Abstract
Sepsis-induced diaphragm dysfunction is a major contributor to respiratory failure in mechanically ventilated patients. There are no pharmacological treatments for this syndrome, but studies suggest that diaphragm weakness is linked to mitochondrial free radical generation. We hypothesized that administration of mitoquinone mesylate (MitoQ), a mitochondrially targeted free radical scavenger, would prevent sepsis-induced diaphragm dysfunction. We compared diaphragm function in 4 groups of male mice: 1) sham-operated controls treated with saline (0.3 mL ip), 2) sham-operated treated with MitoQ (3.5 mg/kg/day given intraperitoneally in saline), 3) cecal ligation puncture (CLP) mice treated with saline, and 4) CLP mice treated with MitoQ. Forty-eight hours after surgery, we assessed diaphragm force generation, myosin heavy chain content, state 3 mitochondrial oxygen consumption (OCR), and aconitase activity. We also determined effects of MitoQ in female mice with CLP sepsis and in mice with endotoxin-induced sepsis. CLP decreased diaphragm specific force generation and MitoQ prevented these decrements (e.g. maximal force averaged 30.2 ± 1.3, 28.0 ± 1.3, 12.8 ± 1.9, and 30.0 ± 1.0 N/cm2 for sham, sham + MitoQ, CLP, and CLP + MitoQ groups, respectively, P < 0.001). CLP also reduced diaphragm mitochondrial OCR and aconitase activity; MitoQ blocked both effects. Similar responses were observed in female mice and in endotoxin-induced sepsis. Moreover, delayed MitoQ treatment (by 6 h) was as effective as immediate treatment. These data indicate that MitoQ prevents sepsis-induced diaphragm dysfunction, preserving force generation. MitoQ may be a useful therapeutic agent to preserve diaphragm function in critically ill patients with sepsis.
NEW & NOTEWORTHY This is the first study to show that mitoquinone mesylate (MitoQ), a mitochondrially targeted antioxidant, treats sepsis-induced skeletal muscle dysfunction. This biopharmaceutical agent is without known side effects and is currently being used by healthy individuals and in clinical trials in patients with various diseases. When taken together, our results suggest that MitoQ has the potential to be immediately translated into treatment for sepsis-induced skeletal muscle dysfunction.
Keywords: diaphragm weakness, mitochondrial dysfunction, MitoQ, mitoquinone mesylate, sepsis
INTRODUCTION
Mechanically ventilated critically ill patients often develop severe diaphragmatic weakness, with several studies reporting reductions in diaphragm force generation for these patients to be less than 25% of values for normal healthy subjects (1–3). This work also indicates that diaphragm weakness and/or diaphragm muscle atrophy is associated with poor outcomes, including increased mortality and a prolonged requirement for mechanical ventilation (1, 2, 4, 5). It is thought that there are two main mechanisms by which diaphragm dysfunction develops in critically ill patients, namely, ventilator-induced diaphragm weakness (termed ventilator-induced diaphragm dysfunction or VIDD) (6, 7) and sepsis (2). Animal models of sepsis have revealed several mechanistic processes by which infections alter muscle function, suggesting that infections elicit increases in muscle mitochondrial superoxide generation and that mitochondrial free radical generation is linked to activation of downstream proteolytic pathways (8–12).
Based on these previous reports, theoretically it should be possible to prevent the development of sepsis-induced diaphragm weakness by administration of pharmacological agents that scavenge mitochondrially produced superoxide and other mitochondrially generated reactive oxygen species (ROS). In fact, in recent work, we found that administration of two mitochondrially targeted antioxidants, SS31 and mitoTEMPOL, was effective in reducing sepsis-induced diaphragm dysfunction (13, 14). Unfortunately, neither of these compounds is currently available for clinical administration. However, there is one mitochondrially targeted antioxidant that is widely ingested by humans around the world (over 300,000 subjects per day) and is known to have no significant side effects. This agent, MitoQ (i.e., mitoquinone mesylate), contains a potent antioxidant (ubiquinone) chemically linked to a mitochondrially targeting component (a lipophilic triphenylphosphonium cation) (15, 16). MitoQ has been shown to suppress renal, hepatic, lung, intestinal barrier, and cardiac dysfunction in animal models of sepsis (17–20). No previous studies, however, have determined if MitoQ can also prevent sepsis-induced skeletal muscle dysfunction.
The purpose of the present study, therefore, was to test the hypothesis that MitoQ administration would reduce the development of sepsis-induced diaphragm weakness. Studies were performed using the cecal ligation puncture (CLP) model of sepsis since this particular model is thought to be clinically relevant, with efficacious treatments in this model predictive of similar responses in patients (21). The primary assessment was to compare diaphragm parameters in four groups of animals, including sham-operated controls, sham-operated animals treated with MitoQ, septic animals, and septic animals given MitoQ.
MATERIALS AND METHODS
Rationale for Experimental Design
The primary goal of this study was to determine if MitoQ administration would prevent sepsis-induced diaphragm weakness. To do so, in our first experiment, we used the CLP (cecal ligation perforation surgery) model of sepsis and compared diaphragm muscle function in animals (mice) with: 1) sham surgery given saline, 2) sham surgery given MitoQ, 3) CLP surgery given saline, and 4) CLP surgery given MitoQ. To assess diaphragm force generating capacity, animals were euthanized at 48 h after sham or CLP surgery and diaphragm force was assessed by mounting these muscles in organ baths and electrically stimulating muscles with supramaximal currents. To assess the effects of sex, this basic experiment was conducted using both male and female mice. We also examined responses in the LPS model of sepsis to determine if MitoQ was also effective in an alternative sepsis model. Since previous studies have shown that sepsis induces muscle myosin heavy chain depletion, mitochondrial dysfunction, and increased production of muscle mitochondrial free radicals, we examined the effects of MitoQ on these other parameters.
Experimental Protocols
Experiments were approved by the University of Kentucky Institutional Animal Care and Use Committee. Male and female CD1 mice (25–30 g) were purchased from Charles River Laboratories, Inc., Wilmington, MA. MitoQ was purchased from MedKoo Biosciences, Inc., Morrisville, NC (Cat. No. 317102).
We first examined four groups of male mice (5 or 6/group): 1) sham-operated, vehicle-treated controls [0.3 mL ip after surgery]; 2) sham-operated + MitoQ (3.5 mg/kg/day administered intraperitoneally in 0.3 mL of saline, administered immediately after surgery); 3) cecal ligation puncture (CLP) + vehicle; and 4) CLP + MitoQ. This dosage of MitoQ was chosen to approximate that achieved in previous work by our group (19). Forty-eight hours postoperatively, animals were euthanized, diaphragms removed for force determinations, with remaining diaphragm frozen at −80°C for biochemical measurements.
Using a similar experimental paradigm, we assessed responses in female animals (n = 3/group) and male animals with endotoxin-induced sepsis (LPS, 8 mg/kg/day in 0.3 mL of saline ip) (n = 3/group). We also examined the effect of delayed MitoQ administration given 6 h after CLP surgery to male mice (n = 4/group). Diaphragm mitochondrial function and aconitase activity were determined for the four groups of male mice (n = 4/group). Finally, we examined plasma and diaphragm MitoQ concentrations at baseline, 4, 8, and 24 h after administration of single dose of MitoQ (3.5 mg/kg, n = 4/group).
Cecal Ligation Puncture-Induced Sepsis
Cecal ligation puncture (CLP) was performed as previously reported (10, 13, 14, 22, 23). Our CLP model induces a low-grade sepsis with extremely low mortality (<10%). Briefly, animals were anesthetized with isoflurane (1.5%–2%), delivered via an anesthetic gas vaporizer. After achieving a steady plane of anesthesia as assessed by no response to tail pressure or toe pinch, the abdomen was prepared by removing fur with a depilatory cream. Once this was accomplished, the bare skin was prepped with betadine and alcohol, followed by incising the skin, and then inducing a 1-cm incision through the abdominal musculature. The cecum was identified and a 1 cm portion was ligated. A 21-gauge sterile needle was used to puncture the ligated cecum through and through, followed by expression of a small amount of feces through the needle hole. The abdominal musculature was then sutured, followed by closure of the skin with surgical staples. For sham surgical procedures, the abdomen was opened and closed without ligation or puncture of the cecum. Immediately following the surgery, all animals were resuscitated with 60 mL/kg of saline subcutaneously. Analgesia was instituted by injecting animals immediately postoperatively with buprenorphine (0.05 mg/kg) and then every 12 h following surgery. Animals were euthanized at 48 h after surgery using an intraperitoneal injection of pentobarbital (150 mg/kg).
Endotoxin (LPS)-Induced Sepsis
Animals were injected intraperitoneally with endotoxin (LPS; 8 mg/kg in 0.3-mL saline) and MitoQ (given as 3.5 mg/kg/day intraperitoneally in 0.3-mL saline) daily for 2 days and euthanized at 48 h after the initial injection using an intraperitoneal injection of pentobarbital (150 mg/kg). For controls, 0.3 mL of saline was injected intraperitoneally instead of MitoQ for 2 days and mice were euthanized at 48 h. Endotoxin (LPS from Escherichia coli serotype 055:B5, Cat. No. L2880) was purchased from Millipore Sigma, Burlington, MA.
Assessment of Diaphragm Strength and Responses to Repetitive Contractions
Diaphragm strips were dissected from the left mid-costal diaphragm as previously reported (13, 14). Strips were placed in organ baths containing Krebs–Henselheit solution (25°C, pH 7.40, 135 mM NaCl, 5 mM KCl, 11.1 mM dextrose, 2.5 mM CaCl2, 1 mM MgSO4, 14.9 mM NaHCO3, 1 mM NaHPO4, 50 U/L of insulin, 95% O2-5% CO2) with one end of the strip tied to a force transducer and the other to the base of the organ bath. Supramaximal currents from a constant current amplifier connected to a Grass S48 stimulator (Grass, West Warwick, RI) were delivered using platinum mesh field electrodes for direct muscle stimulation.
After an equilibration period (15 min), the muscle length was adjusted to Lo. Diaphragm muscle force-frequency relationships were obtained by stimulating the muscle strips with electrical trains applied via muscle field stimulation (1, 10, 20, 50, 100, 150, and 200 Hz) using a train duration of 800 ms and 30 s between adjacent trains. Force generated by the contracting diaphragm was recorded using a BIOPAC transducer/computer system (BIOPAC Systems, Inc., Goleta, CA). Diaphragm specific force generation (force per cross-sectional area) was calculated as previously reported using the method of Close (24).
After an additional 15 min of quiescence, we assessed diaphragm responses to repetitive contractions using supramaximal muscle field stimulation (trains of 330 ms, 40 Hz stimulation, at a rate of 1 train/s over 2 min).
Diaphragm Mitochondrial Function
Diaphragm mitochondrial homogenates were prepared as previously described (25), suspended in mitochondrial assay buffer, and placed in a fiberoptic fluorescence monitoring system (Instech Laboratories, Inc., Plymouth Meeting, PA.). Mitochondrial oxygen consumption (state 3 and state 4), respiratory control ratio (RCR), ADP/O ratios, and ATP production rates were measured as previously described (13, 14). Oxygen consumption rates were normalized to the protein content of diaphragm mitochondrial homogenates.
Diaphragm Aconitase Activity
To determine diaphragm mitochondrial free radical generation, we assessed aconitase activity, which is an index of mitochondrial superoxide generation (26). These measurements were performed using a commercially available kit, the Abcam Aconitase assay (Cambridge, MA), following the manufacturer’s protocol (13, 14).
Myosin Heavy Chain Content in Diaphragm Muscle
To assess myosin heavy chain content in the diaphragm muscles from the experimental groups, previously frozen muscles were homogenized in buffer and protein levels quantified using the Bradford assay (BioRad, Hercules, CA). Following measurement of protein content in the sample, aliquots of homogenates were diluted with an equal volume of Laemmli loading buffer. Equal amounts of protein were then loaded onto Mini-Protean TGX Stain-Free gels (BioRad, Hercules, CA), followed by use of the V3 Western Workflow system and ChemiDoc Touch Imaging System to perform Western blot analyses (BioRad, Hercules, CA). The sc-20641 antibody from Santa Cruz Biotechnology (Dallas, TX) was used to probe for myosin heavy chain (MHC) content. Using Image Lab 5.2.1 software (BioRad, Hercules, CA), MHC densitometry was performed by normalization to the total protein loaded for each lane as previously reported (13). Validation that the sc-20641 antibody does, in fact, measure myosin heavy chain level was assured by demonstrating that this antibody quantitatively detected a myosin heavy chain positive control with Western blotting (SC-364250, mouse skeletal muscle extract, was from Santa Cruz Biotechnology, Dallas, TX).
Measurement of MitoQ Levels
MitoQ plasma and tissue levels were measured using ultra performance liquid chromatography-coupled electrospray ionization tandem mass spectrometry with an ABSciex 6500 Q-Trap mass spectrometer. The structurally related compound idebenone was used as a surrogate internal standard with quantitation of MitoQ accomplished by reference to an off-line calibration. Results are reported as pmol/mL for plasma samples and as µg/mg for tissue samples.
Statistical Analysis
ANOVA was used to compare variables across groups treated with different agents, with post hoc testing (Tukey’s) to determine differences between groups. P values < 0.05 were taken as indicating statistical significance.
RESULTS
Diaphragm Force Generation
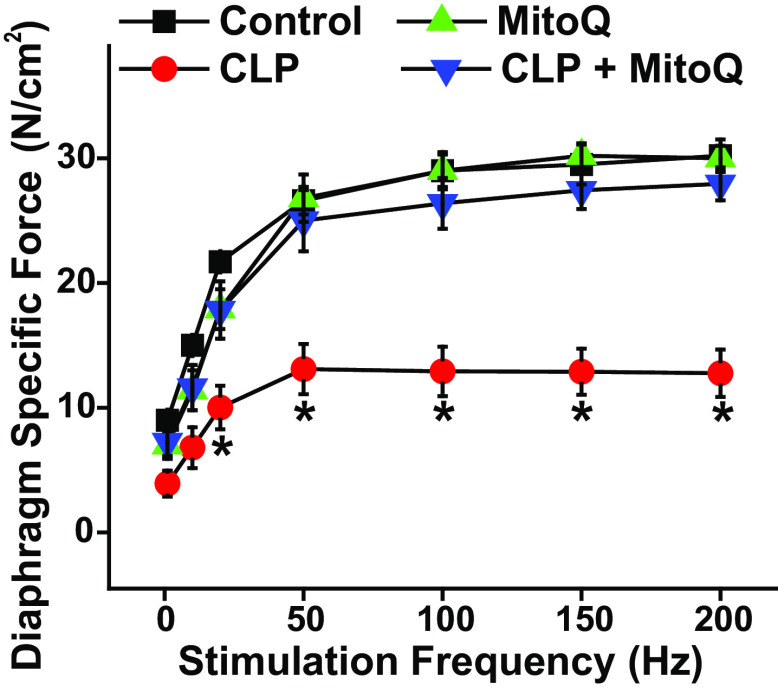
CLP sepsis elicited a large downward shift in the diaphragm force-frequency relationship (Fig. 1). We also found that MitoQ administration at the time of surgery prevented sepsis-induced reductions in diaphragm strength. As an example, stimulation at 200 Hz elicited average diaphragm specific forces of 30.2 ± 1.3, 28.0 ± 1.3, 12.8 ± 1.9, and 30.0 ± 1.0 N/cm2, respectively, for sham, sham + MitoQ, CLP, and CLP + MitoQ groups (P < 0.001 for comparison of the CLP group to the other three conditions). We also calculated the ratio of twitch force (Pt) to maximum force (Po) for the four groups and found this ratio was similar across the four conditions. Specifically, Pt/Po averaged 0.29 ± 0.02, 0.28 ± 0.06, 0.26 ± 0.03, and 0.24 ± 0.05, respectively, for control, CLP, MitoQ, and CLP + MitoQ groups (P = 0.770).
Figure 1.
MitoQ administration ablates sepsis-induced diaphragm weakness. Diaphragm specific force generation (i.e. force per cross-sectional area) in response to increasing stimulation frequencies (n = 5 or 6 animals/group) is shown for sham (black), sham + MitoQ (green), CLP (red), and CLP + MitoQ (blue) groups. CLP induced large reductions in diaphragm force generation in response to 20–200-Hz excitation frequencies when compared with values for sham-operated control animals (P < 0.01 for comparison at each frequency). MitoQ treatment prevented CLP-induced reductions in force, with diaphragm specific force for the CLP + MitoQ group higher than CLP values (P < 0.02 for 20–200 Hz). Force levels for the sham + MitoQ group were similar to those in the sham controls (NS). Data are presented as mean values with error bars representing 1 standard error of the mean. ANOVA was used to compare variables across groups; *statistical significance. CLP, cecal ligation puncture; MitoQ, mitoquinone mesylate.
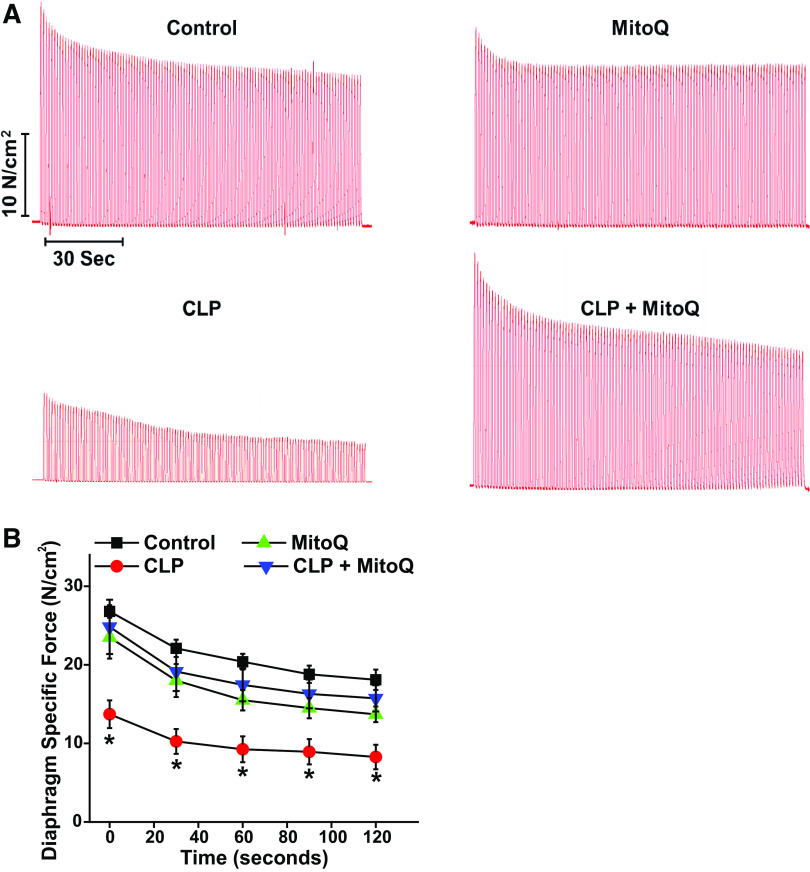
In addition, we measured force generation during repetitive contraction trials (Fig. 2, A and B). We found that force at all time points during these trials was reduced for diaphragms from septic animals as compared with diaphragms from the other three groups (P < 0.005 for all time points). At the end of trials, diaphragm force averaged 18.1 ± 1.3, 15.7 ± 1.6, 8.2 ± 1.5, and 13.7 ± 1.0 N/cm2, respectively, for sham, sham + MitoQ, CLP, and CLP + MitoQ groups (P < 0.001 for comparison to sham and P = 0.007 for comparison of CLP to CLP + MitoQ groups). When expressed as a percentage of initial force, final force averaged 65.0 ± 3.9%, 61.3 ± 4.2%, 57.5 ± 6.2%, and 66.8 ± 6.3%, respectively, for sham, sham + MitoQ, CLP, and CLP + MitoQ groups (NS).
Figure 2.
Effects of MitoQ and sepsis on repetitive contraction trials. During repetitive contraction trials, force generation was significantly lower at all time points for diaphragms from CLP animals (n = 5 or 6 animals/group). Concomitant administration of MitoQ prevented these CLP-induced reductions in force at all time points (representative tracings are shown in A, mean results in B; P < 0.01 for comparison of the sham and CLP groups and P < 0.02 for comparison of the CLP and CLP + MitoQ groups at all time points). Data are presented as mean values with error bars representing 1 standard error of the mean. ANOVA was used to compare variables across groups treated with different agents; *statistical significance. CLP, cecal ligation puncture; MitoQ, mitoquinone mesylate.
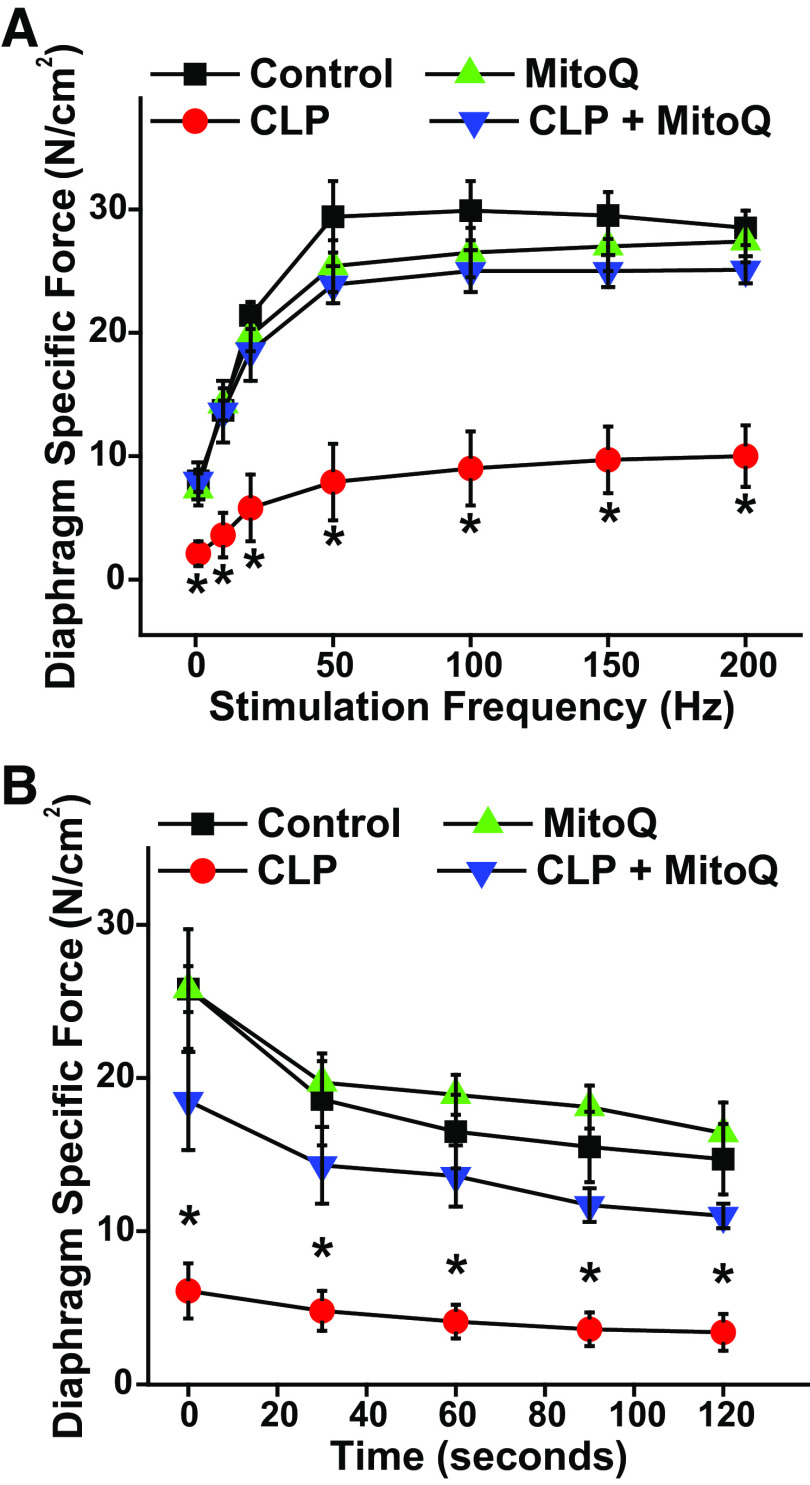
Responses to CLP and MitoQ Administration in Female Mice
Results presented in Figs. 1 and 2 indicate responses obtained when studying male animals. To determine if diaphragm responses to CLP and/or MitoQ are sex specific, we also examined the effects of CLP sepsis and MitoQ in adult female mice. As shown in Fig. 3, the effects of CLP sepsis and MitoQ on female mouse diaphragm strength were almost identical to the responses observed in male mice. In addition, when expressed as absolute force, forces generated by diaphragms from septic female were lower than forces generated by muscles from the other three groups at all points in time during repetitive contraction trials. When expressed as a percentage of initial force, the final force for repetitive contraction trials averaged 56.7 ± 4.2%, 66.7 ± 8.0%, 54.0 ± 12.2%, and 63.3 ± 8.5%, respectively, for sham, sham + MitoQ, CLP, and CLP + MitoQ groups (NS).
Figure 3.
No evidence of sex-related effects of MitoQ administration on CLP sepsis-induced diaphragm weakness. A: diaphragm mean force-frequency curves in response to direct muscle stimulation for muscles taken from female animals in the four experimental groups (n = 3 animals/group). B: diaphragm force over time during repetitive contraction trials for muscles from female animals. Similar to results from studies in male animals (shown in Figs. 1 and 2), CLP sepsis induced large reductions in diaphragm specific force generation compared with controls as well as force during repetitive contraction trials in female animals. MitoQ prevented CLP-induced reductions in diaphragm force-frequency relationships (P < 0.04 for all frequencies) and loss of force during repetitive contraction trials (P < 0.01 for all time points). The effects of both CLP and MitoQ administration on diaphragm function in female mice were comparable to that observed in male mice, suggesting there were no sex effects in these studies. Data are presented as mean values with error bars representing 1 standard error of the mean. ANOVA was used to compare variables across groups treated with different agents; *statistical significance. CLP, cecal ligation puncture; MitoQ, mitoquinone mesylate.
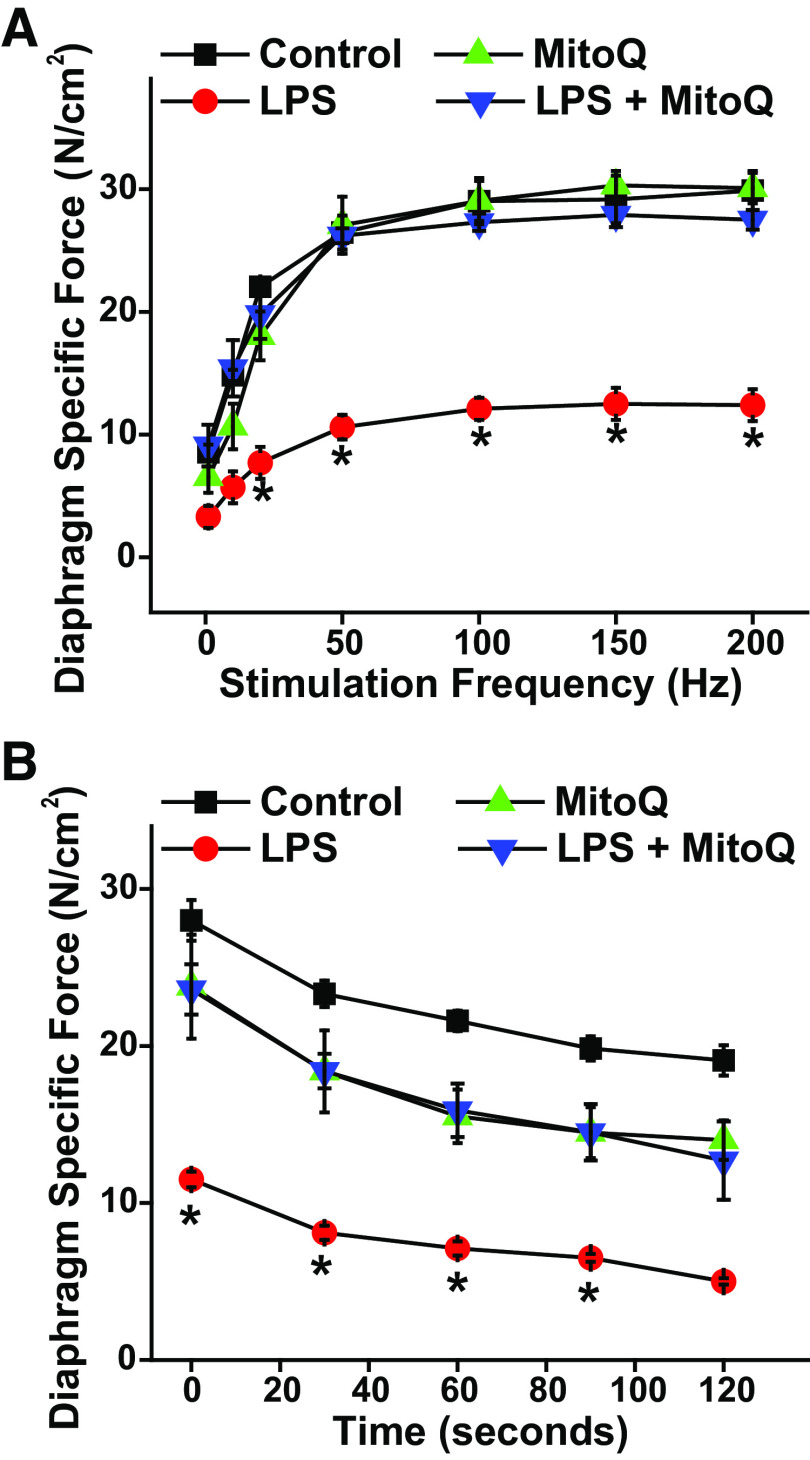
Endotoxin (LPS) Model of Sepsis
We also performed studies using the endotoxin (LPS) model of sepsis to ascertain if MitoQ would have effects in this other model of sepsis that were equivalent to effects observed in the CLP model. We found that LPS administration significantly reduced diaphragm strength, and MitoQ administration to LPS-injected animals prevented this reduction in muscle force generation (Fig. 4A). LPS also elicited a significant reduction in diaphragm force over time during repetitive contraction trials (Fig. 4B) and MitoQ prevented these endotoxin-induced reductions in force generation. When expressed as a percentage of initial force, the final force for repetitive contraction trials averaged 65.0 ± 3.9%, 61.3 ± 4.2%, 43.5 ± 3.5%, and 53.0 ± 6.5%, respectively, for sham, sham + MitoQ, LPS, and LPS + MitoQ groups (NS).
Figure 4.
MitoQ administration restores diaphragm function in endotoxin-induced sepsis. A: diaphragm force-frequency curves in response to direct muscle stimulation for studies in which sepsis was induced by systemic administration of endotoxin (LPS; n = 3 or 6 animals/group). B: diaphragm force over time during repetitive contraction trials for experiments in which LPS was employed to induce sepsis. LPS administration significantly reduced diaphragm force as measured during both force-frequency assessments and during repetitive contraction trials. MitoQ administration prevented LPS-induced reductions in diaphragm force generation (P < 0.002 for comparison of LPS to the other three experimental groups for frequencies 20–200 Hz). The effects of LPS and MitoQ on diaphragm function were similar to the effects of MitoQ on the diaphragm when administered during CLP. Data are presented as mean values with error bars representing 1 standard error of the mean. ANOVA was used to compare variables across groups treated with different agents; *statistical significance. CLP, cecal ligation puncture; MitoQ, mitoquinone mesylate.
Mitochondrial Function
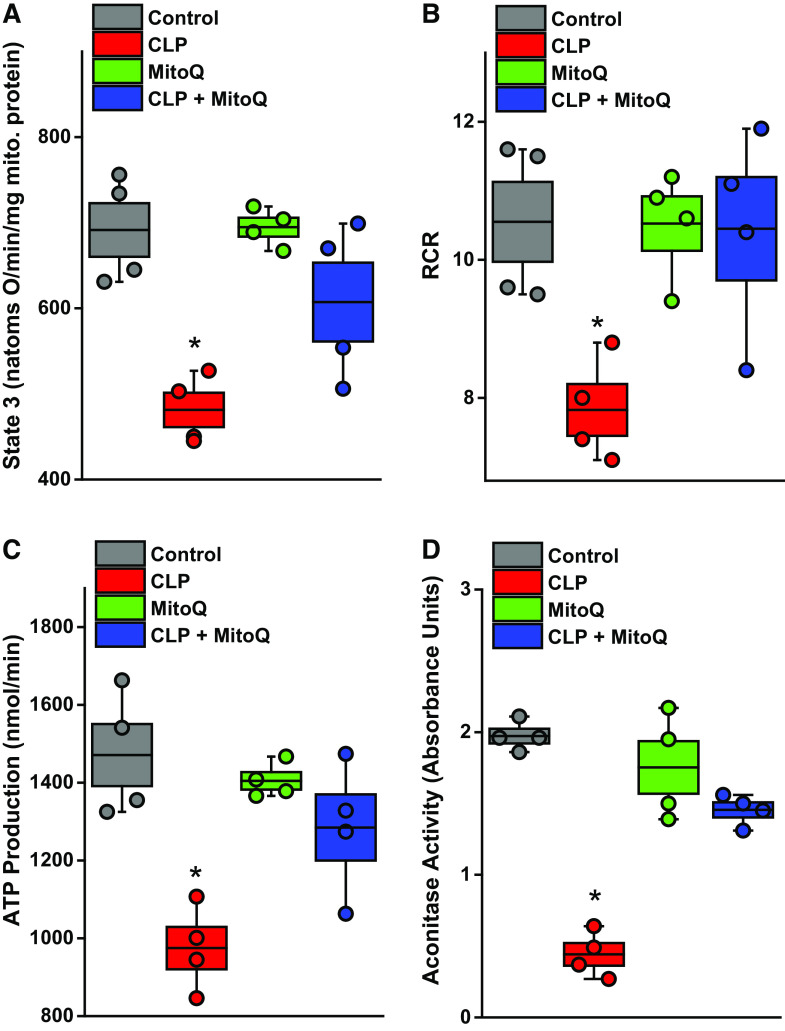
We found that ADP-stimulated oxygen consumption rate (state 3 respiration) was significantly reduced for mitochondria from the CLP group; in addition, we found that MitoQ administration to CLP animals prevented the reduction in state 3 oxygen consumption rate (Fig. 5A, P < 0.05 for comparison of CLP to the other three groups). State 4 rates were similar for the four experimental groups, with the result that the respiratory control ratio (i.e., state 3/state 4 rate ratio) was significantly reduced for the CLP animals. Moreover, MitoQ administration prevented the CLP-induced reduction in the respiratory control ratio (Fig. 5B) (P < 0.02 for comparison of CLP to the other three experimental groups). We also calculated the muscle mitochondrial ATP production rate and found that CLP sepsis reduced ATP production, whereas concomitant MitoQ administration to CLP animals preserved ATP production (Fig. 5C, P < 0.03 for comparison of the CLP to the other three experimental groups). Finally, we measured aconitase activity, an index of mitochondrial superoxide generation, and found that CLP reduced mitochondrial aconitase activity (consistent with an increase in mitochondrial superoxide generation) and MitoQ administration prevented this effect of sepsis (Fig. 5D, P < 0.001 for comparison of CLP to the other three groups).
Figure 5.
MitoQ administration preserved diaphragm mitochondrial function in CLP sepsis. Mitochondrial function was assessed in diaphragm-isolated mitochondrial homogenates by measuring ADP-stimulated respiration (state 3 oxygen consumption rates, A), the respiratory control ratio (RCR; B), and calculating ATP production rates (C) (n = 4 animals/group). CLP induced significant reductions in diaphragm ADP-stimulated oxygen consumption, in the RCR, and in ATP production rates. MitoQ ablated each of these CLP-induced alterations in mitochondrial function, with parameters for the CLP group significantly lower than values for the other three groups (P < 0.05 for ADP stimulated respiration, P < 0.02 for RCR, and P < 0.03 for ATP production rates). D: aconitase activity, an index of mitochondrial free radical generation (greater free radical generation reduces aconitase activity), demonstrating that CLP significantly reduced diaphragm aconitase activity and MitoQ blocked this response (P < 0.001). Data are presented in box and whisker plots with overlapping individual data points. The box represents 1 SE above and below the mean, the mean is shown by the black line in the box, and the whiskers represent the 5%–95% confidence intervals. ANOVA was used to compare variables across groups treated with different agents; *statistical significance. CLP, cecal ligation puncture; MitoQ, mitoquinone mesylate.
Levels of Myosin Heavy Chain
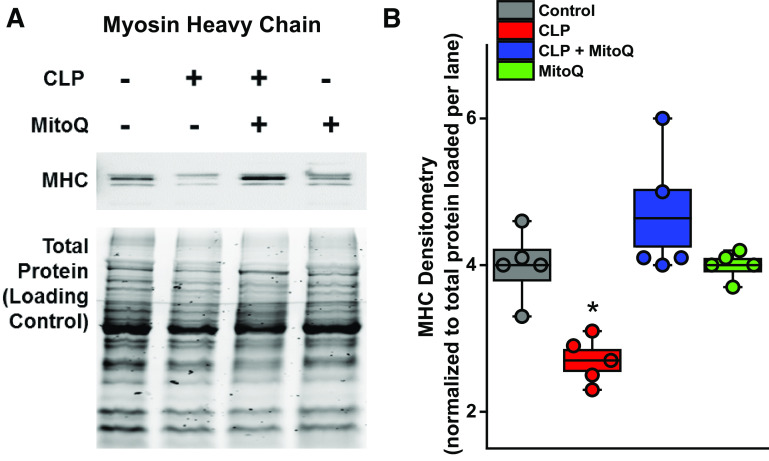
As shown in Fig. 6, A and B, CLP elicited significant reductions in diaphragm myosin heavy chain content compared with sham controls, and MitoQ administration prevented this sepsis-induced reduction (P < 0.01 for these comparisons), with myosin heavy chain remaining at control levels for diaphragms from the CLP + MitoQ-treated group.
Figure 6.
MitoQ blocks diaphragm myosin heavy chain loss induced by sepsis. A representative Western blot for diaphragm myosin heavy chain (MHC) levels is shown in A, whereas group mean data for MHC protein densitometry levels are presented in B (n = 4 animals/group). CLP significantly reduced diaphragm levels of MHC but administration of MitoQ blocked loss of this critical contractile protein induced by sepsis (P < 0.01 for comparison of MHC content for CLP compared with the other three groups). Densitometry data are presented in box and whisker plots with overlapping individual data points. The box represents 1 SE above and below the mean, the mean is shown by the black line in the box, and the whiskers represent the 5%–95% confidence intervals. ANOVA was used to compare variables across groups treated with different agents; *statistical significance. CLP, cecal ligation puncture; MitoQ, mitoquinone mesylate.
Plasma and Muscle MitoQ Levels
To evaluate diaphragm levels of MitoQ, we collected diaphragm tissue and plasma samples at baseline and then again at 4, 8 and 24 h after intraperitoneal injection of a single dose of 3.5 mg/kg of MitoQ. As shown in Table 1, plasma levels rose and peaked at 4 h, with subsequent reductions by 8 and 24 h. Diaphragm levels increased more slowly and remained elevated for 24 h. At the 24-h time point, diaphragm levels were substantially higher than plasma levels, with the diaphragm/plasma level ratio at ∼5,450/1.
Table 1.
Plasma and tissue levels of MitoQ
| MitoQ Levels | ||
|---|---|---|
| Time, h | Plasma, ng/mL | Diaphragm, mcg/gm |
| 0 | 0 | 0 |
| 4 | 41 ± 20 | 6.4 ± 3.2 |
| 8 | 24 ± 13 | 21.5 ± 3.9 |
| 24 | 2 ± 1 | 10.9 ± 7.6 |
Values are means ± SE. MitoQ levels in plasma and diaphragm were assessed by mass spectroscopy at 4, 8, and 24 h after administration of a single 3.5 mg/kg intraperitoneal dose (above). A tissue level of greater than 0.03 mcg/gm is considered adequate to achieve antioxidant effects in mitochondria (n = 3 animals/time point/sample). MitoQ, mitoquinone mesylate.
Delayed Administration of MitoQ
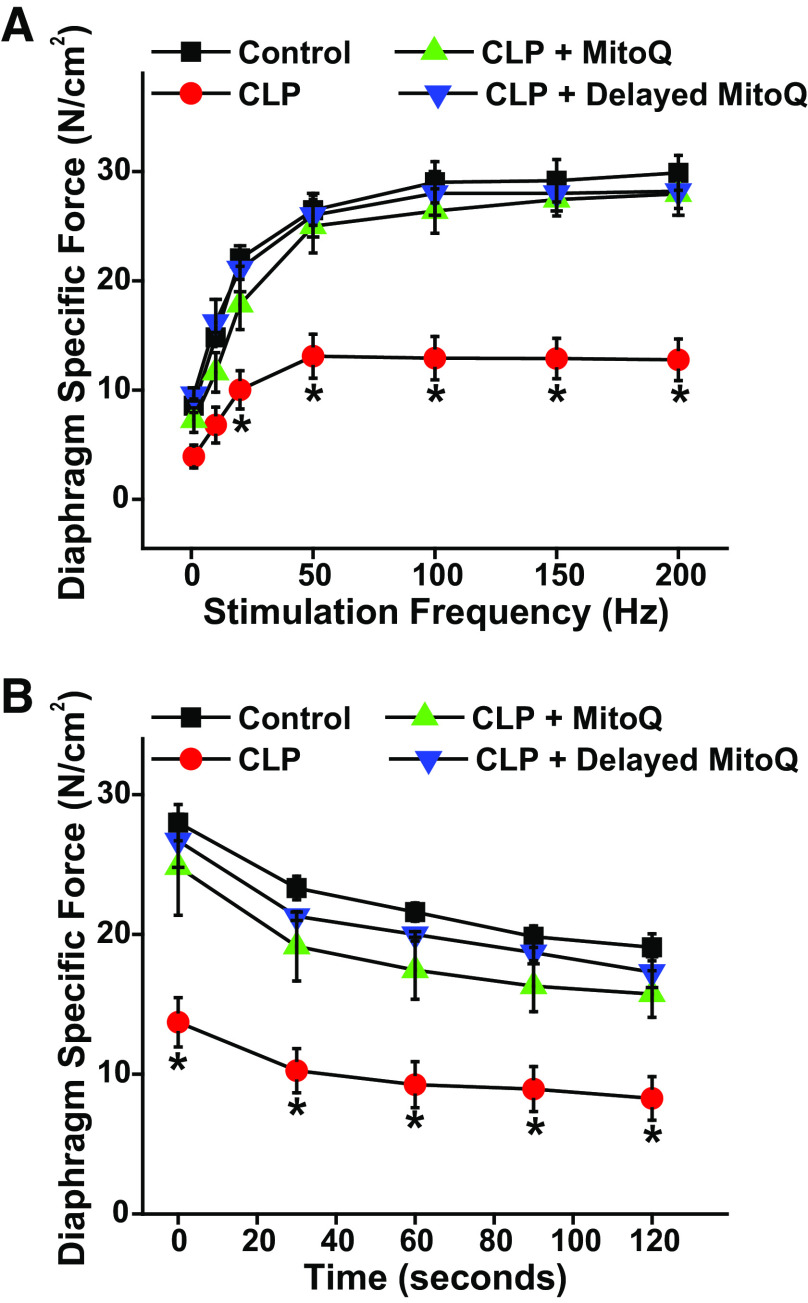
In clinical circumstances, there are inevitable delays between the time at which a patient becomes sick and the time that the individual seeks medical care. We therefore performed a group of studies to ascertain the impact of delaying MitoQ administration until 6 h after CLP surgery. As shown in the Fig. 7A, delayed MitoQ administration was as effective as immediate administration (i.e., at the time of CLP surgery) in preventing CLP sepsis-induced reductions in diaphragm strength. Similar findings were observed for diaphragm force measured during repetitive contraction trials (Fig. 7B), with delayed MitoQ administration again as effective as immediate MitoQ treatment in maintaining force for diaphragms from CLP animals treated with MitoQ (P < 0.02 for comparison of CLP to the other three experimental groups at all time points). When expressed as a percentage of initial force, final force for repetitive contraction trials averaged 65.0 ± 3.9%, 57.5 ± 6.2%, 6.8 ± 6.3%, and 65.7 ± 5.7%, respectively, for sham control, CLP, CLP + immediate MitoQ, and CLP + delayed MitoQ-treated groups (NS).
Figure 7.
Delayed MitoQ administration preserves diaphragm function in CLP sepsis. Data compare the efficacy of delayed administration of MitoQ (6 h after CLP surgery) to the effects of immediate administration of MitoQ (at the time of CLP surgery) (n = 4 or 6 animals/group). A presents diaphragm mean force-frequency curves and B shows diaphragm force over time during repetitive contraction trials. Delayed MitoQ administration was as effective as immediate administration in preventing CLP-induced reductions in diaphragm specific force generation (P < 0.001 for both immediate MitoQ + CLP and delayed MitoQ + CLP as compared with the CLP group for 20–200 Hz stimulation frequencies). Delayed and immediate MitoQ also had similar effects on diaphragm force during repetitive contraction trials (P < 0.02 for both groups compared with the CLP group at all time points). Data are presented as mean values with error bars representing 1 SE of the mean. ANOVA was used to compare variables across groups treated with different agents; *statistical significance. CLP, cecal ligation puncture; MitoQ, mitoquinone mesylate.
DISCUSSION
Close to 1,000,000 patients per year develop respiratory failure in the United States and require more than transient mechanical ventilation. Recent studies indicate that a high percentage of these patients develop significant diaphragm weakness, with diaphragm strength averaging only 20%–25% of the level of normal, healthy subjects in the critically ill, intensive care unit (ICU) population (1–3). Importantly, these studies also indicate that diaphragm weakness is a major determinant of outcomes in ICU patients, representing a major risk factor for mortality and the requirement for prolonged mechanical ventilation (2). Several reports also suggest that diaphragm weakness in ICU patients is linked to infection, with diaphragm pressure generation of infected patients less than half than that observed for noninfected individuals (2).
A number of animal studies have investigated the mechanisms by which infections induce diaphragm weakness. These reports indicate that infections increase skeletal muscle mitochondrial free radical generation (10) and additional reports indicate that increased free radical generation is linked to activation of proteolytic pathways in skeletal muscle, leading to loss of critical muscle proteins and muscle weakness (13, 14, 27). In addition, sepsis has been reported to deplete several mitochondrial proteins in animal models, including cytochrome c and cytochrome b (25).
If these data are correct, administration of mitochondrially targeted antioxidants should prevent loss of critical skeletal muscle contractile proteins and preserve diaphragm force generation in septic animals. In keeping with this theory, we recently demonstrated that two such agents, SS31 and mitoTEMPOL, are capable of preventing sepsis-induced diaphragm dysfunction (13, 14). These two agents, however, are not approved for administration to patients. The only mitochondrially targeted antioxidant widely used by humans as a biopharmaceutical currently is MitoQ. Mitoquinone mesylate (also termed MitoQ) consists of a ubiquinone chemically linked to a lipophilic triphenylphosphonium cation (15, 16). A limited number of previous studies have evaluated the effects of administration of MitoQ in animal models of sepsis. One study found that MitoQ preserved liver and renal function in endotoxin-induced sepsis (18), a study found that MitoQ administration prevented sepsis-induced cardiac dysfunction (19), one found that MitoQ improved intestinal barrier function, and yet another one found that MitoQ attenuated sepsis-induced acute lung injury (17, 20).
The present study is the first to demonstrate that MitoQ administration prevents CLP sepsis-induced physiological abnormalities in skeletal muscle. Most importantly, MitoQ treatment prevented sepsis-induced reductions in diaphragm strength, preserving diaphragm force generation in response to stimulation frequencies from 20 Hz to 200 Hz. Note these measurements used direct field stimulation of muscle, only evaluating sepsis-induced myopathy. We have previously shown that this model of CLP sepsis does not result in neuromuscular transmission failure (13, 14).
We also found that sepsis depressed absolute diaphragm force generation during a repetitive contraction trial and that absolute force generation during these trials for diaphragms from MitoQ-treated, septic animals was similar to that for control animals. In terms of relative force, the percentage change of force over time for muscle from septic animals was similar to muscles from control animals. We believe this represents an abnormality, since the weaker diaphragms of septic animals should have had force decline more slowly over time during repetitive contractions because septic muscles are generating less force than stronger control muscles and thus have a lower rate of usage of high-energy phosphate compounds. As a result, our finding that force normalized to its initial value declined as rapidly in the weaker muscles from septic animals as for controls and MitoQ-treated groups actually indicates a greater than expected fall in force in response to repetitive contractions for muscles from septic animals.
Several studies indicate that the responses to sepsis may be sex specific (28, 29). To address potential sex-related differential effects in skeletal muscle during sepsis, we examined the response of the diaphragm to CLP in female animals and determined if the administration of MitoQ would be more or less efficacious in female as compared with male animals. We found, however, that the responses of female animals to both CLP and MitoQ were nearly identical to that seen in male animals, with both CLP and MitoQ eliciting similar alterations in diaphragm force generation in both sexes. We also examined responses in another model of sepsis, i.e., systemic administration of endotoxin (LPS). We found that MitoQ administration was as efficacious in the LPS model of sepsis as it was in the CLP model.
We assessed diaphragm mitochondrial function by measuring the oxygen consumption rates of diaphragm homogenates. We found that ADP-stimulated oxygen consumption (i.e., state 3 oxygen consumption rate) and ATP generation were significantly depressed for samples from CLP animals and that MitoQ completely prevented these sepsis-induced reductions. These findings are consistent with multiple previous papers demonstrating significant reductions in muscle mitochondrial ATP generation rates in response to sepsis (9, 14, 30, 31). This is the first report to show that MitoQ can prevent this sepsis-induced derangement in the diaphragm.
Myosin heavy chain is a key contractile protein determining muscle force generation, and several previous reports have found that myosin heavy chain is selectively depleted in the muscles of critically ill patients with evidence of critical illness-induced myopathy (32–34). We also found that CLP sepsis elicited significant reductions in diaphragm myosin heavy chain content and MitoQ administration prevented this alteration. As a result, it is reasonable to argue that sepsis-induced reductions in myosin heavy chain levels may be a major cause of sepsis-induced muscle weakness, and the effect of MitoQ to preserve myosin heavy chain may be a major mechanism by which this agent improves strength.
A range of doses of MitoQ has been used in a variety of animal and human studies in the past. The dose chosen for the current study was selected because we estimated it would result in delivery of a MitoQ dose roughly similar to that achieved in our previous animal study in which we examined the effect of MitoQ on cardiac function in an animal model of sepsis (19). To directly determine the effect of once a day dosing of MitoQ at 3.5 mg/kg on tissue levels, we measured diaphragm and plasma levels of MitoQ using mass spectroscopy in the present study. Previous studies have suggested that a tissue level of MitoQ in the 30 pmol/g (i.e., 0.03 µg/gm) range is required to adequately inhibit pathological levels of free radical generation (35). We found, as expected, that diaphragm MitoQ levels were substantially higher than plasma levels at 24 h after single-dose administration, consistent with the fact this agent preferentially accumulates in tissue. More impressively, our data indicate that diaphragm levels remained above the “therapeutic” target range for 24 h after a single dose of MitoQ, suggesting that daily dosing of this agent should be capable of providing therapeutic effects in the diaphragm. We also found that delayed administration of MitoQ (6 h after induction of CLP) was as effective as immediate dosing in preventing diaphragm force loss. Both sets of data support the potential for translation of the present findings to the clinical arena, indicating that once a day dosing of MitoQ, even when delayed, should be capable of blocking free radical-mediated diaphragm dysfunction.
Taken together, the present findings indicate that MitoQ administration is capable of preventing sepsis-induced depletion of a key contractile protein and preserving diaphragm contractile and mitochondrial function. We speculate that MitoQ may therefore have the potential to preserve respiratory muscle function when administered to critically ill patients with sepsis. As a corollary, by reducing diaphragm weakness, this agent may have the potential to improve patient outcomes, shortening the duration of mechanical ventilation and reducing the mortality of critically ill patients.
GRANTS
G. S. Supinski is supported by National Institutes of Health (NIH) Grants R01HL113494 and R01HL141356 and by Department of Veterans Affairs Grant 5I01BX002132. E. A. Schroder is supported by NIH Grant R01HL141356. A. J. Morris is supported by NIH Grant P30GM127211. L. A. P. Callahan is supported by NIH Grants R01HL112085 and R01HL141356.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.S.S. conceived and designed research; G.S.S., E.A.S., L.W., and A.J.M. performed experiments; G.S.S., E.A.S., and A.J.M. analyzed data; G.S.S. and L.A.P.C. interpreted results of experiments; G.S.S. and L.A.P.C. prepared figures; G.S.S. drafted manuscript; G.S.S., E.A.S., L.W., and L.A.P.C. edited and revised manuscript; G.S.S., E.A.S., L.W., A.J.M., and L.A.P.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We express our appreciation to Dr. Jianzhong Chen, a postdoctoral scholar in A. J. Morris’ laboratory for performing the measurements of MitoQ levels in plasma and in tissues.
REFERENCES
- 1.Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi Y-SA, Tobin MJ. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med 167: 120–127, 2003. doi: 10.1164/rccm.200210-1246OC. [DOI] [PubMed] [Google Scholar]
- 2.Supinski GS, Callahan LA. Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care 17: R120, 2013. doi: 10.1186/cc12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson AC, Hughes PD, Louise Harris M, Hart N, Ware RJ, Wendon J, Green M, Moxham J. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med 29: 1325–1331, 2001. doi: 10.1097/00003246-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Dres M, Goligher EC, Heunks LMA, Brochard LJ. Critical illness-associated diaphragm weakness. Intensive Care Med 43: 1441–1452, 2017. doi: 10.1007/s00134-017-4928-4. [DOI] [PubMed] [Google Scholar]
- 5.Laghi F, D'Alfonso N, Tobin MJ. A paper on the pace of recovery from diaphragmatic fatigue and its unexpected dividends. Intensive Care Med 40: 1220–1226, 2014. doi: 10.1007/s00134-014-3340-6. [DOI] [PubMed] [Google Scholar]
- 6.Petrof BJ, Hussain SN. Ventilator-induced diaphragmatic dysfunction: what have we learned? Curr Opin Crit Care 22: 67–72, 2016. doi: 10.1097/MCC.0000000000000272. [DOI] [PubMed] [Google Scholar]
- 7.Powers SK, Wiggs MP, Sollanek KJ, Smuder AJ. Ventilator-induced diaphragm dysfunction: cause and effect. Am J Physiol Regul Integr Comp Physiol 305: R464–R477, 2013. doi: 10.1152/ajpregu.00231.2013. [DOI] [PubMed] [Google Scholar]
- 8.Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech 6: 25–39, 2013. doi: 10.1242/dmm.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callahan LA, Supinski GS. Sepsis-induced myopathy. Crit Care Med 37: S354–S367, 2009. doi: 10.1097/CCM.0b013e3181b6e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Supinski GS, Alimov AP, Wang L, Song X-H, Callahan LA. Calcium-dependent phospholipase A2 modulates infection-induced diaphragm dysfunction. Am J Physiol Lung Cell Mol Physiol 310: L975–L984, 2016. doi: 10.1152/ajplung.00312.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Supinski GS, Alimov AP, Wang L, Song X-H, Callahan LA. Neutral sphingomyelinase 2 is required for cytokine-induced skeletal muscle calpain activation. Am J Physiol Lung Cell Mol Physiol 309: L614–L624, 2015. doi: 10.1152/ajplung.00141.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Supinski GS, Wang W, Callahan LA. Caspase and calpain activation both contribute to sepsis-induced diaphragmatic weakness. J Appl Physiol (1985) 107: 1389–1396, 2009. doi: 10.1152/japplphysiol.00341.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Supinski GS, Wang L, Schroder EA, Callahan LAP. MitoTEMPOL, a mitochondrial targeted antioxidant, prevents sepsis-induced diaphragm dysfunction. Am J Physiol Lung Cell Mol Physiol 319: L228–L238, 2020. doi: 10.1152/ajplung.00473.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Supinski GS, Wang L, Schroder EA, Callahan LAP. SS31, a mitochondrially targeted antioxidant, prevents sepsis-induced reductions in diaphragm strength and endurance. J Appl Physiol (1985) 128: 463–472, 2020. doi: 10.1152/japplphysiol.00240.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy MP. Understanding and preventing mitochondrial oxidative damage. Biochem Soc Trans 44: 1219–1226, 2016. doi: 10.1042/BST20160108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribeiro RF Jr, Dabkowski ER, Shekar KC, Ka OC, Hecker PA, Murphy MP. MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radic Biol Med 117: 18–29, 2018. doi: 10.1016/j.freeradbiomed.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R, Ren T, Zeng J. Mitochondrial coenzyme Q protects sepsis-induced acute lung injury by activating PI3K/Akt/GSK-3β/mTOR pathway in rats. Biomed Res Int 2019: 5240898, 2019. doi: 10.1155/2019/5240898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowes DA, Thottakam BMV, Webster NR, Murphy MP, Galley HF. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic Biol Med 45: 1559–1565, 2008[Erratum inFree Radic Biol Med47: 1098, 2009] doi: 10.1016/j.freeradbiomed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Supinski GS, Murphy MP, Callahan LA. MitoQ administration prevents endotoxin-induced cardiac dysfunction. Am J Physiol Regul Integr Comp Physiol 297: R1095–R1102, 2009. doi: 10.1152/ajpregu.90902.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Zhou Q, Li Y, Zhang Y, Wu Y. MitoQ modulates lipopolysaccharide-induced intestinal barrier dysfunction via regulating Nrf2 signaling. Mediators Inflamm 2020: 3276148, 2020. doi: 10.1155/2020/3276148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW 3rd, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock 24, Suppl 1: 52–57, 2005. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 22.Supinski GS, Callahan LA. β-hydroxy-β-methylbutyrate (HMB) prevents sepsis-induced diaphragm dysfunction in mice. Respir Physiol Neurobiol 196: 63–68, 2014. doi: 10.1016/j.resp.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Supinski GS, Wang L, Song X-H, Moylan JS, Callahan LA. Muscle-specific calpastatin overexpression prevents diaphragm weakness in cecal ligation puncture-induced sepsis. J Appl Physiol (1985) 117: 921–929, 2014. doi: 10.1152/japplphysiol.00975.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Close RI. Dynamic properties of mammalian skeletal muscles. Physiol Rev 52: 129–197, 1972. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- 25.Callahan LA, Supinski GS. Sepsis induces diaphragm electron transport chain dysfunction and protein depletion. Am J Respir Crit Care Med 172: 861–868, 2005. doi: 10.1164/rccm.200410-1344OC. [DOI] [PubMed] [Google Scholar]
- 26.Larsen FJ, Schiffer TA, Ortenblad N, Zinner C, Morales-Alamo D, Willis SJ, Calbet JA, Holmberg HC, Boushel R. High-intensity sprint training inhibits mitochondrial respiration through aconitase inactivation. FASEB J 30: 417–427, 2016. doi: 10.1096/fj.15-276857. [DOI] [PubMed] [Google Scholar]
- 27.Supinski GS, Callahan LA. Calpain activation contributes to endotoxin-induced diaphragmatic dysfunction. Am J Respir Cell Mol Biol 42: 80–87, 2010. doi: 10.1165/rcmb.2008-0275OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosch F, Angele MK, Chaudry IH. Gender differences in trauma, shock and sepsis. Mil Med Res 5: 35, 2018. doi: 10.1186/s40779-018-0182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weng L, Zeng X-Y, Yin P, Wang L-J, Wang C-Y, Jiang W, Zhou M-G, Du B; China Critical Care Clinical Trials G. Sepsis-related mortality in China: a descriptive analysis. Intensive Care Med 44: 1071–1080, 2018. doi: 10.1007/s00134-018-5203-z. [DOI] [PubMed] [Google Scholar]
- 30.Boczkowski J, Lisdero CL, Lanone S, Samb A, Carreras MC, Boveris A, Aubier M, Poderoso JJ. Endogenous peroxynitrite mediates mitochondrial dysfunction in rat diaphragm during endotoxemia. FASEB J 13: 1637–1646, 1999. doi: 10.1096/fasebj.13.12.1637. [DOI] [PubMed] [Google Scholar]
- 31.Callahan LA, Supinski GS. Diaphragm and cardiac mitochondrial creatine kinases are impaired in sepsis. J Appl Physiol (1985) 102: 44–53, 2007. doi: 10.1152/japplphysiol.01204.2005. [DOI] [PubMed] [Google Scholar]
- 32.Derde S, Hermans G, Derese I, Guiza F, Hedstrom Y, Wouters PJ, Bruyninckx F, D'Hoore A, Larsson L, Van den Berghe G, Vanhorebeek I. Muscle atrophy and preferential loss of myosin in prolonged critically ill patients. Crit Care Med 40: 79–89, 2012. doi: 10.1097/CCM.0b013e31822d7c18. [DOI] [PubMed] [Google Scholar]
- 33.Friedrich O. Critical illness myopathy: what is happening? Curr Opin Clin Nutr Metab Care 9: 403–409, 2006. doi: 10.1097/01.mco.0000232900.59168.a0. [DOI] [PubMed] [Google Scholar]
- 34.Friedrich O, Reid MB, Van den Berghe G, Vanhorebeek I, Hermans G, Rich MM, Larsson L. The sick and the weak: neuropathies/myopathies in the critically ill. Physiol Rev 95: 1025–1109, 2015. doi: 10.1152/physrev.00028.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jauslin ML, Meier T, Smith RAJ, Murphy MP. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J 17: 1972–1974, 2003. doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]