Abstract
Objective
Comorbidities influence disease assessment in axial spondyloarthritis (axSpA), but their association with response to TNF inhibitors (TNFi) is unclear. We examined associations between comorbidity history at TNFi initiation and: (i) change in disease indices over time; (ii) binary response definitions; and (iii) time to treatment discontinuation.
Methods
We studied participants starting their first TNFi from a national axSpA register. Comorbidity categories were created from 14 physician-diagnosed conditions and compared against: change in disease indices over time using linear mixed effects models; BASDAI50/2 (50% or 2-unit reduction) and BASDAI < 4 at 6 months using logistic models; and time to treatment discontinuation using Cox models. Models were adjusted for age, gender, BMI, deprivation and education.
Results
In total, 994 were eligible for analysis (68% male, mean age 45 years); 21% had one comorbidity and 11% had ≥2. Baseline disease severity was higher in those with comorbidities across all indices, but absolute improvement over time was comparable for BASDAI and spinal pain. Participants with ≥2 comorbidities had smaller absolute improvement in BASFI and quality of life. This group also had numerically reduced odds of achieving BASDAI50/2 [odds ratio (OR) 0.81; 95% CI: 0.45, 1.45] and BASDAI < 4 (OR 0.57; 95% CI: 0.32, 1.04). Treatment discontinuation was increased in those with two comorbidities [hazard ratio (HR) 1.32; 95% CI: 0.88, 2.00] and ≥3 comorbidities (HR 2.18; 95% CI: 1.20, 3.93) compared with none.
Conclusions
Participants with multiple comorbidities had poorer treatment outcomes, particularly increased treatment discontinuation and poorer improvements in function and quality of life. These results inform clinicians and educate patients about response to the first TNFi given comorbidity burden.
Keywords: axial spondyloarthritis, comorbidities, treatment response, TNF inhibitors, ankylosing spondylitis
Rheumatology key messages
Comorbidity burden is associated with more severe disease at TNF inhibitor initiation.
AxSpA patients with multiple comorbidities experience reduced improvement in function and quality of life.
Rate of treatment discontinuation is over 2-fold higher in those with ≥3 comorbidities.
Introduction
Over half of patients with axial spondyloarthritis (axSpA) have at least one comorbidity [1, 2]. Comorbidities are associated with adverse health outcomes such as poorer function, health-related quality of life, work productivity and mortality [3]. Many, for example cardiovascular and gastrointestinal diseases, can directly influence treatment choice [4]. Comorbidities may also impact management through the way in which axSpA disease activity is assessed. Commonly used patient-reported disease activity indices (e.g. BASDAI and spinal pain) are inflated in the presence of comorbidities [5]. This may lead to treatment escalation (e.g. to biologic DMARDs) that is driven by the impact of comorbidities rather than axSpA inflammation.
Despite their potential to influence disease assessment, research into the impact of comorbidities on treatment response is scarce. This is an important unmet need for two reasons. First, up to half of patients do not respond to their first TNF inhibitor (TNFi) [6], thus identifying predictors or causal factors are informative for clinical practice. Second, if some axSpA disease indices are inflated in the presence of comorbidities, comorbid patients may have persistently high scores—thereby at risk of having treatment discontinued—despite successfully supressing axSpA inflammation. Of the limited number of existing studies [7–9], most rely on binary response definitions, such as a certain degree of improvement (e.g. 50% improvement in BASDAI) or disease state (e.g. AS Disease Activity Score (ASDAS) low disease activity <2.1). These outcomes are problematic in observational data where starting disease activity differs between groups under comparison: those with higher baseline disease activity are simultaneously more able to achieve the former (because there is more ‘room’ for improvement) and less able to achieve the latter (because greater absolute improvement is required), compared with those with lower disease activity. Thus, absolute improvement in disease activity is likely to differ from the binary response derived from the same index. Untangling the role of comorbidities on treatment response calls for more than one approach to defining response.
The aim of this study was to examine the association between comorbidity history at the time of commencing biologic therapy and response to TNFi using three response definitions: (i) absolute change in disease activity and other disease indices; (ii) binary response definitions; and (iii) time to treatment discontinuation.
Methods
Patient population
The British Society for Rheumatology Biologics Register for Ankylosing Spondylitis (BSRBR-AS) is a UK-wide prospective cohort study that recruited biologics-naïve patients fulfilling the ASAS (Assessment of SpondyloArthritis international Society) criteria for axial SpA between December 2012 and December 2017. The study protocol has been previously published [10]. Participants who started their first TNFi were eligible for this analysis (i.e. either individuals from the ‘biologic’ group or from the ‘non-biologic’ group but who subsequently started TNFi); they were followed up at baseline, 3, 6 and 12 months and annually thereafter. Eligible participants were required to have a baseline assessment within a window from 1 year before to 7 days after the TNFi start date, from which the baseline values of time-varying data (i.e. the disease indices) were obtained. All variables beside disease indices were considered time-invariant and could come from any assessment time if missing at baseline. This analysis used the study dataset of December 2018. Ethical approval was obtained from the National Research Ethics Service Committee North East – County Durham and Tees Valley (reference 11/NE/0374) and informed consent was obtained from all participants.
Comorbidity
Participating centres obtained a history of physician-diagnosed comorbidities from medical records, including: ischaemic heart disease, heart failure, stroke, hypertension, diabetes, asthma, chronic obstructive pulmonary disease (COPD), peptic ulcer disease, liver disease, renal disease, depression, cancer, tuberculosis (TB) and demyelinating disease. These conditions were selected through a consensus meeting of clinicians and researchers, based on commonly recorded comorbidities in routine practice. Comorbidity status was defined at baseline. Extra-skeletal manifestations of axSpA (uveitis, psoriasis and inflammatory bowel disease) were not considered comorbidities.
The count of 14 comorbidities was analysed as a categorical variable. This approach provides more information than comparing presence vs absence of comorbidities, and does not assume a linear relationship with the outcome as when using it as a continuous variable [9]. It is, however, limited by sample size; categories of higher comorbidity count were combined where necessary to make analyses feasible.
Outcomes
The outcome of Aim 1 was change in continuous disease indices over follow-up time. Response was assessed in the first 3 years because very few participants had longer follow-up. Disease activity was assessed using BASDAI, ASDAS and spinal pain numerical rating scale (NRS). We also examined functional impairment (BASFI), the AS quality of life questionnaire (ASQoL, which has a range of 0–18 with higher scores indicating poorer quality of life), and the Chalder Fatigue Scale Likert scale (CFQ, which has a range of 0–33 with higher scores indicating greater levels of fatigue [11]).
To demonstrate limitations of the prevailing approach, Aim 2 examined common binary response definitions at 6 months: BASDAI50/2 (50% or 2-unit reduction), ASDAS major improvement (ASDAS-MI, ≥2-unit reduction), and two ‘low disease activity’ definitions (BASDAI < 4 and ASDAS < 2.1). Participants with missing baseline BASDAI or ASDAS were excluded. Where the 6-month assessment was missing but individuals remained on drug, they were considered as responders if they demonstrated response at 3 or 12 months; participants were unlikely to remain on drug if they did not have or lost response, as per UK prescribing guidelines. Participants who discontinued treatment within 6 months for any reason were considered non-responders.
Aim 3 examined time-to-treatment discontinuation, defined as continuous time from TNFi initiation to discontinuation. Censoring was defined by the last study contact (visit or questionnaire) for those who did not discontinue treatment.
Covariates
Covariates were determined a priori including: age, gender, BMI, socioeconomic status (Index of Multiple Deprivation as a continuous variable) and educational attainment (as dummy variables). Baseline disease activity is a controversial covariate. Prior research suggested that comorbidities influence baseline assessment [5], in which case adjusting for it – a causal intermediate (or mediator) – would introduce bias. However, not accounting for baseline disease activity is problematic for binary response definitions (see above). Therefore, baseline disease activity was only included for binary outcome analyses, presented alongside the same models without baseline adjustment.
Statistics
Descriptive statistics were used to describe comorbidity prevalence in this analysis cohort and compare participant with and without comorbidities. For Aim 1, linear mixed effects models with interaction terms between comorbidity categories and categorical time (the assessment closest to each per-protocol follow-up) were used to compare continuous change in disease indices. The interaction term coefficient represents the additional difference in change from baseline (i.e. difference in ΔBASDAI; see online Supplementary Materials Table S1 and Fig. S1, available at Rheumatology online for further explanation). To facilitate interpretation, model-predicted values (with all covariates held at their mean value) were shown graphically, with asterisks indicating statistical significance of the interaction term at the P <0.05 level (all raw model coefficients are provided in online Supplementary Materials, available at Rheumatology online).
For Aim 2, each of the four binary response variables were used in turn as the dependent variable. Odds ratios were reported without, then with, adjustment for baseline values of the disease index of interest, in addition to the above covariates.
For Aim 3, Kaplan–Meier curves were used to show the shape of the survival function for each comorbidity category. The relationship between baseline comorbidity and discontinuation was then examined using Cox models adjusting for covariates. A small number of TNFi initiators did not have any subsequent assessments by visit or questionnaire. These individuals with zero follow-up time could contribute their baseline characteristics to the analysis by having an arbitrarily small follow-up (0.001 days) assigned, which does not affect the overall person-time. Proportional hazards assumption was tested using Schoenfeld residuals; non-proportional covariates were stratified in the model (i.e. allowing equal coefficients across strata but with a baseline hazard unique to each stratum).
For Aims 2 and 3, the overall significance of comorbidity dummy variables was tested using the Wald test.
Sensitivity analyses
Using the above definitions for binary response, individuals who stayed on treatment (i.e. no stop date) but did not have assessments recorded at 3, 6 or 12 months would have missing response values. In the first set of sensitivity analyses, these individuals were assumed to have responded at 6 months if they remained on treatment beyond one year.
Participants with baseline BASDAI <4 would not ordinarily be eligible for TNFi according to NICE guidance. These individuals were excluded in the second set of sensitivity analyses.
Results
Among 2687 participants in the BSRBR-AS, 1145 started on biologics, 17 were excluded for using non-TNFi bDMARD, 134 for having no valid baseline assessment within the required window. Thus, 994 were eligible for the current analysis. TNFi initiators included and excluded from the analysis set were similar in characteristics except the former were more often male (68 versus 60%) (see online Supplementary Materials Table S2, available at Rheumatology online). The analysis cohort was predominantly (68%) male with a mean age of 45 years (Table 1). Participants with comorbidities were older, had higher BMI, and less frequently had university education than those without; they also less frequently used NSAIDs in the past 6 months and had more severe disease across all indices.
Table 1.
Baseline characteristics of 994 participants in the longitudinal analysis cohort
| All participants (n = 994) | axSpA without comorbidities (n = 671) | axSpA with ≥1 comorbidity (n = 323) | P-value | ||
|---|---|---|---|---|---|
| Mean age, years | 44.7 (13.4) | 43.0 (12.7) | 48.3 (14.2) | <0.001 | |
| Males | 679 (68%) | 468 (70%) | 211 (65%) | 0.17 | |
| Meeting modified New York criteria | 597 (60%) | 394 (59%) | 203 (63%) | 0.24 | |
| Mean age at symptom onset, years | 28.6 (11.3) | 28.3 (10.9) | 29.4 (12.1) | 0.13 | |
| Mean symptom duration, years | 16.0 (12.6) | 14.7 (12.0) | 18.9 (13.4) | <0.001 | |
| HLA-B27 positivea | 540 (74%) | 382 (76%) | 158 (71%) | 0.12 | |
| Mean BMI, kg/m2 | 28.1 (5.8) | 27.5 (5.6) | 29.2 (6.1) | <0.001 | |
| Smoking status | Never smoked | 351 (40%) | 251 (42%) | 100 (35%) | 0.093 |
| Ex-smoker | 291 (33%) | 188 (32%) | 103 (36%) | ||
| Current smoker | 241 (27%) | 155 (26%) | 86 (30%) | ||
| Education | Secondary school | 289 (33%) | 184 (31%) | 105 (36%) | 0.022b |
| Apprenticeship | 82 (9%) | 56 (10%) | 26 (9%) | ||
| Further education college | 276 (31%) | 175 (30%) | 101 (35%) | ||
| University degree | 178 (20%) | 138 (23%) | 40 (14%) | ||
| Further degree | 53 (6%) | 36 (6%) | 17 (6%) | ||
| IMD, median (IQR) | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 0.12 | |
| NSAID use in past 6 months | 734 (75%) | 509 (77%) | 225 (70%) | 0.023 | |
| DMARD use in past 6 months | 146 (15%) | 104 (16%) | 42 (13%) | 0.29 | |
| BASDAI, median (IQR) | 6.7 (5.3, 7.8) | 6.5 (5.1, 7.7) | 7.2 (6.0, 8.2) | <0.001 | |
| Spinal pain, median (IQR) | 7.0 (5.0, 8.0) | 7.0 (5.0, 8.0) | 7.0 (6.0, 8.0) | <0.001 | |
| ASDAS, mean (s.d.)a | 2.9 (0.8) | 2.8 (0.8) | 3.0 (0.8) | <0.001 | |
| BASFI, median (IQR) | 6.5 (4.5, 8.1) | 6.1 (4.1, 7.7) | 7.1 (5.4, 8.6) | <0.001 | |
| ASQoL, median (IQR) | 13.0 (9.0, 16.0) | 12.0 (8.0, 15.0) | 14.0 (11.0, 17.0) | <0.001 | |
| Fatigue, median (IQR) | 17.5 (14.0, 21.0) | 17.0 (13.0, 21.0) | 19.0 (15.0, 23.0) | <0.001 | |
| Sleep, median (IQR) | 14.0 (8.0, 18.0) | 13.0 (8.0, 17.0) | 15.0 (10.0, 19.0) | 0.003 | |
Data shown as mean (s.d.) and n (%) unless otherwise indicated. bCuzick non-parametric test for trend. ASDAS: AS disease activity score; ASQoL: AS quality of life questionnaire; BASDAI: Bath AS disease activity index; BASFI: Bath AS functional index; IMD: index of multiple deprivation; IQR: interquartile range.
Depression was the most common comorbidity (15%), followed by hypertension (11%), asthma (8.7%), peptic ulcer disease (2.8%), diabetes mellitus (2.5%), cancer (1.6%), ischaemic heart disease (1.5%), TB (1.5%), renal disease (1.1%), COPD (1.0%), stroke (0.7%), liver disease (0.7%), heart failure (0.6%) and demyelinating disease (0.2%). The distribution of comorbidity count is shown in online Supplementary Materials, Fig. S2, available at Rheumatology online; around 1 in 5 had one comorbidity and 1 in 10 had two or more.
Absolute improvement in continuous outcomes
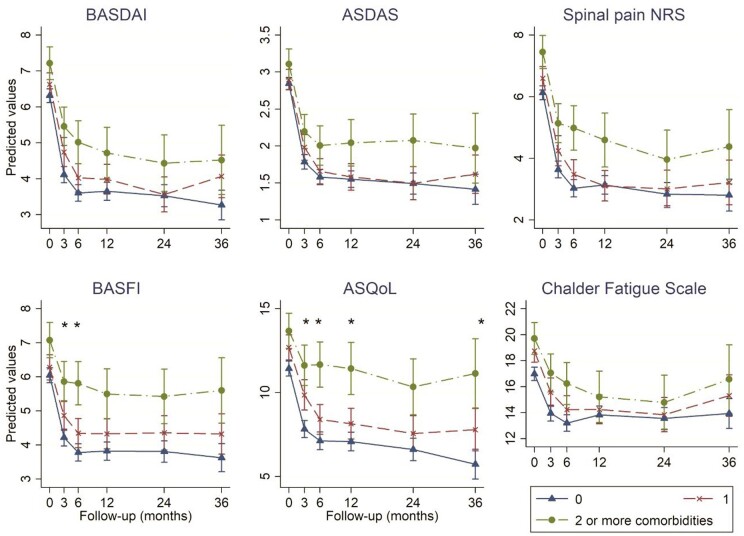
The top category was combined as ≥2 comorbidities due to the small number of participants with ≥3. Baseline disease severity was higher in the group with one comorbidity (vs none), reaching statistical significance for all six indices (Fig. 1 with accompanying coefficients in Supplementary Table S3 and S4, available at Rheumatology online).
Fig. 1.
Change in disease indices according to comorbidity count categories, shown as marginal predicted values from linear mixed models (with all covariates held constant)
Asterisks indicate differences in slope at the P <0.05 level between groups with 0 and ≥2 comorbidities. Full model output and marginal predictions are shown in Supplementary Tables S3 and S4, available at Rheumatology online. ASQoL, AS quality of life questionnaire; NRS, numerical rating scale.
The top three panels of Fig. 1 compare disease activity according to comorbidity count categories. All comparison groups showed an improvement, but there were no statistically significant differences in the absolute change from baseline (i.e. the three lines were not significantly different from parallel). Participants with 0 or 1 comorbidity had similar disease activity trajectories, while those with ≥2 comorbidities had significantly and persistently higher disease activity.
The bottom panels of Fig. 1 show differences in other disease indices. Again, all groups showed an improvement. Although patients with one comorbidity had numerically higher baseline BASFI, ASQoL and CFQ compared with those with none, the two groups had similar absolute improvement (i.e. trajectories were approximately parallel). Compared to those with no comorbidities, participants with ≥2 had smaller absolute improvement in BASFI by 0.6 units at 3 months and 1.0 unit at 6 months (i.e. the gradient of the dot-dash line is less ‘steep’ in the first 6 months). Absolute improvement in ASQoL was similarly smaller in participants with ≥2 comorbidities than none over the first 12 months.
Binary response definitions
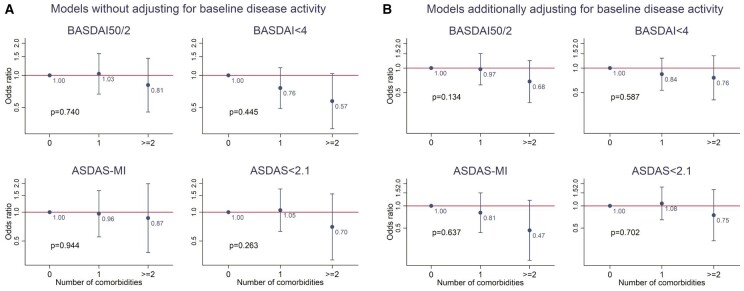
Overall, 51% of participants achieved BASDAI50/2 at 6 months, 52% BASDAI <4, 26% ASDAS-MI and 34% ASDAS <2.1. Given the small number of participants with ≥3 comorbidities, the top category was combined as ≥2 comorbidities. All model coefficients are provided in online Supplementary Materials Table S5, available at Rheumatology online. The difference in odds of achieving each binary response was not statistically significant with increasing number of comorbidities without adjusting for baseline values of each index (Fig. 2A). Effect sizes for BASDAI <4 (43% reduction) and ASDAS <2.1 (30% reduction) were, however, numerically different with potential clinical significance.
Fig. 2.
Associations between comorbidity count categories and binary responses at 6 months without (A) and with (B) adjustment for baseline BASDAI or ASDAS. BASDAI50/2, 50% or 2-unit reduction in BASDAI; ASDAS-MI, major improvement i.e. ≥2-unit reduction
(A) Models without adjusting for baseline disease activity. (B) Models additionally adjusting for baseline disease activity.
The same models that additionally adjusted for baseline values of each index decreased effect sizes for the ‘low disease activity’ definitions, but increased effect sizes for BASDAI50/2 (35% reduction) and ASDAS-MI (53% reduction) (Fig. 2B). This was explained by the effect sizes of baseline BASDAI/ASDAS: as a covariate, each unit higher baseline BASDAI significantly increased odds of achieving BASDAI50/2 [odds ratio (OR) 1.25; 95% CI: 1.12, 1.40] but decreased odds of BASDAI <4 (OR 0.68; 95% CI: 0.60, 0.77); the same was observed for ASDAS and derived response definitions (full model coefficients are shown in online Supplementary Table S5, available at Rheumatology online).
Time-to-treatment discontinuation
Of the 994 participants that started TNFi, 18 did not have any post-baseline follow-up and were each assigned an arbitrarily small follow-up time. The remaining 976 subjects had 1441 person-years of follow-up, with mean of 17 months and median 12 months. A total of 31% of the cohort stopped treatment over the whole study follow-up: 29% of those with no comorbidities, 30% of those with one, 42% two and 68% with ≥3.
Drug survival according to categories of comorbidity count is shown in Fig. 3. Log-rank test confirms a statistically significant difference, particularly notable for those with 2 and ≥3 comorbidities.
Fig. 3.
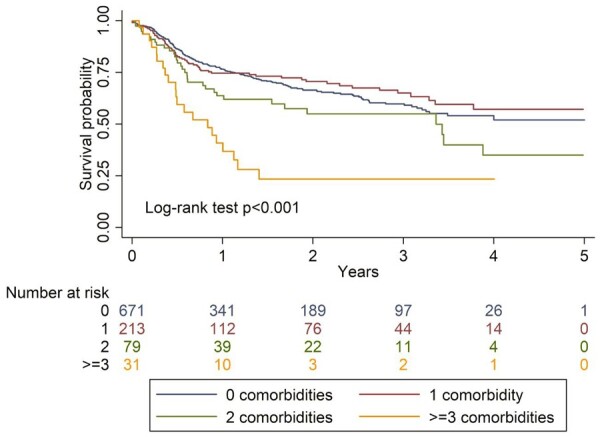
Kaplan–Meier curves comparing drug survival in participants according to categories of comorbidity count
Education violated the proportional hazards assumption and was stratified in the model. Results from the Cox model of categorical comorbidity count and risk of drug discontinuation are shown in Fig. 4 (model coefficients shown in Supplementary Table S6, available at Rheumatology online). Participants with one comorbidity were no more or less likely to discontinue TNFi compared with those with none (Kaplan–Meier estimates cross for these two groups). The hazard of TNFi discontinuation was 32% higher for those with two comorbidities (95% CI: 0.88, 2.00), and over 2-fold higher (HR2.18; 95% CI: 1.20, 3.93) for those with ≥3 comorbidities, compared with none.
Fig. 4.
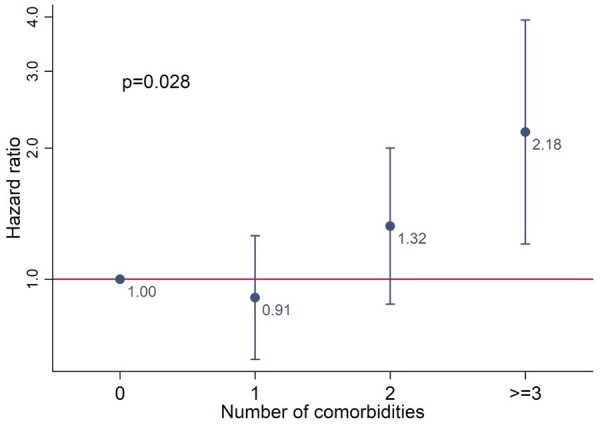
TNF inhibitor discontinuation compared according to the number of baseline comorbidities.
Sensitivity analyses
Analyses of binary outcomes with imputation changed the proportion of responders, particularly for ASDAS-based variables (due to more missing data with ASDAS). However, analyses of binary, continuous or categorical comorbidity count definitions showed no meaningful differences from results of the primary analysis (Supplementary Fig. S3, available at Rheumatology online). Excluding patients with baseline BASDAI < 4 showed similar results for the binary response and time-to-event analyses (data not shown). There were minor changes to precision in the analyses of absolute response, but not overall results (Supplementary Fig. S4, available at Rheumatology online).
Discussion
In this longitudinal study of response to the first TNF inhibitor, participants with greater comorbidity burden had higher disease activity at baseline that persisted throughout follow-up. Participants with multiple (≥2) comorbidities had significantly poorer absolute improvements in function and health-related quality of life. These individuals also had significantly higher rate of TNFi discontinuation compared with those without comorbidities.
Strengths of this study lie in its large sample size, recruited from a broad range of rheumatology centres. Ascertainment of comorbidities was robust, using physician diagnoses from medical records. Use of different outcome variables provided contextual overview that many similar studies lacked; for example, we showed that relying on binary response definitions is problematic in observational studies when comparison groups have different baseline disease activity. There were, however, limitations. Using comorbidity history collected at baseline means that the complex time-varying relationship between comorbidities and disease activity could not be captured. Comorbidity data in registry were not collected specifically for this secondary analysis; however, included comorbidities were representative of important diseases when compared with prior axSpA research [3]. Results from analysis of change over time should be interpreted with one important caveat: participants who did not respond by the first assessment (usually after 3 months) or those who lost response would have had their treatment stopped under NICE guidance; therefore, record of such high disease activity would be censored. Informative censoring should not affect data within the first 3 months [12]. This may help explain the dramatic difference between the relatively unimpressive effect sizes of the response analyses compared with treatment discontinuation. Data on reasons for discontinuation in the BSRBR-AS had limitations that precluded inclusion into primary analyses; reasons did not appear to differ according to the presence of comorbidities (data not shown). We included assessments up to a year before the TNFi start date as baseline because there were variable delays in patients obtaining the drug, and the time when non-biologic group participants subsequently started TNFi rarely coincided with their annual assessment. Three-quarters of patients had assessments within 3 months before TNFi start date; restricting analysis to this subgroup did not change results (data not shown). Lastly, analyses of comorbidity count assume that individual comorbidities affect the outcomes equally. This is unlikely to be true and larger studies are required to examine relative contributions of individual conditions in the context of overall comorbidity burden; for example, COPD may contribute to reduced physical function while depression may affect adherence [13].
Three studies have previously examined the same topic. Iannone [8] et al. showed that mRCDI (modified Rheumatic Disease Comorbidity Index) was significantly correlated (by Spearman’s rank) with the number of biologic switches. Kaplan–Meier curves showed no difference between mRDCI of 1 and 0, while the mRDCI ≥ 2 group separated after ∼1 year. These curves were difficult to interpret as they suggested: (i) discontinuation was assessed at large intervals of discrete time, and (ii) >90% of those with mRDCI 0 or 1, but 0% of those with mRDCI ≥ 2, remained on treatment at end of follow-up (∼60 months). The second study, by Lindström et al. [7], examined risk of TNFi discontinuation in unadjusted Cox models for each of: cardiovascular disease (HR 1.24; 1.08, 1.43), affective disorder (HR 1.81; 1.54, 2.13), chronic lung disease (HR 1.49; 1.22, 1.82), malignancy (HR 1.36; 1.06, 1.74), diabetes (HR 1.36; 0.99, 1.87) and chronic kidney disease (HR 0.79; 0.41, 1.52). The third study used an earlier sample of 335 BSRBR-AS participants to show continuous comorbidity count as a predictor of binary response [9]. The investigators showed each additional comorbidity to reduce odds of achieving clinically important improvement in ASDAS (≥1.1) by 43% (95% CI: 0.37, 0.88) and ASDAS < 2.1 by 40% (95% CI: 0.38, 0.95) within 10 weeks to 9 months of treatment initiation. These prediction models assumed a log-linear relationship between outcomes and each additional comorbidity, which was shown not to be the case in this study.
Another limitation of these prior studies is the fact that they used only binary definitions of response. The decision to adjust for baseline disease activity or not are both fraught with potential bias. By analysing three types of outcomes, this study highlights the importance of interpreting each result in the context of the others. For example, patients with comorbidities may have lower odds of achieving ‘low disease activity’ states, but their degree of improvement from pre-treatment levels, particularly pain, is comparable to those with no comorbidities. Communicating the influence of comorbidities to patients should be more nuanced, depending on whether degree of improvement or a fixed low level of disease activity is more important to the individual. Existing definitions of response as used for prescribing guidelines in the UK, for example, may need to be reconsidered: a patient starting TNFi with BASDAI of 7 will have higher odds (by 25% in this data) of achieving BASDAI50/2, while simultaneously having lower odds of BASDAI < 4 (by 32%), than an identical individual with starting BASDAI of 6. Which definition of response is more relevant for health-economics remains to be determined. Lastly, many comorbid patients would have been ineligible for randomized controlled trials; this study provided unique insights into how therapeutic effects on function and health-related quality of life differed in comorbid patients, highlighting the need to optimize comorbidities, not just axSpA.
In conclusion, participants with multiple (≥2) comorbidities had reduced absolute improvement in function and health-related quality of life compared with those without. Overall, participants with comorbidities generally had reduced odds of achieving binary response definitions compared with those with none, although most effect estimates were small and with uncertainty. They also had significantly higher rate of treatment discontinuation. Taken together, results can be used to better inform clinicians and educate patients about the likelihood of response to the first TNFi given baseline comorbidity status. Further research is needed to identify potentially modifiable comorbidities that may improve response.
Supplementary Material
Acknowledgements
Contribution: S.S.Z. analysed the data and wrote the manuscript, with significant input from all co-authors. G.J.M. and G.T.J. are Chief Investigator and Deputy Chief Investigator, respectively, on BSRBR-AS and designed the study and oversaw its conduct. In the current project, they discussed results and provided input into drafts of the manuscript. We are grateful to the staff of the BSRBR-AS register and to the recruiting staff at the clinical centres, details of which are available at: www.abdn.ac.uk/bsrbr-as.
Funding: The BSRBR-AS is funded by the British Society for Rheumatology (BSR) who have received funding for this from Pfizer, AbbVie and UCB. These companies receive advance copies of manuscripts for comments. They have no input in determining the topics for analysis or work involved in undertaking it.
Disclosure statement: The authors declare no conflicts of interest.
Data availability statement
Data from the British Society for Rheumatology Biologics Register for Ankylosing Spondylitis are available to external investigators, on reasonable request. For information on how to access data, see: http://www.rheumatology.org.uk.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1.Zhao SS, Ermann J, Xu C. et al. Comparison of comorbidities and treatment between ankylosing spondylitis and non-radiographic axial spondyloarthritis in the United States. Rheumatology 2019;58:2025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao SS, Radner H, Siebert S. et al. Comorbidity burden in axial spondyloarthritis: a cluster analysis. Rheumatology 2019;58:1746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao SS, Robertson S, Reich T. et al. Prevalence and impact of comorbidities in axial spondyloarthritis: systematic review and meta-analysis. Rheumatology 2020;59:iv47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.González C, Curbelo Rodríguez R, Torre-Alonso JC. et al. Recommendations for the management of comorbidity in patients with axial spondyloarthritis in clinical practice. Reumatol Clín Engl Ed 201814:346–59. [DOI] [PubMed] [Google Scholar]
- 5.Zhao S, Jones G, Macfarlane G. et al. Association between comorbidities and disease activity in axial spondyloarthritis: results from the BSRBR-AS. Rheumatology 2020; doi:10.1093/rheumatology/keaa768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lord PA, Farragher TM, Lunt M, et al. Predictors of response to anti-TNF therapy in ankylosing spondylitis: results from the British Society for Rheumatology Biologics Register . Rheumatology 2010;49:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindström U, Olofsson T, Wedrén S, Qirjazo I, Askling J.. Impact of extra-articular spondyloarthritis manifestations and comorbidities on drug retention of a first TNF-inhibitor in ankylosing spondylitis: a population-based nationwide study. RMD Open 2018;4:e000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iannone F, Salaffi F, Fornaro M. et al. Influence of baseline modified Rheumatic Disease Comorbidity Index (mRDCI) on drug survival and effectiveness of biological treatment in patients affected with Rheumatoid arthritis, Spondyloarthritis and Psoriatic arthritis in real-world settings. Eur J Clin Invest 2018;48:e13013. [DOI] [PubMed] [Google Scholar]
- 9.Macfarlane GJ, Pathan E, Jones GT, Dean LE.. Predicting response to anti-TNFα therapy among patients with axial spondyloarthritis (axSpA): results from BSRBR-AS. Rheumatology 2020;59:2481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macfarlane GJ, Barnish MS, Jones EA. et al. The British Society for Rheumatology Biologics Registers in Ankylosing Spondylitis (BSRBR-AS) study: protocol for a prospective cohort study of the long-term safety and quality of life outcomes of biologic treatment. BMC Musculoskelet Disord 2015;16:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson C.The Chalder Fatigue Scale (CFQ 11). Occup Med Lond 2015;65:86. [DOI] [PubMed] [Google Scholar]
- 12.Zhao S, Yoshida K, Jones GT. et al. The impact of smoking on response to TNF inhibitors in axial spondyloarthritis: methodological considerations for longitudinal observational studies. Arthritis Care Res 2020;72:591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiMatteo MR, Lepper HS, Croghan TW.. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000;160:2101–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the British Society for Rheumatology Biologics Register for Ankylosing Spondylitis are available to external investigators, on reasonable request. For information on how to access data, see: http://www.rheumatology.org.uk.