Abstract
Preeclampsia (PE) is characterized by maternal hypertension, intrauterine growth restriction, and increased cytolytic natural killer cells (cNKs), which secrete interferon γ (IFNγ). However, the precise role of IFNγ in contributing to PE pathophysiology remains unclear. Using the reduced uterine perfusion pressure (RUPP) rat model of placental ischemia, we tested the hypothesis that neutralization of IFNγ in RUPPs will decrease placental reactive oxygen species (ROS) and improve vascular function resulting in decreased MAP and improved fetal growth. On gestation day (GD) 14, the RUPP procedure was performed and on GDs 15 and 18, a subset of normal pregnant rats (NP) and RUPP rats were injected with 10 μg/kg of an anti-rat IFNγ monoclonal antibody. On GD 18, uterine artery resistance index (UARI) was measured via Doppler ultrasound and on GD 19, mean arterial pressure (MAP) was measured, animals were euthanized, and blood and tissues were collected for analysis. Increased MAP was observed in RUPP rats compared with NP and was reduced in RUPP + anti-IFNγ. Placental ROS was also increased in RUPP rats compared with NP rats and was normalized in RUPP + anti-IFNγ. Fetal and placental weights were reduced in RUPP rats, but were not improved following anti-IFNγ treatment. However, UARI was elevated in RUPP compared with NP rats and was reduced in RUPP + anti-IFNγ. In conclusion, we observed that IFNγ neutralization reduced MAP, UARI, and placental ROS in RUPP recipients. These data suggest that IFNγ is a potential mechanism by which cNKs contribute to PE pathophysiology and may represent a therapeutic target to improve maternal outcomes in PE.
Keywords: interferon γ, preeclampsia, reactive oxygen species, soluble Fms-like tyrosine kinase-1
INTRODUCTION
Preeclampsia (PE) is a hypertensive obstetric disorder that affects 2%–8% of pregnancies worldwide and is a leading cause of maternal and fetal morbidity and mortality (1). The disorder is characterized by new onset hypertension (HTN) occurring after the twentieth week of pregnancy, intrauterine growth restriction (IUGR), and end-organ dysfunction that can manifest as proteinuria, liver dysfunction, and cerebral abnormalities (1). The precise etiology of PE development and progression is not entirely understood, but the most prevalent hypothesis suggests it is initiated by shallow trophoblast invasion and impaired spiral artery remodeling resulting in decreased placental blood flow (2). The ischemic conditions in the placenta lead to an angiogenic and immune imbalance that lead to systemic maternal endothelial dysfunction, which is the major driver of the clinical manifestations of PE (2, 3). The immune imbalance has been demonstrated in several studies which have observed increased levels of inflammatory cytokines interferon γ (IFNγ), tumor necrosis factor α (TNF-α), and interleukin (IL)-6, as well as decreased anti-inflammatory cytokines IL-10 and IL-4 in women with PE compared with normotensive pregnant women (4–6).
IFN γ, a potent proinflammatory cytokine, has been shown to be important early in pregnancy for implantation as well as the spiral artery remodeling that is important in the establishment of placental blood flow (7). However, elevated concentrations of IFNγ have been shown to lead to increased rates of spontaneous abortion and fetal abnormalities in mice, rats, and rabbits suggesting that the balance of this cytokine is important in pregnancy (8–10). Of the two major cell types that produce IFNγ, T Helper (TH1) and decidual natural killer cells (dNKs), the latter make up the majority of lymphocytes in the decidua. In PE, dNKs have been demonstrated to shift toward an activated phenotype characterized by increased IFNγ secretion (11–14). This suggests that they are largely responsible for the increased levels of IFNγ observed in PE (11, 15, 16). Although elevated IFNγ has been documented in women with PE and animal models, the precise role of this cytokine in PE pathophysiology remains largely uninvestigated (17).
Our group has investigated the role of the immune system and inflammatory cytokines in PE using the reduced uterine perfusion pressure (RUPP) rat model of placental ischemia. This model demonstrates many characteristics of PE in women including HTN, IUGR, vascular dysfunction, and immune activation (18–20). In previous studies, we have observed increased NK activation in the circulation and placentas of RUPP rats compared with normal pregnant (NP) animals and increased levels of circulating and placental IFNγ in RUPP rats compared with control rats (21–23). Our most recent publication demonstrated PE-like characteristics and increased circulating and placental IFNγ after intravenous adoptive transfer of RUPP-stimulated NK cells into pregnant rats (22). We have also observed that the increased placental IFNγ observed in the RUPP was normalized following NK cell depletion (21). Data from these two studies indicate that NK cells are a significant source of IFNγ in the RUPP model (21). Although maternal blood pressure and fetal weight were improved following NK cell depletion, we have not directly assessed the role that IFNγ plays in the HTN, IUGR, and vascular dysfunction observed in this model. Therefore, in this study we tested the hypothesis that neutralization of IFNγ in RUPP will both improve vascular function and decrease oxidative stress resulting in decreased mean arterial pressure (MAP) and improved fetal growth. To test this hypothesis, we injected a subset of NP and RUPP rats with 10 μg/kg of anti-rat-IFNγ monoclonal antibody, and evaluated changes in MAP, fetal and placental weight, and uterine artery resistance index (UARI). We also measured levels of soluble Fms-like tyrosine kinase-1 (sFlt-1), circulating and placental vascular endothelial factor (VEGF), placental reactive oxygen species (ROS), and placental endothelial nitric oxide synthase (eNOS) expression and phosphorylation.
MATERIALS AND METHODS
Timed pregnant, 12- to 13-wk-old, Sprague–Dawley rats purchased from Envigo RMS, Inc. (Indianapolis, IN) were used in this study. The animals were delivered to the Center for Comparative Research at the University of Mississippi Medical Center on day 10 or 11 of gestation and weighed ∼250–260 g upon arrival. The animals were housed in a temperature-controlled room (23°C) with a 12:12-h light-dark cycle and maintained on Teklad 8640 diet (Envigo). The rats were group housed until surgery was performed, and rats were randomly assigned to experimental groups. All experimental procedures conducted in this study were in accordance with the National Institute of Health guidelines for use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center.
Reduction in Uterine Perfusion Pressure
On gestation day (GD) 14, a subset of timed pregnant Sprague–Dawley rats, underwent the RUPP surgery under isoflurane anesthesia delivered by an anesthesia apparatus (Ohio Medical Products, Madison, WI) as previously described (23–26). Briefly, a midline incision was made, and a constrictive silver clip (0.203 mm) was placed on the abdominal aorta superior to the iliac bifurcation. To prevent compensatory blood flow via the ovarian arteries, restrictive silver clips (0.100 mm) were applied to the bilateral uterine arcades at the ovarian end. The animals received carprofen immediately after surgery and 24 h postsurgery (5 mg/kg) to control for postoperative pain. Rats were excluded when the procedure resulted in total reabsorption of all fetuses.
IFNγ Neutralization
On GDs 15 and 18, rats received intraperitoneal injections of saline vehicle (NP: n = 9 and RUPP: n = 9) or 10 μg/kg anti-rat IFNγ monoclonal antibody (R&D systems Cat. No. MAB585-500) (NP + anti-IFNγ: n = 9 and RUPP + anti-IFNγ: n = 9). We performed a dose-response analysis using 1 μg/kg, 5 μg/kg, and 10 μg/kg of the anti-rat IFNγ monoclonal antibody to determine the dose used throughout the study.
Measurement of Mean Arterial Pressure in Conscious Rats
Under isoflurane anesthesia, 0.58 mm inner diameter (ID) × 0.99 mm outer diameter (OD) vinyl catheter tubing (Scientific Commodities Inc., Lake Havasu City, AZ) was implanted into the carotid arteries and tunneled to the back of the neck on GD 18 for the measurement of mean arterial pressure. On GD 19, rats were placed in individual restrainers and conscious MAP was monitored with a pressure transducer (Powerlab, AD Instruments, Colorado Springs, CO). MAP was recorded for 30 min after a 30-min stabilization period.
Sample Collection
After MAP measurement, the animals were anesthetized for blood and tissue collection. Total litter size as well as the number of live pups was recorded. Placental and fetal weights were recorded for each dam and averaged. Randomly selected placentas (consisting of the decidua, junctional, and labyrinth zones) were snap frozen in liquid nitrogen and stored at −80°C until analyses.
Placental Reactive Oxygen Species Measurement
Superoxide production in the placenta was measured using the lucigenin technique, as previously described by our laboratory (23, 27, 28). Briefly, placentas chosen at random from all groups were snap frozen in liquid nitrogen immediately after collection and stored at −80°C until further processing. Placentas were homogenized using the Bio-Rad Cell Lysis Kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. The tissue lysate was incubated with lucigenin (Sigma-Aldrich, St. Louis, MO) at a concentration of 5 μM in the presence or absence of 0.2 μM NADPH (Sigma No. N9660). The samples were allowed to equilibrate for 15 min in the dark, and the luminescence was measured for 10 s on a BioTek Plate Reader (BioTek, Winooski, VT). Luminescence was recorded as relative light units per minute (RLUs/min). An assay blank containing lucigenin with no homogenate was subtracted from the reading before transformation of the data. Each sample was run in triplicate and the average was used for data transformation. The protein concentration was measured using a protein assay with BSA standards (Pierce, Rockford, IL). All data were normalized to protein concentration (expressed as RLU/min/mg protein).
Determination of Circulating Total Nitrate/Nitrite
Blood was collected in EDTA tubes and spun at 3,000 g for 10 min at 4°C. The collected plasma was assessed for total nitrate/nitrite in duplicate using the Nitrate/Nitrite Colorimetric Assay Kit (catalog no. 780001, Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions.
Determination of Placental and Circulating Cytokines and Angiogenic Factors
Circulating and placental IFN-γ and IL-6, and placental VEGF were measured using the Bio-Plex Pro Rat Cytokine Immunoassay Kit (Bio-Rad) according to the manufacturer’s instructions. Circulating sFlt-1 levels were measured in collected plasma via ELISA (R&D systems, Minneapolis, MN; Cat. No. MVR100) according to the manufacturer’s instructions. Circulating VEGF was measured in collected serum via ELISA (Boster Biological Technology, Pleasanton, CA; Cat. No. EK054) according to the manufacturer’s instructions. All sample analyses were performed in duplicate. Protein concentration of the placental homogenates was measured using a protein assay with BSA standards. All placental data were normalized to protein (expressed as pg/mg).
Determination of Uterine Artery Resistance Index
On GD 18, rats from all four groups were shaved and all abdominal hair was removed with a depilation cream. Power Doppler velocimetry measurements were performed on anesthetized pregnant dams at an imaging station with a Vevo 770 unit (Visual Sonics, Toronto, Canada) using a 30-Hz transducer and an insonating angle <30° as previously described (24, 29). The peak systolic flow velocity (PSV) and end diastolic flow velocity (EDV) were measured bilaterally. The UARI was calculated using the following formula: UARI = (PSV − EDV)/PSV. UARI was determined using the mean measurements of three wave forms per side.
Western Blot Analysis of Placental eNOS Expression and Phosphorylation
Placental eNOS expression and phosphorylation at S1176 was assessed in placental tissue via Western blot as previously described (24, 29). Briefly, placentas were homogenized in cold RIPA-buffer and protein extracts, separated by SDS-PAGE using a polyacrylamide gel (4%–20%). Protein were transferred onto nitrocellulose membranes (Bio‐Rad) and blocked with Blocking Buffer (LI-COR Biosciences, Lincoln, NE) for 1 h at room temperature. Membranes were incubated overnight at 4°C with primary antibody directed against eNOS (1:250; BD Transduction Laboratories, San Jose, CA. Cat. No. 610296) or Ser1177(human)/Ser1176 (rodent) peNOS (BD Transduction Laboratories Cat. No. 612392). This was followed by anti-mouse IgG IRDye 800Dx conjugated secondary antibody (1:10,000; Rockland, Gilbertsville, PA Cat. No. 610731002) for 1 h at room temperature and scanned using the Odyssey CLx Imager (LI-COR Biosciences, Lincoln, NE). The intensity of specific bands was quantified by densitometry using Image J (National Institutes of Health) and the expression of eNOS or eNOS pS1176 was normalized to β-actin (1:2,000; Millipore Sigma, Darmstadt, Germany Cat. No. A1978).
Western Blot Analysis of Placental Signal Transducer and Activator of Transcription 3
Placentas were homogenized in cold RIPA buffer, and protein extracts were separated by SDS-PAGE using a polyacrylamide gel (Any KD). Protein were transferred onto nitrocellulose membranes (Bio‐Rad) and blocked with blocking buffer (LI-COR Biosciences) for 1 h at room temperature. Membranes were incubated overnight at 4°C with primary antibody directed against signal transducer and activator of transcription 3 (STAT3; 1:1,000; Cell Signaling Technology, Danvers, MA; Cat. No. 9139). This was followed by goat anti-mouse IgG IRDye 800 CW conjugated secondary antibody (1:13,333; LI-COR Biosciences; Cat. No. 926-32210) for 1 h at room temperature and scanned using the Odyssey CLx Imager (LI-COR Biosciences). The intensity of specific bands was quantified by densitometry using Image J (National Institutes of Health) and the expression of STAT3 was normalized to GAPDH (1:1,000; Cell Signaling Cat. No. 2118), which was visualized with the goat anti-rabbit IRDye 680RD secondary antibody (1:13,333; LI-COR Biosciences; Cat. No. 926–68071).
Statistical Analysis
All statistical analyses were performed with GraphPad Prism 8 software (San Diego, CA). All data are expressed as mean ± SEM per group. Statistical analyses of the dose-response data were performed using one-way ANOVA with multiple comparisons followed by Tukey’s post hoc multiple comparison test. All remaining analyses were performed using two-way ANOVA with multiple comparisons followed by Tukey’s post hoc multiple comparison test if a significant interaction occurred. A value of P < 0.05 was considered statistically significant.
RESULTS
Effects of IFNγ Inhibition on Mean Arterial Pressure and Intrauterine Growth Restriction on Pregnant Rats
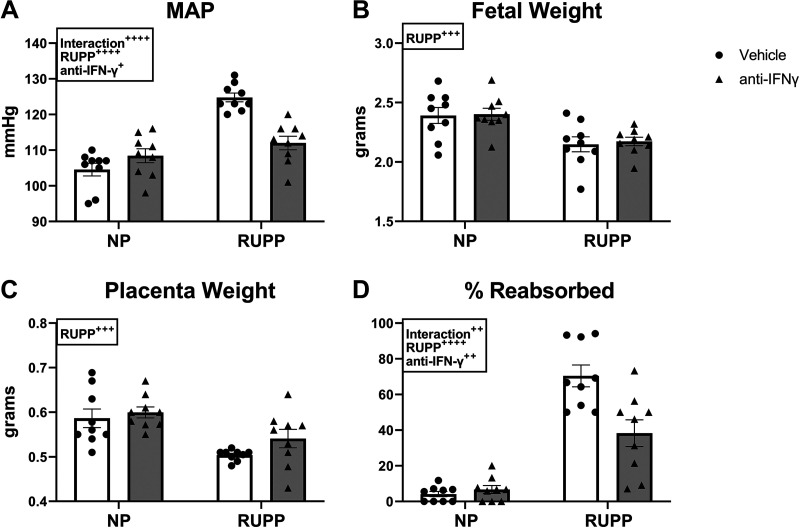
We performed a dose-response experiment using 1, 5, and 10 μg/kg of an anti-rat IFNγ monoclonal antibody to determine which dose would be used throughout the study. Although fetal and placental weight were unchanged by any dose, our results demonstrate that the 10 μg/kg significantly reduced MAP in RUPP rats (P = 0.0447 vs. RUPP) and was thus used throughout the remainder of the study (Supplemental Fig. S1; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.13379729). Following this, all anti-IFNγ treatment groups received the 10 μg/kg dose. We have previously published studies comparing MAP, fetal, and placental weight in NP versus Sham operated rats and reported no differences between the groups (30). Therefore, NP rats served as controls in the current study. MAP was 105 ± 2 mmHg in NP and was unchanged in NP + anti-IFNγ at 108 ± 2 mmHg (P = 0.40 vs. NP). MAP was elevated to 125 ± 1 mmHg in RUPP (P < 0.0001 vs. NP) and was reduced to 112 ± 2 mmHg in RUPP + anti-IFNγ (P < 0.0001 vs. RUPP; Fig. 1A). Mean fetal weight was 2.4 ± 0.07 g in NP and was unchanged in NP + anti-IFNγ at 2.4 ± 0.05. Mean fetal weight was significantly reduced to 2.1 ± 0.06 g in RUPP and was unchanged in RUPP + anti-IFNγ at 2.2 ± 0.04 g (Fig. 1B). Mean placental weight was 0.59 ± 0.02 g in NP and was unchanged in NP + anti-IFNγ at 0.60 ± 0.1 g. Mean placental weight was significantly reduced to 0.50 ± 0.004 g in RUPP and was unchanged at 0.54 ± 0.02 g in RUPP + anti-IFNγ (Fig. 1C). The mean percentage of reabsorbed pups was 4 ± 1% in NP, 7 ± 2% in NP + anti-IFNγ (P = 0.98 vs. NP), and was elevated to 70 ± 6% in RUPP (P < 0.0001 vs. NP). Percentage of reabsorbed pups was reduced in RUPP + anti-IFNγ at 38 ± 7% (P = 0.0004 vs. RUPP) although this was still significantly elevated compared with NP (P = 0.0002 vs. NP; Fig. 1D).
Figure 1.
Effect of interferon γ (IFNγ) neutralization on mean arterial pressure (MAP) and intrauterine growth restriction in pregnant rats. On gestation day (GD) 14, the reduced uterine perfusion pressure (RUPP) procedure was performed on a subset of normal pregnant (NP) rats. On GDs 15 and 18, vehicle or 10 μg/kg of anti-IFNγ antibody was injected intraperitoneally in a subset of NP and RUPP rats. On GD 19, conscious mean arterial pressure (A) was measured and fetal weights (B), placental weights (C), and fetal resorptions (D) were recorded under isoflurane anesthesia. NP: n = 9 rats; RUPP: n = 9 rats; NP + anti-IFNγ: n = 9 rats; RUPP + anti-IFNγ: n = 9 rats. All data are expressed as mean ± SEM. Statistical analyses were performed using two-way ANOVA with multiple comparisons followed by Tukey’s post hoc test. +P < 0.05; ++P < 0.01; +++P < 0.001; ++++P < 0.0001.
Effects of IFNγ Inhibition on Uterine Artery Resistance, Plasma Total Nitrate, and Placental ROS in Pregnant Rats
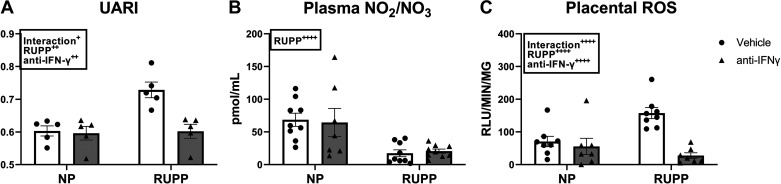
UARI was 0.6 ± 0.02 in NP and 0.6 ± 0.02 in NP + anti-IFNγ (P = 0.995 vs. NP) and was elevated in RUPP at 0.73 ± 0.02 (P = 0.0028 vs. NP). UARI was normalized to 0.6 ± 0.2 in RUPP + anti-IFNγ (P = 0.003 vs. RUPP; Fig. 2A). Plasma levels of total nitrate were 69 ± 10 pmol/mL in NP and 64 ± 22 pmol/mL in NP + anti-IFNγ. Plasma total nitrate was significantly reduced in RUPP + vehicle at 17 ± 5 pmol/mL and did not improve in RUPP + anti-IFNγ (21 ± 3 pmol/mL; Fig. 2B). Baseline placental ROS was 17 ± 6 RLU/min/mg and 16 ± 8 RLU/min/mg in RUPP and RUPP + anti-IFNγ, respectively (P > 0.9999 vs. RUPP, data not shown). NADPH-stimulated placental ROS was 71 ± 20 RLU/min/mg in NP and 56 ± 25 RLU/min/mg in NP + anti-IFNγ (P = 0.93 vs. NP). This was elevated to 157 ± 17 RLU/min/mg in RUPP (P = 0.006 vs. NP) and was reduced to 27 ± 9 RLU/min/mg in RUPP + anti-IFNγ (P < 0.0001 vs. RUPP; Fig. 2C). Addition of NADPH increased RUPP placental ROS generation eightfold (P < 0.0001 vs. RUPP baseline) whereas placental ROS remained in RUPP + anti-IFNγ was similar to baseline (Supplemental Fig. S2).
Figure 2.
Effect of interferon gamma (IFNγ) neutralization on uterine artery resistance index (UARI), plasma total nitrate, and placental reactive oxygen species (ROS). On gestation day (GD) 14, the reduced uterine perfusion pressure (RUPP) procedure was performed on a subset of normal pregnant (NP) rats. On GDs 15 and 18, vehicle or 10 μg/kg of anti-IFNγ antibody was injected intraperitoneally in a subset of NP and RUPP rats. On GD 18, UARI (A) was measured via Doppler ultrasound and on GD 19, blood and placentas were collected under isoflurane anesthesia and frozen for analysis of plasma total nitrate (B) and placental ROS (C). NP: n = 5–9 rats; RUPP: n = 5–9 rats; NP + anti-IFNγ: n = 5–9 rats; RUPP + anti-IFNγ: n = 5–9 rats. All data are expressed as means ± SEM. Statistical analyses were performed using two-way ANOVA with multiple comparisons followed by Tukey’s post hoc test. +P < 0.05; ++P < 0.01; ++++P < 0.0001.
Effects of IFNγ Inhibition on Angiogenic Factors in Pregnant Rats
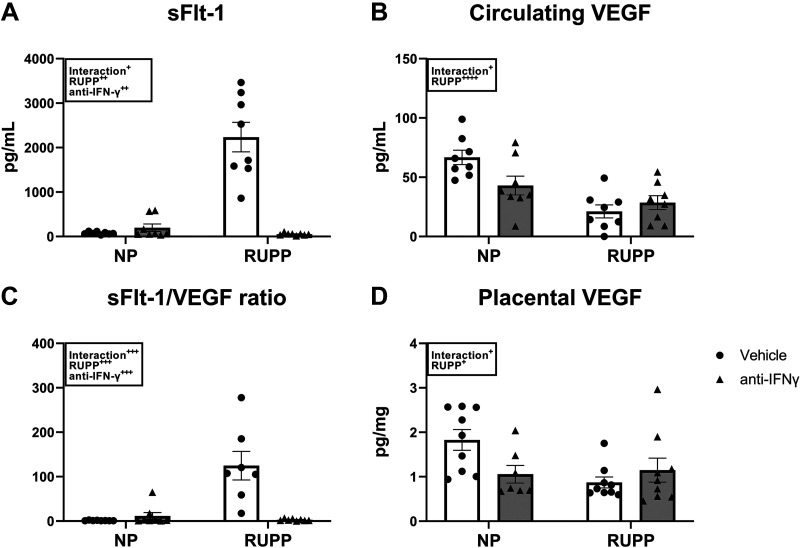
Soluble FMS-like tyrosine kinase-1 (sFlt-1), an anti-angiogenic factor, was 60 ± 10 pg/mL in NP and 200 ± 83 pg/mL in NP + anti-IFNγ (P = 0.94 vs. NP). This was significantly elevated in RUPP at 2,236 ± 333 pg/mL (P < 0.0001 vs. NP) and was normalized to 44 ± 5 pg/mL in RUPP + anti-IFNγ (P < 0.0001 vs. RUPP; Fig. 3A). Circulating VEGF was 67 ± 6 pg/mL in NP and 43 ± 8 pg/mL in NP + anti-IFNγ (P = 0.08 vs. NP). This was significantly reduced in RUPP at 21 ± 5 pg/mL (P = 0.0002 vs. NP) as well as in RUPP + anti-IFNγ at 29 ± 6 pg/mL (P = 0.96 vs. RUPP; Fig. 3B). The mean sFlt-1-to-VEGF ratio was 1 ± 0.2 in NP and 11 ± 8 in NP + anti-IFNγ (P = 0.96 vs. NP). This was significantly elevated at 125 ± 32 in RUPP (P < 0.0001 vs. NP) and was normalized to 3 ± 0.8 in RUPP + anti-IFNγ (P < 0.0001 vs. RUPP; Fig. 3C). Placental VEGF was 1.8 ± 0.2 pg/mg in NP and 1.1 ± 0.2 pg/mg in NP + anti-IFNγ (P = 0.10 vs. NP). Placental VEGF was reduced to 0.9 ± 0.1 pg/mg in RUPP (P = 0.02 vs. NP) and remained unchanged at 1.1 ± 0.3 pg/mg in RUPP + anti-IFNγ (P = 0.79 vs. RUPP; Fig. 3D).
Figure 3.
Effect of interferon γ (IFNγ) neutralization on circulating and placental angiogenic factors. On gestation day (GD) 14, the reduced uterine perfusion pressure (RUPP) procedure was performed on a subset of normal pregnant (NP) rats. On GDs 15 and 18, vehicle or 10 μg/kg of anti-IFNγ antibody was injected intraperitoneally in a subset of NP and RUPP rats. On GD 19, blood and placentas were collected under isoflurane anesthesia and processed for further analysis. Circulating soluble Fms-Like tyrosine kinase-1 (sFlt-1; A) and vascular endothelial growth factor (VEGF; B) were measured via ELISA and the sFlt-1-to-VEGF ratio (C) is shown. Placental VEGF (D) was measured using the Bio-Plex Pro Rat Cytokine Immunoassay Kit. NP: n = 8–9 rats; RUPP: n = 8–9 rats; NP + anti-IFNγ: n = 7–9 rats; RUPP + anti-IFNγ: n = 8–9 rats. All data are expressed as means ± SEM. Statistical analyses were performed using two-way ANOVA with multiple comparisons followed by Tukey’s post hoc test. +P < 0.05; ++P < 0.01; +++P < 0.001; ++++P < 0.0001.
Effects of IFNγ Inhibition on Circulating and Placental Cytokines in Pregnant Rats
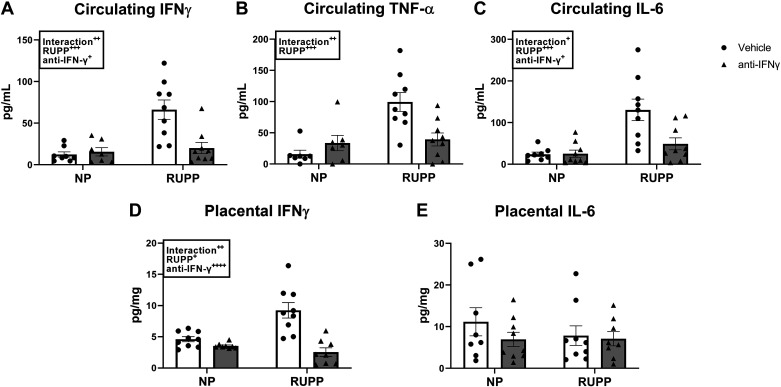
Circulating IFNγ was 12 ± 3 pg/mL in NP and 16 ± 5 pg/mL in NP + anti-IFNγ (P = 0.9999 vs. NP). This was elevated in RUPP to 66 ± 11 pg/mL (P = 0.0002 vs. NP) and was normalized to 20 ± 7 pg/mL in RUPP recipients of anti-IFNγ (P = 0.0009 vs. RUPP; Fig. 4A). Circulating TNF-α was also elevated in RUPP at 99 ± 15 pg/mL (P = 0.0002 vs. NP) compared with 16 ± 6 pg/mL and 34 ± 12 pg/mL in NP and NP + anti-IFNγ, respectively (P = 0.76 vs. NP). Levels of circulating TNF-α were reduced to 39 ± 10 pg/mL in RUPP + anti-IFNγ (P = 0.004 vs. RUPP; Fig. 4B). Similarly, circulating IL-6 was 24 ± 5 pg/mL in NP and 25 ± 9 pg/mL in NP + anti-IFNγ (P > 0.9999 vs. NP). IL-6 was elevated in the circulation of RUPP rats at 130 ± 26 pg/mL (P = 0.0003 vs. NP) and was normalized to 49 ± 14 pg/mL in RUPP + anti-IFNγ (P = 0.005 vs. RUPP; Fig. 4C). In the placenta, IFNγ was 4.6 ± 0.4 pg/mg in NP and 3.5 ± 0.2 pg/mg in NP + anti-IFNγ (P = 0.76 vs. NP). Placental IFNγ was elevated to 9.2 ± 1.2 pg/mg in RUPP (P = 0.0007 vs. NP) and was reduced to 2.5 ± 0.7 pg/mg in RUPP + anti-IFNγ (P < 0.0001 vs. RUPP; Fig. 4D). Finally, placental IL-6 remained unchanged and was 11 ± 3 pg/mg in NP, 6.9 ± 2 pg/mg in NP + anti-IFNγ, 7.8 ± 2 pg/mg in RUPP, and 6.3 ± 2 pg/mg in RUPP + anti-IFNγ (Fig. 4E).
Figure 4.
Effect of interferon γ (IFNγ) neutralization on circulating and placental cytokines. On gestation day (GD) 14, the reduced uterine perfusion pressure (RUPP) procedure was performed on a subset of normal pregnant (NP) rats. On GDs 15 and 18, vehicle or 10 μg/kg of anti-IFNγ antibody was injected intraperitoneally in a subset of NP and RUPP rats. On GD 19, blood and placentas were collected under isoflurane anesthesia and frozen for analysis of circulating IFNγ (A), circulating TNF-α (B), circulating IL-6 (C), placental IFNγ (D), and placental IL-6 (E). NP: n = 7–9 rats; RUPP: n = 8–9 rats; NP + anti-IFNγ: n = 7–9 rats; RUPP + anti-IFNγ: n = 8–9 rats. All data are expressed as means ± SEM. Statistical analyses were performed using two-way ANOVA with multiple comparisons followed by Tukey’s post hoc test. +P < 0.05; ++P < 0.01; +++P < 0.001; ++++P < 0.0001.
Effects of IFNγ Inhibition on Placental Expression and Phosphorylation of eNOS in Pregnant Rats
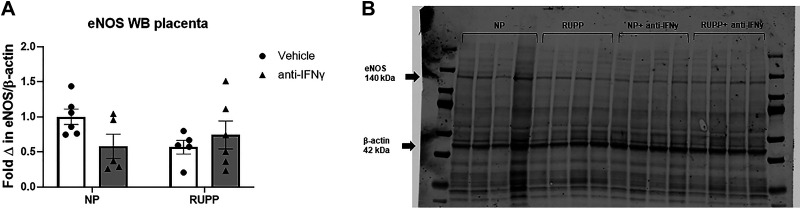
Placental eNOS expression was unchanged in all groups with a mean fold change of 1 ± 0.1 in NP, 0.6 ± 0.1 in NP + anti-IFNγ, 0.6 ± 0.1 in RUPP, and 0.7 ± 0.2 in RUPP + anti-IFNγ (Fig. 5A). A representative blot is shown in Fig. 5B.
Figure 5.
Western blot analysis of placental endothelial nitric oxide synthase (eNOS) expression. On gestation day (GD) 14, the reduced uterine perfusion pressure (RUPP) procedure was performed on a subset of normal pregnant (NP) rats. On GDs 15 and 18, vehicle or 10 μg/kg of anti-IFNγ antibody was injected intraperitoneally in a subset of NP and RUPP rats. On GD 19, placentas were collected following euthanasia and processed for analysis of placental eNOS expression. A: quantified fold change of placental eNOS expression normalized to β-actin. B: a representative blot of eNOS and β-actin expression in all groups is shown. NP: n = 6 rats; RUPP: n = 6 rats; NP + anti-IFNγ: n = 5 rats; RUPP + anti-IFNγ: n = 6 rats. All data are expressed as means ± SEM. Statistical analyses were performed using two-way ANOVA with multiple comparisons followed by Tukey’s post hoc test.
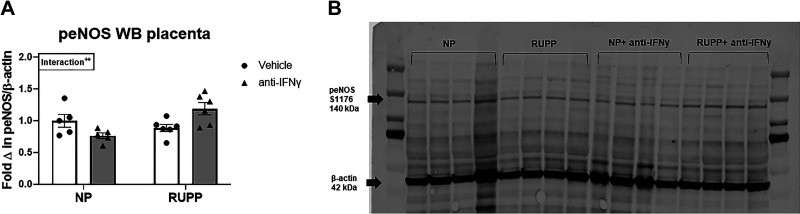
Fold change in placental eNOS pS1176 expression was 1 ± 0.1 in NP, 0.8 ± 0.05 in NP + anti-IFNγ, 0.9 ± 0.1 in RUPP, and 1.2 ± 0.1 in RUPP + anti-IFNγ (P = 0.008 vs. NP + anti-IFNγ; Fig. 6A). A representative blot is shown in Fig. 6B.
Figure 6.
Western blot analysis of placental endothelial nitric oxide synthase (eNOS) S1176 phosphorylation. On gestation day (GD) 14, the reduced uterine perfusion pressure (RUPP) procedure was performed on a subset of normal pregnant (NP) rats. On GDs 15 and 18, vehicle or 10 μg/kg of anti-IFNγ antibody was injected intraperitoneally in a subset of NP and RUPP rats. On GD 19, placentas were collected following euthanization and processed for analysis of placental eNOS expression. A: quantified fold change of placental eNOS expression normalized to β-actin. B: a representative blot of eNOS pS1176 and β-actin expression in all groups is shown. NP: n = 5 rats; RUPP: n = 6 rats; NP + anti-IFNγ: n = 5 rats; RUPP + anti-IFNγ: n = 6 rats. All data are expressed as means ± SEM. Statistical analyses were performed using two-way ANOVA with multiple comparisons followed by Tukey’s post hoc test. ++P < 0.01.
Effects of IFNγ Inhibition on Placental STAT3 Expression in Pregnant Rats
Mean placental STAT3 expression was 1.4 ± 0.1 in NP and trended toward a decrease at 0.99 ± 0.06 in RUPP (interaction P = 0.075). Placental STAT3 was 1.6 ± 0.2 in NP + anti-IFNγ and was significantly elevated in RUPP + anti-IFNγ to 1.8 ± 0.1 (P = 0.02 vs. RUPP; Supplemental Fig. S3A). Representative blots are shown in Supplemental Fig. S3B.
DISCUSSION
Although IFNγ is believed to be an important cytokine in the establishment and maintenance of pregnancy(16), elevated levels of IFNγ have been observed in PE and are implicated in its pathophysiology (17, 31). However, the direct role of this cytokine in PE pathophysiology has not been thoroughly investigated. Therefore, in this study we tested the hypothesis that neutralization of IFNγ in RUPP rats will decrease oxidative stress and improve vascular function resulting in decreased MAP and increased fetal growth. The results and hypothesized mechanism are illustrated in Supplemental Fig. S4. We observed that while fetal growth was not improved, MAP, UARI, and fetal reabsorptions were significantly reduced in RUPP + anti-IFNγ compared with RUPP rats that received vehicle. We also observed that placental ROS and circulating sFlt-1 were significantly reduced. Thus, we have demonstrated that IFNγ plays a direct role in the MAP and vascular dysfunction observed in RUPP rats and may be a therapeutic target to lower blood pressure in women with PE.
Part of the focus that has driven our study of IFNγ in PE is an interest in the role of NK cells in PE pathophysiology and progression. Decidual NK cells (dNKs) make up around 70% of lymphocytes in the decidua and placental bed of human pregnancies and play a supportive role in placentation and spiral artery remodeling through the release of trophoblast chemoattractant and cytokines (32–34). dNKs are also prominent producers of IFNγ, and several studies in animal models have identified IFNγ as an essential cytokine involved in spiral artery remodeling. IFNγ-deficient pregnant mice experienced significant fetal loss and impaired decidual development (35) while in rabbits, IFNγ administration reduced implantation percentage and disrupted the maternal endometrium (36). During normal pregnancy, dNKs decrease in number as pregnancy progresses, and many studies show that they are nearly absent at term. In contrast, women with PE or other pregnancy disorders have increased numbers of dNKs in both the decidua and inner villous layers of the placenta late in pregnancy (14, 37–40). dNKs have been shown to shift toward an activated phenotype characterized by increased cytotoxicity and IFNγ secretion (11–14). Therefore, dNKs are believed to be a major source of the increased in circulating and decidual IFNγ observed in women with PE (6, 41–43). In addition, the T cell imbalance observed in PE can also contribute to increased IFNγ levels. Although normal pregnancy is predominated by T helper (TH) 2 cells, a number of studies suggest that women with preeclampsia have an increase in TH1 cells that can contribute to the increased levels serum levels of Type 1 inflammatory cytokines such as IFNγ and TNF-α (4, 6, 17, 44, 45). Macrophages are also significant immune cells that reside in the placental bed, making up around 20% of the leukocytes in this compartment (46). Similar to T cells, macrophages can display a number of phenotypes including immunomodulatory M2 macrophages and proinflammatory M1 macrophages (47). Studies have shown that decidual M2 macrophages are the predominant phenotype in the second trimester of normal pregnancies, but a predominance of M1 macrophages is observed in preeclamptic women (48–50). M1 macrophages are also known to secrete IFNγ and may represent a source of excess IFNγ in PE (47). Despite its essential role in early pregnancy, excess IFNγ has been shown to be detrimental to pregnancy in mice, rats, and rabbits, and IFNγ has been observed to be increased in animal models of PE and hypertension during pregnancy (8–10, 21, 36, 51, 52). These data suggest that further research examining the role IFNγ may play in PE pathophysiology is needed.
Previous studies using the RUPP model have reflected a number of these phenomena observed in women with PE. A 2011 study by Wallace et al. (53) found that RUPP rats have increased numbers of T helper cells that secreted increased levels of the type 1 cytokine TNF-α compared with NP T Helper cells. Although IFNγ was not measured in that study, our group has shown increased circulating levels of IFNγ in RUPPs, and this T helper imbalance is a potential source of this cytokine (21, 22). Our laboratory has also examined the phenotype of RUPP NK cells isolated from the circulation and placenta. RUPP rats demonstrate a shift in both circulating and placental NK cells toward an activated phenotype reflective of that seen in circulating and decidual NKs of preeclamptic women, and isolated RUPP placental NK cells also displayed increased cytotoxicity in vitro which is further suggestive of their activated phenotype (11, 12, 21, 23). NK cell depletion in RUPP rats improved MAP and fetal growth and resulted in reduced placental IFNγ (21). Our recent adoptive transfer experiments have also demonstrated that RUPP-stimulated placental NK cells cause a PE-like phenotype in normal pregnant recipient animals, and this adoptive transfer was accompanied by increased levels of placental IFNγ (22). The results of these studies suggest that IFNγ is a mechanism by which activated NK cells contribute to RUPP pathophysiology and PE. However, despite these and other studies demonstrating an association between PE and elevated IFNγ, there have been few studies examining how this cytokine is directly influencing PE pathophysiology (17). In this study, we demonstrated that IFNγ inhibition in the RUPP model of PE is sufficient to decrease MAP, circulating sFlt-1, and placental oxidative stress. These data support the hypothesis that excessive IFNγ secretion is a mechanism by which immune activation contributes to PE pathophysiology. This study has also shed light into possible mechanisms by which IFNγ contributes to MAP and vascular function in PE.
The vascular dysfunction characteristic of PE may be due in large part to the imbalance in proangiogenic and antiangiogenic factors that has been observed (54–56). Increased sFlt-1 and decreased levels of free VEGF and placental growth factor (PLGF) have been consistently observed in women with PE when compared with women with normal pregnancies, and this is reflected in several PE animal models (57–61). sFlt-1 is a splice variant of the Flt-1 receptor, which binds both VEGF and PLGF (62). This variant lacks the transmembrane and cytoplasmic domains and is secreted into the maternal circulation where it binds VEGF and PLGF and prevents them from reaching their cellular receptors (63, 64). Circulating levels of sFlt-1 are correlated with PE severity and overexpression or infusion of sFlt-1 in pregnant animals causes a PE-like phenotype with HTN, IUGR, increased placental ROS production, and vascular dysfunction (57, 59, 65, 66). In addition, a small clinical study found that women who had sFlt-1 removed via apheresis showed an improvement in renal function and an extension in the length of their pregnancies (67). The role of sFlt-1 in reducing free VEGF is believed to be the major mechanism of its contribution to PE pathophysiology. Although largely known for its angiogenic properties, VEGF plays important roles in promoting endothelial cell viability, dilating vasculature, and promoting placental development (68–70). Moreover, a well-recognized side effect of anti-VEGF therapy is increased risk of hypertension development, further highlighting VEGF’s importance in endothelial and vascular function (68, 71–73). These and other studies indicate that excessive sFlt-1 and the imbalance between sFlt-1 and VEGF is an important mediator of the endothelial dysfunction that leads to the hypertension and IUGR observed in PE. Thus, the reduction of sFlt-1 observed in the RUPP + anti-IFNγ rats is one potential mechanism that may have led to an improvement in MAP and UARI in this group.
In our current study, we observed that the increased ratio of sFlt-1 to VEGF observed in RUPP rats compared with NP was significantly improved in RUPP + anti-IFNγ. Looking into the individual components of the ratio, we saw that while circulating VEGF was significantly reduced in both RUPP and RUPP + anti-IFNγ rats, sFlt-1 was significantly increased in RUPP and was normalized in RUPP + anti-IFNγ rats. This serves to link IFNγ to sFlt-1 production in RUPP rats and possibly women with PE. Furthermore, in vitro administration of IFNγ has been shown to induce sFlt-1 production from isolated human corneal cells, human umbilical vein endothelial cells (HUVECs), and human trophoblasts (54–56). These data suggest that decreased sFlt-1 production is a potential mechanism by which IFNγ neutralization in RUPP rats resulted in an improvement in MAP and UARI.
Increased oxidative stress is also observed in women with PE compared with normotensive pregnant women and has been implicated in PE pathophysiology and progression (74). There is evidence that the excess ROS observed in women with PE is associated with the vascular dysfunction and hypertension (75). In addition, experiments in PE animal models have demonstrated increased ROS and show that antioxidants can improve MAP and fetal outcomes in these models, pointing to a direct role for ROS in contributing to PE pathophysiology (28, 76, 77). Excessive placental ROS is detrimental because these highly reactive molecules cause lipid peroxidation, endothelial activation, and apoptosis of trophoblasts (78, 79). ROS can also be secreted into the maternal circulation, further contributing to systemic endothelial dysfunction and inflammation (80). In this study, we observed increased levels of placental ROS in RUPPs and reduced levels in RUPP + anti-IFNγ, independent of changes in placental cytokines, and this suggests a direct role for circulating IFNγ in placental ROS generation. IFNγ has been recognized to induce increased ROS production by acting as an activator of NADPH oxidase (NOX) isoforms 1 and 2, both of which are producers of superoxide (81, 82). NOX2 is mainly present on macrophages and neutrophils, whereas NOX1 is a prominent isoform present on placental trophoblasts and is a major source of superoxide during pregnancy (81, 83–85). Moreover, additional analysis of placental ROS generation revealed that the NADPH-dependent increase in placental ROS seen in RUPPs was abolished in RUPP + anti-IFNγ. This suggests that IFNγ-induced NOX activation is a potential mechanism of placental ROS generation in RUPP rats.
Impaired vascular function and placental perfusion is believed to be another contributing factor to increased placental ROS observed in PE (3, 86–88). Studies have shown that impaired trophoblast invasion and maternal vascular dysfunction can lead to insufficient placental perfusion, and the resultant ischemia/reperfusion injuries can promote increased ROS production in the placenta (78, 80). The RUPP procedure results in a 40% reduction in placental perfusion as well as augmented vascular reactivity (19, 89–93). This impaired vascular function is reflected by the increased UARI observed in this group (94–96). In this study, we found that IFNγ inhibition lowered UARI in RUPP + anti-IFNγ rats suggesting an improvement in placental perfusion. This may be another contributing factor to the significantly reduced placental ROS observed after IFNγ inhibition during placental ischemia.
We observed that IFNγ inhibition increased STAT3 expression in the placentas of RUPP + anti-IFNγ compared with RUPP rats. STAT3 is a mediator of the signaling pathway whereby IL-10 suppresses proinflammatory cytokine production (97). Importantly, IFNγ has been suggested to inhibit the anti-inflammatory actions of IL-10 by disrupting STAT3-mediated signaling (98). In addition, reduced STAT3 expression has also been observed in the placentas of women with PE and the Nω-nitro-l-arginine methyl ester-induced rat model of PE (99, 100). As studies have demonstrated a role for STAT3 to protect against heart, lung, and endothelial damage, a number of investigators have suggested that the reduced placental STAT3 levels observed in PE may contribute to reduced expression of prosurvival signals resulting in increased apoptosis of trophoblasts (99–104).
Circulating TNF-α was also increased in RUPP and normalized in RUPP + anti-IFNγ, and may represent an additional mechanism by which IFNγ blockade improved RUPP pathophysiology. A number of studies have reported significantly elevated levels of TNF-α in the circulation and immune cells of preeclamptic women compared with healthy, gestation-matched controls (4, 105–107). There have been several studies investigating this cytokine in PE, which have identified several potential mechanisms by which it can contribute to PE pathophysiology. TNF-α infusion in pregnant rats, mice, and baboons causes a PE-like phenotype, which was accompanied by increased sFlt-1 production (20, 60, 108). Moreover, RUPP rats display elevated levels of TNF-α in the circulation, and TNF-α blockade abolishes the elevation of sFlt-1 observed in RUPP rats (60, 109, 110). These studies suggest that the reduced circulating TNF-α observed in the RUPP + anti-IFNγ may also contribute to the reduced sFlt-1 levels seen in this group. In addition, infusion of TNF-α into normal pregnant rats has been shown to cause elevated renal vascular resistance which suggests a role for TNF-α in PE and RUPP-associated vascular dysfunction (20). Thus the reduced circulating TNF-α in the RUPP + anti-IFNγ may contribute to the reduced UARI observed in this group.
Finally, IL-6, a potent inflammatory cytokine, was also increased in the circulation of RUPP rats and was diminished in RUPP recipients of anti-IFNγ. An in vitro study in corneal epithelial cells has shown that IFNγ can induce IL-6 production, and IL-6 has been identified as a mediator of hypertension in PE with studies showing that infusion of IL-6 into pregnant animals can cause increased MAP (111, 112). In addition, IL-6 is also linked to oxidative stress as this cytokine is a potent inducer of ROS in epithelial cells (113). This may be an additional mechanism by which IFNγ neutralization decreased MAP and placental ROS in the treated RUPP.
Although the results of this study identified a role for IFNγ as a potential mediator of PE-associated MAP and vascular dysfunction, there are some limitations to the study. In this study, we observed decreased NO bioavailability in RUPP compared with NP without any changes in eNOS expression or phosphorylation at S1176, raising additional questions about the mechanism of this reduction. We also observed that anti-IFNγ treatment did not increase plasma total nitrate indicating that IFNγ is not the driving factor for the reduced NO bioavailability in RUPP rats. We did not include Sham-operated controls in this study. This was because of our previous study demonstrating no differences in MAP, fetal, or placental weight between Sham operated and NP controls. We also did not include a control IgG antibody treatment in this study.
Perspectives and Significance
This study has shown that IFNγ neutralization in RUPP rats is associated with decreased sFlt-1, increased circulating VEGF, and improved MAP and UARI. The results of this study identify IFNγ as a mediator of several known contributing factors of PE and could serve to facilitate development of novel therapeutics for PE treatment. Importantly, these data suggest that blockade of IFN in PE women may be effective in improving maternal outcomes by reducing blood pressure and normalizing vascular function.
SUPPLEMENTAL DATA
Supplemental Figs. S1–S4: https://doi.org/10.6084/m9.figshare.13379729.
GRANTS
This work was funded by National Institutes of Health Grants F31HL149257 (to O. K. Travis), R01DK109133 (to J. M. Williams), and R00HL130456 and R01HL151407 (to D. C. Cornelius).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
O.K.T., G.A.T., J.M.W., and D.C.C. conceived and designed research; O.K.T., G.A.T., C.G., S.S., H.T.N., M.T.C., T.D.J., A.K.B., G.W.B., A.N.S., J.M.W., and D.C.C. performed experiments; O.K.T., G.A.T., C.G., S.S., G.W.B., A.N.S., J.M.W., and D.C.C., analyzed data; O.K.T., G.A.T., C.G., S.S., H.T.N., G.W.B., J.M.W., and D.C.C. interpreted results of experiments; O.K.T., G.A.T., and D.C.C. and prepared figures; O.K.T. and G.A.T. drafted manuscript; O.K.T., G.A.T., C.G., S.S., H.T.N., M.T.C., T.D.J., A.K.B., and D.C.C. edited and revised manuscript; O.K.T., G.A.T., C.G., S.S., H.T.N., M.T.C., T.D.J., A.K.B. J.M.W., and D.C.C. approved final version of manuscript.
REFERENCES
- 1.American College of Obstetricians and Gynecologists' Committee on Practice Bulletin Opinion. Gestational hypertension and preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol 135: e237–e260, 2020. doi: 10.1097/AOG.0000000000003891. [DOI] [PubMed] [Google Scholar]
- 2.Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag 7: 467–474, 2011. doi: 10.2147/VHRM.S20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez-Aranguren LC, Prada CE, Riano-Medina CE, Lopez M. Endothelial dysfunction and preeclampsia: role of oxidative stress. Front Physiol 5: 372, 2014. doi: 10.3389/fphys.2014.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Udenze I, Amadi C, Awolola N, Cc M. The role of cytokines as inflammatory mediators in preeclampsia. Pan Afr Med J 20: 219, 2015. doi: 10.11604/pamj.2015.20.219.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW Jr, Wallace K, LaMarca B. The role of inflammation in the pathology of preeclampsia. Clin Sci (Lond) 130: 409–419, 2016. doi: 10.1042/CS20150702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinheiro MB, Martins-Filho OA, Mota APL, Alpoim PN, Godoi LC, Silveira ACO, Teixeira-Carvalho A, Gomes KB, Dusse LM. Severe preeclampsia goes along with a cytokine network disturbance towards a systemic inflammatory state. Cytokine 62: 165–173, 2013. doi: 10.1016/j.cyto.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 7.Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, Croy BA. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol 171: 2937–2944, 2003. doi: 10.4049/jimmunol.171.6.2937. [DOI] [PubMed] [Google Scholar]
- 8.Li ZY, Chao HH, Liu HY, Song ZH, Li LL, Zhang YJ, Yang Y, Peng JP. IFN-gamma induces aberrant CD49b(+) NK cell recruitment through regulating CX3CL1: a novel mechanism by which IFN-gamma provokes pregnancy failure. Cell Death Dis 5: e1512, 2014. doi: 10.1038/cddis.2014.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu HY, Liu ZK, Chao H, Li Z, Song Z, Yang Y, Peng JP. High-dose interferon-gamma promotes abortion in mice by suppressing Treg and Th17 polarization. J Interferon Cytokine Res 34: 394–403, 2014. doi: 10.1089/jir.2013.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Si LF, Zhang SY, Gao CS, Chen SL, Zhao J, Cheng XC. Effects of IFN-gamma on IL-18 expression in pregnant rats and pregnancy outcomes. Asian-Australas J Anim Sci 26: 1399–1405, 1970. doi: 10.5713/ajas.2013.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukui A, Yokota M, Funamizu A, Nakamua R, Fukuhara R, Yamada K, Kimura H, Fukuyama A, Kamoi M, Tanaka K, Mizunuma H. Changes of NK cells in preeclampsia. Am J Reprod Immunol 67: 278–286, 2012. doi: 10.1111/j.1600-0897.2012.01120.x. [DOI] [PubMed] [Google Scholar]
- 12.Fukui A, Funamizu A, Yokota M, Yamada K, Nakamua R, Fukuhara R, Kimura H, Mizunuma H. Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. J Reprod Immunol 90: 105–110, 2011. doi: 10.1016/j.jri.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Borzychowski AM, Croy BA, Chan WL, Redman CWG, Sargent IL. Changes in systemic type 1 and type 2 immunity in normal pregnancy and pre-eclampsia may be mediated by natural killer cells. Eur J Immunol 35: 3054–3063, 2005. doi: 10.1002/eji.200425929. [DOI] [PubMed] [Google Scholar]
- 14.Du M, Wang W, Huang L, Guan X, Lin W, Yao J, Li L. Natural killer cells in the pathogenesis of preeclampsia: a double-edged sword. J Matern Fetal Neonatal Med 1–8, 2020. doi: 10.1080/14767058.2020.1740675. [DOI] [PubMed] [Google Scholar]
- 15.Jabrane-Ferrat N, Siewiera J. The up side of decidual natural killer cells: new developments in immunology of pregnancy. Immunology 141: 490–497, 2014. doi: 10.1111/imm.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy SP, Tayade C, Ashkar AA, Hatta K, Zhang J, Croy BA. Interferon gamma in successful pregnancies. Biol Reprod 80: 848–859, 2009. doi: 10.1095/biolreprod.108.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng Z, Zhang L, Tang Q, Xu Y, Liu S, Li H. Circulating levels of IFN-gamma, IL-1, IL-17 and IL-22 in pre-eclampsia: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 248: 211–221, 2020. doi: 10.1016/j.ejogrb.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 18.Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol 303: H1–H8, 2012. doi: 10.1152/ajpheart.00117.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2001. doi: 10.1161/01.HYP.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 20.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension 46: 1022–1025, 2005. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 21.Elfarra J, Amaral LM, McCalmon M, Scott JD, Cunningham MW Jr, Gnam A, Ibrahim T, LaMarca B, Cornelius DC. Natural killer cells mediate pathophysiology in response to reduced uterine perfusion pressure. Clin Sci (Lond) 131: 2753–2762, 2017. doi: 10.1042/CS20171118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Travis OK, Baik C, Tardo GA, Amaral L, Jackson C, Greer M, Giachelli C, Ibrahim T, Herrock OT, Williams JM, Cornelius DC. Adoptive transfer of placental ischemia stimulated natural killer cells causes a preeclampsia-like phenotype in pregnant rats. Am J Reprod Immunol 85: e13386, 2021. doi: 10.1111/aji.13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Travis OK, White D, Baik CH, Giachelli C, Thompson W, Stubbs C, Greer M, Lemon JP, Williams JM, Cornelius DC. Interleukin-17 signaling mediates cytolytic natural killer cell activation in response to placental ischemia. Am J Physiol Regul Integr Comp Physiol 318: R1036–R1046, 2020. doi: 10.1152/ajpregu.00285.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amaral LM, Cornelius DC, Harmon A, Moseley J, Martin JN Jr, LaMarca B. 17-Hydroxyprogesterone caproate significantly improves clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension 65: 225–231, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension 52: 1168–1172, 2008. doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornelius DC, Amaral LM, Wallace K, Campbell N, Thomas AJ, Scott J, Herse F, Wallukat G, Dechend R, LaMarca B. Reduced uterine perfusion pressure T-helper 17 cells cause pathophysiology associated with preeclampsia during pregnancy. Am J Physiol Regul Integr Comp Physiol 311: R1192–R1199, 2016. doi: 10.1152/ajpregu.00117.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Travis OK, White D, Pierce WA, Ge Y, Stubbs CY, Spradley FT, Williams JM, Cornelius DC. Chronic infusion of interleukin-17 promotes hypertension, activation of cytolytic natural killer cells, and vascular dysfunction in pregnant rats. Physiol Rep 7: e14038, 2019. doi: 10.14814/phy2.14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shields CA, McCalmon M, Ibrahim T, White DL, Williams JM, LaMarca B, Cornelius DC. Placental ischemia-stimulated T-helper 17 cells induce preeclampsia-associated cytolytic natural killer cells during pregnancy. Am J Physiol Regul Integr Comp Physiol 315: R336–R343, 2018. doi: 10.1152/ajpregu.00061.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amaral LM, Faulkner JL, Elfarra J, Cornelius DC, Cunningham MW, Ibrahim T, Vaka VR, McKenzie J, LaMarca B. Continued investigation into 17-OHPC: results from the preclinical RUPP rat model of preeclampsia. Hypertension 70: 1250–1255, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elfarra JT, Cottrell JN, Cornelius DC, Cunningham MW Jr, Faulkner JL, Ibrahim T, Lamarca B, Amaral LM. 17-Hydroxyprogesterone caproate improves T cells and NK cells in response to placental ischemia; new mechanisms of action for an old drug. Pregnancy Hypertens 19: 226–232, 2020. doi: 10.1016/j.preghy.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raghupathy R, Al-Azemi M, Azizieh F. Intrauterine growth restriction: cytokine profiles of trophoblast antigen-stimulated maternal lymphocytes. Clin Dev Immunol 2012: 734865, 2012., doi: 10.1155/2012/734865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 12: 1065–1074, 2006. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 33.Sharma S. Natural killer cells and regulatory T cells in early pregnancy loss. Int J Dev Biol 58: 219–229, 2014. doi: 10.1387/ijdb.140109ss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bulmer JN, Williams PJ, Lash GE. Immune cells in the placental bed. Int J Dev Biol 54: 281–294, 2010. doi: 10.1387/ijdb.082763jb. [DOI] [PubMed] [Google Scholar]
- 35.Ashkar AA, Croy BA. Interferon-gamma contributes to the normalcy of murine pregnancy. Biol Reprod 61: 493–502, 1999. doi: 10.1095/biolreprod61.2.493. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z, Sun QH, Yang Y, Liu JM, Peng JP. Effect of IFNgamma on caspase-3, Bcl-2 and Bax expression, and apoptosis in rabbit placenta. Cytokine 24: 201–209, 2003. doi: 10.1016/j.cyto.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Hara CCP, França EL, Fagundes DLG, de Queiroz AA, Rudge MVC, Honorio-França AC, Calderon IMP. Characterization of natural killer cells and cytokines in maternal placenta and fetus of diabetic mothers. J Immunol Res 2016: 7154524, 2016. doi: 10.1155/2016/7154524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King A, Wellings V, Gardner L, Loke YW. Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Hum Immunol 24: 195–205, 1989. doi: 10.1016/0198-8859(89)90060-8. [DOI] [PubMed] [Google Scholar]
- 39.Bulmer JN, Morrison L, Longfellow M, Ritson A, Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod 6: 791–798, 1991. doi: 10.1093/oxfordjournals.humrep.a137430. [DOI] [PubMed] [Google Scholar]
- 40.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol 2: 656–663, 2002. [Erratum in Nat Rev Immunol 2: 975, 2002]. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 41.Banerjee S, Smallwood A, Moorhead J, Chambers AE, Papageorghiou A, Campbell S, Nicolaides K. Placental expression of interferon-gamma (IFN-gamma) and its receptor IFN-gamma R2 fail to switch from early hypoxic to late normotensive development in preeclampsia. J Clin Endocrinol Metab 90: 944–952, 2005. doi: 10.1210/jc.2004-1113. [DOI] [PubMed] [Google Scholar]
- 42.Laresgoiti-Servitje E, Gomez-Lopez N, Olson DM. An immunological insight into the origins of pre-eclampsia. Hum Reprod Update 16: 510–524, 2010. [Erratum in Hum Reprod Update 17: 291, 2011]. doi: 10.1093/humupd/dmq007. [DOI] [PubMed] [Google Scholar]
- 43.Arriaga-Pizano L, Jimenez-Zamudio L, Vadillo-Ortega F, Martinez-Flores A, Herrerias-Canedo T, Hernandez-Guerrero C. The predominant Th1 cytokine profile in maternal plasma of preeclamptic women is not reflected in the choriodecidual and fetal compartments. J Soc Gynecol Investig 12: 335–342, 2005. doi: 10.1016/j.jsgi.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Saito S, Sakai M. Th1/Th2 balance in preeclampsia. J Reprod Immunol 59: 161–173, 2003. doi: 10.1016/S0165-0378(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 45.Aggarwal R, Jain AK, Mittal P, Kohli M, Jawanjal P, Rath G. Association of pro- and anti-inflammatory cytokines in preeclampsia. J Clin Lab Anal 33: e22834, 2019. doi: 10.1002/jcla.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao Y, Xu X-H, Jin L. Macrophage polarization in physiological and pathological pregnancy. Front Immunol 10: 792–792, 2019. doi: 10.3389/fimmu.2019.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vishnyakova P, Elchaninov A, Fatkhudinov T, Sukhikh G. Role of the monocyte-macrophage system in normal pregnancy and preeclampsia. Int J Mol Sci 20: 3695, 2019. doi: 10.3390/ijms20153695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang SW, Cho EH, Choi SY, Lee YK, Park JH, Kim MK, Park JY, Choi HJ, Lee JI, Ko HM, Park SH, Hwang HS, Kang YS. DC-SIGN expression in Hofbauer cells may play an important role in immune tolerance in fetal chorionic villi during the development of preeclampsia. J Reprod Immunol 124: 30–37, 2017. doi: 10.1016/j.jri.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Ma Y, Ye Y, Zhang J, Ruan CC, Gao PJ. Immune imbalance is associated with the development of preeclampsia. Medicine (Baltimore) 98: e15080, 2019. doi: 10.1097/MD.0000000000015080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schonkeren D, van der Hoorn ML, Khedoe P, Swings G, van Beelen E, Claas F, van Kooten C, de Heer E, Scherjon S. Differential distribution and phenotype of decidual macrophages in preeclamptic versus control pregnancies. Am J Pathol 178: 709–717, 2011. doi: 10.1016/j.ajpath.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tinsley JH, Chiasson VL, South S, Mahajan A, Mitchell BM. Immunosuppression improves blood pressure and endothelial function in a rat model of pregnancy-induced hypertension. Am J Hypertens 22: 1107–1114, 2009. doi: 10.1038/ajh.2009.125. [DOI] [PubMed] [Google Scholar]
- 52.Haddad EK, Duclos AJ, Antecka E, Lapp WS, Baines MG. Role of interferon-gamma in the priming of decidual macrophages for nitric oxide production and early pregnancy loss. Cell Immunol 181: 68–75, 1997. doi: 10.1006/cimm.1997.1199. [DOI] [PubMed] [Google Scholar]
- 53.Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, Wilson M, Martin JN Jr, LaMarca B. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension 57: 949–955, 2011. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kommineni VK, Nagineni CN, William A, Detrick B, Hooks JJ. IFN-gamma acts as anti-angiogenic cytokine in the human cornea by regulating the expression of VEGF-A and sVEGF-R1. Biochem Biophys Res Commun 374: 479–484, 2008. doi: 10.1016/j.bbrc.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, Devey LR, Wigmore SJ, Abbas A, Hewett PW, Ahmed A. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation 115: 1789–1797, 2007. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 56.Cudmore MJ, Ramma W, Cai M, Fujisawa T, Ahmad S, Al-Ani B, Ahmed A. Resveratrol inhibits the release of soluble fms-like tyrosine kinase (sFlt-1) from human placenta. Am J Obstet Gynecol 206: e253.e10–e253.e15, 2012. doi: 10.1016/j.ajog.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee Kim Y, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol 190: 1541–1547, 2004. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 58.Robinson CJ, Johnson DD, Chang EY, Armstrong DM, Wang W. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol 195: 255–259, 2006. doi: 10.1016/j.ajog.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 59.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sunderland NS, Thomson SE, Heffernan SJ, Lim S, Thompson J, Ogle R, McKenzie P, Kirwan PJ, Makris A, Hennessy A. Tumor necrosis factor alpha induces a model of preeclampsia in pregnant baboons (Papio hamadryas). Cytokine 56: 192–199, 2011. doi: 10.1016/j.cyto.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 61.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res 95: 884–891, 2004. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 62.Eddy AC, Bidwell GL III, George EM. Pro-angiogenic therapeutics for preeclampsia. Biol Sex Differ 9: 36, 2018. doi: 10.1186/s13293-018-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA 90: 10705–10709, 1993. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shibuya M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis 9: 225–230, 2006. doi: 10.1007/s10456-006-9055-8. [DOI] [PubMed] [Google Scholar]
- 65.Bridges JP, Gilbert JS, Colson D, Gilbert SA, Dukes MP, Ryan MJ, Granger JP. Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens 22: 564–568, 2009. doi: 10.1038/ajh.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, Hankins GD, Saade GR. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol 196: 396.e1–396.e7, 2007; discussion 396.e7. doi: 10.1016/j.ajog.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 67.Thadhani R, Hagmann H, Schaarschmidt W, Roth B, Cingoez T, Karumanchi SA, Wenger J, Lucchesi KJ, Tamez H, Lindner T, Fridman A, Thome U, Kribs A, Danner M, Hamacher S, Mallmann P, Stepan H, Benzing T. Removal of soluble fms-like tyrosine kinase-1 by dextran sulfate apheresis in preeclampsia. J Am Soc Nephrol 27: 903–913, 2016. doi: 10.1681/ASN.2015020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pandey AK, Singhi EK, Arroyo JP, Ikizler TA, Gould ER, Brown J, Beckman JA, Harrison DG, Moslehi J. Mechanisms of VEGF (vascular endothelial growth factor) inhibitor-associated hypertension and vascular disease. Hypertension 71: e1–e8, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eichmann A, Simons M. VEGF signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol 24: 188–193, 2012. doi: 10.1016/j.ceb.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maynard SE, Karumanchi SA. Angiogenic factors and preeclampsia. Semin Nephrol 31: 33–46, 2011. doi: 10.1016/j.semnephrol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis 49: 186–193, 2007. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 72.Moshfeghi AA, Rosenfeld PJ, Puliafito CA, Michels S, Marcus EN, Lenchus JD, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: twenty-four-week results of an uncontrolled open-label clinical study. Ophthalmology 113: 2002.e1–2002.e12, 2006. doi: 10.1016/j.ophtha.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 73.Robinson ES, Khankin EV, Karumanchi SA, Humphreys BD. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: mechanisms and potential use as a biomarker. Semin Nephrol 30: 591–601, 2010. doi: 10.1016/j.semnephrol.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raijmakers MT, Dechend R, Poston L. Oxidative stress and preeclampsia: rationale for antioxidant clinical trials. Hypertension 44: 374–380, 2004. doi: 10.1161/01.HYP.0000141085.98320.01. [DOI] [PubMed] [Google Scholar]
- 75.Mannaerts D, Faes E, Cos P, Briede JJ, Gyselaers W, Cornette J, Gorbanev Y, Bogaerts A, Spaanderman M, Van Craenenbroeck E, Jacquemyn Y. Oxidative stress in healthy pregnancy and preeclampsia is linked to chronic inflammation, iron status and vascular function. PLoS One 13: e0202919, 2018. doi: 10.1371/journal.pone.0202919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoffmann DS, Weydert CJ, Lazartigues E, Kutschke WJ, Kienzle MF, Leach JE, Sharma JA, Sharma RV, Davisson RL. Chronic tempol prevents hypertension, proteinuria, and poor feto-placental outcomes in BPH/5 mouse model of preeclampsia. Hypertension 51: 1058–1065, 2008. doi: 10.1161/HYPERTENSIONAHA.107.107219. [DOI] [PubMed] [Google Scholar]
- 77.Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens 21: 1152–1156, 2008. doi: 10.1038/ajh.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aouache R, Biquard L, Vaiman D, Miralles F. Oxidative stress in preeclampsia and placental diseases. Int J Mol Sci 19: 1496, 2018. doi: 10.3390/ijms19051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu X, Guo X, Wassall CD, Kemple MD, Unthank JL, Kassab GS. Reactive oxygen species cause endothelial dysfunction in chronic flow overload. J Appl Physiol (1985) 110: 520–527, 2011. doi: 10.1152/japplphysiol.00786.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tenorio MB, Ferreira RC, Moura FA, Bueno NB, de Oliveira ACM, Goulart MOF. Cross-talk between oxidative stress and inflammation in preeclampsia. Oxid Med Cell Longev 2019: 8238727, 2019. doi: 10.1155/2019/8238727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hodny Z, Reinis M, Hubackova S, Vasicova P, Bartek J. Interferon gamma/NADPH oxidase defense system in immunity and cancer. Oncoimmunology 5: e1080416, 2016. doi: 10.1080/2162402X.2015.1080416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuwano Y, Kawahara T, Yamamoto H, Teshima-Kondo S, Tominaga K, Masuda K, Kishi K, Morita K, Rokutan K. Interferon-gamma activates transcription of NADPH oxidase 1 gene and upregulates production of superoxide anion by human large intestinal epithelial cells. Am J Physiol Cell Physiol 290: C433–C443, 2006. doi: 10.1152/ajpcell.00135.2005. [DOI] [PubMed] [Google Scholar]
- 83.Bevilacqua E, Gomes SZ, Lorenzon AR, Hoshida MS, Amarante-Paffaro AM. NADPH oxidase as an important source of reactive oxygen species at the mouse maternal-fetal interface: putative biological roles. Reprod Biomed Online 25: 31–43, 2012. doi: 10.1016/j.rbmo.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 84.Hernandez I, Fournier T, Chissey A, Therond P, Slama A, Beaudeux JL, Zerrad-Saadi A. NADPH oxidase is the major source of placental superoxide in early pregnancy: association with MAPK pathway activation. Sci Rep 9: 13962, 2019. doi: 10.1038/s41598-019-50417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cui XL, Brockman D, Campos B, Myatt L. Expression of NADPH oxidase isoform 1 (Nox1) in human placenta: involvement in preeclampsia. Placenta 27: 422–431, 2006. doi: 10.1016/j.placenta.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Myatt L, Webster RP. Vascular biology of preeclampsia. J Thromb Haemost 7: 375–384, 2009. doi: 10.1111/j.1538-7836.2008.03259.x. [DOI] [PubMed] [Google Scholar]
- 87.Roberts JM. Preeclampsia: what we know and what we do not know. Semin Perinatol 24: 24–28, 2000. doi: 10.1016/s0146-0005(00)80050-6. [DOI] [PubMed] [Google Scholar]
- 88.Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol 283: R29–R45, 2002. doi: 10.1152/ajpregu.00762.2001. [DOI] [PubMed] [Google Scholar]
- 89.Giardina JB, Cockrell KL, Granger JP, Khalil RA. Low-salt diet enhances vascular reactivity and Ca(2+) entry in pregnant rats with normal and reduced uterine perfusion pressure. Hypertension 39: 368–374, 2002. doi: 10.1161/hy02t2.102806. [DOI] [PubMed] [Google Scholar]
- 90.Barron LA, Giardina JB, Granger JP, Khalil RA. High-salt diet enhances vascular reactivity in pregnant rats with normal and reduced uterine perfusion pressure. Hypertension 38: 730–735, 2001. doi: 10.1161/01.hyp.38.3.730. [DOI] [PubMed] [Google Scholar]
- 91.Reho JJ, Toot JD, Peck J, Novak J, Yun YH, Ramirez RJ. Increased myogenic reactivity of uterine arteries from pregnant rats with reduced uterine perfusion pressure. Pregnancy Hypertens 2: 106–114, 2012. doi: 10.1016/j.preghy.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eder DJ, McDonald MT. A role for brain angiotensin ii in experimental pregnancy-induced hypertension in laboratory rats. Clin Exp Hypertens B 6: 431–451, 1987. doi: 10.3109/10641958709023492. [DOI] [Google Scholar]
- 93.Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension 35: 367–372, 2000. doi: 10.1161/01.hyp.35.1.367. [DOI] [PubMed] [Google Scholar]
- 94.Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol 232: R27–R44, 2017. doi: 10.1530/JOE-16-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brandao AH, Pereira LM, Goncalves AC, Reis ZS, Leite HV, Cabral AC. Comparative study of endothelial function and uterine artery doppler velocimetry between pregnant women with or without preeclampsia development. J Pregnancy 2012: 909315, 2012. doi: 10.1155/2012/909315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Teulings N, Wood AM, Sovio U, Ozanne SE, Smith GCS, Aiken CE. Independent influences of maternal obesity and fetal sex on maternal cardiovascular adaptation to pregnancy: a prospective cohort study. Int J Obes 44: 2246–2255, 2020. doi: 10.1038/s41366-020-0627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Murray PJ. STAT3-mediated anti-inflammatory signalling. Biochem Soc Trans 34: 1028–1031, 2006. doi: 10.1042/BST0341028. [DOI] [PubMed] [Google Scholar]
- 98.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity 31: 539–550, 2009. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Z, Wang X, Wang J, Zhang L. The decreased expression of Stat3 and p-Stat3 in preeclampsia-like rat placenta. J Mol Histol 49: 175–183, 2018. doi: 10.1007/s10735-018-9757-4. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Z, Yang X, Zhang L, Duan Z, Jia L, Wang P, Shi Y, Li Y, Gao J. Decreased expression and activation of Stat3 in severe preeclampsia. J Mol Histol 46: 205–219, 2015. doi: 10.1007/s10735-015-9613-8. [DOI] [PubMed] [Google Scholar]
- 101.Hilfiker-Kleiner D, Hilfiker A, Drexler H. Many good reasons to have STAT3 in the heart. Pharmacol Ther 107: 131–137, 2005. doi: 10.1016/j.pharmthera.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 102.Kano A, Wolfgang MJ, Gao Q, Jacoby J, Chai GX, Hansen W, Iwamoto Y, Pober JS, Flavell RA, Fu XY. Endothelial cells require STAT3 for protection against endotoxin-induced inflammation. J Exp Med 198: 1517–1525, 2003. doi: 10.1084/jem.20030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zouein FA, Altara R, Chen Q, Lesnefsky EJ, Kurdi M, Booz GW. Pivotal importance of STAT3 in protecting the heart from acute and chronic stress: new advancement and unresolved issues. Front Cardiovasc Med 2: 36, 2015. doi: 10.3389/fcvm.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang X, Shan P, Jiang G, Zhang SS, Otterbein LE, Fu XY, Lee PJ. Endothelial STAT3 is essential for the protective effects of HO-1 in oxidant-induced lung injury. FASEB J 20: 2156–2158, 2006. [Erratum in FASEB J 21: 630, 2007]. doi: 10.1096/fj.06-5668fje. [DOI] [PubMed] [Google Scholar]
- 105.Trisnawati E, Nontji W, Nurasni S. Tumour necrosis factor-alpha (TNF-alpha) serum levels in preeclampsia pregnant women and pregnant women at risk with preeclampsia. Enferm Clin 30: 27–30, 2020. doi: 10.1016/j.enfcli.2019.07.021. [DOI] [Google Scholar]
- 106.Saito S, Umekage H, Sakamoto Y, Sakai M, Tanebe K, Sasaki Y, Morikawa H. Increased T-helper-1-type immunity and decreased T-helper-2-type immunity in patients with preeclampsia. Am J Reprod Immunol 41: 297–306, 1999. doi: 10.1111/j.1600-0897.1999.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 107.Darmochwal-Kolarz D, Leszczynska-Gorzelak B, Rolinski J, Oleszczuk J. T helper 1- and T helper 2-type cytokine imbalance in pregnant women with pre-eclampsia. Eur J Obstet Gynecol Reprod Biol 86: 165–170, 1999. doi: 10.1016/S0301-2115(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 108.Bobek G, Surmon L, Mirabito KM, Makris A, Hennessy A. Placental Regulation of Inflammation and Hypoxia after TNF-α Infusion in Mice. Am J Reprod Immunol 74: 407–418, 2015. doi: 10.1111/aji.12417. [DOI] [PubMed] [Google Scholar]
- 109.Murphy SR, LaMarca BB, Parrish M, Cockrell K, Granger JP. Control of soluble fms-like tyrosine-1 (sFlt-1) production response to placental ischemia/hypoxia: role of tumor necrosis factor-alpha. Am J Physiol Regul Integr Comp Physiol 304: R130–R135, 2013. doi: 10.1152/ajpregu.00069.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Parrish MR, Murphy SR, Rutland S, Wallace K, Wenzel K, Wallukat G, Keiser S, Ray LF, Dechend R, Martin JN, Granger JP, LaMarca B. The effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am J Hypertens 23: 911–916, 2010. doi: 10.1038/ajh.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lamarca B, Brewer J, Wallace K. IL-6-induced pathophysiology during pre-eclampsia: potential therapeutic role for magnesium sulfate? Int J Interferon Cytokine Mediat Res 2011: 59–64, 2011. doi: 10.2147/IJICMR.S16320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Terasaka Y, Miyazaki D, Yakura K, Haruki T, Inoue Y. Induction of IL-6 in transcriptional networks in corneal epithelial cells after herpes simplex virus type 1 infection. Invest Ophthalmol Vis Sci 51: 2441–2449, 2010. doi: 10.1167/iovs.09-4624. [DOI] [PubMed] [Google Scholar]
- 113.Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Bohm M, Nickenig G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res 94: 534–541, 2004. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]