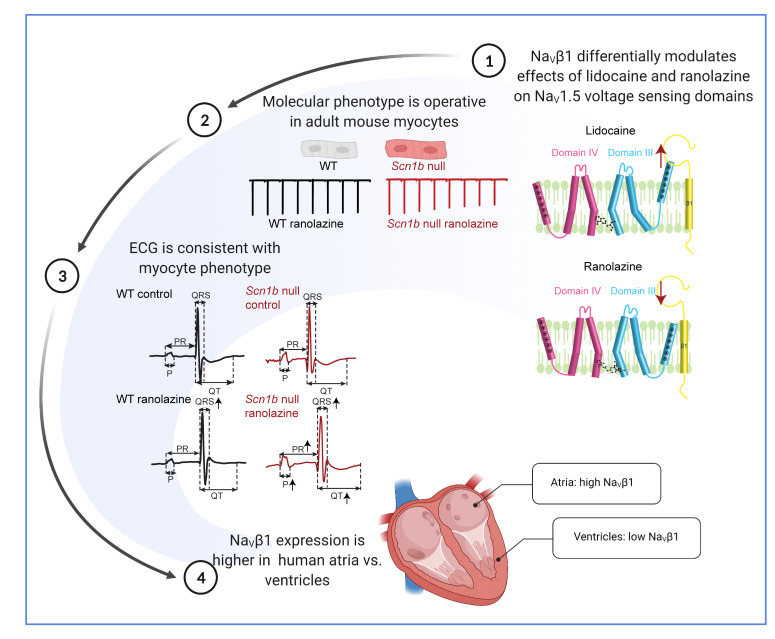
Abstract
Native myocardial voltage-gated sodium (NaV) channels function in macromolecular complexes comprising a pore-forming (α) subunit and multiple accessory proteins. Here, we investigated the impact of accessory NaVβ1 and NaVβ3 subunits on the functional effects of 2 well-known class Ib antiarrhythmics, lidocaine and ranolazine, on the predominant NaV channel α subunit, NaV1.5, expressed in the mammalian heart. We showed that both drugs stabilized the activated conformation of the voltage sensor of domain-III (DIII-VSD) in NaV1.5. In the presence of NaVβ1, the effect of lidocaine on the DIII-VSD was enhanced, whereas the effect of ranolazine was abolished. Mutating the main class Ib drug-binding site, F1760, affected but did not abolish the modulation of drug block by NaVβ1/β3. Recordings from adult mouse ventricular myocytes demonstrated that loss of Scn1b (NaVβ1) differentially affected the potencies of lidocaine and ranolazine. In vivo experiments revealed distinct ECG responses to i.p. injection of ranolazine or lidocaine in WT and Scn1b-null animals, suggesting that NaVβ1 modulated drug responses at the whole-heart level. In the human heart, we found that SCN1B transcript expression was 3 times higher in the atria than ventricles, differences that could, in combination with inherited or acquired cardiovascular disease, dramatically affect patient response to class Ib antiarrhythmic therapies.
Keywords: Cardiology, Therapeutics
Keywords: Arrhythmias, Drug therapy, Sodium channels
Introduction
Inward Na+ currents (INa–) carried by voltage-gated (NaV) channels underlie the initiation and propagation of action potentials in the atria and ventricles (1). Functional NaV channels reflect the assembly of the 4 homologous domains (DI–DIV) in the pore-forming (α) subunit that are connected by intracellular linkers. Each domain contains 6 transmembrane segments (S1–S6). S1–S4 form the voltage-sensing domains (VSDs). The VSDs undergo conformational changes upon membrane depolarization, which open the pore (S5–S6), enabling inward Na+ flux (2). Native myocardial NaV channels function in macromolecular protein complexes, containing many regulatory and anchoring proteins that differentially affect channel function and localization based on the cell type (3). NaVβ subunits are essential components of these macromolecular complexes. There are 5 different types of NaVβ subunits, NaVβ1, NaVβ1B, NaVβ2, NaVβ3, and NaVβ4. NaVβ1, NaVβ1B, and NaVβ3 interact with the NaV α subunits noncovalently; NaVβ2 and NaVβ4 are linked covalently through the formation of disulfide bonds (4). NaVβ1, NaVβ2, NaVβ3, and NaVβ4 are transmembrane proteins, whereas NaVβ1B is secreted (5). Consistent with a crucial role for NaVβ subunits in maintaining normal heart function, variants in the genes encoding NaVβ subunits have been linked to cardiac rhythm disorders, including Brugada syndrome, long QT syndrome, and sick sinus syndrome (4). However, recent evidence suggests that SCN1B may not be a monogenic cause of Brugada or sudden arrhythmic death syndrome (6, 7). NaVβ1 and NaVβ1B, splice variants of SCN1B, are the dominant NaVβ subunits in the mammalian heart (8).
Although NaVβ subunits were first cloned from a rat brain in the 1990s (9), the molecular interactions between NaVα-NaVβ subunits have remained elusive until recently. The cryo-electron microscopy structures of the NaV1.4-NaVβ1 and the NaV1.7-NaVβ1-NaVβ2 complexes suggest that NaVβ1 coassembles with NaV α subunits near the domain-III VSD (DIII-VSD) (10–12). However, the recent structure of NaV1.5 revealed that NaVβ1 interacts with the predominant cardiac NaV α subunit at a distinct site or sites that are characterized by weaker binding and an unresolvable NaV1.5-NaVβ1 complex (13). This difference in comparison with channels encoded by other NaV α subunits is partially due to the unique N-linked glycosylation of NaV1.5 that hinders its interaction with the Ig domain of NaVβ1 (13). Intriguingly, NaVβ1 and NaVβ3 are highly homologous except in the Ig domains. Previously, optical tracking of the NaV1.5 VSDs using voltage-clamp fluorometry (VCF) revealed that the NaVβ3 subunit modulates both the DIII and the DIV-VSDs, whereas NaVβ1 only modulates the DIV-VSD conformational dynamics (14, 15). Fluorescence quenching experiments showed that the DIII-VSD is in close proximity to NaVβ3 but not NaVβ1 (14). These results suggest that NaVβ1 and NaVβ3 regulate the NaV1.5 DIII-VSD differently.
The conformational changes in the VSDs are not only important for regulating channel gating; they are also essential for modulating channel interactions with drugs, including those that bind to the pore domain, such as local anesthetics (16). Previously, VCF and gating current recordings showed that when lidocaine blocks NaV1.4 channels, it stabilizes the DIII-VSD in its activated conformation (17). Moreover, we recently demonstrated that alteration of DIII-VSD conformational changes caused by long QT syndrome 3 variants leads to channels with different mexiletine sensitivities (18, 19).
Class I antiarrhythmics modulate cardiomyocyte excitability via NaV channel targeting. Class Ib molecules, such as lidocaine, ranolazine, and mexiletine, specifically modulate the late component of INa, resulting in shortening of the action potential duration in ventricular cardiomyocytes (20). Lidocaine has long been used to manage ventricular arrhythmias in hospital settings (21). Ranolazine has been shown to be effective in controlling various cases of atrial fibrillation (AF) (22–24), particularly paroxysmal AF (25, 26). Recently, the RAID trial demonstrated that ranolazine also marginally lowered the risk of recurrent ventricular tachycardia and ventricular fibrillation in high-risk patients with implanted cardioverter-defibrillators (27). Although both drugs are commonly prescribed for several arrhythmias, their efficacies are highly variable. Thus, it remains an important task to understand the determinants of channel-drug interactions that contribute to this variability.
In the experiments presented here, we aimed to understand the molecular mechanisms whereby noncovalently bound NaVβ subunits modulate the interaction of class Ib antiarrhythmics with myocardial NaV1.5 channels. We further investigated the physiological significance of this modulation by assessing ranolazine and lidocaine drug blockade of native NaV currents in mouse ventricular myocytes, probing the mRNA expression levels of NaVβ subunits in human hearts and detailing the in vivo electrophysiological phenotypes evident in the cardiac-specific Scn1b-null mouse (28). Our results showed a critical role for β subunits in differentially modulating the efficacy of lidocaine and ranolazine, implying that patient-to-patient differences in β subunit expression are likely to have a significant impact on therapeutic outcomes.
Results
Both lidocaine and ranolazine alter NaV1.5 DIII-VSD dynamics.
Previous studies demonstrated that lidocaine shifts the activation of the DIII-VSD in rat NaV1.4 channels encoded by Scn4a and prominent in skeletal muscle in the hyperpolarizing direction (16, 29, 30). Recent findings showed that a class Ib antiarrhythmic, mexiletine, which is similar in structure to lidocaine, also affects the DIII-VSD conformation in NaV1.5 channels (18, 19). The DIII-VSD effect also determines the tonic and use-dependent properties of class Ib drugs (18, 19). Taken together, these observations suggest that factors that alter drug effects on the DIII-VSD would be expected to have an impact on therapeutic efficacy.
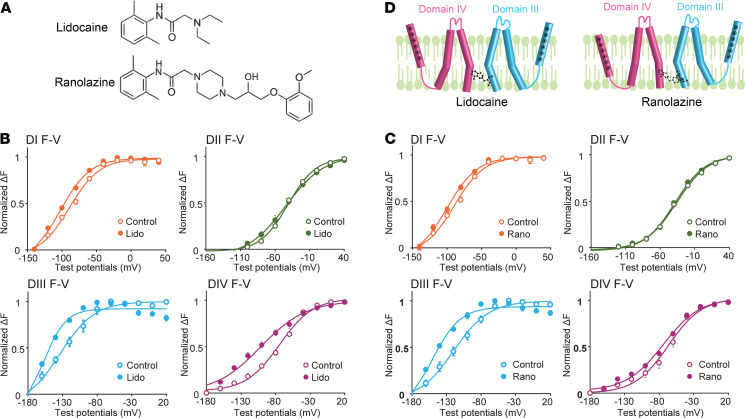
To explore this hypothesis, we first used VCF to assess the effects of 2 class Ib antiarrhythmics, lidocaine and ranolazine (Figure 1A), on the DIII-VSD in heterologously expressed human NaV1.5 channels, which are encoded by SCN5A, the predominant NaV α subunit expressed in the mammalian heart (Figure 1, B and C). When we expressed the NaV1.5 α subunit alone in Xenopus oocytes, we observed a hyperpolarizing shift (ΔV1/2 = –24.8 ± 9.4 mV, P = 0.03) in the DIII fluorescence-voltage (F-V) curve on exposure to 10 mM lidocaine, and a similar shift (ΔV1/2 = –30.7 ± 7.5 mV, P = 0.05) on application of 4 mM ranolazine, suggesting that both lidocaine and ranolazine stabilize the DIII-VSD in its activated conformation (Figure 1, B–D). The observation of similar effects on the DIII-VSD caused by both drugs is not surprising because they share similar molecular structures (Figure 1A), shown previously to interact with residue F1760 in DIV-S6 (20, 31). In addition, however, the effects of lidocaine and ranolazine are not identical. Lidocaine, for example, induced a hyperpolarizing shift in the DIV F-V curve, an effect not observed with ranolazine (Figure 1, B–D), suggesting that despite sharing common binding motifs on the NaV1.5 α subunit, the distinct chemical structures of lidocaine and ranolazine (Figure 1A) uniquely regulate DIV-VSD dynamics.
Figure 1. Class Ib antiarrhythmics lidocaine and ranolazine alter NaV1.5 VSD conformations.
(A) Chemical structures of lidocaine and ranolazine. (B) Voltage dependence of the steady-state fluorescence (F-V curves) from the 4 domains (DI-V215C, DII-S805C, DIII-M1296C, DIV-S1618C) of NaV1.5 before and after 10 mM lidocaine. Lidocaine was used at 10 mM to produce a robust tonic block (TB). In the presence of lidocaine, fluorescence was measured when TB reached more than 70%. Lidocaine induced a hyperpolarizing shift in both the DIII and DIV F-V curves. (C) F-V curves from the 4 domains of NaV1.5 before and after 4 mM ranolazine. Similar to the lidocaine experiment (B), in the presence of ranolazine, the fluorescence was measured when TB reached more than 70%. Ranolazine caused a hyperpolarizing shift in the DIII but not in the DIV F-V curve. (D) Schematic showing effects of lidocaine and ranolazine on the DIII- and DIV-VSDs; note that each VSD is represented by a single S4 segment for clarity. Lidocaine caused both the DIII and the DIV VSDs to stabilize in the activated conformation, whereas ranolazine only stabilized the DIII-VSD in the activated position. Each data set represents mean ± SEM values from 4–6 cells.
NaVβ1 and NaVβ3 differentially modulate lidocaine/ranolazine effects on the DIII-VSD.
We have previously shown that both NaVβ1 and NaVβ3 alter DIII-VSD dynamics during NaV1.5 channel gating (14). Thus, we hypothesized that these NaVβ subunits will also alter the effects of class Ib antiarrhythmics on the DIII-VSD. To test this hypothesis, we coexpressed NaV1.5 with the NaVβ1 or NaVβ3 subunit and measured DIII-VSD and DIV-VSD conformational changes before and after lidocaine or ranolazine application.
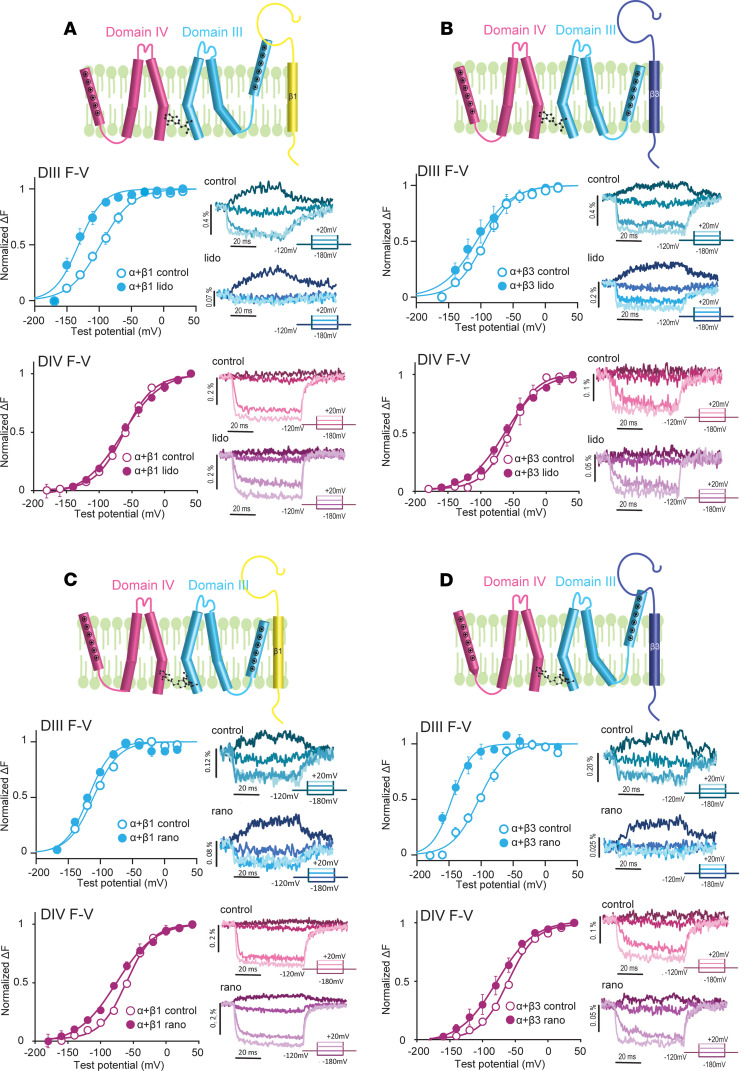
When we coexpressed NaV1.5 with NaVβ1, we observed distinct DIII-VSD responses to lidocaine and ranolazine. Lidocaine induced a greater hyperpolarizing shift (ΔV1/2 = –57.6 ± 10.2 mV, P = 0.01) in DIII-FV (Figure 2A) when NaVβ1 was present compared with the NaV1.5 α subunit expressed alone. Exposure to ranolazine, in marked contrast, did not result in a significant DIII-FV shift (ΔV1/2 = –12.8 ± 16.8 mV, P = 0.53) (Figure 2C), suggesting that the DIII-VSD was free to move in NaV1.5 channels in the presence of NaVβ1 to recover to the resting state. Although the presence of NaVβ1 increased the lidocaine effect on the DIII-VSD, NaVβ1 coexpression eliminated the ranolazine effect.
Figure 2. Coexpression with NaVβ1 or NaVβ3 differentially modulates the effect of lidocaine and ranolazine on the DIII-VSD.
(A) In the presence of NaVβ1, the hyperpolarizing shift in the DIII F-V curve produced by lidocaine was enhanced compared with the Nav1.5 α subunit expressed alone. In marked contrast, the DIV F-V curve was not affected by lidocaine with NaVβ1 present. (B) In contrast with NaVβ1 (A), the hyperpolarized shift in the DIII F-V curve induced by lidocaine was eliminated when NaVβ3 was coexpressed. Similar to NaVβ1, however, the DIV F-V curve was minimally affected by lidocaine. (C) In the presence of NaVβ1, the effect of ranolazine on the DIII F-V curve was eliminated, whereas the DIV F-V was slightly hyperpolarized. (D) In contrast with NaVβ1 (C), the hyperpolarized shift in the DIII F-V curve caused by ranolazine was enhanced when NaVβ3 was coexpressed. In the presence of NaVβ3, ranolazine also caused a small hyperpolarizing shift in the DIV F-V curve. Each data set represents mean ± SEM values from 4–6 cells.
Strikingly, coexpression with the NaVβ3 subunit resulted in opposite effects on lidocaine and ranolazine interaction with the DIII-VSD. Upon lidocaine block, the DIII F-V curve was minimally shifted to more hyperpolarized potentials (ΔV1/2 = –25.3 ± 10.9 mV, P = 0.13) (Figure 2B), while the ranolazine effect on the DIII-VSD was potentiated, resulting in a larger hyperpolarizing shift in the DIII F-V (ΔV1/2 = 58.0 ± 4.7 mV, P < 0.001) (Figure 2D).
Additionally, coexpression of NaVβ1 or NaVβ3 with NaV1.5 both eliminated the hyperpolarizing shift in the DIV F-V curve that was observed with the NaV1.5 α subunit expressed alone (Figure 2, A and B), suggesting that the NaVβ1 and NaVβ3 subunits similarly altered lidocaine’s effect on the DIV-VSD.
These results demonstrated that NaVβ subunits differentially regulated lidocaine and ranolazine interactions with the DIII-VSD in heterologously expressed NaV1.5 channels. Specifically, NaVβ1 enhanced the effect of lidocaine but decreased the effect of ranolazine on the DIII-VSD activation, whereas NaVβ3 coexpression had the opposite effects on both drugs. The altered drug interactions with the DIII-VSD resulted in an enhanced block by lidocaine and reduced block by ranolazine when the NaV1.5 α subunit was coexpressed with NaVβ1 compared with NaVβ3 (Figure 3C).
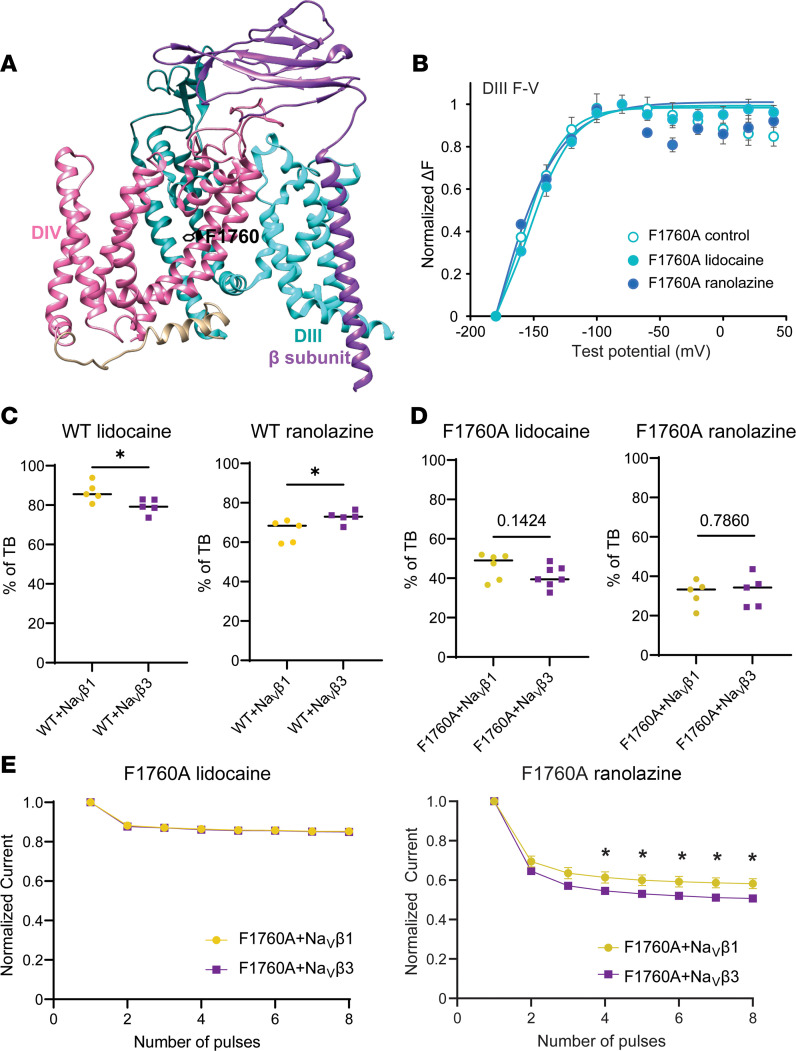
Figure 3. Altering the key local anesthetics’ binding site F1760 did not completely abolish NaVβ1/β3 modulations of ranolazine block.
(A) Cryo-electron microscopy structure of human NaV1.4(11) (Protein Data Bank 6AGF) showing the relative locations of the F1760 residue, DIII, DIV, and NaVβ1. (B) Mutating the main local anesthetic binding residue F1760 to alanine (A) greatly reduced the hyperpolarizing shift in the DIII-VSD upon 10 mM lidocaine, as well as 4 mM ranolazine, observed in the WT channel (Figures 1 and 2). (C) Percentage of TB induced by 10 mM lidocaine and 4 mM ranolazine in the WT channel. The presence of NaVβ3 reduced lidocaine TB but enhanced ranolazine TB compared with the α-NaVβ1 complex. (D) Percentage of TB induced by 10 mM lidocaine and 4 mM ranolazine in the F1760A channel. In contrast to WT, NaVβ1 and NaVβ3 no longer exerted a significant effect on lidocaine and ranolazine TB. (E) UDB by lidocaine and ranolazine in F1760A channel coexpressed with NaVβ1 or NaVβ3. There was no change in lidocaine UDB comparing coexpression with NaVβ1 and NaVβ3. However, the presence of NaVβ1 caused a reduced ranolazine UDB compared with NaVβ3, a phenomenon that is similar to NaVβ1’s effects on the WT channel. Each data set represents mean ± SEM values from 3–6 cells. Unpaired 2-tailed Student’s t test was used to test significance (C–E). *P < 0.05.
To determine whether the differential modulation of lidocaine and ranolazine block by NaVβ1 and NaVβ3 is dependent on the main local anesthetic binding site F1760 (20, 31) (Figure 3A), we assessed drug blockade of the F1760A-mutant NaV1.5 channel in the presence of NaVβ1 or NaVβ3. As expected, the F1760A-mutant channels exhibited much reduced block by lidocaine and ranolazine compared with the WT channels (Figure 3, C and D). However, application of 10 mM lidocaine or 4 mM ranolazine still caused significant tonic block (TB) and use-dependent block (UDB) of the F1760A channels (Figure 3, D and E). TB reflects resting-state drug block, while UDB requires prior channel opening (32). In contrast to the WT channels, the hyperpolarizing shift in the DIII F-V upon lidocaine or ranolazine block was not observed with the F1760A-mutant channels (Figure 3B). The F1760A mutation also eliminated NaVβ1 and NaVβ3 modulation of TB by lidocaine and ranolazine as well as UDB by lidocaine (Figure 3, D and E). However, despite the absence of a major drug-binding site, coexpression of NaVβ3 still caused stronger UDB by ranolazine compared with NaVβ1 (Figure 3E). These results suggest that the effects of NaVβ1/β3 on lidocaine and ranolazine block are affected by the F1760 anesthetic binding site but are not completely dependent on it.
Loss of Scn1b expression in mouse cardiomyocytes does not affect NaV channel gating.
To further investigate how noncovalent NaVβ1/β1B subunits affect the cardiomyocyte response to class Ib antiarrhythmics, we utilized the cardiac-specific Scn1b-null mouse model (Scn1bfl/fl/Myh6-cre) described previously (28). First, we compared INa in left ventricular (LV) myocytes acutely dissociated from adult Scn1b cardiac-specific null and WT mice. Peak INa density was increased by 28% in Scn1b-null compared with WT LV myocytes (Scn1b-null: 81.3 ± 3.6 pA/pF, WT: 63.9 ± 5.2 pA/pF, P = 0.017). An increase in INa density in cardiac-specific Scn1b-null isolated from juvenile mice was previously reported (28). Consistent with the increase in current density, we also observed increased Scn5a transcript expression in the ventricles (and atria) of the Scn1b-null compared with WT mice (Supplemental Figure 1; Supplemental material available online with this article; https://doi.org/10.1172/jci.insight.143092DS1). Other than increasing peak current density, Scn1b deletion did not measurably alter other NaV channel gating properties in ventricular cardiomyocytes (Figure 4A), including the voltage dependences of channel activation (Figure 4B), steady-state inactivation (Figure 4B), and/or the kinetics of channel recovery from inactivation (Figure 4C). Notably, deleting Scn1b did not measurably alter the expression of other NaVβ subunits (Supplemental Figure 1). These results, although contrary to previously reported effects of NaVβ1 on INa in heterologous expression systems, are consistent with results obtained in studies on global and cardiac-specific Scn1b-null mice (28, 33).
Figure 4. INa gating is similar in Scn1b-null and WT mouse LV myocytes.
(A) Representative recordings of INa in WT and Scn1b-null mouse LV myocytes revealed similar kinetics of activation and inactivation. However, the average peak current density was slightly (~28%) higher in Scn1b-null compared with WT. (B) Loss of NaVβ1 in Scn1b-null mouse LV myocytes did not affect the voltage dependences of INa activation or steady-state inactivation. (C) Loss of NaVβ1 in Scn1b-null mouse LV myocytes also did not affect the time course of INa recovery from inactivation. Each data set represents mean ± SEM values from 6–9 cells.
Increased block of INa by ranolazine but reduced block by lidocaine in adult Scn1b-null mouse ventricular myocytes.
Even though NaV channel gating was not measurably affected in cardiac-specific Scn1b-null myocytes, we went on to determine whether the loss of Scn1b affects the responses of native NaV channels to class Ib antiarrhythmics. We examined the effects of lidocaine and ranolazine on TB and UDB of INa in LV myocytes isolated from WT and cardiac-specific Scn1b-null mice.
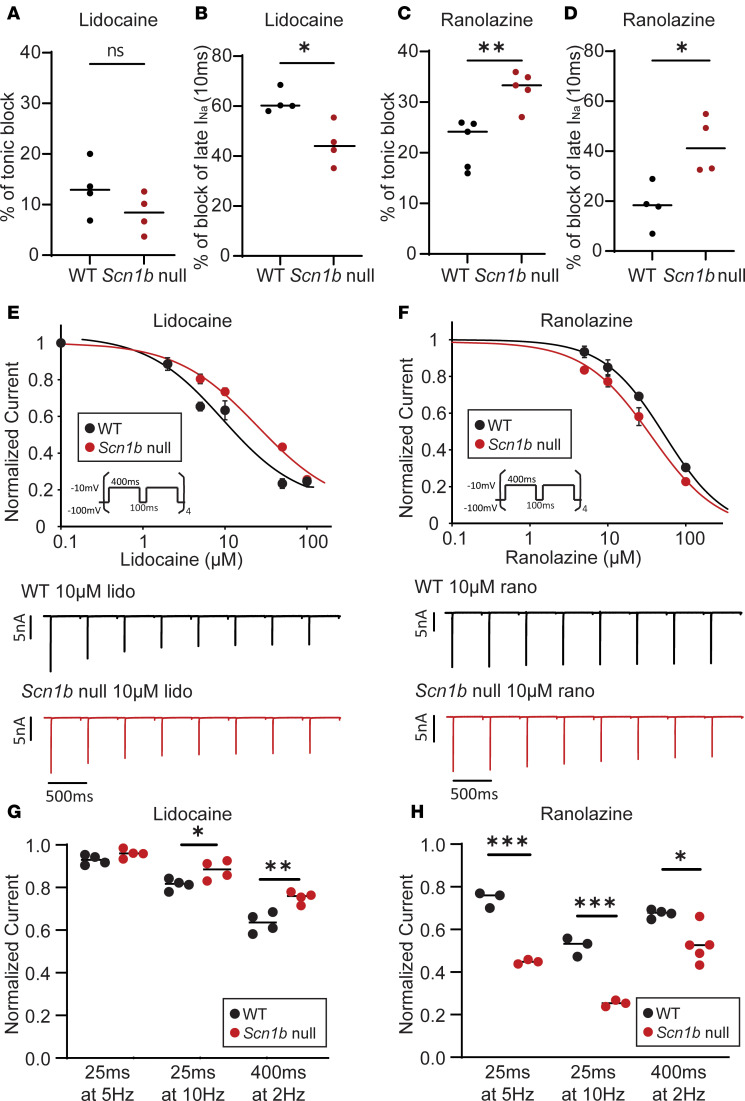
The TB produced by 100 μM lidocaine was similar in WT and Scn1b-null LV myocytes (Figure 5A). In marked contrast, the block of late INa by lidocaine was significantly reduced in Scn1b-null LV myocytes (Figure 5B). There was also an approximately 3-fold reduction in lidocaine UDB in Scn1b-null compared with WT LV myocytes (WT: EC50UDB = 9.3 μM, Scn1b-null EC50UDB = 24.8 μM) (Figure 5E). Conversely, ranolazine increased TB, late INa block, and UDB in Scn1b-null compared with WT adult mouse LV myocytes (Figure 5, C, D, and F) (WT: EC50UDB = 53.3 μM, Scn1b-null EC50UDB = 36.0 μM). The differences in UDB by lidocaine between WT and Scn1b-null myocytes depended on the frequency and duration of the depolarizing pulses (Figure 5G). In response to 10 μM lidocaine, INa from Scn1b-null showed decreased UDB compared with WT myocytes at 10 Hz (25 ms duration) and 2 Hz (400 ms duration) but not 5 Hz (25 ms duration) (Figure 5G). In contrast, in response to 10 μM ranolazine, INa in WT myocytes showed increased UDB compared with Scn1b-null myocytes at all 3 frequencies (Figure 5H). Both lidocaine and ranolazine are known to cause a hyperpolarizing shift in the voltage dependence of steady-state inactivation of cardiac INa (24, 34), indicating that drug binding promotes channel inactivation at more hyperpolarized membrane potentials. Therefore, we also compared the voltage dependence of INa inactivation in WT and Scn1b-null LV myocytes before and after lidocaine or ranolazine application. These experiments revealed 100 μM lidocaine induced a hyperpolarizing shift in INa inactivation in WT LV myocytes and a smaller shift in Scn1b-null LV myocytes (Supplemental Figure 2A). Conversely, 100 μM ranolazine induced a comparable leftward shift in the voltage dependence of inactivation of INa in WT and Scn1b-null LV myocytes (Supplemental Figure 2B).
Figure 5. Scn1b-null LV myocytes show reduced lidocaine but enhanced ranolazine responses.
(A) TB of INa by 100 μM lidocaine was slightly reduced in Scn1b-null compared with WT mouse LV myocytes. (B) Percentage of late INa block by 100 μM lidocaine was markedly lower in Scn1b-null compared with WT mouse LV myocytes. Late INa was measured 30 ms after the onset of the depolarizing voltage step. (C) TB of INa by 100 μM ranolazine was greater in Scn1b-null compared with WT mouse LV myocytes. (D) Percentage of late INa block by 100 μM ranolazine was greater in Scn1b-null compared with WT mouse LV myocytes. (E) Dose-response curve (top) and example traces (bottom) for UDB of INa by lidocaine. UDB was examined by measuring INa evoked in response to 8 repetitive (400 ms duration) depolarizations presented at 2 Hz, which determines the initial rate of UDB. The EC50 for UDB of INa by lidocaine was lower in WT compared with Scn1b-null suggesting that NaVβ1 enhances the sensitivity to lidocaine. (F) Dose-response curve (top) and example traces (bottom) for UDB of INa by ranolazine. In contrast to lidocaine, the EC50 for UDB by ranolazine was higher in WT compared with Scn1b-null, suggesting NaVβ1 reduces the effects of ranolazine. (G) Frequency-dependent UDB block of INa by 10 μM lidocaine in WT and Scn1b-null LV myocytes. UDB was assessed by measuring INa evoked by repetitive depolarizing pulses at 5 Hz (25 ms, 40 pulses), 10 Hz (25 ms, 40 pulses), and 2 Hz (400 ms, 8 pulses). Normalized currents indicate INa(last-pulse)/INa(first-pulse). (H) Frequency-dependent UDB block of INa by 10 μM ranolazine in WT and Scn1b-null LV myocytes. Each data set represents mean ± SEM of data from 3–5 cells. Unpaired 2-tailed Student’s t test. *P < 0.05; **P < 0.01; ***P < 0.001.
Overall, these cellular studies revealed that in adult mouse LV myocytes, the cardiac deletion of Scn1b resulted in reduced lidocaine UDB but increased ranolazine UDB. These results are consistent with our VCF data (Figures 1 and 2), suggesting that the presence of NaVβ1 subunits enhanced lidocaine’s effects but reduced ranolazine’s effects on the NaV1.5 DIII-VSD. The reduced effects on the DIII-VSD are also consistent with the decreased UDB of INa observed in LV myocytes (Figure 5).
Ranolazine and lidocaine induced distinct ECG phenotypes in WT and Scn1b-null mice.
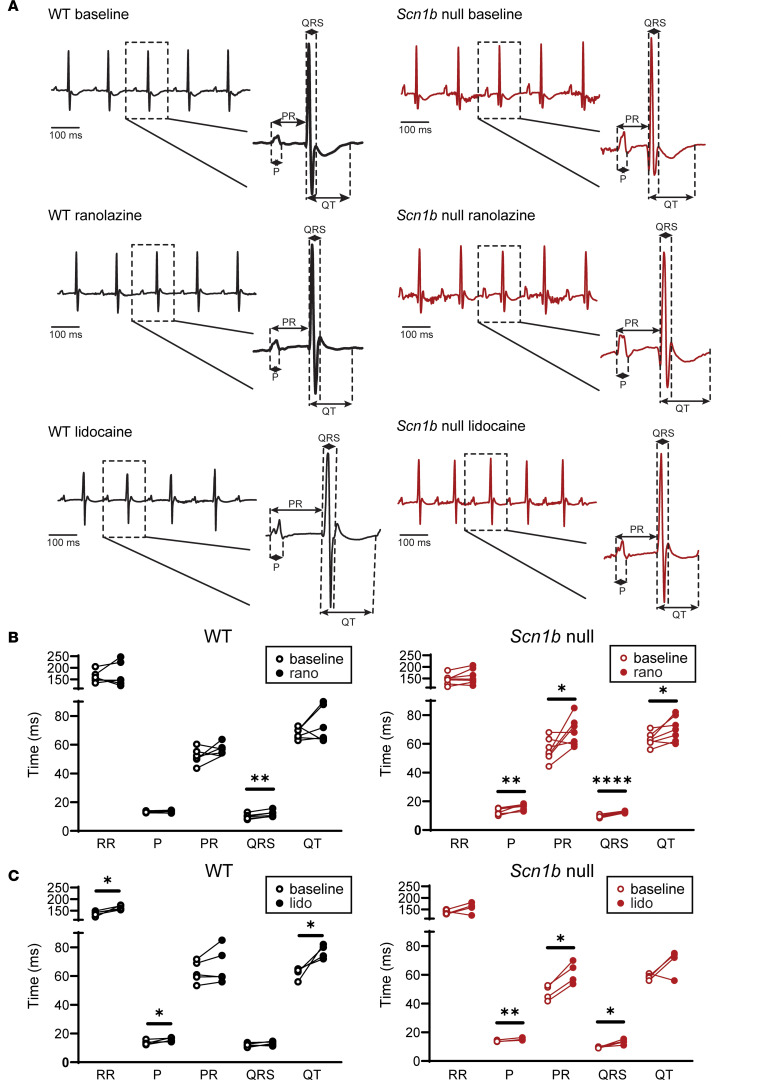
To understand how NaVβ1/β1B modulate antiarrhythmic responses at the whole-heart level, we measured surface ECGs in anesthetized WT and Scn1b-null mice before and after i.p. injection of lidocaine or ranolazine (Figure 6 and Supplemental Table 1). From the raw ECG data, we quantified several parameters that describe overall heart electrical functioning, including RR intervals, providing a measure of heart rates; P wave intervals, representing atrial conduction; PR intervals, characterizing atrial-ventricular conduction; QRS intervals, revealing ventricle conduction; and QT and ST intervals, corresponding to ventricular repolarization.
Figure 6. ECG recordings from WT and Scn1b-null mice before and after ranolazine or lidocaine injections.
(A) Representative ECG recordings obtained from WT and Scn1b-null mice at baseline, postranolazine, and postlidocaine are presented. The postranolazine and postlidocaine data were recorded 10 minutes after the i.p. injections of ranolazine or lidocaine. P wave durations, PR, QRS, and QT intervals were measured as indicated in the insets. (B) Comparison of ECG parameters measured in WT (left panel) and Scn1b-null (right panel) mice at baseline and 10 minutes after i.p. injections of ranolazine injection. Ranolazine markedly prolonged the P wave duration and the PR interval in Scn1b-null but not in WT mice. (C) Comparison of ECG parameters measured in WT (left panel) and Scn1b-null (right panel) mice at baseline and 10 minutes after i.p. injections of lidocaine. Lidocaine markedly prolonged the RR interval, P wave duration, and QT interval in WT mice. In Scn1b-null mice, lidocaine also prolonged the P wave duration and resulted in marked prolongation of the PR and QRS intervals. Each data set represents data from 4–7 mice. The ECG parameters and statistical comparisons are shown in Supplemental Table 1.
We found that 20 mg/kg ranolazine caused QRS prolongation in both WT and Scn1b-null mice (Figure 6, A and B), but that P wave and PR interval prolongation only occurred in the Scn1b-null mice (Figure 6B). The QT interval, but not the ST interval, was also prolonged by ranolazine in Scn1b-null mice (Figure 6B and Supplemental Figure 3). These results suggest that Scn1b deletion enhanced the inhibitory effect of ranolazine on cardiac conduction. Similar to the effects observed at the single myocyte level (Figures 4 and 5) that the loss of Scn1b enhanced TB and UDB of INa by ranolazine, loss of NaVβ1/β1B in Scn1b-null mice promoted ranolazine block, manifesting as P wave, PR, and QRS interval prolongation.
We observed that 30 mg/kg lidocaine administration increased P wave duration in WT and Scn1b-null mice (Figure 6, A and C). In addition, lidocaine induced prolongation of RR, QT, and ST intervals in WT but not Scn1b-null mice (Figure 6C and Supplemental Figure 3). In contrast, lidocaine increased PR and QRS intervals in Scn1b-null mice (Figure 6C). Lidocaine injection, therefore, resulted in distinct functional effects in the 2 genotypes. We conducted control experiments in which we measured ECGs before and after injection of PBS solution. Comparison of baseline and post-PBS data showed that ECG parameters remained constant (Supplemental Figure 4).
SCN1B is differentially expressed in human atria and ventricles.
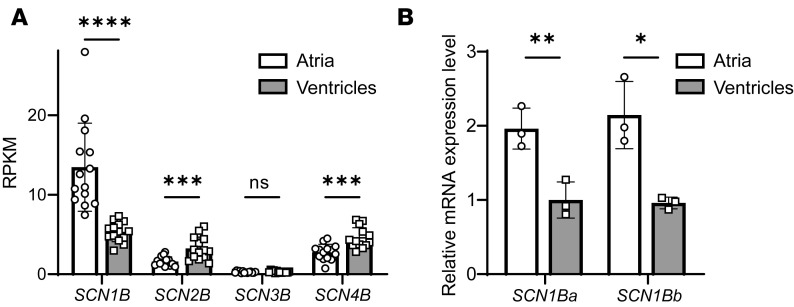
The observation that loss of Scn1b alters the ability of class Ib antiarrhythmics to block NaV channels in the mouse heart suggests that the differential expression of SCN1B might play an important role in regulating antiarrhythmic drug responses in humans. To begin to explore this hypothesis, we examined mRNA expression levels of the genes SCN1B, SCN2B, SCN3B, and SCN4B encoding NaVβ subunits in human heart tissue in a recently published RNA-Seq library (35). These analyses revealed that in the human heart, SCN1B is the most abundant of the NaVβ subunits at the transcript level and that SCN1B transcript expression is much higher in atria than in ventricles (Figure 7A). In contrast, the expression levels of the SCN2B and SCN4B transcripts are higher in the ventricles than in the atria (Figure 7A). To validate the RNA-Seq findings and to determine whether both SCN1B splice variants, SCN1BA (NaVβ1) and SCN1BB (NaVβ1B), are differentially expressed in human atria and ventricles, we performed quantitative RT-PCR analyses on the same tissue samples used in the RNA-Seq analyses. These experiments revealed that the relative expression levels of the 2 SCN1B splice variants were significantly higher in the atria compared with the ventricles (Figure 7B). Additional analyses revealed that, although expression of SCN1BA was approximately 100-fold higher than SCN1BB, the expression levels of the 2 (SCN1BA and SCN1BB) splice variants were similar in human right and left atria and in the LV and right ventricle (RV) (Supplemental Figure 5).
Figure 7. Regional differences in SCN1B expression in human atria and ventricles.
(A) Extracted RNA-Seq data, expressed as reads per kilobase of exon per million mapped reads, from analyses of sequencing data obtained from matched (n = 8) human ventricular and atrial tissue samples (44). The SCN1B transcript was the most abundant of the NaVβ subunits expressed in human atria and ventricles. In addition, SCN1B expression was approximately 3-fold higher in human atria compared with ventricles, whereas both SCN2B and SCN4B were approximately 2-fold higher in human ventricles than atria. (B) The differential expression of SCN1B in human atria and ventricles was confirmed by quantitative PCR (qPCR) analyses of the same paired human atrial and ventricular tissue samples analyzed by RNA-Seq. In addition, qPCR analyses using primers that distinguish the 2 SCN1B variants, SCN1Ba and SCN1Bb, revealed that the relative expression levels of both SCN1Ba and SCN1Bb transcripts were higher in the atria than the ventricles. Paired 2-tailed Student’s t test was used to test significance. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Discussion
Although class Ib antiarrhythmics have considerable therapeutic potential, they are not broadly prescribed because of proarrhythmic risks in some patients and ineffectiveness in others (36, 37). Patient or disease variability in class Ib drug response suggests that there are external factors that modulate drug interactions with the channel (37). In this study, we investigated the role of NaV channel accessory subunits NaVβ1 and NaVβ3 in regulating class Ib antiarrhythmic–mediated effects on NaV1.5 channels. We demonstrated that at a molecular level, NaVβ1 or NaVβ3 subunit coexpression differentially altered the effects of lidocaine and ranolazine on the NaV1.5 DIII-VSD. NaVβ1 enhanced lidocaine but inhibited ranolazine modulation of the DIII-VSD. Conversely, NaVβ3 eliminated lidocaine modulation but increased the effect of ranolazine on the DIII-VSD. Differential molecular interactions between NaV1.5 DIII-VSD and class Ib antiarrhythmic drugs caused by NaVβ1 subunit expression in a heterologous system translated to distinct drug blockade of NaV channels in WT versus Scn1b cardiac-specific null mouse cardiomyocytes. We further demonstrated differential effects of lidocaine and ranolazine on the ECG phenotypes of WT and Scn1b-null mice.
NaVβ1 and NaVβ3 subunits alter NaV1.5 channel pharmacology via the DIII-VSD.
The DIII-VSD plays an important role in regulating NaV1.5 channel gating. It is involved in both activation and inactivation of NaV channels (38, 39). Recent studies showed a correlation between DIII-VSD deactivation and the slow component of recovery from inactivation, which suggests that an activated form of the DIII-VSD stabilizes inactivation (39). Given that class Ib antiarrhythmics promote DIII-VSD activation, they may subsequently promote inactivation to induce greater levels of UDB. We recently demonstrated that DIII-VSD activation determines NaV1.5 channel blockade by mexiletine and affects the sensitivity of LQT3 variants to this drug (18), demonstrating a clear connection between the conformation of the DIII-VSD and class Ib drug potency.
We previously demonstrated that NaVβ3 directly modulates the DIII-VSD, whereas NaVβ1 does not (14). Recent channel structures suggest that NaVβ1 associates with NaV1.7 and NaV1.4 through the DIII-VSD, an interaction that is not conserved in NaV1.5 (10, 12, 13). In light of these structural and functional data, it is plausible that NaVβ3 interacts with NaV1.5 through similar sites as illustrated in the NaV1.4/ NaV1.7-β1 complex, while NaVβ1 occupies a different site. Two distinct interaction mechanisms will result in the differential modulation of the DIII-VSD by NaVβ1 and NaVβ3. Here, we showed that coexpression of the NaVβ1 or NaVβ3 subunit differentially modulated the ability of class Ib antiarrhythmics to stabilize the DIII-VSD in the activated conformation, providing further evidence that in contrast to other NaV channel α subunits, NaVβ1 and NaVβ3 have distinct interactions with NaV1.5. The effect of lidocaine on the DIII-VSD has been previously shown (14) to regulate UDB, a critical feature of class Ib drugs, which renders them most potent when myocytes are being excited repeatedly during an arrhythmic event. We observed that ranolazine and lidocaine similarly affected NaV1.5 α subunit–encoded channels expressed in the absence of the NaVβ subunits. However, with the NaVβ1 subunit present, the lidocaine effect on the DIII-VSD was enhanced, whereas the ranolazine effect was blunted. Conversely, NaVβ3 enhanced ranolazine-induced DIII-VSD stabilization while inhibiting the effect of lidocaine. The differential regulation of the DIII-VSD resulted in distinct effects on the potencies of lidocaine and ranolazine depending on which NaVβ was present. Thus, despite the similarity of the chemical structures (Figure 1), the therapeutic responses to lidocaine and ranolazine were differentially modified by the coexpression of the NaVβ1 or the NaVβ3 subunit.
NaVβ1/β1B modulates class Ib antiarrhythmic responses from molecular to the whole-heart level.
In Xenopus oocytes, with VCF and cut-open voltage clamp recordings, we demonstrated that NaVβ1 coexpression enhanced lidocaine’s but inhibited ranolazine’s effect on the DIII-VSD, which resulted in an increased lidocaine but decreased ranolazine block. In addition, we observed increased UDB and late INa block by lidocaine but opposite effects with ranolazine in WT compared with Scn1b-null mouse LV myocytes. This cellular difference further led to distinct phenotypes of the in vivo ECG recordings in response to lidocaine and ranolazine injections. For example, in response to ranolazine injection, the P wave duration and PR interval were prolonged in the Scn1b-null mice but not in the WT mice. This difference in ECG parameters reflects the cellular phenotype of enhanced ranolazine block of INa in Scn1b-null compared with WT LV myocytes. However, not all the ECG changes can be explained by the differences observed in myocyte INa recordings of atrial and ventricular myocytes, and they may be caused by NaVβ1-mediated effects on other regions of the myocardium, such as the sinoatrial and atrioventricular nodes (40). Alternatively, NaVβ1 may affect drug interactions with other ion channels that regulate cardiac excitation, as discussed further below.
Differential expression of NaVβ1/β1B in human atria and ventricles and chamber-specific drug responses.
Ranolazine was proposed as a candidate for atrial specific therapy for AF (24, 41). Studies in the canine heart showed that atrial and ventricular cardiomyocytes have distinct responses to ranolazine (24). In the atria, ranolazine prolongs the action potential duration measured at 90% repolarization (APD90) and effective refractory period (24). In contrast, in the ventricle, ranolazine shortens the APD90. Here, we demonstrated higher SCN1Ba (NaVβ1) and SCN1Bb (NaVβ1B) subunit mRNA expression levels in human atria compared with ventricles. While we showed that NaVβ1 coexpression attenuated ranolazine block of INa in both Xenopus oocytes and mouse LV myocytes, higher levels of NaVβ1 in human atria may result in similar attenuation of the effects of ranolazine, thus contributing to decreased ranolazine blockade compared with ventricles. Notably, ranolazine is also a blocker of KCNH2–encoded (HERG-encoded) repolarizing IKr channels (22). If ranolazine blockade of INa is reduced in atria, modulation of IKr may dominate, resulting in prolongation of APD specifically in atria. Thus, the heterogeneous expression of NaVβ1 may play a role in the chamber-specific ranolazine response.
NaVβ subunit modulation of class Ib drug effects may underlie disease-specific drug responses.
A search of NCBI’s Gene Expression Omnibus (GEO) profile database (42) revealed that SCN1B is upregulated in ischemic cardiomyopathies in human heart [GEO GDS651 and GDS1362 (43)] and mouse heart failure models [GEO GDS411, GDS427 (44), and GDS3660 (45)] (Supplemental Figure 6). These data suggest that the expression of NaVβ subunits can be altered in disease remodeling of the cardiac tissue. Since late INa was found to be increased in the failing heart, class Ib drugs have become potential therapeutic approaches in targeting heart failure–related arrhythmias (46). As we have demonstrated that NaVβ can differentially modulate class Ib effects, upregulation of NaVβ1 in failing tissue may alter the patient response to NaV channel–targeting antiarrhythmic therapies.
Differential effects of NaVβ subunits on lidocaine and ranolazine interactions reflect distinct molecular drug-pore interactions.
Previous work postulated that both lidocaine and ranolazine bind to common residues in the NaV1.5 channel pore (20, 31). However, we observed that lidocaine modulated both the DIII- and DIV-VSDs of NaV1.5, whereas ranolazine only affected the DIII-VSD when NaV1.5 was expressed without NaVβ subunits, suggesting that these compounds may present in different orientations in the channel pore. Recent molecular dynamics simulations shed light on the detailed binding conformations of lidocaine and ranolazine within the NaV1.5 channel (47). Interestingly, these studies revealed that 2 lidocaine molecules can bind to the pore concurrently, one to the F1760 site and the other to the central pore (47). In contrast, ranolazine binds to the F1760 residue and possesses a more flexible linear structure, allowing it to interact with a larger area ranging from the fenestration to the selectivity filter (47). Moreover, ranolazine has a pKa of 7.2 (48) and lidocaine has a pKa of 7.9 (49). At physiological pH, therefore, a higher percentage of ranolazine molecules are expected to be uncharged compared with lidocaine. This difference will determine the relative percentages of the drug molecules entering in the hydrophobic pathway through the fenestration versus the hydrophilic pathway via the intracellular gate. The presence of NaVβ1 or NaVβ3 modulates DIII-VSD and DIV-VSD dynamics, which can allosterically affect the conformation of the pore and fenestrations. We demonstrated that NaVβ1/β3’s modulation of ranolazine block was not entirely dependent on the main local anesthetic binding site, F1760, suggesting the changes in the DIII-VSD conformation due to NaVβ modulation are essential for determining drug accessibility to the pore, independent of binding. In contrast, eliminating the F1760 binding site abolished the effects of NaVβ1/β3 coexpression on lidocaine block, further suggesting that lidocaine acts through mechanisms distinct from those of ranolazine. Because lidocaine and ranolazine have different stoichiometries, orientations within the pore, and entrance pathways, it is plausible that changing channel VSD and pore conformations in the presence of NaVβ subunits can result in opposite effects on DIII-VSD interactions with these drugs.
NaVβ subunit modulation of antiarrhythmic drug outcome beyond NaV channels.
Aside from NaV channels, NaVβ subunits have been shown to modulate expression and gating of various potassium channels, including the voltage-gated KV 4.3 and the inward-rectifying Kir 2.1 channels, resulting in modifications of 2 essential cardiac currents, the fast transient outward K+ current (Ito,f) and the inward rectifier current (IK1–) (50–52). Although unexplored to date, it also seems highly likely that the presence of NaVβ subunits can affect KV and Kir channel pharmacology as well. Here, we showed that although single cardiac-specific Scn1b-null LV myocytes displayed attenuated inhibition of INa by lidocaine compared with WT cells, enhanced PR and QRS interval prolongation was observed in Scn1b-null animals with lidocaine, suggesting contributions from other cardiac ionic currents.
Limitations.
To assess the drug effects on VSD dynamics, we conducted the VCF experiments in Xenopus oocytes. Because this is a heterologous expression system and the experiments were performed at 19°C, extrapolating the results to mammalian physiology can be difficult. However, we observed consistent drug-mediated modulatory effects in oocytes and in mouse LV myocytes, observations that support the hypothesis that the molecular mechanisms we identified with VCF are operative in mammalian systems.
We were only able to show that the expression of SCN1B was enriched in the atria compared with ventricles in the human heart at the transcript level. Although we attempted to examine protein expression levels directly, none of the available anti-NaVβ1 antibodies detect NaVβ1 proteins in mouse or human cardiac tissues. Since it is currently not possible to perform gene editing or to use siRNA-mediated knockdown strategies in native human myocytes, we were not able to explore the functional effects of NaVβ1 on class Ib drugs in human myocytes directly.
Conclusions.
In summary, we have demonstrated roles for noncovalently linked NaVβ subunits in regulating antiarrhythmic drug effects from molecular interactions to whole-heart phenotypes. Our results elucidated the differential regulation of NaV1.5 channels by 2 class Ib agents, lidocaine and ranolazine, by NaVβ1 and NaVβ3 subunits. The unique expression profile of NaVβ subunits in the human heart suggests chamber-dependent responses to these 2 compounds. Our findings provide crucial insights into strategies for improving the clinical outcomes of patients treated with class Ib agents for different forms of arrhythmias. Moreover, NaVβ1 expression is upregulated in heart failure, and it remains unexplored whether NaVβ subunit expression is affected in other heart pathologies. This knowledge will be highly valuable in establishing disease-specific approaches to personalize arrhythmia treatment with lidocaine and ranolazine because known changes in β subunit expression will have predictable effects on therapeutic outcomes.
Methods
Experimental animals.
Adult (8–15 weeks old) male and female WT and cardiac-specific Scn1b-null C57BL/6J mice were used in the experiments here. Cardiac-specific Scn1b-null mice were generated by crossing Scn1bfl mice (27) with B6.FVBTg(Myh6-cre)2182Mds/J mice (The Jackson Laboratory), which express Cre recombinase driven by the α-myosin heavy chain promoter. Mice were genotyped by PCR analyses of genomic (tail) DNA using primers targeting sequences external to the loxP sites, as well as primers targeting Cre recombinase, as previously described (27). Because of the breeding strategy required to generate cardiac-specific Scn1b-null C57BL/6J mice, WT littermates were not generated. The WT mice used in the experiments presented here, therefore, were not littermate controls; rather they were WT C57BL/6J mice from our colony. Additional control experiments, however, were conducted on Scn1bfl and B6.FVBTg(Myh6-cre)2182Mds/J mice, which were determined to be indistinguishable electrophysiologically from WT C57BL/6J animals. Xenopus oocyte harvests were performed as described previously (53).
Cut-open VCF.
VCF experiments were conducted using 4 previously developed NaV1.5 channel constructs (DI: V215C, DII: S805C, DIII: M1296C, DIV: S1618C) (53). Capped mRNAs were synthesized with the mMESSAGE mMACHINE T7 transcription kit (Life Technologies, Thermo Fisher Scientific) from the linearized pMAX vectors. VCF construct mRNA was injected alone or coinjected with SCN1B or SCN3B mRNA into Xenopus oocytes as previously described (14). VCF experiments were performed 4–6 days after injection. The recording setup and labeling protocol used were described previously (14, 53–55). Lidocaine hydrochloride and ranolazine dihydrochloride were dissolved in extracellular recording solution and then further diluted to 10 mmol/L and 4 mmol/L, respectively. Both drugs were manually perfused into the extracellular solution chamber in the cut-open voltage clamp setup. Fluorescence signals and currents were analyzed as previously described (18). V1/2 values reported were quantified from the Boltzmann function fit, y = 1/(1 + exp [V – V1/2]/k). Because the DIII F-V curve, especially under drug treatment conditions, did not saturate at the lowest voltage recorded (–160 mV), the fit was performed by fixing 0 at –200 mV. Because of the lack of saturation at the most negative potentials measured, the estimated V1/2 for DIII F-V is likely to be higher than the actual V1/2 value.
ECG recordings.
Surface ECG recordings were obtained as previously described from mice anesthetized by i.p. injection of avertin (0.25 mg/kg; MilliporeSigma) (56). Baseline ECGs were recorded, and animals were weighed. For injections, drugs were dissolved in 250 μL PBS. Lidocaine or ranolazine was then injected at a dosage of 30 mg/kg or 20 mg/kg, respectively. Different animals were used for lidocaine or ranolazine injections. Between recordings, mice were kept on a heating pad maintained at 37°C ± 0.5°C. Postinjection ECGs were recorded at 5 minutes, 10 minutes, 15 minutes, 20 minutes, and 30 minutes. Peak responses were observed at 10 minutes, which was subsequently selected as the time point for ECG analysis.
RR, PR, and QT intervals, as well as P and QRS durations, were measured and compiled using Clampfit 10.3 (Molecular Devices) and GraphPad Prism. Note that QT intervals shown in figures were not corrected because several recent studies have shown that QT intervals in anesthetized mice do not vary with heart rate (57, 58). Similar differences were revealed, however, when corrected QT intervals were compared (Supplemental Table 1).
Isolation of adult mouse cardiomyocytes.
Myocytes were isolated from adult (8 to 12 weeks old) WT or Scn1b-null mice as previously described (59). Briefly, hearts were isolated and perfused retrogradely through the aorta with a Ca2+-free Earle’s balanced salt solution containing 0.8 mg/mL type II collagenase (Worthington). After perfusion, the LV free wall was dissected and minced. The tissue pieces were then triturated to provide individual LV myocytes. Dispersed cells were then filtered and resuspended in Medium199 (Gibco, Thermo Fisher Scientific), plated on laminin-coated (MilliporeSigma) glass coverslips, and maintained in a 95% air/5% CO2 incubator at 37°C.
Whole-cell NaV current (INa) recordings were obtained from isolated LV myocytes at room temperature (22°C–24°C) within 5–6 hours of isolation using a Dagan 3900A amplifier interfaced to a Digidata 1332A A/D converter (Axon) using pClamp 10.2 (Axon). Recording pipettes contained the following (in mmol/L): 120 glutamic acid, 120 CsOH, 10 HEPES, 0.33 MgCl2, 20 tetraethylammonium chloride (TEACl), 4 Mg-ATP, 5 glucose, and 5 EGTA (pH adjusted to 7.3 with CsOH); pipette resistances were 1.5–3.0 MΩ. The bath solution contained the following (in mM): 20 mM NaCl, 110 mM TEACl, 4 KCl, 2 MgCl2, 1 CaCl2, 10 HEPES, and 10 glucose (pH 7.4; 300 mOsm).
Electrophysiological data were acquired at 10–20 KHz, and signals were low-pass-filtered at 5 kHz before digitization and storage. After the formation of a giga-seal (>1 GΩ) and establishment of the whole-cell configuration, brief (10 ms) ± 10 mV voltage steps from a holding potential (HP) of –70 mV were presented to allow measurements of whole-cell membrane capacitances (Cm), input resistances (Rin), and series resistances (Rs). In each cell, Cm and Rs were compensated electronically by approximately 85%; voltage errors resulting from uncompensated Rs were less than 2 mV and were not corrected. Leak currents were always less than 50 pA and were not corrected. Whole-cell INa were evoked in response to 40 ms voltage steps to potentials between –60 and +40 mV from an HP of –100 mV in 10 mV increments at 15-second intervals.
Electrophysiological data were compiled and analyzed using Clampfit 10.3 (Molecular Devices) and GraphPad Prism.
Quantitative reverse-transcription PCR.
Total RNA (2 μg) isolated from individual matched (n = 6) human right atria, left atria, RV, and LV tissue samples was reverse-transcribed into cDNA with a high-capacity cDNA kit. Transcript analysis was conducted with SYBR green using a 7900HT Fast Real-Time PCR system (all from Applied Biosystems, Thermo Fisher Scientific). Data were analyzed using the Ct relative quantification method using the GAPDH and hypoxanthine guanine phosphoribosyl transferase I (HPRT) genes as endogenous controls.
Statistics.
Results are presented as mean ± SEM. The number of animals and the number of cells used in each experiment are provided in the figure legends. Comparisons of differences between WT and Scn1b-null cells/animals under control conditions and before and after drug treatments were performed using a paired 2-tailed Student’s t test (Microsoft Excel). In comparisons of more than 2 groups, 1-way ANOVA was used followed by multiple comparisons. The P values shown were corrected for multiple hypothesis testing using Dunnett’s correction method. P values less than 0.05 were considered significant.
Study approval.
All animals were handled in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and all experimental protocols were approved by the Washington University IACUC.
Author contributions
WZ contributed to designing studies, conducting experiments, acquiring data, analyzing data, and writing the manuscript. WW, PA, and RLM contributed to conducting experiments and acquiring data. LLI contributed to providing experimental animals and editing the manuscript. JMN and JRS contributed to designing studies and editing the manuscript.
Supplementary Material
Acknowledgments
Human cardiac tissue samples were provided by the Translational Cardiovascular Biobank and Repository, supported by the Washington University Institute for Clinical and Translational Sciences, recipient of a Clinical and Translational Sciences Award (UL1 RR024992) from the NIH National Center for Research Resources.
Financial support was provided by the American Heart Association (predoctoral fellowship 15PRE25080073 to WZ); NIH National Heart, Lung, and Blood Institute (R01 HL136553 to JRS); NIH National Heart, Lung, and Blood Institute (R01 HL-034161 and R01 HL-142520 to JMN).
Version 1. 06/22/2021
In-Press Preview
Version 2. 08/09/2021
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2021, Zhu et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: JCI Insight. 2021;6(15):e143092.https://doi.org/10.1172/jci.insight.143092.
Contributor Information
Wandi Zhu, Email: wzhu5@bwh.harvard.edu.
Wei Wang, Email: david_wang89@hotmail.com.
Paweorn Angsutararux, Email: angsutararux.p@wustl.edu.
Rebecca L. Mellor, Email: rmellor@wustl.edu.
Lori L. Isom, Email: lisom@umich.edu.
Jeanne M. Nerbonne, Email: jnerbonne@wustl.edu.
Jonathan R. Silva, Email: jonsilva@wustl.edu.
References
- 1. Zipes DP, Jalife J, eds. Cardiac Electrophysiology: From Cell to Bedside: Sixth Edition. Saunders; 2013. [Google Scholar]
- 2.Gellens ME, et al. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc Natl Acad Sci U S A. 1992;89(2):554–558. doi: 10.1073/pnas.89.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abriel H. Cardiac sodium channel Nav1.5 and interacting proteins: physiology and pathophysiology. J Mol Cell Cardiol. 2010;48(1):2–11. doi: 10.1016/j.yjmcc.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 4.Calhoun JD, Isom LL. The role of non-pore-forming β subunits in physiology and pathophysiology of voltage-gated sodium channels. Hands Exp Pharmacol. 2014;221:51–89. doi: 10.1007/978-3-642-41588-3_4. [DOI] [PubMed] [Google Scholar]
- 5.Patino GA, et al. Voltage-gated Na+ channel 1B: a secreted cell adhesion molecule involved in human epilepsy. J Neurosci. 2011;31(41):14577–14591. doi: 10.1523/JNEUROSCI.0361-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rehm HL, et al. ClinGen — the clinical genome resource. N Engl J Med. 2015;372(23):2235–2242. doi: 10.1056/NEJMsr1406261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray B, et al. Lack of genotype-phenotype correlation in Brugada syndrome and sudden arrhythmic death syndrome families with reported pathogenic SCN1B variants. Heart Rhythm. 2018;15(7):1051–1057. doi: 10.1016/j.hrthm.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Yuan L, et al. Investigations of the Navβ1b sodium channel subunit in human ventricle; functional characterization of the H162P Brugada syndrome mutant. Am J Physiol Heart Circ Physiol. 2014;306(8):H1204–H1212. doi: 10.1152/ajpheart.00405.2013. [DOI] [PubMed] [Google Scholar]
- 9.Isom LL, et al. Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science. 1992;256(5058):839–842. doi: 10.1126/science.1375395. [DOI] [PubMed] [Google Scholar]
- 10.Yan Z, et al. Structure of the Nav1.4-β1 complex from electric eel. Cell. 2017;170(3):470–482. doi: 10.1016/j.cell.2017.06.039. [DOI] [PubMed] [Google Scholar]
- 11.Pan X, et al. Structure of the human voltage-gated sodium channel Nav1.4 in complex with β1. Science. 2018;362(6412):eaau2486. doi: 10.1126/science.aau2486. [DOI] [PubMed] [Google Scholar]
- 12.Shen H, et al. Structures of human Nav1.7 channel in complex with auxiliary subunits and animal toxins. Science. 2019;363(6433):1303–1308. doi: 10.1126/science.aaw2493. [DOI] [PubMed] [Google Scholar]
- 13.Jiang D, et al. Structure of the cardiac sodium channel. Cell. 2020;180(1):122–134. doi: 10.1016/j.cell.2019.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu W, et al. Mechanisms of noncovalent β subunit regulation of Na V channel gating. J Gen Physiol. 2017;149(8):813–831. doi: 10.1085/jgp.201711802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salvage SC, et al. Gating control of the cardiac sodium channel Nav1.5 by its β3-subunit involves distinct roles for a transmembrane glutamic acid and the extracellular domain. J Biol Chem. 2019;294(51):19752–19763. doi: 10.1074/jbc.RA119.010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muroi Y, Chanda B. Local anesthetics disrupt energetic coupling between the voltage-sensing segments of a sodium channel. J Gen Physiol. 2009;133(1):1–15. doi: 10.1085/jgp.200810103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheets MF, Hanck DA. Outward stabilization of the S4 segments in domains III and IV enhances lidocaine block of sodium channels. J Physiol. 2007;582(1):317–334. doi: 10.1113/jphysiol.2007.134262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu W, et al. Predicting patient response to the antiarrhythmic mexiletine based on genetic variation: personalized medicine for long QT syndrome. Circ Res. 2019;124(4):539–552. doi: 10.1161/CIRCRESAHA.118.314050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno JD, et al. A molecularly detailed NaV1.5 model reveals a new class I antiarrhythmic target. JACC Basic to Transl Sci. 2019;4(6):736–751. doi: 10.1016/j.jacbts.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ragsdale DS, et al. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci U S A. 1996;93(17):9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gianelly R, et al. Effect of lidocaine on ventricular arrhythmias in patients with coronary heart disease. N Engl J Med. 1967;277(23):1215–1219. doi: 10.1056/NEJM196712072772301. [DOI] [PubMed] [Google Scholar]
- 22.Antzelevitch C, et al. Electrophysiologic basis for the antiarrhythmic actions of ranolazine. Heart Rhythm. 2011;8(8):1281–1290. doi: 10.1016/j.hrthm.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerra F, et al. Ranolazine for rhythm control in atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol. 2017;227:284–291. doi: 10.1016/j.ijcard.2016.11.103. [DOI] [PubMed] [Google Scholar]
- 24.Burashnikov A, et al. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116(13):1449–1457. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antzelevitch C, et al. Electrophysiologic basis for the antiarrhythmic actions of ranolazine. Hear Rhythm. 2011;8(8):1281–1290. doi: 10.1016/j.hrthm.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez RJ, et al. Mechanisms by which ranolazine terminates paroxysmal but not persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2019;12(10):e005557. doi: 10.1161/CIRCEP.117.005557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zareba W, et al. Ranolazine in high-risk patients with implanted cardioverter-defibrillators: the RAID trial. J Am Coll Cardiol. 2018;72(6):636–645. doi: 10.1016/j.jacc.2018.04.086. [DOI] [PubMed] [Google Scholar]
- 28.Lin X, et al. Scn1b deletion leads to increased tetrodotoxin-sensitive sodium current, altered intracellular calcium homeostasis and arrhythmias in murine hearts. J Physiol. 2014;00(6):1–1407. doi: 10.1113/jphysiol.2014.277699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arcisio-Miranda M, et al. Molecular mechanism of allosteric modification of voltage-dependent sodium channels by local anesthetics. J Gen Physiol. 2010;136(5):541–554. doi: 10.1085/jgp.201010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanck DA, et al. Using lidocaine and benzocaine to link sodium channel molecular conformations to state-dependent antiarrhythmic drug affinity. Circ Res. 2009;105(5):492–499. doi: 10.1161/CIRCRESAHA.109.198572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ragsdale DS, et al. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265(5179):1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- 32.Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez-Santiago LF, et al. Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J Mol Cell Cardiol. 2007;43(5):636–47. doi: 10.1016/j.yjmcc.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu W, et al. Conservation and divergence in NaChBac and NaV1.7 pharmacology reveals novel drug interaction mechanisms. Sci Rep. 2020;10(1):10730. doi: 10.1038/s41598-020-67761-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson EK, et al. Regional differences in mRNA and lncRNA expression profiles in non-failing human atria and ventricles. Sci Rep. 2018;8(1):13919. doi: 10.1038/s41598-018-32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimetbaum P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation. 2012;125(2):381–389. doi: 10.1161/CIRCULATIONAHA.111.019927. [DOI] [PubMed] [Google Scholar]
- 37.Mazzanti A, et al. Gene-specific therapy with mexiletine reduces arrhythmic events in patients with long QT syndrome type 3. J Am Coll Cardiol. 2016;67(9):1053–1058. doi: 10.1016/j.jacc.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cha A, et al. Voltage sensors in domains III and IV, but not I and II, are immobilized by Na+ channel fast inactivation. Neuron. 1999;22(1):73–87. doi: 10.1016/S0896-6273(00)80680-7. [DOI] [PubMed] [Google Scholar]
- 39.Hsu EJ, et al. Regulation of Na+ channel inactivation by the DIII and DIV voltage-sensing domains. J Gen Physiol. 2017;149(3):389–403. doi: 10.1085/jgp.201611678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domínguez JN, et al. Temporal and spatial expression pattern of beta1 sodium channel subunit during heart development. Cardiovasc Res. 2005;65(4):842–850. doi: 10.1016/j.cardiores.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 41.Zygmunt AC, et al. Mechanisms of atrial-selective block of Na+ channels by ranolazine: I. Experimental analysis of the use-dependent block. AJP Hear Circ Physiol. 2011;301(4):H1606–H1614. doi: 10.1152/ajpheart.00242.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barrett T, et al. NCBI GEO: archive for functional genomics data sets — update. Nucleic Acids Res. 2013;41(Database issue):D991–D995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kittleson MM, et al. Gene expression analysis of ischemic and nonischemic cardiomyopathy: shared and distinct genes in the development of heart failure. Physiol Genomics. 2005;21(3):299–307. doi: 10.1152/physiolgenomics.00255.2004. [DOI] [PubMed] [Google Scholar]
- 44.Blaxall BC, et al. Differential myocardial gene expression in the development and rescue of murine heart failure. Physiol Genomics. 2003;15(2):105–114. doi: 10.1152/physiolgenomics.00087.2003. [DOI] [PubMed] [Google Scholar]
- 45.Wang T, et al. Particulate matter induces cardiac arrhythmias via dysregulation of carotid body sensitivity and cardiac sodium channels. Am J Respir Cell Mol Biol. 2012;46(4):524–531. doi: 10.1165/rcmb.2011-0213OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horvath B, Bers DM. The late sodium current in heart failure: pathophysiology and clinical relevance. ESC Heart Fail. 2014;1(1):26–40. doi: 10.1002/ehf2.12003. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen PT, et al. Structural basis for antiarrhythmic drug interactions with the human cardiac sodium channel. Proc Natl Acad Sci U S A. 2019;116(8):2945–2954. doi: 10.1073/pnas.1817446116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peters CH, et al. Effects of the antianginal drug, ranolazine, on the brain sodium channel NaV1.2 and its modulation by extracellular protons. Br J Pharmacol. 2013;169(3):704–716. doi: 10.1111/bph.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu H, et al. Common molecular determinants of flecainide and lidocaine block of heart Na+ channels: evidence from experiments with neutral and quaternary flecainide analogues. J Gen Physiol. 2003;121(3):199–214. doi: 10.1085/jgp.20028723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deschênes I, et al. Post-transcriptional gene silencing of KChIP2 and Navβ1 in neonatal rat cardiac myocytes reveals a functional association between Na and Ito currents. J Mol Cell Cardiol. 2008;45(3):336–346. doi: 10.1016/j.yjmcc.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deschênes I, Tomaselli GF. Modulation of Kv4.3 current by accessory subunits. FEBS Lett. 2002;528(1–3):183–188. doi: 10.1016/s0014-5793(02)03296-9. [DOI] [PubMed] [Google Scholar]
- 52.Edokobi N, Isom LL. Voltage-gated sodium channel β1/β1B subunits regulate cardiac physiology and pathophysiology. Front Physiol. 2018;9:351. doi: 10.3389/fphys.2018.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varga Z, et al. Direct measurement of cardiac Na+ channel conformations reveals molecular pathologies of inherited mutations. Circ Arrhythm Electrophysiol. 2015;8(5):1228–1239. doi: 10.1161/CIRCEP.115.003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rudokas MW, et al. The Xenopus oocyte cut-open vaseline gap voltage-clamp technique with fluorometry. J Vis Exp. 2014;(85):1–11. doi: 10.3791/51040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu W, et al. Molecular motions that shape the cardiac action potential: insights from voltage clamp fluorometry. Prog Biophys Mol Biol. 2016;120(1–3):3–17. doi: 10.1016/j.pbiomolbio.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Marionneau C, et al. Distinct cellular and molecular mechanisms underlie functional remodeling of repolarizing K+ currents with left ventricular hypertrophy. Circ Res. 2008;102(111):1406–1415. doi: 10.1161/CIRCRESAHA.107.170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Speerschneider T, Thomsen MB. Physiology and analysis of the electrocardiographic T wave in mice. Acta Physiol (Oxf) 2013;209(4):262–271. doi: 10.1111/apha.12172. [DOI] [PubMed] [Google Scholar]
- 58.Roussel J, et al. The Complex QT/RR relationship in mice. Sci Rep. 2016;6(1):25388. doi: 10.1038/srep25388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brunet S, et al. Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J Physiol. 2004;559(pt 1):103–120. doi: 10.1113/jphysiol.2004.063347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.