Keywords: anesthesia, chemosensory, mechanosensory, renal nerves, renal reflex
Abstract
Activation of renal sensory nerves by chemo- and mechanosensitive stimuli produces changes in efferent sympathetic nerve activity (SNA) and arterial blood pressure (ABP). Anesthesia and sex influence autonomic function and cardiovascular hemodynamics, but it is unclear to what extent anesthesia and sex impact SNA and ABP responses to renal sensory stimuli. We measured renal, splanchnic, and lumbar SNA and ABP in male and female Sprague-Dawley rats during contralateral renal infusion of capsaicin and bradykinin or during elevation in renal pelvic pressure. Responses were evaluated with a decerebrate preparation or Inactin, urethane, or isoflurane anesthesia. Intrarenal arterial infusion of capsaicin (0.1–30.0 μM) increased renal SNA, splanchnic SNA, or ABP but decreased lumbar SNA in the Inactin group. Intrarenal arterial infusion of bradykinin (0.1–30.0 μM) increased renal SNA, splanchnic SNA, and ABP but decreased lumbar SNA in the Inactin group. Elevated renal pelvic pressure (0–20 mmHg, 30 s) significantly increased renal SNA and splanchnic SNA but not lumbar SNA in the Inactin group. In marked contrast, SNA and ABP responses to every renal stimulus were severely blunted in the urethane and decerebrate groups and absent in the isoflurane group. In the Inactin group, the magnitude of SNA responses to chemo- and mechanosensory stimuli were not different between male and female rats. Thus, chemo- and mechanosensitive stimuli produce differential changes in renal, splanchnic, and lumbar SNA. Experimentally, future investigations should consider Inactin anesthesia to examine sympathetic and hemodynamic responses to renal sensory stimuli.
NEW & NOTEWORTHY The findings highlight the impact of anesthesia, and to a lesser extent sex, on sympathetic efferent and hemodynamic responses to chemosensory and mechanosensory renal stimuli. Sympathetic nerve activity (SNA) and arterial blood pressure (ABP) responses were present in Inactin-anesthetized rats but largely absent in decerebrate, isoflurane, or urethane preparations. Renal chemosensory stimuli differentially changed SNA: renal and splanchnic SNA increased, but lumbar SNA decreased. Future investigations should consider Inactin anesthesia to study SNA and hemodynamic responses to renal sensory nerve activation.
INTRODUCTION
The renal nerves are comprised of both afferent (sensory) and efferent sympathetic fibers that modulate renal function, central hemodynamics, and arterial blood pressure (ABP). Renal sensory nerves are activated by ischemia, selective chemical stimuli (e.g., capsaicin, bradykinin, and substance P), and increases in renal pelvic pressure (1–6). Activation of renal sensory nerves elicits reflexive changes in efferent sympathetic nerve activity (SNA) and ABP to maintain homeostasis (5–7). Such sympathetic responses can also pathologically contribute to hypertension and renal disease etiologies. In humans, SNA is elevated in hypertension (8–11) and renal denervation lowers both muscle SNA (12, 13) and ABP (14, 15). Of note, efferent SNA is elevated in animal models of neurogenic hypertension, and renal denervation or selective renal afferent denervation lowers neurogenic pressor activity and ABP in these models (16–19). Thus, the degree by which efferent responses to renal sensory stimuli contribute to disease is an area of high importance.
The majority of published studies have explored renal-reflex responses using anesthetized preparations, yet anesthesia can differentially impact central autonomic function and the regulation of SNA (2, 20–28). For example, chemical or electrical stimulation of the nucleus tractus solitarii, paraventricular nucleus (PVN), or area postrema produces differential pressor responses in anesthetized versus unanesthetized rat preparations (22, 23, 28). Pentobarbital sodium, urethane, and chloralose anesthesia attenuate baroreflex responses and differentially modulate renal SNA (20, 21, 24, 25, 27). In regard to renal-reflex responses, we reported that anesthesia differentially alters renal afferent activity to sensory stimuli (2). Renal chemokine infusion or elevated renal pelvic pressure increased renal afferent activity in decerebrate or Inactin-anesthetized rats. However, such sensory responses were significantly blunted in rats anesthetized with urethane or isoflurane. The extent to which renal sensory stimuli subsequently alter efferent SNA and hemodynamics was not evaluated in this context because sectioning the renal nerve was required to preferentially isolate and record afferent activity.
A second major experimental factor not assessed in previous studies is the influence of sex on SNA and hemodynamic responses to activation of renal sensory nerves. In humans, the prevalence of cardiovascular disease and hypertension are lower for young women versus men (29–33). Similarly, multiple experimental forms of neurogenic hypertension show a sex dependence reflected by an attenuated hypertension or attenuated sympathetic contribution in female versus male rodents (34–37). Moreover, muscle SNA and/or pressor responses to handgrip exercise are also attenuated in women versus men (38, 39), and centrally evoked pressor responses are attenuated in young female versus male animals (40, 41). Yet, the extent by which sex alters SNA and hemodynamic responses to activation of renal sensory nerves has not been examined previously.
Thus, the purpose of the present study was to assess how anesthesia and sex modulate efferent SNA and hemodynamic responses to activation of renal sensory fibers. Since our prior study indicated that the largest and most sensitive renal afferent responses were observed with Inactin or a decerebrate preparation, we hypothesized that these preparations would produce the largest SNA and ABP changes to renal chemokine infusion and/or elevated renal pelvic pressure. Furthermore, we hypothesized that SNA and ABP responses would be attenuated in female versus male rodents.
METHODS
Animals
All experimental procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Male and female Sprague-Dawley rats (250–400 g; Charles River Laboratories) were pair-housed in a temperature-controlled room (22 ± 1°C) with a 12:12-h dark-light cycle (lights on at 0700–1900) and given ad libitum access to deionized water and 0.1% NaCl chow diet (Research Diets, D17020). Animal surgery began at 1000, and data collection started 2.5–3 h later.
General Procedures
Rats were anesthetized with isoflurane (2–3% in 100% O2) and prepared for simultaneous recording of ABP and lumbar, renal, and splanchnic SNA as described previously (42–44). Briefly, depth of anesthesia was assessed by the absence of corneal reflexes and paw withdrawal responses to foot pinch. Brachial artery and femoral vein catheters (PE50 tubing) were implanted to monitor ABP (BPM-832 Dual Pressure Monitor; CWE Inc.) and infuse saline (0.25 mL/h iv), respectively. Animals were artificially ventilated via tracheotomy to maintain end-expiratory CO2 between 3.5% and 4.5% and O2 between 35% and 45% (Gemini Respiratory Gas Analyzer; CWE Inc.). Body temperature was continuously monitored and maintained at 37 ± 0.5°C with a rectal thermometer and a circulating water pad. Through a ventral midline incision, a heat-stretched catheter [0.037 outer diameter (OD) × 0.023 inner diameter (ID), Micro-Renathane; Braintree Scientific] was placed at the entrance of the right renal pelvis (contralateral to the renal SNA recording) via the ureter and exteriorized at the level of the bladder. The presence of urine flow indicated proper placement and catheter patency. The lumbar sympathetic nerve was isolated, placed on bipolar stainless steel electrodes, and insulated with Kwik-Sil (WPI, Sarasota, FL). Then, animals were placed in a stereotaxic frame. The right kidney was approached through a retroperitoneal incision, and the adrenal artery that branches off the right renal artery was cannulated with heat-stretched Micro-Renathane tubing as described previously (2, 6). Next, the left renal and splanchnic nerves were isolated through a left retroperitoneal incision by gentle retraction of the kidney. Nerves were placed on separate sets of bipolar electrodes and insulated with Kwik-Sil. Nerve signals were amplified (10,000×) and filtered (0.3–1.0 kHz) with a differential AC amplifier (A-M Systems, Sequim, WA), monitored on a Tektronix TDS2004 digital oscilloscope, and then digitized at 2 kHz with a Micro1401 and Spike2 software (Cambridge Electronic Design Inc.). The quality of the SNA recording was confirmed at the start of each experiment by the presence of cardiac-related bursts, a sympathoinhibition during acute phenylephrine injection (4 μg/kg iv; Sigma P6126), and a signal-to-noise ratio ≥ 2:1. Lumbar, renal, and splanchnic SNA were rectified and integrated (1-s time constant). The nerve signal was calculated by subtracting the noise obtained after ganglionic blockade (30 mg/kg iv hexamethonium; Sigma H0879). Values were normalized to baseline values set at 100%. The nerves were not sectioned distal to the recording electrodes to simultaneously assess hemodynamic responses. However, the noise obtained after ganglionic blockade was similar to that after nerve transection, thereby suggesting that afferent nerve activity was negligible in these preparations.
Anesthesia and Midcollicular Decerebration
After all surgical procedures were completed, isoflurane anesthesia was either maintained (1.8–2.0% in 100% O2) or replaced by Inactin (120 mg/kg, 0.2 mL/min iv; Sigma T133) or urethane (1.2 g/kg, 0.2 mL/min iv; Sigma U2500). Depth of anesthesia was assessed by the absence of corneal reflexes and paw withdrawal responses to foot pinch. A fourth group of animals received a midcollicular decerebration as described previously (45, 46). Briefly, the carotid arteries were ligated with 4-0 silk suture. After a craniotomy, the cerebral cortex was aspirated to visualize the colliculi. Complete coronal transection of the brain was performed with a microspatula inserted between the superior and inferior colliculi and confirmed postmortem. Then, isoflurane anesthesia was terminated. Pancuronium bromide was administered as needed (0.1 mg/kg iv) in all decerebrate animals to facilitate stable ventilation and end-tidal CO2 levels. For all groups, animals stabilized for a minimum of 30 min after surgical procedures. In total, 56 rats (27 males; 29 females) were assessed. Animals were excluded if ABP was <60 mmHg (n = 4) or if no urine was produced through the ureteral catheter (n = 2).
Experimental Protocol
Numbers of animals (including sex) per group are reported in the figure legends and Table 1. Each stimulus paradigm was tested in a randomized order. Chemokine concentrations and renal pelvic pressure steps were validated previously (2). Intrarenal artery infusion of capsaicin (0.1–30.0 μΜ; Sigma M2028) or saline vehicle was performed (0.05 mL over 15 s) via the adrenal artery catheter every 6 min in a randomized order (2). Intrarenal artery infusion of bradykinin (0.1–30.0 μΜ; Sigma B3259) or saline vehicle was performed (0.05 mL over 15 s) via the adrenal artery catheter every 3 min in a randomized order. Both capsaicin and bradykinin were flushed through the catheter with 0.1 mL of saline at 0.4 mL/min. SNA was rectified/integrated and averaged in 1-s bins. A rolling 5-s average was calculated, and the peak responses were compared to a 30-s baseline segment. Capsaicin and bradykinin responses were not tested in a small subset of animals lacking a renal-branching adrenal artery. Increased renal pelvic pressure was performed by connecting the ureteral-pelvic catheter to a pressure transducer and column of water by Y connector. Pelvic pressure was elevated (0, 1, 2, 5, 10, and 20 mmHg) for 30 s, followed by a 2-min recovery period. At the end of the pressure steps the Y connector was disconnected. Data were averaged in 1-s bins. A 3-s rolling average was calculated to compare the peak response versus a 30-s baseline.
Table 1.
Baseline mean ABP, heart rate, renal SNA, splanchnic SNA, and lumbar SNA
| n | Mean ABP, mmHg | Heart Rate, beats/min | Renal SNA, μV | Splanchnic SNA, μV | Lumbar SNA, μV | |
|---|---|---|---|---|---|---|
| Inactin | Total 16 | 97 ± 3* | 343 ± 7* | 1.601 ± 0.543* | 1.480 ± 0.242* | 0.484 ± 0.087* |
| Male 8 | 89 ± 4 | 342 ± 10 | 2.745 ± 0.942 | 1.983 ± 0.383 | 0.671 ± 0.140 | |
| Female 8 | 105 ± 3# | 343 ± 10 | 0.457 ± 0.071# | 0.977 ± 0.184# | 0.298 ± 0.057# | |
| Decerebrate | Total 16 | 124 ± 5 | 512 ± 9 | 3.672 ± 0.498 | 3.533 ± 0.662 | 2.014 ± 0.169 |
| Male 8 | 124 ± 11 | 515 ± 14 | 4.582 ± 0.706 | 3.614 ± 0.883 | 2.076 ± 0.259 | |
| Female 8 | 135 ± 5 | 510 ± 12 | 2.876 ± 0.602 | 3.462 ± 1.030 | 1.960 ± 0.239 | |
| Isoflurane | Total 13 | 96 ± 3* | 328 ± 10* | 2.477 ± 0.375 | 1.681 ± 0.288* | 1.137 ± 0.109* |
| Male 6 | 90 ± 4 | 342 ± 12 | 2.213 ± 0.6267 | 2.100 ± 0.559 | 1.010 ± 0.165002 | |
| Female 7 | 101 ± 4 | 315 ± 15 | 2.704 ± 0.474 | 1.321 ± 0.198 | 1.246 ± 0.143 | |
| Urethane | Total 11 | 136 ± 3 | 395 ± 14* | 2.677 ± 0.636 | 1.435 ± 0.237* | 0.777 ± 0.188* |
| Male 6 | 135 ± 5 | 398 ± 12 | 2.153 ± 0.447 | 1.246 ± 0.360 | 0.843 ± 0.319 | |
| Female 5 | 138 ± 4 | 393 ± 18 | 3.307 ± 1.323 | 1.661 ± 0.303 | 0.700 ± 0.143 |
All data presented as means ± SE. ABP, arterial blood pressure; SNA, sympathetic nerve activity. Baseline values were analyzed across groups with a 1-way ANOVA and Dunnett’s post hoc test compared with decerebrate group (*P < 0.05). Sex differences within each group were analyzed with a Student’s t test (#P < 0.05).
Statistical Analysis
All data were analyzed with GraphPad Prism v.9.0 software. Data were tested for normality with Shapiro–Wilk test and equal variance with a Bartlett’s test. Data that did not pass normality were log transformed and tested for significance by ANOVA with a Dunnett’s post hoc test or a repeated-measures two-way ANOVA with a Bonferroni post hoc test. Decerebrate group data for renal and splanchnic SNA were not able to be transformed for parametric statistical testing and were assessed nonparametrically by Friedman’s test of repeated measures with a Dunn’s post hoc analysis. Nonparametric conclusions were not different from ANOVA values, which are presented for simplicity. All data are presented as means ± SE. P values <0.05 were statistically significant.
RESULTS
Baseline Hemodynamics and Sympathetic Nerve Activity
Table 1 summarizes baseline hemodynamics and SNA across Inactin, decerebrate, urethane, and isoflurane groups. Baseline mean ABP and heart rate were significantly different across groups (F3,51 = 23.10, P < 0.0001, ANOVA and F3,51 = 79.21, P < 0.0001, ANOVA, respectively). Mean ABP was higher in decerebrate and urethane groups versus Inactin or isoflurane groups (Table 1). Heart rate was higher in decerebrate versus Inactin, isoflurane, and urethane groups. Heart rate was also higher in urethane versus Inactin and isoflurane groups (Table 1). Baseline SNA voltages were also significantly different across groups for renal (F3,51 = 7.203, P = 0.0004, ANOVA), splanchnic (F3,51 = 5.304, P = 0.0029, ANOVA), and lumbar (F3,51 = 24.59, P < 0.0001, ANOVA) SNA. Renal baseline voltages were significantly higher in the decerebrate versus Inactin group (Table 1). Splanchnic baseline voltages were significantly higher in decerebrate versus Inactin, isoflurane, and urethane groups (Table 1). Lumbar baseline voltages were significantly higher in decerebrate versus Inactin, urethane, and isoflurane groups (Table 1).
In the Inactin group, mean ABP was significantly higher in female versus male rats, yet heart rate was not different (Table 1). No significant differences were observed in mean ABP or heart rate between male and female rats in the decerebrate, urethane, or isoflurane preparations. Furthermore, female rats exhibited significantly lower voltages than male rats for renal (P = 0.029), splanchnic (P = 0.033), and lumbar nerve activity in the Inactin group (Table 1). No differences in baseline voltage were observed between sexes within decerebrate, urethane, or isoflurane groups.
Sympathetic and Hemodynamic Responses to Intrarenal Capsaicin Infusion
To assess the extent by which anesthesia influences SNA and ABP responses to renal chemosensory stimuli, the transient receptor potential vanilloid 1 (TRPV1) agonist capsaicin was infused (0.05 mL, 15 s) into the right renal artery via an adrenal arterial catheter. Figure 1 illustrates SNA and ABP responses to intrarenal arterial infusion with 10 μΜ capsaicin. Renal SNA, splanchnic SNA, and ABP promptly increased in Inactin-anesthetized rats. In marked contrast, these responses were blunted or even absent in decerebrate, urethane, and isoflurane groups.
Figure 1.
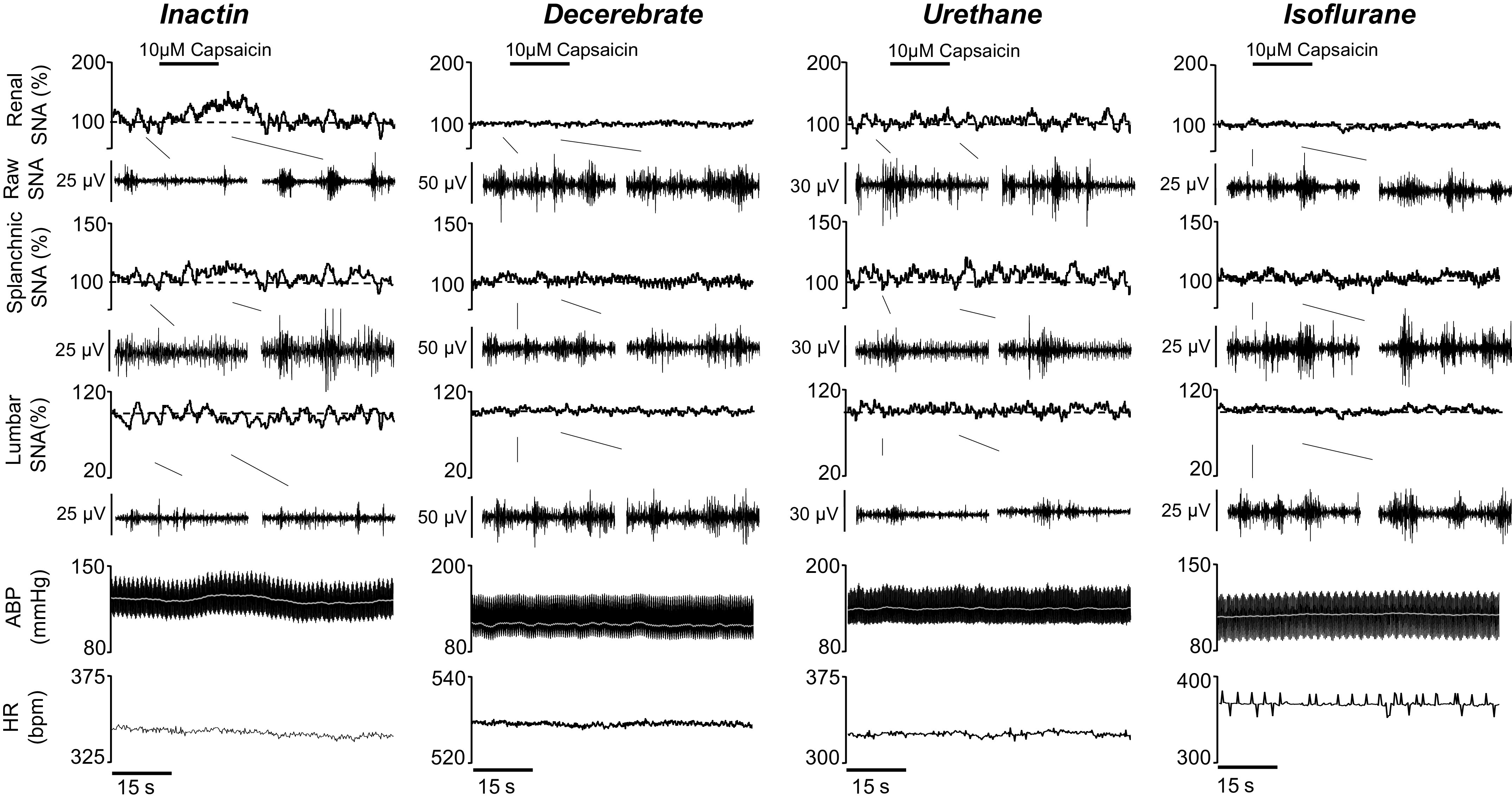
Example of rectified/integrated (∫) renal sympathetic nerve activity (SNA), splanchnic SNA, and lumbar SNA, raw SNA, heart rate [HR; beats/min (bpm)], arterial blood pressure (ABP), and mean ABP (white line) during intrarenal arterial infusion with 10.0 μΜ capsaicin (black line; 0.05 mL, 0.2 mL/min). Raw SNA tracings (0.5 s) highlight baseline and peak capsaicin responses.
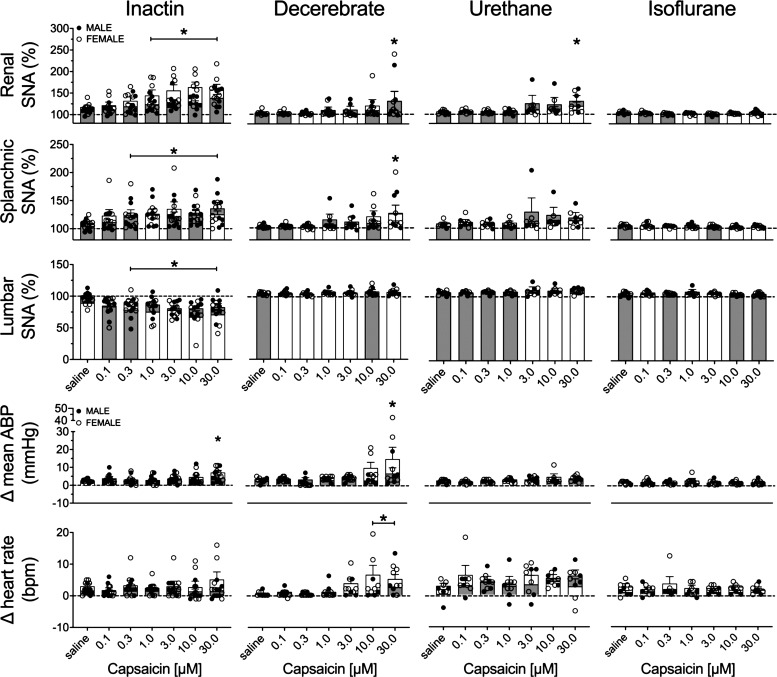
Figure 2 illustrates summary data of the peak change of all variables for both male and female rats. The threshold sensitivity for responses was defined as the lowest concentration required to produce a statistically significant difference from saline within each group. In the Inactin group, capsaicin infusion increased renal SNA (F6,84 = 8.819, P < 0.0001, ANOVA) and splanchnic SNA (F6,84 = 5.109, P = 0.0002, ANOVA) but decreased lumbar SNA (F6,78 = 4.644, P = 0.0004, ANOVA). The threshold concentration was 1.0 μM for renal, 0.3 μM for splanchnic, and 0.3 μM for lumbar SNA (Fig. 2). In the decerebrate group, significant changes were detected for renal SNA (F6,54 = 3.904, P = 0.0026, ANOVA) and splanchnic SNA (F6,54 = 4.683, P = 0.0007, ANOVA) but not for lumbar SNA (F6,54 = 1.204, P = 0.3183, ANOVA). The threshold concentrations for renal and splanchnic SNA were both 30.0 μM. In the urethane group, a significant effect was observed during capsaicin infusion for renal SNA (F6,48 = 3.546, P = 0.0055, ANOVA) but not for splanchnic SNA (F6,48 = 1.291, P = 0.2794, ANOVA) or lumbar SNA (F6,42 = 1.035 P = 0.4163, ANOVA). The threshold concentration for renal SNA was 30.0 μM. In the isoflurane group, no significant effects were observed for renal SNA (F6,48 = 2.026, P = 0.0803, ANOVA), splanchnic SNA (F6,48 = 1.009, P = 0.4306, ANOVA), or lumbar SNA (F6,48 = 0.4716, P = 0.8260, ANOVA).
Figure 2.
Means ± SE and individual data points of the peak change in renal sympathetic nerve activity (SNA), splanchnic SNA, lumbar SNA, heart rate [beats/min (bpm)], and mean arterial blood pressure (ABP) to intra-arterial renal infusion of capsaicin (0.1–30.0 μΜ). Male (M; gray bars) and female (F; white bars) animals are indicated for Inactin (n = 15; 8 M, 7 F), decerebrate (n = 11; 5 M, 6 F), urethane (n = 9; 4 M, 5 F), and isoflurane (n = 9; 4 M, 5 F) groups. One-way ANOVA with Dunnett’s post hoc test identified the lowest threshold concentration significantly different from saline within each group (*P < 0.05).
Intrarenal artery infusion of capsaicin significantly altered mean ABP in the Inactin (F6,84 = 3.286, P < 0.0059, ANOVA) and decerebrate (F6,54 = 4.229, P = 0.015, ANOVA) groups. A Dunnett’s post hoc test detected a significant increase in the mean ABP for both Inactin (+5 mmHg) and decerebrate (+11 mmHg) groups with 30.0 μΜ capsaicin (Fig. 2). Mean ABP was unaltered in the urethane (F6,48 = 2.141, P = 0.0656, ANOVA) and isoflurane (F6,48 = 0.1707, P = 0.9834, ANOVA) groups. Moreover, heart rate was unchanged during capsaicin infusion in the Inactin (F6,84 = 0.8744, P < 0.5174, ANOVA), urethane (F6,48 = 1.140, P = 0.3542, ANOVA), and isoflurane (F6,48 = 0.1663, P = 0.9845, ANOVA) groups. However, heart rate was significantly altered in the decerebrate group (F6,54 = 4.092, P < 0.0001, ANOVA). A Dunnett’s post hoc test detected a significant increase in heart rate (3 beats/min) at 10 μΜ and 30 μΜ capsaicin concentrations.
Intrarenal capsaicin infusion consistently evoked SNA and ABP responses over several concentrations in the Inactin group, and a subsequent analysis was performed to assess sex differences in these responses. Of note, there were no sex differences in any variable within the decerebrate, urethane, or isoflurane groups (analysis not shown). A two-way ANOVA of renal SNA responses indicated a significant main effect for concentration (F6,78 = 8.995, P < 0.0001, ANOVA) and sex (F1,13 = 6.366, P = 0.0255, ANOVA) but no interaction (F6,78 = 0.8870, P = 0.5086, ANOVA). SNA responses to 10 μΜ capsaicin were significantly greater in female versus male rats. Analysis of splanchnic SNA by two-way ANOVA indicated a significant effect for concentration (F6,78 = 4.947, P = 0.002, ANOVA) but not for sex (F1,13 = 0.2251, P = 0.6430, ANOVA) and no interaction (F6,78 = 0.9829, P = 0.4427, ANOVA). For lumbar SNA, a two-way ANOVA indicated a significant main effect for concentration (F6,72 = 4.637, P = 0.0005, ANOVA) and an interaction (F6,72 = 2.423, P = 0.0344, ANOVA) but no significant effect for sex (F1,12 = 1.029, P = 0.3305, ANOVA).
The changes in mean ABP and heart rate were similarly assessed. A significant effect was observed for concentration (F6,78 = 3.825, P = 0.0021, ANOVA) but not for sex (F1,13 = 0.2591, P = 0.6193, ANOVA) or an interaction (F6,78 = 2.126, P = 0.0596, ANOVA). For heart rate, there was no significant effect for concentration (F6,78 = 0.9301, P = 0.4783, ANOVA) or sex (F1,13 = 0.4289, P = 0.5239, ANOVA) or interaction (F6,78 = 1.217, P = 0.3065, ANOVA).
Sympathetic and Hemodynamic Responses to Intrarenal Bradykinin Infusion
To further assess the extent to which anesthesia influences chemosensitive responses, bradykinin was infused (0.05 mL, 15 s) into the right renal artery via an adrenal arterial catheter. Figure 3 demonstrates that intrarenal artery infusion of 10 μM bradykinin in an Inactin-anesthetized animal increases renal SNA, splanchnic SNA, and ABP but decreases lumbar SNA. Again, these responses were blunted in the urethane group and absent in the decerebrate and isoflurane groups.
Figure 3.
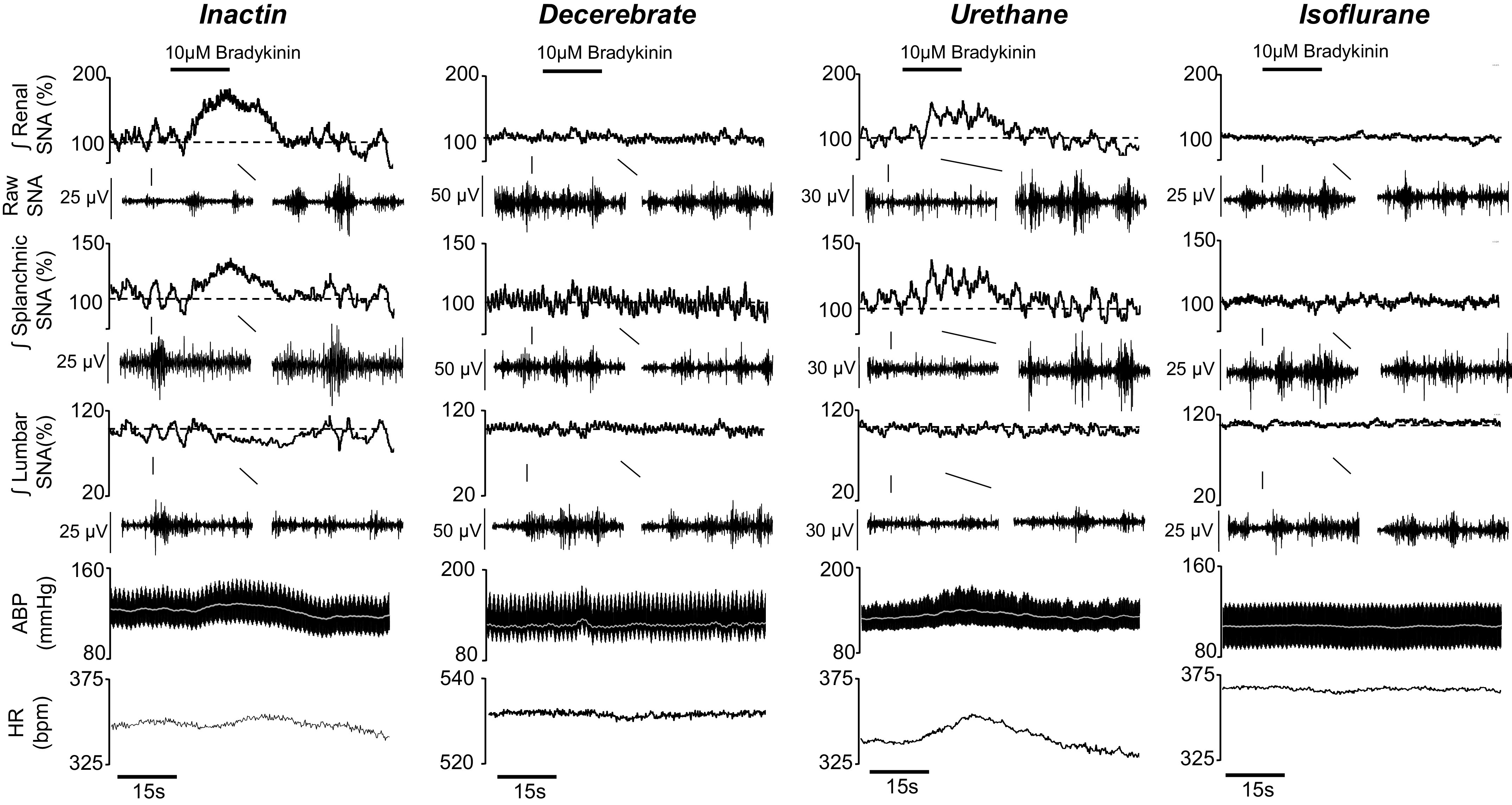
Example of rectified/integrated (∫) renal sympathetic nerve activity (SNA), splanchnic SNA, lumbar SNA, raw SNA, heart rate [HR; beats/min (bpm)], arterial blood pressure (ABP), and mean ABP (white line) during arterial renal infusion with 10.0 μΜ bradykinin (black line; 0.05 mL, 0.2 mL/min). Raw SNA tracings (0.5 s) highlight baseline and peak bradykinin responses.
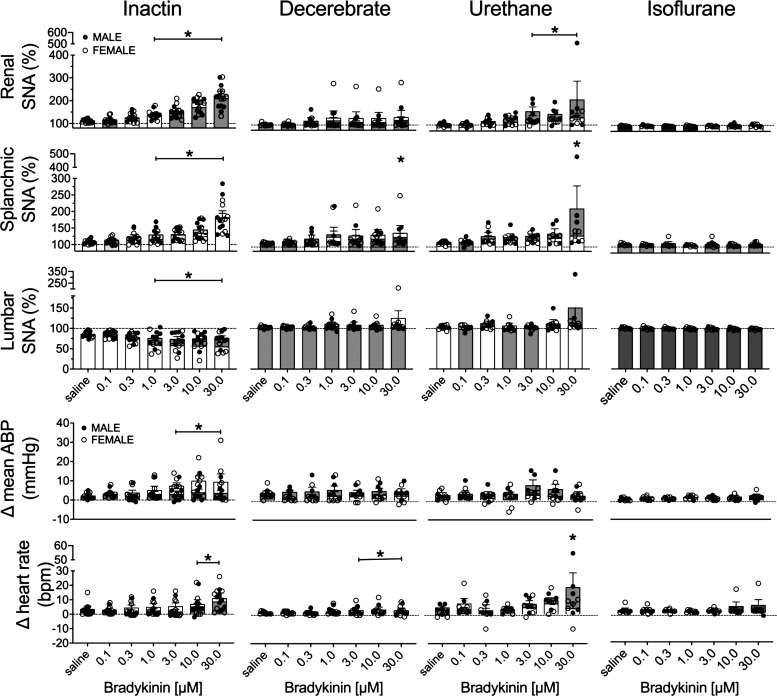
Figure 4 illustrates summary data of the peak change in all variables across anesthetic groups for both male and female rats. The threshold sensitivity was again assessed by ANOVA. In the Inactin group, bradykinin infusion increased renal SNA (F6,84 = 50.32, P < 0.0001, ANOVA) and splanchnic SNA (F6,84 = 37.26, P < 0.0001, ANOVA) but decreased lumbar SNA (F6,72 = 6.628, P < 0.0001, ANOVA). The threshold sensitivity was 1.0 μM for renal, splanchnic, and lumbar SNA (Fig. 4). In the decerebrate group, bradykinin infusion increased splanchnic SNA (F6,60 = 2.631, P = 0.0248, ANOVA) but did not alter renal SNA (F6,60 = 2.070, P = 0.0701, ANOVA) or lumbar SNA (F6,60 = 1.735, P = 0.1285, ANOVA). The threshold sensitivity for splanchnic SNA was 30.0 μΜ. In the urethane group, bradykinin infusion significantly increased renal SNA (F6,54 = 4.317, P = 0.0013, ANOVA) and splanchnic SNA (F6,54 = 3.917, P = 0.0025, ANOVA) but did not alter lumbar SNA (F6,54 = 1.715, P = 0.1353, ANOVA). The threshold concentration for renal and splanchnic SNA was 3.0 μM and 30.0 μΜ, respectively. For splanchnic SNA, a statistical analysis determined one case to be an outlier during 30.0 μΜ bradykinin infusion. Removal of the data point or removal of this animal from the entire data set did not alter statistical significance, and it is included in the summary data (Fig. 4). In the isoflurane group, no significant effects were observed for renal SNA (F6,56 = 0.5915, P = 0.7357, ANOVA), splanchnic SNA (F6,56 = 0.5088, P = 0.7991, ANOVA), or lumbar SNA (F6,48 = 1.117, P = 0.3666, ANOVA).
Figure 4.
Means ± SE and individual data points of the peak change in renal sympathetic nerve activity (SNA), splanchnic SNA, lumbar SNA, heart rate [beats/min (bpm)], and mean arterial blood pressure (ABP) to intra-arterial renal infusion of bradykinin (0.1–30.0 μΜ). Male (M; gray bars) and female (F; white bars) animals are indicated for Inactin (n = 15; 8 M, 7 F), decerebrate (n = 11; 5 M, 6 F), urethane (n = 9; 4 M, 5 F), and isoflurane (n = 9; 4 M, 5 F) groups. One-way ANOVA with Dunnett’s post-hoc test identified the lowest threshold concentration significantly different from saline within each group (*P < 0.05).
Intrarenal infusion of bradykinin significantly increased mean ABP in the Inactin group (F6,84 = 3.466, P < 0.0041, ANOVA). A Dunnett’s post hoc test detected a significant increase in mean ABP of 5–7 mmHg at 3.0–30.0 μΜ bradykinin (Fig. 4).
Mean ABP was unaltered in the decerebrate (F6,60 = 1.108, P = 0.3686, ANOVA), urethane (F6,54 = 1.054, P = 0.4012, ANOVA), and isoflurane (F6,56 = 1.346, P = 0.2528, ANOVA) groups. Intrarenal infusion of bradykinin also significantly increased heart rate in the Inactin (F6,84 = 9.403, P < 0.0001, ANOVA), decerebrate (F6,60 = 4.711, P = 0.0005, ANOVA), and urethane (F6,48 = 3.212, P = 0.0098, ANOVA) groups but not the isoflurane group (F6,48 = 1.223, P = 0.3111, ANOVA). Post hoc testing detected a significant increase in heart rate between 10.0 and 30.0 μΜ for the Inactin group, between 3.0 and 30.0 μΜ for the decerebrate group, and at 30.0 μM for the urethane group.
Intrarenal bradykinin infusion consistently evoked SNA and ABP responses over several concentrations in the Inactin group, and a subsequent analysis was performed to assess sex differences in these responses. Of note, there were no sex differences in any variable within the decerebrate, urethane, or isoflurane groups (analysis not shown). A two-way ANOVA of renal SNA indicated a significant main effect for concentration (F6,78 = 52.01, P < 0.0001, ANOVA) but not for sex (F1,13 = 0.2286, P = 0.6405, ANOVA) and no interaction (F6,78 = 0.8158, P = 0.5509, ANOVA). Analysis of splanchnic SNA by two-way ANOVA indicated a significant effect for concentration (F6,78 = 38.03, p < 0.0001, ANOVA) but not for sex (F1,13 = 0.1797, P = 0.6786, ANOVA) and no interaction (F6,78 = 1.375, P = 0.2350, ANOVA). Finally, assessment of lumbar SNA by two-way ANOVA indicated a significant effect for concentration (F6,66 = 6.522, P < 0.0001, ANOVA) but not for sex (F1,11 = 3.422, P = 0.0914, ANOVA) and no interaction (F6,66 = 1.712, P = 0.1319, ANOVA).
The changes in mean ABP and heart rate were assessed by two-way ANOVA. A significant effect for concentration (F6,78 = 3.475, P = 0.0043, ANOVA) but not for sex (F1,13 = 3.275, P = 0.0935, ANOVA) and no interaction (F6,78 = 1.599, P = 0.1585, ANOVA) were observed for the change in mean ABP. For heart rate, a significant effect for concentration (F6,78 = 9.105, P < 0.0001, ANOVA) but not for sex (F1,13 = 0.2541, P = 0.6226, ANOVA) and no interaction (F6,78 = 0.9700, P = 0.4512, ANOVA) were observed.
Sympathetic and Hemodynamic Responses to Elevated Renal Pelvic Pressure
A final set of experiments assessed SNA and hemodynamic responses to increased renal pelvic pressure (0, 1, 2, 5, 10, and 20 mmHg, 30 s each) in Inactin, decerebrate, urethane, and isoflurane groups. Figure 5 illustrates responses to a 20-mmHg elevation in renal pelvic pressure. In Inactin-anesthetized rats, renal and splanchnic SNA increased but lumbar SNA and ABP were unaltered. These responses were absent in the decerebrate, urethane, and isoflurane groups.
Figure 5.
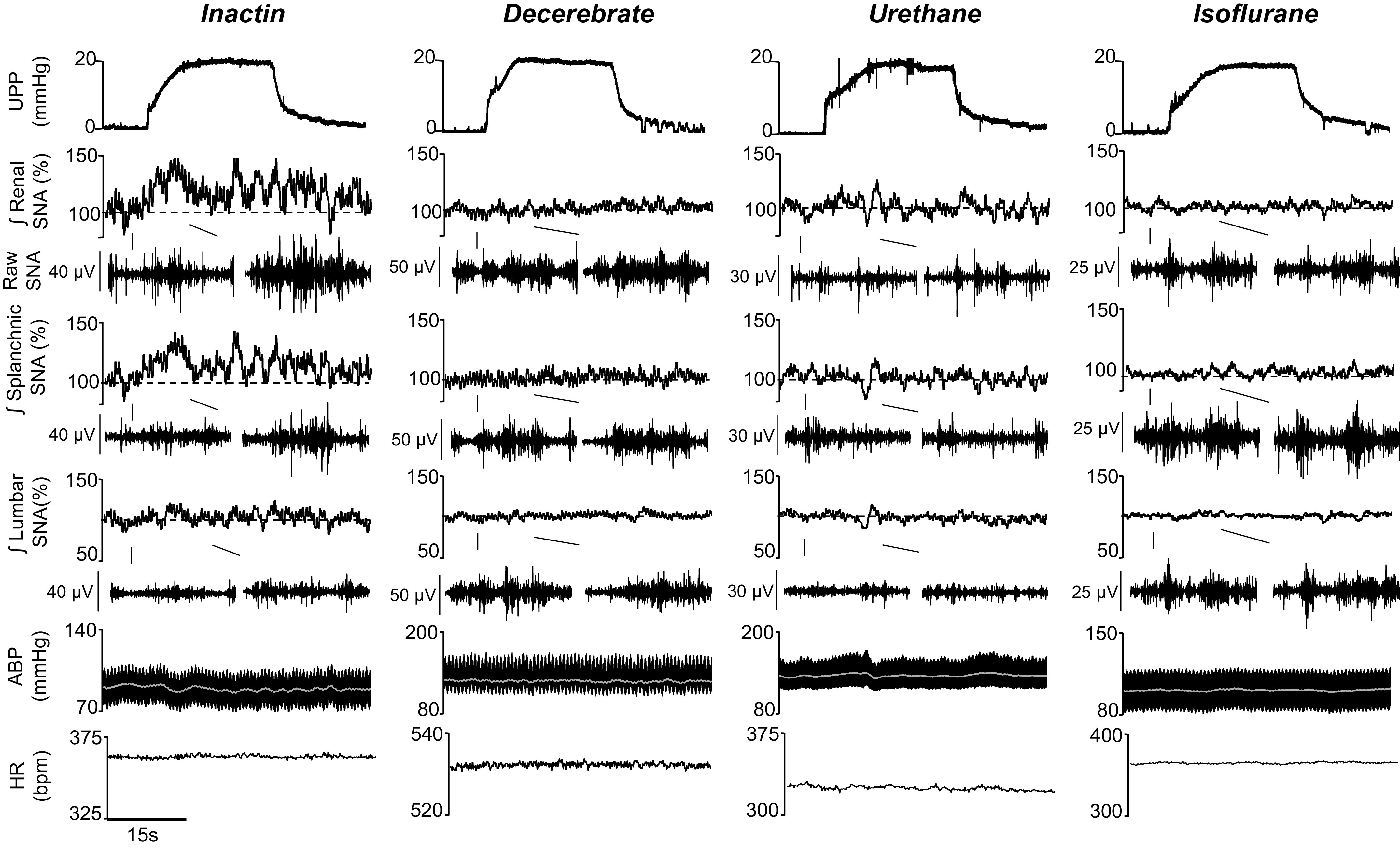
Example of rectified/integrated (∫) renal sympathetic nerve activity (SNA), splanchnic SNA, lumbar SNA, heart rate [HR; beats/min (bpm)], arterial blood pressure (ABP), and mean ABP (white line) during 30-s elevations in renal pelvic pressure (20 mmHg). Inactin anesthesia produced qualitatively greater increases in SNA vs. decerebrate, urethane, and isoflurane groups. Raw SNA tracings (0.5 s) highlight baseline and peak responses.
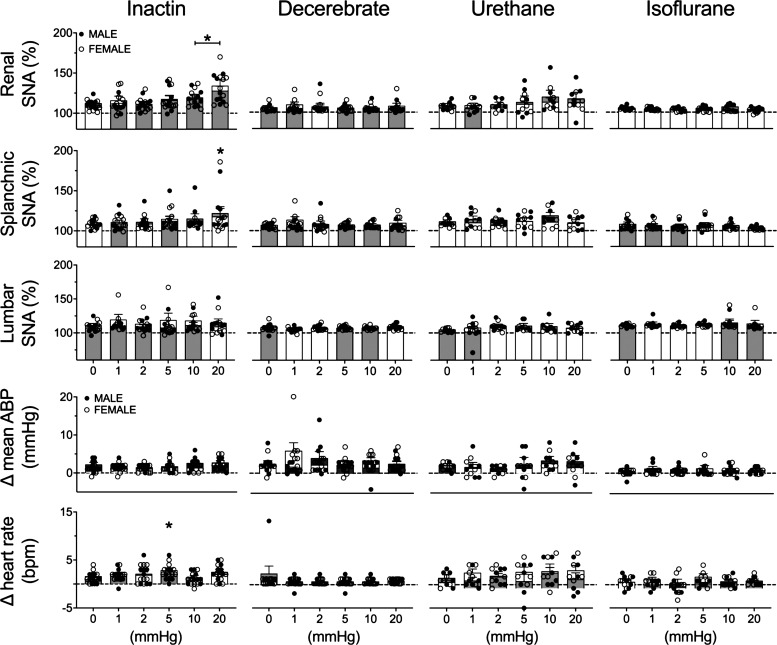
Figure 6 shows summary data for both male and female animals. The threshold sensitivity was assessed by ANOVA and defined as the lowest pressure to significantly increase SNA versus control (0 mmHg) pressure step. In the Inactin group, a significant effect of elevated pelvic pressure was observed for renal SNA (F5,75 = 11.42, P < 0.0001, ANOVA) and splanchnic SNA (F5,75 = 2.466, P = 0.0401, ANOVA) but not for lumbar SNA (F5,65 = 0.6543, P = 0.6593, ANOVA). The threshold sensitivity was 10 and 20 mmHg for renal and splanchnic SNA, respectively (Fig. 6). In the decerebrate group, increased pelvic pressure did not significantly alter renal SNA (F5,75 = 0.5748, P = 0.7191, ANOVA), splanchnic SNA (F5,75 = 0.7696, P = 0.5746, ANOVA), or lumbar SNA (F5,75 = 2.213, P = 0.0617, ANOVA). In the urethane group, increased pelvic pressure did not alter renal SNA (F5,50 = 2.112, P = 0.0794, ANOVA), splanchnic SNA (F5,50 = 1.289, P = 0.2836, ANOVA), or lumbar SNA (F5,50 = 0.9969, P = 0.4293, ANOVA). Similarly in the isoflurane group, no significant effects were observed for renal SNA (F5,60 = 1.145, P = 0.3471, ANOVA), splanchnic SNA (F5,60 = 1.875, P = 0.1121, ANOVA), or lumbar SNA (F5,60 = 1.271, P = 0.2883, ANOVA).
Figure 6.
Means ± SE and individual data points of the peak change in renal sympathetic nerve activity (SNA), splanchnic SNA, lumbar SNA, heart rate [beats/min (bpm)], and mean arterial blood pressure (ABP) to elevated renal pelvic pressure (0–20 mmHg). Male (M; gray bars) and female (F; white bars animals are indicated for Inactin (n = 16; 8 M, 8 F), decerebrate (n = 16; 8 M, 8 F), urethane (n = 11; 6 M, 5 F), and isoflurane (n = 13; 7 M, 6 F) groups. One-way ANOVA with Dunnett’s post hoc test identified the lowest threshold concentration significantly different from saline within each group (*P < 0.05).
In the Inactin group, elevated pelvic pressure did not produce significant changes in mean ABP (F5,75 = 1.640, P = 0.1598, ANOVA) but did significantly alter heart rate (F6,75 = 2.797, P = 0.0227, ANOVA). Post hoc testing indicated a significant increase in heart rate at 5 mmHg only. Increased renal pelvic pressure did not change ABP or heart rate in decerebrate, urethane, or isoflurane groups (Fig. 6).
Since elevated pelvic pressures consistently evoked SNA and ABP responses in the Inactin group, a subsequent analysis was performed to assess the extent by which sex influences sympathetic responses in this group. Of note, there were no sex differences in any variable within the decerebrate, urethane, or isoflurane groups (analysis not shown). A two-way ANOVA of renal SNA responses revealed a significant main effect for time (pressure) (F5,70 = 11.13, P < 0.0001, ANOVA) but not for sex (F1,14 = 0.1383, P = 0.7156, ANOVA) and no interaction (F5,70 = 0.8413, P = 0.5250, ANOVA). A two-way ANOVA of splanchnic SNA responses revealed a significant main effect for pressure (F5,70 = 2.382, P < 0.0470, ANOVA) but not for sex (F1,14 = 0.04889, P = 0.8282, ANOVA) and no interaction (F5,70 = 0.4912, P = 0.7818, ANOVA). A two-way ANOVA of lumbar SNA responses revealed no significant effects for pressure (F5,60 = 1.613, P < 0.1703, ANOVA) or sex (F1,12 = 0.6893, P = 0.4226, ANOVA) or interaction (F5,60 = 1.613, P = 0.1703, ANOVA). A two-way ANOVA of mean ABP indicated no significant effect for pressure (F5,70 = 1.727, P = 0.1397, ANOVA) or sex (F1,14 = 2.455, P = 0.1395, ANOVA) or interaction (F5,70 = 1.797, P = 0.1248, ANOVA). For heart rate, no significant effects were observed for pressure (F5,70 = 1.727, P = 0.1397, ANOVA) or sex (F1,14 = 2.455, P = 0.1395, ANOVA) and no interaction (F5,70 = 1.797, P = 0.1248, ANOVA).
DISCUSSION
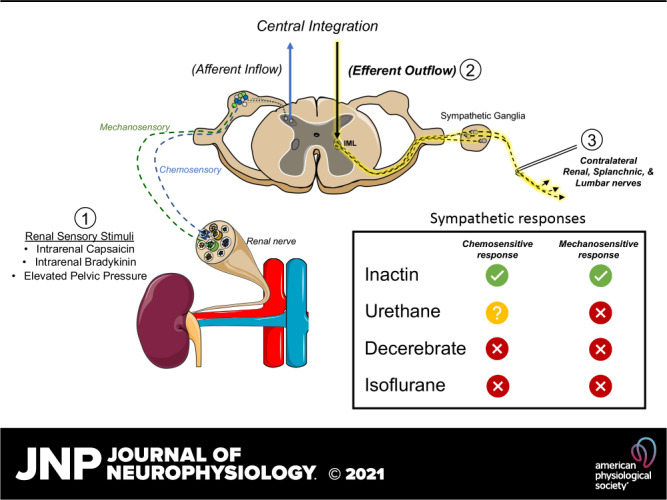
The present study examined the extent to which anesthesia and sex influence efferent SNA and hemodynamic responses to renal chemosensory and mechanosensory stimuli. First, intrarenal artery infusion of capsaicin or bradykinin produced renal and splanchnic sympathoexcitation in Inactin-anesthetized animals. Such responses were largely attenuated in decerebrate and urethane groups and absent in the isoflurane group. Interestingly, renal chemosensory stimuli produced a differential sympathetic response profile in which renal and splanchnic SNA increased but lumbar SNA decreased. Second, elevation of renal pelvic pressure significantly increased renal and splanchnic SNA at 10–20 mmHg only in the Inactin group. Again, these responses were absent in decerebrate, urethane, and isoflurane groups. Third, sex did not consistently influence SNA responses to intrarenal infusion of chemokines or elevated renal pelvic pressure. Overall, SNA and hemodynamic responses to renal chemosensory and mechanosensory stimuli were attenuated by decerebration, urethane, and isoflurane but unaffected by sex. Therefore, future investigations should consider using Inactin anesthesia to study SNA and hemodynamic responses during activation of renal sensory nerves or in renovascular models of hypertension.
Chemosensitive renal sensory nerves respond to renal ischemia, the chemical composition of urine in the renal pelvis, and various local factors including substance P and bradykinin (3, 6, 47, 48). The mechanisms by which these chemosensitive afferent nerves are activated remain to be identified, but a role for TRPV1-containing fibers has been proposed (49, 50). TRPV1 channels are expressed in renal sensory nerve endings (19, 51, 52), and renal administration of TRPV1 agonists increases renal afferent nerve activity (1, 2, 49). To first assess whether anesthesia can influence efferent autonomic responses to renal sensory nerve activation, we infused the TRPV1 agonist capsaicin into the renal artery. Intrarenal arterial capsaicin infusion produced concentration-dependent increases in renal and splanchnic SNA but decreases in lumbar SNA in the Inactin group. A 30 μM dose of capsaicin produced a small but significant increase in ABP in this group. In contrast, SNA and hemodynamic responses were absent in decerebrate, urethane, and isoflurane groups. In our prior investigation, intrarenal arterial infusion of capsaicin significantly increased renal afferent nerve activity in Inactin, decerebrate, urethane, and isoflurane preparations (2). However, Inactin and decerebration yielded the most sensitive and largest afferent responses, whereas urethane and isoflurane groups exhibited attenuated responses. Here, the lack of reflexive efferent sympathetic responses to intrarenal arterial capsaicin infusion may reflect 1) an anesthetic-dependent reduction of sensory input into the brain in urethane and isoflurane groups, 2) the influence of anesthetics on central integration or synaptic transmission, or 3) a combination of both. For unanesthetized decerebrate preparations, a lack of efferent SNA response indicates that decerebration may sever critical forebrain structures that underlie the reflexive changes in SNA and ABP to intrarenal capsaicin infusion. Nevertheless, our data suggest that reflex responses to intra-arterial capsaicin infusion were observed in Inactin versus decerebrate, urethane, and isoflurane preparations.
As a secondary chemosensory stimulus, bradykinin was infused into the renal artery to activate renal afferent fibers. Bradykinin is an endogenous vasoactive peptide that can stimulate prostaglandin synthesis, modulate immune cell function, and sensitize nociceptors (53). As a renal chemosensitive stimulus, intrapelvic bradykinin infusion increases renal afferent nerve activity (47). Intrarenal artery infusion of bradykinin produces renal nerve-dependent pressor responses and changes in vascular resistance (6). In the present study, intra-arterial bradykinin infusion into Inactin-anesthetized rats produced concentration-dependent increases in renal and splanchnic SNA, as well as increases in ABP and heart rate. Lumbar SNA significantly decreased. At higher bradykinin concentrations, renal and splanchnic sympathoexcitatory responses were also observed in the urethane group. Such responses were absent in decerebrate and isoflurane groups. Of note, these data support observations in conscious rats in which intra-arterial bradykinin infusion elevated ABP and differentially altered renal, mesenteric, and hindlimb vascular resistance (6). Here, the preservation of bradykinin-evoked responses in only the Inactin group underscores evidence that anesthesia differentially influences autonomic function and suggests that an Inactin preparation permits the study of chemosensory reflex responses when anesthesia is unavoidable.
Renal sensory nerves also exhibit mechanosensitive properties and are activated by abrupt changes in renal perfusion pressure or elevations in renal pelvic pressure (54, 55). Published data from our laboratory and others demonstrate that mechanosensitive afferent nerves are exquisitely sensitive to changes in renal pelvic pressures as low as 2–5 mmHg (2, 55). In the present study, elevating renal pelvic pressure (1–20 mmHg; 30 s) produced pressure-dependent increases in renal and splanchnic SNA at 10 and 20 mmHg, respectively, and no change in lumbar SNA, ABP, or heart rate with Inactin anesthesia. Efferent responses were absent in decerebrate, urethane, and isoflurane preparations. These data support a pattern of contralateral renal sympathoexcitation to increased renal pelvic pressure. Our data contrast early studies indicating a renal pressure-dependent sympathoinhibitory reflex that lowers renal SNA to promote natriuresis and diuresis involved in fluid/electrolyte balance (4). A key difference between early studies and observations here were longer (10–20 min) pressure steps of >20 mmHg in rats. The present experiments employed short (30 s) pressure steps at ≤20 mmHg. The rationale for avoiding high and prolonged pressure steps was the possibility of activating chemosensory fibers in the renal pelvis by compressing the renal parenchyma and impeding urine or renal capillary flow within the renal medulla (48, 56). Another experimental distinction between our work and others is that earlier studies sectioned the renal nerve at the level of the aorta to simultaneously record afferent nerve activity with contralateral renal functional or contralateral efferent nerve activity (4, 57, 58). The degree of renal innervation to the stimulated kidney is unclear and as such compromises afferent input and likely renal reflex responses. Overall, efferent reflex responses to mechanosensitive stimuli are clearly influenced by anesthesia, and evaluating these responses likely requires careful consideration of both the pressure duration and the magnitude of the stimulus.
SNA responses to chemosensory and mechanosensory stimuli were consistently impaired or absent in decerebrate and isoflurane preparations. Decerebrate animals exhibit pronounced increases in renal afferent nerve activity to intrarenal capsaicin infusion or increased pelvic pressure (2). The lack of any change in SNA or ABP in the decerebrate group indicates that decerebration likely severs forebrain nuclei critical for efferent and hemodynamic responses. Although the central nervous system circuitry for such responses is not defined, the hypothalamic paraventricular nucleus (PVN) may contribute. For example, electrical stimulation of renal afferent nerves increases Fos-labeled neurons in the brain stem and forebrain, including the PVN (59). PVN neurons are excited by renal afferent nerve stimulation (60, 61), and PVN lesion attenuates hemodynamic responses to renal afferent nerve stimulation (62). Furthermore, it is not yet known whether central integration of chemosensitive and mechanosensitive information utilize similar or distinct autonomic nuclei. In the isoflurane group, reflex responses were similarly impaired for both SNA and hemodynamics. This surprising finding is consistent with a suppressive role of isoflurane anesthesia on autonomic function and is supported by studies demonstrating that volatile anesthetic agents functionally modify postsynaptic receptors such as the GABAA receptor (63, 64). Thus, the lack of efferent responses with decerebrate or isoflurane preparations suggests that sensory input from the kidney requires an intact central nervous system and highlights the negative impact of isoflurane anesthesia in assessing autonomic function, respectively.
Sex as a biological variable impacts autonomic function, renal function, and cardiovascular hemodynamics (65, 66). In this study, sex as a factor was only analyzed in the Inactin group, as responses in other preparations were absent. SNA and ABP responses to elevated renal pelvic pressure or intrarenal infusion of capsaicin or bradykinin did not consistently differ between female and male groups. However, baseline renal SNA was significantly lower in female versus male rats. Since SNA responses were normalized to baseline values (100%), the absolute voltage SNA response is likely attenuated in female versus male mice. This attenuated response would be consistent with attenuated centrally evoked pressor responses in female versus male rodents (40, 67) and attenuated muscle SNA responses to handgrip in human female versus male subjects (38, 39). Numerous experimental models as well as clinical hypertension are influenced by sex (29, 68–70), and key autonomic regulatory centers express estrogen receptors that could be protected by sex hormones (71–73). Thus, whether the sex-dependent nature in these neurogenic models reflects reduced activation of renal sensory nerves versus a sex-dependent modulation of central autonomic circuits remains to be investigated.
GRANTS
The research was supported by NIH NHLBI Grants R01 HL-145875 (S.D.S.) and R01 HL-152680 (S.D.S.) and postdoctoral NIH F32 DK-123994 (L.J.D.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.J.D. and S.D.S. conceived and designed research; L.J.D. and S.D.S. performed experiments; L.J.D. analyzed data; L.J.D. and S.D.S. interpreted results of experiments; L.J.D. prepared figures; L.J.D. drafted manuscript; L.J.D. and S.D.S. edited and revised manuscript; L.J.D. and S.D.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Alan Sved for helpful discussions.
REFERENCES
- 1.Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y, Osborn JW. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 68: 1415–1423, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLalio LJ, Stocker SD. Impact of anesthesia, sex, and circadian cycle on renal afferent nerve sensitivity. Am J Physiol Heart Circ Physiol 320: H117–H132, 2021. doi: 10.1152/ajpheart.00675.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katholi RE, Hageman GR, Whitlow PL, Woods WT. Hemodynamic and afferent renal nerve responses to intrarenal adenosine in the dog. Hypertension 5: I149–I154, 1983. doi: 10.1161/01.hyp.5.2_pt_2.i149. [DOI] [PubMed] [Google Scholar]
- 4.Kopp UC, Olson LA, DiBona GF. Renorenal reflex responses to mechano- and chemoreceptor stimulation in the dog and rat. Am J Physiol Renal Physiol 246: F67–F77, 1984. doi: 10.1152/ajprenal.1984.246.1.F67. [DOI] [PubMed] [Google Scholar]
- 5.Recordati GM, Moss NG, Waselkov L. Renal chemoreceptors in the rat. Circ Res 43: 534–543, 1978. doi: 10.1161/01.RES.43.4.534. [DOI] [PubMed] [Google Scholar]
- 6.Smits JF, Brody MJ. Activation of afferent renal nerves by intrarenal bradykinin in conscious rats. Am J Physiol Regul Integr Comp Physiol 247: R1003–R1008, 1984. doi: 10.1152/ajpregu.1984.247.6.R1003. [DOI] [PubMed] [Google Scholar]
- 7.Katholi RE, Whitlow PL, Hageman GR, Woods WT. Intrarenal adenosine produces hypertension by activating the sympathetic nervous system via the renal nerves in the dog. J Hypertens 2: 349–359, 1984. [PubMed] [Google Scholar]
- 8.Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension 11: 3–20, 1988. doi: 10.1161/01.HYP.11.1.3. [DOI] [PubMed] [Google Scholar]
- 9.Fisher JP, Paton JF. The sympathetic nervous system and blood pressure in humans: implications for hypertension. J Hum Hypertens 26: 463–475, 2012. doi: 10.1038/jhh.2011.66. [DOI] [PubMed] [Google Scholar]
- 10.Johansson M, Elam M, Rundqvist B, Eisenhofer G, Herlitz H, Lambert G, Friberg P. Increased sympathetic nerve activity in renovascular hypertension. Circulation 99: 2537–2542, 1999. doi: 10.1161/01.CIR.99.19.2537. [DOI] [PubMed] [Google Scholar]
- 11.Miyajima E, Yamada Y, Yoshida Y, Matsukawa T, Shionoiri H, Tochikubo O, Ishii M. Muscle sympathetic nerve activity in renovascular hypertension and primary aldosteronism. Hypertension 17: 1057–1062, 1991. doi: 10.1161/01.HYP.17.6.1057. [DOI] [PubMed] [Google Scholar]
- 12.Grassi G, Seravalle G, Brambilla G, Trabattoni D, Cuspidi C, Corso R, Pieruzzi F, Genovesi S, Stella A, Facchetti R, Spaziani D, Bartorelli A, Mancia G. Blood pressure responses to renal denervation precede and are independent of the sympathetic and baroreflex effects. Hypertension 65: 1209–1216, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04823. [DOI] [PubMed] [Google Scholar]
- 13.Hausberg M, Kosch M, Harmelink P, Barenbrock M, Hohage H, Kisters K, Dietl KH, Rahn KH. Sympathetic nerve activity in end-stage renal disease. Circulation 106: 1974–1979, 2002. doi: 10.1161/01.CIR.0000034043.16664.96. [DOI] [PubMed] [Google Scholar]
- 14.Kandzari DE, Böhm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, Brar S, Cohen SA, Fahy M, Pilcher G, Kario K; SPYRAL HTN-ON MED Trial Investigators. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 391: 2346–2355, 2018. doi: 10.1016/S0140-6736(18)30951-6. [DOI] [PubMed] [Google Scholar]
- 15.Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp AS, Watkinson AF, Schmieder RE, Schmid A, Choi JW, East C, Walton A, Hopper I, Cohen DL, Wilensky R, Lee DP, Ma A, Devireddy CM, Lea JP, Lurz PC, Fengler K, Davies J, Chapman N, Cohen SA, DeBruin V, Fahy M, Jones DE, Rothman M, Böhm M; SPYRAL HTN-OFF MED trial investigators. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet 390: 2160–2170, 2017. doi: 10.1016/S0140-6736(17)32281-X. [DOI] [PubMed] [Google Scholar]
- 16.Banek CT, Gauthier MM, Baumann DC, Van Helden D, Asirvatham-Jeyaraj N, Panoskaltsis-Mortari A, Fink GD, Osborn JW. Targeted afferent renal denervation reduces arterial pressure but not renal inflammation in established DOCA-salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol 314: R883–R891, 2018. doi: 10.1152/ajpregu.00416.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foss JD, Fink GD, Osborn JW. Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension 61: 806–811, 2013.doi: 10.1161/HYPERTENSIONAHA.111.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foss JD, Wainford RD, Engeland WC, Fink GD, Osborn JW. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol 308: R112–R122, 2015. doi: 10.1152/ajpregu.00427.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ong J, Kinsman BJ, Sved AF, Rush BM, Tan RJ, Carattino MD, Stocker SD. Renal sensory nerves increase sympathetic nerve activity and blood pressure in 2-kidney 1-clip hypertensive mice. J Neurophysiol 122: 358–367, 2019. doi: 10.1152/jn.00173.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barringer DL, Buñag RD. Differential anesthetic depression of chronotropic baroreflexes in rats. J Cardiovasc Pharmacol 15: 10–15, 1990. doi: 10.1097/00005344-199001000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Fluckiger JP, Sonnay M, Boillat N, Atkinson J. Attenuation of the baroreceptor reflex by general anesthetic agents in the normotensive rat. Eur J Pharmacol 109: 105–109, 1985. doi: 10.1016/0014-2999(85)90545-X. [DOI] [PubMed] [Google Scholar]
- 22.Hegarty AA, Hayward LF, Felder RB. Sympathetic responses to stimulation of area postrema in decerebrate and anesthetized rats. Am J Physiol Heart Circ Physiol 268: H1086–H1095, 1995. doi: 10.1152/ajpheart.1995.268.3.H1086. [DOI] [PubMed] [Google Scholar]
- 23.Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol Regul Integr Comp Physiol 256: R1325–R1330, 1989. doi: 10.1152/ajpregu.1989.256.6.R1325. [DOI] [PubMed] [Google Scholar]
- 24.Matsukawa K, Ninomiya I. Anesthetic effects on tonic and reflex renal sympathetic-nerve activity in awake cats. Am J Physiol Regul Integr Comp Physiol 256: R371–R378, 1989. doi: 10.1152/ajpregu.1989.256.2.R371. [DOI] [PubMed] [Google Scholar]
- 25.Morita H, Nishida Y, Uemura N, Hosomi H. Effect of pentobarbital anesthesia on renal sympathetic nerve activity in the rabbit. J Auton Nerv Syst 20: 57–64, 1987. doi: 10.1016/0165-1838(87)90081-6. [DOI] [PubMed] [Google Scholar]
- 26.Shimokawa A, Kunitake T, Takasaki M, Kannan H. Differential effects of anesthetics on sympathetic nerve activity and arterial baroreceptor reflex in chronically instrumented rats. J Auton Nerv Syst 72: 46–54, 1998. doi: 10.1016/S0165-1838(98)00084-8. [DOI] [PubMed] [Google Scholar]
- 27.Stornetta RL, Guyenet PG, McCarty RC. Autonomic nervous system control of heart rate during baroreceptor activation in conscious and anesthetized rats. J Auton Nerv Syst 20: 121–127, 1987. doi: 10.1016/0165-1838(87)90109-3. [DOI] [PubMed] [Google Scholar]
- 28.Vlahakos D, Gavras I, Gavras H. α-Adrenoceptor agonists applied in the area of the nucleus tractus solitarii in the rat: effect of anesthesia on cardiovascular responses. Brain Res 347: 372–375, 1985. doi: 10.1016/0006-8993(85)90202-1. [DOI] [PubMed] [Google Scholar]
- 29.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics—2019 Update: a report from the American Heart Association. Circulation. 139: e56–e528, 2019. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 30.Burt VL, Cutler JA, Higgins M, Horan MJ, Labarthe D, Whelton P, Brown C, Roccella EJ. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population: data from the health examination surveys, 1960 to 1991. Hypertension 26: 60–69, 1995[Erratum inHypertension27: 1192, 1996]. doi: 10.1161/01.HYP.26.1.60. [DOI] [PubMed] [Google Scholar]
- 31.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension 53: 571–576, 2009. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hogarth AJ, Mackintosh AF, Mary DA. Gender-related differences in the sympathetic vasoconstrictor drive of normal subjects. Clin Sci (Lond) 112: 353–361, 2007. doi: 10.1042/CS20060288. [DOI] [PubMed] [Google Scholar]
- 33.Wiinberg N, Høegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens 8: 978–986, 1995. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 34.Crofton JT, Share L. Gonadal hormones modulate deoxycorticosterone-salt hypertension in male and female rats. Hypertension 29: 494–499, 1997. doi: 10.1161/01.HYP.29.1.494. [DOI] [PubMed] [Google Scholar]
- 35.Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension 44: 405–409, 2004. doi: 10.1161/01.HYP.0000142893.08655.96. [DOI] [PubMed] [Google Scholar]
- 36.Reckelhoff JF, Zhang H, Srivastava K, Granger JP. Gender differences in hypertension in spontaneously hypertensive rats: role of androgens and androgen receptor. Hypertension 34: 920–923, 1999. doi: 10.1161/01.HYP.34.4.920. [DOI] [PubMed] [Google Scholar]
- 37.Xue B, Johnson AK, Hay M. Sex differences in angiotensin II-induced hypertension. Braz J Med Biol Res 40: 727–734, 2007. doi: 10.1590/S0100-879X2007000500018. [DOI] [PubMed] [Google Scholar]
- 38.Ettinger SM, Silber DH, Collins BG, Gray KS, Sutliff G, Whisler SK, McClain JM, Smith MB, Yang QX, Sinoway LI. Influences of gender on sympathetic nerve responses to static exercise. J Appl Physiol (1985) 80: 245–251, 1996. doi: 10.1152/jappl.1996.80.1.245. [DOI] [PubMed] [Google Scholar]
- 39.Jarvis SS, VanGundy TB, Galbreath MM, Shibata S, Okazaki K, Reelick MF, Levine BD, Fu Q. Sex differences in the modulation of vasomotor sympathetic outflow during static handgrip exercise in healthy young humans. Am J Physiol Regul Integr Comp Physiol 301: R193–R200, 2011. doi: 10.1152/ajpregu.00562.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cherney A, Edgell H, Krukoff TL. NO mediates effects of estrogen on central regulation of blood pressure in restrained, ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 285: R842–R849, 2003. doi: 10.1152/ajpregu.00035.2003. [DOI] [PubMed] [Google Scholar]
- 41.Shi Z, Hansen KM, Bullock KM, Morofuji Y, Banks WA, Brooks VL. Resistance to the sympathoexcitatory effects of insulin and leptin in late pregnant rats. J Physiol 597: 4087–4100, 2019. doi: 10.1113/JP278282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinsman BJ, Simmonds SS, Browning KN, Stocker SD. Organum vasculosum of the lamina terminalis detects NaCl to elevate sympathetic nerve activity and blood pressure. Hypertension 69: 163–170, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simmonds SS, Lay J, Stocker SD. Dietary salt intake exaggerates sympathetic reflexes and increases blood pressure variability in normotensive rats. Hypertension 64: 583–589, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stocker SD, Lang SM, Simmonds SS, Wenner MM, Farquhar WB. Cerebrospinal fluid hypernatremia elevates sympathetic nerve activity and blood pressure via the rostral ventrolateral medulla. Hypertension 66: 1184–1190, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dobson KL, Harris J. A detailed surgical method for mechanical decerebration of the rat. Exp Physiol 97: 693–698, 2012. doi: 10.1113/expphysiol.2012.064840. [DOI] [PubMed] [Google Scholar]
- 46.Meehan CF, Mayr KA, Manuel M, Nakanishi ST, Whelan PJ. Decerebrate mouse model for studies of the spinal cord circuits. Nat Protoc 12: 732–747, 2017. doi: 10.1038/nprot.2017.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kopp UC, Smith LA. Role of prostaglandins in renal sensory receptor activation by substance-P and bradykinin. Am J Physiol Regul Integr Comp Physiol 265: R544–R551, 1993. doi: 10.1152/ajpregu.1993.265.3.R544. [DOI] [PubMed] [Google Scholar]
- 48.Recordati G, Moss NG, Genovesi S, Rogenes P. Renal chemoreceptors. J Auton Nerv Syst 3: 237–251, 1981. doi: 10.1016/0165-1838(81)90066-7. [DOI] [PubMed] [Google Scholar]
- 49.Kopp UC, Smith LA. Inhibitory renorenal reflexes—a role for substance-P or other capsaicin-sensitive neurons. Am J Physiol Regul Integr Comp Physiol 260: R232–R239, 1991. doi: 10.1152/ajpregu.1991.260.1.R232. [DOI] [PubMed] [Google Scholar]
- 50.Xie C, Wang DH. Ablation of transient receptor potential vanilloid 1 abolishes endothelin-induced increases in afferent renal nerve activity. Hypertension 54: 1298–1305, 2009. doi: 10.1161/HYPERTENSIONAHA.109.132167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ditting T, Tiegs G, Rodionova K, Reeh PW, Neuhuber W, Freisinger W, Veelken R. Do distinct populations of dorsal root ganglion neurons account for the sensory peptidergic innervation of the kidney? Am J Physiol Renal Physiol 297: F1427–F1434, 2009. doi: 10.1152/ajprenal.90599.2008. [DOI] [PubMed] [Google Scholar]
- 52.Feng NH, Lee HH, Shiang JC, Ma MC. Transient receptor potential vanilloid type 1 channels act as mechanoreceptors and cause substance P release and sensory activation in rat kidneys. Am J Physiol Renal Physiol 294: F316–F325, 2008. doi: 10.1152/ajprenal.00308.2007. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, Ehnert C, Brenner GJ, Woolf CJ. Bradykinin and peripheral sensitization. Biol Chem 387: 11–14, 2006. doi: 10.1515/BC.2006.003. [DOI] [PubMed] [Google Scholar]
- 54.Aström A, Crafoord J. Afferent and efferent activity in the renal nerves of cats. Acta Physiol Scand 74: 69–78, 1968. doi: 10.1111/j.1748-1716.1968.tb04215.x. [DOI] [PubMed] [Google Scholar]
- 55.Kopp UC, Smith LA, Pence AL. Na+-K+-ATPase inhibition sensitizes renal mechanoreceptors activated by increases in renal pelvic pressure. Am J Physiol Regul Integr Comp Physiol 267: R1109–R1117, 1994. doi: 10.1152/ajpregu.1994.267.4.R1109. [DOI] [PubMed] [Google Scholar]
- 56.Moss NG, Karastoianova IV. Static and dynamic responses of renal chemoreceptor neurons to intrapelvic pressure increases in the rat. J Auton Nerv Syst 63: 107–114, 1997. doi: 10.1016/S0165-1838(96)00132-4. [DOI] [PubMed] [Google Scholar]
- 57.Kopp UC, Smith LA, DiBona GF. Renorenal reflexes—neural components of ipsilateral and contralateral renal responses. Am J Physiol Renal Physiol 249: F507–F517, 1985. doi: 10.1152/ajprenal.1985.249.4.F507. [DOI] [PubMed] [Google Scholar]
- 58.Kopp UC, Smith LA, DiBona GF. Impaired renorenal reflexes in spontaneously hypertensive rats. Hypertension 9: 69–75, 1987. doi: 10.1161/01.HYP.9.1.69. [DOI] [PubMed] [Google Scholar]
- 59.Solano-Flores LP, Rosas-Arellano MP, Ciriello J. Fos induction in central structures after afferent renal nerve stimulation. Brain Res 753: 102–119, 1997. doi: 10.1016/s0006-8993(96)01497-7. [DOI] [PubMed] [Google Scholar]
- 60.Caverson MM, Ciriello J. Renal and cardiovascular afferent inputs to hypothalamic paraventriculo-spinal neurons. Neurosci Lett 95: 167–172, 1988. doi: 10.1016/0304-3940(88)90651-9. [DOI] [PubMed] [Google Scholar]
- 61.Ciriello J. Afferent renal inputs to paraventricular nucleus vasopressin and oxytocin neurosecretory neurons. Am J Physiol Regul Integr Comp Physiol 275: R1745–R1754, 1998. doi: 10.1152/ajpregu.1998.275.6.R1745. [DOI] [PubMed] [Google Scholar]
- 62.Caverson MM, Ciriello J. Contribution of paraventricular nucleus to afferent renal nerve pressor response. Am J Physiol Regul Integr Comp Physiol 254: R531–R543, 1988. doi: 10.1152/ajpregu.1988.254.3.R531. [DOI] [PubMed] [Google Scholar]
- 63.Grasshoff C, Antkowiak B. Effects of isoflurane and enflurane on GABAA and glycine receptors contribute equally to depressant actions on spinal ventral horn neurones in rats. Br J Anaesth 97: 687–694, 2006. doi: 10.1093/bja/ael239. [DOI] [PubMed] [Google Scholar]
- 64.Jones MV, Brooks PA, Harrison NL. Enhancement of gamma‐aminobutyric acid‐activated Cl− currents in cultured rat hippocampal neurones by three volatile anaesthetics. J Physiol 449: 279–293, 1992. doi: 10.1113/jphysiol.1992.sp019086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hay M, Xue B, Johnson AK. Yes! Sex matters: sex, the brain and blood pressure. Curr Hypertens Rep 16: 458, 2014. doi: 10.1007/s11906-014-0458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sabolić I, Asif AR, Budach WE, Wanke C, Bahn A, Burckhardt G. Gender differences in kidney function. Pflugers Arch 455: 397–429, 2007. doi: 10.1007/s00424-007-0308-1. [DOI] [PubMed] [Google Scholar]
- 67.Gingerich S, Krukoff TL. Estrogen in the paraventricular nucleus attenuates l-glutamate–induced increases in mean arterial pressure through estrogen receptor β and NO. Hypertension 48: 1130–1136, 2006. doi: 10.1161/01.HYP.0000248754.67128.ff. [DOI] [PubMed] [Google Scholar]
- 68.Milner TA, Drake CT, Lessard A, Waters EM, Torres-Reveron A, Graustein B, Mitterling K, Frys K, Iadecola C. Angiotensin II-induced hypertension differentially affects estrogen and progestin receptors in central autonomic regulatory areas of female rats. Exp Neurol 212: 393–406, 2008. doi: 10.1016/j.expneurol.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ 3: 7, 2012. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xue B, Badaue-Passos D Jr, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK. Sex differences and central protective effect of 17β-estradiol in the development of aldosterone/NaCl-induced hypertension. Am J Physiol Heart Circ Physiol 296: H1577–H1585, 2009. doi: 10.1152/ajpheart.01255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saleh TM, Connell B. Role of oestrogen in the central regulation of autonomic function. Clin Exp Pharmacol Physiol 34: 827–832, 2007. doi: 10.1111/j.1440-1681.2007.04663.x. [DOI] [PubMed] [Google Scholar]
- 72.Stern JE, Zhang W. Preautonomic neurons in the paraventricular nucleus of the hypothalamus contain estrogen receptor β. Brain Res 975: 99–109, 2003. doi: 10.1016/S0006-8993(03)02594-0. [DOI] [PubMed] [Google Scholar]
- 73.Vanderhorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor‐α and ‐β immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol 488: 152–179, 2005. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]