Rheumatology key message
Response to JAK inhibition suggests that interferon hyperactivation underlies psoriatic arthritis in Down syndrome.
Dear Editor, People with Down syndrome (DS), the condition caused by trisomy 21 (T21), display increased prevalence of several autoimmune conditions relative to the general population, including autoimmune thyroid disease (AITD), celiac disease, autoimmune skin conditions and arthropathies [1]. Although it is now well established that T21 causes hyperactivation of IFN and downstream Janus kinase (JAK) signalling [2, 3], the therapeutic value of this observation remains to be defined. Here, we describe the first reported case of an individual with DS who was effectively treated with the JAK inhibitor tofacitinib as a first-line therapy for severely debilitating PsA.
Although tofacitinib is approved by the United States Food and Drug Administration for the treatment of PsA in the typical population, it is considered less effective than first-line targeted agents that inhibit TNF-α, IL17, IL12 and/or IL23 signalling [4]. However, given that T21 causes elevated IFN and JAK signalling, we reasoned that tofacitinib would be a preferable first-line treatment for PsA in DS. Informed consent for research was obtained in accordance with the Declaration of Helsinki and specific consent obtained for this report. This study was approved by the Colorado Multiple Institutional Review Board. This patient is a 27-year-old woman with DS, psoriasis, hypothyroidism, celiac disease and a history of self-resolving atrial septal defect. The participant initially presented to her primary care physician (PCP) with left shoulder joint pain that persisted despite exercise, ibuprofen and heat therapy. Her exam and workup were largely unremarkable except for high levels of CRP. With continued conservative management, her joint pain worsened over four months to involve her left hand, elbow and knee, especially in the morning, resulting in mobility limitations that caused her to be homebound. As the signs of arthritis worsened, so did her psoriasis.
The participant therefore re-presented to her PCP who reported significant swelling in her left metacarpophalangeal and proximal interphalangeal joints, which was also evident on hand radiographs. Inflammatory markers were further elevated with a CRP of 32.4 mg/l (normal <10 mg/l), ESR of 40 mm/h (normal <19 mm/h) and positivity for ANA. She tested negative for RF and CCP antibodies, with all other labs within normal limits.
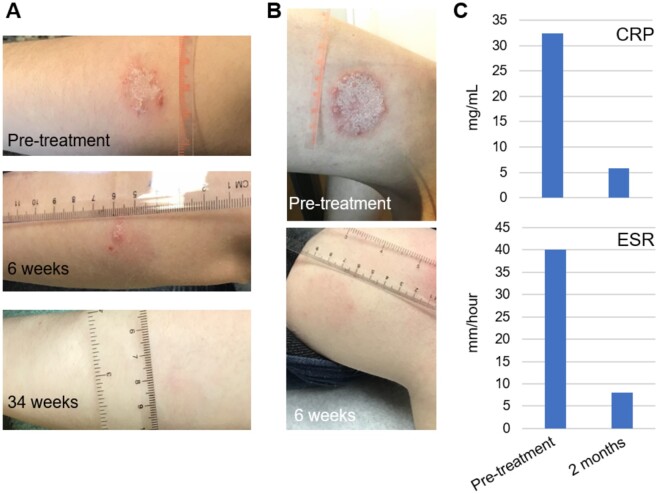
A subsequent rheumatology referral resulted in a diagnosis of PsA, given psoriasis history, current presentation of inflammatory joint disease, and the presence of dactylitis of the third and fourth digits on the left hand on exam (CASPAR score: 4) [5]. At this point, the individual was prescribed tofacitinib 5 mg oral tablet twice daily. The participant reported rapid improvement in arthropathy symptoms, regaining the ability to walk long distances after several weeks of immobility and resumed her normal moderate intensity exercise activities within one month. Complete resolution of her arthritis, without adverse reactions, was noted after two months during a telehealth follow-up visit. Furthermore, the participant reported that her psoriasis also cleared rapidly and had largely disappeared by six weeks (Fig. 1A and 1B), with a concurrent normalization of inflammatory markers with a CRP of 5.8 mg/l and ESR of 8 mm/h (Fig. 1C) at two months.
Fig. 1.
Treatment of PsA in Down syndrome with tofacitinib
(A) Resolution of psoriasis on left arm over treatment course pre-tofacitinib, 6 weeks and 34 weeks post-treatment. (B) Resolution of psoriasis on left leg over treatment course pre-tofacitinib and 6 weeks post-treatment. (C) Normalization of inflammatory markers 2 months post-treatment.
The aetiology of PsA in the general population involves dysregulation of innate and adaptive immunity, including activation of plasmacytoid dendritic cells (pDCs), production of Type I (α,β) IFNs, polarization of CD4+ T cells towards the Th17 state, and hyperactivation of cytotoxic CD8+ T cells, concomitant with dysregulated production of TNF-α, IL17, IL12, IL22, IL23 and IFNγ [6]. In fact, current published guidelines recommend IL17 and IL23 inhibitors as first-line agents for this inflammatory arthropathy [7]. Of note, many of these processes are dysregulated in individuals with DS, even in the absence of overt clinical autoimmunity, including hyperactive pDCs [3], polarization of CD4+ T cells towards IL17- and IL22-producing states [8], hyperactivation of CD8+ T cells [8], and elevated circulating levels of TNF-α, diverse IL17 isoforms, IL22 and IFNγ [9]. All of these phenomena could be potentially explained by the fact that four IFN receptors are encoded on chromosome 21 (IFNAR1, IFNAR2, IFNGR2 and IL10RB), thus leading to hypersensitivity to IFN ligands, increased JAK signalling and an overall pro-inflammatory, autoimmunity-prone state [2, 3, 8, 9].
While further studies are required to elucidate the mechanisms leading to increased autoimmunity in DS, this report adds to a growing body of evidence pointing to increased IFN and JAK signalling as contributing factors. The fact that the participant responded remarkably well to tofacitinib, a JAK inhibitor that inhibits signalling downstream of all four IFN receptors encoded on chromosome 21, encourages further study. While tofacitinib is not recommended as a first-line agent for PsA in the typical population, it may be the preferable option for those with DS affected by inflammatory joint diseases and autoimmune skin conditions.
Acknowledgements
We are grateful to members of the Crnic Institute’s Human Trisome Project research participants and their families.
Funding: This work was supported by the NIH Office of the Director, the National Institute of Allergy and Infectious Diseases (NIAID), and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) through grants R01AI150305 and R61AR077495 as part of the NIH INCLUDE Project. Additional funding was provided by the Linda Crnic Institute for Down Syndrome, the Global Down Syndrome Foundation, the Anna and John J. Sie Foundation, and the Human Immunology and Immunotherapy Initiative (HI3), at the University of Colorado School of Medicine.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
Data will be shared on reasonable request to the corresponding author, subject to privacy policies.
References
- 1.Bull MJ, the Committee on Genetics. Health supervision for children with Down syndrome. Pediatrics 2011;128:393–406. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan KD, Lewis HC, Hill AA. et al. Trisomy 21 consistently activates the interferon response. Elife 2016;5:e16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waugh KA, Araya P, Pandey A. et al. Mass cytometry reveals global immune remodeling with multi-lineage hypersensitivity to type I interferon in Down Syndrome. Cell Rep 2019;29:1893–908.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuo CM, Tung TH, Wang SH, Chi CC.. Efficacy and safety of tofacitinib for moderate-to-severe plaque psoriasis: a systematic review and meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol 2018;32:355–62. [DOI] [PubMed] [Google Scholar]
- 5.Taylor W, Gladman D, Helliwell P. et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- 6.Bravo A, Kavanaugh A.. Bedside to bench: defining the immunopathogenesis of psoriatic arthritis. Nat Rev Rheumatol 2019;15:645–56. [DOI] [PubMed] [Google Scholar]
- 7.Singh JA, Guyatt G, Ogdie A. et al. Special Article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol 2019;71:5–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araya P, Waugh KA, Sullivan KD. et al. Trisomy 21 dysregulates T cell lineages toward an autoimmunity-prone state associated with interferon hyperactivity. Proc Natl Acad Sci USA 2019;116:24231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powers RK, Culp-Hill R, Ludwig MP. et al. Trisomy 21 activates the kynurenine pathway via increased dosage of interferon receptors. Nat Commun 2019;10:4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be shared on reasonable request to the corresponding author, subject to privacy policies.