EJ, Agnelli G, Bertrand M, Califf RM, Clement DL, Creager MA, Easton JD, Gavin JR 3rd, Greenland P, Hankey G, Hanrath P, Hirsch AT, Meyer J, Smith SC, Sullivan F, Weber MA; Prevention of Atherothrombotic Disease N. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med 163: 884–892, 2003. doi: 10.1001/archinte.163.8.884. [DOI] [PubMed] [Google Scholar]
- 3.Spronk S, White JV, Bosch JL, Hunink MG. Impact of claudication and its treatment on quality of life. Semin Vasc Surg 20: 3–9, 2007. doi: 10.1053/j.semvascsurg.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362: 801–809, 1993. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 5.Anderson CP, Pekas EJ, Sy P. Microvascular dysfunction in peripheral artery disease: is heat therapy a viable treatment? Int J Environ Res Public Health 18: 2384, 2021. doi: 10.3390/ijerph18052384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gimbrone MA Jr, Garcia-Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118: 620–636, 2016. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brevetti G, Schiano V, Chiariello M. Endothelial dysfunction: a key to the pathophysiology and natural history of peripheral arterial disease? Atherosclerosis 197: 1–11, 2008. doi: 10.1016/j.atherosclerosis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 8.BöGer RH, Bode-BöGer SM, Thiele W, Junker W, Alexander K, FröLich JC, Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation 95: 2068–2074, 1997. doi: 10.1161/01.CIR.95.8.2068. [DOI] [PubMed] [Google Scholar]
- 9.Allen JD, Miller EM, Schwark E, Robbins JL, Duscha BD, Annex BH. Plasma nitrite response and arterial reactivity differentiate vascular health and performance. Nitric Oxide 20: 231–237, 2009. doi: 10.1016/j.niox.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundberg JO, Carlstrom M, Larsen FJ, Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc Res 89: 525–532, 2011. doi: 10.1093/cvr/cvq325. [DOI] [PubMed] [Google Scholar]
- 11.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 51: 784–790, 2008. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velmurugan S, Gan JM, Rathod KS, Khambata RS, Ghosh SM, Hartley A, Van Eijl S, Sagi-Kiss V, Chowdhury TA, Curtis M, Kuhnle GG, Wade WG, Ahluwalia A. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr 103: 25–38, 2016. doi: 10.3945/ajcn.115.116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker MA, Bailey TG, McIlvenna L, Allen JD, Green DJ, Askew CD. Acute dietary nitrate supplementation improves flow mediated dilatation of the superficial femoral artery in healthy older males. Nutrients 11: 954, 2019. doi: 10.3390/nu11050954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bock JM, Treichler DP, Norton SL, Ueda K, Hughes WE, Casey DP. Inorganic nitrate supplementation enhances functional capacity and lower-limb microvascular reactivity in patients with peripheral artery disease. Nitric Oxide 80: 45–51, 2018. doi: 10.1016/j.niox.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, Vanbruggen M, Privette G, Yim E, Kraus WE, Allen JD. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol (1985) 110: 1582–1591, 2011. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardman RL, Jazaeri O, Yi J, Smith M, Gupta R. Overview of classification systems in peripheral artery disease. Semin Intervent Radiol 31: 378–388, 2014. doi: 10.1055/s-0034-1393976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rienks JN, Vanderwoude AA, Maas E, Blea ZM, Subudhi AW. Effect of beetroot juice on moderate-intensity exercise at a constant rating of perceived exertion. Int J Exerc Sci 8: 277–286, 2015. [PMC free article] [PubMed] [Google Scholar]
- 18.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 19: 333–337, 2008. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol (1985) 115: 325–336, 2013. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]
- 20.McIlvenna LC, Monaghan C, Liddle L, Fernandez BO, Feelisch M, Muggeridge DJ, Easton C. Beetroot juice versus chard gel: a pharmacokinetic and pharmacodynamic comparison of nitrate bioavailability. Nitric Oxide 64: 61–67, 2017. doi: 10.1016/j.niox.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Burleigh MC, Sculthorpe N, Henriquez FL, Easton C. Nitrate-rich beetroot juice offsets salivary acidity following carbohydrate ingestion before and after endurance exercise in healthy male runners. PLoS One 15: e0243755, 2020. doi: 10.1371/journal.pone.0243755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SY, Pekas EJ, Headid RJ 3rd, Son WM, Wooden TK, Song J, Layec G, Yadav SK, Mishra PK, Pipinos II. Acute mitochondrial antioxidant intake improves endothelial function, antioxidant enzyme activity, and exercise tolerance in patients with peripheral artery disease. Am J Physiol Heart Circ Physiol 319: H456–H467, 2020. doi: 10.1152/ajpheart.00235.2020. [DOI] [PubMed] [Google Scholar]
- 23.Headid RJ 3rd, Pekas EJ, Wooden TK, Son WM, Layec G, Shin J, Park SY. Impacts of prolonged sitting with mild hypercapnia on vascular and autonomic function in healthy recreationally active adults. Am J Physiol Heart Circ Physiol 319: H468–H480, 2020. doi: 10.1152/ajpheart.00354.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc 23: 402–408, 1991. [PubMed] [Google Scholar]
- 25.Ryan TE, Erickson ML, Brizendine JT, Young HJ, McCully KK. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. J Appl Physiol (1985) 113: 175–183, 2012. doi: 10.1152/japplphysiol.00319.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishiyama SK, Zhao J, Wray DW, Richardson RS. Vascular function and endothelin-1: tipping the balance between vasodilation and vasoconstriction. J Appl Physiol (1985) 122: 354–360, 2017. doi: 10.1152/japplphysiol.00772.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan GM, Feinn R. Using effect size-or why the P value is not enough. J Grad Med Educ 4: 279–282, 2012. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol (1985) 107: 1144–1155, 2009. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 29.Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation 108: 2093–2098, 2003. doi: 10.1161/01.cir.0000095273.92468.d9. [DOI] [PubMed] [Google Scholar]
- 30.Loffredo L, Pignatelli P, Cangemi R, Andreozzi P, Panico MA, Meloni V, Violi F. Imbalance between nitric oxide generation and oxidative stress in patients with peripheral arterial disease: effect of an antioxidant treatment. J Vasc Surg 44: 525–530, 2006. doi: 10.1016/j.jvs.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 31.Pipinos II, Judge AR, Zhu Z, Selsby JT, Swanson SA, Johanning JM, Baxter BT, Lynch TG, Dodd SL. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Radic Biol Med 41: 262–269, 2006. doi: 10.1016/j.freeradbiomed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Jones T, Dunn EL, Macdonald JH, Kubis HP, McMahon N, Sandoo A. The effects of beetroot juice on blood pressure, microvascular function and large-vessel endothelial function: a randomized, double-blind, placebo-controlled pilot study in healthy older adults. Nutrients 11: 1792, 2019. doi: 10.3390/nu11081792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asgary S, Afshani MR, Sahebkar A, Keshvari M, Taheri M, Jahanian E, Rafieian-Kopaei M, Malekian F, Sarrafzadegan N. Improvement of hypertension, endothelial function and systemic inflammation following short-term supplementation with red beet (Beta vulgaris L.) juice: a randomized crossover pilot study. J Hum Hypertens 30: 627–632, 2016. doi: 10.1038/jhh.2016.34. [DOI] [PubMed] [Google Scholar]
- 34.Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension 65: 320–327, 2015. doi: 10.1161/hypertensionaha.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green DJ, Dawson EA, Groenewoud HM, Jones H, Thijssen DH. Is flow-mediated dilation nitric oxide mediated? A meta-analysis. Hypertension 63: 376–382, 2014. doi: 10.1161/hypertensionaha.113.02044. [DOI] [PubMed] [Google Scholar]
- 36.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995. doi: 10.1161/01.CIR.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 37.Ninfali P, Angelino D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia 89: 188–199, 2013. doi: 10.1016/j.fitote.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Clifford T, Howatson G, West DJ, Stevenson EJ. The potential benefits of red beetroot supplementation in health and disease. Nutrients 7: 2801–2822, 2015. doi: 10.3390/nu7042801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pekas EJ, Shin J, Headid RJ, Son WM, Layec G, Yadav SK, Scott SD, Sy P. Combined anthocyanins and bromelain supplement improves endothelial function and skeletal muscle oxygenation status in adults: a double-blind placebo-controlled randomised crossover clinical trial. Br J Nutr 125: 161–171, 2021. doi: 10.1017/S0007114520002548. [DOI] [PubMed] [Google Scholar]
- 40.Erbs S, Gielen S, Linke A, Mobius-Winkler S, Adams V, Baither Y, Schuler G, Hambrecht R. Improvement of peripheral endothelial dysfunction by acute vitamin C application: different effects in patients with coronary artery disease, ischemic, and dilated cardiomyopathy. Am Heart J 146: 280–285, 2003. doi: 10.1016/S0002-8703(03)00184-4. [DOI] [PubMed] [Google Scholar]
- 41.Tropea T, Renshall LJ, Nihlen C, Weitzberg E, Lundberg JO, David AL, Tsatsaris V, Stuckey DJ, Wareing M, Greenwood SL, Sibley CP, Cottrell EC. Beetroot juice lowers blood pressure and improves endothelial function in pregnant eNOS-/- mice: importance of nitrate-independent effects. J Physiol 598: 4079–4092, 2020. doi: 10.1113/jp279655. [DOI] [PubMed] [Google Scholar]
- 42.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7: 156–167, 2008. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 43.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123: 2263–2273, 2011. doi: 10.1161/CIRCULATIONAHA.110.981738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Secomb TW. Hemodynamics. Compr Physiol 6: 975–1003, 2016. doi: 10.1002/cphy.c150038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gresele P, Migliacci R, Arosio E, Bonizzoni E, Minuz P, Violi F, Nxs G; NCX 4016-X-208 Study Group. Effect on walking distance and atherosclerosis progression of a nitric oxide-donating agent in intermittent claudication. J Vasc Surg 56: 1622–1628, 2012. 1628 e1-5. doi: 10.1016/j.jvs.2012.05.064. [DOI] [PubMed] [Google Scholar]
- 46.Patel SK, Surowiec SM. Intermittent claudication. In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2020. https://www.ncbi.nlm.nih.gov/books/NBK430778/ [PubMed] [Google Scholar]
- 47.Kerr P, Tam R, Plane F. Endothelium. In: Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists, edited by Fitridge R and Thompson M.. Adelaide, Australia: University of Adelaide Press, 2011. https://www.ncbi.nlm.nih.gov/books/NBK534260/ [PubMed] [Google Scholar]
- 48.Sandoo A, van Zanten JJ, Metsios GS, Carroll D, Kitas GD. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J 4: 302–312, 2010. doi: 10.2174/1874192401004010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kusters YH, Barrett EJ. Muscle microvasculature's structural and functional specializations facilitate muscle metabolism. Am J Physiol Endocrinol Metab 310: E379–E387, 2016. doi: 10.1152/ajpendo.00443.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar D, Branch BG, Pattillo CB, Hood J, Thoma S, Simpson S, Illum S, Arora N, Chidlow JH Jr, Langston W, Teng X, Lefer DJ, Patel RP, Kevil CG. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci USA 105: 7540–7545, 2008. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hendgen-Cotta UB, Luedike P, Totzeck M, Kropp M, Schicho A, Stock P, Rammos C, Niessen M, Heiss C, Lundberg JO, Weitzberg E, Kelm M, Rassaf T. Dietary nitrate supplementation improves revascularization in chronic ischemia. Circulation 126: 1983–1992, 2012. doi: 10.1161/CIRCULATIONAHA.112.112912. [DOI] [PubMed] [Google Scholar]
- 52.Hoon MW, Fornusek C, Chapman PG, Johnson NA. The effect of nitrate supplementation on muscle contraction in healthy adults. Eur J Sport Sci 15: 712–719, 2015. doi: 10.1080/17461391.2015.1053418. [DOI] [PubMed] [Google Scholar]
- 53.Park SY, Wong A, Son WM, Pekas EJ. Effects of heated water-based versus land-based exercise training on vascular function in individuals with peripheral artery disease. J Appl Physiol (1985) 128: 565–575, 2020. doi: 10.1152/japplphysiol.00744.2019. [DOI] [PubMed] [Google Scholar]
- 54.Park S-Y, Kwak Y-S, Pekas EJ. Impacts of aquatic walking on arterial stiffness, exercise tolerance, and physical function in patients with peripheral artery disease: a randomized clinical trial. J Appl Physiol (1985) 127: 940–949, 2019. doi: 10.1152/japplphysiol.00209.2019. [DOI] [PubMed] [Google Scholar]
- 55.Omar SA, Webb AJ, Lundberg JO, Weitzberg E. Therapeutic effects of inorganic nitrate and nitrite in cardiovascular and metabolic diseases. J Intern Med 279: 315–336, 2016. doi: 10.1111/joim.12441. [DOI] [PubMed] [Google Scholar]
- 56.Zweier JL, Li H, Samouilov A, Liu X. Mechanisms of nitrite reduction to nitric oxide in the heart and vessel wall. Nitric Oxide 22: 83–90, 2010. doi: 10.1016/j.niox.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505, 2003. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 58.Nagababu E, Ramasamy S, Abernethy DR, Rifkind JM. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J Biol Chem 278: 46349–46356, 2003. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 59.Miller AJ, Luck JC, Kim DJ, Leuenberger UA, Proctor DN, Sinoway LI, Muller MD. Blood pressure and leg deoxygenation are exaggerated during treadmill walking in patients with peripheral artery disease. J Appl Physiol (1985) 123: 1160–1165, 2017. doi: 10.1152/japplphysiol.00431.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol 50: 1–13, 2007. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 61.Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 25: 359–364, 2002. doi: 10.1291/hypres.25.359. [DOI] [PubMed] [Google Scholar]
- 62.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large A. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 63.Kaya M, Balasubramanian V, K-J. Li J. Augmentation index in the assessment of wave reflections and systolic loading. Comput Biol Med 113: 103418, 2019. doi: 10.1016/j.compbiomed.2019.103418. [DOI] [PubMed] [Google Scholar]
- 64.Zachariah JP. Pulse wave reflection in children: amplification through the lifecourse. J Hypertens 35: 1363–1365, 2017. doi: 10.1097/HJH.0000000000001357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks AP, van der Kuip DA, Hofman A, Witteman JC. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke 32: 454–460, 2001. doi: 10.1161/01.str.32.2.454. [DOI] [PubMed] [Google Scholar]
- 66.Zahner GJ, Gruendl MA, Spaulding KA, Schaller MS, Hills NK, Gasper WJ, Grenon SM. Association between arterial stiffness and peripheral artery disease as measured by radial artery tonometry. J Vasc Surg 66: 1518–1526, 2017. doi: 10.1016/j.jvs.2017.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hughes WE, Ueda K, Treichler DP, Casey DP. Effects of acute dietary nitrate supplementation on aortic blood pressure and aortic augmentation index in young and older adults. Nitric Oxide 59: 21–27, 2016. doi: 10.1016/j.niox.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 68.Limberg JK, Casey DP, Trinity JD, Nicholson WT, Wray DW, Tschakovsky ME, Green DJ, Hellsten Y, Fadel PJ, Joyner MJ, Padilla J. Assessment of resistance vessel function in human skeletal muscle: guidelines for experimental design, Doppler ultrasound, and pharmacology. Am J Physiol Heart Circ Physiol 318: H301–H325, 2020. doi: 10.1152/ajpheart.00649.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gliemann L, Mortensen SP, Hellsten Y. Methods for the determination of skeletal muscle blood flow: development, strengths and limitations. Eur J Appl Physiol 118: 1081–1094, 2018. doi: 10.1007/s00421-018-3880-5. [DOI] [PubMed] [Google Scholar]
- 70.Dezsi CA, Szentes V. The real role of beta-blockers in daily cardiovascular therapy. Am J Cardiovasc Drugs 17: 361–373, 2017. doi: 10.1007/s40256-017-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Armayor GM, Lopez LM. Lisinopril: a new angiotensin-converting enzyme inhibitor. Drug Intell Clin Pharm 22: 365–372, 1988. [Erratum in Drug Intell Clin Pharm 22: 920, 1988]. doi: 10.1177/106002808802200501. [DOI] [PubMed] [Google Scholar]
- 72.Shionoiri H, Ueda S, Minamisawa K, Minamisawa M, Takasaki I, Sugimoto K, Gotoh E, Ishii M. Pharmacokinetics and pharmacodynamics of benazepril in hypertensive patients with normal and impaired renal function. J Cardiovasc Pharmacol 20: 348–357, 1992. doi: 10.1097/00005344-199209000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Ripley E, Hirsch A. Fifteen years of losartan: what have we learned about losartan that can benefit chronic kidney disease patients? Int J Nephrol Renovasc Dis 3: 93–98, 2010. doi: 10.2147/ijnrd.s7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luc K, Schramm-Luc A, Guzik TJ, Mikolajczyk TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol 70: 809–824, 2019. doi: 10.26402/jpp.2019.6.01. [DOI] [PubMed] [Google Scholar]
- 75.Nogueira L, Trisko BM, Lima-Rosa FL, Jackson J, Lund-Palau H, Yamaguchi M, Breen EC. Cigarette smoke directly impairs skeletal muscle function through capillary regression and altered myofibre calcium kinetics in mice. J Physiol 596: 2901–2916, 2018. doi: 10.1113/JP275888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol 23: 471–478, 2011. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Senthelal S, Li J, Goyal A, Bansal P, Thomas MA. Arthritis. In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2021. https://www.ncbi.nlm.nih.gov/books/NBK518992/ [Google Scholar]
- 78.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 79.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension 57: 363–369, 2011. doi: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]
- 80.Dumville JC, Lee AJ, Smith FB, Fowkes FG. The health-related quality of life of people with peripheral arterial disease in the community: the Edinburgh Artery Study. Br J Gen Pract 54: 826–831, 2004. [PMC free article] [PubMed] [Google Scholar]