Abstract
Arterial pCO2 elevations increase minute ventilation via activation of chemosensors within the carotid body (CB) and brainstem. Although the roles of CB chemoafferents in the hypercapnic (HC) ventilatory response have been investigated, there are no studies reporting the role of these chemoafferents in the ventilatory responses to a HC challenge or the responses that occur upon return to room air, in freely moving mice. This study found that an HC challenge (5% CO2, 21% O2, 74% N2 for 15 min) elicited an array of responses, including increases in frequency of breathing (accompanied by decreases in inspiratory and expiratory times), and increases in tidal volume, minute ventilation, peak inspiratory and expiratory flows, and inspiratory and expiratory drives in sham-operated (SHAM) adult male C57BL6 mice, and that return to room air elicited a brief excitatory phase followed by gradual recovery of all parameters toward baseline values over a 15-min period. The array of ventilatory responses to the HC challenge in mice with bilateral carotid sinus nerve transection (CSNX) performed 7 days previously occurred more slowly but reached similar maxima as SHAM mice. A major finding was responses upon return to room air were dramatically lower in CSNX mice than SHAM mice, and the parameters returned to baseline values within 1–2 min in CSNX mice, whereas it took much longer in SHAM mice. These findings are the first evidence that CB chemoafferents play a key role in initiating the ventilatory responses to HC challenge in C57BL6 mice and are essential for the expression of post-HC ventilatory responses.
NEW & NOTEWORTHY This study presents the first evidence that carotid body chemoafferents play a key role in initiating the ventilatory responses, such as increases in frequency of breathing, tidal volume, and minute ventilation that occur in response to a hypercapnic gas challenge in freely moving C57BL6 mice. Our study also demonstrates for the first time that these chemoafferents are essential for the expression of the ventilatory responses that occur upon return to room air in these mice.
Keywords: carotid sinus chemoafferents, C57BL6 mice, hypercapnia, ventilatory parameters
INTRODUCTION
Peripheral and central chemosensory structures relay signals to respiratory circuitry within the brainstem to monitor and control ventilatory output in response to changes in arterial blood gas chemistry (1–5). Glomus (type I) cells of the carotid body (CB) respond to decreases in blood O2, increases in blood CO2, and decreases in blood pH, which results from the carbonic anhydrase-catalyzed production of protons due to enhanced bioavailability of circulating CO2 (6–13), by depolarizing and releasing neurotransmitters (14–23). These neurotransmitters excite closely apposed chemosensory afferent nerve terminals whose fibers course through the carotid sinus nerve (CSN) to synapse in the commissural nucleus tractus solitarius (cNTS) within the brainstem (8, 9, 11–13, 24). This input from CSN chemoafferents triggers cardiorespiratory responses (e.g., an increase in minute ventilation) that are designed to restore arterial blood gas homeostasis (9, 19, 25). The role of CB chemoafferents in eliciting ventilatory responses to hypoxic (HX) and hypercapnic (HC) gas challenges has been explored in humans (26–34), and a variety of animals, including dogs (35–37), rats (38–53), and mice (54–56). The HX ventilatory response (e.g., the increase in minute ventilation) is vitally dependent upon the CB-CSN complex, whereas the HC ventilatory response is dependent on the CB-CSN complex (1, 5, 35, 57, 58) and brainstem structures, including the retrotrapezoid nucleus (RTN) (59–66).
Wild-type and genetically engineered mice are widely used to investigate mechanisms by which HX gas challenges elicit CB-dependent and CB-independent ventilatory responses (22, 23, 55, 67–81). Only one study has directly addressed the role of the CB-CSN complex in the ventilatory responses to a HC gas challenge (43). In that study, ventilatory responses of urethane-anesthetized adult male C57BL/6CrSlc mice to HX (e.g., 10% O2, 90% N2) and hyperoxic (100% O2)-hypercapnic (5% CO2) gas challenges were established before and after bilateral CSN transection (CSNX). The authors found that ventilatory responses elicited by both challenges were reduced after bilateral CSNX. Our laboratory has reported that the ventilatory responses elicited by HX gas challenges were markedly reduced in freely moving adult male C57BL6 mice with bilateral CSNX (55). Moreover, we show that the robust post-HX changes in ventilation observed in sham-operated (SHAM) mice upon return to room air were virtually absent in CSNX mice (55). We used the C57BL6 mouse strain because it is widely used as a healthy mouse model to study the physiology of cardiorespiratory systems and is used to generate genetically engineered mice to study mechanisms involved in cardiorespiratory control processes (55, 77, 82–86). The C57BL6 mouse is also used extensively by sleep-apnea researchers because it displays irregular breathing patterns (e.g., apneas and sighs) during sleep and wakefulness and disordered breathing upon return to room air after HX exposures (68, 87–94). Many of the neurochemical (91–97) and genetic (85, 88–90, 93, 98–101) factors underlying the breathing patterns of C57BL6 mice, including greater degree of sleep disordered breathing and responses to HX challenges, have been identified. In addition, the potential role of structural differences in the carotid bodies in C57BL6 mice compared to other strains has also been studied (82, 102, 103).
Despite the extensive use of the C57BL6 mouse to study cardiorespiratory processes, nothing is known about the role of the CB-CSN complex in the ventilatory responses to HC gas challenge or those that occur upon return to room air in freely moving C57BL6 mice. Thus, the overall goal of the present study was to provide those findings in this mouse strain, which is of vital importance to understanding genetic aspects of breathing. The underlying hypothesis was that the absence of the CSN-CB complex would impact the ventilatory responses during and following a HC gas challenge, therefore providing initial data designed to uncover and understand the interplay between peripheral chemoreceptor input and central circuitry processing of the HC ventilatory response in freely moving C57BL6 mice. The specific objective of this study was to compare the ventilatory and thermoregulatory responses that occur during and following a 15-min normoxic-HC gas challenge (5% CO2, 21% O2, 74% N2) in freely moving sham-operated (SHAM) adult male C57BL6 mice and in mice in which both CSNs were transected 7 days previously (CSNX mice). The data demonstrate that the loss of CSN chemoafferent input to the brainstem has important effects on the ventilatory responses elicited by HC challenge and the ventilatory responses that occur upon return to room air in freely moving C57BL6 mice.
METHODS
Permissions
All procedures were carried out in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 80-23) revised in 1996 (https://www.nap.edu/catalog/5140/guide-for-the-care-and-use-of-laboratory-animals). The protocols were approved by the Animal Care and Use Committees of the University of Virginia and Case Western Reserve University.
Mice
C57BL6 male mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were delivered pathogen free and housed under specific pathogen-free conditions with a 12-h light-dark cycle.
Surgical Transection of the Carotid Sinus Nerve
Male C57BL6 mice were anesthetized with isoflurane (2% in room air) and a midline neck incision was made to reveal the left and right CSN (Fig. 1) as detailed previously (16). In control mice, each CSN was isolated, but not transected (sham-operated (SHAM) mice), whereas in CSNX mice, each CSN was isolated and transected immediately as the CSN branched from the glossopharyngeal nerve. The mice were allowed 7 days to recover from the surgery before use in the plethysmography experiments. It is important to note that the CSNX surgery was not verified in these mice by a hypoxic or sodium cyanide (NaCN) challenge. However, in other C57BL6 mice subjected to SHAM or bilateral CSNX surgeries during the same week, the ventilatory responses during a 15-min HX (10% O2, 90% N2) challenge in SHAM (n = 5) and CSNX mice (n = 8) were studied 7 days postsurgery. In brief, we found that the cumulative arithmetic change in MV in SHAM mice was 811 ± 73 mL/min as compared with 44 ± 8 mL/min in the CSNX mice (P < 0.05). The greatly diminished ventilatory responses following hypoxia exposure were consistent with our previous publication (55).
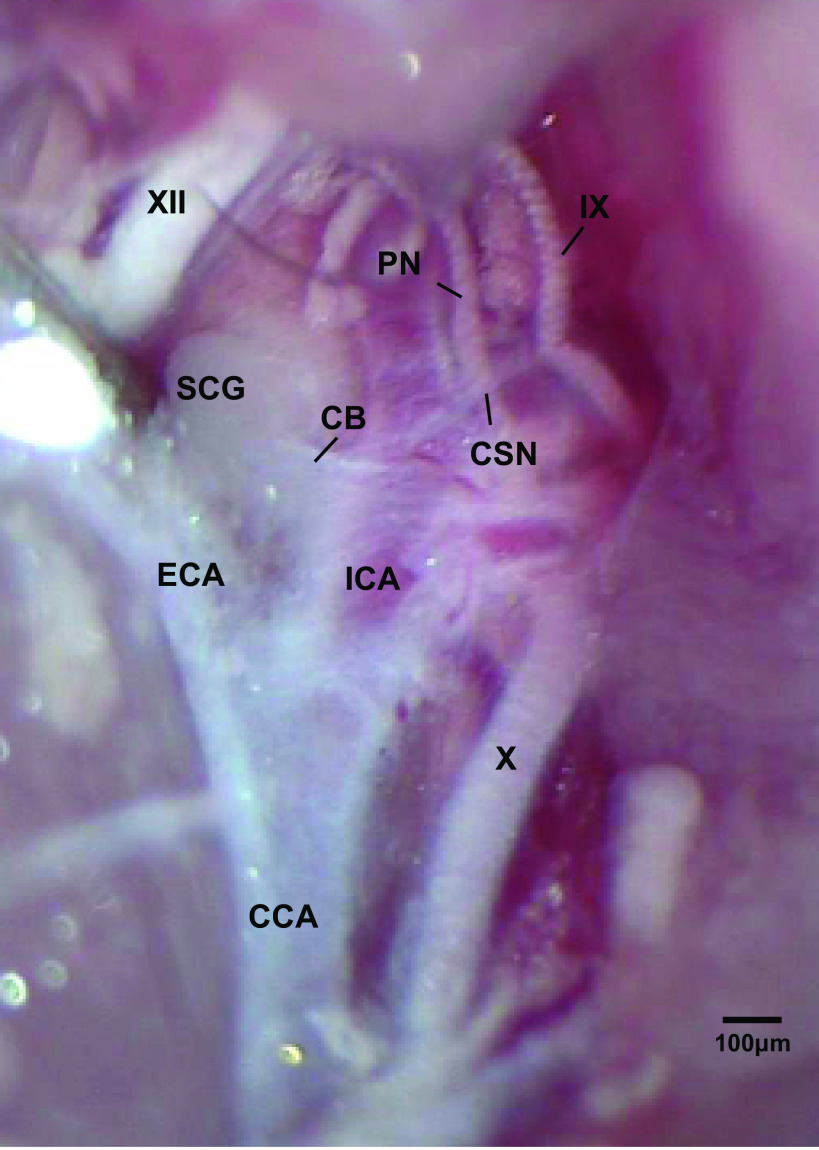
Figure 1.
Photograph in a C57BL6 adult male mouse of the carotid sinus nerve (CSN) branching off the glossopharyngeal nerve (IX) and entering the carotid body (CB). The pharyngeal nerve (PN), hypoglossal nerve (XII), superior cervical ganglion (SCG), vagus nerve (X), common carotid artery (CCA), and internal (ICA) and external (ECA) carotid arteries are also shown. This dissection was done on the left side of the animal. The scale bar is 100 μm.
Whole Body Plethysmography
Seven days postsurgery, the SHAM and CSNX mice were placed in unrestrained, individual whole body plethysmography chambers [Buxco Small Animal Whole Body Plethysmography, Data Sciences International (DSI), a division of Harvard Biosciences, Inc., St. Paul, MN] to continuously monitor ventilatory parameters (55, 68, 75–77, 84). The array of ventilatory parameters was recorded to provide an in-depth analysis of the differences in resting breathing patterns and responses to the HC gas challenge in SHAM and bilateral CSNX mice (55, 68, 75–77, 84, 96, 104). Directly recorded parameters were 1) frequency of breathing (Freq), 2) tidal volume (TV), 3) inspiratory time (Ti, duration of inspiration) and expiratory time (Te, duration of expiration), and 4) peak inspiratory flow (PIF) and peak expiratory flow (PEF). The calculated parameters were 1) minute ventilation (MV; i.e., Freq × TV) and 2) inspiratory drive (TV/Ti) and expiratory drive (TV/Te). The provided software (Fine Pointe, BUXCO) constantly corrected digitized values for changes in chamber temperature and humidity. A rejection algorithm was included in the breath-by-breath analysis to exclude episodes of nasal breathing. Pressure changes associated with the respiratory waveform were converted to volumes (TV, PIF, and PEF) using algorithms of Epstein and colleagues (38, 68, 105). Factoring in chamber temperature and humidity, the cycle analyzers filtered the acquired signals, and a host of proprietary algorithms (Fine Pointe, BUXCO) generated an array of box flow data that identified a breath. From this array, minimum and maximum box flow values were determined. Minimum and maximum box flows were multiplied by the compensation factor provided by the selected algorithm, thus producing the TV, PIF, and PEF parameters.
The detailed analyses of the patterns of breathing and the individual components of respiratory function provided by our commercial [Data Sciences International (DSI)] plethysmography system (https://www.datasci.com/products/software/ponemah/analysis-modules/pulmonary-air-flow-and-airway-resistance) were intended for us to gain an in-depth understanding of how HC challenge affects breathing in freely moving C57BL6 mice and how CSNX alters the response pattern to HC challenge. We were specifically looking to establish whether the responses of individual parameters would provide a deeper insight into how HC challenge affects breathing and how the loss of the CB-CSN complex affects the responses of individual parameters, such as PIF and PEF. It should be noted that PIF and PEF are measures of inspiratory and expiratory effort in humans (106, 107) and experimental animals, such as rats (108) and mice (109, 110), and is a function of central drive, functional capacity of muscles driving inspiration and passive/active expiration, and resistance to airflow in the upper and lower airway (14, 107–110).
Hypercapnic Gas Challenge
SHAM and CSNX mice were placed in individual plethysmography chambers in which they could freely move around and allowed 45–60 min to acclimatize to the new environment. Mice breathed room air during the acclimatization period. They were then exposed to a normoxic-HC gas challenge (5% CO2, 21%O2, 76% N2) for 15 min after which time they were reexposed to room air for an additional 15 min.
Body Temperature Recordings
SHAM (n = 9, 80.4 ± 0.4 days of age, 24.7 ± 0.2 g body weight) and CSNX mice (n = 9, 79.9 ± 0.3 days of age, 24.5 ± 0.2 g body weight) had their body temperatures recorded using a thermistor probe (YS 451, Yellow Springs Instruments, Yellow Springs, OH) connected to a battery-operated thermometer unit (YSI 400) placed in a work station (Coy, Grass Lake, MI), with room air flowing through, as described previously (55). This workstation allowed access to the mice and recording equipment via air-tight arm holes. After 5 min, the thermistor probe was inserted 1.5–2.0 cm into the rectum to record core body temperature. The mice were removed and a normoxic-HC environment (5% CO2, 21% O2, 74% N2) was established in the workstation using a Pro:Ox: Model 350 unit (Biospherix, Lacona, NY). The mice were next placed back in the workstation once the HC environment was established, and body temperature was recorded at 5- and 15-min time-points during HC challenge. The mice were again removed from the workstation, and a room air environment was rapidly induced by fully opening the workstation. The mice were once again placed back in the workstation, and body temperature was recorded at 5- and 15-min time-points.
Statistics
All recorded and derived parameters are presented as means ± SE. The following steps were taken to determine the total responses during the hypercapnic gas challenge and return to room air. For each mouse, we summed the 15 values (1 per min) recorded before the HC challenge, and the 15 values recorded both during the HC challenge and subsequent return to room air. We then calculated the cumulative response during HC gas challenge by the formula, total response (% change) = [(sum of values during HC challenge − sum of values before HC challenge)/sum of values before HC challenge] × 100. We also calculated the cumulative response following return to room air by the formula, total response (%change) = [(sum of values during return to room air − sum of values before HC challenge)/sum of values before HC challenge] × 100. We then determined the mean ± SE of the group data. All data were analyzed by one-way or two-way ANOVA followed by Student’s modified t test with Bonferroni corrections for multiple comparisons between means (38, 75–77).
RESULTS
Baseline Values
General information about the adult male C57BL6 mice that were used in this study and their resting ventilatory parameters recorded over the 5-min period immediately before exposure to the HC challenge are presented in Table 1. There were 16 mice in each of the sham-operated (SHAM) and carotid sinus nerve-transected (CSNX) groups. The ages and weights of the two groups were similar to one another. Resting TV, MV, PEF, and expiratory drive were significantly lower in CSNX mice than in SHAM mice. There were no between group differences in any of the other ventilatory parameters.
Table 1.
Resting parameters in sham-operated (SHAM) male mice and in those with bilateral transection of the carotid sinus nerve (CSNX)
| Parameter | SHAM | CSNX |
|---|---|---|
| Number of mice | 16 | 16 |
| Age, days | 78.0 ± 0.2 | 80.8 ± 2.2 |
| Body weight, g | 25.3 ± 0.2 | 25.2 ± 0.2 |
| Frequency, breaths/min | 192 ± 5 | 185 ± 6 |
| Tidal volume, mL | 0.209 ± 0.006 | 0.178 ± 0.008* |
| Minute ventilation, mL/min | 40.1 ± 1.7 | 32.7 ± 1.6* |
| Inspiratory time, s | 0.115 ± 0.002 | 0.114 ± 0.003 |
| Expiratory time, s | 0.229 ± 0.006 | 0.243 ± 0.007 |
| Inspiratory drive, mL/s | 1.85 ± 0.07 | 1.58 ± 0.07 |
| Expiratory drive, mL/s | 0.93 ± 0.03 | 0.74 ± 0.03* |
| Peak inspiratory flow, mL/s | 2.96 ± 0.10 | 2.78 ± 0.10 |
| Peak expiratory flow, mL/s | 2.09 ± 0.10 | 1.72 ± 0.0.11* |
Values are means ± SE. CSNX, C57BL6 male mice with bilateral carotid sinus nerve transection; SHAM, sham-operated male C57BL6 mice.
*P < 0.05, CSNX versus SHAM.
Changes in Body Temperature
The changes in body temperature recorded before, during, and after the HC gas challenge in SHAM and CSNX mice are summarized in Table 2. The SHAM and CSNX mice had equivalent body temperatures before the HC gas challenge. Also, exposure to the HC challenge or return to room air did not affect body temperature in either SHAM or CSNX mice at any time point.
Table 2.
Changes in body temperature during a hypercapnic gas challenge and upon return to room air
| Body Temperature, °C |
|||||
|---|---|---|---|---|---|
| Hypercapnia |
Room air |
||||
| Group | Pre | 5 min | 15 min | 5 min | 15 min |
| SHAM | 36.5 ± 0.1 | 36.5 ± 0.1 | 36.4 ± 0.1 | 36.4 ± 0.1 | 36.5 ± 0.1 |
| Delta (°C) | −0.02 ± 0.04 | −0.10 ± 0.04 | −0.08 ± 0.04 | +0.03 ± 0.03 | |
| CSNX | 36.4 ± 0.1 | 36.3 ± 0.1 | 36.5 ± 0.1 | 36.4 ± 0.1 | 36.4 ± 0.1 |
| Delta (°C) | −0.09 ± 0.06 | −0.06 ± 0.06 | −0.01 ± 0.06 | +0.02 ± 0.06 | |
Values are means ± SE. There were 9 mice in each group. Note, there were no significant changes in body temperature or any between-group differences at any time point (P > 0.05, for all comparisons). CSNX, C57BL6 male mice with bilateral carotid sinus nerve transection; SHAM, sham-operated male C57BL6 mice.
Ventilatory Responses during and following the Hypercapnic Gas Challenge
Frequency of breathing.
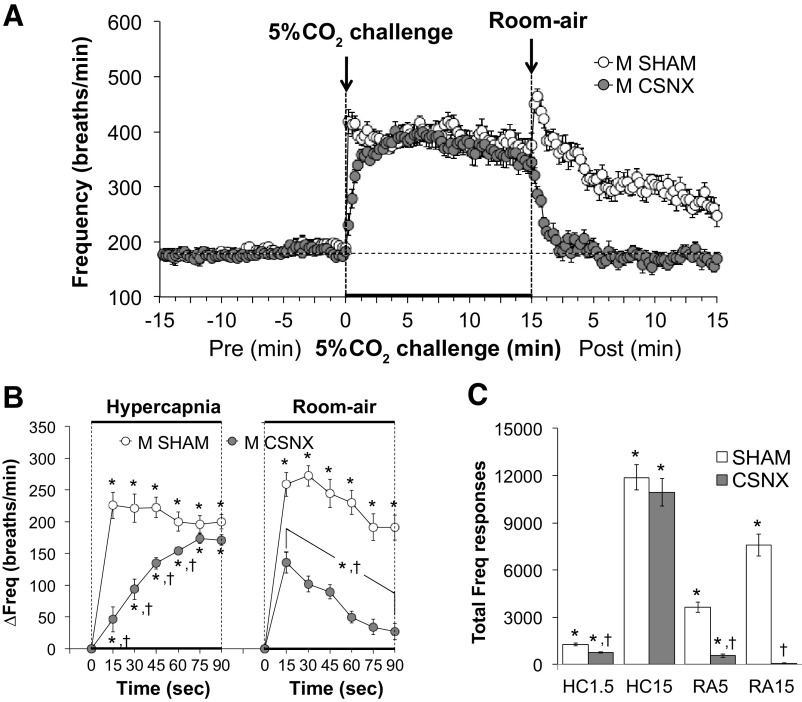
Frequency of breathing (Freq) values before, during, and following exposure to a 15-min HC challenge (5% CO2, 21% O2, 74% N2) in SHAM and CSNX mice are shown in Fig. 2. HC challenge elicited a robust and sustained increase in Freq in both groups (Fig. 2A) with the initial response to hypercapnia being significantly delayed in CSNX mice over the first 60 s (Fig. 2B). The return toward baseline values upon switching back to room air occurred more rapidly in CSNX mice compared with SHAM mice (Fig. 2B). As seen in Fig. 2C, the total increases in Freq were smaller in CSNX than SHAM mice over the first 1.5 min (HC1.5) of the HC challenge, but not the entire 15-min HC challenge (HC15). The total increases in Freq in CSNX mice were significantly smaller compared with SHAM mice over the first 5 min (RA5) and entire 15 min (RA15) of return to room air (Fig. 2C).
Figure 2.
A: frequency of breathing (Freq) values before and during a hypercapnic gas challenge (5% CO2, 21% O2, 74% N2), and upon return to room air in sham-operated male C57BL6 (SHAM) mice and in male C57BL6 mice with bilateral transection of the carotid sinus nerve (CSNX). B: arithmetic changes in Freq in SHAM and CSNX mice during the first 90 s of exposure to the hypercapnic gas challenge and the first 90 s upon return to room air. C: total changes in Freq in SHAM and CSNX mice during the first 1.5 min (HC1.5) and the entire 15 min (HC15) hypercapnic gas challenge (HC), and during the first 5 min (RA5) and the entire 15 min (RA15) return to room air (RA). The data are shown as means ± SE. *P < 0.05, significant response. †P < 0.05, CSNX versus SHAM.
Tidal volume.
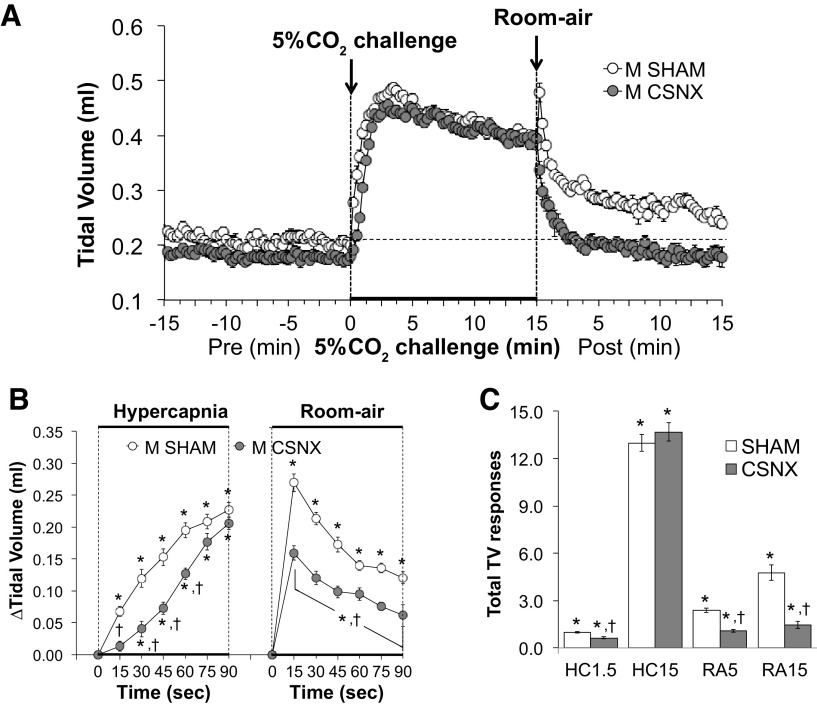
Tidal volume (TV) values before, during, and following exposure to a 15-min HC challenge (5% CO2, 21%O2, 74% N2) in SHAM and CSNX mice are shown in Fig. 3. HC gas challenge elicited a robust and sustained increase in TV in both groups (Fig. 3A) with the initial response being significantly delayed in CSNX mice over the first 60 s (Fig. 3B). The return toward baseline values upon switching back to room air occurred more rapidly in CSNX mice than in SHAM mice (Fig. 3B). As seen in Fig. 3C, the total increases in TV were significantly smaller in CSNX than SHAM mice over the first 1.5 min (HC1.5) of the HC gas challenge, but not the entire 15-min HC challenge (HC15). The total increases in TV in the CSNX mice were significantly smaller compared with the SHAM mice over the first 5 min (RA5) and entire 15 min (RA15) of return to room air (Fig. 3C).
Figure 3.
A: tidal volume (TV) values before and during a hypercapnic gas challenge (5% CO2, 21% O2, 74% N2), and upon return to room air in sham-operated male C57BL6 (SHAM) mice and in male C57BL6 mice with bilateral transection of the carotid sinus nerve (CSNX). B: arithmetic changes in TV in SHAM and CSNX mice during the first 90 s of exposure to the hypercapnic gas challenge and the first 90 s upon return to room air. C: total changes in TV in SHAM and CSNX mice during the first 1.5 min (HC1.5) and the entire 15 min (HC15) hypercapnic gas challenge (HC), and during the first 5 min (RA5) and the entire 15 min (RA15) return to room air (RA). The data are shown as means ± SE. *P < 0.05, significant response. †P < 0.05, CSNX versus SHAM.
Minute ventilation.
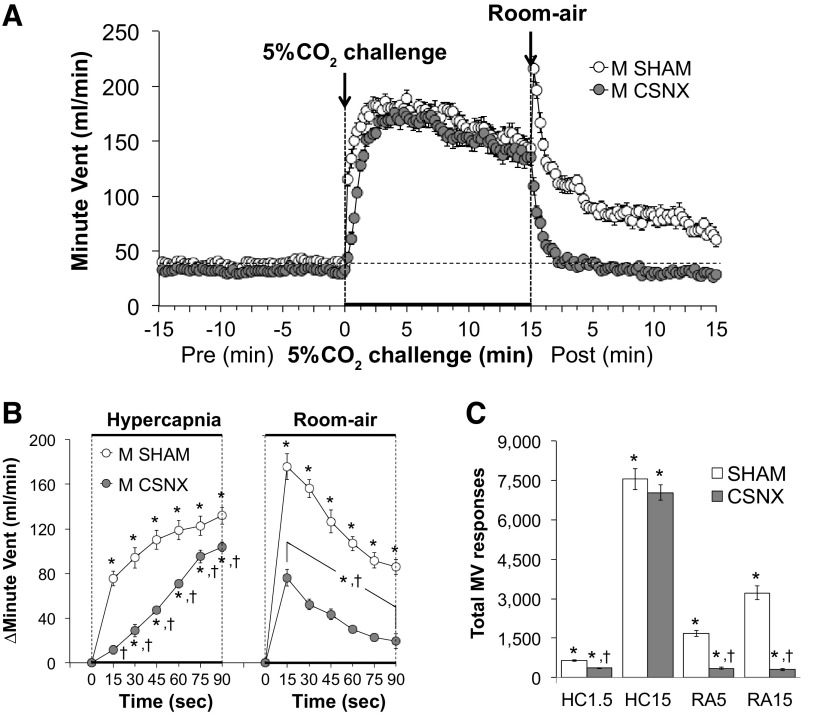
Minute ventilation (MV) values before, during, and following exposure to a 15-min HC challenge (5% CO2, 21% O2, 74% N2) in SHAM and CSNX mice are shown in Fig. 4. HC challenge elicited a robust and sustained increase in MV in both groups (Fig. 4A) with the initial response being significantly delayed in CSNX mice compared with SHAM mice (Fig. 4B). The return toward baseline values upon switching back to room air occurred more rapidly in CSNX mice than in SHAM mice (Fig. 4B). As seen in Fig. 4C, the total increases in MV were significantly smaller in CSNX than SHAM mice over the first 1.5 min (HC1.5) of the HC gas challenge, but not the entire 15-min HC challenge (HC15). Additionally, the total increases in MV in CSNX mice were significantly smaller compared with the SHAM mice over the first 5 min (RA5) and entire 15 min (RA15) of return to room air (Fig. 4C).
Figure 4.
A: minute ventilation (MV) values before and during a hypercapnic gas challenge (5% CO2, 21% O2, 74% N2), and upon return to room air in sham-operated male C57BL6 (SHAM) mice and in male C57BL6 mice with bilateral transection of the carotid sinus nerve (CSNX). B: arithmetic changes in MV in SHAM and CSNX mice during the first 90 s of exposure to the hypercapnic gas challenge and the first 90 s upon return to room air. C: total changes in MV in SHAM and CSNX mice during the first 1.5 min (HC1.5) and the entire 15 min (HC15) hypercapnic gas challenge (HC), and during the first 5 min (RA5) and the entire 15 min (RA15) return to room air (RA). The data are shown as means ± SE. *P < 0.05, significant response. †P < 0.05, CSNX versus SHAM.
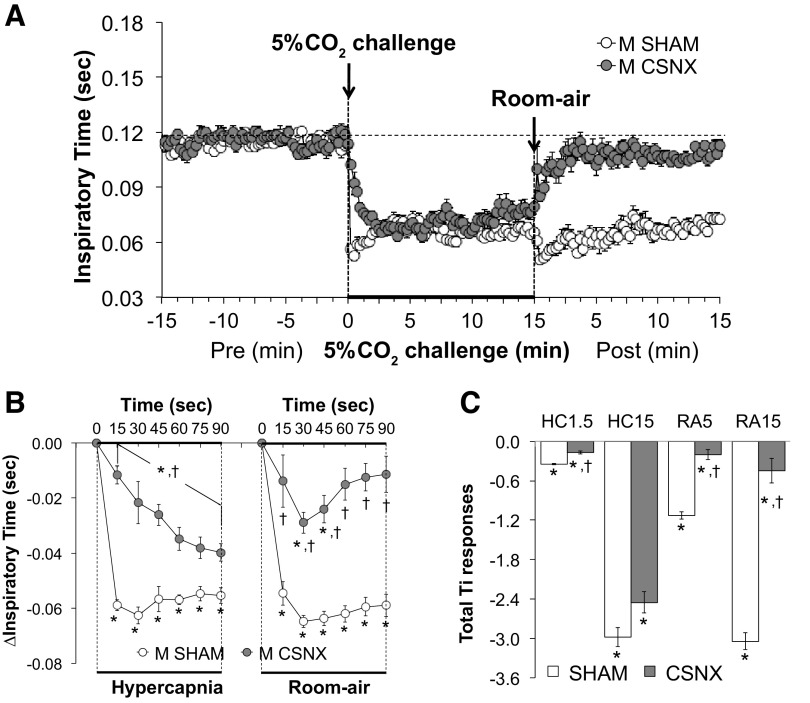
Inspiratory time.
Inspiratory time (Ti) values before, during, and following exposure to a 15-min HC challenge (5% CO2, 21% O2, 74% N2) in SHAM and CSNX mice are shown in Fig. 5. HC challenge elicited a robust and sustained decrease in Ti in both groups (Fig. 5A) with the initial response being significantly delayed in CSNX mice (Fig. 5B). The return toward baseline values upon switching back to room air occurred more rapidly in CSNX mice than in SHAM mice (Fig. 5B). As seen in the Fig. 5C, the total decreases in Ti were significantly smaller in CSNX than SHAM mice over the first 1.5 min (HC1.5) of the HC gas challenge, but not the entire 15-min HC challenge (HC15). Also, the total decreases in Ti in CSNX mice were significantly smaller compared with the SHAM mice over the first 5 min (RA5) and entire 15 min (RA15) of return to room air (Fig. 5C).
Figure 5.
A: inspiratory time (Ti) values before and during a hypercapnic gas challenge (5% CO2, 21% O2, 74% N2), and upon return to room air in sham-operated male C57BL6 (SHAM) mice and in male C57BL6 mice with bilateral transection of the carotid sinus nerve (CSNX). B: arithmetic changes in Ti in SHAM and CSNX mice during the first 90 s of exposure to the hypercapnic gas challenge and the first 90 s upon return to room air. C: total changes in Ti in SHAM and CSNX mice during the first 1.5 min (HC1.5) and the entire 15 min (HC15) hypercapnic gas challenge (HC), and during the first 5 min (RA5) and the entire 15 min (RA15) return to room air (RA). The data are shown as means ± SE. *P < 0.05, significant response. †P < 0.05, CSNX versus SHAM.
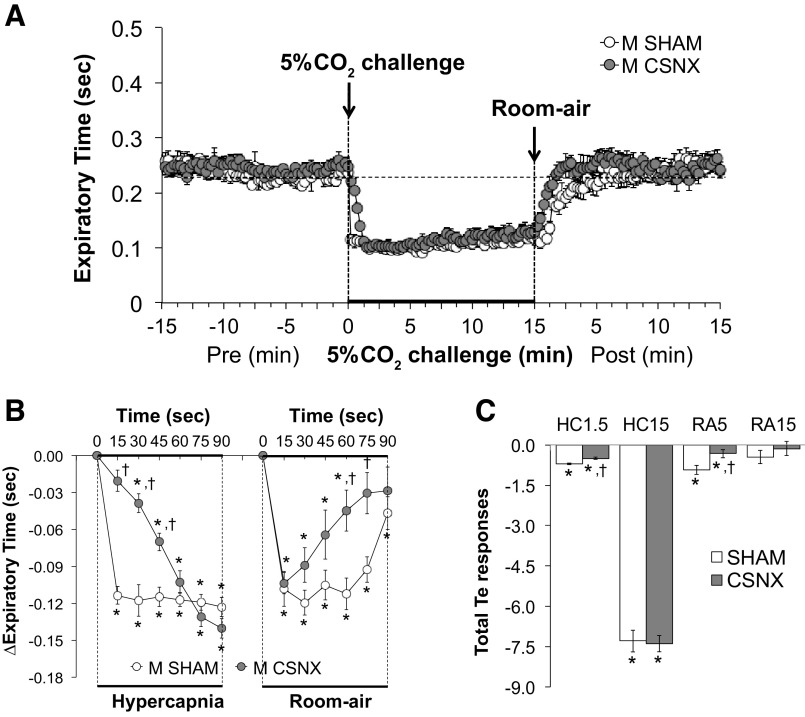
Expiratory time.
Expiratory time (Te) values before, during, and following exposure to a 15-min HC challenge (5% CO2, 21% O2, 74% N2) in SHAM and CSNX mice are shown in Fig. 6. HC challenge elicited a robust and sustained decrease in Te in both groups (Fig. 6A) with the initial response being significantly delayed in CSNX mice over the first 45 s (Fig. 6B). The return toward baseline values upon switching back to room air occurred more rapidly in CSNX mice than in SHAM mice; however, only the 60-s and 75-s time points were significant (Fig. 6B). As seen in Fig. 6C, the total decreases in Te were significantly smaller in CSNX than SHAM mice over the first 1.5 min (HC1.5) of the HC gas challenge, but not the entire 15-min HC challenge (HC15). In addition, the total decreases in Te in CSNX mice were significantly smaller compared with the SHAM mice over the first 5 min (RA5), however not the entire 15 min (RA15) of return to room air (Fig. 6C).
Figure 6.
A: expiratory time (Te) values before and during a hypercapnic gas challenge (5% CO2, 21% O2, 74% N2), and upon return to room air in sham-operated male C57BL6 (SHAM) mice and in male C57BL6 mice with bilateral transection of the carotid sinus nerve (CSNX). B: arithmetic changes in Te in SHAM and CSNX mice during the first 90 s of exposure to the hypercapnic gas challenge and the first 90 s upon return to room air. C: total changes in Te in SHAM and CSNX mice during the first 1.5 min (HC1.5) and the entire 15 min (HC15) hypercapnic gas challenge (HC), and during the first 5 min (RA5) and the entire 15 min (RA15) return to room air (RA). The data are shown as means ± SE. *P < 0.05, significant response. †P < 0.05, CSNX versus SHAM.
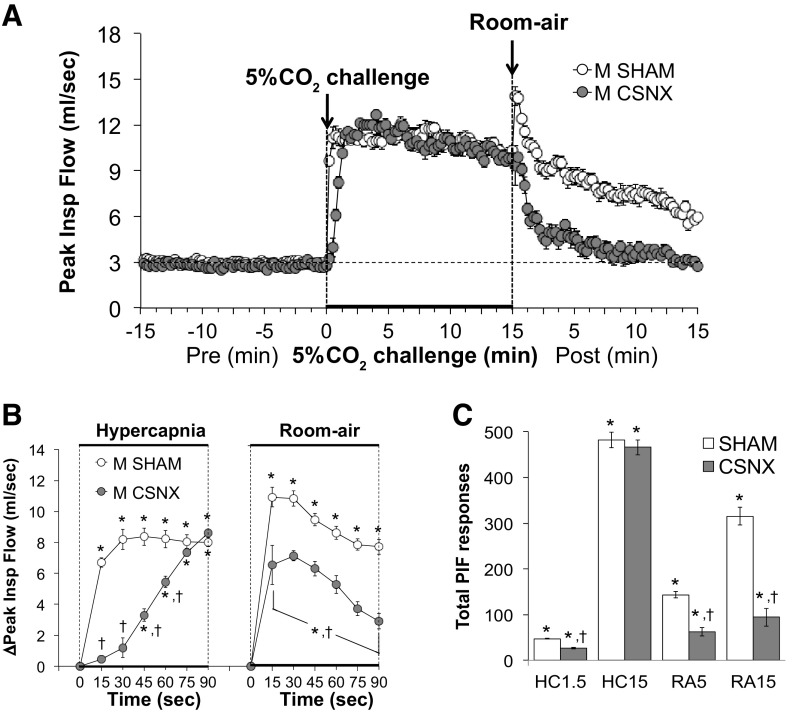
Peak inspiratory flow.
Peak inspiratory flow (PIF) values before, during, and following exposure to a 15-min HC challenge (5% CO2, 21% O2, 74% N2) in SHAM and CSNX mice are shown in Fig. 7. HC challenge elicited a robust and sustained increase in PIF in both groups (Fig. 7A) with the initial response being significantly delayed in CSNX mice over the first 60 s (Fig. 7B). The return toward baseline values upon switching back to room air occurred more rapidly in CSNX mice than in SHAM mice (Fig. 7B). As seen in Fig. 7C, the total increases in PIF were significantly smaller in CSNX than SHAM mice over the first 1.5 min (HC1.5) of the HC gas challenge, but not the entire 15-min HC challenge (HC15). The total increases in PIF in CSNX mice were significantly smaller compared with the SHAM mice over the first 5 min (RA5) and entire 15 min (RA15) of return to room air (Fig. 7C).
Figure 7.
A: peak inspiratory flow (PIF) values before and during a hypercapnic gas challenge (5% CO2, 21% O2, 74% N2), and upon return to room air in sham-operated male C57BL6 (SHAM) mice and in male C57BL6 mice with bilateral transection of the carotid sinus nerve (CSNX). B: arithmetic changes in PIF in SHAM and CSNX mice during the first 90 s of exposure to the hypercapnic gas challenge and the first 90 s upon return to room air. C: total changes in PIF in SHAM and CSNX mice during the first 1.5 min (HC1.5) and the entire 15 min (HC15) hypercapnic gas challenge (HC), and during the first 5 min (RA5) and the entire 15 min (RA15) return to room air (RA). The data are shown as means ± SE. *P < 0.05, significant response. †P < 0.05, CSNX versus SHAM.
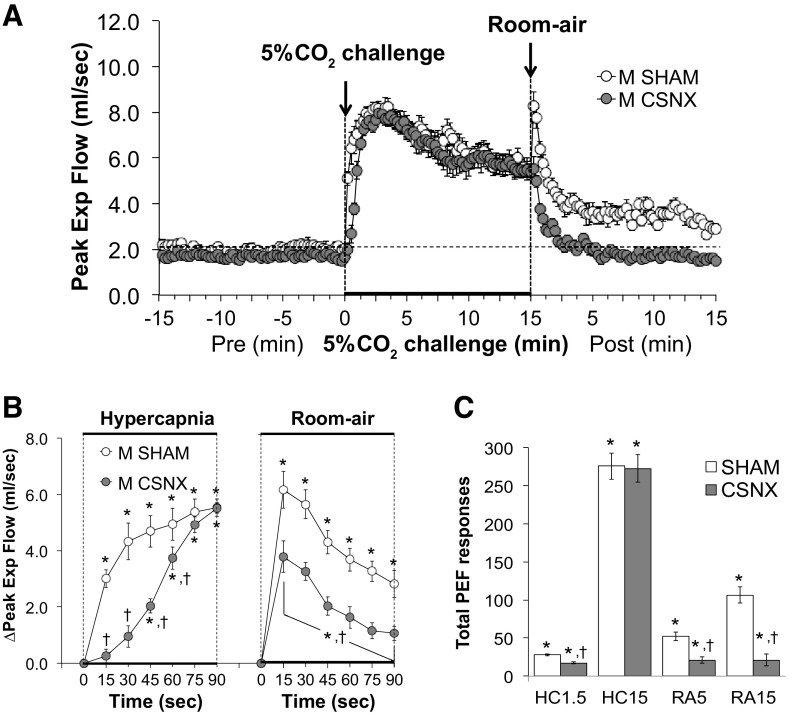
Peak expiratory flow.
Peak expiratory flow (PEF) values before, during, and following exposure to a 15-min HC challenge (5% CO2, 21% O2, 74% N2) in SHAM and CSNX mice are shown in Fig. 8. HC challenge elicited a robust and sustained increase in PEF in both groups (Fig. 8A) with the initial response being delayed in CSNX mice over the initial 60 s (Fig. 8B). The return toward baseline values upon switching back to room air occurred more rapidly in CSNX mice than in SHAM mice (Fig. 8B). As seen in Fig. 8C, the total increases in PEF were significantly smaller in CSNX than SHAM mice over the first 1.5 min (HC1.5) of the HC gas challenge, but not the entire 15-min HC challenge (HC15). In addition, the total increases in PEF in CSNX mice were significantly smaller compared with the SHAM mice over the first 5 min (RA5) and entire 15 min (RA15) of return to room air (Fig. 8C).
Figure 8.
A: peak expiratory flow (PEF) values before and during a hypercapnic gas challenge (5% CO2, 21% O2, 74% N2), and upon return to room air in sham-operated male C57BL6 (SHAM) mice and in male C57BL6 mice with bilateral transection of the carotid sinus nerve (CSNX). B: arithmetic changes in PEF in SHAM and CSNX mice during the first 90 s of exposure to the hypercapnic gas challenge and the first 90 s upon return to room air. C: total changes in PEF in SHAM and CSNX mice during the first 1.5 min (HC1.5) and the entire 15 min (HC15) hypercapnic gas challenge (HC), and during the first 5 min (RA5) and the entire 15 min (RA15) return to room air (RA). The data are shown as means ± SE. *P < 0.05, significant response. †P < 0.05, CSNX versus SHAM.
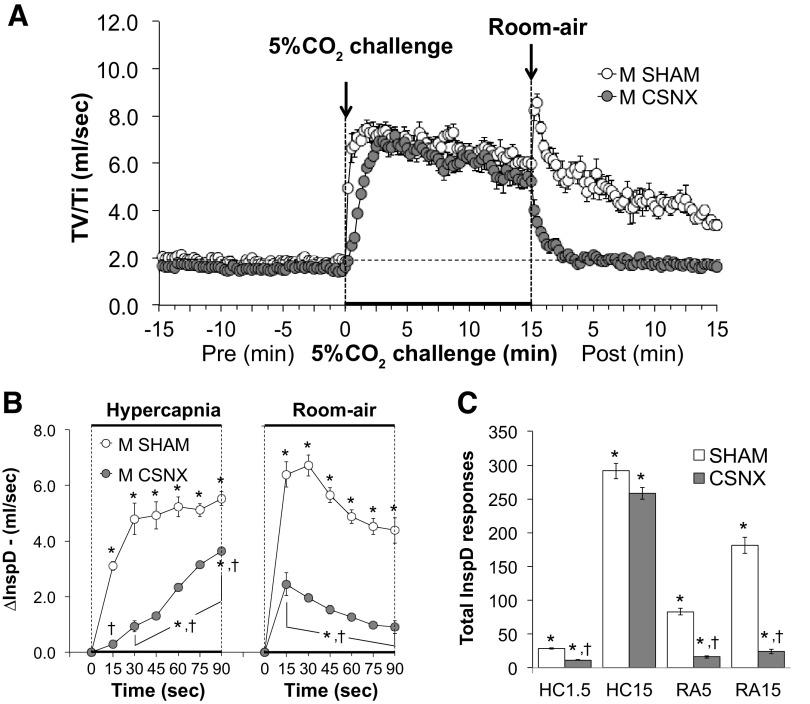
Inspiratory drive.
Inspiratory drive (TV/Ti) values before, during, and following exposure to a 15-min HC challenge (5% CO2, 21% O2, 74% N2) in SHAM and CSNX mice are shown in Fig. 9. HC challenge elicited a robust and sustained increase in inspiratory drive in both groups (Fig. 9A) with initial response being significantly delayed in CSNX mice (Fig. 9B). The return toward baseline values upon switching back to room air occurred more rapidly in CSNX mice than in SHAM mice (Fig. 9B). As seen in Fig. 9C, the total increases in inspiratory drive were significantly smaller in CSNX than SHAM mice over the first 1.5 min (HC1.5) of HC challenge, but not the entire 15-min HC challenge (HC15). Total increases in inspiratory drive in CSNX mice were significantly smaller than in SHAM mice over the first 5 min (RA5) and entire 15 min (RA15) of return to room air (Fig. 9C).
Figure 9.
A: inspiratory drive (InspD or TV/Ti) values before and during a hypercapnic gas challenge (5% CO2, 21% O2, 74% N2), and upon return to room air in sham-operated male C57BL6 (SHAM) mice and in male C57BL6 mice with bilateral transection of the carotid sinus nerve (CSNX). B: arithmetic changes in InspD in SHAM and CSNX mice during the first 90 s of exposure to the hypercapnic gas challenge and the first 90 s upon return to room air. C: total changes in InspD in SHAM and CSNX mice during the first 1.5 min (HC1.5) and the entire 15 min (HC15) hypercapnic gas challenge (HC), and during the first 5 min (RA5) and the entire 15 min (RA15) return to room air (RA). The data are shown as means ± SE. *P < 0.05, significant response. †P < 0.05, CSNX versus SHAM.
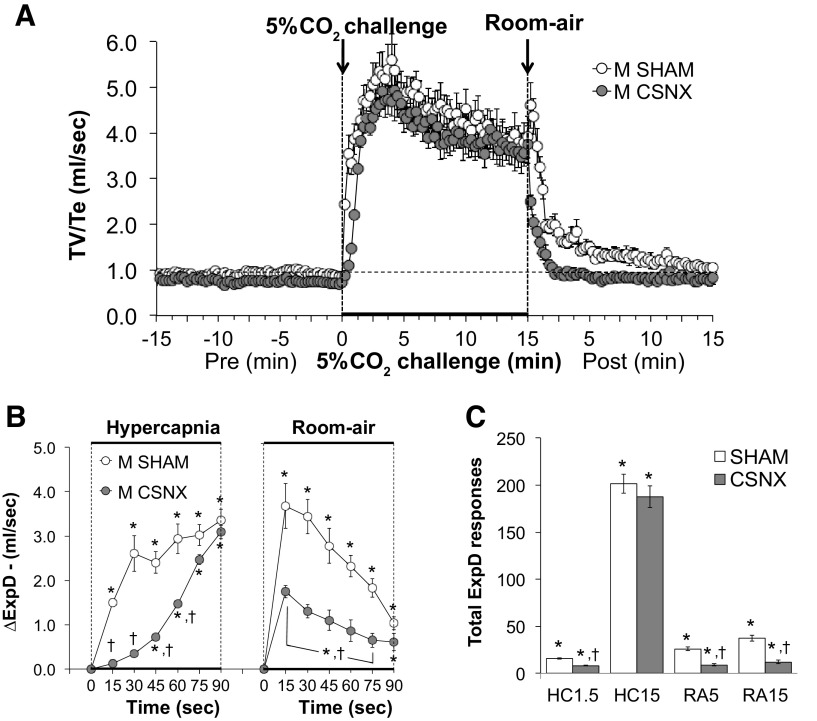
Expiratory drive.
Expiratory drive (TV/Te) values before, during, and following exposure to a 15-min HC challenge (5% CO2, 21% O2, 74% N2) in SHAM and CSNX mice are shown in Fig. 10. HC challenge elicited a robust and sustained increase in expiratory drive in both groups (Fig. 10A) with the initial response being significantly delayed in CSNX mice over the first 60 s (Fig. 10B). The return toward baseline values upon switching back to room air occurred more rapidly in CSNX mice than in SHAM mice over the first 75 s (Fig. 10B). As seen in Fig. 10C, the total increases in expiratory drive were significantly smaller in CSNX than SHAM mice over the first 1.5 min (HC1.5) of the HC gas challenge, but not the entire 15-min HC challenge (HC15). In addition, the total increases in expiratory drive in CSNX mice were significantly smaller compared with the SHAM mice over the first 5 min (RA5) and entire 15 min (RA15) of return to room air (Fig. 10C).
Figure 10.
A: expiratory drive (ExpD or TV/Te) values before and during a hypercapnic gas challenge (5% CO2, 21% O2, 74% N2), and upon return to room-air in sham-operated male C57BL6 (SHAM) mice and in male C57BL6 mice with bilateral transection of the carotid sinus nerve (CSNX). B: arithmetic changes in ExpD in SHAM and CSNX mice during the first 90 s of exposure to the hypercapnic gas challenge and the first 90 s upon return to room air. C: total changes in ExpD in SHAM and CSNX mice during the first 1.5 min (HC1.5) and the entire 15 min (HC15) hypercapnic gas challenge (HC), and during the first 5 min (RA5) and the entire 15 min (RA15) return to room air (RA). The data are shown as means ± SE. *P < 0.05, significant response. †P < 0.05, CSNX versus SHAM.
DISCUSSION
Our study shows that a loss of CSN chemoafferents is not benign in adult male C57BL6 mice and suggests the presence of ongoing chemoafferent activity in these mice. Resting TV was significantly lower in CSNX mice compared with SHAM mice 7 days after bilateral CSN transection, whereas resting Freq was not. Resting PEF and expiratory drive values were also lower in CSNX mice compared to SHAM, therefore challenging the expectation that expiration is a passive process in these mice. Conversely, a previous study showed that resting values in adult male C57BL6 mice with CSNX were not different from SHAM mice 10–12 days after surgery (55). As such, our data suggests that adaptive processes between 7 and 10 days after CSNX allow for full return of resting ventilatory function.
The lack of effect of HC challenge on mouse body temperature has been reported previously (24, 111). However, there are no recordings of body temperature post-HC challenge. Our results are consistent with previous studies and show that the body temperatures of SHAM and CSNX mice are similar and display minor changes during and after HC challenge. Therefore, changes in metabolic drive are unlikely to contribute to the differences in ventilatory responses between SHAM and CSNX mice that we see in our study (38, 55).
The initial (90 s) responses to HC challenge occurred more slowly in CSNX than SHAM mice, although the responses achieved similar plateaus at the end of the 15-min HC challenge in both groups. This suggests that elevations in blood pCO2 stimulate CSN chemoafferents before signaling the brain to activate central neurons that drive hypercapnic signaling, such as those in the retrotrapezoid nucleus (RTN) (59–66). Activation of central neurons involves a series of steps: 1) entry of CO2 into cerebrospinal fluid, 2) carbonic-anhydrase-catalyzed reaction of CO2 and H2O to form carbonic acid (H2CO3), 3) spontaneous breakdown of H2CO3 to HCO3− and protons (H+), and 4) H+-mediated activation of neurons via binding to acid-sensing ion channels (65, 66). Additionally, it is well-known that central and peripheral chemoreceptive systems interact with one another (112–115) and CB chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2 (115). The slower initial responses we see in CSNX mice to HC challenge may also be the result of losing active connections between the CB and RTN, and thus diminishing sensitivity of central CO2/H+-sensing neurons via downregulation of the expression of acid-sensing ion channels (59–64, 116, 117). In rats, the RTN contains chemosensitive glutamatergic neurons, which express the paired-like homeobox 2 b gene (Phox2b), and these Phox2b expressing neurons are in a circuit linking the CB, CSN chemoafferents, and chemosensitive NTS projections to the RTN (60, 118). Thus, CO2-sensitive RTN neurons comprise a site of convergence for several inputs controlling ventilation (119), and such circuitry most likely exists in the mouse RTN as well. Our findings that CSNX and SHAM mice reached similar ventilatory plateaus at the end of the 15-min HC challenge suggests that central chemosensitive neurons in CSNX mice eventually mount a robust ventilatory response despite possible changes in activation and expression pattern of proton-sensitive ion channels in the RTN.
The ventilatory responses upon return to room air in SHAM and CSNX mice were markedly different. In SHAM mice, switching from HC to room air resulted in a brief increase in Freq, TV, MV, PIF, PEF, and inspiratory and expiratory drives that was followed by a gradual return toward baseline over the 15-min period. The increases in Freq were associated with sustained decreases in Ti, whereas Te displayed a rapid (within 5 min) return to baseline in SHAM mice. The mechanism(s) behind this difference in the temporal responses of Ti and Te is unknown, but it is evident that processes promoting active breathing (i.e., inspiration) are sustained, whereas those that stimulate expiration are corrected following reintroduction of room air so that exhalation returns to a passive process. In contrast, the ventilatory responses in CSNX mice involved the immediate return of all parameters to baseline. As such, the return to room air responses in SHAM animals is heavily dependent on signaling at the CB-CSN complex. The mechanisms involved in activation of the CB-CSN upon return to room air in SHAM mice or why it takes some parameters over 15 min to return to baseline values are unknown. We have evidence that the generation of S-nitrosothiols (e.g., L-S-nitrosocysteine and L-S-nitrosoglutathione) in the blood and CB plays a key role in CB chemoafferent-mediated increases in ventilation upon return to room air after hypoxic (HX) gas challenge. This evidence includes the following: 1) post-HX ventilatory responses are reduced in CSNX mice and in mice in which red blood cell hemoglobin cannot generate S-nitrosothiols (55), 2) post-HX responses are augmented in mice lacking the enzyme that degrades L-S-nitrosoglutathione (77), and 3) arterial injections of L-S-nitrosocysteine increase MV by activating CSN chemoafferents via mechanisms involving modulation of voltage-gated K+-channels (84). Whether S-nitrosothiols mediate CSN-dependent post-HC responses remain to be established.
Conclusions
In summary, CB chemoafferents initiate the rapid increase in ventilatory in response to HC challenge in freely moving adult male C57BL6 mice, and the CB chemoafferents are also required for the ventilatory responses (e.g., changes in Freq, TV, MV, Ti, Te, PIF, and PEF) that occur upon return to room air. The slower onset of the rise in breathing in CSNX mice suggests that the CB-CSN has a vital role in eliciting the initial ventilatory responses to HC challenge and that the CB chemosensitive glomus cells’ response to increases in blood CO2 is activated before brainstem (e.g., RTN) systems are recruited. The loss of CSN input to the brainstem NTS may decrease RTN sensitivity to H+/CO2 stimuli via changes in activation and expression pattern of proton-sensitive ion channels (112–115, 120).
The sustained increase in frequency of breathing upon return to room air following HC challenge in SHAM male C57BL6 mice is consistent with our previous findings (68). In CSNX mice, the ventilatory responses upon return to room air were markedly diminished suggesting that the CB-CSN complex is crucial to generate these responses. We provided evidence that generation of circulating S-nitrosothiols and their ability to activate small diameter unmyelinated C-fibers in the CSN is responsible for the post-HX room air responses seen in rats and mice (55, 77, 84). We speculate that HC challenge may generate circulating S-nitrosothiols that mediate the ventilatory responses that occur upon return to room air via activation of CSN small diameter unmyelinated C-fibers. Other mechanisms such as reorganization of central chemoreceptor circuitry and/or altered hemodynamic responses in CSNX mice may also be involved in eliciting the room air responses. Additionally, there is compelling evidence that CSNX causes substantial acute changes in the expression of functional proteins, such as glutamatergic [N-methyl-d-aspartate (NMDA)] receptor subunits, tryptophan hydroxylase and serotonin transporters, in key nuclei/structures in the brainstem of goats (121, 122). Whatever the mechanisms, the present study suggests that return to room air following HC challenge elicits robust ventilatory responses involving the CB-CSN complex.
The robust ventilatory responses elicited by HC challenge in both SHAM and CSNX C57BL6 mice are consistent with responses reported in various strains of adult mice (68, 73, 91) and rats (120, 123, 124). Ventilatory systems display considerable plasticity following sensory denervation (7, 125, 126), and it is possible that the peripheral and central systems involved in processing the ventilatory responses to HC challenge in C57BL6 mice may have adapted to the loss of CB chemoafferent input to the brainstem. These adaptations may have accounted for the relatively slower rise to maximum ventilation to occur in CSNX mice during HC gas challenge and may have contributed to the reduced responses upon return to room air. Mouradian et al. (120) reported that the ventilatory responses to HC challenge in male Brown Norway, Dahl Salt-Sensitive, or Sprague–Dawley rats with bilateral CSNX were similar to those of sham-operated (SHAM) rats, although the initial exposures (i.e., over the first 90 s) to HC challenge were not reported nor were the return to room air responses. Consistent with our present findings, we reported that the initial increase in MV elicited by HC challenge in juvenile (P25) Sprague–Dawley rats with bilateral CSNX was slower than in SHAM rats and that the post-HC room air responses were diminished in CSNX P25 rats (105). The complex interactions between the CB and central (e.g., RTN) chemoreceptors with respect to the control of breathing and the ventilatory responses to HX, HC, and combined hypoxic-hypercapnic (HX-HC) challenges were addressed by Smith et al. (112) and Forster and Smith (57) and by our group (105). Briefly the data suggests that there can be a positive interaction between the CB and central chemoreceptors, such that carotid chemoafferents increase the responsiveness of central CO2/H+ chemoreceptors (57, 112).
Our findings that the loss of CSN chemoafferents has major effects on the ventilatory responses that occur during the initial 90 s of the HC challenge and after HC challenge should be considered with respect to peripheral and central neural systems that control breathing in other mammalian species, especially humans. Our data showing that the initial rise in MV is delayed in CSNX mice are consistent with evidence in other species showing that the ventilatory responses to inhaled CO2 have a rapid component involving the CB-CSN and a slower component involving central chemoreflex circuitry (127–129). The relative roles of these peripheral and central chemoreflex systems are similar in humans, although the exact importance and temporal recruitment of the systems and the nature of their interactivity are subject to debate (28, 130–134). Currently in the literature, there are no data looking at the ventilatory responses that occur upon return to room air after HC challenge or the roles of peripheral/central chemoreceptor systems in humans. However, studies in humans have found that HC challenge increases cerebral blood flow in synchrony with end-tidal CO2 values, whereas post-HC recovery of blood flow upon return to room air breathing is delayed in relation to end-tidal CO2 (135–137). The potential role of the CB-CSN in these post-HC responses in humans is unknown.
With respect to study limitations, the C57BL6 mouse is widely used to study ventilatory processes despite this strain expressing a greater degree of disordered breathing at rest and in response to HX and HC challenges than other strains (68, 87–89, 91–94, 96, 97). Although female C57BL6 mice have higher levels of disordered breathing than other female mouse strains (75–77), the role of the CB-CSN in their ventilatory responses has not been studied. Therefore, the value of the present findings in male C57BL6 mice will be put into better perspective when studies in female C57BL6 mice are performed.
Future studies are needed to address whether loss of the CB-CSN complex affects ventilatory responses to HC challenge at different stages of postnatal development, which is a vital contributor in the expression of these responses (138, 139). Moreover, Netick et al. (140) characterized the ventilatory responses during the awake-sleep cycle in cats and found that responses were diminished during rapid-eye movement (REM) sleep. The issue as to how the CB-CSN complex participates in the ventilatory responses to HC challenge during various stages of the awake-sleep cycle in C57BL6 mice is something we hope to address. Currently, we are investigating the role of the CB-CSN complex in the initial response to HC challenge and upon return to room air in other mouse strains, since strain differences in responsiveness to HC challenge exist in mice (141) and rats (124). For example, Tankersley et al. (141) reported that ventilatory responses to 5% and 8% HC gas challenges were greater in male C57BL6 mice than in male mice of the C3H/HeJ strain.
GRANTS
This work was supported by a component of a program grant from the National Institutes of Health (PO1HL101871) to S.J.L. (Project 3: Cellular S-nitrosothiol signaling in respiratory biology).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.M.G., S.S., and S.J.L. conceived and designed research; P.M.G. and S.J.L. performed experiments; P.M.G., S.S., and S.J.L. analyzed data; P.M.G., S.S., and S.J.L. interpreted results of experiments; P.M.G. and S.J.L. prepared figures; P.M.G., S.S., and S.J.L. drafted manuscript; P.M.G., S.S., and S.J.L. edited and revised manuscript; P.M.G., S.S., and S.J.L. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of S. Sundararajan: Division of Neonatology, Department of Pediatrics, University of Maryland School of Medicine, Baltimore, Maryland 21201.
REFERENCES
- 1.Forster RE 2nd.Carbonic anhydrase and the carotid body. Adv Exp Med Biol 337: 137–147, 1993. doi: 10.1007/978-1-4615-2966-8_20. [DOI] [PubMed] [Google Scholar]
- 2.Lahiri S, Forster RE 2nd.. CO2/H+ sensing: peripheral and central chemoreception. Int J Biochem Cell Biol 35: 1413–1435, 2003. doi: 10.1016/s1357-2725(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 3.Lahiri S, Roy A, Baby SM, Hoshi T, Semenza GL, Prabhakar NR. Oxygen sensing in the body. Prog Biophys Mol Biol 91: 249–286, 2006. doi: 10.1016/j.pbiomolbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 4.O'Regan RG, Majcherczyk S. Role of peripheral chemoreceptors and central chemosensitivity in the regulation of respiration and circulation. J Exp Biol 100: 23–40, 1982. doi: 10.1242/jeb.100.1.23. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RJ, Teppema LJ. Integration of central and peripheral respiratory chemoreflexes. Compr Physiol 6: 1005–1041, 2016. doi: 10.1002/cphy.c140040. [DOI] [PubMed] [Google Scholar]
- 6.Kikuta S, Iwanaga J, Kusukawa J, Tubbs RS. Carotid sinus nerve: a comprehensive review of its anatomy, variations, pathology, and clinical applications. World Neurosurg 127: 370–374, 2019. doi: 10.1016/j.wneu.2019.04.064. [DOI] [PubMed] [Google Scholar]
- 7.Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol 2: 141–219, 2012. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peers C, Buckler KJ. Transduction of chemostimuli by the type I carotid body cell. J Membr Biol 144: 1–9, 1995. doi: 10.1007/bf00238411. [DOI] [PubMed] [Google Scholar]
- 9.Prabhakar NR. Oxygen sensing by the carotid body chemoreceptors. J Appl Physiol (1985) 88: 2287–2295, 2000. doi: 10.1152/jappl.2000.88.6.2287. [DOI] [PubMed] [Google Scholar]
- 10.Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev 90: 675–754, 2010. doi: 10.1152/physrev.00012.2009. [DOI] [PubMed] [Google Scholar]
- 11.Tse A, Yan L, Lee AK, Tse FW. Autocrine and paracrine actions of ATP in rat carotid body. Can J Physiol Pharmacol 90: 705–711, 2012. doi: 10.1139/y2012-054. [DOI] [PubMed] [Google Scholar]
- 12.Eyzaguirre C, Zapata P. Perspectives in carotid body research. J Appl Physiol Respir Environ Exerc Physiol 57: 931–957, 1984. doi: 10.1152/jappl.1984.57.4.931. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74: 829–898, 1994. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- 14.Bairam A, Carroll JL. Neurotransmitters in carotid body development. Respir Physiol Neurobiol 149: 217–232, 2005. doi: 10.1016/j.resp.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Conde SV, Monteiro EC, Sacramento JF. Purines and carotid body: new roles in pathological conditions. Front Pharmacol 8: 913, 2017. doi: 10.3389/fphar.2017.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eyzaguirre C. Chemical and electric transmission in the carotid body chemoreceptor complex. Biol Res 38: 341–345, 2005. doi: 10.4067/s0716-97602005000400005. [DOI] [PubMed] [Google Scholar]
- 17.Iturriaga R, Alcayaga J. Neurotransmission in the carotid body: transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Brain Res Brain Res Rev 47: 46–53, 2004. doi: 10.1016/j.brainresrev.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Leonard EM, Salman S, Nurse CA. Sensory processing and integration at the carotid body tripartite synapse: neurotransmitter functions and effects of chronic hypoxia. Front Physiol 9: 225, 2018. doi: 10.3389/fphys.2018.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nurse CA. Neurotransmission and neuromodulation in the chemosensory carotid body. Auton Neurosci 120: 1–9, 2005. doi: 10.1016/j.autneu.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Nurse CA, Piskuric NA. Signal processing at mammalian carotid body chemoreceptors. Semin Cell Dev Biol 24: 22–30, 2013. doi: 10.1016/j.semcdb.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Prabhakar NR. O2 sensing at the mammalian carotid body: why multiple O2 sensors and multiple transmitters? Exp Physiol 91: 17–23, 2006. doi: 10.1113/expphysiol.2005.031922. [DOI] [PubMed] [Google Scholar]
- 22.Prabhakar NR, Peng YJ, Yuan G, Nanduri J. Reactive oxygen radicals and gaseous transmitters in carotid body activation by intermittent hypoxia. Cell Tissue Res 372: 427–431, 2018. doi: 10.1007/s00441-018-2807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prabhakar NR, Peng YJ, Nanduri J. Recent advances in understanding the physiology of hypoxic sensing by the carotid body. F1000Res 7: F1000 Faculty Rev-1900, 2018. doi: 10.12688/f1000research.16247.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li A, Nattie E. Serotonin transporter knockout mice have a reduced ventilatory response to hypercapnia (predominantly in males) but not to hypoxia. J Physiol 586: 2321–2329, 2008. doi: 10.1113/jphysiol.2008.152231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nurse CA. Neurotransmitter and neuromodulatory mechanisms at peripheral arterial chemoreceptors. Exp Physiol 95: 657–667, 2010. doi: 10.1113/expphysiol.2009.049312. [DOI] [PubMed] [Google Scholar]
- 26.Bellville JW, Whipp BJ, Kaufman RD, Swanson GD, Aqleh KA, Wiberg DM. Central and peripheral chemoreflex loop gain in normal and carotid body-resected subjects. J Appl Physiol Respir Environ Exerc Physiol 46: 843–853, 1979. doi: 10.1152/jappl.1979.46.4.843. [DOI] [PubMed] [Google Scholar]
- 27.Dahan A, Nieuwenhuijs D, Teppema L. Plasticity of central chemoreceptors: effect of bilateral carotid body resection on central CO2 sensitivity. PLoS Med 4: e239, 2007. doi: 10.1371/journal.pmed.0040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fatemian M, Nieuwenhuijs DJF, Teppema LJ, Meinesz S, Mey AGL, Dahan A, Robbins PA. The respiratory response to carbon dioxide in humans with unilateral and bilateral resections of the carotid bodies. J Physiol 549: 965–973, 2003. doi: 10.1113/jphysiol.2003.042259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honda Y, Hashizume I, Kimura H, Severinghuas JW. Bilateral carotid body resection in man enhances hypoxic tachycardia. Jpn J Physiol 38: 917–928, 1988. doi: 10.2170/jjphysiol.38.917. [DOI] [PubMed] [Google Scholar]
- 30.Honda Y. Role of carotid chemoreceptors in control of breathing at rest and in exercise: studies on human subjects with bilateral carotid body resection. Jpn J Physiol 35: 535–544, 1985. doi: 10.2170/jjphysiol.35.535. [DOI] [PubMed] [Google Scholar]
- 31.Honda Y, Tanaka M. Respiratory and cardiovascular activities in carotid body resected humans. Adv Exp Med Biol 337: 359–364, 1993. doi: 10.1007/978-1-4615-2966-8_50. [DOI] [PubMed] [Google Scholar]
- 32.Lugliani R, Whipp BJ, Seard C, Wasserman K. Effect of bilateral carotid-body resection on ventilatory control at rest and during exercise in man. N Engl J Med 285: 1105–1111, 1971. doi: 10.1056/NEJM197111112852002. [DOI] [PubMed] [Google Scholar]
- 33.Swanson GD, Whipp BJ, Kaufman RD, Aqleh KA, Winter B, Bellville JW. Effect of hypercapnia on hypoxic ventilatory drive in carotid body-resected man. J Appl Physiol Respir Environ Exerc Physiol 45: 871–877, 1978. doi: 10.1152/jappl.1978.45.6.971. [DOI] [PubMed] [Google Scholar]
- 34.Timmers HJ, Wieling W, Karemaker JM, Lenders JW. Denervation of carotid baro- and chemoreceptors in humans. J Physiol 553: 3–11, 2003. doi: 10.1113/jphysiol.2003.052415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2. J Physiol 588: 2455–2471, 2010. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouverot P, Candas V, Libert JP. Role of the arterial chemoreceptors in ventilatory adaptation to hypoxia of awake dogs and rabbits. Respir Physiol 17: 209–219, 1973. doi: 10.1016/0034-5687(73)90062-5. [DOI] [PubMed] [Google Scholar]
- 37.Rodman JR, Curran AK, Henderson KS, Dempsey JA, Smith CA. Carotid body denervation in dogs: eupnea and the ventilatory response to hyperoxic hypercapnia. J Appl Physiol (1985) 91: 328–335, 2001[Erratum inJ Appl Physiol91: following table of contents, 2001]. doi: 10.1152/jappl.2001.91.1.328. [DOI] [PubMed] [Google Scholar]
- 38.Baby SM, Gruber RB, Young AP, MacFarlane PM, Teppema LJ, Lewis SJ. Bilateral carotid sinus nerve transection exacerbates morphine-induced respiratory depression. Eur J Pharmacol 834: 17–29, 2018. doi: 10.1016/j.ejphar.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiocchio SR, Hilton SM, Tramezzani JH, Willshaw P. Loss of peripheral chemoreflexes to hypoxia after carotid body removal in the rat. Respir Physiol 57: 235–246, 1984. doi: 10.1016/0034-5687(84)90096-3. [DOI] [PubMed] [Google Scholar]
- 40.Czyzyk-Krzeska MF, Trzebski A. Respiratory-related discharge pattern of sympathetic nerve activity in the spontaneously hypertensive rat. J Physiol 426: 355–368, 1990. doi: 10.1113/jphysiol.1990.sp018142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golder FJ, Dax S, Baby SM, Gruber R, Hoshi T, Ideo C, Kennedy A, Peng S, Puskovic V, Ritchie D, Woodward R, Wardle RL, Van Scott MR, Mannion JC, MacIntyre DE. Identification and characterization of GAL-021 as a novel breathing control modulator. Anesthesiology 123: 1093–1104, 2015. doi: 10.1097/ALN.0000000000000844. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi F, Yoshida A, Fukuda Y, Honda Y. The ventilatory response to hypoxia in the anesthetized rat. Pflugers Arch 396: 121–127, 1983. doi: 10.1007/BF00615516. [DOI] [PubMed] [Google Scholar]
- 43.Izumizaki M, Pokorski M, Homma I. Role of the carotid bodies in chemosensory ventilatory responses in the anesthetized mouse. J Appl Physiol (1985) 97: 1401–1407, 2004. doi: 10.1152/japplphysiol.00025.2004. [DOI] [PubMed] [Google Scholar]
- 44.Jacono FJ, Peng YJ, Nethery D, Faress JA, Lee Z, Kern JA, Prabhakar NR. Acute lung injury augments hypoxic ventilatory response in the absence of systemic hypoxemia. J Appl Physiol (1985) 101: 1795–1802, 2006. doi: 10.1152/japplphysiol.00100.2006. [DOI] [PubMed] [Google Scholar]
- 45.Kongo M, Yamamoto R, Kobayashi M, Nosaka S. Hypoxia inhibits baroreflex vagal bradycardia via a central action in anaesthetized rats. Exp Physiol 84: 47–56, 1999. doi: 10.1111/j.1469-445x.1999.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 46.Martin-Body RL, Robson GJ, Sinclair JD. Respiratory effects of sectioning the carotid sinus glossopharyngeal and abdominal vagal nerves in the awake rat. J Physiol 361: 35–45, 1985. doi: 10.1113/jphysiol.1985.sp015631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin-Body RL, Robson GJ, Sinclair JD. Restoration of hypoxic respiratory responses in the awake rat after carotid body denervation by sinus nerve section. J Physiol 380: 61–73, 1986. doi: 10.1113/jphysiol.1986.sp016272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin-Body RL. Brain transections demonstrate the central origin of hypoxic ventilatory depression in carotid body-denervated rats. J Physiol 407: 41–52, 1988. doi: 10.1113/jphysiol.1988.sp017402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roux JC, Peyronnet J, Pascual O, Dalmaz Y, Pequignot JM. Ventilatory and central neurochemical reorganisation of O2 chemoreflex after carotid sinus nerve transection in rat. J Physiol 522: 493–501, 2000. doi: 10.1111/j.1469-7793.2000.t01-4-00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ryan AT, Ward DA, Megirian D. Sleep-waking patterns of intact and carotid sinus nerve-transected rats during hypoxic-CO2 breathing. Exp Neurol 80: 337–348, 1983. doi: 10.1016/0014-4886(83)90287-x. [DOI] [PubMed] [Google Scholar]
- 51.Ryan AT, Megirian D. Sleep-wake patterns of intact and carotid sinus nerve sectioned rats during hypoxia. Sleep 5: 1–10, 1982. doi: 10.1093/sleep/5.1.1. [DOI] [PubMed] [Google Scholar]
- 52.Sapru HN, Krieger AJ. Carotid and aortic chemoreceptor function in the rat. J Appl Physiol Respir Environ Exerc Physiol 42: 344–348, 1977. doi: 10.1152/jappl.1977.42.3.344. [DOI] [PubMed] [Google Scholar]
- 53.Weil JV, Stevens T, Pickett CK, Tatsumi K, Dickinson MG, Jacoby CR, Rodman DM. Strain-associated differences in hypoxic chemosensitivity of the carotid body in rats. Am J Physiol Lung Cell Mol Physiol 274: L767–L774, 1998. doi: 10.1152/ajplung.1998.274.5.L767. [DOI] [PubMed] [Google Scholar]
- 54.Gassmann M, Pfistner C, Doan VD, Vogel J, Soliz J. Impaired ventilatory acclimatization to hypoxia in female mice overexpressing erythropoietin: unexpected deleterious effect of estradiol in carotid bodies. Am J Physiol Regul Integr Comp Physiol 299: R1511–R1520, 2010. doi: 10.1152/ajpregu.00205.2010. [DOI] [PubMed] [Google Scholar]
- 55.Gaston B, May WJ, Sullivan S, Yemen S, Marozkina NV, Palmer LA, Bates JN, Lewis SJ. Essential role of hemoglobin beta-93-cysteine in post-hypoxia facilitation of breathing in conscious mice. J Appl Physiol (1985) 116: 1290–1299, 2014. doi: 10.1152/japplphysiol.01050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soliz J, Joseph V, Soulage C, Becskei C, Vogel J, Pequignot JM, Ogunshola O, Gassmann M. Erythropoietin regulates hypoxic ventilation in mice by interacting with brainstem and carotid bodies. J Physiol 568: 559–571, 2005. doi: 10.1113/jphysiol.2005.093328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Forster HV, Smith CA. Contributions of central and peripheral chemoreceptors to the ventilatory response to CO2/H+. J Appl Physiol (1985) 108: 989–994, 2010. doi: 10.1152/japplphysiol.01059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iturriaga R. Carotid body chemoreception: the importance of CO2-HCO3- and carbonic anhydrase. Biol Res 26: 319–329, 1993. [PubMed] [Google Scholar]
- 59.Cummins EP, Strowitzki MJ, Taylor CT. Mechanisms and consequences of oxygen and carbon dioxide sensing in mammals. Physiol Rev 100: 463–488, 2020. doi: 10.1152/physrev.00003.2019. [DOI] [PubMed] [Google Scholar]
- 60.Guyenet PG. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol (1985) 105: 404–416, 2008. doi: 10.1152/japplphysiol.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guyenet PG, Bayliss DA. Neural control of breathing and CO2 homeostasis. Neuron 87: 946–961, 2015. doi: 10.1016/j.neuron.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J Comp Neurol 518: 3883–3906, 2010. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol 586: 2043–2048, 2008. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guyenet PG, Stornetta RL, Abbott SB, Depuy SD, Kanbar R. The retrotrapezoid nucleus and breathing. Adv Exp Med Biol 758: 115–122, 2012. doi: 10.1007/978-94-007-4584-1_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol 59: 299–331, 1999. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 66.Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526, 2004. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- 67.Gao L, Ortega-Sáenz P, López-Barneo J. Acute oxygen sensing-role of metabolic specifications in peripheral chemoreceptor cells. Respir Physiol Neurobiol 265: 100–111, 2019. doi: 10.1016/j.resp.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 68.Getsy PM, Davis J, Coffee GA, May WJ, Palmer LA, Strohl KP, Lewis SJ. Enhanced non-eupneic breathing following hypoxic, hypercapnic or hypoxic-hypercapnic gas challenges in conscious mice. Respir Physiol Neurobiol 204: 147–159, 2014. doi: 10.1016/j.resp.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He L, Chen J, Dinger B, Fidone S. Characteristics of carotid body chemosensitivity in the mouse. Baseline studies for future experiments with knockout animals. Adv Exp Med Biol 475: 697–704, 2000. doi: 10.1007/0-306-46825-5_69. [DOI] [PubMed] [Google Scholar]
- 70.He L, Chen J, Dinger B, Sanders K, Sundar K, Hoidal J, Fidone S. Characteristics of carotid body chemosensitivity in NADPH oxidase-deficient mice. Am J Physiol Cell Physiol 282: C27–C33, 2002. doi: 10.1152/ajpcell.2002.282.1.C27. [DOI] [PubMed] [Google Scholar]
- 71.He L, Chen J, Dinger B, Sanders K, Sundar K, Hoidal J, Fidone S. Carotid body chemoreceptor activity in mice deficient in selected subunits of NADPH oxidase. Adv Exp Med Biol 536: 41–46, 2003. doi: 10.1007/978-1-4419-9280-2_5. [DOI] [PubMed] [Google Scholar]
- 72.Kline DD, Buniel MC, Glazebrook P, Peng YJ, Ramirez-Navarr A, Prabhakar NR, Kunze DL. Kv1.1 deletion augments the afferent hypoxic chemosensory pathway and respiration. J Neurosci 25: 3389–3399, 2005. doi: 10.1523/JNEUROSCI.4556-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kline DD, Prabhakar NR. Peripheral chemosensitivity in mutant mice deficient in nitric oxide synthase. Adv Exp Med Biol 475: 571–579, 2000. doi: 10.1007/0-306-46825-5_55. [DOI] [PubMed] [Google Scholar]
- 74.Ortega-Sáenz P, Caballero C, Gao L, López-Barneo J. Testing acute oxygen sensing in genetically modified mice: plethysmography and amperometry. Methods Mol Biol 1742: 139–153, 2018. doi: 10.1007/978-1-4939-7665-2_13. [DOI] [PubMed] [Google Scholar]
- 75.Palmer LA, deRonde K, Brown-Steinke K, Gunter S, Jyothikumar V, Forbes MS, Lewis SJ. Hypoxia-induced changes in protein s-nitrosylation in female mouse brainstem. Am J Respir Cell Mol Biol 52: 37–45, 2015. doi: 10.1165/rcmb.2013-0359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palmer LA, May WJ, deRonde K, Brown-Steinke K, Gaston B, Lewis SJ. Hypoxia-induced ventilatory responses in conscious mice: gender differences in ventilatory roll-off and facilitation. Respir Physiol Neurobiol 185: 497–505, 2013. doi: 10.1016/j.resp.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palmer LA, May WJ, deRonde K, Brown-Steinke K, Bates JN, Gaston B, Lewis SJ. Ventilatory responses during and following exposure to a hypoxic challenge in conscious mice deficient or null in S-nitrosoglutathione reductase. Respir Physiol Neurobiol 185: 571–581, 2013. doi: 10.1016/j.resp.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peng YJ, Zhang X, Nanduri J, Prabhakar NR. Therapeutic targeting of the carotid body for treating sleep apnea in a pre-clinical mouse model. Adv Exp Med Biol 1071: 109–114, 2018. doi: 10.1007/978-3-319-91137-3_14. [DOI] [PubMed] [Google Scholar]
- 79.Pérez-García MT, Colinas O, Miguel-Velado E, Moreno-Domínguez A, López-López JR. Characterization of the Kv channels of mouse carotid body chemoreceptor cells and their role in oxygen sensing. J Physiol 557: 457–471, 2004. doi: 10.1113/jphysiol.2004.062281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pichard LE, Crainiceanu CM, Pashai P, Kostuk EW, Fujioka A, Shirahata M. Role of BK channels in murine carotid body neural responses in vivo. Adv Exp Med Biol 860: 325–333, 2015. doi: 10.1007/978-3-319-18440-1_37. [DOI] [PubMed] [Google Scholar]
- 81.Wang J, Hogan JO, Wang R, White C, Kim D. Role of cystathionine-γ-lyase in hypoxia-induced changes in TASK activity, intracellular [Ca2+] and ventilation in mice. Respir Physiol Neurobiol 246: 98–106, 2017. doi: 10.1016/j.resp.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 82.Campen MJ, Tagaito Y, Li J, Balbir A, Tankersley CG, Smith P, Schwartz A, O'Donnell CP. Phenotypic variation in cardiovascular responses to acute hypoxic and hypercapnic exposure in mice. Physiol Genomics 20: 15–20, 2004. doi: 10.1152/physiolgenomics.00197.2003. [DOI] [PubMed] [Google Scholar]
- 83.Campen MJ, Tagaito Y, Jenkins TP, Balbir A, O'Donnell CP. Heart rate variability responses to hypoxic and hypercapnic exposures in different mouse strains. J Appl Physiol (1985) 99: 807–813, 2005. doi: 10.1152/japplphysiol.00039.2005. [DOI] [PubMed] [Google Scholar]
- 84.Gaston B, Smith L, Bosch J, Seckler J, Kunze D, Kiselar J, Marozkina N, Hodges CA, Wintrobe P, McGee K, Morozkina TS, Burton ST, Lewis T, Strassmaier T, Getsy P, Bates JN, Lewis SJ. Voltage-gated potassium channel proteins and stereoselective S-nitroso-L-cysteine signaling. JCI Insight 5: e134174, 2020. doi: 10.1172/jci.insight.134174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tankersley CG, Irizarry R, Flanders S, Rabold R. Circadian rhythm variation in activity, body temperature, and heart rate between C3H/HeJ and C57BL/6J inbred strains. J Appl Physiol (1985) 92: 870–877, 2002. doi: 10.1152/japplphysiol.00904.2001. [DOI] [PubMed] [Google Scholar]
- 86.Tewari SG, Bugenhagen SM, Wang Z, Schreier DA, Carlson BE, Chesler NC, Beard DA. Analysis of cardiovascular dynamics in pulmonary hypertensive C57BL6/J mice. Front Physiol 4: 355, 2013. doi: 10.3389/fphys.2013.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han F, Strohl KP. Inheritance of ventilatory behavior in rodent models. Respir Physiol 121: 247–256, 2000. doi: 10.1016/s0034-5687(00)00132-8. [DOI] [PubMed] [Google Scholar]
- 88.Han F, Subramanian S, Dick TE, Dreshaj IA, Strohl KP. Ventilatory behavior after hypoxia in C57BL/6J and A/J mice. J Appl Physiol (1985) 91: 1962–1970, 2001. doi: 10.1152/jappl.2001.91.5.1962. [DOI] [PubMed] [Google Scholar]
- 89.Han F, Subramanian S, Price ER, Nadeau J, Strohl KP. Periodic breathing in the mouse. J Appl Physiol (1985) 92: 1133–1140, 2002. doi: 10.1152/japplphysiol.00785.2001. [DOI] [PubMed] [Google Scholar]
- 90.Tagaito Y, Polotsky VY, Campen MJ, Wilson JA, Balbir A, Smith PL, Schwartz AR, O'Donnell CP. A model of sleep-disordered breathing in the C57BL/6J mouse. J Appl Physiol (1985) 91: 2758–2766, 2001. doi: 10.1152/jappl.2001.91.6.2758. [DOI] [PubMed] [Google Scholar]
- 91.Yamauchi M, Dostal J, Kimura H, Strohl KP. Effects of buspirone on posthypoxic ventilatory behavior in the C57BL/6J and A/J mouse strains. J Appl Physiol (1985) 105: 518–526, 2008. doi: 10.1152/japplphysiol.00069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamauchi M, Ocak H, Dostal J, Jacono FJ, Loparo KA, Strohl KP. Post-sigh breathing behavior and spontaneous pauses in the C57BL/6J (B6) mouse. Respir Physiol Neurobiol 162: 117–125, 2008. doi: 10.1016/j.resp.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamauchi M, Dostal J, Strohl KP. Post-hypoxic unstable breathing in the C57BL/6J mouse: effects of acetazolamide. Adv Exper Med Biol 605: 75–79, 2008. doi: 10.1007/978-0-387-73693-8_13. [DOI] [PubMed] [Google Scholar]
- 94.Yamauchi M, Kimura H, Strohl KP. Mouse models of apnea: strain differences in apnea expression and its pharmacologic and genetic modification. Adv Exp Med Biol 669: 303–307, 2010. doi: 10.1007/978-1-4419-5692-7_62. [DOI] [PubMed] [Google Scholar]
- 95.Groeben H, Meier S, Tankersley CG, Mitzner W, Brown RH. Heritable and pharmacological influences on pauses and apneas in inbred mice during anesthesia and emergence. Exp Lung Res 31: 839–853, 2005. doi: 10.1080/01902140600586458. [DOI] [PubMed] [Google Scholar]
- 96.Moore MW, Chai S, Gillombardo CB, Carlo A, Donovan LM, Netzer N, Strohl KP. Two weeks of buspirone protects against posthypoxic ventilatory pauses in the C57BL/6J mouse strain. Respir Physiol Neurobiol 183: 35–40, 2012. doi: 10.1016/j.resp.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 97.Price ER, Han F, Dick TE, Strohl KP. 7-nitroindazole and posthypoxic ventilatory behavior in the A/J and C57BL/6J mouse strains. J Appl Physiol (1985) 95: 1097–1104, 2003. doi: 10.1152/japplphysiol.00166.2003. [DOI] [PubMed] [Google Scholar]
- 98.Tankersley CG. Selected contribution: variation in acute hypoxic ventilatory response is linked to mouse chromosome 9. J Appl Physiol (1985) 90: 1615–1622, 2001. doi: 10.1152/jappl.2001.90.4.1615. [DOI] [PubMed] [Google Scholar]
- 99.Tankersley CG. Genetic aspects of breathing: on interactions between hypercapnia and hypoxia. Respir Physiol Neurobiol 135: 167–178, 2003. doi: 10.1016/s1569-9048(03)00035-1. [DOI] [PubMed] [Google Scholar]
- 100.Tankersley CG, Fitzgerald RS, Kleeberger SR. Differential control of ventilation among inbred strains of mice. Am J Physiol Regul Integr Comp Physiol 267: R1371–R1377, 1994. doi: 10.1152/ajpregu.1994.267.5.R1371. [DOI] [PubMed] [Google Scholar]
- 101.Tankersley CG, Elston RC, Schnell AH. Genetic determinants of acute hypoxic ventilation: patterns of inheritance in mice. J Appl Physiol (1985) 88: 2310–2318, 2000. doi: 10.1152/jappl.2000.88.6.2310. [DOI] [PubMed] [Google Scholar]
- 102.Yamaguchi S, Balbir A, Schofield B, Coram J, Tankersley CG, Fitzgerald RS, O'Donnell CP, Shirahata M. Structural and functional differences of the carotid body between DBA/2J and A/J strains of mice. J Appl Physiol (1985) 94: 1536–1542, 2003. doi: 10.1152/japplphysiol.00739.2002. [DOI] [PubMed] [Google Scholar]
- 103.Yamaguchi S, Balbir A, Okumura M, Schofield B, Coram J, Tankersley CG, Fitzgerald RS, O'Donnell CP, Shirahata M. Genetic influence on carotid body structure in DBA/2J and A/J strains of mice. Adv Exp Med Biol 580: 105–109, 2006. doi: 10.1007/0-387-31311-7_16. [DOI] [PubMed] [Google Scholar]
- 104.Solberg LC, Valdar W, Gauguier D, Nunez G, Taylor A, Burnett S, Arboledas-Hita C, Hernandez-Pliego P, Davidson S, Burns P, Bhattacharya S, Hough T, Higgs D, Klenerman P, Cookson WO, Zhang Y, Deacon RM, Rawlins JN, Mott R, Flint J. A protocol for high-throughput phenotyping, suitable for quantitative trait analysis in mice. Mamm Genome 17: 129–146, 2006. doi: 10.1007/s00335-005-0112-1. [DOI] [PubMed] [Google Scholar]
- 105.Getsy PM, Coffee GA, Lewis SJ. The role of carotid sinus nerve input in the hypoxic-hypercapnic ventilatory response in juvenile rats. Front Physiol 11: 613786, 2020. doi: 10.3389/fphys.2020.613786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clark AR, Hollingworth AM. The relationship between powder inhaler resistance and peak inspiratory conditions in healthy volunteers—implications for in vitro testing. J Aerosol Med 6: 99–110, 1993. doi: 10.1089/jam.1993.6.99. [DOI] [PubMed] [Google Scholar]
- 107.Sharma G, Mahler DA, Mayorga VM, Deering KL, Harshaw O, Ganapathy V. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis 4: 217–224, 2017. doi: 10.15326/jcopdf.4.3.2017.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shoemaker A, Steelman K, Srbu R, Bell HJ. Disparity in the effect of morphine on eupnea and gasping in anesthetized spontaneously breathing adult rats. Am J Physiol Regul Integr Comp Physiol 319: R526–R540, 2020. doi: 10.1152/ajpregu.00031.2020. [DOI] [PubMed] [Google Scholar]
- 109.Burns DP, Murphy KH, Lucking EF, O'Halloran KD. Inspiratory pressure-generating capacity is preserved during ventilatory and non-ventilatory behaviours in young dystrophic mdx mice despite profound diaphragm muscle weakness. J Physiol 597: 831–848, 2019. doi: 10.1113/JP277443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Quindry JC, Ballmann CG, Epstein EE, Selsby JT. Plethysmography measurements of respiratory function in conscious unrestrained mice. J Physiol Sci 66: 157–164, 2016. doi: 10.1007/s12576-015-0408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ray RS, Corcoran AE, Brust RD, Soriano LP, Nattie EE, Dymecki SM. Egr2-neurons control the adult respiratory response to hypercapnia. Brain Res 1511: 115–125, 2013. doi: 10.1016/j.brainres.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith CA, Forster HV, Blain GM, Dempsey JA. An interdependent model of central/peripheral chemoreception: evidence and implications for ventilatory control. Respir Physiol Neurobiol 173: 288–297, 2010. doi: 10.1016/j.resp.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smith CA, Jameson LC, Mitchell GS, Musch TI, Dempsey JA. Central-peripheral chemoreceptor interaction in awake cerebrospinal fluid-perfused goats. J Appl Physiol Respir Environ Exerc Physiol 56: 1541–1549, 1984. doi: 10.1152/jappl.1984.56.6.1541. [DOI] [PubMed] [Google Scholar]
- 114.Smith CA, Rodman JR, Chenuel BJ, Henderson KS, Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol (1985) 100: 13–19, 2006. doi: 10.1152/japplphysiol.00926.2005. [DOI] [PubMed] [Google Scholar]
- 115.Smith CA, Blain GM, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2: role of carotid body CO2. J Physiol 593: 4225–4243, 2015. doi: 10.1113/JP270114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Detweiler ND, Vigil KG, Resta TC, Walker BR, Jernigan NL. Role of acid-sensing ion channels in hypoxia- and hypercapnia-induced ventilatory responses. PLoS One 13: e0192724, 2018. doi: 10.1371/journal.pone.0192724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hirata Y, Suzuki Y, Tominaga M, Oku Y. TRPM8 channel is involved in the ventilatory response to CO2 mediating hypercapnic Ca2+ responses. Respir Physiol Neurobiol 263: 20–25, 2019. doi: 10.1016/j.resp.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 118.Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26: 10305–10314, 2006. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol 572: 503–523, 2006. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mouradian GC, Forster HV, Hodges MR. Acute and chronic effects of carotid body denervation on ventilation and chemoreflexes in three rat strains. J Physiol 590: 3335–3347, 2012. doi: 10.1113/jphysiol.2012.234658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miller JR, Neumueller S, Muere C, Olesiak S, Pan L, Hodges MR, Forster HV. Changes in neurochemicals within the ventrolateral medullary respiratory column in awake goats after carotid body denervation. J Appl Physiol (1985) 115: 1088–1098, 2013. doi: 10.1152/japplphysiol.00293.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miller JR, Neumueller S, Muere C, Olesiak S, Pan L, Bukowy JD, Daghistany AO, Hodges MR, Forster HV. Changes in glutamate receptor subunits within the medulla in goats after section of the carotid sinus nerves. J Appl Physiol (1985) 116: 1531–1542, 2014. doi: 10.1152/japplphysiol.00216.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dwinell MR, Forster HV, Petersen J, Rider A, Kunert MP, Cowley AW Jr., Jacob HJ. Genetic determinants on rat chromosome 6 modulate variation in the hypercapnic ventilatory response using consomic strains. J Appl Physiol (1985) 98: 1630–1638, 2005. doi: 10.1152/japplphysiol.01148.2004. [DOI] [PubMed] [Google Scholar]
- 124.Hodges MR, Forster HV, Papanek PE, Dwinell MR, Hogan GE. Ventilatory phenotypes among four strains of adult rats. J Appl Physiol (1985) 93: 974–983, 2002. doi: 10.1152/japplphysiol.00019.2002. [DOI] [PubMed] [Google Scholar]
- 125.Forster HV. Plasticity in the control of breathing following sensory denervation. J Appl Physiol (1985) 94: 784–794, 2003. doi: 10.1152/japplphysiol.00602.2002. [DOI] [PubMed] [Google Scholar]
- 126.Hodges MR, Forster HV. Respiratory neuroplasticity following carotid body denervation: central and peripheral adaptations. Neural Regen Res 7: 1073–1079, 2012. doi: 10.3969/j.issn.1673-5374.2012.14.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Berkenbosch A, DeGoede J, Ward DS, Olievier CN, VanHartevelt J. Dynamic response of peripheral chemoreflex loop to changes in end-tidal CO2. J Appl Physiol (1985) 64: 1779–1785, 1988. doi: 10.1152/jappl.1988.64.5.1779. [DOI] [PubMed] [Google Scholar]
- 128.DeGoede J, Berkenbosch A, Ward DS, Bellville JW, Olievier CN. Comparison of chemoreflex gains obtained with two different methods in cats. J Appl Physiol (1985) 59: 170–179, 1985. doi: 10.1152/jappl.1985.59.1.170. [DOI] [PubMed] [Google Scholar]
- 129.Swanson GD, Bellville JW. Step changes in end-tidal CO2: methods and implications. J Appl Physiol (1985) 39: 377–385, 1975. doi: 10.1152/jappl.1975.39.3.377. [DOI] [PubMed] [Google Scholar]
- 130.Donoghue S, Fatemian M, Balanos GM, Crosby A, Liu C, O'Connor D, Talbot NP, Robbins PA. Ventilatory acclimatization in response to very small changes in PO2 in humans. J Appl Physiol (1985) 98: 1587–1591, 2005. doi: 10.1152/japplphysiol.01019.2004. [DOI] [PubMed] [Google Scholar]
- 131.Fatemian M, Dahan A, Meinesz S, van der Mey A, Robbins PA. Modeling the ventilatory response to variations in end-tidal PCO2 in patients who have undergone bilateral carotid body resection. Adv Exp Med Biol 499: 275–277, 2001. doi: 10.1007/978-1-4615-1375-9_43. [DOI] [PubMed] [Google Scholar]
- 132.Pedersen ME, Fatemian M, Robbins PA. Identification of fast and slow ventilatory responses to carbon dioxide under hypoxic and hyperoxic conditions in humans. J Physiol 521: 273–287, 1999. doi: 10.1111/j.1469-7793.1999.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ursino M, Magosso E, Avanzolini G. An integrated model of the human ventilatory control system: the response to hypercapnia. Clin Physiol 21: 447–464, 2001. doi: 10.1046/j.1365-2281.2001.00349.x. [DOI] [PubMed] [Google Scholar]
- 134.Wood HE, Fatemian M, Robbins PA. Non-dimensional quantification of the interactions between hypoxia, hypercapnia and exercise on ventilation in humans. Adv Exp Med Biol 605: 245–248, 2008. doi: 10.1007/978-0-387-73693-8_43. [DOI] [PubMed] [Google Scholar]
- 135.Kreft B, Tzschätzsch H, Schrank F, Bergs J, Streitberger KJ, Wäldchen S, Hetzer S, Braun J, Sack I. Time-resolved response of cerebral stiffness to hypercapnia in humans. Ultrasound Med Biol 46: 936–943, 2020. doi: 10.1016/j.ultrasmedbio.2019.12.019. [DOI] [PubMed] [Google Scholar]
- 136.Liu P, De Vis JB, Lu H. Cerebrovascular reactivity (CVR) MRI with CO2 challenge: a technical review. Neuroimage 187: 104–115, 2019. doi: 10.1016/j.neuroimage.2018.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Verbree J, Bronzwaer AS, Ghariq E, Versluis MJ, Daemen MJ, van Buchem MA, Dahan A, van Lieshout JJ, van Osch MJ. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol (1985) 117: 1084–1089, 2014. doi: 10.1152/japplphysiol.00651.2014. [DOI] [PubMed] [Google Scholar]
- 138.Dzal YA, Sprenger RJ, Milsom WK. Postnatal changes in O2 and CO2 sensitivity in rodents. Respir Physiol Neurobiol 272: 103313, 2020. doi: 10.1016/j.resp.2019.103313. [DOI] [PubMed] [Google Scholar]
- 139.Sprenger RJ, Kim AB, Dzal YA, Milsom WK. Comparison of the CO2 ventilatory response through development in three rodent species: effect of fossoriality. Respir Physiol Neurobiol 264: 19–27, 2019. doi: 10.1016/j.resp.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 140.Netick A, Dugger WJ, Symmons RA. Ventilatory response to hypercapnia during sleep and wakefulness in cats. J Appl Physiol Respir Environ Exerc Physiol 56: 1347–1354, 1984. doi: 10.1152/jappl.1984.56.5.1347. [DOI] [PubMed] [Google Scholar]
- 141.Tankersley CG, Fitzgerald RS, Mitzner WA, Kleeberger SR. Hypercapnic ventilatory responses in mice differentially susceptible to acute ozone exposure. J Appl Physiol (1985) 75: 2613–2619, 1993. doi: 10.1152/jappl.1993.75.6.2613. [DOI] [PubMed] [Google Scholar]