Abstract
Objectives
Antibodies against anti-CD74 are related to axial spondyloarthritis (axSpA). The objectives were (i) to study IgA anti-CD74 in radiographic (r)-axSpA patients in the Backbone cohort and to calculate the sensitivity and specificity of anti-CD74, (ii) to study the fluctuation of IgA anti-CD74 levels in prospectively collected samples, and (iii) to explore the relation between IgA anti-CD74 and radiographic spinal changes.
Methods
IgA anti-CD74 was analysed by ELISA in 155 patients with r-axSpA and age- and sex-matched controls. BASDAI, ASDAS, BASFI and BASMI were assessed and spinal radiographs were scored for r-axSpA-related changes with mSASSS. Previously donated samples, before inclusion in the Backbone study, were identified in the Medical Biobank of Northern Sweden.
Results
A total of 155 patients comprising 69% men and 31% women, age [mean (s.d.)] 55.5 (11.4) years and 152 (98.1%) HLA-B27 positive, were included. The plasma level of IgA anti-CD74 was significantly higher in the patients [median (interquartile range), 12.9 (7.9–17.9) U/ml] compared with controls [10.9 (7.2–14.6) U/ml, P = 0.003]. IgA anti-CD74 was above the cut-off level of 20 U/ml in 36/155 (23.2%) patients and in 15/151 (9.9%) controls (P = 0.002). Multivariable logistic regression analyses revealed ≥1 syndesmophyte associated with IgA anti-CD74 (odds ratio 5.64; 95% CI: 1.02, 35.58; P = 0.048) adjusted for hsCRP, smoking, BMI, sex and age. No distinct pattern of IgA anti-CD74 over time was revealed.
Conclusion
Plasma levels of IgA anti-CD74 were increased in r-axSpA and independently associated with radiographic spinal changes, which suggests that IgA anti-CD74 could play a role in the pathogenies of r-axSpA.
Keywords: radiographic axial spondyloarthritis ankylosing spondylitis, IgA anti-CD74, outcomes research
Rheumatology key messages
IgA anti-CD74 plasma level was significantly higher in patients with r-axSpA compared with controls.
IgA anti-CD74 plasma level was associated with r-axSpA-related changes in the spine.
There was no difference in IgA anti-CD74 concentrations between HLA-B27 positive and HLA-B27 negative patients or controls.
Introduction
Ankylosing spondylitis (AS) is a chronic rheumatic inflammatory disease that belongs to the spondyloarthritides (SpA) [1]. Axial (ax)SpA with predominant involvement of the spine and sacroiliac joints is divided into radiographic (r)-axSpA and non-radiographic (nr)-axSpA depending on whether or not there is typical radiographic structural changes in the sacroiliac joints [2]. Recently, it was suggested that AS and r-axSpA can be used as interchangeable terms [3], and therefore we will use the name r-axSpA in this report. Due to the lack of specific tests, the diagnosis of axSpA in early phases is challenging and there often remains a considerable delay to diagnosis [4, 5]. HLA-B27 plays a role in the diagnosis and prognosis of axSpA. Only about 1.3–6% of the HLA-B27 positive persons develop axSpA and the prevalence of HLA-B27 varies in different populations, with about 15% of the population in northern Scandinavia [1, 6–8]. Also, the HLA-B27 occurrence differs between nr-axSpA and r-axSpA and between different cohorts around the world [9–11]. CRP or ESR reflect merely systemic inflammation and are thus not disease-specific. MRI of the sacroiliac joints is a crucial tool to identify inflammation but is also not disease-specific since signs of inflammations may also be found in ice-hockey players and runners [12, 13].
CD74, known as HLA class II invariant chain in humans, is an essential protein in antigen presentation. CD74 is involved in the intracellular assembly of the major histocompatibility complex (MHC) class II and prevents premature binding of peptides to MHC II [14]. When surface-expressed, activation of CD74 may initiate a signal pathway that leads to cell proliferation and the production of pro-inflammatory cytokines such as TNFα [15]. The potential involvement of CD74 in axSpA pathogenesis and diagnosis has been suggested since autoantibodies against the class II-associated invariant chain peptide (CLIP) domain of CD74 are associated with axSpA. Several studies have analysed IgG, IgG4 or IgA anti-CD74 antibodies in different axSpA cohorts, and mostly found significantly higher serum levels of anti-CD74 antibodies in axSpA patients compared with persons without known axSpA. The isotype of the anti-CD74 antibodies with the best predictive value and the prevalence of the antibodies in relation to disease duration has been inconsistent [16–21]. However, knowledge about the association of anti-CD74 antibodies with disease characteristics and severity of r-axSpA is scarce.
The aims of this investigation were (i) to study IgA anti-CD74 in a cohort of patients with r-axSpA with a very high percentage of HLA-B27 in comparison with matched controls from the same geographical area and to calculate the sensitivity and specificity of anti-CD74, (ii) to study, in prospectively collected samples, the fluctuation of plasma levels of IgA anti-CD74 over time in r-axSpA in comparison with the fluctuation in controls, and (iii) to explore the relation between IgA anti-CD74 and disease-related characteristics including radiographic changes in the spine in the patients with r-axSpA.
Methods
Patients and controls
A total of 155 patients from Västerbotten County, northern Sweden, that fulfilled the modified New York criteria for AS [22] were included in the Backbone study, 2016–2017, at the Department of Rheumatology at Norrlands University Hospital (NUS). The Backbone study examines severity and comorbidities in r-axSpA. Exclusion criteria were dementia, pregnancy, other inflammatory rheumatic diseases and difficulties in understanding Swedish. The 155 r-axSpA patients were checked if they previously had participated in the Northern Sweden Health and Disease Study (NSHDS) and donated blood samples (after overnight fasting) to the Medical Biobank of Northern Sweden before the recruitment to Backbone. The NSHDS cohort consists of over 135 000 participants, is population-based and started in 1985. All inhabitants in Västerbotten County are asked to take part in NSHDS at 40, 50 and 60 years of age. Controls to the r-axSpA patients were persons from the NSHDS, matched for age, sex, date of blood sampling (pre-Backbone controls ±1 year and Backbone controls ±7 years), area of inhabitance (rural or urban), and with no known malignant disease. A total of 151 controls were matched to the 155 patients in Backbone (group C). Altogether, we identified 106/155 (68%) patients who had donated 168 plasma samples after the symptom onset but before 2016–2017 with corresponding controls (group B), and 16/155 (10.3%) individuals who had donated 22 blood samples, with corresponding controls, before the onset of any symptom and referred to as pre-symptomatic individuals (group A). Serum samples of the pre-symptomatic individuals and controls were found in the rubella and virus biobank at NUS. If the r-axSpA patient had donated >1 sample in group B the corresponding control samples was chosen from a control person that had donated >1 sample.
Clinical assessment and questionnaires
At inclusion in Backbone, the patients with r-axSpA underwent clinical examination and answered questionnaires regarding lifestyle habits, medication and disease-related data. Mobility was measured by the BASMI [23]. Disease activity and physical function were assessed using the BASDAI [24], the Ankylosing Spondylitis Disease Activity Score based on CRP (ASDAS-CRP) [25] and the BASFI [26].
Radiography
Spinal radiographic alterations were graded from the lateral projection of spinal radiographs and scored according to the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS). Briefly, the anterior corners of vertebra C2–T1 and T12–S1 are graded in mSASSS with a score between 0 and 3 (0 = normal, 1 = erosion, sclerosis, or squaring, 2 = syndesmophyte, 3 = bridging syndesmophyte). The overall scoring scale ranges from 0 to 72, with 72 representing complete ankylosis [27]. A mSASSS score ≥2 at a vertebral corner was considered as having a syndesmophyte. Severe spinal radiographic changes were defined as ≥3 consecutive inter-vertebral bridges in the cervical spine and/or the lumbar spine, similar to the definition of severe in the Bath Ankylosing Spondylitis Radiology Index [28].
Laboratory assessment
Blood samples were drawn in the morning after overnight fasting. ESR, high sensitivity CRP (hsCRP) analysed consecutively and HLA-B27 were analysed using standard laboratory techniques. The plasma/sera level of IgA autoantibodies against CD74 was measured, in samples that had been frozen at −80°C, with ELISA (AESKULISA SpA Detect, AESKU Diagnostics, Wendelsheim, Germany) according to the manufacturer’s instructions. The limit of detection in the ELISA was 2.7 U/ml and the cut-off level was determined as 20 U/ml.
Statistical analysis
All data were analysed using IBM SPSS Statistics 25 (IBM Corp., Armonk, NY, USA) and R 3.6.2 [R Core Team (2019), Vienna, Austria]. Descriptive statistics are presented as mean with (s.d.), median with interquartile range (IQR), or absolute number with percentages. When comparing cases and controls, the Wilcoxon signed-rank test or Student’s paired t-test was applied and when comparing groups with AS, the Mann–Whitney U-test or an independent t-test was used for continuous variables depending on the distribution of the variable. A χ2 test or Fisher's exact test was applied for categorical data. Correlation analysis was performed using Spearman’s rank correlation test. Univariable and multivariable linear regression analyses were used to identify the association between IgA antibodies against CD74 and AS related factors and demographics as covariates. Independent variables hypothesized to be of importance for anti-CD74 were considered for the multivariable models in addition to age and sex. Multivariable logistic regression analyses were run with ≥1 syndesmophyte or severe radiographic alterations in the spine as dependent variables and IgA anti-CD74 and known risk factors for radiographic changes; age, sex, BMI, smoking and hsCRP were independent variables. Also, BASMI and BASFI were modelled using linear regression adjusting for age, sex, BMI, smoking and hsCRP. Differences in age trend on IgA anti-CD74 levels between patients and controls were longitudinally modelled using a linear mixed-effect model with a random intercept for subjects and a random intercept for each matched case and control pair. The mixed-effect models were adjusted for BMI and smoking. Due to its skewed distribution, IgA anti-CD74 levels, hsCRP and mSASSS were log-transformed when used in the regression models. The variability in measurements of IgA anti-CD74 levels was analysed by calculation of the intraclass correlation using an intercept-only mixed effect model on the longitudinal logarithmic measurements stratified by cases and controls. To have a characteristic was coded 1 and to not have a characteristic was coded 0 in dichotomous variables. Female sex was coded 1 and male sex 0. All tests were two-tailed and P ≤ 0.05 was considered statistically significant.
Results
Characteristics of the patients with r-axSpA at inclusion in Backbone
A total of 155 patients comprising 69% men and 31% women with a mean age of 55.5 (11.4) years and a mean symptom duration of 31.8 (11.9) years were included. Patient characteristics are presented in Table 1. HLA-B27 was present in 152 (98.1%) patients. Mean mSASSS was 18.0 (20.7) and 86 (56.2%) had ≥1 syndesmophyte.
Table 1.
Descriptive characteristics of 155 patients with radiographic axial spondyloarthritis
| Characteristic | Value (n = 155) |
|---|---|
| Sex, n (%) | |
| Women | 48 (31.0) |
| Men | 107 (69.0) |
| Age, mean (s.d.), years | 55.5 (11.4) |
| BMI, mean (s.d.), kg/m2 | 27.9 (5.3) |
| Ever smoker, n (%) | 71 (45.8) |
| AS related variables | |
| Symptom duration, mean (s.d.), years | 31.8 (11.9) |
| HLA-B27 positive, n (%) | 152 (98.1) |
| ESR, median (IQR), mm/h | 11.0 (5.0–20.0) |
| hsCRP, median (IQR), mg/l | 2.7 (1.0–6.0) |
| History of anterior uveitis, n (%) | 80 (51.6) |
| History of peripheral arthritis, n (%) | 83 (53.5) |
| ASDAS-CRP score, median (IQR) | 1.8 (1.3–2.2) |
| BASDAI score, median (IQR) | 3.5 (2.2–5.3) |
| BASFI score, median (IQR) | 2.6 (1.4–4.2) |
| BASMI score, median (IQR) | 4.0 (3.0–5.4) |
| NSAID, regular use, n (%) | 95 (61.3) |
| csDMARD, n (%) | 19 (12.3) |
| bDMARD, n (%) | 27 (17.4) |
| csDMARD and/or bDMARD, n (%) | 38 (24.5) |
| Prednisolone, n (%) | 13 (8.4) |
| mSASSS scorea, median (IQR) | 18 (1–30.5) |
| ≥1 syndesmophytea, n (%) | 86 (56.2) |
| Severe spinal radiographic changesa,b, n (%) | 32 (20.9) |
Two missing. b≥3 consecutive inter-vertebral bridges, cervical and/or lumbar spine. ASDAS: Ankylosing Spondylitis Disease Activity Score; bDMARD: biologic DMARD; csDMARD: conventional synthetic DMARD; hsCRP: high sensitivity CRP; IQR: interquartile range; mSASSS: Modified Stoke Ankylosing Spondylitis Score.
Comparisons between the patients with r-axSpA at inclusion in Backbone and the controls
The patients with r-axSpA were numerically slightly but still significantly older than the matched controls, while there was no difference in sex distribution (Table 2). HLA-B27 was positive in 152/155 (98.1%) of the r-axSpA patients in comparison with 30/117 (25.6%) of the controls (P < 0.001). The plasma level of IgA anti-CD74 was significantly higher in the patients [median (IQR), 12.9 (7.9–17.9) U/ml] compared with the controls [10.9 (7.2–14.6) U/ml; P = 0.003]. Using a cut-off level of 20 U/ml, according to the ELISA kit instructions, IgA anti-CD74 was above the cut-off level in 36/155 (23.2%) patients with r-axSpA and 15/151 (9.9%) controls (P = 0.002). Thus, the sensitivity with a cut-off level at 20 U/ml was 0.23 with a specificity of 0.90. Based on the IgA anti-CD74 values of the patients and the controls in this study, the area under the curve was 0.58 (95% CI: 0.51, 0.64). Using the Youden index the cut-off level of 11.75 U/ml resulted in the best balance between sensitivity (0.57) and specificity (0.56) (Supplementary Fig. 1, available at Rheumatology online). Using the cut-off level of 11.75 U/ml, IgA anti-CD74 was above this cut-off level in 89/155 (57.4%) patients with r-axSpA and in 66/151 (43.7%) controls (P = 0.016).
Table 2.
Comparisons of IgA anti-CD74, HLA-B27, smoking and demographics between patients with radiographic axial spondyloarthritis and matched controls
| Characteristic |
r-axSpA
(n = 155) |
Controls
(n = 151) |
P-value |
|---|---|---|---|
| Sex, n (%) | |||
| Women | 48 (31.0) | 48 (31.8) | 0.9 |
| Men | 107 (69.0) | 103 (68.2) | |
| Age, mean (s.d.), years | |||
| All | 55.5 (11.45) | 53.0 (9.0) | <0.001 |
| Women | 57.7 (10.6) | 53.7 (8.9) | <0.001 |
| Men | 54.5 (11.7) | 52.6 (9.0) | <0.001 |
| Ever smoker, n (%) | 71 (45.8) | 59 (39.3)a | 0.25 |
| HLA-B27 positive, n (%) | |||
| All | 152 (98.1) | 30 (25.6)b | <0.001 |
| Women | 47 (97.9) | 11 (28.9) | <0.001 |
| Men | 105 (98.1) | 19 (24.0) | <0.001 |
| IgA anti-CD74 | |||
| All, median (IQR), U/ml | 12.9 (7.9–17.9) | 10.9 (7.2–14.6) | 0.003 |
| mean (s.d.), U/ml | 14.9 (10.1) | 12.7 (7.8) | |
| Women, median (IQR), U/ml | 13.2 (8.4–20.4) | 9.6 (6.6–13.9) | 0.011 |
| Men, median (IQR), U/ml | 12.9 (8.7–16.6) | 11.5 (8.1–15.9) | 0.076 |
| IgA anti-CD74+ (cut-off 20 U/ml), n (%) | |||
| All | 36 (23.2) | 15 (9.9) | 0.002 |
| Women | 13 (27.1) | 5 (10.4) | 0.036 |
| Men | 23 (21.5) | 10 (9.7) | 0.019 |
| r-axSpA | Controls | |||
|---|---|---|---|---|
| HLA-B27 status | B27+ | B27− | B27+ | B27− |
| IgA anti-CD74+ | 36 | 0 | 1 | 13 |
| IgA anti-CD74− | 116 | 3 | 29 | 74 |
| Total | 152 | 3 | 30 | 87 |
| P-valuec | 0.45 | 0.11 | ||
| IgA anti-CD74, median (IQR), U/ml | 13.0 (8.7–18.8) | 11.8 (6.6–12.2) | 11.0 (7.2–14.2) | 11.4 (7.6–18.9) |
| P-value | 0.34 | 0.38 | ||
One missing. bHLA-B27 was analysed in 117 controls, 38 women and 79 men. cFisher’s exact test. IQR: interquartile range; r-axSpA; radiographic axial spondyloarthritis.
There was no significant association between HLA-B27 status and IgA anti-CD74 status (cut-off 20 U/ml) in either patients or controls. Additionally, there was no difference in IgA anti-CD74 concentrations between HLA-B27 positive and HLA-B27 negative patients or controls.
No significant correlation between age and IgA anti-CD74 level was found in the r-axSpA patients (rs = −0.089, P = 0.27) but in the controls (rs = 0.17, P = 0.038).
IgA anti-CD74 in patients with r-axSpA at inclusion in Backbone
Correlation analyses revealed that plasma level of IgA anti-CD74 was significantly correlated with ESR (rs = 0.25, P = 0.002), hsCRP (rs = 0.21, P = 0.007) and platelets (rs = 0.16, P = 0.043), with a tendency with mSASSS (rs = 0.14, P = 0.079). No significant associations were found between IgA anti-CD74 level and symptom duration, BMI, ASDAS-CRP, BASDAI, BASMI or BASFI. Concerning medication, patients on conventional synthetic DMARDs and/or biologic DMARDs had numerically slightly higher IgA anti-CD74 compared with patients without DMARDs [median (IQR) 14.2 (10.4–21.9) vs 12.8 (8.3–16.4) U/ml; P = 0.084]. The same pattern was found between patients on prednisolone vs not on prednisolone [18.9 (11.2–36.9) vs 12.2 (8.7–16.5) U/ml; P = 0.020] whereas patients on regular use of NSAID had lower IgA anti-CD74 levels compared with non-regular users [11.8 (8.7–16.3) vs 14.0 (8.9–21.5) U/ml; P = 0.032]. Ever smokers tended to have higher IgA anti-CD74 levels compared with never smokers [13.2 (10.2–20.4) vs 12.2 (8.3–16.3) U/ml; P = 0.093]. Furthermore, patients with severe spinal radiographic changes had significantly higher IgA anti-CD74 levels than those without severe changes [14.0 (11.3–22.2) vs 11.9 (8.2–16.6) U/ml; P = 0.046]. No significant differences in IgA anti-CD74 levels were found between sexes, patients with a history of anterior uveitis, peripheral arthritis, or overweight or not.
Multivariable linear regression analyses demonstrating factors associated with IgA anti-CD74 levels in patients with r-axSpA
In multivariable linear regression analyses, the logarithmic plasma levels of IgA anti-CD74 showed significant associations with ever smoker, ESR, mSASSS and use of prednisolone, and inversely with age and regular treatment with NSAIDs (R2 = 0.26) (Table 3). The results of univariable regression analyses are shown in Supplementary Table 1, available at Rheumatology online.
Table 3.
Multivariable linear regression analysis in 155 patients with radiographic axial spondyloarthritis (r-axSpA) with logarithmic plasma level of IgA anti-CD74 concentrations as dependent variable and r-axSpA related and demographics as covariates
| Logarithmic plasma level of IgA anti-CD74, U/ml |
||||
|---|---|---|---|---|
| Covariate | Β | 95% CI | Β, standardized | P-value |
| Age, years | −0.005 | −0.008, −0.001 | −0.24 | 0.006 |
| Sex | −0.039 | −0.121, 0.043 | −0.07 | 0.35 |
| BMI, kg/m2 | 0.005 | −0.002, 00.012 | 0.11 | 0.13 |
| Ever smoker | 0.080 | 0.009, 0.152 | 0.17 | 0.028 |
| NSAID, regular use | −0.081 | −0.154, −0.009 | −0.16 | 0.028 |
| csDMARD and/or bDMARD | 0.056 | −0.028, 0.14 | 0.10 | 0.19 |
| Prednisolone | 0.192 | 0.064, 0.320 | 0.22 | 0.004 |
| ESR, mm/h | 0.005 | 0.002, 0.008 | 0.23 | 0.003 |
| mSASSS, score | 0.002 | 0.002, −0.004 | 0.17 | 0.041 |
| R2 0.26 | ||||
bDMARD: biologic DMARD; csDMARD: conventional synthetic DMARD; mSASSS: Modified Sroke Ankylosing Spondylitis Score.
Multivariable logistic regression analyses with radiographic outcomes in patients with r-axSpA
Multivariable logistic regression analyses with ≥1 syndesmophyte as the dependent variable and log IgA anti-CD74 as an independent variable adjusted for other known risk factors for radiographic changes in the spine in r-axSpA showed an odds ratio (OR) of 5.64 (95% CI: 1.02, 35.58; P = 0.048) for log IgA anti-CD74. Multivariable logistic regression analyses with the presence of severe radiographic changes as the dependent variable and log IgA anti-CD74 as an independent variable adjusted for other known risk factors for radiographic alterations displayed an OR of 8.11 (95% CI: 1.13, 67.31; P = 0.037) for log IgA anti-CD74. Thus, our results indicate that IgA anti-CD74 is associated with r-axSpA-related changes in the spine (Table 4).
Table 4.
Multivariable logistic regression analyses with presence of ≥1 syndesmophyte or severe radiographic alterationsa as dependent variables
| ≥1 syndesmophyte |
Severe radiographic changes |
|||||
|---|---|---|---|---|---|---|
| Covariates | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Age, years | 1.10 | 1.06, 1.15 | <0.001 | 1.09 | 1.05, 1.15 | <0.001 |
| Sex | 0.16 | 0.06, 0.38 | <0.001 | 0.18 | 0.05, 0.57 | 0.002 |
| BMI, kg/m2 | 0.98 | 0.92, 1.06 | 0.672 | 1.03 | 0.95, 1.12 | 0.495 |
| Ever smoker | 1.03 | 0.47, 2.27 | 0.932 | 0.82 | 0.33, 2.01 | 0.668 |
| Log hsCRP, mg/l | 1.64 | 0.87, 1.80 | 0.236 | 1.19 | 0.88, 2.00 | 0.182 |
| Log IgA anti-CD74, U/ml | 5.64 | 1.02, 35.58 | 0.048 | 8.11 | 1.13, 67.31 | 0.037 |
Defined as ≥3 consecutive inter-vertebral bridges in the cervical spine and/or the lumbar spine. Female sex was coded 1 and male sex 0. hsCRP: high sensitivity CRP.
The fluctuation of plasma levels of IgA anti-CD74 over time in r-axSpA in comparison with the fluctuation in controls
Mixed-effects analysis of log IgA anti-CD74 levels, adjusting for BMI and ever smoker (at inclusion in Backbone), revealed an increasing trend in relation to age in the controls (β = 0.11; 95% CI: 0.01, 0.2; P = 0.031). In contrast, the patients with r-axSpA displayed a more variable pattern (age-group interaction: β = −0.15; 95% CI: −0.27, −0.02; P = 0.025). The r-axSpA patients had slightly but not significantly higher baseline IgA anti-CD74 levels with an estimated difference of β = 0.02 (95% CI: −0.04, 0.08; P = 0.502). Log IgA anti-CD74 levels in group B in patients with r-axSpA and controls concerning age are shown in Fig. 1. The intraclass correlation for the longitudinal measurements was 0.63 for cases and 0.70 for controls where the difference between cases and controls is explained by a higher residual variance among cases compared with controls (0.026 vs 0.017). On the logarithmic scale the variability was comparable between the two groups (s.d. cases: 0.30; s.d. controls: 0.29).
Fig. 1.
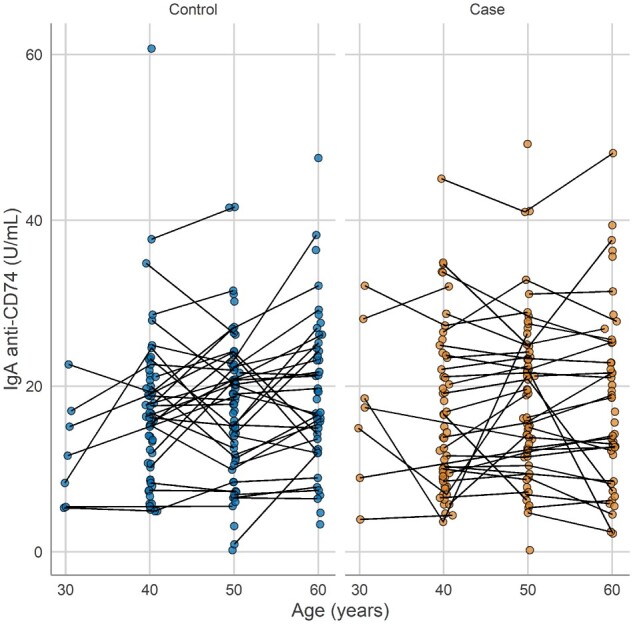
Plasma levels of IgA anti-CD74 in patients with radiographic axial spondyloarthritis and matched controls over age in group B
Association between plasma levels of IgA anti-CD74 over time and the severity of r-axSpA
Multivariable regression, adjusted for age, sex, BMI, ever smoking and log CRP, showed that the overall levels and trend in log IgA anti-CD74 levels in group B and C (group C corresponds with the Backbone) in the patients with r-axSpA had no significant influence on mSASSS, presence of ≥1 syndesmophyte or severe radiographic spinal changes, BASMI or BASFI at inclusion in Backbone. The full results are presented in Supplementary Table 2, available at Rheumatology online. In Fig. 2, IgA anti-CD74 levels with age for the r-axSpA patients with ≥1 measurement of IgA anti-CD74 [106/155 (68%) in group B and C] are shown. IgA anti-CD74 was analysed in the longitudinally collected samples from each of these 106 r-axSpA patients (2–4 samples/patient, altogether in 274 samples).
Fig. 2.
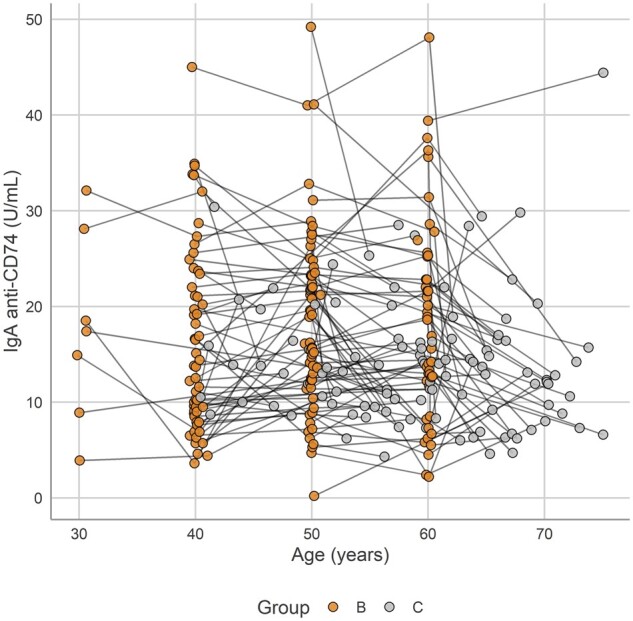
Plasma levels of IgA anti-CD74 in patients with radiographic axial spondyloarthritis over age in group B and C
IgA anti-CD74 in pre-symptomatic individuals
Concerning the pre-symptomatic individuals, 16/155 (10.3%), 5 men and 11 women, had donated 22 blood samples, with corresponding samples from controls (group A). No difference in the levels of IgA anti-CD74 was found [median (IQR), in pre-symptomatic individuals 20.8 (16.7–28.8) U/ml vs 25.2 (17.5–37.2) U/ml in controls] or in age [in pre-symptomatic individuals 27.7 (25.0–33.9) years vs 27.8 (25.1–33.1) years in controls].
Discussion
In this study on patients with r-axSpA participating in the Backbone study, which investigates severity and comorbidities in r-axSpA, we found that the plasma level of IgA anti-CD74 and the frequency of IgA anti-CD74 positives were significantly increased compared with age- and sex-matched controls from the same geographical area. We did not find any difference in sex concerning the plasma level or frequency of IgA anti-CD74 positives (threshold 20 U/ml) in patients or controls. The plasma IgA anti-CD74 levels fluctuated over time in both r-axSpA patients and controls with no distinct pattern. Furthermore, the levels over time did not predict radiographic spinal changes later at the inclusion in Backbone whereas IgA anti-CD74 was significantly associated with radiographic spinal changes at the inclusion in Backbone.
The HLA-B27 positive rate was high in the Backbone cohort (98%), which mirrors the high HLA-B27 frequency in the general population in this part of Sweden. Also, about one-fourth of the controls were HLA-B27 positive. We did not find a relation between HLA-B27 status and IgA anti-CD74 status in patients or controls. Previous studies on axSpA patients are inconsistent. Witte et al. reported recently that in one of their two cohorts studied, significantly more HLA-B27 negative patients compared with HLA-B27 positive patients had IgA anti-CD74s [29], whereas no such association was found in the InterSpA [21].
Using the cut-off of 20 U/ml according to the ELISA kit instructions, we found IgA anti-CD74 in 23% of the patients with r-axSpA compared with 10% of the controls. In comparison with the current study, Riechers et al. reported that IgA anti-CD74 was present in 36% of patients with r-axSpA, out of which 90% were HLA-B27 positive, and in 47% of patients with nr-axSpA, out of which 81% carried HLA-B27 [21]. De Winter et al. measured IgA anti-CD74 in a cohort with r-axSpA and the Spondyloarthritis Caught Early (SPACE) cohort and found the frequency to be 28% and 55%, respectively. The frequency was low in healthy controls (5%) but higher in the control group with chronic back pain (37%) [18]. Thus, the pattern is somewhat different in the diverse studies, which might be explained by different symptom duration, demographics, and cut-off levels of IgA anti-CD74, among others.
Concerning the sensitivity and specificity of IgA anti-CD74, we achieved a sensitivity of 0.23 with a specificity of 0.90 (cut-off plasma level of 20 U/ml), which indicates a good specificity but a low sensitivity. By use of our calculated cut-off level (11.75 U/ml), the sensitivity was 0.57 with a specificity of 0.56, indicating modest sensitivity and specificity of the test in our patients with mostly longstanding r-axSpA. Thus, the overall sensitivity/specificity found by these two different cut-offs was only moderate.
We also analysed, to the best of our knowledge, for the first time plasma levels of IgA anti-CD74 in prospectively collected samples. About 70% of the patients had participated earlier in life in the NSHDS and donated blood samples (frozen at −80°C) where the earliest sample was donated in 1989. The levels fluctuated over time in both patients and controls with no distinct development pattern over time in either group. In addition, we analysed serum/plasma levels of IgA anti-CD74 in 16 pre-symptomatic individuals who later developed r-axSpA and matched controls. The earliest donated and frozen sample was from 1976. No significant difference in the IgA anti-CD74 level was found between the pre-symptomatic persons and their controls.
Furthermore, we studied the relation between IgA anti-CD74 levels and clinical characteristics, laboratory measures of inflammation, medication, disease activity, physical function and spinal radiographic changes at inclusion in the Backbone study. In multivariable logistic regression analyses, we found that log IgA anti-CD74 was significantly associated with spinal radiographic changes assessed both as the presence of syndesmophytes and as the presence of severe radiographic changes adjusted for other known risk factors for radiographic changes in the spine. In accordance, in multivariable linear regression analysis mSASSS, ever smoker, ESR, use of prednisolone and inversely with age and regular treatment with NSAIDs were significantly associated with log IgA anti-CD74 as the dependent variable. Thus, we demonstrated clear relationships between IgA anti-CD74 levels and the osteoproliferative process in the spine in patients with r-axSpA. In line with our findings, Witte et al. recently showed that the axSpA patients with IgA anti-CD74 had significantly higher sacroiliitis scores compared with patients without IgA anti-CD74 and patients with IgA anti-CD74 had higher mSASSS compared with patients without IgA anti-CD74 [29]. Unfortunately, we did not find a predictive value of the IgA anti-CD74 level analysed in the prospectively collected samples and radiographic spinal changes.
The limitations of this study include that some of the samples have been frozen for many years, which may have influenced the results, though the same influence should have occurred in the control samples since they were handled in the same way. The outcomes of severity such as radiographic changes were only evaluated once, which limits the possibility to evaluate changes over time in relation to the IgA anti-CD74 levels. Also, we lack MRI data on the spine that could support the association between IgA anti-CD74 and the radiographic changes. The CI intervals in the logistic regression analyses with radiographic severity outcomes were broad, meaning that the variation in IgA anti-CD74 levels was large. To improve the power a higher number of patients and controls would have been preferable.
In summary, we found that the plasma levels of IgA anti-CD74 were elevated and the frequency of IgA anti-CD74 positivity increased in this cohort of well-characterized patients with r-axSpA with a high rate of HLA-B27. IgA anti-CD74 levels were associated with the radiographic changes independent of hsCRP, smoking, BMI, age and sex, which implies that IgA anti-CD74 could play a part in the pathogenies of r-axSpA.
Ethics
All participants gave their written informed consent. The study was approved by the Ethics Review Board at Umeå University, Umeå, Sweden (Dnr 2016/208–31), and carried out in accordance with the Declaration of Helsinki.
Supplementary Material
Acknowledgements
We are grateful to all participants in the study. We wish to thank the research nurses at the University Hospital of Umeå, Viktoria von Zweigbergk and Jeanette Beckman Rehnman, for assisting with the project. L.D. analysed samples, did statistical analyses, participated in interpretation of data, and drafted part of the manuscript. G.G. contributed to statistical analyses, interpretation of data, and drafted part of the manuscript. U.H. contributed to the analysis and interpretation of data and revised the manuscript critically for important intellectual content. K.L. contributed to the analysis and interpretation of data and revised the manuscript critically for important intellectual content. M.G. contributed to the acquisition of data and revised the manuscript critically for important intellectual content. X.B. contributed to the analysis and interpretation of data and revised the manuscript critically for important intellectual content. T.W. analysed samples, contributed to analysis and interpretation of data, and revised the manuscript critically for important intellectual content. H.F.dE. was responsible for study design, recruitment of patients, data collection, did statistical analyses, interpretation of data, and drafted part of the manuscript. All authors have critically reviewed the manuscript, approved the final version to be published, and agreed to be accountable for all aspects of the work.
Funding: This work was supported by grants from The Swedish Research Council [2016–02035]; the County of Västerbotten (agreement concerning research and education of doctors) [ALFVLL-640251]; King Gustaf Vth 80-year Foundation [FAI-2017–0454]; Västerbotten’s Association Against Rheumatism; The Swedish Association Against Rheumatism; The Norrland’s Heart Foundation; and Mats Kleberg’s Foundation. T.W. was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy—EXC 2155, Project No. 390874280.
Disclosure statement: T.W. holds a patent on the commercial exploitation of the measurement of antibodies against CD74.
Data availability statement
The data sets generated and/or analysed during the current study are not publicly available due to the General Data Protection Regulation (GDPR). Researchers with a specific question regarding the study are encouraged to contact the corresponding author (H.F.dE.).
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1.Dougados M, Baeten D.. Spondyloarthritis. Lancet 2011;377:2127–37. [DOI] [PubMed] [Google Scholar]
- 2.Rudwaleit M, van der Heijde D, Landewe R. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 3.Boel A, Molto A, van der Heijde D. et al. Do patients with axial spondyloarthritis with radiographic sacroiliitis fulfil both the modified New York criteria and the ASAS axial spondyloarthritis criteria? Results from eight cohorts. Ann Rheum Dis 2019;78:1545–9. [DOI] [PubMed] [Google Scholar]
- 4.Redeker I, Callhoff J, Hoffmann F. et al. Determinants of diagnostic delay in axial spondyloarthritis: an analysis based on linked claims and patient-reported survey data. Rheumatology (Oxford) 2019;58:1634–8. [DOI] [PubMed] [Google Scholar]
- 5.Danve A, Deodhar A.. Axial spondyloarthritis in the USA: diagnostic challenges and missed opportunities. Clin Rheumatol 2019;38:625–34. [DOI] [PubMed] [Google Scholar]
- 6.Bjelle A, Cedergren B, Dahlqvist SR.. HLA B27 in the population of northern Sweden. Scand J Rheumatol 1982;11:23–6. [DOI] [PubMed] [Google Scholar]
- 7.Gran JT, Mellby AS, Husby G.. The prevalence of HLA-B27 in Northern Norway. Scand J Rheumatol 1984;13:173–6. [DOI] [PubMed] [Google Scholar]
- 8.van der Linden SM, Valkenburg HA, de Jongh BM, Cats A.. The risk of developing ankylosing spondylitis in HLA-B27 positive individuals. A comparison of relatives of spondylitis patients with the general population. Arthritis Rheum 1984;27:241–9. [DOI] [PubMed] [Google Scholar]
- 9.Hammoudeh M, Abdulaziz S, Alosaimi H. et al. Challenges of diagnosis and management of axial spondyloarthritis in North Africa and the Middle East: an expert consensus. J Int Med Res 2016;44:216–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziade NR.HLA B27 antigen in Middle Eastern and Arab countries: systematic review of the strength of association with axial spondyloarthritis and methodological gaps. BMC Musculoskelet Disord 2017;18:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez-Medina C, Molto A, Claudepierre P, Dougados M.. Clinical manifestations, disease activity and disease burden of radiographic versus non-radiographic axial spondyloarthritis over 5 years of follow-up in the DESIR cohort. Ann Rheum Dis 2020;79:209–16. [DOI] [PubMed] [Google Scholar]
- 12.Weber U, Jurik AG, Zejden A. et al. Frequency and anatomic distribution of magnetic resonance imaging features in the sacroiliac joints of young athletes: exploring “Background Noise” toward a data-driven definition of sacroiliitis in early spondyloarthritis. Arthritis Rheumatol 2018;70:736–45. [DOI] [PubMed] [Google Scholar]
- 13.de Winter J, de Hooge M, van de Sande M. et al. Magnetic resonance imaging of the sacroiliac joints indicating sacroiliitis according to the assessment of spondyloarthritis international society definition in healthy individuals, runners, and women with postpartum back pain. Arthritis Rheumatol 2018;70:1042–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroder B.The multifaceted roles of the invariant chain CD74—More than just a chaperone. Biochim Biophys Acta 2016;1863:1269–81. [DOI] [PubMed] [Google Scholar]
- 15.Starlets D, Gore Y, Binsky I. et al. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood 2006;107:4807–16. [DOI] [PubMed] [Google Scholar]
- 16.Baerlecken NT, Nothdorft S, Stummvoll GH. et al. Autoantibodies against CD74 in spondyloarthritis. Ann Rheum Dis 2014;73:1211–4. [DOI] [PubMed] [Google Scholar]
- 17.Baraliakos X, Baerlecken N, Witte T, Heldmann F, Braun J.. High prevalence of anti-CD74 antibodies specific for the HLA class II-associated invariant chain peptide (CLIP) in patients with axial spondyloarthritis. Ann Rheum Dis 2014;73:1079–82. [DOI] [PubMed] [Google Scholar]
- 18.de Winter JJ, van de Sande MG, Baerlecken N. et al. Anti-CD74 antibodies have no diagnostic value in early axial spondyloarthritis: data from the spondyloarthritis caught early (SPACE) cohort. Arthritis Res Ther 2018;20:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Liao X, Shi G.. Autoantibodies in spondyloarthritis, focusing on anti-CD74 antibodies. Front Immunol 2019;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziade NR, Mallak I, Merheb G. et al. Added value of anti-CD74 autoantibodies in axial spondylo arthritis in a population with low HLA-B27 prevalence. Front Immunol 2019;10:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riechers E, Baerlecken N, Baraliakos X. et al. Sensitivity and specificity of autoantibodies against CD74 in nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2019;71:729–35. [DOI] [PubMed] [Google Scholar]
- 22.van der Linden S, Valkenburg HA, Cats A.. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 23.Jenkinson TR, Mallorie PA, Whitelock HC. et al. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index. J Rheumatol 1994;21:1694–8. [PubMed] [Google Scholar]
- 24.Garrett S, Jenkinson T, Kennedy LG. et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 25.van der Heijde D, Lie E, Kvien TK. et al. ; Assessment of SpondyloArthritis international Society (ASAS). ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:1811–8. [DOI] [PubMed] [Google Scholar]
- 26.Calin A, Garrett S, Whitelock H. et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994;21:2281–5. [PubMed] [Google Scholar]
- 27.Creemers MC, Franssen MJ, van't Hof MA. et al. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 2005;64:127–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacKay K, Mack C, Brophy S, Calin A.. The Bath Ankylosing Spondylitis Radiology Index (BASRI): a new, validated approach to disease assessment. Arthritis Rheum 1998;41:2263–70. [DOI] [PubMed] [Google Scholar]
- 29.Witte T, Kohler M, Georgi J. et al. IgA antibodies against CD74 are associated with structural damage in the axial skeleton in patients with axial spondyloarthritis. Clin Exp Rheumatol 2020;38:1127–31. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and/or analysed during the current study are not publicly available due to the General Data Protection Regulation (GDPR). Researchers with a specific question regarding the study are encouraged to contact the corresponding author (H.F.dE.).


