Abstract
Context
Various herbal medicines are thought to be useful in the management of cardiometabolic disease and its risk factors. Ganoderma lucidum (Curtis) P. Karst. (Ganodermataceae), also known as Lingzhi, has received considerable attention for various indications, including some related to the prevention and treatment of cardiovascular and metabolic disease by ameliorating major cardiovascular risk factors.
Objective
This review focuses on the major studies of the whole plant, plant extract, and specific active compounds isolated from G. lucidum in relation to the main risk factors for cardiometabolic disease.
Methods
References from major databases including PubMed, Web of Science, and Google Scholar were compiled. The search terms used were Ganoderma lucidum, Lingzhi, Reishi, cardiovascular, hypoglycaemic, diabetes, dyslipidaemia, antihypertensive, and anti-inflammatory.
Results
A number of in vitro studies and in vivo animal models have found that G. lucidum possesses antioxidative, antihypertensive, hypoglycaemic, lipid-lowering, and anti-inflammatory properties, but the health benefits in clinical trials are inconsistent. Among these potential health benefits, the most compelling evidence thus far is its hypoglycaemic effects in patients with type 2 diabetes or hyperglycaemia.
Conclusions
The inconsistent evidence about the potential health benefits of G. lucidum is possibly because of the use of different Ganoderma formulations and different study populations. Further large controlled clinical studies are therefore needed to clarify the potential benefits of G. lucidum preparations standardised by known active components in the prevention and treatment of cardiometabolic disease.
Keywords: Antihypertensive, antioxidant, dyslipidaemia, hypoglycaemic, Lingzhi, Reishi
Introduction
Cardiovascular disease (CVD) is highly prevalent, with ischaemic heart disease and stroke being the two leading causes of mortality throughout the world (World Health Organization 2021). Metabolic syndrome is characterised by a cluster of conditions including insulin resistance, central obesity, hypertension, dyslipidaemia, and low-grade chronic inflammation (Eckel et al. 2005). Several drug treatments for CVD have been derived from plant sources, such as digoxin and reserpine. Herbal medicines are now becoming more popular, representing a potentially cost-effective class of substances for combating CVD if safe and effective therapies can be identified. The common herbal medicines used in the West include Asian ginseng, astragalus, flaxseed oil, garlic, ginkgo, grape seeds, green tea, hawthorn, milk thistle, and soy (Liperoti et al. 2017). Herbal formulae are widely used in the clinic in China for hypertension, dyslipidaemia, coronary heart disease, and heart failure (Liu and Huang 2016).
Ganoderma (Ganodermataceae) is a kind of woody mushroom that can be found all over the world. Individual members of the species are identified according to different characteristics, such as shape and colour (red, black, blue/green, white, yellow, and purple) of the fruiting bodies, host specificity, and geographical origin (Upton 2000; Wachtel-Galor et al. 2011). Ganoderma lucidum (Curtis) P. Karst. (Curtis 1781), known as Lingzhi in China and Reishi in Japan, has been used in traditional Chinese medicine (TCM) for over 2000 years for a broad range of indications including improving general health, well-being, and longevity (Bishop et al. 2015; Klupp et al. 2015).
A variety of commercial products from G. lucidum, such as powders, dietary supplements, and tea (Wachtel-Galor et al. 2011), are available. They have been shown to possess a range of activities against CVD, including effects on lipids, blood pressure, obesity, diabetes, and antioxidant and radical scavenging properties (Liu and Tie 2019; Meng and Yang 2019; Winska et al. 2019). However, scientific evidence supporting the beneficial medical properties of G. lucidum is still inconclusive (Hapuarachchi et al. 2016). Many of the commercial products from G. lucidum may not have undergone effective standardisation, so it is difficult to compare results from different studies with different products. Many different herbal supplements or nutraceutical commercial products bearing the names Lingzhi, Reishi, or Ganoderma, etc., contain extracts from various parts of G. lucidum, often in combination with other herbal components. Ganopoly™ (Encore Health), which is a product containing water-soluble G. lucidum polysaccharides, has been used in some animal and clinical studies.
Methods
In this review, the major studies of the whole plant, plant extract, and specific active compounds isolated from G. lucidum in relation to the main risk factors for CVD with particular emphasis on the more recent studies, are summarised. Electronic literature searches were performed using PubMed, Web of Science, and Google Scholar (published from 1961 to 2021). The search terms used were Ganoderma lucidum, Lingzhi, Reishi, cardiovascular, hypoglycaemic, diabetes, dyslipidaemia, antihypertensive, and anti-inflammatory. A total of 4224 articles were identified. The bibliographies of all relevant articles thus located were also scanned for further relevant references. S.W.C and B.T. extracted all articles independently based on the relevance, quality, and strength of the studies; only a shortlist of 115 studies or representative findings are discussed below.
Active constituents of G. lucidum
G. lucidum is thought to have numerous different biologically active constituents, the main ones being various triterpenes, polysaccharides, and proteins (Ahmad 2018; Ahmad et al. 2013). The pharmacologically active compounds are present in different amounts in various parts of the mushroom such as the fruiting bodies, mycelium and spores.
Triterpenes
Terpenes are a large and diverse group of naturally occurring compounds derived from the branched C5 carbon skeleton of isoprene. Triterpenes are a subclass of terpenes and are derived from squalene, a C30 hydrocarbon (Abdullah et al. 2012). They can be classified based on the number of cyclic structures making up the compounds. Up to now, more than 150 triterpenes have been identified from the spores, fruiting bodies, and mycelia of G. lucidum (Xia et al. 2014; Baby et al. 2015). The methods of extraction of triterpenes usually involve methanol, ethanol, chloroform, ether, acetone, or a mixture of these solvents. The extracts can be further purified by various separation methods such as normal and reverse-phase high-performance liquid chromatography (HPLC) (Chen et al. 1999). The majority of triterpenes identified are ganoderic acids and lucidenic acids; other important triterpenes include ganodermic acids, ganoderals, and ganoderiols (Wachtel-Galor et al. 2011). The strong bitterness of G. lucidum originates from the triterpenoid compounds and the bitterness depends on the strain, cultivation conditions and manufacturing processes (Seo et al. 2009). Triterpenoids have been reported to exhibit various biological activities including anti-hypertensive, lipid-lowering, anti-acetylcholinesterase, antioxidant, and anticancer activities, etc. (Abdullah et al. 2012; Chen et al. 2017).
Polysaccharides and peptidoglycans
G. lucidum polysaccharides are macromolecules with a molecular mass of above 500 kDa. Many different polysaccharides, including (1→3), (1→6)-α/β-glucans, α-d-glucans, α-d-mannans, and polysaccharide-protein complexes, have been identified from the spores, fruiting bodies and mycelia of G. lucidum. These compounds are reported to have immunomodulatory and anticancer activities (Xu et al. 2011; Kao et al. 2013). Glucose, together with xylose, mannose, galactose, and fucose in different conformations, forms the major component of the polysaccharide molecules. Polysaccharides are the major component by weight among all constituents in the spores. Several of the mushroom polysaccharide compounds have proceeded through Phase I, II, and III clinical trials and have been used in some Asian countries to treat various cancers and other diseases (Wasser 2010). The contents of polysaccharides differ among commercial Lingzhi products (Wachtel-Galor et al. 2011). A polysaccharide-based product extracted from the spores of G. lucidum originally named ‘Ji 731 Injection’ was used since 1973 in China for treating myopathy (Zeng et al. 2018). The drug was renamed ‘Ji Sheng Injection’ in 1985 and subsequently ‘Polysaccharidum of G. lucidum Karst Injection’ (Lin Bao Duo Tang Zhu She Ye) and is still used for intramuscular injection for various types of immune-mediated muscle diseases. Various bioactive peptidoglycans possessing antiviral (Li et al. 2005) and immunomodulating activities (Zhang et al. 2019), such as ganoderans A, B, and C, have also been isolated from G. lucidum.
Bioactive proteins
Several bioactive proteins from G. lucidum have been reported. One of these is a polypeptide called Lingzhi-8 (LZ-8) which consists of 110 amino acids with a molecular mass of 12 kDa. It has an immunoglobulin-like structure and was the first immunomodulatory protein isolated from the mushroom in 1989 (Hsu and Cheng 2018). Another protein from the fruiting bodies of G. lucidum is ganodermin, which has a molecular mass of 15 kDa and has antifungal activity.
Health benefits of G. lucidum
Antioxidant effects
Free radicals are unstable and highly reactive chemical entities which contain one or more unpaired electrons and can be uncharged or charged. Free radicals are beneficial to the cell signalling and immune system, as well as maintenance of normal body functioning. However, excessive formation and/or insufficient removal of reactive oxygen species (ROS) and reactive nitrogen species (RNS), known as ‘oxidative stress’, may modulate the blood vessel wall, creating an environment that facilitates the progression of atherosclerosis, and leading to various illnesses, such as heart disease, diabetes and cancer (Johansen et al. 2005; Ullah et al. 2016).
In vitro studies demonstrated that several constituents of G. lucidum, in particular triterpenoids and polysaccharides, exhibit antioxidant activity, reducing power, scavenging and chelating abilities (Mau et al. 2002; Saltarelli et al. 2009; Wu and Wang 2009; Liu et al. 2010; Sarmadi and Ismail 2010; Kozarski et al. 2011; Ferreira et al. 2015; Krishna et al. 2016). In contrast, polysaccharide extracts of G. lucidum have superoxide and hydroxyl radical scavenging activities but do not have antioxidative activity as measured by detecting malondialdehyde (MDA) contents of liver microsomes (Liu et al. 1997). It has been demonstrated that the phenolic compounds from the fresh fruiting bodies of G. lucidum exhibit strong 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity but low superoxide dismutase (SOD) activity. The study also showed that DPPH radical scavenging activity and SOD activity were positively correlated with phenolic compounds including caffeic acid, catechin, ferulic acid, gallic acid, myricetin, naringin, pyrogallol, protocatechuic acid, homogentisic acid, and quercetin, as well as total phenolic compounds (Kim et al. 2008). A study comparing the antioxidant activities of four of the most widely known mushrooms, including G. lucidum, demonstrated that polysaccharide extracts exhibited a strong correlation between the reducing power and the total amount of phenols and α-glucans, while a correlation between the reducing power and the amount of total polysaccharides and proteins was not found (Kozarski et al. 2012).
In vivo studies have shown that G. lucidum increases the activity of the antioxidant enzymes SOD and catalase (CAT), which are involved in removing harmful ROS (Cherian et al. 2009; Yurkiv et al. 2015; Vitak et al. 2017; Rahman et al. 2018). In an ischaemia and reperfusion isolated perfused rat heart model, administration of G. lucidum extract (400 mg/kg for 15 days) exhibited antioxidant properties and the author concluded that the cardioprotective properties of G. lucidum extract are related to its antioxidant effects (Lasukova et al. 2015). A study in rats showed that G. lucidum ethanol extract (250 mg/kg body weight) ameliorated the cardiotoxicity of adriamycin by reducing the increase in lipid peroxidation and reversing the decrease in the antioxidant enzymes, glutathione peroxidase (GPx), glutathione-S-transferase (GST), SOD and CAT in the heart tissue (Rajasekaran and Kalaimagal 2012). The cardioprotective effect of G. lucidum may be attributed to the antioxidant chemicals triterpenes and polysaccharides (Wachtel-Galor et al. 2004b). In a carotid-artery-ligation mouse model, daily oral G. lucidum (300 mg/kg/day) prevented neointimal thickening 2 weeks after ligation. Furthermore, subcutaneous injections of ganoderma triterpenoid (GT) crude extract (300 mg/kg/day) abolished ligation-induced neointima formation. The authors concluded that GTs prevent atherogenesis by eliminating disturbed flow-induced oxidative stress through inhibiting the induction of a series of atherogenic factors, as well as inflammation (Hsu et al. 2018).
A short-term supplementation study over 10 days in healthy subjects showed an improvement in antioxidant status (Wachtel-Galor et al. 2004a), but a longer double-blind, placebo-controlled, cross-over intervention study over 4 weeks with a commercially available encapsulated Lingzhi preparation (1.44 g Lingzhi/day; equivalent to 13.2 g fresh mushroom/day) showed no significant effects in a range of biomarkers for antioxidant status, cardiovascular risk, DNA damage, immune status, and inflammation (Wachtel-Galor et al. 2004b). A placebo-controlled cross-over study in 42 healthy subjects examined the antioxidation and hepatoprotective efficacy of triterpenoids and polysaccharide-enriched G. lucidum, which was taken as a 225 mg capsule containing 7% triterpenoid-ganoderic acid (A, B, C, C5, C6, D, E and G), 6% polysaccharide peptides with a few essential amino acids and trace elements, once daily for 6 consecutive months (Chiu et al. 2017). The treatment showed an improvement in total antioxidant capacity, total thiols and glutathione content in plasma, significantly enhanced activities of antioxidant enzymes (SOD, CAT, GPx and glucose-6-phosphate dehydrogenase), and reduced the levels of thiobarbituric acid reactive substances, 8-hydroxy-deoxy-guanosine and hepatic marker enzymes, glutamic-oxaloacetic transaminase and glutamic-pyruvic transaminase. Mild fatty liver detected by abdominal ultrasonic examination was reversed to normal with G. lucidum treatment.
Hypoglycaemic activity
Hyperglycaemia may increase the susceptibility to lipid peroxidation and modulate glucose metabolism in the body, which ultimately contributes to the increased incidence of atherosclerosis or further accelerates its progression (Giugliano et al. 1996; Poznyak et al. 2020). Insulin treatment is essential for people with type 1 diabetes. In type 2 diabetes mellitus (T2DM), lifestyle modification is recommended. If lifestyle modification is not sufficient in achieving glycemic control, patients should be treated initially with metformin (American Diabetes Association 2020). Metformin belongs to the biguanide class of drugs, which originate from the plant goat’s rue or French lilac (Galega officinalis, Linnaeus, [Fabaceae]) (Witters 2001). Recently, the glucagon-like peptide 1 (GLP-1) receptor agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors, which were developed from phlorizin, a natural compound isolated from the bark of apple roots (Tomlinson et al. 2017), have been considered suitable for first-line treatment in some patients with T2DM who have concomitant cardiac or renal disease, in order to improve cardiovascular outcome benefits (Davies et al. 2018).
The hypoglycaemic effects of various extracts from G. lucidum have been studied in different animal models of diabetes and in in vitro experiments to identify mechanisms (Ma et al. 2015; Wang et al. 2016; Winska et al. 2019). The main in vitro, animal and clinical studies investigating the hypoglycaemic effects of G. lucidum are summarised in Tables 1–3, respectively.
Table 1.
In vitro studies on the hypoglycaemic effects of G. lucidum.
| References | Model | Interventions | Findings |
|---|---|---|---|
| Zhang et al. 2003 | Alloxan-induced pancreatic islet damage | Gl-PS polysaccharides from the fruiting body of G. lucidum | Gl-PS showed a protective effect |
| Fatmawati et al. 2009 | Human aldose reductase activity | Methanol extracts of 17 medicinal and edible mushrooms | G. lucidum showed the highest aldose reductase inhibitory activity |
| Fatmawati et al. 2010 | Human aldose reductase activity | Ganoderic acid Df isolated from the fruiting body of G. lucidum | Ganoderic acid Df showed potent human aldose reductase inhibitory activity |
| Fatmawati et al. 2011a | Human α-glucosidase activity | Chloroform extract of the fruiting body of G. lucidum | Ganoderol B identified as an active α-glucosidase inhibitor |
| Pan et al. 2015 | PTP1B activity | FYGL proteoglycan isolated from G. lucidum | Competitive inhibitor of PTP1B |
| Yang et al. 2018a | Liver tissues of ob/ob mice and HepG2 cells | FYGL proteoglycan isolated from G. lucidum | Inhibited PTP1B overexpression, improved IRS1 phosphorylation, activated PI3K/Akt cascades, increased phosphorylation of GSK3β, enhanced insulin-stimulated glycogen synthesis |
| Yang et al. 2018b | Rat myoblast L6 cells | FYGL proteoglycan isolated from G. lucidum | Increased insulin-stimulated glucose uptake, inhibited PTP1B expression, increased IRS1 phosphorylation, activated PI3K/Akt, increased phosphorylation of AMPK and up-regulated expression of GLUT4 |
Akt: protein kinase B; AMPK: adenosine monophosphate-activated protein kinase; FYGL: Fudan-Yueyang Ganoderma lucidum; GLUT4: glucose transporter type 4; GSK3β: glycogen synthase kinase-3β; IRS1: insulin receptor substrate 1; PI3K: phosphatidylinositol-3 kinase; PTP1B: protein tyrosine phosphatase 1B.
Table 2.
Animal studies on the hypoglycaemic effects of G. lucidum.
| References | Animal model | Interventions | Findings |
|---|---|---|---|
| Hikino et al. 1985 | Normal and alloxan-induced hyperglycaemic mice | Water extracts (104 mg/kg crude drug equivalent, i.p.) of the fruiting bodies of G. lucidum for 7 or 27 h | Reduced plasma glucose and 2 glycans, ganoderans A and B, with hypoglycaemic action isolated |
| Hikino et al. 1989 | Normal and glucose-loaded mice | Ganoderan B | Increased insulin and altered enzyme activities |
| Kino et al. 1990 | Autoimmune diabetes model in non-obese mice | Ling Zhi-8 immunomodulatory protein (10.3 − 12.6 mg/kg twice weekly) from 4 weeks of age, followed up to 42 weeks of age | Prevented development of autoimmune diabetes by immunosuppressive mechanism |
| Zhang et al. 2003 | Alloxan-induced diabetic mice | Pre-treatment with intragastric Gl-PS (50 − 200 mg/kg) for 10 days | Gl-PS partly protected beta cells from necrosis |
| Zhang & Lin 2004 | Normal fasted mice | Gl-PS (25 − 100 mg/kg) given by single intraperitoneal injections | Reduced serum glucose and increased insulin levels |
| He et al. 2006 | Streptozotocin-induced diabetic mice | Gl-PS (125 and 250 mg/kg) given for 8 weeks | Reduced serum glucose, increased insulin levels and delayed progression of diabetic renal disease |
| Seto et al. 2009 | Genetically obese/diabetic (+db/+db) and lean (+db/+m) mice | Water extract of G. lucidum (0.003, 0.03 and 0.3 g/kg) for 4 weeks, oral gavage | Extract reduced serum glucose and liver PEPCK expression |
| Li et al. 2011 | Streptozotocin-induced diabetic mice | Gl-PS at low (50 mg/kg) and high (150 mg/kg) dose for 28 days | Reduced serum glucose, increased insulin levels and improvements in blood lipids |
| Teng et al. 2011 | Streptozotocin-induced diabetic mice | FYGL proteoglycan from G. lucidum (50 and 150 mg/kg, oral dose) for up to 4 weeks | Reduced plasma glucose with effect comparable with metformin |
| Teng et al. 2012 | Streptozotocin-induced diabetic rats | FYGL proteoglycan from G. lucidum (40 and 120 mg/kg, oral dose) for 30 days | Reduced plasma glucose, increased insulin and inhibited PTP1B |
| Zheng et al. 2012 | Streptozotocin-induced diabetic rats | Low-molecular-weight Gl-PS (200 mg/kg) orally for 8 weeks | Reduced serum glucose appeared related to protection of pancreatic β-cells |
| Xiao et al. 2012 | Streptozotocin-induced diabetic mice | Polysaccharides from G. lucidum (50 or 100 mg/kg/day) given for 7 days | Reduced fasting serum glucose and insulin levels |
| Pan et al. 2013 | Obese/diabetic (+db/+db) mice | FYGL proteoglycan from G. lucidum (75, 250, or 450 mg/kg) for 8 weeks | Reduced HbA1c, increased insulin and C-peptide levels, increased glucokinase and lowered PEPCK activities |
| Sarker 2015 | Rats with alloxan- or corticosteroid-induced diabetes | A petroleum ether extract and a methanol extract of G. lucidum (200, 400, 600 and 800 mg/kg/day) for 7 days | Reduced fasting and postprandial plasma glucose and HbA1c, increased plasma insulin levels and improved lipid profile |
| Xiao et al. 2017 | Streptozotocin-induced diabetic mice | F31 polysaccharide from G. lucidum (50 mg/kg/day) | Decreased fasting serum glucose, fasting serum insulin and liver glucose regulatory enzymes |
| Ratnaningtyas et al. 2018 | Alloxan-induced diabetic rats | Ethanol extract of G. lucidum powdered fruiting bodies (250, 500 and 1000 mg/kg) for 14 days | Dose-dependent reduction in blood glucose, reduction in HbA1c, and increase in insulin |
| Bach et al. 2018 | Streptozotocin-induced diabetic rats | Hydroethanolic extract of G. lucidum (1 mL/kg/day) for 30 days | Reduced plasma glucose and lipid levels |
FYGL: Fudan-Yueyang Ganoderma lucidum; HbA1c: Glycosylated Haemoglobin Level; Gl-PS: Ganoderma lucidum polysaccharides; PEPCK: phosphoenolpyruvate carboxykinase; PTP1B: protein tyrosine phosphatase.
Table 3.
Human studies on the hypoglycaemic effects of G. lucidum.
| References | Subjects | Interventions | Findings |
|---|---|---|---|
| Gao et al. 2004b | 62 patients with T2DM | Multi-centered randomised controlled trial of GanopolyTM 1800 mg 3 times daily versus placebo for 12 weeks | Reduced HbA1c and fasting and postprandial plasma glucose levels with GanopolyTM |
| Wang et al. 2008 | 46 patients with T2DM | Randomised, double-blind, placebo-controlled dry extract of G. lucidum 3000 mg or placebo for 12 weeks | No changes in fasting glucose or HbA1c but the plasma glucose area under the curve during a meal tolerance test was reduced more with G. lucidum extract |
| Chu et al. 2012 | 23 subjects with borderline elevations of blood pressure and/or cholesterol | Randomised, double-blind, cross-over study with a Lingzhi product 1.44 g daily or placebo for 12 weeks | No significant effect on HbA1c, fasting plasma glucose, blood pressure or lipids. Plasma insulin and HOMA-IR reduced with Lingzhi compared to placebo |
| Klupp et al. 2016 | 84 patients with T2DM and metabolic syndrome | Randomised controlled trial of G. lucidum 3 g/day or G. lucidum plus Cordyceps sinensis capsules, versus placebo for 16 weeks | No significant effect on HbA1c, fasting plasma glucose, blood pressure or lipids |
HbA1c: Glycosylated Haemoglobin Level; HOMA-IR: homeostasis model assessment-insulin resistance; T2DM: type 2 diabetes mellitus.
Hypoglycaemic activity of triterpenoids
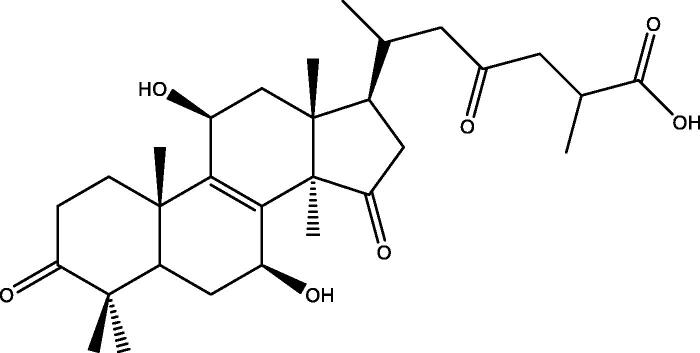
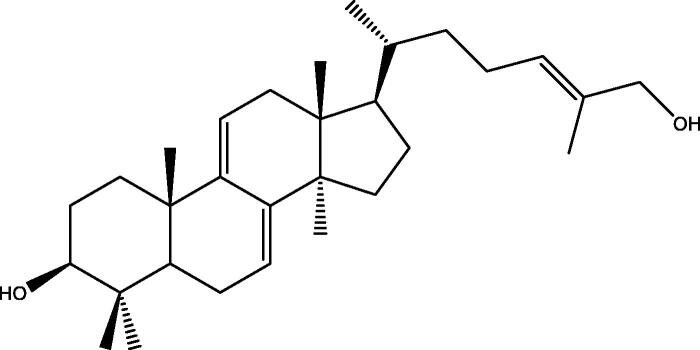
A series of in vitro studies by Fatmawati and colleagues have identified that methanol extract from the fruiting bodies of G. lucidum has a strong inhibitory effect on human aldose reductase activity. Ganoderic acid Df (Figure 1), a lanostane-type triterpenoid, exhibited potent aldose reductase inhibitory activity with an IC50 value of 22.8 µM (Fatmawati et al. 2009, 2010). Fatmawati et al. (2011a) subsequently demonstrated that ganoderol B (Figure 2), which was isolated from a chloroform extract of G. lucidum, was effective in inhibiting α-glucosidase activity with an IC50 value of 119.8 µM and the inhibitory effect was stronger than that of acarbose, which is commonly used as a medication to inhibit α-glucosidase in patients with T2DM. Structure-activity studies were performed to identify the structural requirements of lanostane-type triterpenoids from G. lucidum, which were necessary to increase α-glucosidase inhibitory activity (Fatmawati et al. 2013).
Figure 1.
Chemical structure of ganoderic acid Df. The hydroxyl group at C-11 and the carbonyl group at C-15 along with the hydroxyl group at C-7 are thought to be important for inhibition of aldose reductase (Fatmawati et al. 2010, 2011b).
Figure 2.
Chemical structure of ganoderol B. The hydroxyl group at C-3 and the double-bond in the side chain are thought to be important for α-glucosidase inhibitory activity (Fatmawati et al. 2013).
Hypoglycaemic activity of proteoglycans/peptidoglycans
Inhibition of PTP1B activity has been regarded as a potential therapy for T2DM for many years (Johnson et al. 2002). Fudan-Yueyang-G. lucidum (FYGL), which is a water soluble macromolecular proteoglycan extracted from the fruiting bodies of G. lucidum, inhibits PTP1B activity with an IC50 value of 5.12 ± 0.05 µg/mL (Teng et al. 2011). FYGL enhances glycogen synthesis and inhibits the expression of glycogen synthase kinase-3β (GSK3β) in liver tissues of ob/ob mice and HepG2 cells probably via modulating insulin receptor substrate 1 (IRS1)/phosphatidylinositol-3 kinase (PI3K)/protein kinase B (Akt)/AMP-activated protein kinase (AMPK)/GSK3β cascades (Yang et al. 2018a). In rat myoblast PTP1B-transfected L6 cells, FYGL improves insulin resistance by regulating IRS1-glucose transporter type 4 (GLUT4) cascades in the insulin signalling pathway (Yang et al. 2018b). In streptozotocin-induced T2DM mice, FYGL reduces plasma glucose levels with an effect comparable with metformin and rosiglitazone, via inhibiting the PTP1B expression and activity, and consequently modulating the tyrosine phosphorylation level of the insulin receptor (IR) 13-subunit (Teng et al. 2011, 2012). In addition, FYGL improves the plasma biochemistry indexes associated with T2DM-accompanied metabolic disorders, including free fatty acids, triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) (Teng et al. 2012). Further mechanistic studies in db/db mice found that the hypoglycaemic effect of FYGL is associated with its ability to enhance insulin secretion, decrease hepatic glucose output, and increase adipose and skeletal muscle glucose disposal (Pan et al. 2013, 2014). In normal and alloxan-induced hyperglycaemic mice, water extraction yielded from the fruiting bodies of G. lucidum and the two peptidoglycans, ganoderans A and B, subsequently produced through fractionation have all shown hypoglycaemic activity (Hikino et al. 1985). Administration of ganoderan B increases plasma insulin levels in normal and glucose-loaded mice; it also increases the activities of hepatic glucokinase, phosphofructokinase and glucose-6-phosphate dehydrogenase, decreases hepatic glucose-6-phosphatase (G6Pase) and glycogen synthetase activities and does not affect the activities of hexokinase and glycogen phosphorylase (GP) (Hikino et al. 1989).
Hypoglycaemic activity of Ganoderma polysaccharides
Hypoglycaemic effects of polysaccharides from G. lucidum (Gl-PS) have been demonstrated in several in vitro and in vivo studies. Gl-PS showed a protective effect against alloxan-induced damage to pancreatic islets in vitro. Pre-treatment with intragastric Gl-PS (50-200 mg/kg) for 10 days produced hypoglycaemic effects via its scavenging ability to protect the pancreatic β-cells from alloxan-induced necrosis (Zhang et al. 2003). Gl-PS (25-100 mg/kg) given by single intraperitoneal injections to normal fasted mice reduced serum glucose levels after 3 and 6 h in a dose-dependent manner and increased insulin levels from 1 h after administration via enhancing Ca2+ influx into pancreatic β cells (Zhang and Lin 2004). Furthermore, administration of Gl-PS produced hypoglycaemic effects and an improvement in lipid profile in streptozotocin-induced diabetic mice (He et al. 2006; Li et al. 2011; Zheng et al. 2012). It has been suggested that the hypoglycaemic effect is mainly through preventing apoptosis of pancreatic β-cells and enhancing β-cells regeneration (Zheng et al. 2012), and a modulation of serum insulin and hepatic mRNA levels of several key enzymes involved in gluconeogenesis and/or glycogenolysis, including GP, fructose-1,6-bisphosphatase (FBPase), phosphoenolpyruvate carboxykinase (PEPCK), and G6Pase (Xiao et al. 2012). Xiao et al. (2017) isolated F31, a β-heteropolysaccharide with a weight-average molecular weight of 15.9 kDa, from Gl-PS. The mechanism of action of Gl-PS F31 may be associated with down-regulation of the hepatic glucose regulated enzyme mRNA levels via AMPK activation, improvement of insulin resistance, and reduction of epididymal fat/body weight ratio (Xiao et al. 2017). An integrative analysis of transcriptomics and proteomics data from the liver from F31-treated diabetic db/db mice found that genes in the glycolysis and gluconeogenesis pathways, insulin pathway, and lipid metabolism pathways showed significantly different expression compared to the untreated mice and that microRNAs probably participated in the regulation of the genes involved in glucose metabolism (Xiao et al. 2018).
Hypoglycaemic activity of Ganoderma extracts
Some other studies used extracts of G. lucidum in which the active constituents were not clearly identified. A water-extract of G. lucidum given to lean (+db/+m) and genetically obese/diabetic (+db/+db) mice lowered the serum glucose level in + db/+db mice after one week of treatment and in + db/+m mice after 4 weeks, through the down-regulation of the hepatic PEPCK gene expression (Seto et al. 2009). A study in alloxan-and steroid-induced diabetic rats showed that a petroleum ether extract and a methanol extract of G. lucidum given orally at 200, 400, 600 and 800 mg/kg/day for 7 days reduced plasma glucose levels, increased insulin sensitivity, and decreased lipid levels, and the suspected bioactive chemicals were polysaccharides available in the extracts (Sarker 2015). A hypoglycaemic effect was also observed following administration of an alcoholic extract of G. lucidum (250, 500, and 1000 mg/kg) given for 14 days in alloxan-induced diabetic rats (Ratnaningtyas et al. 2018). Another recent study in streptozotocin-induced diabetic rats showed that a hydroethanolic extract of G. lucidum containing β-glucan, proteins, and phenols, reduced plasma glucose and lipid levels through preservation of pancreatic islets (Bach et al. 2018).
Hypoglycaemic activity of Ganoderma proteins
Ling Zhi-8 (LZ-8), an immunomodulatory protein isolated from the mycelial extract of G. lucidum, prevented the development of autoimmune diabetes by reducing antigen-induced antibody formation in non-obese diabetic mice (Kino et al. 1990). In a model of transplanted allogeneic pancreatic rat islets, LZ-8 delayed the rejection process of allografted islets (van der Hem et al. 1995).
Evidence from clinical studies
Clinical studies of the hypoglycaemic/antidiabetic effects of G. lucidum products are very limited. In a placebo-controlled study in 62 patients with T2DM, administration of Ganopoly™ at 1800 mg three times daily for 12 weeks reduced fasting and postprandial plasma glucose levels, as well as HbA1c (Gao et al. 2004b). Administration of a dry extract of G. lucidum (3 g) in addition to regular oral hypoglycaemic agents for 12 weeks did not affect fasting glucose or HbA1c; however, the plasma glucose area under the curve during a meal tolerance test was reduced more significantly in patients taking G. lucidum (Wang et al. 2008). A randomised, double-blind, placebo-controlled, cross-over study with placebo-controlled run-in and cross-over periods of a Lingzhi product at a dose of 1.44 g daily for 12 weeks was performed in subjects with borderline elevations of blood pressure and/or cholesterol. There were reductions in plasma insulin and homeostasis model assessment-insulin resistance with Lingzhi compared to placebo. The subjects in this study had normal plasma glucose levels and it was speculated that the effects on insulin and insulin resistance would be greater in subjects with impaired glucose tolerance or T2DM (Chu et al. 2012). However, in a more recent study in 84 patients with T2DM and metabolic syndrome, administration of G. lucidum alone or combined with Cordyceps sinensis [now called Ophiocordyceps sinensis (Berk.) Sacc. (Ophiocordycipitaceae)], over 16 weeks, did not show any improvement in hyperglycaemia and cardiovascular risk factors (Klupp et al. 2016). It is noteworthy that different extracts of G. lucidum will have different components, therefore it may not be appropriate to compare the results from different studies.
Effects on dyslipidaemia
Dyslipidaemia which is characterised by decreased levels of HDL-C and accompanied with increased levels of TG, apo B, and small dense LDL particles, is an important modifiable risk factor for the development of atherosclerosis and CVD. Guidelines for the treatment of lipid disorders recommend initiating treatment with the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors or statins (Grundy et al. 2019; Mach et al. 2020). Statins have their origin in products isolated from fungi (Endo 2004).
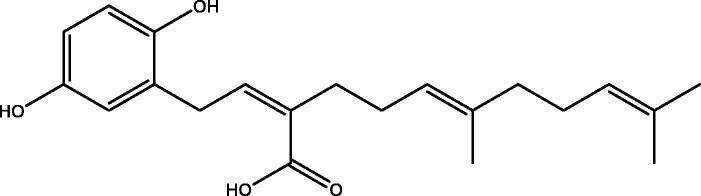
In vitro studies showed that polysaccharides and oxygenated triterpenoids from G. lucidum have a very broad spectrum of biological activities and pharmacological effects. Some types of ganoderic acid might reduce cholesterol by inhibiting HMG-CoA reductase, like the statin drugs (Shiao 2003). Compounds isolated from fruiting bodies of G. lucidum including ganolucidic acid eta, ganoderenic acid K, and the farnesyl hydroquinones (ganomycin J and ganomycin B), showed strong inhibitory activity against HMG-CoA reductase (Figure 3) (Chen et al. 2017).
Figure 3.
Chemical structure of ganomycin B. Ganomycin B showed strong inhibitory activity against HMG-CoA reductase with an IC50 of 14.3 μM (Chen et al. 2017).
The cholesterol-lowering properties of G. lucidum have been demonstrated in a series of in vitro and ex vivo studies, and in hamsters and minipigs (Berger et al. 2004). The organic fractions containing oxygenated lanosterol derivatives inhibited cholesterol synthesis in T9A4 hepatocytes. The investigators found that both 2.5 and 5% dried G. lucidum reduced hepatic microsomal ex-vivo HMG-CoA reductase activity. In hamsters, administration of 5.0% dried G. lucidum decreased TC and HDL-C but not LDL-C, whereas in minipigs, 2.5% dried G. lucidum reduced all these parameters.
The improvements in the lipid profile in some diabetic animal models and in patients with T2DM treated with G. lucidum products may be related to the improvement in glycemic control, rather than a direct effect on lipid metabolism as hyperglycaemia is often associated with elevated TG and reduced HDL-C (Taskinen and Borén 2015). In a randomised, double-blind, cross-over study in 26 patients with borderline elevations of blood pressure and/or cholesterol, administration of Lingzhi (1.44 g extract/d) for 12 weeks produced a non-significant trend for reduction in TG and increase in HDL-C (Chu et al. 2012). Those changes could have been related to improvements in insulin resistance as these lipid abnormalities, hypertension, central obesity and insulin resistance cluster together in the metabolic syndrome.
Antihypertensive effects
The most recent guidelines for the management of hypertension recommend initiating antihypertensive drug therapy in most patients with a combination of two different drugs from the classes of thiazide diuretics, calcium channel blockers, angiotensin converting enzyme (ACE) inhibitors, or angiotensin receptor blocker (ARBs) (Whelton et al. 2018; Williams et al. 2018).
Triterpenes and G. lucidum proteins have been demonstrated to possess potent ACE-inhibitory properties in vitro (Abdullah et al. 2012; Mohamad Ansor et al. 2013). Mohamad Ansor et al. (2013) reported that the protein fractions from the mycelia of G. lucidum contain highly potent anti-ACE proteins with IC50 values below 200 μg/mL. Furthermore, three small peptides with ACE-inhibitory activity, including Gln-Leu-Val-Pro (QLVP), Gln-Asp-Val-Leu (QDVL), and Gln-Leu-Asp-Leu (QLDL), were recently isolated from G. lucidum mycelia (Wu et al. 2019). Notably, QLVP worked in a mixed-type manner against ACE and has an IC50 value of 127.9 µmol/L.
A transverse aortic constriction (TAC) mouse model of pressure overload-induced cardiomyopathy and heart failure revealed that administration of oral Ganoderma spore oil every other day for 14 days normalised ejection fraction, corrected the fractional shortening and reduced left ventricular hypertrophy. The cardioprotective effect is associated with reduced expression of circular RNA circ-Foxo3, which plays a role in the pathogenesis of heart failure (Xie et al. 2016).
An early uncontrolled trial in Japanese showed that supplementation with G. lucidum extract (240 mg daily) for 6 months reduced blood pressure in hypertensive patients but not borderline hypertensive or normotensive patients (Kanmatsuse et al. 1985). In a double-blind, randomised, placebo-controlled study in 160 patients with confirmed coronary heart disease (CHD), treatment with G. lucidum polysaccharides (Ganopoly™) for 12 weeks improved the symptoms of CHD and reduced average blood pressure from 142.5/96.4 mmHg to 135.1/92.8 mmHg, whereas there was no significant blood pressure reduction in the control group (Gao et al. 2004a). Serum TC also decreased significantly with Ganopoly™ therapy, but not in the control group.
Anti-inflammatory effects
Inflammation is a physiological response to harmful stimuli that are physical, chemical, or biological in nature. A number of inflammatory markers, such as high-sensitivity C-reactive protein (hsCRP), interleukin (IL)-6, IL-1, and tumour necrosis factor (TNF)-α, have been shown to be associated with obesity, metabolic syndrome, and an elevated risk of chronic diseases (Pravenec et al. 2011; Dallmeier et al. 2012). Elevated circulating levels of hsCRP and IL-6 predict the development of T2DM through diminishing insulin sensitivity (Guarner & Rubio-Ruiz 2015). Obesity-induced inflammation has been implicated as a risk factor in the pathogenesis of T2DM, insulin resistance, CVD, and metabolic syndrome (Kumar et al. 2019).
There are several in vitro studies showing the anti-inflammatory effect of G. lucidum extracts. The triterpene extract from G. lucidum reduced the secretion of TNF-α and IL-6, and inflammatory mediator nitric oxide (NO) and prostaglandin E(2) (PGE2) from lipopolysaccharide (LPS)-activated murine macrophages via inhibition of nuclear factor-κB (NF-κB) and activator protein 1 (AP-1) signalling (Dudhgaonkar et al. 2009). G. lucidum sterols downregulated the mRNA expressions of NO, TNF-α, IL-1β, and IL-6, and attenuated LPS-induced cell polarisation by modulating mitogen-activated protein kinase (MAPK) and NF-κB pathways (Xu et al. 2021). Furthermore, G. lucidum ethanol extract reduced the excessive production of NO, PGE2, and pro-inflammatory cytokines, IL-1β, and TNF-α via inhibition of the NF-κB and toll-like receptor signalling pathways in LPS-stimulated BV2 microglial cells (Yoon et al. 2013).
In an in vivo study, administration of water extract of G. lucidum (2 g/kg, s.c.) 1 h prior to applying carrageenan reduced both the first and second phases of carrageenan-induced inflammation (Lin et al. 1993). It has been demonstrated that both ethyl acetate and 70% methanol extracts of G. lucidum (500 and 1000 mg/kg) produced anti-inflammatory effects against carrageenan-induced acute and formalin-induced chronic inflammation in mice and the effect was comparable to that of the standard reference drug, diclofenac (10 mg/kg) (Sheena et al. 2003).
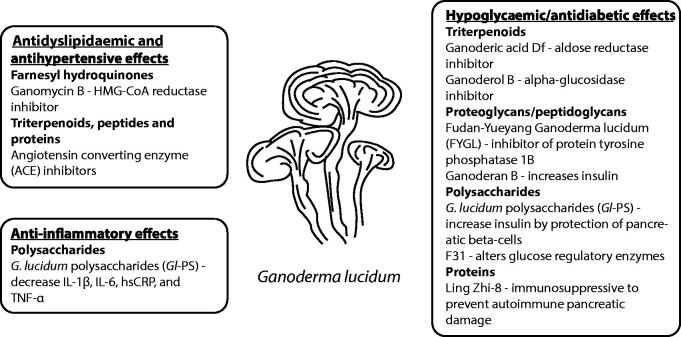
The anti-inflammatory effect of G. lucidum supplementation has been investigated in several small scale trials. In a clinical trial involving 45 ST-elevation myocardial infarction (STEMI) and non-STEMI patients, the polysaccharides of G. lucidum (750 mg/day in 3 divided doses for 90 days) decreased the levels of IL-1 and TNF-α, as well as the MDA levels (Sargowo et al. 2019). In a recent randomised closed-label clinical trial involving 38 patients with atrial fibrillation, consumption of polysaccharides of G. lucidum (PT Sahabat Lingkungan Hidup, Surabaya, Indonesia), 3 times a day for 90 days, reduced significantly the systolic and diastolic blood pressure, heart rate, LDL-C, IL-1β, IL-6, hsCRP, and TNF-α, compared to placebo-treated patients (Rizal et al. 2020). These data suggest that G. lucidum polysaccharide peptides may have beneficial effects against factors involved in the pathogenesis of atherosclerosis and atrial fibrillation. The main active compounds which have been shown to influence some of the major risk factors for CVD are shown in Figure 4.
Figure 4.
Potential mechanisms for cardiovascular disease prevention and therapy with constituents of Ganoderma lucidum.
Adverse effects
G. lucidum is generally regarded as safe and is listed in the safest drug class (Class 1 Drug) in the American Herbal Products Association Botanical Safety Handbook with no known herb-drug interactions (McGuffin et al. 1997). Recent human clinical trials with G. lucidum have included laboratory safety parameters such as hepatic, renal, and hematological biomarkers and no pathological abnormality or serious adverse event has been reported (Klupp et al. 2015, 2016). Mild symptomatic adverse effects such as dry mouth, sore throat, and nausea have been reported occasionally. A case of hepatotoxicity related to G. lucidum mushroom powder was reported from Hong Kong in 2004, but this was thought to be due to the excipient ingredients (Yuen et al. 2004). Another case of fatal fulminant hepatitis in a patient taking Lingzhi in powder form was reported from Thailand in 2007 (Wanmuang et al. 2007). Such cases do need careful assessment before attributing the effects to G. lucidum components, but they also illustrate the need to be vigilant with herbal treatments.
It is important to be cautious when taking herbal supplements in combination with conventional medications, particularly those that are very sensitive to herb or drug interactions such as warfarin. Most herbal supplements are contraindicated in patients taking warfarin. G. lucidum may have a mild antithrombotic effect itself in high doses and this could increase the effect of other anticoagulant or antiplatelet medications, including aspirin (Kumaran et al. 2011), resulting in an increased risk of bruising or bleeding. In patients taking other prescription medications, it is generally better to separate the intake of those medications and G. lucidum products by at least two hours in case there is any interference with drug absorption.
Conclusions
G. lucidum has a reputation for many beneficial effects from a historical perspective and its safety has largely been established by empirical observation. The beneficial effects are supported by several in vitro studies and studies in animals, but clinical trials in humans in the cardiovascular field are limited. Secondly, the use of different products in the clinical trials makes it difficult to compare the results. In the prevention and treatment of CVD, the hypoglycaemic effects of G. lucidum are the best established properties from the in vitro and animal studies, but these benefits have not been confirmed in recent clinical trials. Components from G. lucidum herbal materials have been identified with lipid-lowering and antihypertensive effects and compounds with specific mechanisms of action have been isolated. Nevertheless, the content of these components and their bioavailability in different G. lucidum formulations are uncertain and clinical trials in these areas have been inadequate. Further studies are needed to isolate all the active ingredients with known biological activity, and to characterise their bioavailability for specific indications before clinical trials pertaining to the use of G. lucidum products for relevant clinical benefits are conducted. Clinical trials should be performed in subjects with abnormal baseline levels of cardiovascular risk factors that are being targeted so that improvements can be seen more readily.
Acknowledgments
We are grateful for the support from the Faculty of Medicine, Macau University of Science and Technology.
Funding Statement
This work was funded by the Institutional Development Grant of Caritas Institute of Higher Education, grant number IDG200114.
Author contributions
Conceptualisation - S.W.C. and B.T.; Writing - Original draft preparation – S.W.C., B.T. and P.C.; Writing - Review and editing S.W.C., B.T., P.C. and C.W.K.L.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abdullah N, Ismail SM, Aminudin N, Shuib AS, Lau BF.. 2012. Evaluation of selected culinary-medicinal mushrooms for antioxidant and ACE inhibitory activities. Evid Based Complement Alternat Med. 2012:464238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad MF. 2018. Ganoderma lucidum: persuasive biologically active constituents and their health endorsement. Biomed Pharmacother. 107:507–519. [DOI] [PubMed] [Google Scholar]
- Ahmad MF, Ahmad FA, Azad ZAA, Alam MI, Ansari JA, Panda BP.. 2013. Edible mushrooms as health promoting agent. Adv Sci Focus. 1(3):189–196. [Google Scholar]
- American Diabetes Association . 2020. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 43(Supplement 1):S98–S110. [DOI] [PubMed] [Google Scholar]
- Baby S, Johnson AJ, Govindan B.. 2015. Secondary metabolites from Ganoderma. Phytochemistry. 114:66–101. [DOI] [PubMed] [Google Scholar]
- Bach EE, Hi EMB, Martins AMC, Nascimento PAM, Wadt NSY.. 2018. Hypoglicemic and hypolipedimic effects of Ganoderma lucidum in streptozotocin-induced diabetic rats. Medicines (Basel). 5(3):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A, Rein D, Kratky E, Monnard I, Hajjaj H, Meirim I, Piguet-Welsch C, Hauser J, Mace K, Niederberger P.. 2004. Cholesterol-lowering properties of Ganoderma lucidum in vitro, ex vivo, and in hamsters and minipigs. Lipids Health Dis. 3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop KS, Kao CH, Xu Y, Glucina MP, Paterson RR, Ferguson LR.. 2015. From 2000 years of Ganoderma lucidum to recent developments in nutraceuticals. Phytochemistry. 114:56–65. [DOI] [PubMed] [Google Scholar]
- Chen DH, Shiou WY, Wang KC, Huang SY, Shie YT, Tsai CM, Shie JF, Chen KD.. 1999. Chemotaxonomy of triterpenoid pattern of HPLC of Ganoderma lucidum and Ganoderma tsugae. Jnl Chinese Chemical Soc. 46(1):47–51. [Google Scholar]
- Chen B, Tian J, Zhang J, Wang K, Liu L, Yang B, Bao L, Liu H.. 2017. Triterpenes and meroterpenes from Ganoderma lucidum with inhibitory activity against HMGs reductase, aldose reductase and α-glucosidase. Fitoterapia. 120:6–16. [DOI] [PubMed] [Google Scholar]
- Cherian E, Patani G, Sudheesh NP, Janardhanan KK.. 2009. Free-radical scavenging and mitochondrial antioxidant activities of Reishi-Ganoderma lucidum (Curt: Fr) P. Karst and Arogyapacha-Trichopus zeylanicus Gaertn extracts. J Basic Clin Physiol Pharmacol. 20(4):289–308. [DOI] [PubMed] [Google Scholar]
- Chiu HF, Fu HY, Lu YY, Han YC, Shen YC, Venkatakrishnan K, Golovinskaia O, Wang CK.. 2017. Triterpenoids and polysaccharide peptides-enriched Ganoderma lucidum: a randomized, double-blind placebo-controlled crossover study of its antioxidation and hepatoprotective efficacy in healthy volunteers. Pharm Biol. 55(1):1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu TT, Benzie IF, Lam CW, Fok BS, Lee KK, Tomlinson B.. 2012. Study of potential cardioprotective effects of Ganoderma lucidum (Lingzhi): results of a controlled human intervention trial. Br J Nutr. 107(7):1017–1027. [DOI] [PubMed] [Google Scholar]
- Curtis W. 1781. Flora Londinensis, or, Plates and descriptions of such plants as grow wild in the environs of London. London: for the author and B. White, [1775]-1777-1798. [Google Scholar]
- Dallmeier D, Larson MG, Vasan RS, Keaney JF, Fontes JD, Meigs JB, Fox CS, Benjamin EJ.. 2012. Metabolic syndrome and inflammatory biomarkers: a community-based cross-sectional study at the Framingham Heart Study. Diabetol Metab Syndr. 4(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB.. 2018. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 41(12):2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudhgaonkar S, Thyagarajan A, Sliva D.. 2009. Suppression of the inflammatory response by triterpenes isolated from the mushroom Ganoderma lucidum. Int Immunopharmacol. 9(11):1272–1280. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ.. 2005. The metabolic syndrome. Lancet. 365(9468):1415–1428. [DOI] [PubMed] [Google Scholar]
- Endo A. 2004. The origin of the statins. 2004. Atheroscler Suppl. 5(3):125–130. [DOI] [PubMed] [Google Scholar]
- Fatmawati S, Kondo R, Shimizu K.. 2013. Structure-activity relationships of lanostane-type triterpenoids from Ganoderma Lingzhi as α-glucosidase inhibitors. Bioorg Med Chem Lett. 23(21):5900–5903. [DOI] [PubMed] [Google Scholar]
- Fatmawati S, Kurashiki K, Takeno S, Kim Y, Shimizu K, Sato M, Imaizumi K, Takahashi K, Kamiya S, Kaneko S, et al. . 2009. The inhibitory effect on aldose reductase by an extract of Ganoderma lucidum. Phytother Res. 23(1):28–32. [DOI] [PubMed] [Google Scholar]
- Fatmawati S, Shimizu K, Kondo R.. 2010. Ganoderic acid Df, a new triterpenoid with aldose reductase inhibitory activity from the fruiting body of Ganoderma lucidum. Fitoterapia. 81(8):1033–1036. [DOI] [PubMed] [Google Scholar]
- Fatmawati S, Shimizu K, Kondo R.. 2011a. Ganoderol B: a potent alpha-glucosidase inhibitor isolated from the fruiting body of Ganoderma lucidum. Phytomedicine. 18(12):1053–1055. [DOI] [PubMed] [Google Scholar]
- Fatmawati S, Shimizu K, Kondo R.. 2011b. Structure-activity relationships of ganoderma acids from Ganoderma lucidum as aldose reductase inhibitors. Bioorg Med Chem Lett. 21(24):7295–7297. [DOI] [PubMed] [Google Scholar]
- Ferreira IC, Heleno SA, Reis FS, Stojkovic D, Queiroz MJ, Vasconcelos MH, Sokovic M.. 2015. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry. 114:38–55. [DOI] [PubMed] [Google Scholar]
- Gao Y, Chen G, Dai X, Ye J, Zhou S.. 2004a. A phase I/II study of Ling Zhi mushroom Ganoderma lucidum (W.Curt.:Fr.) Lloyd (Aphyllophoromycetideae) extract in patients with coronary heart disease. Int J Med Mushr. 6(4):327–334. [Google Scholar]
- Gao Y, Lan J, Dai X, Ye J, Zhou S.. 2004b. A phase I/II study of Ling Zhi mushroom Ganoderma lucidum (W.Curt.:Fr.) Lloyd (Aphyllophoromycetideae) extract in patients with type II diabetes mellitus. Int J Med Mushrooms. 6(1):33–39. [Google Scholar]
- Giugliano D, Ceriello A, Paolisso G.. 1996. Oxidative stress and diabetic vascular complications. Diabetes Care. 19(3):257–267. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. . 2019. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the manage ment of blood cholesterol: Executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 139(25):e1046–e1081. [DOI] [PubMed] [Google Scholar]
- Guarner V, Rubio-Ruiz ME. 2015. Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. In: Yashin AI, Jazwinski SM, editors. Aging and health - a systems biology perspective. Basel: Karger; p. 99–106. [DOI] [PubMed] [Google Scholar]
- Hapuarachchi KK, Wen TC, Jeewon R, Wu XL, Kang JC.. 2016. Mycosphere essays 15. Ganoderma lucidum - are the beneficial medical properties substantiated? Mycosphere. 7(6):687–715. [Google Scholar]
- He CY, Li WD, Guo SX, Lin SQ, Lin ZB.. 2006. Effect of polysaccharides from Ganoderma lucidum on streptozotocin-induced diabetic nephropathy in mice. J Asian Nat Prod Res. 8(8):705–711. [DOI] [PubMed] [Google Scholar]
- Hikino H, Ishiyama M, Suzuki Y, Konno C.. 1989. Mechanisms of hypoglycemic activity of ganoderan B: a glycan of Ganoderma lucidum fruit bodies. Planta Med. 55(5):423–428. [DOI] [PubMed] [Google Scholar]
- Hikino H, Konno C, Mirin Y, Hayashi T.. 1985. Isolation and hypoglycemic activity of ganoderans A and B, glycans of Ganoderma lucidum fruit Bodies1. Planta Med. 51(4):339–340. [DOI] [PubMed] [Google Scholar]
- Hsu KD, Cheng KC.. 2018. From nutraceutical to clinical trial: frontiers in Ganoderma development. Appl Microbiol Biotechnol. 102(21):9037–9051. [DOI] [PubMed] [Google Scholar]
- Hsu PL, Lin YC, Ni H, Mo FE.. 2018. Ganoderma triterpenoids exert antiatherogenic effects in mice by alleviating disturbed flow-induced oxidative stress and inflammation. Oxid Med Cell Longev. 2018:3491703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JS, Harris AK, Rychly DJ, Ergul A.. 2005. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TO, Ermolieff J, Jirousek MR.. 2002. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat Rev Drug Discov. 1(9):696–709. [DOI] [PubMed] [Google Scholar]
- Kanmatsuse K, Kajiwara N, Hayashi K, Shimogaichi S, Fukinbara I, Ishikawa H, Tamura T.. 1985. [Studies on Ganoderma lucidum. I. Efficacy against hypertension and side effects]. Yakugaku Zasshi. 105(10):942–947. ]. [DOI] [PubMed] [Google Scholar]
- Kao CHJ, Jesuthasan AC, Bishop KS, Glucina MP, Ferguson LR.. 2013. Anticancer activities of Ganoderma lucidum: active ingredients and pathways. FFHD. 3(2):48–65. [Google Scholar]
- Kim MY, Seguin P, Ahn JK, Kim JJ, Chun SC, Kim EH, Seo SH, Kang EY, Kim SL, Park YJ, et al. . 2008. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J Agric Food Chem. 56(16):7265–7270. [DOI] [PubMed] [Google Scholar]
- Kino K, Mizumoto K, Sone T, Yamaji T, Watanabe J, Yamashita A, Yamaoka K, Shimizu K, Ko K, Tsunoo H.. 1990. An immunomodulating protein, Ling Zhi-8 (LZ-8) prevents insulitis in non-obese diabetic mice. Diabetologia. 33(12):713–718. [DOI] [PubMed] [Google Scholar]
- Klupp NL, Chang D, Hawke F, Kiat H, Cao H, Grant SJ, Bensoussan A.. 2015. Ganoderma lucidum mushroom for the treatment of cardiovascular risk factors. Cochrane Database Syst Rev. 2:CD007259. Art. No.: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klupp NL, Kiat H, Bensoussan A, Steiner GZ, Chang DH.. 2016. A double-blind, randomised, placebo-controlled trial of Ganoderma lucidum for the treatment of cardiovascular risk factors of metabolic syndrome. Sci Rep. 6:29540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarski M, Klaus A, Niksic M, Jakovljevic D, Helsper J, Van Griensven L.. 2011. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 129(4):1667–1675. [Google Scholar]
- Kozarski M, Klaus A, Nikšić M, Vrvić MM, Todorović N, Jakovljević D, Van Griensven LJ.. 2012. Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor. J Food Compost Anal. 26(1–2):144–153. [Google Scholar]
- Krishna KV, Karuppuraj V, Perumal K.. 2016. Antioxidant activity and folic acid content in indigenous isolates of Ganoderma lucidum. Asian Jour Pharmac Anal. 6(4):213–215. [Google Scholar]
- Kumar DP, Koka S, Li C, Rajagopal S.. 2019. Inflammatory mediators in obesity. Mediators Inflamm. 2019:9481819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran S, Palani P, Nishanthi R, Kaviyarasan V.. 2011. Studies on screening, isolation and purification of a fibrinolytic protease from an isolate (VK12) of Ganoderma lucidum and evaluation of its antithrombotic activity. Med Mycol J. 52(2):153–162. [DOI] [PubMed] [Google Scholar]
- Lasukova TV, Maslov LN, Arbuzov AG, Burkova VN, Inisheva LI.. 2015. Cardioprotective activity of Ganoderma lucidum extract during total ischemia and reperfusion of isolated heart. Bull Exp Biol Med. 158(6):739–741. [DOI] [PubMed] [Google Scholar]
- Li Z, Liu J, Zhao Y.. 2005. Possible mechanism underlying the antiherpetic activity of a proteoglycan isolated from the mycelia of Ganoderma lucidum in vitro. J Biochem Mol Biol. 38(1):34–40. [DOI] [PubMed] [Google Scholar]
- Li F, Zhang Y, Zhong Z.. 2011. Antihyperglycemic effect of Ganoderma lucidum polysaccharides on streptozotocin-induced diabetic mice. Int J Mol Sci. 12(9):6135–6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JM, Lin CC, Chiu HF, Yang JJ, Lee SG.. 1993. Evaluation of the anti-inflammatory and liver-protective effects of Anoectochilus formosanus, Ganoderma lucidum and Gynostemma pentaphyllum in rats. Am J Chin Med. 21(1):59–69. [DOI] [PubMed] [Google Scholar]
- Liperoti R, Vetrano DL, Bernabei R, Onder G.. 2017. Herbal medications in cardiovascular medicine. J Am Coll Cardiol. 69(9):1188–1199. [DOI] [PubMed] [Google Scholar]
- Liu C, Huang Y.. 2016. Chinese Herbal medicine on cardiovascular diseases and the mechanisms of action. Front Pharmacol. 7:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Ooi VEC, Chang ST.. 1997. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 60(10):763–771. [DOI] [PubMed] [Google Scholar]
- Liu Q, Tie L.. 2019. Preventive and therapeutic effect of Ganoderma (Lingzhi) on diabetes. Adv Exp Med Biol. 1182:201–215. [DOI] [PubMed] [Google Scholar]
- Liu W, Wang H, Pang X, Yao W, Gao X.. 2010. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int J Biol Macromol. 46(4):451–457. [DOI] [PubMed] [Google Scholar]
- Ma H-T, Hsieh J-F, Chen S-T.. 2015. Anti-diabetic effects of Ganoderma lucidum. Phytochemistry. 114:109–113. [DOI] [PubMed] [Google Scholar]
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. . 2020. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 41(1):111–188. [DOI] [PubMed] [Google Scholar]
- Mau JL, Lin HC, Chen CC.. 2002. Antioxidant properties of several medicinal mushrooms. J Agric Food Chem. 50(21):6072–6077. [DOI] [PubMed] [Google Scholar]
- McGuffin M, Hobbs C, Upton R, Goldberg A.. 1997. American Herbal Products Association’s botanical safety handbook. Boca Raton (FL): CRC Press. [Google Scholar]
- Meng J, Yang B.. 2019. Protective effect of Ganoderma (Lingzhi) on cardiovascular system. Adv Exp Med Biol. 1182:181–199. [DOI] [PubMed] [Google Scholar]
- Mohamad Ansor N, Abdullah N, Aminudin N.. 2013. Anti-angiotensin converting enzyme (ACE) proteins from mycelia of Ganoderma lucidum (Curtis) P. Karst. BMC Complement Altern Med. 13:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D, Wang L, Chen C, Hu B, Zhou P. 2015. Isolation and characterization of a hyperbranched proteoglycan from Ganoderma lucidum for anti-diabetes. Carbohydr Polym. 117:106–114. [DOI] [PubMed] [Google Scholar]
- Pan D, Zhang D, Wu J, Chen C, Xu Z, Yang H, Zhou P.. 2013. Antidiabetic, antihyperlipidemic and antioxidant activities of a novel proteoglycan from Ganoderma lucidum fruiting bodies on db/db mice and the possible mechanism. PLoS One. 8(7):e68332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D, Zhang D, Wu J, Chen C, Xu Z, Yang H, Zhou P.. 2014. A novel proteoglycan from Ganoderma lucidum fruiting bodies protects kidney function and ameliorates diabetic nephropathy via its antioxidant activity in C57BL/6 db/db mice. Food Chem Toxicol. 63:111–118. [DOI] [PubMed] [Google Scholar]
- Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN.. 2020. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. 21(5):1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravenec M, Kajiya T, Zidek V, Landa V, Mlejnek P, Simakova M, Silhavy J, Malinska H, Oliyarnyk O, Kazdova L, et al. . 2011. Effects of human C-reactive protein on pathogenesis of features of the metabolic syndrome. Hypertension. 57(4):731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MA, Abdullah N, Aminudin N.. 2018. Evaluation of the antioxidative and hypo-cholesterolemic effects of Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (Agaricomycetes), in ameliorating cardiovascular disease. Int J Med Mushrooms. 20(10):961–969. [DOI] [PubMed] [Google Scholar]
- Rajasekaran M, Kalaimagal C.. 2012. Cardioprotective effect of a medicinal mushroom, Ganoderma lucidum against adriamycin induced toxicity. Int J Pharmacol. 8(4):252–258. [Google Scholar]
- Ratnaningtyas NI, Hernayanti H, Andarwanti S, Ekowati N, Purwanti ES, Sukmawati D.. 2018. Effects of Ganoderma lucidum extract on diabetic rats. J Bio Bio Edu. 10(3):642–647. [Google Scholar]
- Rizal A, Sandra F, Fadlan MR, Sargowo D.. 2020. Ganoderma lucidum polysaccharide peptide reduce inflammation and oxidative stress in patient with atrial fibrillation. Indones Biomed J. 12(4):384–389. [Google Scholar]
- Saltarelli R, Ceccaroli P, Iotti M, Zambonelli A, Buffalini M, Casadei L, Vallorani L, Stocchi V.. 2009. Biochemical characterisation and antioxidant activity of mycelium of Ganoderma lucidum from Central Italy. Food Chem. 116(1):143–151. [Google Scholar]
- Sargowo D, Aissy CR, Kalsum U, Nugroho FW, Kamila PA, Irawan D, Sitio M, Adrian LH, Pratama AR, Arifin Y, et al. . 2019. The role of polysaccharide peptide of Ganoderma lucidum as a potent protective vascular endothelial cell, anti inflammation, and antioxidant in STEMI and NSTEMI patients. In: Khotimah H, Budianto WY, editors. International conference on bioinformatics and nanomedicine from natural resources for biomedical research. Melville (NY): Amer Inst Physics. [Google Scholar]
- Sarker MMR. 2015. Antihyperglycemic, insulin-sensitivity and anti-hyperlipidemic potential of Ganoderma lucidum, a dietary mushroom, onalloxan-and glucocorticoid-induced diabetic Long-Evans rats. FFHD. 5(12):450–466. [Google Scholar]
- Sarmadi BH, Ismail A.. 2010. Antioxidative peptides from food proteins: a review. Peptides. 31(10):1949–1956. [DOI] [PubMed] [Google Scholar]
- Seo HW, Hung TM, Na M, Jung HJ, Kim JC, Choi JS, Kim JH, Lee HK, Lee I, Bae K, et al. . 2009. Steroids and triterpenes from the fruit bodies of Ganoderma lucidum and their anti-complement activity. Arch Pharm Res. 32(11):1573–1579. [DOI] [PubMed] [Google Scholar]
- Seto SW, Lam TY, Tam HL, Au AL, Chan SW, Wu JH, Yu PH, Leung GP, Ngai SM, Yeung JH, et al. . 2009. Novel hypoglycemic effects of Ganoderma lucidum water-extract in obese/diabetic (+db/+db) mice. Phytomedicine. 16(5):426–436. [DOI] [PubMed] [Google Scholar]
- Sheena N, Ajith TA, Janardhanan KK.. 2003. Anti-inflammatory and anti-nociceptive activities of Ganoderma lucidum occurring in South India. Pharm Biol. 41(4):301–304. [DOI] [PubMed] [Google Scholar]
- Shiao MS. 2003. Natural products of the medicinal fungus Ganoderma lucidum: occurrence, biological activities, and pharmacological functions. Chem Rec. 3(3):172–180. [DOI] [PubMed] [Google Scholar]
- Taskinen M-R, Borén J.. 2015. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis. 239(2):483–495. [DOI] [PubMed] [Google Scholar]
- Teng BS, Wang CD, Yang HJ, Wu JS, Zhang D, Zheng M, Fan ZH, Pan D, Zhou P.. 2011. A protein tyrosine phosphatase 1B activity inhibitor from the fruiting bodies of Ganoderma lucidum (Fr.) Karst and its hypoglycemic potency on streptozotocin-induced type 2 diabetic mice. J Agric Food Chem. 59(12):6492–6500. [DOI] [PubMed] [Google Scholar]
- Teng BS, Wang CD, Zhang D, Wu JS, Pan D, Pan LF, Yang HJ, Zhou P.. 2012. Hypoglycemic effect and mechanism of a proteoglycan from Ganoderma lucidum on streptozotocin-induced type 2 diabetic rats. Eur Rev Med Pharmacol Sci. 16(2):166–175. [PubMed] [Google Scholar]
- Tomlinson B, Hu M, Zhang Y, Chan P, Liu ZM.. 2017. Evaluation of the pharmacokinetics, pharmacodynamics and clinical efficacy of empagliflozin for the treatment of type 2 diabetes. Expert Opin Drug Metab Toxicol. 13(2):211–223. [DOI] [PubMed] [Google Scholar]
- Ullah A, Khan A, Khan I.. 2016. Diabetes mellitus and oxidative stress - a concise review. Saudi Pharm J. 24:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton R. 2000. American herbal pharmacopoeia and therapeutic compendium: Reishi mushroom, Ganoderma lucidum. Standards of analysis, quality control, and therapeutics. Scotts Valley (CA): American Herbal Pharmacopoeia. [Google Scholar]
- van der Hem LG, van der Vliet JA, Bocken CF, Kino K, Hoitsma AJ, Tax WJ.. 1995. Ling Zhi-8: studies of a new immunomodulating agent. Transplantation. 60(5):438–443. [PubMed] [Google Scholar]
- Vitak TY, Wasser SP, Nevo E, Sybirna NO.. 2017. Enzymatic system of antioxidant protection of erythrocytes in diabetic rats treated with medicinal mushrooms Agaricus brasiliensis and Ganoderma lucidum (Agaricomycetes). Int J Med Mushrooms. 19(8):697–708. [DOI] [PubMed] [Google Scholar]
- Wachtel-Galor S, Szeto YT, Tomlinson B, Benzie IF.. 2004a. Ganoderma lucidum ('Lingzhi'); acute and short-term biomarker response to supplementation. Int J Food Sci Nutr. 55(1):75–83. [DOI] [PubMed] [Google Scholar]
- Wachtel-Galor S, Tomlinson B, Benzie IF.. 2004b. Ganoderma lucidum ("Lingzhi"), a Chinese medicinal mushroom: biomarker responses in a controlled human supplementation study. Ganoderma lucidum. 91:263–269. [DOI] [PubMed] [Google Scholar]
- Wachtel-Galor S, Yuen J, Buswell JA, Benzie IFF.. 2011. Chapter 9, Ganoderma lucidum (Lingzhi or Reishi): a medicinal mushroom. In: Benzie IFF, Wachtel-Galor S, editors. Herbal medicine: biomolecular and clinical aspects. 2nd ed. Boca Raton (FL): CRC Press/Taylor & Francis. [PubMed] [Google Scholar]
- Wang CW, Tschen JSM, Sheu WHH.. 2008. Ganoderma lucidum on metabolic control in type 2 diabetes subjects - a double blinded placebo control study. J Intern Med. 19(1):54–60. [Google Scholar]
- Wang PC, Zhao S, Yang BY, Wang QH, Kuang HX.. 2016. Anti-diabetic polysaccharides from natural sources: a review. Carbohydr Polym. 148:86–97. [DOI] [PubMed] [Google Scholar]
- Wanmuang H, Leopairut J, Kositchaiwat C, Wananukul W, Bunyaratvej S.. 2007. Fatal fulminant hepatitis associated with Ganoderma lucidum (Lingzhi) mushroom powder. J Med Assoc Thai. 90(1):179–181. [PubMed] [Google Scholar]
- Wasser SP. 2010. Medicinal mushroom science: history, current status, future trends, and unsolved problems. Int J Med Mushr. 12(1):1–16. [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2018. 2018. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation. 138(17):e484–e594. [DOI] [PubMed] [Google Scholar]
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. . 2018. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 39(33):3021–3104. [DOI] [PubMed] [Google Scholar]
- Winska K, Maczka W, Gabryelska K, Grabarczyk M.. 2019. Mushrooms of the genus Ganoderma used to treat diabetes and insulin resistance. Molecules. 24(22):4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witters LA. 2001. The blooming of the French lilac. J Clin Invest. 108(8):1105–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2021. Cardiovascular diseases (CVDs). [accessed 2021 Jul 15]. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
- Wu Q, Li Y, Peng K, Wang XL, Ding Z, Liu L, Xu P, Liu GQ.. 2019. Isolation and characterization of three antihypertension peptides from the mycelia of Ganoderma lucidum (Agaricomycetes). J Agric Food Chem. 67(29):8149–8159. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang D.. 2009. A new class of natural glycopeptides with sugar moiety-dependent antioxidant activities derived from Ganoderma lucidum fruiting bodies. J Proteome Res. 8(2):436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Q, Zhang HZ, Sun XF, Zhao HJ, Wu LF, Zhu D, Yang GH, Shao YY, Zhang XX, Mao X, et al. . 2014. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules. 19(11):17478–17535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Wu QP, Cai W, Tan JB, Yang XB, Zhang JM.. 2012. Hypoglycemic effects of Ganoderma lucidum polysaccharides in type 2 diabetic mice. Arch Pharm Res. 35(10):1793–1801. [DOI] [PubMed] [Google Scholar]
- Xiao C, Wu Q, Xie Y, Tan J, Ding Y, Bai L.. 2018. Hypoglycemic mechanisms of Ganoderma lucidum polysaccharides F31 in db/db mice via RNA-seq and iTRAQ. Food Funct. 9(12):6495–6507. [DOI] [PubMed] [Google Scholar]
- Xiao C, Wu Q, Zhang J, Xie Y, Cai W, Tan J.. 2017. Antidiabetic activity of Ganoderma lucidum polysaccharides F31 down-regulated hepatic glucose regulatory enzymes in diabetic mice. J Ethnopharmacol. 196:47–57. [DOI] [PubMed] [Google Scholar]
- Xie YZ, Yang F, Tan W, Li X, Jiao C, Huang R, Yang BB.. 2016. The anti-cancer components of Ganoderma lucidum possesses cardiovascular protective effect by regulating circular RNA expression. Oncoscience. 3(7–8):203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Chen X, Zhong Z, Chen L, Wang Y.. 2011. Ganoderma lucidum polysaccharides: Immunomodulation and potential anti-tumor activities. Am J Chin Med. 39(1):15–27. [DOI] [PubMed] [Google Scholar]
- Xu J, Xiao C, Xu H, Yang S, Chen Z, Wang H, Zheng B, Mao B, Wu X.. 2021. Anti-inflammatory effects of Ganoderma lucidum sterols via attenuation of the p38 MAPK and NF-κB pathways in LPS-induced RAW 264.7 macrophages. Food Chem Toxicol. 150:112073. [DOI] [PubMed] [Google Scholar]
- Yang Z, Chen C, Zhao J, Xu W, He Y, Yang H, Zhou P.. 2018a. Hypoglycemic mechanism of a novel proteoglycan, extracted from Ganoderma lucidum, in hepatocytes. Eur J Pharmacol. 820:77–85. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wu F, He Y, Zhang Q, Zhang Y, Zhou G, Yang H, Zhou P.. 2018b. A novel PTP1B inhibitor extracted from Ganoderma lucidum ameliorates insulin resistance by regulating IRS1-GLUT4 cascades in the insulin signaling pathway. Food Funct. 9(1):397–406. [DOI] [PubMed] [Google Scholar]
- Yoon HM, Jang KJ, Han MS, Jeong JW, Kim GY, Lee JH, Choi YH.. 2013. Ganoderma lucidum ethanol extract inhibits the inflammatory response by suppressing the NF-κB and toll-like receptor pathways in lipopolysaccharide-stimulated BV2 microglial cells. Exp Ther Med. 5(3):957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen MF, Ip P, Ng WK, Lai CL.. 2004. Hepatotoxicity due to a formulation of Ganoderma lucidum (Lingzhi). J Hepatol. 41(4):686–687. [DOI] [PubMed] [Google Scholar]
- Yurkiv B, Wasser SP, Nevo E, Sybirna NO.. 2015. Antioxidant effects of medicinal mushrooms Agaricus brasiliensis and Ganoderma lucidum (higher Basidiomycetes): evidence from animal studies. Int J Med Mushrooms. 17(10):943–955. [DOI] [PubMed] [Google Scholar]
- Zeng P, Guo Z, Zeng X, Hao C, Zhang Y, Zhang M, Liu Y, Li H, Li J, Zhang L.. 2018. Chemical, biochemical, preclinical and clinical studies of Ganoderma lucidum polysaccharide as an approved drug for treating myopathy and other diseases in China. J Cell Mol Med. 22(7):3278–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HN, He JH, Yuan L, Lin ZB.. 2003. In vitro and in vivo protective effect of Ganoderma lucidum polysaccharides on alloxan-induced pancreatic islets damage. Life Sci. 73(18):2307–2319. [DOI] [PubMed] [Google Scholar]
- Zhang HN, Lin ZB.. 2004. Hypoglycemic effect of Ganoderma lucidum polysaccharides. Acta Pharmacol Sin. 25(2):191–195. [PubMed] [Google Scholar]
- Zhang S, Pang G, Chen C, Qin J, Yu H, Liu Y, Zhang X, Song Z, Zhao J, Wang F, et al. . 2019. Effective cancer immunotherapy by Ganoderma lucidum polysaccharide-gold nanocomposites through dendritic cell activation and memory T cell response. Carbohydr Polym. 205:192–202. [DOI] [PubMed] [Google Scholar]
- Zheng J, Yang B, Yu Y, Chen Q, Huang T, Li D.. 2012. Ganoderma lucidum polysaccharides exert anti-hyperglycemic effect on streptozotocin-induced diabetic rats through affecting β-cells. Comb Chem High Throughput Screen. 15(7):542–550. [DOI] [PubMed] [Google Scholar]