Abstract
Spaceflight-associated neuro-ocular syndrome (SANS) develops during long-duration (>1 mo) spaceflight presumably because of chronic exposure to a headward fluid shift that occurs in weightlessness. We aimed to determine whether reversing this headward fluid shift with acute application of lower body negative pressure (LBNP) can influence outcome measures at the eye. Intraocular pressure (IOP) and subfoveal choroidal thickness were therefore evaluated by tonometry and optical coherence tomography (OCT), respectively, in 14 International Space Station crewmembers before flight in the seated, supine, and 15° head-down tilt (HDT) postures and during spaceflight, without and with application of 25 mmHg LBNP. IOP in the preflight seated posture was 14.4 mmHg (95% CI, 13.5–15.2 mmHg), and spaceflight elevated this value by 1.3 mmHg (95% CI, 0.7–1.8 mmHg, P < 0.001). Acute exposure to LBNP during spaceflight reduced IOP to 14.2 mmHg (95% CI, 13.4–15.0 mmHg), which was equivalent to that of the seated posture (P > 0.99), indicating that venous fluid redistribution by LBNP can influence ocular outcome variables during spaceflight. Choroidal thickness during spaceflight (374 µm, 95% CI, 325–423 µm) increased by 35 µm (95% CI, 25–45 µm, P < 0.001), compared with the preflight seated posture (339 µm, 95% CI, 289–388 µm). Acute use of LBNP during spaceflight did not affect choroidal thickness (381 µm, 95% CI, 331–430 µm, P = 0.99). The finding that transmission of reduced venous pressure by LBNP did not decrease choroidal thickness suggests that engorgement of this tissue during spaceflight may reflect changes that are secondary to the chronic cerebral venous congestion associated with spaceflight.
NEW & NOTEWORTHY Spaceflight induces a chronic headward fluid shift that is believed to underlie ocular changes observed in astronauts. The present study demonstrates, for the first time, that reversing this headward fluid shift via application of lower body negative pressure (LBNP) during spaceflight may alter the ocular venous system, as evidenced by a decrease in intraocular pressure. This finding indicates that LBNP has the potential to be an effective countermeasure against the headward fluid shift during spaceflight, which may then be beneficial in preventing or reversing associated ocular changes.
Keywords: capillary filtration, choroid, headward fluid shift, intraocular pressure, spaceflight neuro-ocular syndrome
INTRODUCTION
Spaceflight-associated neuro-ocular syndrome (SANS), which can develop in astronauts exposed to long-duration spaceflight (>1 mo), presents with signs that include optic disc edema, chorioretinal folds, flattening of the posterior globe of the eye, and hyperopic shifts in vision (1–3). The primary factor contributing to this condition is hypothesized to be the chronic headward fluid shift that occurs in the absence of head-to-foot gravitational forces (4). Optic disc edema resolves in astronauts during the months after return from spaceflight when the only intervention is exposure to upright postures in a gravitational environment (3). Therefore, reversing the headward fluid shift during spaceflight may be an effective countermeasure to prevent or resolve SANS findings while a mission is in progress.
It was initially assumed that the weightlessness-induced headward fluid shift resulted in a pathological elevation of intracranial pressure (ICP) and that this must be a key mechanism underlying SANS development (1). However, invasive measurements of ICP during acute weightlessness in parabolic flight are lower than those of the supine posture, suggesting that ICP during spaceflight is unlikely to be elevated to clinically pathological levels, as occurs in idiopathic intracranial hypertension (5). Although ICP is thought to remain relatively low, its potential role in SANS continues to be evaluated because the mean daily value in spaceflight may still be greater than on Earth, where two-thirds of each day is typically spent in the upright posture (6).
More recently, the potential role of cerebral venous congestion in SANS has gained attention (7). With exposure to the headward fluid shift in chronic weightlessness, internal jugular vein (IJV) pressure and area are increased, and flow patterns are altered (7). Although studies are limited, increases in intraocular pressure (IOP) (∼5 mmHg) have been documented during the first day of spaceflight (8–11); however, these values normalized to within the range of preflight seated values (10–16 mmHg) by the fourth day in weightlessness (2). The initial elevation in IOP could be due to an influence of cerebral venous congestion on episcleral venous pressure, a major contributor to IOP (12). Evidence regarding the time course of choroidal thickening during long-duration spaceflight is limited to two reports, which suggest that the choroid thickens progressively during the first 30 days of the spaceflight (1, 3) and remains stable for at least the next 5 mo of the mission. Similar to the weightlessness-induced increase in IOP, choroidal thickening may potentially be explained by cerebral venous congestion that interferes with outflow through the vortex veins.
Lower body negative pressure (LBNP) can reverse the headward fluid shift during spaceflight by drawing fluid toward the lower body from the central circulation (7, 13). To investigate the use of LBNP as a SANS countermeasure strategy (2), we tested the hypothesis that the redistribution of venous fluid generated by acute exposure to 25 mmHg LBNP can influence ocular outcome variables during spaceflight by measuring IOP and choroidal thickness in crewmembers during long-duration missions aboard the International Space Station (ISS). IOP and choroidal thickness were selected as outcome measures because each can be affected by changes in venous pressure (14–17). Although neither of these variables has been directly linked to SANS, observing that LBNP can oppose the spaceflight-induced headward fluid shift to influence an outcome at the eye would suggest that this countermeasure strategy may mitigate ocular changes that define the condition (e.g., optic disc edema). To our knowledge, this study represents the first time that effects of LBNP on ocular outcomes have been examined during spaceflight.
MATERIALS AND METHODS
Subjects
Fourteen ISS crewmembers (3 females) participated in the “Fluid Shifts Study,” a multi-institution international study. The cohort averaged 45 ± 6 (SD) yr of age and had a mean body mass index of 26.4 ± 2.8 kg/m2. Eight individuals had no previous spaceflight experience, whereas the other six individuals had logged an average of 234 ± 106 days in space before the mission in which testing occurred. All subjects underwent preflight and postflight eye examinations by a NASA Flight Medicine Clinic optometrist (MedB1.10, https://lsda.jsc.nasa.gov/MRID) and had medical histories that certified eligibility for spaceflight. Informed consent was obtained from each subject, and data were collected between 2014 and 2020 in accordance with the study protocol approved by the NASA Johnson Space Center Institutional Review Board and the Human Research Multilateral Review Board. This research adhered to the Declaration of Helsinki, although it is not a clinical trial.
Ground and Spaceflight Study Conditions
Preflight experiments were performed one time per subject in the Cardiovascular and Vision Laboratory at the NASA Johnson Space Center on an average of 109 days (range, 53–220 days) before launch. Each subject was studied in the seated, supine, and 15° head-down tilt (HDT) postures (in this order) during a single session, and consistent body position within and across subjects was achieved with the use of a medical tilt table (OT-9003 SPC, Omni Technologies, Tri W-G, Valley City, ND) and digital inclinometer (Acumar ACU001, Lafayette Instrument Company, Lafayette, IN). The 15° HDT condition was included to induce a greater headward fluid shift than was anticipated for the seated to supine transition. Therefore, this approach provided the potential for observing a broad range of effects on outcome variables on the ground that could assist in interpreting results from the spaceflight conditions. Data collection occurred after 10–15 min of quiet rest in each posture.
During spaceflight on the ISS, data were collected in the normal weightlessness environment in the Columbus module and ∼10–20 min into the sessions of continuous exposure to LBNP (25 mmHg) in the Russian Zvezda Service module using the Russian Chibis-M LBNP system (18). Crewmembers were scheduled to have 60 min of LBNP exposure during a single session; optical coherence tomography (OCT) data collection occurred ∼15 min into the session and IOP data collection occurred immediately following OCT. Subjects then remained inside the LBNP device after IOP data collection for additional measures that are not presented here. The decision to set the LBNP level to 25 mmHg was made for safety reasons in collaboration with our Russian coinvestigators who were responsible for medical monitoring during the Fluid Shifts Study. Since then, this pressure has been shown on Earth to decrease IJV area, IJV pressure, and ICP (19) without causing syncope. On average, collection of spaceflight data without LBNP occurred on flight day (FD) 46 (range, FD33–FD63) and FD142 (range, FD91–FD175), and data collection with LBNP occurred on FD54 (range, FD40–FD67) and FD165 (range, FD141–FD181). All data were collected during the first half of the day. For experiments performed on the ISS, crewmembers collected the data while investigators at Johnson Space Center who had access to a live video camera feed of the module cabin provided real-time guidance via two-way verbal communication. All crewmembers tasked with operating the instruments during data collection while aboard the ISS received hands-on training before flight.
Intraocular Pressure Measurements
During preflight data collection, IOP was measured in triplicate in the left eye using the Icare Pro tonometer (Icare, Vantaa, Finland), which does not require anesthetization of the cornea. This instrument contains an inclination sensor that allows for measurements to be collected accurately in seated and supine postures (20); subjects looked directly up at the ceiling during HDT so that the instrument could be held at the angle used for supine measurements. Inflight measurements were collected in the left eye using the Tono-Pen Avia tonometer (Reichert Technologies, Depew, NY) following local anesthetization of the cornea with proparacaine hydrochloride (0.5%). An inflight data take was considered complete upon collection of a least three measurements that were within ±1 mmHg of each other. The accuracy of the Tono-Pen Avia is independent of the effects of gravity (21). Data were collected on the ground by members of NASA’s Cardiovascular and Vision Laboratory and on the ISS by astronauts.
Optical Coherence Tomography and Choroidal Thickness Measurements
Spectral-domain OCT images were acquired from the left eye using the Spectralis OCT1 and OCT2 imaging systems (Heidelberg Engineering, Heidelberg, Germany) on Earth and on the ISS. Images collected on Earth in the seated posture served as references for the AutoRescan follow-up feature that was used to ensure that subsequent scans collected on Earth and on the ISS were acquired at the same retinal location within each subject. Choroidal thickness was measured from a single B-scan aligned through the fovea and the optic disc. To maximize signal-to-noise, the final image was the average of 100 B-scans that were collected using enhanced-depth imaging (22). Two independent observers manually segmented the chorioscleral border using Eye Explorer software (Heidelberg Engineering, Heidelberg, Germany). The mean distance from that border to Bruch’s membrane was automatically calculated across a 3 mm region centered under the fovea using a custom MATLAB script (MathWorks, Natick, MA). If the observers’ measurements of an image differed by ≥ 10%, then the two observers and at least one additional scientist reviewed that image, as a group, to reassess the location of the chorioscleral border. By employing this strategy, the two values contributing to each measurement of choroidal thickness in this study were brought within 10% of each other. All OCT data from one subject were excluded because of poor image quality that prevented delineation of the chorioscleral border.
Cardiovascular Measurements
On Earth, blood pressure and heart rate were measured during each posture using an automated blood pressure device (Dinamap XL, General Electric, Fairfield, CT) within 5 min of both IOP and OCT data collection. During spaceflight, blood pressure and heart rate were measured without LBNP (Tonoport V, General Electric, Fairfield, CT) and during use of LBNP (Gamma, Heine Optotechnik, Gilching, Germany, until replacement in 2017 by the KMA-01 System, Russia); as on Earth, these measurements occurred within 5 min of the IOP and OCT data collections. Mean arterial pressure (MAP) was calculated as the sum of one-third of the systolic blood pressure and two-thirds of the diastolic blood pressure. MAP recorded at the brachial artery differs from that of the eye in the seated and HDT positions because of the hydrostatic pressure column (23), and we did not adjust for this difference. However, the supine posture (6) and both spaceflight (24) conditions are hydrostatically neutral in this context, making the brachial MAP a reasonable surrogate for that of the eye.
Statistical Analysis
Statistical analyses were performed using Stata software (Version 16, StatCorp, College Station, TX) with an emphasis on characterizing the observed effects with modeled means and 95% confidence intervals (CI). Statistically significant differences were determined against a two-tailed null hypothesis of no-differences with α = 0.05. All model assumptions were evaluated before reporting effects, and the five observations that produced standardized residuals exceeding ±3 were eliminated to meet model assumptions (IOP: two observations within the seated posture for 1 subject; choroidal thickness: HDT, ∼FD150 spaceflight, and ∼FD150 LBNP for 1 subject). Although those outlying data points are included in the figures, the values do not influence the plotted means or 95% CIs. IOP was measured repeatedly within each time point to increase precision. For each outcome variable, the confidence intervals of the mean of the data for the two time points (∼FD50 and ∼FD150) within each spaceflight condition were virtually indistinguishable; therefore, these measurements were included in the statistical model such that a single value for the spaceflight condition and a single value for the spaceflight with LBNP condition were generated.
We submitted each of our continuously scaled outcomes to separate statistical mixed-models. Each mixed-model had a priori fixed effects parameters that compared: 1) seated posture data to those of all other condition/time combinations and 2) the inflight LBNP data to those of all other condition/time combinations. Given the number of pairwise contrasts performed per outcome, we used Bonferroni adjustments to control for inflated Type I error. All models included a random Y-intercept to accommodate the within-subjects experimental design and, where appropriate, the repeated observations within time. All data are presented as the marginal means ±95% CI unless otherwise noted.
RESULTS
LBNP Reduces IOP in Spaceflight
IOP measurements collected during acute posture changes on Earth, which generate headward fluid shifts of varying magnitudes, established the range of values for these subjects to which measurements collected during spaceflight, without or with LBNP, could be compared. IOP averaged 14.4 mmHg (95% CI, 13.5–15.2 mmHg) in the seated posture on Earth, increased by 1.2 mmHg (95% CI, 0.6–1.9 mmHg, P = 0.001) after transition to the supine posture, and increased by 4.3 mmHg (95% CI, 3.6–4.9 mmHg, P < 0.001) after transition to the HDT posture (Fig. 1, Table 1). IOP was 15.6 mmHg (95% CI, 14.9–16.4 mmHg) during spaceflight before use of LBNP, which was 1.3 mmHg (95% CI, 0.7–1.8 mmHg, P < 0.001) greater than that of the seated posture on Earth and not different from that of the supine posture on Earth (15.6 mmHg, 95% CI, 14.8–16.4 mmHg). Acute application of LBNP reduced IOP by 1.4 mmHg (95% CI, 0.9–1.9 mmHg, P < 0.001), resulting in an IOP that did not differ from that of the seated posture (difference of 0.2 mmHg, 95% CI, −0.4 to 0.8 mmHg, P > 0.99).
Figure 1.
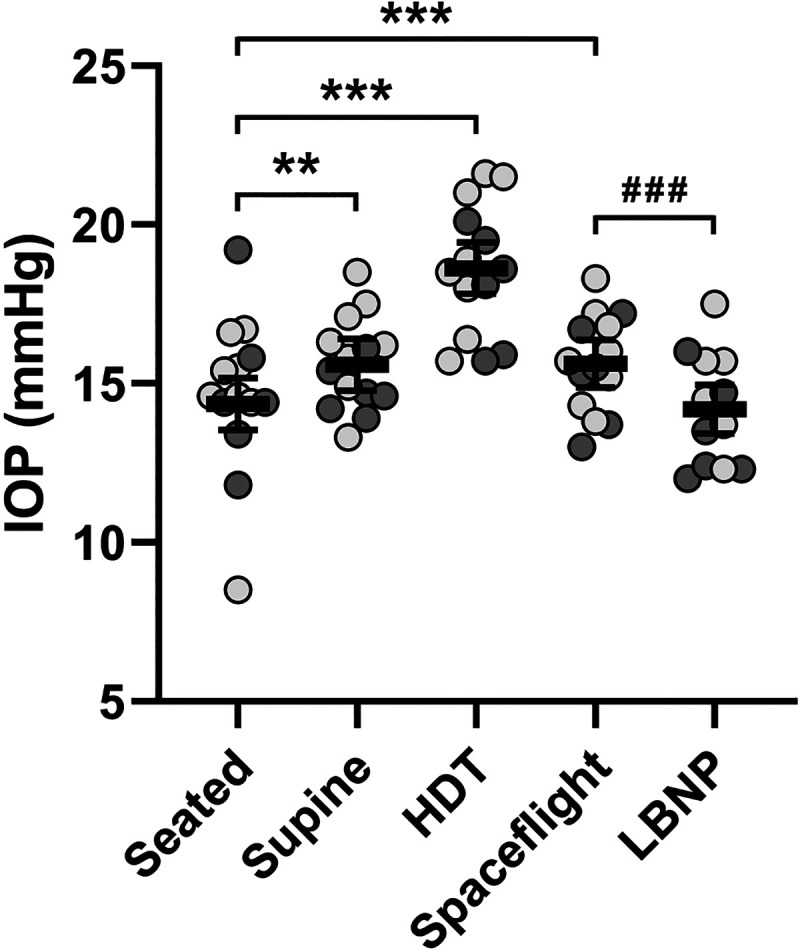
Reduction of intraocular pressure by LBNP-induced acute fluid shifts during spaceflight. IOP elevates with acute, head-lowering posture changes on Earth. In comparison with the seated posture, long-duration spaceflight results in a mild elevation of IOP that can be countered with acute application of LBNP. Mean values (bars) of individual subject left eye data (light circles, no prior spaceflight experience; dark circles, prior spaceflight experience) are shown, and error bars represent 95% CI; n = 14 subjects. Statistical significance is indicated by **P < 0.01 and ***P < 0.001 for comparison to the seated posture and by ###P < 0.001 for comparison to the LBNP condition (mixed model analysis). CI, confidence interval; HDT, head-down tilt; IOP, intraocular pressure; LBNP, lower body negative pressure.
Table 1.
Ocular and cardiovascular measurements by experimental condition
| Preflight |
Spaceflight |
||||
|---|---|---|---|---|---|
| Seated | Supine | HDT | Without LBNP | LBNP | |
| IOP, mmHg | 14.4 (13.5–15.2) | 15.6 (14.8–16.4) | 18.6 (17.8–19.4) | 15.6 (14.9–16.4) | 14.2 (13.4–15.0) |
| Choroidal thickness, µm | 339 (289–388) | 340 (290–390) | 344 (294–393) | 374 (325–423) | 381 (331–430) |
| Systolic BP, mmHg | 117 (113–122) | 114 (109–118) | 119 (115–124) | 124 (120–128) | 119 (115–123) |
| Diastolic BP, mmHg | 78 (74–83) | 72 (67–76) | 71 (67–76) | 78 (74–82) | 63 (59–68) |
| MAP, mmHg | 93 (88–97) | 86 (82–91) | 87 (83–92) | 93 (89–97) | 82 (78–86) |
| Heart rate, beats/min | 59 (56–62) | 54 (51–57) | 53 (50–56) | 59 (56–62) | 70 (67–73) |
Marginal means for ocular and cardiovascular measures for each posture on the ground and during spaceflight, without and with application of LBNP. 95% CI is shown in parenthesis. Hand-calculated differences of the means may diverge slightly from the estimates of the statistical model reported in the results. BP, blood pressure; CI, confidence interval; HDT, head-down tilt; IOP, intraocular pressure; LBNP, lower body negative pressure; MAP, mean arterial pressure.
LBNP Does Not Affect Choroidal Thickness during Spaceflight
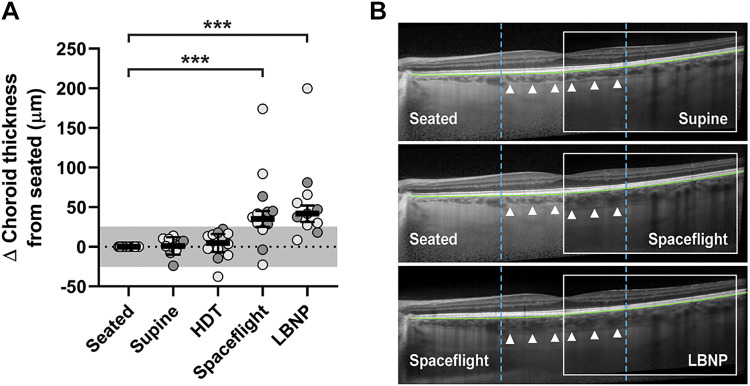
Choroidal thickness measurements were collected based on the same rationale as the abovementioned IOP measurements. On Earth, choroidal thickness in the seated posture (339 µm, 95% CI, 289–388 µm) did not change after transition to the supine posture (difference of 1 µm, 95% CI, −10 to 12 µm, P > 0.99) or HDT posture (difference of 5 µm, 95% CI, −7 to 16 µm, P > 0.99). Choroidal thickness during spaceflight (374 µm, 95% CI, 325–423 µm) was 35 µm (95% CI, 25–45 µm, P < 0.001) greater than for the seated posture on Earth (Fig. 2A, Table 1). However, application of LBNP did not change choroidal thickness during spaceflight (difference of 7 µm between spaceflight and LBNP conditions, 95% CI, −2 to 16 µm, P = 0.99). Images documenting the third greatest spaceflight-induced increase in choroidal thickness observed in this study are shown (Fig. 2B).
Figure 2.
Choroidal thickness is not affected by LBNP-induced acute fluid shifts during spaceflight. A: choroidal thickness does not change following acute posture changes on Earth or with acute application of LBNP during spaceflight. However, choroidal thickness increases during the chronic fluid shift experienced during long-duration spaceflight, as compared with the seated position on Earth. Mean values (bars) of individual subject left eye data (light circles, no prior spaceflight experience; dark circles, prior spaceflight experience) are shown and error bars represent 95% CI; n = 13 subjects. Statistical significance is indicated by ***P < 0.001 for comparison with the seated posture (mixed model analysis). The shaded area represents the predefined range (± 25.5 µm) of normal variation. B: OCT images from a single subject who exhibited the third greatest spaceflight-induced increase in choroidal thickness. Bruch’s membrane is segmented (green line), the chorioscleral border is indicated (white triangles), and the 3 mm subfoveal region in which retinal thickness was calculated is bounded (dashed blue lines). Choroidal thickness in the seated posture (top left, middle left) is no different from that of the supine posture (top inset) and is thinner than that of spaceflight (middle inset). Choroidal thickness during spaceflight (bottom left, middle inset) is not altered by application of LBNP (bottom inset). CI, confidence interval; HDT, head-down tilt; LBNP, lower body negative pressure; OCT, optical coherence tomography.
Cardiovascular Measurements during Fluid Shifts
Cardiovascular measurements were collected to confirm that LBNP during spaceflight did not cause hypotension (Table 1). Although MAP was 93 mmHg while seated, this value decreased by 7 mmHg (95% CI, 2–11 mmHg, P = 0.01) after transition to the supine posture and tended to be lower in the HDT posture (5 mmHg change, 95% CI, 1–10 mmHg, P = 0.07). During spaceflight, MAP was 93 mmHg (95% CI, 89–97 mmHg) and LBNP caused MAP to decrease by 11 mmHg (95% CI, 8–14 mmHg, P < 0.001). Average heart rate was 59 beats/min (95% CI, 56–62 beats/min) in the seated posture; this value decreased by 5 beats/min (95% CI, 2–8 beats/min, P = 0.003) in the supine posture and by 6 beats/min (95% CI, 3–9 beats/min, P < 0.001) in the HDT posture. The heart rate of 59 beats/min (95% CI, 56–62 beats/min) recorded during spaceflight without LBNP increased by 11 beats/min (95% CI, 8–13 beats/min, P < 0.001) during application of LBNP. For both MAP and heart rate, the values from the spaceflight condition were not different from those of the seated posture (for each comparison, P > 0.99).
DISCUSSION
Intraocular pressure and choroidal thickness respond differently to LBNP during spaceflight. The observed decrease in IOP suggests that venous fluid redistribution by LBNP can influence ocular outcome variables. Unlike IOP, weightlessness-induced choroidal thickening was not attenuated by LBNP, suggesting that choroidal engorgement reflects changes that are secondary to the chronic cerebral venous congestion associated with spaceflight.
We hypothesize that this effect of LBNP on IOP is primarily caused by a decrease in episcleral venous pressure, in accordance with the Goldmann equation (17). Specifically, we are proposing that the fall in episcleral venous pressure is a result of LBNP-induced sequestration of venous blood in the splanchnic and peripheral leg circulation that reduces cerebral venous volume and pressure. Consistent with this hypothesis, we have previously shown that the use of LBNP during long-duration spaceflight reduces IJV pressure and area (7), downstream of ocular outflow. Given that the headward fluid shift is hypothesized to be the primary initiator of SANS, the observation that LBNP exposure produces a physiological effect at the eye suggests a potential for this approach to have utility as a SANS countermeasure, even though the magnitude of change is within the normal variation for the measurement (25, 26). Further study is needed to determine whether the effect of LBNP on the eye, as revealed by measuring IOP, can prevent or reverse the development of SANS signs (e.g., optic disc edema). Although optimizing the duration and/or magnitude of the LBNP exposure would be necessary to mitigate SANS findings, it is encouraging that the present regimen does not drive IOP to pathologically low levels.
The changes in IOP observed during the ground and inflight phases of this study are consistent with a persistent headward fluid shift of venous blood occurring during chronic weightlessness. The reliable effects on IOP produced by brief postural changes and by application of LBNP are similar to those described in previous ground studies (27–29). The likeness of IOP measurements acquired during spaceflight without LBNP to those collected on the ground in the supine posture is consistent with previous reports on posture, fluid shifts, and hypothesized pressure distributions (4, 6, 30). Furthermore, the findings in the present study do not conflict with prior reports indicating that IOP normalizes during the first days of spaceflight to be equivalent to the seated posture (2); those data were interpreted such that a return of this measurement to within ∼1 mmHg of baseline would have been considered rectified. Although we did not document the initial increase in IOP upon entry into weightlessness, stable values were recorded across the FD50 and FD150 data collections (15.4 mmHg and 16.1 mmHg, respectively). In the present study, we did not investigate mechanisms for how IOP normalizes throughout the first week of spaceflight. However, we speculate that this effect could have resulted from a change in equilibrium between the anterior chamber pressure and episcleral venous pressure (36) and/or a reflexive innervation of the episcleral vascular network (37) that allowed for increased aqueous outflow. The observation that the decrease in IOP that occurs spontaneously during the initial adaptation to spaceflight (9) is similar in magnitude to that which is associated with 25 mmHg LBNP is considered coincidental.
Choroidal thickness increased during long-duration spaceflight from preflight values in excess of the predefined 25.5 µm threshold of normal variability for this measure (31), and exposure to LBNP did not reverse this change. The finding that the choroid expands in sustained weightlessness is consistent with other studies (3, 32, 33), and the null effect of brief LBNP exposure is similar to the observation that acute posture changes did not significantly alter choroidal thickness. Although a link between choroidal thickness changes and SANS findings has not definitively been established to date, choroidal expansion is associated with the development of choroidal folds (34) and computational modeling suggests that choroidal expansion can generate biomechanical strain on the optic nerve head (35). However, we previously observed that after ∼35 days of spaceflight, only 1 of 14 subjects showed Frisén grade edema, and the increase in choroidal thickness of that subject was similar to the cohort mean (33).
There are at least three possible explanations, which are not mutually exclusive, for why the exposure to LBNP may decrease IOP but not choroidal thickness. First, while convergent, the aqueous and choroidal outflow pathways are not identical. If LBNP decreases IOP by reducing episcleral venous congestion resulting from the headward fluid shift, then the effect of LBNP also should be transmitted to the choroid via the vortex veins because both outflow pathways connect through the superior ophthalmic vein and drain into the cavernous sinus (38). However, LBNP may also lower IOP by acting on the unconventional aqueous outflow pathway that has little convergence with choroidal outflow (38, 39); estimates of the fraction of aqueous outflow that this represents vary from 4% to 60% (40). Second, chronic choroidal vessel distension could gradually stretch the collagen lamella such that it does not immediately rebound with reduced intravascular pressure (41). Third, chronically elevated hydrostatic pressure within the superior ophthalmic vein and cerebral capillaries may promote capillary filtration across the permeable choroidal vasculature (42) and gradually produce an accumulation of extravascular fluid beyond what is typical in 1 g conditions for normal eyes (43). If capillary filtration contributes to choroidal engorgement during spaceflight, then LBNP would need to be of sufficient duration to decrease cerebral venous congestion such that the overall capillary pressure favors movement of extravascular fluid back into the vessels. Measurements of choroidal thickness occurred ∼15 min after starting LBNP in the present study, which may have been too early to observe an effect. On Earth, there may be an additional factor(s) related to hydrostatic pressure or the gravitational vector that prevents capillary filtration, underlying the difference in choroidal engorgement observed during actual weightlessness versus head-down tilt bed rest (33). Vessel distension and enhanced capillary filtration represent potential mechanisms underlying increased choroidal thickness that would occur as secondary effects of cerebral venous congestion.
We previously reported an analysis of choroidal thickness across a 3 mm region centered under the fovea that found a nonsignificant increase of 7 µm following a seated to supine posture change (31). In contrast, other studies report that acute postural changes cause statistically significant increases in choroidal thickness; however, several of those analyses used a single-point measurement strategy (44–46) that cannot capture the physiologic variability in choroidal thickness across the posterior pole (30). Like multipoint thickness measurements, choroidal area measurements can account for variability across the tissue, but this approach may cause small changes to be overemphasized. For example, a previous study reported that a posture change from seated to supine caused a nonsignificant increase in choroid area of 0.03 mm2 over an ∼6 mm region (30), which suggests that a thickness change of only 5 µm developed. However, in the same experiment, moving from the seated to the prone posture resulted in a statistically significant change of 0.05 mm2 that represented a thickness change of only 8 µm. We previously modeled the precision of our measurement variability of choroidal thickness to determine when a difference in choroidal thickness between postures exceeds the measurement variability. In subjects studied over multiple days and analyzed by multiple readers, we determined that a change in choroidal thickness >25.5 µm has <5% chance of being due to these sources of variability, and therefore, likely represents a physiologically meaningful result (31). Knowing the precision of the measurement provides assurance that our interpretation of the data from the present study is correct. In short, when detecting very small changes in choroidal thickness, differences between analysis approaches may underlie the inconsistent interpretations reported in the literature, rather than differences in physiology.
All subjects successfully completed LBNP sessions during spaceflight without any indication of presyncope. The increase in heart rate observed here with 25 mmHg LBNP was similar to that reported previously with 30 mmHg LBNP at 6–8 wk of spaceflight (47). Although this increase in heart rate likely prevented a substantial fall in MAP that would be sufficient to cause syncope, the decrease in systolic blood pressure suggests that the use of LBNP created stress on the cardiovascular system. Given that our measurements occurred ∼15–30 min into use of LBNP, it is unknown whether stress imposed on the cardiovascular system would be greater if longer duration exposures to LBNP are used. If LBNP is to be considered as a SANS countermeasure, the cardiovascular system must be able to accommodate the associated level of stress.
Limitations
This study had several limitations. First, different tonometers were used for preflight versus inflight IOP measurements. When this study began, the Tono-Pen Avia was already flight certified and in use for medical monitoring on the ISS, and only the iCare Pro was available to NASA’s Cardiovascular and Vision Laboratory. Although a side-by-side evaluation of these tonometers in 21 healthy subjects (48) showed that the measurements are comparable, it is possible that a small difference in the absolute IOP measurement between the iCare Pro and TonoPen may have impacted the interpretation of ground versus spaceflight comparisons. Second, the instrument used for blood pressure and heart rate measurements differed between tests conducted on the ground (Dinamap), during spaceflight before LBNP (Tonoport), and during spaceflight with LBNP (Gamma and KMA-01 Systems). The Tonoport was flight certified and in use in the Columbus Module, whereas the Gamma and KMA-01 Systems were certified for flight and for use with the LBNP apparatus in the Zvezda Module; these devices are assumed comparable. Third, due to scheduling constraints, performing spaceflight experiments without and with LBNP on the same day was not possible because of differing schedules for the Columbus and Zvezda modules. However, latencies between these testing sessions were minimized. Fourth, scheduling constraints during spaceflight made testing subjects at precisely the same time of day for all study time points impossible. However, all testing on Earth and on the ISS occurred during the first half of the day. Finally, as in all spaceflight research, the number of subjects included in this study was relatively small.
Conclusions
Acute exposure to LBNP during spaceflight reduces the weightlessness-induced increase in IOP, indicating that this approach has the potential to be an effective countermeasure against the chronic headward fluid shift within the venous compartment that occurs during spaceflight. More study is needed to determine whether this approach can prevent or reverse the development of SANS. Choroidal engorgement during spaceflight was not reversed by a 15-min exposure to LBNP, suggesting this structural alteration may predominantly reflect changes within the tissue that are secondary to the chronic cerebral venous congestion associated with spaceflight.
GRANTS
This study was supported by National Aeronautics and Space Administration Grants NNJ11ZSA002NA (to M.B.S.), NNX13AK30G (to S.A.D.), and NNX13AJ12G (to A.R.H.).
DISCLOSURES
A.R.H. has a NASA grant supporting the study of self-generated lower body negative pressure for deep-space missions. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
B.R.M., S.M.C.L., D.J.E., J.H.K.L., I.V.A., S.A.D., A.R.H., M.B.S., and S.S.L. conceived and designed research; B.R.M., S.M.C.L., D.J.E., M.B.S., and S.S.L. performed experiments; S.H.G., B.R.M., S.M.C.L., R.J.P.-S., and S.S.L. analyzed data; S.H.G., B.R.M., S.M.C.L., D.J.E., J.H.K.L., A.R.H., and S.S.L. interpreted results of experiments; S.H.G. prepared figures; S.H.G., B.R.M., and S.S.L. drafted manuscript; S.H.G., B.R.M., S.M.C.L., K.M.-G., D.J.E., J.H.K.L., R.J.P.-S., I.V.A., S.A.D., A.R.H., and S.S.L. edited and revised manuscript; S.H.G., B.R.M., S.M.C.L., K.M.-G., D.J.E., J.H.K.L., R.J.P.-S., I.V.A., S.A.D., A.R.H., M.B.S., and S.S.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the crewmembers who participated in this study, members of the Cardiovascular and Vision Laboratory for technical assistance, the Research Operations and Integration (ROI) Element of NASA’s Human Research Program for support on study logistics and implementation, and our international partners.
REFERENCES
- 1.Mader TH, Gibson CR, Pass AF, Kramer LA, Lee AG, Fogarty J, Tarver WJ, Dervay JP, Hamilton DR, Sargsyan A, Phillips JL, Tran D, Lipsky W, Choi J, Stern C, Kuyumjian R, Polk JD. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology 118: 2058–2069, 2011. doi: 10.1016/j.ophtha.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Stenger MB, Tarver WJ, Brunstetter T, Gibson CR, Laurie SS, Lee SMC, Macias BR, Mader TH, Otto CA, Smith SM, Zwart SR; NASA. Human Research Program Evidence Report: Risk of Spaceflight Associated Neuro-ocular Syndrome (SANS) (Online). NASA Johnson Space Center. https://humanresearchroadmap.nasa.gov/evidence/reports/SANS.pdf?rnd=0.434276635495143 [2018 Feb 15]. [Google Scholar]
- 3.Macias BR, Patel NB, Gibson CR, Samuels BC, Laurie SS, Otto C, Ferguson CR, Lee SMC, Ploutz-Snyder R, Kramer LA, Mader TH, Brunstetter T, Stenger MB. Association of long-duration spaceflight with anterior and posterior ocular structure changes in astronauts and their recovery. JAMA Ophthalmol 138: 553–559, 2020. doi: 10.1001/jamaophthalmol.2020.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L-F, Hargens AR. Spaceflight-induced intracranial hypertension and visual impairment: pathophysiology and countermeasures. Physiol Rev 98: 59–87, 2018. doi: 10.1152/physrev.00017.2016. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed RM, Wilkinson M, Parker GD, Thurtell MJ, Macdonald J, McCluskey PJ, Allan R, Dunne V, Hanlon M, Owler BK, Halmagyi GM. Transverse sinus stenting for idiopathic intracranial hypertension: a review of 52 patients and of model predictions. AJNR Am J Neuroradiol 32: 1408–1414, 2011. doi: 10.3174/ajnr.A2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawley JS, Petersen LG, Howden EJ, Sarma S, Cornwell WK, Zhang R, Whitworth LA, Williams MA, Levine BD. Effect of gravity and microgravity on intracranial pressure. J Physiol 595: 2115–2127, 2017. doi: 10.1113/JP273557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall-Goebel K, Laurie SS, Alferova IV, Arbeille P, Auñón-Chancellor SM, Ebert DJ, Lee SMC, Macias BR, Martin DS, Pattarini JM, Ploutz-Snyder R, Ribeiro LC, Tarver WJ, Dulchavsky SA, Hargens AR, Stenger MB. Assessment of jugular venous blood flow stasis and thrombosis during spaceflight. JAMA Netw Open 2: e1915011, 2019. doi: 10.1001/jamanetworkopen.2019.15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draeger J, Schwartz R, Groenhoff S, Stern C. [Self tonometry during the German 1993 Spacelab D2 mission]. Ophthalmologe 91: 697–699, 1994. [PubMed] [Google Scholar]
- 9.Draeger J, Schwartz R, Groenhoff S, Stern C. Self-tonometry under microgravity conditions. Aviat Space Environ Med 66: 568–570, 1995. [PubMed] [Google Scholar]
- 10.Mader TH, Gibson CR, Caputo M, Hunter N, Taylor G, Charles J, Meehan RT. Intraocular pressure and retinal vascular changes during transient exposure to microgravity. Am J Ophthalmol 115: 347–350, 1993. doi: 10.1016/S0002-9394(14)73586-X. [DOI] [PubMed] [Google Scholar]
- 11.Huang AS, Stenger MB, Macias BR. Gravitational Influence on Intraocular Pressure. J Glaucoma 28: 756–764, 2019. doi: 10.1097/IJG.0000000000001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenwood M, Berdahl J, Ibach M. New technology and current understanding of episcleral venous pressure. Curr Ophthalmol Rep 6: 86–92, 2018. doi: 10.1007/s40135-018-0168-1. [DOI] [Google Scholar]
- 13.Charles JB, Lathers CM. Summary of lower body negative pressure experiments during space flight. J Clin Pharmacol 34: 571–583, 1994. doi: 10.1002/j.1552-4604.1994.tb02009.x. [DOI] [PubMed] [Google Scholar]
- 14.Cagiltay E, Akay F, Demir O, Aydın E, Akmaz B, Pamuk B. The increment of choroidal thickness in euthyroid graves’ ophthalmopathy: is it an early sign of venous congestion? J Ophthalmol 2018: 5891531, 2018. doi: 10.1155/2018/5891531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rey A, Castillo L, Dyrda A, Maseras X, Jürgens I. Subfoveal choroidal thickness changes in carotid cavernous fistula following spontaneous resolution. BMC Ophthalmol 16: 63, 2016. doi: 10.1186/s12886-016-0240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinohara Y, Kashima T, Akiyama H, Kishi S. Alteration of choroidal thickness in a case of carotid cavernous fistula: a case report and a review of the literature. BMC Ophthalmol 13: 75, 2013. doi: 10.1186/1471-2415-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brubaker RF. Goldmann’s equation and clinical measures of aqueous dynamics. Exp Eye Res 78: 633–637, 2004. doi: 10.1016/j.exer.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Yarmanova EN, Kozlovskaya IB, Khimoroda NN, Fomina EV. Evolution of Russian microgravity countermeasures. Aerosp Med Hum Perform 86: A32–A37, 2015. doi: 10.3357/AMHP.EC05.2015. [DOI] [PubMed] [Google Scholar]
- 19.Watkins W, Hargens AR, Seidl S, Clary EM, Macias BR. Lower-body negative pressure decreases noninvasively measured intracranial pressure and internal jugular vein cross-sectional area during head-down tilt. J Appl Physiol (1985) 123: 260–266, 2017. doi: 10.1152/japplphysiol.00091.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakakura S. Icare® rebound tonometers: review of their characteristics and ease of use. Clin Ophthalmol 12: 1245–1253, 2018. doi: 10.2147/OPTH.S163092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pattinson TJ, Gibson CR, Manuel FK, Bishop SL, March WF. The effects of betaxolol hydrochloride ophthalmic solution on intraocular pressures during transient microgravity. Aviat Space Environ Med 70: 1012–1017, 1999. [PubMed] [Google Scholar]
- 22.Spaide RF, Koizumi H, Pozzoni MC, Pozonni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 146: 496–500, 2008. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 23.Caprioli J, Coleman AL; Blood Flow in Glaucoma Discussion. Blood pressure, perfusion pressure, and glaucoma. Am J Ophthalmol 149: 704–712, 2010. doi: 10.1016/j.ajo.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Hargens AR, Richardson S. Cardiovascular adaptations, fluid shifts, and countermeasures related to space flight. Respir Physiol Neurobiol 169, Suppl 1: S30–S33, 2009. doi: 10.1016/j.resp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Brody S, Erb C, Veit R, Rau H. Intraocular pressure changes: the influence of psychological stress and the Valsalva maneuver. Biol Psychol 51: 43–57, 1999. doi: 10.1016/s0301-0511(99)00012-5. [DOI] [PubMed] [Google Scholar]
- 26.Pointer JS. The diurnal variation of intraocular pressure in non-glaucomatous subjects: relevance in a clinical context. Ophthalmic Physiol Opt 17: 456–465, 1997. doi: 10.1016/S0275-5408(97)00036-7. [DOI] [PubMed] [Google Scholar]
- 27.Macias BR, Liu JHK, Grande-Gutierrez N, Hargens AR. Intraocular and intracranial pressures during head-down tilt with lower body negative pressure. Aerosp Med Hum Perform 86: 3–7, 2015. doi: 10.3357/AMHP.4044.2015. [DOI] [PubMed] [Google Scholar]
- 28.Marshall-Goebel K, Mulder E, Bershad E, Laing C, Eklund A, Malm J, Stern C, Rittweger J. Intracranial and intraocular pressure during various degrees of head-down tilt. Aerosp Med Hum Perform 88: 10–16, 2017. doi: 10.3357/AMHP.4653.2017. [DOI] [PubMed] [Google Scholar]
- 29.Prata TS, De Moraes CGV, Kanadani FN, Ritch R, Paranhos A. Posture-induced intraocular pressure changes: considerations regarding body position in glaucoma patients. Surv Ophthalmol 55: 445–453, 2010. doi: 10.1016/j.survophthal.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Anderson AP, Swan JG, Phillips SD, Knaus DA, Kattamis NT, Toutain-Kidd CM, Zegans ME, Fellows AM, Buckey JC. Acute effects of changes to the gravitational vector on the eye. J Appl Physiol (1985) 120: 939–946, 2016. doi: 10.1152/japplphysiol.00730.2015. [DOI] [PubMed] [Google Scholar]
- 31.Marshall-Goebel K, Macias BR, Laurie SS, Lee SMC, Ebert DJ, Kemp DT, Miller AE, Greenwald SH, Martin DS, Young M, Hargens AR, Levine BD, Stenger MB. Mechanical countermeasures to headward fluid shifts. J Appl Physiol (1985) 130: 1766–1777, 2021. doi: 10.1152/japplphysiol.00863.2020.[33856253] [DOI] [PubMed] [Google Scholar]
- 32.Mader TH, Gibson CR, Otto CA, Sargsyan AE, Miller NR, Subramanian PS, Hart SF, Lipsky W, Patel NB, Lee AG. Persistent asymmetric optic disc swelling after long-duration space flight: implications for pathogenesis. J Neuroophthalmol 37: 133–139, 2017. doi: 10.1097/WNO.0000000000000467. [DOI] [PubMed] [Google Scholar]
- 33.Laurie SS, Lee SMC, Macias BR, Patel N, Stern C, Young M, Stenger MB. Optic disc edema and choroidal engorgement in astronauts during spaceflight and individuals exposed to bed rest. JAMA Ophthalmol 138: 165–172, 2020. doi: 10.1001/jamaophthalmol.2019.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agrawal M, Tripathy K. Choroidal folds (Online). In: StatPearls. Treasure Island, FL: StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK557772/. [PubMed] [Google Scholar]
- 35.Feola AJ, Nelson ES, Myers J, Ethier CR, Samuels BC. The impact of choroidal swelling on optic nerve head deformation. Invest Ophthalmol Vis Sci 59: 4172–4181, 2018. doi: 10.1167/iovs.18-24463. [DOI] [PubMed] [Google Scholar]
- 36.Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol J 4: 52–59, 2010. doi: 10.2174/1874364101004010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SS, Robinson MR, Weinreb RN. Episcleral venous pressure and the ocular hypotensive effects of topical and intracameral prostaglandin analogs. J Glaucoma 28: 846–857, 2019. doi: 10.1097/IJG.0000000000001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.e-Anatomy: Radiologic Anatomy Atlas of The Human Body (Online). IMAIOS. https://www.imaios.com/en/e-Anatomy. [2020 Jun 26].
- 39.Johnson M, McLaren JW, Overby DR. Unconventional aqueous humor outflow: a review. Exp Eye Res 158: 94–111, 2017. doi: 10.1016/j.exer.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fautsch MP, Johnson DH. Aqueous humor outflow: what do we know? Where will it lead us? Invest Ophthalmol Vis Sci 47: 4181–4187, 2006. doi: 10.1167/iovs.06-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cyron CJ, Humphrey JD. Growth and remodeling of load-bearing biological soft tissues. Meccanica 52: 645–664, 2017. doi: 10.1007/s11012-016-0472-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bill A, Sperber G, Ujiie K. Physiology of the choroidal vascular bed. Int Ophthalmol 6: 101–107, 1983. doi: 10.1007/BF00127638. [DOI] [PubMed] [Google Scholar]
- 43.Agrawal R, Gupta P, Tan K-A, Cheung CMG, Wong T-Y, Cheng C-Y. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci Rep 6: 21090, 2016. doi: 10.1038/srep21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shinojima A, Iwasaki K-I, Aoki K, Ogawa Y, Yanagida R, Yuzawa M. Subfoveal choroidal thickness and foveal retinal thickness during head-down tilt. Aviat Space Environ Med 83: 388–393, 2012. doi: 10.3357/asem.3191.2012. [DOI] [PubMed] [Google Scholar]
- 45.Laurie SS, Vizzeri G, Taibbi G, Ferguson CR, Hu X, Lee SMC, Ploutz-Snyder R, Smith SM, Zwart SR, Stenger MB. Effects of short-term mild hypercapnia during head-down tilt on intracranial pressure and ocular structures in healthy human subjects. Physiol Rep 5: e13302, 2017. doi: 10.14814/phy2.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balasubramanian S, Tepelus T, Stenger MB, Lee SMC, Laurie SS, Liu JHK, Feiveson AH, Sadda SR, Huang AS, Macias BR. Thigh cuffs as a countermeasure for ocular changes in simulated weightlessness. Ophthalmology 125: 459–460, 2018. doi: 10.1016/j.ophtha.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 47.Campbell MR, Charles JB. Historical review of lower body negative pressure research in space medicine. Aerosp Med Hum Perform 86: 633–640, 2015. doi: 10.3357/AMHP.4246.2015. [DOI] [PubMed] [Google Scholar]
- 48.Barkana Y, Gutfreund S. Measurement of the difference in intraocular pressure between the sitting and lying body positions in healthy subjects: direct comparison of the Icare Pro with the Goldmann applanation tonometer, Pneumatonometer and Tonopen XL. Clin Exp Ophthalmol 42: 608–614, 2014. doi: 10.1111/ceo.12272. [DOI] [PubMed] [Google Scholar]