Abstract
The peptide hormone amylin reduces food intake and body weight and is an attractive candidate target for novel pharmacotherapies to treat obesity. However, the short half-life of native amylin and amylin analogs like pramlintide limits these compounds’ potential utility in promoting sustained negative energy balance. Here, we evaluate the ability of the novel long-acting amylin/calcitonin receptor agonist ZP5461 to reduce feeding and body weight in rats, and also test the role of calcitonin receptors (CTRs) in the dorsal vagal complex (DVC) of the hindbrain in the energy balance effects of chronic ZP5461 administration. Acute dose-response studies indicate that systemic ZP5461 (0.5–3 nmol/kg) robustly suppresses energy intake and body weight gain in chow- and high-fat diet (HFD)-fed rats. When HFD-fed rats received chronic systemic administration of ZP5461 (1–2 nmol/kg), the compound initially produced reductions in energy intake and weight gain but failed to produce sustained suppression of intake and body weight. Using virally mediated knockdown of DVC CTRs, the ability of chronic systemic ZP5461 to promote early reductions in intake and body weight gain was determined to be mediated in part by activation of DVC CTRs, implicating the DVC as a central site of action for ZP5461. Future studies should address other dosing regimens of ZP5461 to determine whether an alternative dose/frequency of administration would produce more sustained body weight suppression.
Keywords: amylin, dorsal vagal complex, hindbrain, obesity, pharmacotherapy
INTRODUCTION
Amylin is a peptide hormone that is thought to be a promising candidate for the development of novel pharmacotherapies to target obesity (1–4). Numerous studies have demonstrated that amylin and amylin agonists decrease food intake and body weight in humans as well as rodent models (5–11). Amylin is cosecreted with insulin from pancreatic β-cells and acts centrally to exert its energy balance effects. Specifically, a sizeable body of literature has demonstrated that amylin acts within key areas of the dorsal vagal complex (DVC), in particular the area postrema (AP) and adjacent nucleus tractus solitarius (NTS), to suppress feeding (for review, see Refs. 1, 2, 4, 12, and 13). Direct administration of amylin into the AP reduces food intake, and systemic administration of amylin and amylin receptor agonists has been shown to increase cFos expression in the AP (14–16), including in neurons that express the components of the amylin receptor (17). Furthermore, intra-AP delivery of an amylin receptor antagonist increases feeding (18), and lesion of the AP or AP/NTS attenuates the anorexigenic effects of a peripheral injection of amylin or an amylin receptor agonist (14, 19, 20), demonstrating the importance of endogenous amylin receptor signaling in this site for the control of food intake. Although the AP is a circumventricular organ and therefore is accessible to circulating substances including hormones like amylin, amylin can also cross the blood-brain barrier (21, 22), suggesting that peripheral amylin may be able to access areas more protected by the blood-brain barrier including the NTS. Collectively, these previous findings demonstrate the pharmacological and physiological relevance of the DVC for the hypophagic and weight-reducing effects of amylin.
Despite strong evidence for the ability of amylin and amylin agonists to promote negative energy balance, there is a paucity of amylin-based pharmacotherapies for obesity treatment. For example, the amylin analog pramlintide is Food and Drug Administration (FDA)-approved for the treatment of type 1 and type 2 diabetes mellitus (23, 24). Although it is not currently FDA-approved for treating obesity, pramlintide has been shown to induce weight loss in humans (8, 9, 25). Individuals with obesity taking pramlintide either on its own or in conjunction with behavioral modifications targeting weight loss achieve approximately a 3%–4% reduction in body weight (placebo-corrected) over ∼4 mo (25, 26). However, pramlintide administration has also been associated with negative side effects such as nausea (8, 25, 26). To date, an important challenge in targeting the amylin system for treatment of metabolic diseases such as obesity is the short half-life of amylin, which is under 15 min in rats (27). Pramlintide also has a relatively short half-life of ∼20–50 min in humans (27–29), thus making it necessary for patients to administer the drug just before meals. Identifying amylin-based compounds with longer biological action, as well as action on not only the amylin receptor complex [collectively composed of one of two G protein-coupled calcitonin receptors (CTRa, b) heterodimerized to one of three receptor-activating modifying protein (RAMPs 1, 2, 3) (30, 31)], but also the calcitonin receptor alone may represent an important strategy for improving the utility of amylin-based pharmacotherapies.
Here, we test the hypothesis that a novel long-acting amylin/calcitonin receptor agonist, ZP5461 (32, 33), suppresses food intake and body weight in rats. Furthermore, we begin to probe the potential sites of action of this compound by investigating whether virally mediated knockdown of amylin receptors in the DVC blunts the ability of the ZP5461 to suppress feeding and body weight. Results indicate robust intake- and body weight-suppressive effects of ZP5461 and support the importance of DVC amylin receptor signaling for normal energy balance control.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats (Charles River) were individually housed in hanging wire cages in a temperature- and humidity-controlled environment, on a 12 h:12 h light:dark cycle. Rats were maintained either on standard chow (Purina 5001) or a 60% high-fat diet (HFD; Research Diets, D12492). All experimental procedures were conducted with the approval of the University of Pennsylvania Institutional Animal Care and Use Committee.
Dose-Response Studies
First, the effect of peripherally delivered ZP5461 on feeding and body weight was evaluated in chow-fed rats. Shortly before lights-off, each rat (n = 14) received a 1 mL/kg sc injection of ZP5461 (0–3 nmol/kg) or vehicle (0.75% l-histidine, 3.6% d-mannitol in diH2O, pH 7.0). Chow intake was measured at 1, 3, 6, 24, 48, and 72 h postinjection to the nearest 0.1 g. Crumbs were accounted for in all food intake measurements. Body weight change was measured every 24 h for 72 h postinjection. Each rat received all treatments according to a within-subjects, Latin square design, with at least 7 days between injections. One rat was excluded from further analyses due to technical issues with intake and body weight measurements, resulting in a final n = 13 for this experiment.
A similar experiment was conducted in a separate group of rats (n = 16) to evaluate the effects of systemic ZP5461 on intake and body weight of rats maintained on HFD. Rats were maintained on HFD for ∼1 mo before testing to induce dietary obesity. Again, in a within-subjects Latin square design, rats received subcutaneous injection of ZP5461 (0–3 nmol/kg) or vehicle (1 mL/kg) just before lights-off, and intake (accounting for spillage) and body weight were measured at the times noted in the chow intake study.
Virally Mediated Knockdown of DVC Amylin/Calcitonin Receptors
Upon arrival, rats were singly housed in hanging wire cages and immediately placed on a 60% HFD. They were maintained on this diet for 4 wk before surgery to induce dietary obesity. Rats were anesthetized with an intramuscular injection of ketamine (90 mg/kg; Butler Schein), xylazine (2.7 mg/kg; Midwest Veterinary Supply), and acepromazine (0.64 mg/kg; Midwest Veterinary Supply) (KAX cocktail). Animals were body weight-matched and divided into two cohorts: control adeno-associated virus (AAV-Control; AAV1-Cb7-CI-eGFP, lot no. CS1039, Penn Vector Core; n = 13) or an amylin/calcitonin receptor knockdown adeno-associated virus (AAV-CTR-KD; AAV1-U6-shRCalc93-CB7-EGFP-SV40, lot no. V4389MI-R-C, Penn Vector Core; n = 17) (34). Rats received bilateral viral infusions (2.5e13 viral particles/mL, 200 nL, 100 nL/s) targeted to the medial NTS of the DVC (resulting in spread and transfection of the AAV within both the NTS and AP) using a microinfusion pump and according to the following coordinates: 1.0 mm posterior to the occipital suture and 7.9 mm ventral to the skull, along the midline (35). Postoperative analgesia (2 mg/kg meloxicam) was administered subcutaneously for 2 days, and animals were allowed to recover for 2 wk. Food intake and body weight were recorded every 48 h.
Examination of DVC CTR Contribution to the Anorectic Effects of Peripherally Administered ZP5461
At the onset of the experimental phase, viral cohorts were divided into separate treatment groups, receiving either vehicle or ZP5461 compound. Group sizes were as follows: AAV-Control, vehicle-treated (n = 6); AAV-Control, ZP5461-treated (n = 7); AAV-CTR-KD, vehicle-treated (n = 8); and AAV-CTR-KD, ZP5461-treated (n = 9). Separated by 96 h, animals received two consecutive intraperitoneal injections of either vehicle or 1 nmol/kg/mL ZP5461, followed by two subcutaneous injections of either vehicle or 1 nmol/kg/mL ZP5461, and finally three subcutaneous injections of vehicle or 2 nmol/kg/mL ZP5461 (Fig. 3D). All injections, daily food intake readings, and body weight measurements occurred 30 min before dark onset. Twenty-four-hour food intakes and body weights were recorded for the entire duration of the experiment. Upon completion, rats were anesthetized with a KAX cocktail followed by decapitation. Brains were quickly removed, flash frozen in isopentane over dry ice, and then stored at −80°C. Inguinal, epididymal, retroperitoneal, and perirenal fat pads were removed and weighed postmortem from AAV-Control and AAV-CTR-KD rats.
Quantification of CTR Expression in the DVC
Medial DVC-enriched bilateral micro-punches (1 mm) were taken for qPCR analysis. Coronal sections at the level of the area postrema were slide mounted for visual verification of green fluorescent protein (GFP) expression as previously described (36). Total RNA was extracted from DVC micro-punches using RNeasy kit (Qiagen, Germanton, MD) and cDNA was synthesized using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). TaqMan gene expression kits and PCR reagents (ThermoFisher Scientific, Waltham, MA) were used to quantify relative mRNA levels of amylin receptor (CTR; Rn00587525_m1; ThermoFisher Scientific) relative to the housekeeping gene GAPDH (Rn01775763_g1; ThermoFisher Scientific). Relative mRNA expression was calculated using the comparative threshold cycle (Ct) method as previously described (37).
Statistical Analyses
For all experiments, P ≤ 0.05 was considered statistically significant. Statistical analyses were completed using Statistica (StatSoft) and Prism 8.4 (GraphPad). For each dose-response study, food intake data were analyzed via repeated measures ANOVA accounting for effects of time and ZP5461 dose; data for body weight change were also analyzed in this way. Significant effects in an overall ANOVA were probed using Student–Newman–Keuls post hoc analyses. For the DVC CTR-KD study, individual two-way ANOVA with Newman–Keuls post hoc analyses applied to weight and food intake data at each timepoint. Total changes in body weight and differences in fat pad weight were analyzed by two-way ANOVA with Newman–Keuls post hoc tests. A single animal from the AAV-CTR-KD cohort was not included in the final data analysis; ROUT analysis (false discovery rate = 1%) determined this animal was a statistical outlier. Based on histological verification of viral expression, a single animal exhibited no GFP expression and therefore was excluded from analysis. All control animals were included. There was a single error in data collection in which the food intake was not recorded due to experimenter error on a single day. Rather than excluding entirely, the previous day and following day were averaged to account for the missing data point. Quantitative PCR analyses were carried out using an unpaired t test. Data are shown as means ± SE.
RESULTS
Systemic Administration of ZP5461 Dose Dependently Suppresses Food Intake and Body Weight in Rats
The novel, long-acting amylin receptor agonist ZP5461 was first evaluated for its effects on feeding and body weight gain in chow-fed rats. Subcutaneous injection of ZP5461 produced a dose-dependent reduction in both chow intake and body weight gain over the 72 h postinjection. Beginning at 6 h postinjection, the higher doses of ZP5461 (2 nmol/kg and 3 nmol/kg) significantly decreased chow intake compared with vehicle (Fig. 1A; main effect of drug: F4,48 = 92.77, P < 0.05; time × drug interaction: F20,240 = 116.17, P < 0.05; post hoc analyses, vehicle versus 2 nmol/kg or 3 nmol/kg, all P < 0.05). At 24 h, 48 h, and 72 h postinjection, significant intake- and body weight-suppressive effects of ZP5461 were observed with all doses tested compared with the response after vehicle treatment (Fig. 1B; for food intake post hoc analyses, vehicle different from all doses of ZP5461 at these times, all P < 0.05; for body weight change, main effect of drug: F4,48 = 76.78, P < 0.05; time × drug interaction: F8,96 = 10.87, P < 0.05; post hoc analyses, vehicle significantly different from all ZP5461 doses at these times, all P < 0.05).
Figure 1.
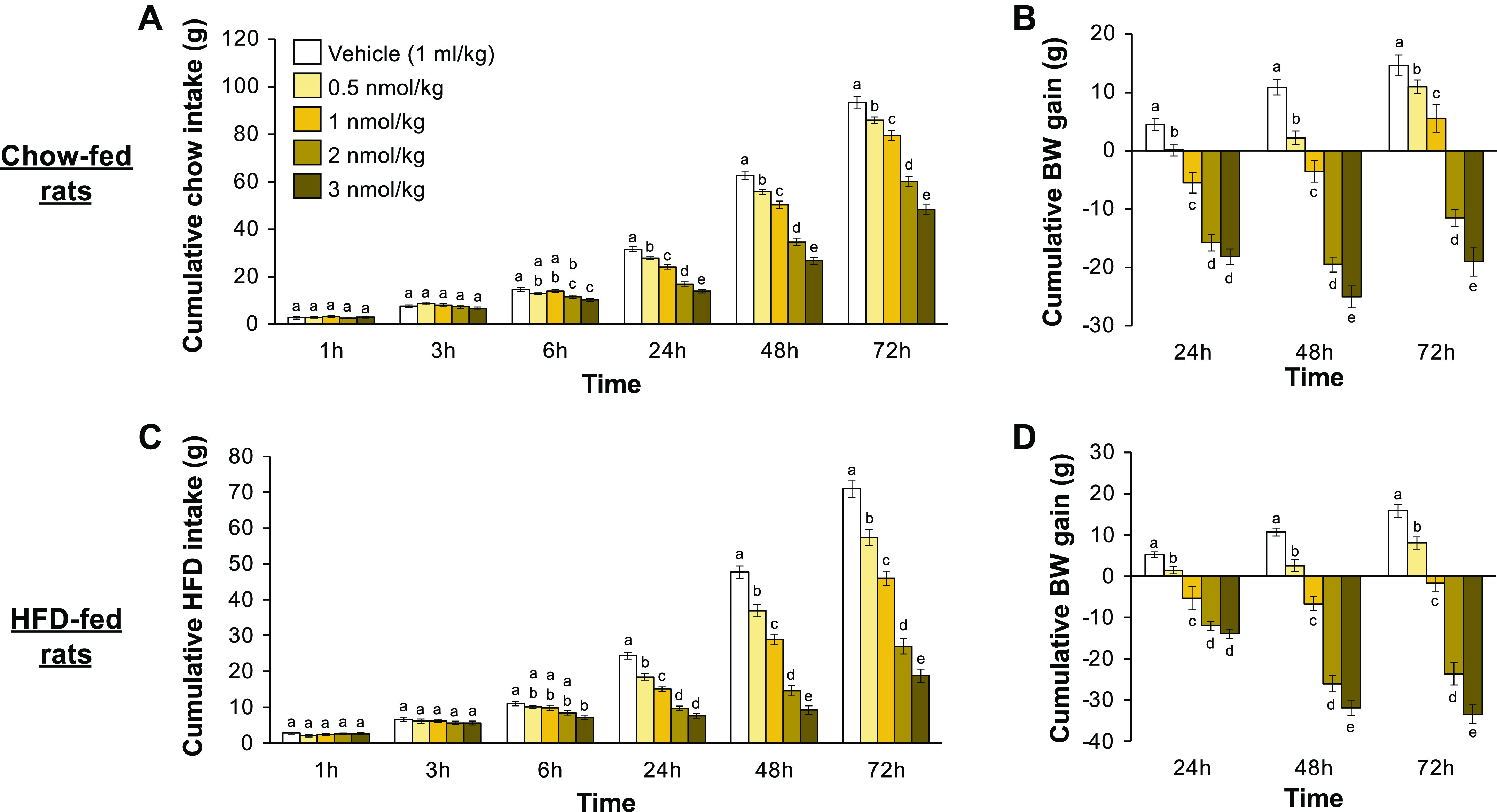
ZP5461 produces a prolonged suppression of food intake and body weight gain in chow-fed and HFD-fed rats. In chow-fed male rats (n = 13), subcutaneous injection of ZP5461 reduced food intake beginning at 6 h postinjection and lasting through the final 72 h reading (A). Body weight gain was also reduced by ZP5461 in these rats at all times tested (B). In HFD-fed rats (n = 16), similar results were seen after subcutaneous injection of ZP5461. Again, food intake was suppressed by ZP5461 beginning at 6 h postinjection and at all subsequent times tested (C), and body weight gain was decreased by ZP5461 at 24, 48, and 72 h postinjection (D). Data are shown as means ± SE. Data were analyzed via repeated measures ANOVA, and statistically significant results were probed with Student–Newman–Keuls post hoc analyses. Within a time bin, bars with different letters are significantly different from each other. BW, body weight; HFD, high-fat diet.
To assess whether ZP5461 also produces hypophagia and body weight loss in rats fed an obesogenic diet, a separate group of rats was maintained on a palatable 60% HFD before and throughout testing. The same doses of ZP5461 used in the chow intake study, again administered subcutaneously, were tested for effects on HFD intake and body weight change in these rats. Here, similar effects of ZP5461 were observed, with the highest dose of the compound (3 nmol/kg), producing suppression of HFD intake at 6 h postinjection (Fig. 1C; main effect of drug: F4,60 = 99.92, P < 0.05; time × drug interaction: F20,300 = 124.12, P < 0.05; post hoc analyses, vehicle versus 3 nmol/kg, P < 0.05), and all doses of ZP5461 reducing HFD intake and body weight change at 24 h, 48 h, and 72 h postinjection compared with vehicle (Fig. 1D; for food intake post hoc analyses, vehicle different from all doses of ZP5461 at these times, all P < 0.05; for body weight change, main effect of drug: F4,60 = 110.80, P < 0.05; time × drug interaction: F8,120 = 51.51, P < 0.05; post hoc analyses, vehicle significantly different from all ZP5461 doses at these times, all P < 0.05). Collectively, these results demonstrate that the novel amylin receptor agonist ZP5461 potently suppresses food intake and weight gain in chow-fed and in HFD-fed rats, and importantly, that a single injection of ZP5461 produces prolonged effects on these parameters up to 72 h postinjection.
Next, we assessed whether the energy intake-suppressive effects of a given dose of ZP5461 varied between chow- and HFD-fed rats at 24, 48, or 72 h postinjection. As shown in Fig. 2, the percent suppression of intake was higher for HFD-fed rats compared with chow-fed rats for all doses of drug at each of these times (drug × time × diet interaction, F6,162 = 6.57, P < 0.05; post hoc analyses, significant differences between chow and HFD for all drug dose/time combinations, all P < 0.05).
Figure 2.
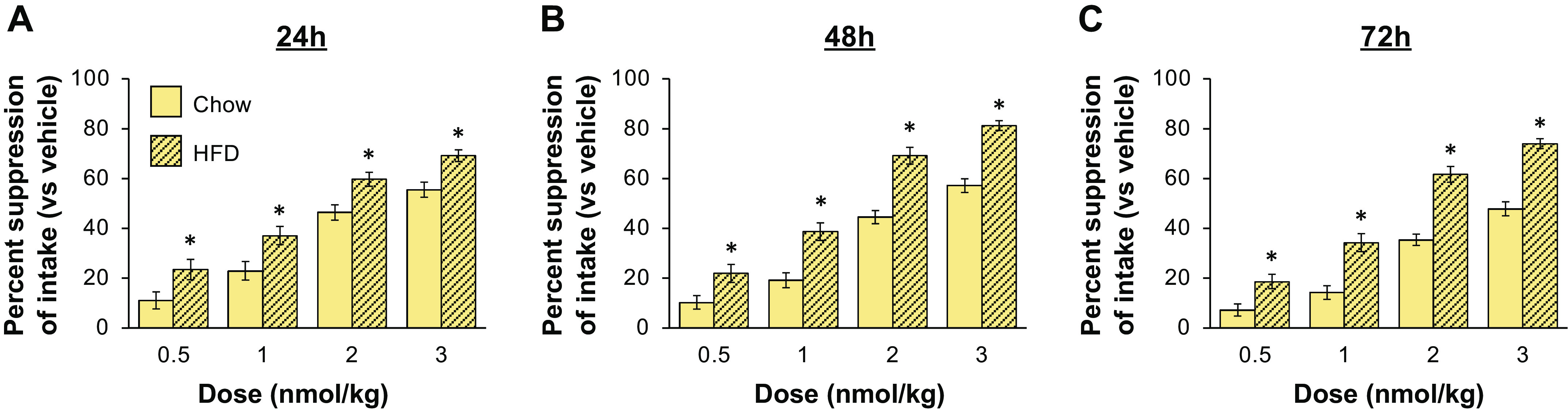
ZP5461 produces a greater percent suppression of energy intake in HFD-fed rats compared with chow-fed rats. Percent suppression of energy intake for each dose/diet combination was calculated using energy intake under vehicle conditions for that diet. At 24 h (A), 48 h (B), and 72 h (C) postinjection, the percent suppression of energy intake by ZP5461 was greater for HFD-fed rats at all doses of drug tested (n = 13–16 per diet condition). Data were analyzed using repeated measures ANOVA with Student–Newman–Keuls post hoc analyses. Data are shown as means ± SE. *P < 0.05. HFD, high-fat diet.
Ability of ZP5461 to Suppress Cumulative Body Weight Change and Food Intake Is Dependent on CTR Expression in the DVC but Is Not Sustained with Chronic Administration
Animals were body weight-matched (P = 0.228) and divided into two viral cohorts. Each group received bilateral DVC infusion of either AAV1-Cb7-CI-eGFP (AAV-Control; n = 13) or AAV1-U6-shRCalc93-CB7-EGFP (AAV-CTR-KD; n = 17). Every 48 h for 15 days post-AAV administration (24 h recording on day 15), body weight (Fig. 3A) and food intake (Fig. 3B) were recorded. During these 2 wk, there was a significant interaction between virus across time to increase body weight (AAV × time: F8,224 = 4.929, P < 0.0001) and food intake (AAV × time: F7,196 = 4.812, P < 0.0001). By day 14, animals receiving AAV-CTR-KD were significantly hyperphagic compared with AAV-Control. Total body weight change across the 2-wk post-AAV period was not significantly different (Fig. 3C).
Figure 3.
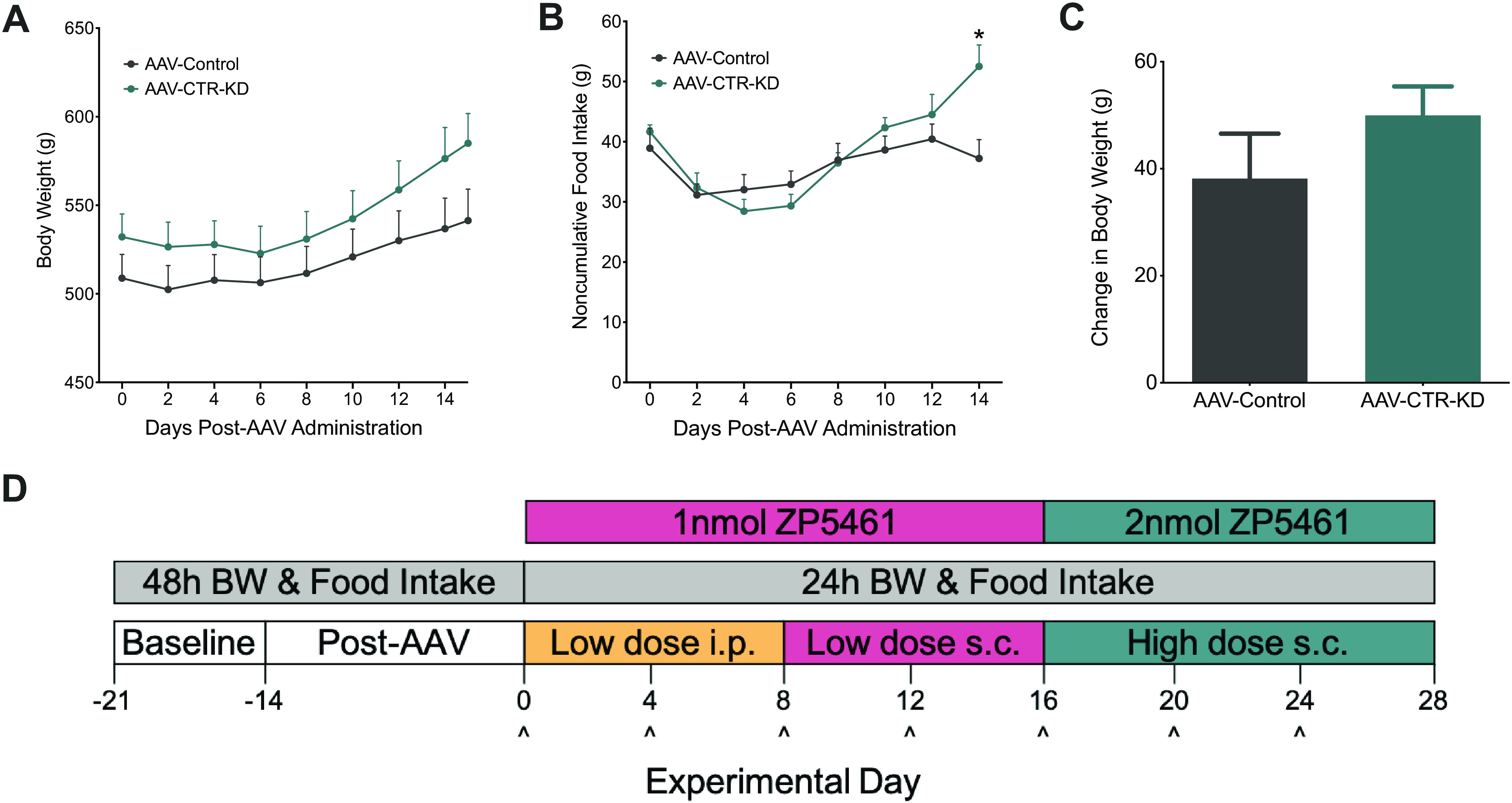
Baseline body weight and food intake following viral administration into the DVC. In HFD-fed male rats, body weight and food intake were recorded every 48 h for 2 wk after DVC administration of either control (AAV-Control; n = 13) or CTR knockdown (AAV-CTR-KD; n = 17) virus. A: 48 h (and 24 h) body weight was not significantly different between the viral cohorts. B: AAV-CTR-KD consumed significantly more HFD at 2 wk after AAV administration. C: over the 15-day pretreatment period, total change in body weight was not impacted by viral administration. D: experimental design of the injection timeline for low- and high-dose ZP5461 administration, ^injection day. Data expressed as means ± SE, *P < 0.05. Data analyzed by two-way ANOVA with Newman–Keuls post hoc analyses (A, B) or unpaired t test (C). BW, body weight; CTR, calcitonin receptor; DVC, dorsal vagal complex; HFD, high-fat diet; i.p., intraperitoneally; sc, subcutaneously.
Immediately following the post-AAV period, we conducted a behavioral assessment of the efficacy of chronically administered ZP5461 to suppress food intake and body weight in AAV-Control compared with AAV-CTR-KD across a span of 4 wk (Fig. 3D). For sake of clarity, significant main effects and interactions from ANOVAs for this study are summarized in Supplemental Table S1; see https://doi.org/10.6084/m9.figshare.14932857.v1. Throughout the experiment, daily body weight (Fig. 4A) and the total body weight change (Fig. 4A, inset) were not significantly different between the AAV-control animals receiving ZP5461 or vehicle. The AAV-Control, ZP5461 animals weighed significantly less than the AAV-CTR-KD, vehicle animals from day 3 to day 20 (Fig. 4A). We observed no main effect of drug treatment on daily body weight; however, on treatment days 3–7, there was a significant main effect of viral treatment. Assessment of daily change in body weight (Fig. 4B) revealed an initial ability of ZP5461 to suppress body weight gain, and this effect was attenuated in AAV-CTR-KD rats. We observed a more pronounced and sustained effect of ZP5461 to suppress cumulative body weight change, and again this was dependent on CTR expression in the DVC (Fig. 4C), as AAV-CTR-KD rats treated with ZP5461 had a significantly higher cumulative body weight change than AAV-Control rats treated with ZP5461 from day 1 to day 20 with the exception of day 18. We observed significant reductions in daily noncumulative (Fig. 5A) and cumulative (Fig. 5B) food intake following ZP5461 during portions of the low-dose treatment phase of the experiment. This appeared to be at least partially dependent on CTR expression in the DVC, as noncumulative food intake in ZP5461-treated AAV-CTR-KD rats was higher than that of ZP5461-treated AAV-Control rats on several days. However, by the fourth round of injections, ZP5461 administration failed to suppress HFD food intake.
Figure 4.
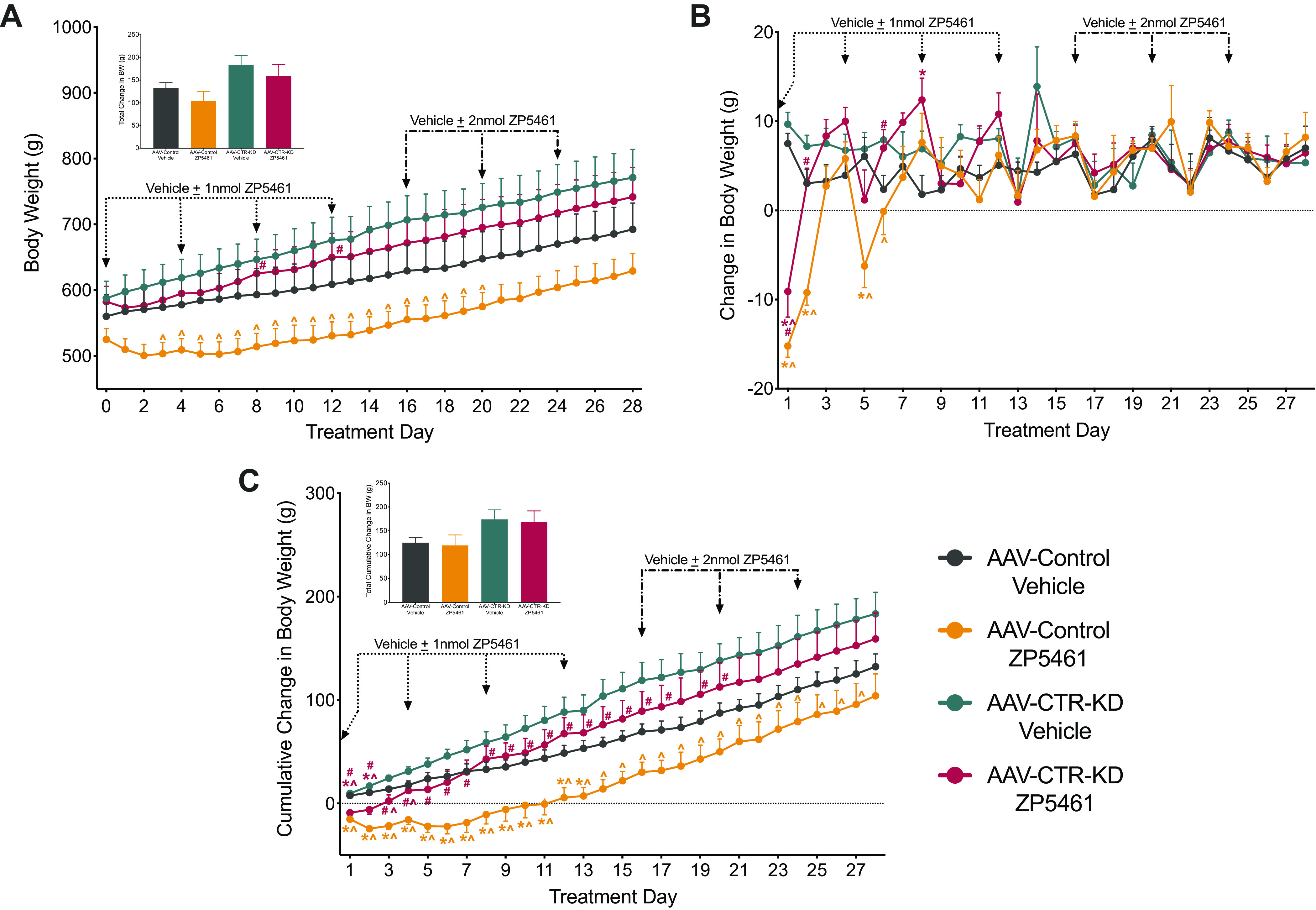
Chronic administration of ZP5461 does not alter long-term body weight in HFD-fed rats, irrespective of CTR-KD. ZP5461 was peripherally administered every 96 h for 4 wk, first at 1 nmol/kg/mL (dotted arrow) and then at 2 nmol/kg/mL (dashed arrow). A: body weight change across 4 wk of injection treatments; total change in body weight from treatment day 0 to day 28 (A, inset). B: daily noncumulative change in body weight and cumulative change in body weight across the experiment (C); total cumulative change in body weight from treatment day 0 to day 28 (C, inset). Data represented as means ± SE. Control virus, AAV-Control; CTR knockdown virus, AAV-CTR-KD. AAV-Control, vehicle (n = 6), AAV-Control, ZP5461 (n = 7), AAV-CTR-KD, vehicle (n = 8), AAV-CTR-KD, ZP5461 (n = 9). Data analyzed by two-way ANOVA at each time point with Newman–Keuls post hoc analyses. Color of the significance symbols indicates associated cohort; * vs. AAV-Control, vehicle-treated; ^ vs. AAV-CTR-KD, vehicle-treated; # vs. AAV-Control, ZP5461-treated. CTR, calcitonin receptor; HFD, high-fat diet.
Figure 5.
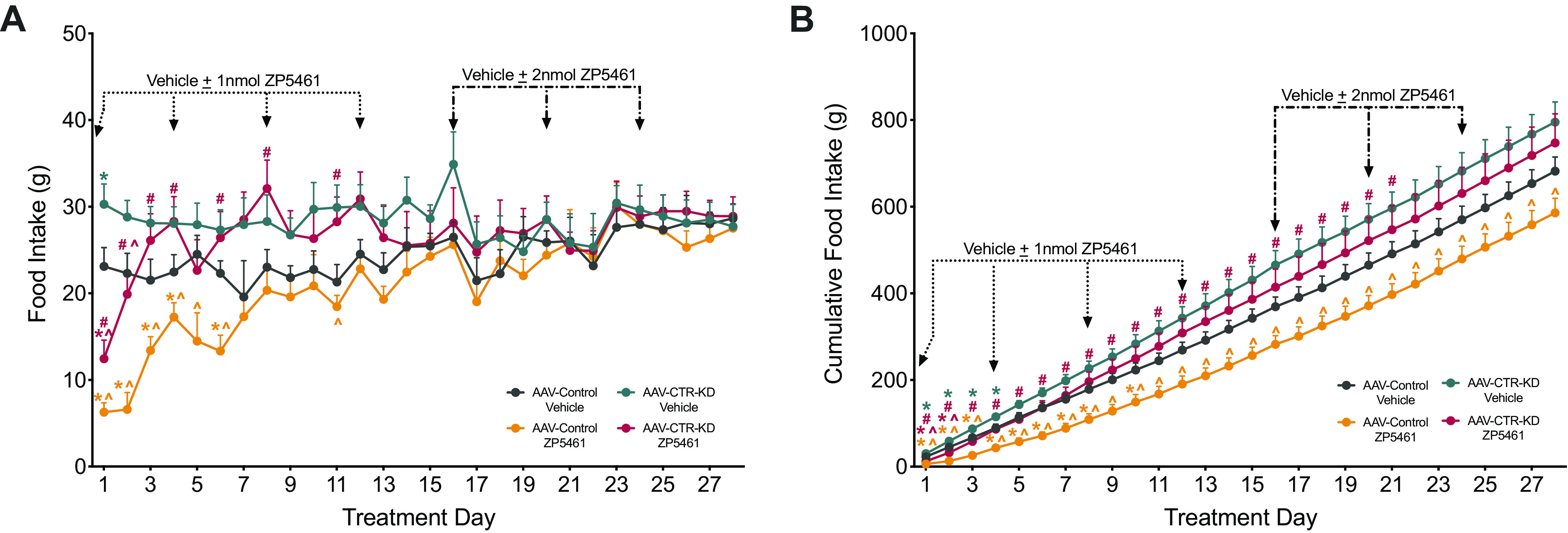
Chronic administration of ZP5461 fails to produce long-term suppression of food intake, irrespective of CTR expression in the DVC. Every 96 hours for 4 wk, animals received peripheral administration of ZP5461, first at 1nmol/kg/mL (dotted line) and then at 2 nmol/kg/mL (dashed line). A: daily noncumulative food intake measurements across 4 wk of injection treatments. B: cumulative food intake. Data represented as means ± SE. Control virus, AAV-Control; CTR knockdown virus, AAV-CTR-KD. AAV-Control, vehicle (n = 6), AAV-Control, ZP5461 (n = 7), AAV-CTR-KD, vehicle (n = 8), AAV-CTR-KD, ZP5461 (n = 9). Data analyzed by two-way ANOVA at each time point with Newman–Keuls post hoc analyses. Color of the significance symbols indicates associated cohort; * vs. AAV-Control, vehicle-treated; ^ vs. AAV-CTR-KD, vehicle-treated; # vs. AAV-Control, ZP5461-treated. CTR, calcitonin receptor; DVC, dorsal vagal complex.
Upon completion of the chronic administration experiment, we euthanized the animals to assess changes in fat pad mass and quantification of DVC CTR mRNA expression. We observed a main effect of viral treatment to increase inguinal (AAV: F1,26 = 6.57, P = 0.02), retroperitoneal (AAV: F1,26 = 4.29, P = 0.05), and perirenal (AAV: F1,26 = 5.21, P = 0.03) fat pad mass, with no changes observed in epididymal fat pad mass (Fig. 6A). ZP5461 treatment significantly reduced retroperitoneal fat pad mass (drug: F1,26 = 4.50, P = 0.04). We did not detect a significant interaction between AAV-treatment and ZP5461 to affect fat pad weight. We observed an ∼77% reduction in CTR mRNA expression in DVC for rats receiving AAV-CTR-KD viral infusion relative to rats infused with the AAV-Control (Fig. 6B).
Figure 6.
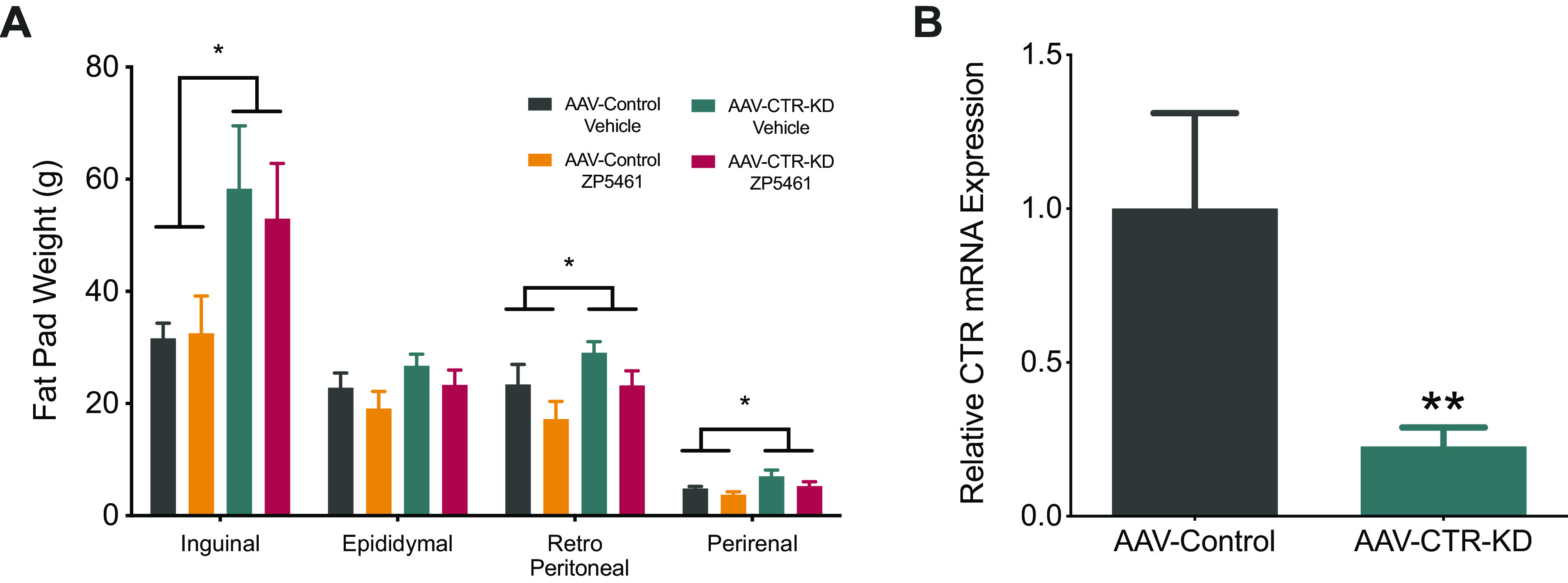
AAV-CTR-KD rats exhibited reduced DVC CTR expression and increased fat mass. At the completion of the chronic experiment, fat pads mass and CTR expression in the DVC was determined. A: there was a significant main effect of AV to increase inguinal, retroperitoneal, and perirenal fat pad mass in AAV-CTR-KD animals (n = 17) compared with AAV-Control rats (n = 13), regardless of ZP5461 administration. B: qPCR analysis of CTR expression from DVC micro-punches demonstrated a significant reduction (∼77%) of CTR gene expression in AAV-CTR-KD rats compared with AAV-Control. Data represented as means ± SE. Data analyzed by either two-way ANOVA (A) or unpaired t test (B), *P < 0.05, **P < 0.01. CTR, calcitonin receptor; DVC, dorsal vagal complex.
DISCUSSION
Pharmacotherapeutic strategies targeting the amylin system have potential clinical utility in treating obesity (1–4), but a limitation of native amylin and the only existing FDA-approved amylin analog, pramlintide, is their relatively short half-life (27–29). Here, we evaluated feeding and body weight suppression in rats after administering a novel long-acting amylin/calcitonin receptor agonist, ZP5461. Acute dose-response studies indicated that the compound produced a prolonged suppression of food intake and body weight in rats maintained on chow or, separately, on a high-fat diet. In addition, ZP5461 produced a greater suppression of energy intake in HFD-fed rats compared with chow-fed animals. Further studies began to probe the mechanisms by which ZP5461 might produce these hypophagic and body weight-suppressive effects by examining the impact of DVC calcitonin receptor knockdown on the ability of ZP5461 to promote energy balance. The DVC was selected as the area of focus for this experiment, given the known relevance of the AP and adjacent NTS for the energy balance effects of amylin and amylin agonists (14, 17–20). Here, we sought to test the efficacy of chronic ZP5461 administration in diet-induced obese rats and determine whether the anorectic effects of ZP5461 are partially dependent on CTR expression in the DVC. Viral administration of AAV-CTR-KD resulted in an approximate 77% reduction in DVC-CTR mRNA expression. As expected, during the initial 2-wk period post-AAV administration, AAV-CTR-KD significantly increased food intake. This hyperphagia was transient, emerging for only a few days at ∼2 wk post-AAV. The first two rounds of ZP5461 treatment effectively suppressed 24-h food intake and body weight in both the Control and CTR-KD cohorts. We observed a significant attenuation of the intake- and body-weight suppressive effects of ZP5461 in the AAV-CTR-KD rats supporting our hypothesis that CTR expression in the DVC mediates ZP5461 action. However, assessment across the entire experiment revealed no sustained effect of ZP5461 to suppress body weight or food intake in the AAV-Control or AAV-CTR-KD rats, suggesting ZP5461 is not sufficient for sustained weight loss at this dose and frequency. Terminal fat pad analysis supports this conclusion; AAV-CTR-KD significantly increased inguinal, retroperitoneal, and perirenal mass, but ZP5461 did not decrease fat pad mass in either viral cohort.
The observation that DVC-CTR knockdown partially attenuates the early body weight gain-suppressive effects of ZP5461 supports the hypothesis that the DVC amylin/calcitonin receptors serve as a site of action for systemic ZP5461. Nuclei of the DVC express the components of the amylin receptor (17, 38, 39) and are known to be critical sites of action for the ability of systemic amylin and amylin agonists to promote negative energy balance (14, 19, 20, 40). This partial blunting of the effects of ZP5461 by DVC CTR knockdown may be attributable to the fact that AAV administration does not completely abolish DVC CTR expression, and perhaps remaining CTRs are sufficient to maintain a portion of the response. Moreover, other central nervous system (CNS) sites, including mesolimbic and hypothalamic nuclei, express amylin receptor components and have been established as sites of action for the anorectic effects of amylin/calcitonin agonists (41–47), raising the possibility that ZP5461 may be able to access other relevant nuclei to exert its effects. Therefore, future studies will need to examine the possible contributions of other CNS amylin/calcitonin receptor populations in the effects of ZP5461 on feeding and weight gain.
It is also important to note that the AAV-mediated knockdown of DVC CTR alone produced hyperphagia and increases in fat pad weight. These food intake results are consistent with data from mice with CTR depletion in the AP/NTS that demonstrate the importance of this receptor population for the hypophagic response to systemic amylin and the amylin/CTR agonist salmon calcitonin (43). Our results also shed light onto the role of these receptors in fat pad weight, although the mechanisms by which DVC CTR contribute to or control fat pad weight remain unclear. Amylin influences sympathetic nervous activation of brown adipose tissue (48, 49), but it is unknown whether sympathetic innervation of white adipose is also altered by amylin receptor activation. Furthermore, it remains an open question whether DVC CTR activity may engage other neurohormonal mechanisms to affect fat pad weight.
The lack of an effect of ZP5461 on feeding or body weight gain at later times in our chronic administration study was unexpected, given the findings of our initial acute dose-response studies. These first experiments used within-subjects designs, and thus rats received repeated injections of ZP5461 or vehicle, yet ZP5461 produced robust suppression of intake and body weight gain in the acute studies. The differences in the effects of ZP5461 between the acute and chronic studies may relate to the dosing and timing of chronically administered ZP5461. We only tested one dosing/timing treatment regimen of ZP5461 in our chronic experiment, and perhaps a different dose of the compound or a different time course between each administration of compound would have produced a more sustained suppression of feeding and body weight. The development of drug tolerance following repeated drug administration mirrors a similar study examining the efficacy of a long-acting amylin analog in body weight suppression (50). It will also be important to assess whether ZP5461 produces side effects like nausea. Although many studies suggest that amylin receptor agonists do not produce nausea/malaise (34, 46, 51–53), pramlintide can produce nausea in some individuals (8, 25, 26). Critically, however, this nausea does not appear to be required for weight loss, as individuals who did and did not experience nausea during pramlintide treatment had comparable degrees of body weight reduction (25, 26). The potential contribution of these variables to the anorectic and weight-suppressive effects of ZP5461 remains to be addressed in future studies.
Perspectives and Significance
Pramlintide, currently the only FDA-approved amylin-based pharmacotherapy, is used to treat diabetes, but its short half-life is an important consideration for its potential utility in treating obesity (27–29). Like pramlintide, other amylin analogs such as davalintide, and amylin/calcitonin receptor agonists like salmon calcitonin promote negative energy balance (54–58), suggesting that the amylin/calcitonin receptor system is a viable target for pharmacotherapies to reduce feeding and body weight. A longer-acting amylin/calcitonin receptor agonist that is able to decrease energy intake and body weight represents a critical step in identifying novel potential treatments for obesity. The present findings suggest that systemic administration of ZP5461 produces robust suppression of food intake and weight gain in rats in a dose-responsive manner. However, repeated administration of the compound fails to produce a sustained reduction in energy intake and body weight, perhaps a fact attributed to the dose and timing of injections tested. Nonetheless, it is still encouraging that ZP5461 produces pronounced weight loss and provides an opportunity for future combinatorial pharmacotherapies that include amylin/calcitonin receptor agonist for treating obesity. As current findings also revealed that the DVC CTRs are a relevant site of action for acute energy balance effects of ZP5461, future studies with combination therapies may wish to explore further the neural substrates of the DVC that can be simultaneously targeted along with those targeted by ZP5461 to enhance energy intake and body weight loss.
SUPPLEMENTAL DATA
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.14932857.v1.
GRANTS
Support for these studies was provided by an investigator-initiated sponsored award from Zealand Pharma and Boehringer-Ingelheim (to M.R.H. and E.G.M-B.) and National Institutes of Health Grant-DK105155 (to M.R.H.).
DISCLOSURES
J.S. is employed by Zealand Pharma A/S. T.B-P. is employed by Boehringer-Ingelheim Pharma GmbH & Co. KG. R. J. was previously employed by Zealand Pharma A/S. M.R.H. receives separate financial support from Boehringer-Ingelheim and Eli Lilly & Co. that was not used for these studies.
AUTHOR CONTRIBUTIONS
J.S., T.B-P., R.J., M.R.H., and E.G.M-B. conceived and designed research; L.M.S., L.E.M., R.L., K.K-L., S.M.F., and E.G.M-B. performed experiments; L.M.S., M.R.H., and E.G.M-B. analyzed data; L.M.S., M.R.H., and E.G.M-B. interpreted results of experiments; L.M.S. and E.G.M-B. prepared figures; L.M.S., M.R.H., and E.G.M-B. drafted manuscript; L.M.S., L.E.M., R.L., K.K-L., S.M.F., J.S., T.B-P., R.J., M.R.H., and E.G.M-B. edited and revised manuscript; L.M.S., L.E.M., R.L., K.K-L., S.M.F., J.S., T.B-P., R.J., M.R.H., and E.G.M-B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Bradford Hamilton for valuable insight on this project, as well as Jack Chen, Alexis Corini, Misgana Ghidewon, Anh Cao, and Dr. David Reiner for helpful technical assistance in these experiments.
REFERENCES
- 1.Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: pharmacology, physiology, and clinical potential. Pharmacol Rev 67: 564–600, 2015. doi: 10.1124/pr.115.010629. [DOI] [PubMed] [Google Scholar]
- 2.Mietlicki-Baase EG, Hayes MR. Amylin activates distributed CNS nuclei to control energy balance. Physiol Behav 136: 39–46, 2014. doi: 10.1016/j.physbeh.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams DM, Nawaz A, Evans M. Drug therapy in obesity: a review of current and emerging treatments. Diabetes Ther 11: 1199–1216, 2020. doi: 10.1007/s13300-020-00816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zakariassen HL, John LM, Lutz TA. Central control of energy balance by amylin and calcitonin receptor agonists and their potential for treatment of metabolic diseases. Basic Clin Pharmacol Toxicol 127: 163–177, 2020. doi: 10.1111/bcpt.13427. [DOI] [PubMed] [Google Scholar]
- 5.Chapman I, Parker B, Doran S, Feinle-Bisset C, Wishart J, Strobel S, Wang Y, Burns C, Lush C, Weyer C, Horowitz M. Effect of pramlintide on satiety and food intake in obese subjects and subjects with type 2 diabetes. Diabetologia 48: 838–848, 2005. doi: 10.1007/s00125-005-1732-4. [DOI] [PubMed] [Google Scholar]
- 6.Lutz TA. The role of amylin in the control of energy homeostasis. Am J Physiol Regul Integr Comp Physiol 298: R1475–R1484, 2010. doi: 10.1152/ajpregu.00703.2009. [DOI] [PubMed] [Google Scholar]
- 7.Lutz TA, Del Prete E, Scharrer E. Reduction of food intake in rats by intraperitoneal injection of low doses of amylin. Physiol Behav 55: 891–895, 1994. doi: 10.1016/0031-9384(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 8.Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, Koda JE, Weyer C. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 17: 1736–1743, 2009. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA 105: 7257–7262, 2008. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rushing PA, Hagan MM, Seeley RJ, Lutz TA, Woods SC. Amylin: a novel action in the brain to reduce body weight. Endocrinology 141: 850–853, 2000. doi: 10.1210/endo.141.2.7378. [DOI] [PubMed] [Google Scholar]
- 11.Smith SR, Blundell JE, Burns C, Ellero C, Schroeder BE, Kesty NC, Chen KS, Halseth AE, Lush CW, Weyer C. Pramlintide treatment reduces 24-h caloric intake and meal sizes and improves control of eating in obese subjects: a 6-wk translational research study. Am J Physiol Endocrinol Metab 293: E620–E627, 2007. doi: 10.1152/ajpendo.00217.2007. [DOI] [PubMed] [Google Scholar]
- 12.Boccia L, Gamakharia S, Coester B, Whiting L, Lutz TA, Le Foll C. Amylin brain circuitry. Peptides 132: 170366, 2020. doi: 10.1016/j.peptides.2020.170366. [DOI] [PubMed] [Google Scholar]
- 13.Kern KA, Mietlicki-Baase EG. Distributed amylin receptor signaling and its influence on motivated behavior. Physiol Behav 222: 112958, 2020. doi: 10.1016/j.physbeh.2020.112958. [DOI] [PubMed] [Google Scholar]
- 14.Braegger FE, Asarian L, Dahl K, Lutz TA, Boyle CN. The role of the area postrema in the anorectic effects of amylin and salmon calcitonin: behavioral and neuronal phenotyping. Eur J Neurosci 40: 3055–3066, 2014. doi: 10.1111/ejn.12672. [DOI] [PubMed] [Google Scholar]
- 15.Riediger T, Zuend D, Becskei C, Lutz TA. The anorectic hormone amylin contributes to feeding-related changes of neuronal activity in key structures of the gut-brain axis. Am J Physiol Regul Integr Comp Physiol 286: R114–R122, 2004. doi: 10.1152/ajpregu.00333.2003. [DOI] [PubMed] [Google Scholar]
- 16.Rowland NE, Crews EC, Gentry RM. Comparison of Fos induced in rat brain by GLP-1 and amylin. Regul Pept 71: 171–174, 1997. doi: 10.1016/s0167-0115(97)01034-3. [DOI] [PubMed] [Google Scholar]
- 17.Liberini CG, Boyle CN, Cifani C, Venniro M, Hope BT, Lutz TA. Amylin receptor components and the leptin receptor are co-expressed in single rat area postrema neurons. Eur J Neurosci 43: 653–661, 2016. doi: 10.1111/ejn.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mollet A, Gilg S, Riediger T, Lutz TA. Infusion of the amylin antagonist AC 187 into the area postrema increases food intake in rats. Physiol Behav 81: 149–155, 2004. doi: 10.1016/j.physbeh.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Lutz TA, Mollet A, Rushing PA, Riediger T, Scharrer E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. Int J Obes Relat Metab Disord 25: 1005–1011, 2001. doi: 10.1038/sj.ijo.0801664. [DOI] [PubMed] [Google Scholar]
- 20.Lutz TA, Senn M, Althaus J, Del Prete E, Ehrensperger F, Scharrer E. Lesion of the area postrema/nucleus of the solitary tract (AP/NTS) attenuates the anorectic effects of amylin and calcitonin gene-related peptide (CGRP) in rats. Peptides 19: 309–317, 1998. doi: 10.1016/S0196-9781(97)00292-1. [DOI] [PubMed] [Google Scholar]
- 21.Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides 19: 883–889, 1998. doi: 10.1016/s0196-9781(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 22.Banks WA, Kastin AJ, Maness LM, Huang W, Jaspan JB. Permeability of the blood-brain barrier to amylin. Life Sci 57: 1993–2001, 1995. doi: 10.1016/0024-3205(95)02197-q. [DOI] [PubMed] [Google Scholar]
- 23.Chawla PS, Kochar MS. What's new in clinical pharmacology and therapeutics. WMJ 105: 24–29, 2006. [PubMed] [Google Scholar]
- 24.Younk LM, Mikeladze M, Davis SN. Pramlintide and the treatment of diabetes: a review of the data since its introduction. Expert Opin Pharmacother 12: 1439–1451, 2011. doi: 10.1517/14656566.2011.581663. [DOI] [PubMed] [Google Scholar]
- 25.Smith SR, Aronne LJ, Burns CM, Kesty NC, Halseth AE, Weyer C. Sustained weight loss following 12-month pramlintide treatment as an adjunct to lifestyle intervention in obesity. Diabetes Care 31: 1816–1823, 2008. doi: 10.2337/dc08-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aronne L, Fujioka K, Aroda V, Chen K, Halseth A, Kesty NC, Burns C, Lush CW, Weyer C. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo-controlled, dose-escalation study. J Clin Endocrinol Metab 92: 2977–2983, 2007. doi: 10.1210/jc.2006-2003. [DOI] [PubMed] [Google Scholar]
- 27.Young A. Tissue expression and secretion of amylin. Adv Pharmacol 52: 19–45, 2005. doi: 10.1016/S1054-3589(05)52002-7. [DOI] [PubMed] [Google Scholar]
- 28.McQueen J. Pramlintide acetate. Am J Health Syst Pharm 62: 2363–2372, 2005. doi: 10.2146/ajhp050341. [DOI] [PubMed] [Google Scholar]
- 29.Young A. Clinical studies. Adv Pharmacol 52: 289–320, 2005. doi: 10.1016/S1054-3589(05)52018-0. [DOI] [PubMed] [Google Scholar]
- 30.Christopoulos G, Perry KJ, Morfis M, Tilakaratne N, Gao Y, Fraser NJ, Main MJ, Foord SM, Sexton PM. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol 56: 235–242, 1999. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- 31.Hay DL, Christopoulos G, Christopoulos A, Sexton PM. Amylin receptors: molecular composition and pharmacology. Biochem Soc Trans 32: 865–867, 2004. doi: 10.1042/BST0320865. [DOI] [PubMed] [Google Scholar]
- 32.Lam S. American Diabetes Association - 76th Scientific Sessions (June 10-14, 2016 - New Orleans, Louisiana, USA). Drugs Today (Barc) 52: 361–366, 2016. doi: 10.1358/dot.2016.52.6.2516437. [DOI] [PubMed] [Google Scholar]
- 33.Skarbaliene J, Just R. Anti-Diabetic Effects of Novel Long-Acting Amylin Analogues ZP4982 and ZP5461 in ZDF Rats (Poster), the American Diabetes Association’s (ADA) 76th Scientific Sessions (Online). New Orleans, LA, 2016. https://static1.squarespace.com/static/58983777d1758e28995640b4/t/5d4953643b48f80001ed9c5a/1565086566205/Jolanta+Skarbaliene+ADA+poster+Anti-diabetic+effects+of+novel+long-acting+amylin+analogues.pdf. [2021 May, 20]. [Google Scholar]
- 34.Mietlicki-Baase EG, Reiner DJ, Cone JJ, Olivos DR, McGrath LE, Zimmer DJ, Roitman MF, Hayes MR. Amylin modulates the mesolimbic dopamine system to control energy balance. Neuropsychopharmacology 40: 372–385, 2015. doi: 10.1038/npp.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. London: Academic Press, 2005. [Google Scholar]
- 36.Alhadeff AL, Mergler BD, Zimmer DJ, Turner CA, Reiner DJ, Schmidt HD, Grill HJ, Hayes MR. Endogenous glucagon-like peptide-1 receptor signaling in the nucleus tractus solitarius is required for food intake control. Neuropsychopharmacology 42: 1471–1479, 2017. doi: 10.1038/npp.2016.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med 12: 917–924, 2006. [Erratum in Nat Med 16: 237, 2010]. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 38.Barth SW, Riediger T, Lutz TA, Rechkemmer G. Peripheral amylin activates circumventricular organs expressing calcitonin receptor a/b subtypes and receptor-activity modifying proteins in the rat. Brain Res 997: 97–102, 2004. doi: 10.1016/j.brainres.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 39.Becskei C, Riediger T, Zund D, Wookey P, Lutz TA. Immunohistochemical mapping of calcitonin receptors in the adult rat brain. Brain Res 1030: 221–233, 2004. doi: 10.1016/j.brainres.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Roth JD, Maier H, Chen S, Roland BL. Implications of amylin receptor agonism: integrated neurohormonal mechanisms and therapeutic applications. Arch Neurol 66: 306–310, 2009. doi: 10.1001/archneurol.2008.581. [DOI] [PubMed] [Google Scholar]
- 41.Baisley SK, Baldo BA. Amylin receptor signaling in the nucleus accumbens negatively modulates mu-opioid-driven feeding. Neuropsychopharmacology 39: 3009–3017, 2014. doi: 10.1038/npp.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baldo BA, Kelley AE. Amylin infusion into rat nucleus accumbens potently depresses motor activity and ingestive behavior. Am J Physiol Regul Integr Comp Physiol 281: R1232–R1242, 2001. doi: 10.1152/ajpregu.2001.281.4.R1232. [DOI] [PubMed] [Google Scholar]
- 43.Coester B, Koester-Hegmann C, Lutz TA, Le Foll C. Amylin/calcitonin receptor-mediated signaling in POMC neurons influences energy balance and locomotor activity in chow-fed male mice. Diabetes 69: 1110–1125, 2020. doi: 10.2337/db19-0849. [DOI] [PubMed] [Google Scholar]
- 44.Le Foll C, Johnson MD, Dunn-Meynell AA, Boyle CN, Lutz TA, Levin BE. Amylin-induced central IL-6 production enhances ventromedial hypothalamic leptin signaling. Diabetes 64: 1621–1631, 2015. doi: 10.2337/db14-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lutz TA, Coester B, Whiting L, Dunn-Meynell AA, Boyle CN, Bouret SG, Levin BE, Le Foll C. Amylin selectively signals onto POMC neurons in the arcuate nucleus of the hypothalamus. Diabetes 67: 805–817, 2018. doi: 10.2337/db17-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mietlicki-Baase EG, Rupprecht LE, Olivos DR, Zimmer DJ, Alter MD, Pierce RC, Schmidt HD, Hayes MR. Amylin receptor signaling in the ventral tegmental area is physiologically relevant for the control of food intake. Neuropsychopharmacology 38: 1685–1697, 2013. doi: 10.1038/npp.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nashawi H, Gustafson TJ, Mietlicki-Baase EG. Palatable food access impacts expression of amylin receptor components in the mesocorticolimbic system. Exp Physiol 105: 1012–1024, 2020. doi: 10.1113/EP088356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan K, Li Q, Pan D, Liu H, Li P, Hai R, Du C. Effects of amylin on food intake and body weight via sympathetic innervation of the interscapular brown adipose tissue. Nutr Neurosci: 1–13, 2020.doi: 10.1080/1028415X.2020.1752998. [DOI] [PubMed] [Google Scholar]
- 49.Fernandes-Santos C, Zhang Z, Morgan DA, Guo DF, Russo AF, Rahmouni K. Amylin acts in the central nervous system to increase sympathetic nerve activity. Endocrinology 154: 2481–2488, 2013. doi: 10.1210/en.2012-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brings A, Borghardt JM, Skarbaliene J, Baader-Pagler T, Deryabina MA, Rist W, Scheuerer S. Modeling energy intake and body weight effects of a long-acting amylin analogue. J Pharmacokinet Pharmacodyn 45: 215–233, 2018. doi: 10.1007/s10928-017-9557-6. [DOI] [PubMed] [Google Scholar]
- 51.Lutz TA, Geary N, Szabady MM, Del Prete E, Scharrer E. Amylin decreases meal size in rats. Physiol Behav 58: 1197–1202, 1995. doi: 10.1016/0031-9384(95)02067-5. [DOI] [PubMed] [Google Scholar]
- 52.Morley JE, Suarez MD, Mattamal M, Flood JF. Amylin and food intake in mice: effects on motivation to eat and mechanism of action. Pharmacol Biochem Behav 56: 123–129, 1997. doi: 10.1016/S0091-3057(96)00168-2. [DOI] [PubMed] [Google Scholar]
- 53.Rushing PA, Seeley RJ, Air EL, Lutz TA, Woods SC. Acute 3rd-ventricular amylin infusion potently reduces food intake but does not produce aversive consequences. Peptides 23: 985–988, 2002. doi: 10.1016/S0196-9781(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 54.Liberini CG, Koch-Laskowski K, Shaulson E, McGrath LE, Lipsky RK, Lhamo R, Ghidewon M, Ling T, Stein LM, Hayes MR. Combined Amylin/GLP-1 pharmacotherapy to promote and sustain long-lasting weight loss. Sci Rep 9: 8447, 2019. 8447doi: 10.1038/s41598-019-44591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lutz TA, Tschudy S, Rushing PA, Scharrer E. Amylin receptors mediate the anorectic action of salmon calcitonin (sCT). Peptides 21: 233–238, 2000. doi: 10.1016/s0196-9781(99)00208-9. [DOI] [PubMed] [Google Scholar]
- 56.Mack CM, Smith PA, Athanacio JR, Xu K, Wilson JK, Reynolds JM, Jodka CM, Lu MG, Parkes DG. Glucoregulatory effects and prolonged duration of action of davalintide: a novel amylinomimetic peptide. Diabetes Obes Metab 13: 1105–1113, 2011. doi: 10.1111/j.1463-1326.2011.01465.x. [DOI] [PubMed] [Google Scholar]
- 57.Mack CM, Soares CJ, Wilson JK, Athanacio JR, Turek VF, Trevaskis JL, Roth JD, Smith PA, Gedulin B, Jodka CM, Roland BL, Adams SH, Lwin A, Herich J, Laugero KD, Vu C, Pittner R, Paterniti JR, Hanley M, Ghosh S, Parkes DG. Davalintide (AC2307), a novel amylin-mimetic peptide: enhanced pharmacological properties over native amylin to reduce food intake and body weight. Int J Obes (Lond) 34: 385–395, 2010. doi: 10.1038/ijo.2009.238. [DOI] [PubMed] [Google Scholar]
- 58.Reidelberger RD, Kelsey L, Heimann D. Effects of amylin-related peptides on food intake, meal patterns, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 282: R1395–R1404, 2002. doi: 10.1152/ajpregu.00597.2001. [DOI] [PubMed] [Google Scholar]


