Keywords: intestine, MCT-1, microRNA, SCFA absorption, SMCT-1
Abstract
Short-chain fatty acids (SCFAs) produced by bacterial fermentation of dietary fiber exert myriad of beneficial effects including the amelioration of inflammation. SCFAs exist as anions at luminal pH; their entry into the cells depends on the expression and function of monocarboxylate transporters. In this regard, sodium-coupled monocarboxylate transporter-1 (SMCT-1) is one of the major proteins involved in the absorption of SCFA in the mammalian colon. However, very little is known about the mechanisms of regulation of SMCT-1 expression in health and disease. MicroRNAs (miRs) are known to play a key role in modulating gene expression. In silico analysis showed miR-29a, b, and c with highest context score and its binding region was conserved among mammals. The 3′-untranslated region (UTR) of human SMCT-1 gene was cloned into pmirGLO vector upstream of luciferase reporter and transiently transfected with miR-29a, b, and c mimics into Caco-2 and/or T-84 cells. The presence of UTR of this gene significantly decreased luciferase activity compared with empty vector. Cotransfection with miR-29a, b, or c resulted in further decrease in 3′-UTR activity of SMCT-1 luciferase constructs. Mimic transfection significantly decreased SMCT-1 protein expression without altering mRNA expression. Furthermore, the expression of miR-29a and c were significantly lower in mouse colon compared with small intestine, consistent with higher levels of SMCT-1 protein in the colon. Our studies demonstrated a novel finding in which miR-29a, b, and c downregulate SMCT-1 expression in colonic epithelial cells and may partly explain the differential expression of these transporters along the length of the gastrointestinal (GI) tract.
NEW & NOTEWORTHY Our study for the first time reports the posttranscriptional regulation of SMCT-1 by miR-29a, b, and c in colonic epithelial cells. We also demonstrate that the expression of these microRNAs is lower in the mouse proximal and distal colon which partially explains the higher expression level of SMCT-1 in the colon compared with small intestine.
INTRODUCTION
MicroRNAs are short (18–24 nt) noncoding RNAs that modulate gene expression by binding to the 3′-untranslated region of messenger RNA (mRNA). MicroRNAs elicit action via two mechanisms by which they regulate the gene expression: 1) mRNA degradation when there is exact match with 3′-untranslated region (UTR) region and 2) translational repression in the case of imperfect complementarity (1, 2). miRNAs are known to play important roles in cell differentiation, proliferation, apoptosis, and signal transduction (3). Studies have shown that miRNAs play a critical role in the regulation of inflammation in chronic inflammatory diseases, including inflammatory bowel disease (IBD) (4). In addition, miRNAs regulate colonic epithelial cell-derived chemokine expression and colonic health (5).
Short-chain fatty acids (SCFAs) are known to enhance colonic health and epithelial integrity. SCFAs such as acetate, propionate, and butyrate are produced in a relative proportion of 6:3:1 in the colonic lumen by anerobic fermentation (6). At luminal pH, the SCFAs exist as anions and require a carrier-mediated transport system to be absorbed into the colonocytes. Two main monocarboxylate transporters, monocarboxylate transporter-1 (MCT-1 or SLC16A1) and sodium-dependent monocarboxylate transporter-1 (SMCT-1 or SLC5A8, solute carrier family 5 member 8) have been shown to play important roles in SCFA absorption into the intestinal epithelial cells (IECs) (7, 8). We and others have shown the downregulation of MCT-1 and SMCT-1 in inflammatory conditions associated with decreased SCFA absorption and metabolism (9, 10). Therefore, it is important to understand the molecular mechanisms of regulation of these transporters in health and disease. Regulation of MCT-1-mediated SCFA absorption via transcriptional and posttranslational mechanisms has been extensively studied (9, 11). However, the studies focused at the regulation of SMCT-1 function and expression remain very limited.
Recent reports demonstrated that specific miRs to regulate the expression of tight junction proteins such as occludin (12), and intestinal ion and nutrient transporters such as CFTR (a chloride channel) (13), downregulated in adenoma (DRA) (Cl−/HCO3− exchanger) (14), Na+-K+-Cl− cotransporter (13), and pepT1 (an oligopeptide transporter) (15). Additionally, a recent study also showed microRNA (miR-29a, b, and c) mediated regulation of MCT-1 in pancreatic β-cells (16).
However, miRNA-mediated regulation of SMCT-1 has not been investigated. Therefore, the current study was designed to determine the role of microRNAs in regulating SMCT-1 in intestinal epithelial cells. We also examined if differential expression of miRs can explain the region-specific expression of SMCT-1 along the length of the intestine. Our novel findings demonstrated that ectopically increasing miR-29a, b, and c levels in intestinal epithelial cells by transient transfection downregulated SMCT-1 expression. We further demonstrated that miR-29a, b, and c regulate SMCT-1 by binding to its 3′-UTR leading to translational repression. Collectively, these data show potential of antigomiRs against miR-29a, b, and c to play a viable therapeutic role in inflammatory conditions where SMCT-1 expression is decreased.
MATERIALS AND METHODS
Cell Culture
Caco-2 and T-84 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). Caco-2 cells were cultured in Eagle’s minimum essential medium (EMEM) and T-84 cells were cultured in Dulbecco’s modified Eagle’s medium F-12 (1:1; DMEM F-12) and DMEM (high glucose). The culture medium for both the cell types was supplemented with 10% and 5% fetal bovine serum, respectively, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 50 µg/mL gentamicin. The cells were maintained at 37°C in a 5% CO2–95% air environment in T-75 (75 cm2) plastic flasks. The cells between passage 25 and 45 were utilized for the present study.
Cloning of 3′-UTR of SMCT-1 and 3′-UTR of MCT-1 in pmirGLO Vector
A 1062-bp fragment of the SMCT-1 3′-UTR and 2043-bp fragment of the MCT-1 3′-UTR (positive control) was amplified by PCR and purified using Qiaquick Gel extraction kit (Qiagen, Frederick, MD). Purified PCR products of 3′-UTR SMCT-1 and 3′-UTR of MCT-1 were cloned separately into multiple cloning site of pmirGLO Dual Luciferase miRNA target expression vector (Promega, Madison, WI) downstream of the firefly luciferase gene. The primer sequences flanked by DraI and XhoI sites for SMCT-1; SacI and SalI for MCT-1 used for PCR amplification are presented in Table 1. The resulting plasmids pmirGLO-3′-UTR-SMCT-1 and pmirGLO-3′-UTR-MCT-1 were used to transform the competent JM-109 cells (Promega); single colonies were picked from ampicillin agar plates and plasmid DNA was prepared using a plasmid miniprep kit (Qiagen). The fidelity of the construct was confirmed by sequencing.
Table 1.
Primers used for cloning 3′-UTR-SMCT-1 in pmirGLO vector and real time PCR
| Human |
| SMCT-1 3′-UTR |
| Forward: 5′-CACTTTTAAAAGCTGCTCTGATACTAGATATCC-3′ |
| Reverse: 5′-ACTCTCGAGTGGATCATCAAGTCCATAAATACC-3′ |
| MCT-1 3′-UTR |
| Forward: 5′-ACTGAGCTCATCCATGGGAAATT-3′ |
| Reverse: 5′-ACTGTCGACTTCCCTCACCAACAGTAATCCTCA-3′ |
| microRNA-Primers (Forward) human and mouse |
| miR-29a 5′-TAGCACCATCTGAAATCGGTTA-3′ |
| miR-29b 5′-TAGCACCATTTGAAATCAGTGTT-3′` |
| miR-29c 5′-TAGCACCATTTGAAATCGGTTA-3′ |
| U6B 5′-CGCAAGGATGACACGCAAATTCG-3′ |
| Real-time PCR primers |
| SMCT-1 |
| Forward: 5′-CCCTATCAGTCCAGGGCATA-3′ |
| Reverse: 5′-CCCAGGGCAATAAACAGAAA-3′ |
| MCT-1 |
| Forward: 5′-TCTGTGTCTATGCGGGATTCTT-3′ |
| Reverse: 5′-TTGAGCCGACCTAAAAGTGGT-3′ |
| GAPDH |
| Forward: 5′-GAAATCCCATCACCATCTTCC-3′ |
| Reverse: 5′-AAATGAGCCCCAGCCTTCT-3′ |
Restriction sites are underlined. MCT-1, monocarboxylate transporter-1; SMCT-1, sodium-coupled monocarboxylate transporter-1; UTR, untranslated region.
Site-Directed Mutagenesis
The miR-29a, b, and c binding seed sequence of SMCT-1–3′-UTR with desired mutations was custom synthesized by GenScript (see Fig.7A and mutations were confirmed by sequencing).
In silico analysis.
Potential miRNAs targeting SMCT-1–3′-UTR were predicted utilizing common prediction algorithm TargetScan (http://www.targetscan.org/) (17). Three microRNAs with highest context score and context score percentile were selected for the experiments.
Transient transfection and 3′-UTR activity.
Caco2 and T-84 cells were transiently transfected with 1.5 µg of pmirGLO-3′-UTR-SMCT-1 alone or in combination with 20 nM of different microRNA mimics or negative control miRNA (Sigma, St. Louis, MO) as previously described by us (14). Briefly, cells grown in T150 flask to 70% confluency were trypsinized and suspended in complete growth medium without antibiotic. Transfection mixture (100 mL optiMEM, 1 mL of RNAiMax reagent, and 20 nM of mimic/negative control) prepared according to the manufacturer’s instructions were added to the cell suspension and plated into 24-well plates. We utilized Lipofectamine RNAiMax (Invitrogen Life Technologies, Carlsbad, CA) to transfect both the cell lines. Cells were harvested 48 h after transfection and lysed in passive lysis buffer (Promega). The luciferase activity was determined using the Dual Luciferase Assay Kit (Promega) and a GLOMAX 20/20 Luminometer (Promega) equipped with double injectors. Effect of 3′-UTR activity on the reporter gene was calculated as a ratio of firefly luciferase to renilla luciferase and was expressed as percentage of control.
RNA extraction and real time PCR.
To quantitate the SMCT-1 mRNA, total RNA from Caco2 or T84 cells was extracted with Qiazol using miRNeasy mini kit (Qiagen, Frederick, MD) according to the manufacturer’s instructions as previously described (14). Briefly, extracted RNA was amplified by Brilliant SYBR Green qRT-PCR Master Mix kit (Agilent Technologies, Santa Clara, CA) using gene-specific primers for SMCT-1, MCT-1, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Table 1). The relative mRNA levels of SMCT-1 and MCT-1 were expressed as percentage of control normalized to GAPDH used as internal control gene.
Quantification of mature miR expression.
Quantification of mature miRNA expression was done as described previously (14). Briefly, 1 µg of total RNA was reverse transcribed to produce cDNA using the NCod miRNA first-strand cDNA synthesis kit (Invitrogen) in a 10-µL reaction according to the manufacturer’s instructions. Mature microRNA levels were quantified by Express SYBR Green qPCR supermix; Universal reverse primer provided in the NCode miRNA first-strand cDNA synthesis kit (Invitrogen) and the specific microRNA forward primers for miR-29a, b, and c and small RNA U6 (used as housekeeping gene) were obtained from MWG operon Eurofins (Huntsville, AL); primer sequences are listed in Table 1. Real-time PCR amplification and data capture were performed using the Stratagene Mx3005P (Agilent Technologies, Santa Clara, CA).
Western blotting.
Cell lysates were prepared after 48 h of transient transfection with mimics as previously described (14). Briefly, media was aspirated, and cells were washed with ice-cold 1X PBS and lysed in 1X cell lysis buffer (Cell Signaling, Danvers, MA) and 1X protease cocktail inhibitor mixture (Roche, Indianapolis, IN). Lysed cells were sonicated, and the lysate was centrifuged at 7,000 rpm for 7 min at 4°C. Concentration of the protein was determined by the Bradford assay. Cell lysates (75–100 µg) were loaded on 7.5% SDS-polyacrylamide gels and transblotted to nitrocellulose membranes to examine the protein levels of SMCT-1, MCT-1, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a loading control. Nonfat dry milk (5%) was used as a blocking buffer for 1 h. Membranes were then probed with human SMCT-1 (1:100 dilution), a kind gift from Dr. Vadivel Ganapathy. SMCT-1 antibody was validated by immunofluorescence as described earlier (18). MCT-1 was probed by utilizing polyclonal MCT-1 antibody (1:100 dilution), purchased from Alpha Diagnostics (San Antonio, Texas) and validated by peptide competition studies by us (19). GAPDH antibody (1:10,000, dilution) was purchased from Sigma (St. Louis, MO). The membranes were washed four times with the wash buffer containing 1X PBS and 0.1% Tween-20 for 5 min. Later, the membranes were probed with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibody (1:2,000 dilution) for 1 h and the bands were visualized using Enhanced Chemiluminiscence detection reagents (Bio-Rad, Hercules, CA). ImageJ software was used for Densitometric analysis of the relative band intensities.
Statistical analysis.
All data were analyzed by Prism (Prism Graph Pad Software). Results are expressed as means ± SEM and represent the data from three to six independent experiments. One-way ANOVA with Tukey’s multiple comparison test and unpaired t test were used for statistical analysis. P < 0.05 was considered as statistically significant.
RESULTS
In Silico Analysis: Identification of Potential miRNAs Targeting the SMCT-1 3´UTR
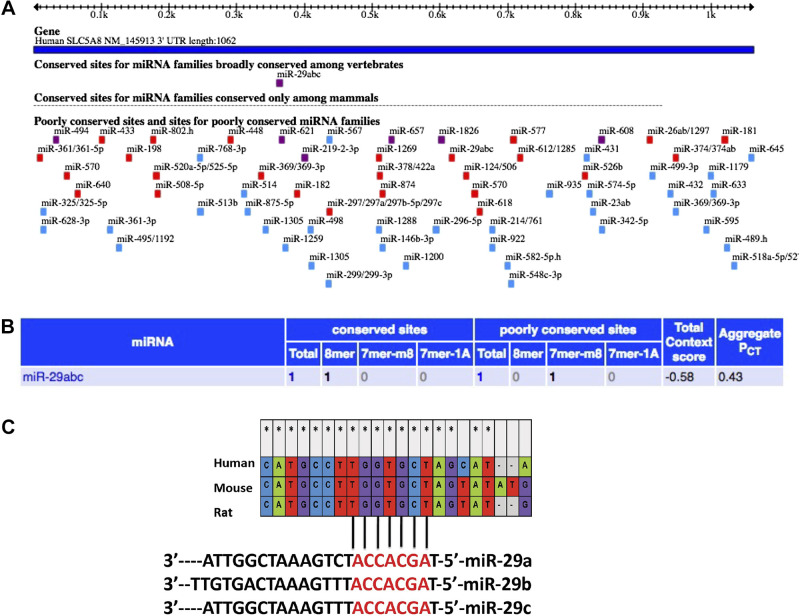
Data from Targetscan release 5.2 (Fig. 1A) predicted the potential microRNAs that could bind to the 3′-UTR of SMCT-1. Results from the algorithm indicated that miR-29a, b, and c were the only three microRNAs, which were broadly conserved among mammals and could bind to the 3'-UTR of SMCT-1 with the highest context score (−0.58) (Fig. 1B). MicroRNAs whose binding sites on the 3′-UTR are conserved among different species and are considered to have a functional importance. Mega 6 software data showed that the binding regions of miR-29a, b, and c on SMCT-1 3′-UTR were conserved among human, rat, and mouse (Fig. 1C).
Figure 1.
In silico analysis: potential microRNA-binding sites in 3′-UTR of SMCT-1 and the high context score candidate binding sites conserved in 3′-UTR of SMCT-1 (SLC5A8) in different species: A: miRNAs: the SLC5A8 3′-UTR, and the location of predicted miRNA seed sites, mammalian conservation. B: table information consists of highest context score candidates, source in Ref. 19. C: miR-29abc binding sites on SLC5A8 3′-UTR in various mammalian species using mega six software. miRNAs, microRNAs; SMCT-1, sodium-coupled monocarboxylate transporter-1; UTR, untranslated region. *Nucleotides that are conserved in human, mice, and rat.
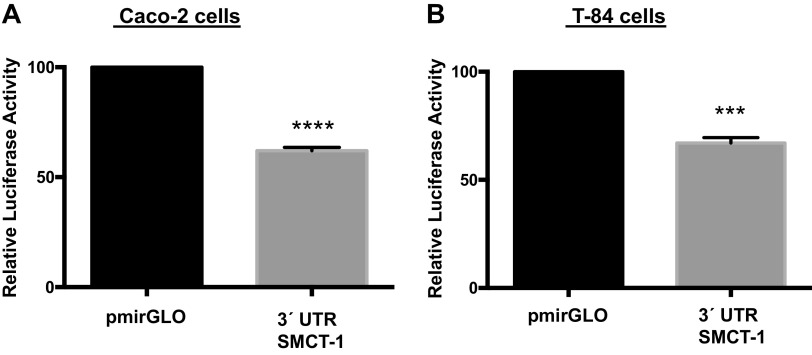
Transient transfection of SMCT 3′-UTR decreased reporter luciferase activity in intestinal epithelial cells.
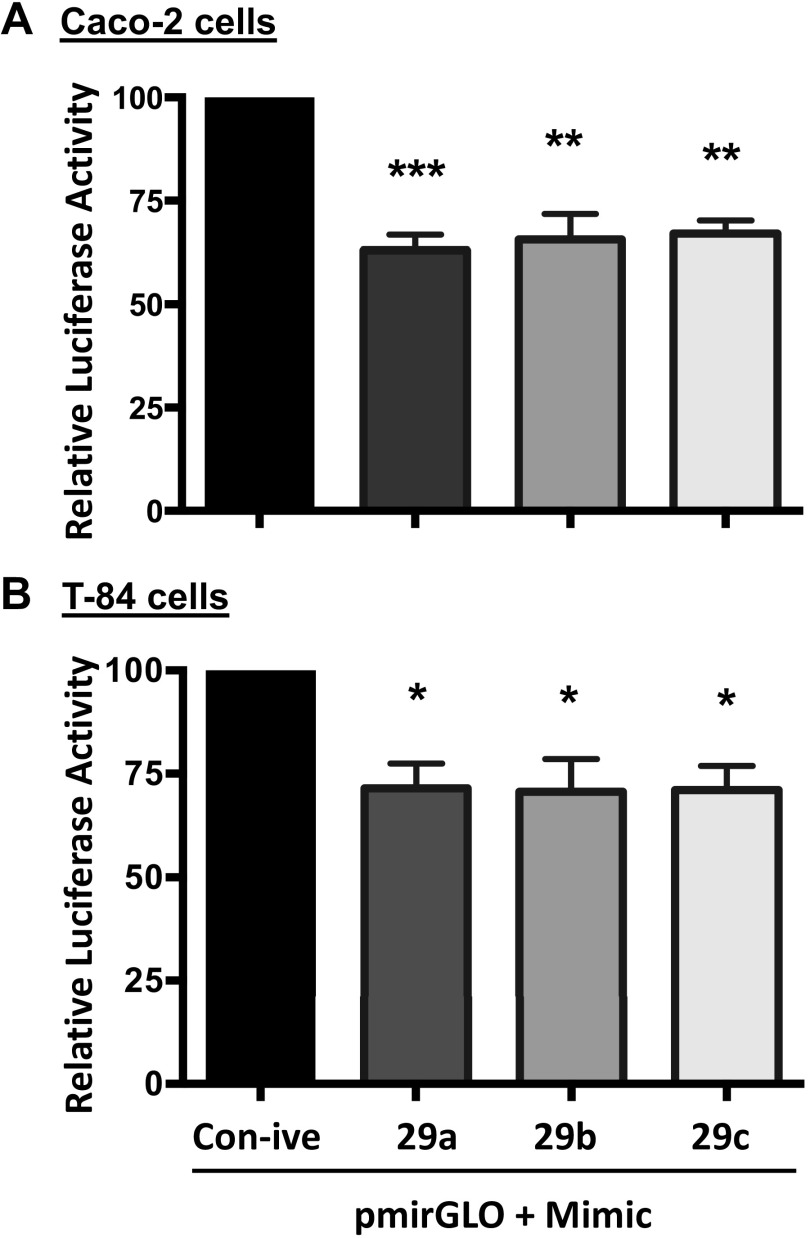
To verify the in silico analysis data, 1062-bp fragment of the region was commercially synthesized by Genscript (Piscataway, NJ) and cloned into a pmiRGLO Dual Luciferase miRNA target expression vector. Caco-2 and T-84 cells were transiently transfected with pmirGLO empty vector or pmirGLO-3′-UTR-SMCT-1. Any change in reporter luciferase activity on transfection of pmirGLO-3′-UTR-SMCT-1 into IECs would signify the likely interaction of SMCT-1 3′-UTR with binding partners. As shown in Fig. 2, A and B, transfection of pmirGLO-3′-UTR-SMCT-1 resulted in a significant decrease in relative luciferase activity in Caco-2 (38%) and T-84 (33%) cells. A decrease in reporter luciferase activity indicates the binding of possible regulators, for example, microRNAs or RNA-binding proteins to the 3′-UTR of SMCT-1. We used MCT-1 as a positive control since its regulation by miR-29a, b, and c has already been shown in pancreatic β-cells(16). A similar reduction in relative luciferase activity was observed when transfected with pmirGLO-3′-UTR-MCT-1 in Caco-2 (36%) and T-84 (45%) cells (Supplemental Fig. S1; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.14489637.v1). Cotransfection of 3′-UTR SMCT-1 with mimic 29a, b, and c showed a further significant decrease in relative luciferase activity compared with pmirGLO-3′-UTR-SMCT-1 cotransfected with a negative control in Caco2 (37%, 35%, and 33%) and T-84 (29%, 30%, and 29%) cells (Fig. 3, A and B), indicating a functional role of miR-29a, b, and c in regulating SMCT-1 expression via the SMCT-1 3′-UTR. A similar pattern of a significant decrease in relative luciferase activities was observed when pmirGLO-3´UTR-MCT-1 was cotransfected with miR mimic of 29a, b, and c (43%, 43%, and 48%) in Caco-2 cells and 51%, 49%, and 53% in T-84 cells (Supplemental Fig. S2).
Figure 2.
Transient transfection of 3′-UTR of SMCT-1 in intestinal epithelial cells decreased the reporter luciferase activity. A and B: relative luciferase activity after 48 h transient transfection of pmirGLO and 3′-UTR SMCT-1 in Caco-2 and T-84 cells, respectively. All the results are means ± SEM of four independent experiments. ***P < 0.001, ****P < 0.0001 vs. pmirGLO. SMCT-1, sodium-coupled monocarboxylate transporter-1; UTR, untranslated region.
Figure 3.
Co-transfection of microRNA mimics that target SMCT-1 3′-UTR decreased the luciferase reporter activity: Caco2 cells (A) and T-84 cells (B) were cotransfected with microRNA mimics that target 3′-UTR of SMCT-1 or negative control along with SMCT-1 3′-UTR or pmirGLO. At 48 h posttransfection, luciferase activities were measured and normalized with respective renilla luciferase activities. Results are means ± SEM of four independent experiments. Significant difference between 3′-UTR-SMCT-1 + control negative vs. pmirGLO-3′-UTR- SMCT-1 + mimic-29a, b, and c. ***P < 0.001, **P < 0.01, and *P < 0.05. SMCT-1, sodium-coupled monocarboxylate transporter-1; UTR, untranslated region.
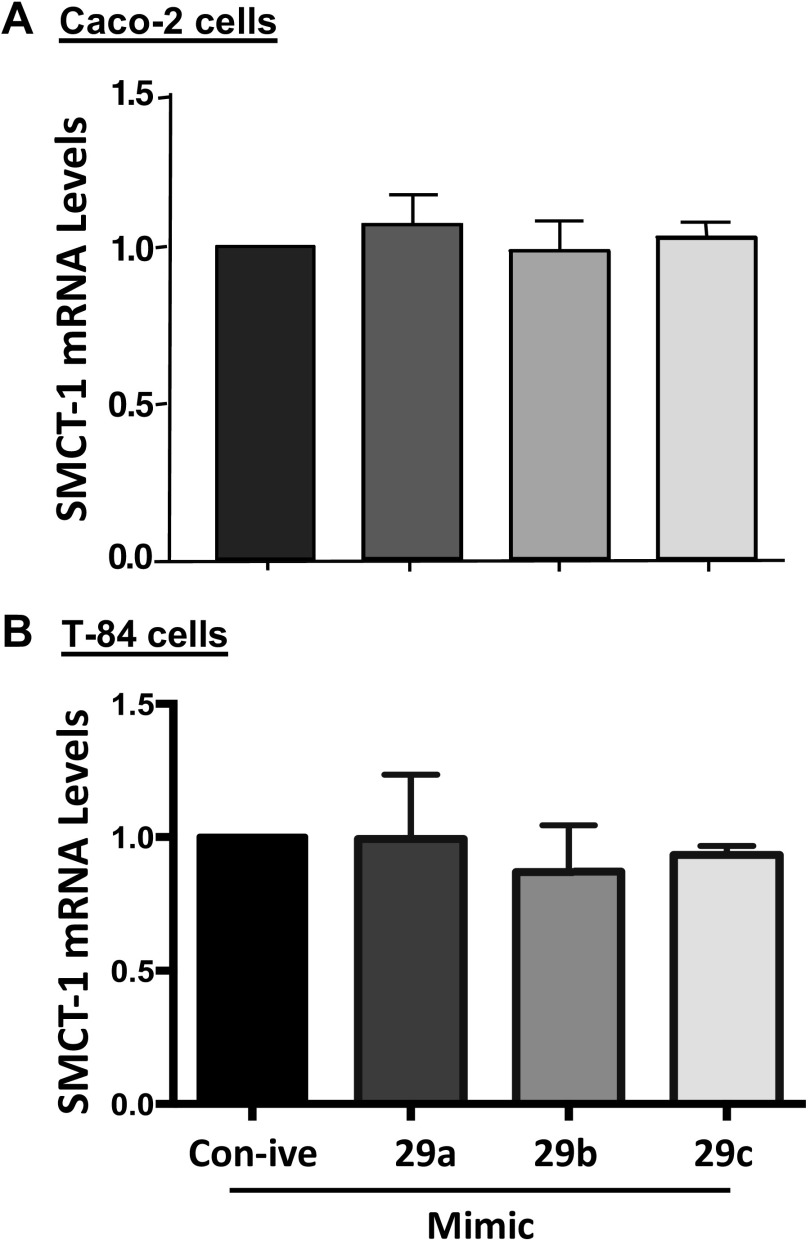
miR-29a, b, and c Did Not Alter SMCT-1 mRNA Levels but Decreased SMCT-1 Protein Levels
To study the effect of miR-29a, b, and c on SMCT-1 mRNA levels, we transiently transfected Caco-2 and T-84 cells with mimics of microRNAs 29a, b, and c and total RNA was isolated 48 h posttransfection. The effect of the miR-29a, b, and c mimics on SMCT-1 mRNA levels was assessed by real-time PCR. As shown in Fig. 4, A and B, transiently transfected Caco-2 and T-84 cells with mimics of microRNAs 29a, b, and c did not alter SMCT-1 mRNA levels and MCT-1 mRNA levels (Supplemental Fig. S3, A and B). This indicates that miR-29a, b, and c do not destabilize SMCT-1 or MCT-1 transcripts.
Figure 4.
microRNA mimic-29a, b, and c transfection did not alter SMCT-1 mRNA levels in intestinal epithelial cells. RNA was extracted from Caco-2 (A) and T-84 (B), 48 h posttransfection with mimics of microRNAs 29a, b and c that target SMCT-1 3′-UTR. SMCT-1 mRNA levels were measured by quantitative real-time PCR as described in materials and methods. Values of mRNA levels for SMCT-1 were normalized against GAPDH mRNA levels. Results are means ± SEM of four independent experiments. SMCT-1, sodium-coupled monocarboxylate transporter-1; UTR, untranslated region.
Next, we evaluated the SMCT-1 protein expression after overexpression of miR-29a, b, and c. Lysates were made 48 h after transient transfection of miR-29a, b, and c in Caco-2 and T-84 cells. As shown in Fig. 5, A and B, transient transfection of mimic 29a, b, and c significantly decreased SMCT-1 protein expression ∼65% in Caco-2 cells and ∼85% in T-84 cells. Similar observations were obtained in MCT-1 mRNA and protein levels in both the cell lines (Supplemental Figs. S3 and S4).
Figure 5.
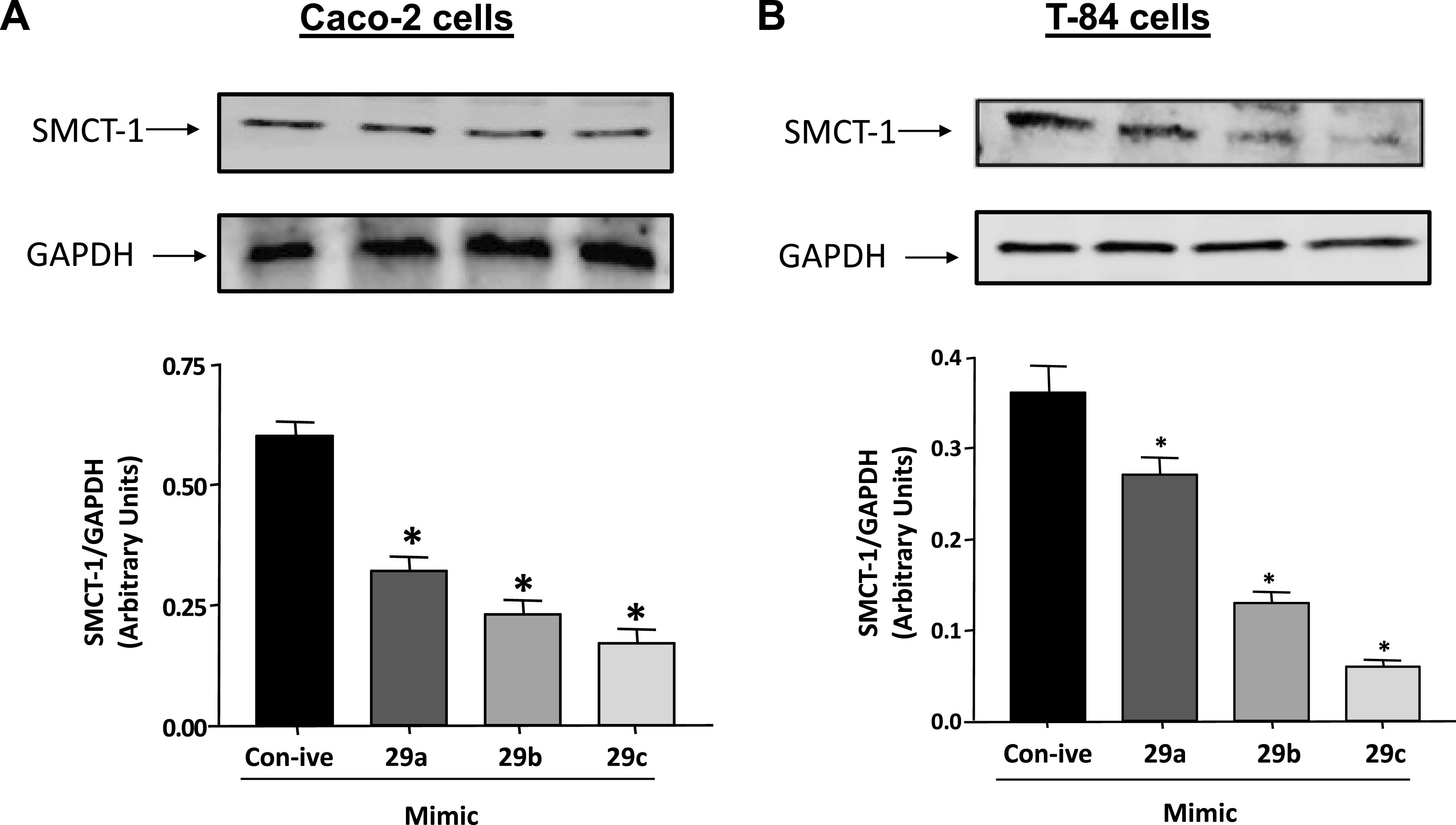
mimic-29a, b, and c transfection decreased SMCT-1 protein expression. Cell lysates of Caco-2 (A) and T-84 (B) transiently transfected with control negative and mimic-29a, b, and c were subjected to 10% SDS-PAGE followed by transfer to nitrocellulose membrane. The blot was probed with anti-SMCT-1 or anti-GAPDH antibody. Densitometric analysis of the relative band intensities using ImageJ. Results represent means ± SEM of five different experiments. *Significant differences between control negative vs. mimic-29a, b, and c transfected groups (*P < 0.05). SMCT-1, sodium-coupled monocarboxylate transporter-1.
miR-29a, b, and c Levels Are Differentially Expressed along the Length of the Mouse Intestine
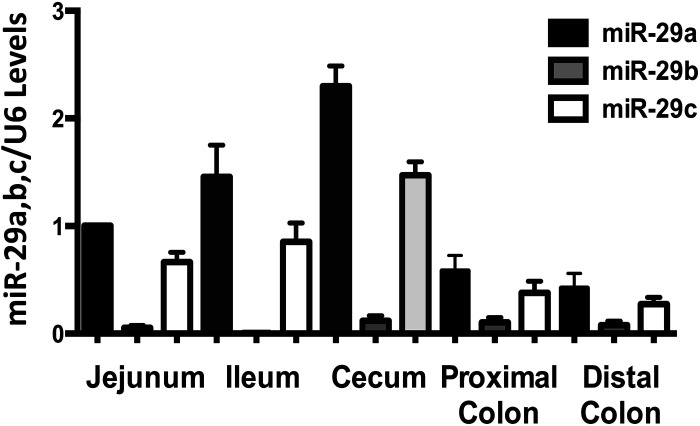
Earlier studies from our group have shown the higher expression of SMCT-1 protein in the mouse large intestine compared with the small intestine (20). We also determined the miR-29a, b, and c levels in different regions of the mouse intestine. Opposite to the SMCT-1 protein expression, miR-29a and c were less abundant in the large intestine compared with the small intestine. The results in Fig. 6 shows miR-29a and c are highly expressed in jejunum and ileum compared with proximal and distal colon.
Figure 6.
MicroRNA-29a, b, and c expression along the length of mouse intestine. MicroRNA 29a, b, and c levels in small and large intestine of mice were measured using real-time PCR. RNA extracted from different regions of mouse intestinal mucosa was reverse transcribed and miR-29a, b, and c specific forward primers and a universal reverse primer utilized (n = 3).
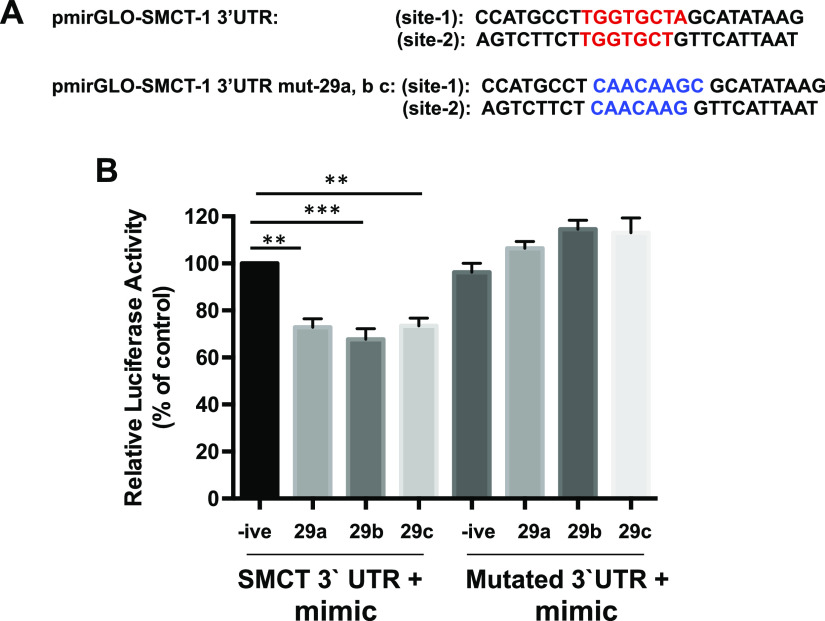
Mutating miR-29a, b, and c Binding Region of 3′-UTR of SMCT-1 Abolished the Effects of miR-29a, b, and c on SMCT-1 3′-UTR Reporter Luciferase Activity
To validate the hypothesis that miR-29a, b, and c exert their effects by binding to 3′-UTR of SMCT-1, we used 3′-UTR of SMCT-1 in which the binding regions of the miR-29a, b, and c are mutated (Fig. 7A). Caco-2 cells were transiently cotransfected with mimic-29a, b, and c along with pmirGLO-SMCT-1 3′-UTR or mutated pmirGLO-SMCT-1 3′-UTR and the cells were harvested after 48 h and relative luciferase activity was measured. Mutation in miR-29a, b, and c binding region in the 3′-UTR of SMCT-1 abrogated the decrease in relative luciferase activity, whereas the unmutated 3′-UTR of SMCT-1 exhibited a decline in the relative luciferase activity Fig. 7B.
Figure 7.
Mutating miR-29a, b, and c binding region in 3′-UTR of SMCT-1 abolished the effects of its effects on relative luciferase activity. A: miR-29a, b, and c binding region on SMCT-1 3′-UTR is denoted in red color and respective mutated sequence is in blue color. B: relative luciferase activities in transiently cotransfected cells with miR-29a, b, and c along with 3′-UTR of SMCT-1 or mutated 3′-UTR of SMCT-1. Results are means SEM of four independent experiments. Significant difference between pmirGLO-3′-UTR of SMCT-1 negative control and pmirGLO-3′-UTR of SMCT-1 mimic-29a and c (**P < 0.01) and mimic-29b (***P < 0.001). SMCT-1, sodium-coupled monocarboxylate transporter-1; UTR, untranslated region.
DISCUSSION
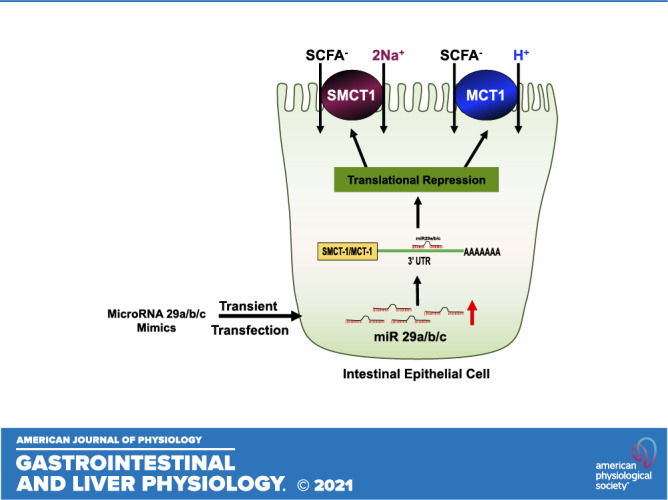
Our current study demonstrates that miR-29a, b, and c regulate SMCT-1 expression. Our data show that: 1) 3′-UTR of SMCT possess a highly conserved region for miR-29a, b, and c binding (in silico analysis); 2) among all the predicted microRNA candidates that can potentially bind to 3′-UTR of SMCT; miR-29a, b, and c have highest context score (in silico); 3) miR-29a, b, and c bind to 3′-UTR of SMCT but do not alter SMCT-1 mRNA levels. We further showed that miR-29a, b, and c decrease SMCT-1 protein levels by translational repression. To our knowledge, our findings are the first to demonstrate microRNA-mediated regulation of SMCT-1, a short-chain fatty acid transporter.
Short-chain fatty acids, such as butyrate, produced by colonic luminal bacterial fermentation of dietary fiber have been shown to exert several beneficial effects including maintenance of colon epithelial health, barrier integrity, stimulation of electrolyte absorption, and suppression of inflammatory signals (10, 21). Since at luminal pH butyrate exists as an anion, it needs to be transported into the cell by a carrier-mediated process to perform its biological functions. Butyrate is suggested to be absorbed into the colonocytes by two distinct SCFA transporters: the H+-coupled MCT-1 (22, 23) and the Na+-coupled SMCT-1 (24, 25). Both MCT-1 and SMCT-1 have been shown to be downregulated under inflammatory conditions (10, 20).
Also, enhanced susceptibility of SMCT-1 KO mice fed with low-fiber diet to dextran sodium sulfate (DSS)-induced colitis has been reported (26). Furthermore, we have earlier shown TNF-α-induced dysregulation of SMCT-1 expression and activity (20). Our current studies further define the molecular mechanisms regulating the expression of this key SCFA transporter. Interestingly, miRNAs have been shown to play a key role in gastrointestinal disorders, such as irritable bowel syndrome (IBS), IBD (including both ulcerative colitis and Crohn’s disease), and colitis-associated cancer (27). miR-29a has also been shown to be significantly upregulated in patients with active ulcerative colitis (UC) (28) and associated with increased intestinal permeability in patients with IBS and IBD (29).
A recent study showed microRNA-29 cluster mediated regulation of MCT-1 in pancreatic β-cells (16), showing miRNA-mediated regulation of this SCFA transporter. We have utilized MCT-1 expression regulation by miR-29a, b, and c as a positive control throughout our study. In the present study, we demonstrated that miR-29a, b, and c downregulated SMCT-1 expression via translational repression. We first cloned 3′-UTR of SMCT-1 in pmirGLO vector consisting of dual luciferase reporter gene (firefly and renilla). The transfection of 3′-UTR of SMCT-1 in pmirGLO vector in Caco-2 and T-84 cells showed a significant decrease in luciferase activity, suggesting the binding of regulatory elements such as microRNAs and/or RNA binding proteins to the 3′-UTR of SMCT-1. To confirm this observation, we performed in silico analysis of the 3′-UTR sequence of SMCT-1 using TargetScan (17). Of note, among all the predicted microRNA candidates identified, miR-29a, b, and c showed the highest context score and percentile. Furthermore, the seed sequence for miR-29a, b, and c binding was found to be highly conserved in human, mice, and rat, suggesting that these microRNAs might have functional role in regulating the expression of SMCT-1. The direct quantitative interaction between miRNA and 3′-UTR of SMCT-1 was evident from the significant decrease in luciferase activity after cotransfection of the mimics for miR-29a, b, and c along with the pmirGLO-SMCT-1–3′-UTR. These data provided further evidence that miR-29a, b, and c could potentially target endogenous SMCT-1, resulting in decreased butyrate absorption by the colonocytes. Overexpression of miR-29a, b, and c produced a significant decrease in the endogenous SMCT-1 protein levels without altering SMCT-1 mRNA levels. This confirmed that miR-29a, b, and c are able to target naturally occurring SMCT-1 protein in both the intestinal epithelial cell lines, Caco-2 and T-84, indicating that these data were not cell line specific. These data also provided the evidence for the absence of perfect complementarity in the binding site between microRNA and 3′-UTR of SMCT-1 as in our current study miR-29a, b, and c regulated SMCT-1 expression by translational repression, as lack of sufficient complementarity between mRNA and miRNA has been shown to lead to translational repression rather than mRNA degradation (30, 31). Furthermore, our studies demonstrated that mutations in the miR-29a, b, and c binding region in 3′-UTR of SMCT-1, abolished the decrease in reporter activity caused by miR-29a, b, and c overexpression. These data suggest that direct interaction of miR-29a, b, and c to 3′-UTR of SMCT-1 is essential for exerting its role in SMCT-1 regulation.
Our group has previously shown that in both mouse and human intestine, SMCT-1 mRNA and protein expression was highest in distal colon, followed by proximal colon and ileum (20). Differentially expressed microRNAs along the length of intestinal regions have been reported to possibly regulate the region-specific expression of target genes (32). To this end, we found that the expression of miR-29a and c was also significantly lower in the colon as compared with the small intestine. Our current findings, therefore, can partially explain the possible role of these microRNAs in governing the region-specific expression of SMCT-1 as previously observed by us. In this regard, we have previously shown that MCT-1 expression is also significantly higher in colon compared with the small intestine. Since miR 29a, b, and c has been shown to downregulate the expression of MCT-1, it is likely that region-specific expression of MCT-1 along the length of the intestine is also controlled by miR-29 cluster.
Collectively, the data obtained in our current study demonstrated a novel regulatory pathway for downregulation of SMCT-1 via miR-29a, b, and c by mechanisms involving translational repression. The enhanced expression of miR-29a, b, and c under inflammatory conditions can be targeted using a variety of miRNA repressors including the use of: 1) antigomirs, specifically designed against the seed sequence of miRNAs; 2) synthetic anti-miRNA oligonucleotides (AMOs) that can suppress the miRNA function by the virtue of possessing reverse complementary sequences to their target miRNAs; or 3) the locked nucleic acid (LNA) AMOs. Interestingly, LNA-AMOs exhibit increased resistance against nucleases and/or increase binding affinity. Such approaches hold promise, as there are multiple miRNA-based drugs that are currently in clinical trials (33). For example, Miravirsen is an LNA-AMO that has been optimized to target and inhibit miR-122, as treatment for hepatitis C. It has entered phase 2a dose-finding clinical trial (34). In contrast, ABX464, a small molecular compound by Abivax is currently in preparation for a phase 2b clinical trial for ulcerative colitis and phase 2a for Crohn’s disease, as it enhances miR-124 to reduce the symptoms of inflammatory bowel disease for patients refractory to anti-TNF biologics and corticosteroids (33). We speculate that targeting miR-29a, b, and c using antigomiR-29a, b, and c could be an effective therapeutic tool for treating gut inflammatory conditions, where SMCT-1 expression is downregulated.
SUPPLEMENTAL DATA
Supplemental material: https://doi.org/10.6084/m9.figshare.14489637.v1.
GRANTS
These studies were supported by the Department of Veterans Affairs (VA), Veterans Heath Administration, Office of Research and Development, Biomedical Laboratory Research and Development: Merit Review Award: BX002011 (P.K.D.), BX002867 (S.S.), BX000152 (W.A.A.), VA CDA2 Award BX004719 (A.K.), and VA Senior Research Career Scientist Award: 1IK6BX005242 (P.K.D.) & Research Career Scientist award BX005243 (W.A.A.). The studies were also supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK54016 and DK92441 (P.K.D.), and partially by R01 DK98170 (R.K.G.) and R01 DK109709 (W.A.A.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.N.A. and P.K.D. conceived and designed research; A.N.A., S.P., A.K., and D.J. performed experiments; A.N.A. and S.P. analyzed data; A.N.A., S.S., R.K.G., and W.A.A. interpreted results of experiments; A.N.A. prepared figures; A.N.A, drafted manuscript; A.N.A., A.K., D.J., A.B., S.S., R.K.G., W.A.A., and P.K.D. edited and revised manuscript; A.N.A. approved final version of manuscript.
REFERENCES
- 1.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev 18: 504–511, 2004. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kong YW, Cannell IG, de Moor CH, Hill K, Garside PG, Hamilton TL, Meijer HA, Dobbyn HC, Stoneley M, Spriggs KA, Willis AE, Bushell M. The mechanism of micro-RNA-mediated translation repression is determined by the promoter of the target gene. Proc Natl Acad Sci USA 105: 8866–8871, 2008. doi: 10.1073/pnas.0800650105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Jiang Y, Zhang H, Greenlee AR, Han Z. Overexpressed miR-494 down-regulates PTEN gene expression in cells transformed by anti-benzo(a)pyrene-trans-7,8-dihydrodiol-9,10-epoxide. Life Sci 86: 192–198, 2010. doi: 10.1016/j.lfs.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Wu F, Dassopoulos T, Cope L, Maitra A, Brant SR, Harris ML, Bayless TM, Parmigiani G, Chakravarti S. Genome-wide gene expression differences in Crohn's disease and ulcerative colitis from endoscopic pinch biopsies: insights into distinctive pathogenesis. Inflamm Bowel Dis 13: 807–821, 2007. doi: 10.1002/ibd.20110. [DOI] [PubMed] [Google Scholar]
- 5.Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, Brant SR, Chakravarti S, Kwon JH. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology 135: 1624–1635.e24,2008. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 6.D'Argenio G, Mazzacca G. Short-chain fatty acid in the human colon. Relation to inflammatory bowel diseases and colon cancer. Adv Exp Med Biol 472: 149–158, 1999. doi: 10.1007/978-1-4757-3230-6_13. [DOI] [PubMed] [Google Scholar]
- 7.Bhutia YD, Babu E, Ramachandran S, Yang S, Thangaraju M, Ganapathy V. SLC transporters as a novel class of tumour suppressors: identity, function and molecular mechanisms. Biochem J 473: 1113–1124, 2016. doi: 10.1042/BJ20150751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol 16: 461–478, 2019. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 9.Borthakur A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: involvement of NF-kappaB pathway. J Cell Biochem 103: 1452–1463, 2008. doi: 10.1002/jcb.21532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thibault R, De Coppet P, Daly K, Bourreille A, Cuff M, Bonnet C, Mosnier JF, Galmiche JP, Shirazi-Beechey S, Segain JP. Down-regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology 133: 1916–1927, 2007. doi: 10.1053/j.gastro.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 11.Cuff MA, Lambert DW, Shirazi-Beechey SP. Substrate-induced regulation of the human colonic monocarboxylate transporter, MCT1. J Physiol 539: 361–371, 2002. doi: 10.1113/jphysiol.2001.014241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology 141: 1323–1333, 2011. doi: 10.1053/j.gastro.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillen AE, Gosalia N, Leir SH, Harris A. MicroRNA regulation of expression of the cystic fibrosis transmembrane conductance regulator gene. Biochem J 438: 25–32, 2011. doi: 10.1042/BJ20110672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anbazhagan AN, Priyamvada S, Kumar A, Maher DB, Borthakur A, Alrefai WA, Malakooti J, Kwon JH, Dudeja PK. Translational repression of SLC26A3 by miR-494 in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 306: G123–G131, 2014. doi: 10.1152/ajpgi.00222.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalmasso G, Nguyen HT, Yan Y, Laroui H, Charania MA, Obertone TS, Sitaraman SV, Merlin D. MicroRNA-92b regulates expression of the oligopeptide transporter PepT1 in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 300: G52–G59, 2011. doi: 10.1152/ajpgi.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pullen TJ, da Silva Xavier G, Kelsey G, Rutter GA. miR-29a and miR-29b contribute to pancreatic beta-cell-specific silencing of monocarboxylate transporter 1 (Mct1). Mol Cell Biol 31: 3182–3194, 2011. doi: 10.1128/MCB.01433-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 27: 91–105, 2007. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gopal E, Miyauchi S, Martin PM, Ananth S, Roon P, Smith SB, Ganapathy V. Transport of nicotinate and structurally related compounds by human SMCT1 (SLC5A8) and its relevance to drug transport in the mammalian intestinal tract. Pharm Res 24: 575–584, 2007. doi: 10.1007/s11095-006-9176-1. [DOI] [PubMed] [Google Scholar]
- 19.Gill RK, Saksena S, Alrefai WA, Sarwar Z, Goldstein JL, Carroll RE, Ramaswamy K, Dudeja PK. Expression and membrane localization of MCT isoforms along the length of the human intestine. Am J Physiol Cell Physiol 289: C846–C852, 2005. doi: 10.1152/ajpcell.00112.2005. [DOI] [PubMed] [Google Scholar]
- 20.Borthakur A, Anbazhagan AN, Kumar A, Raheja G, Singh V, Ramaswamy K, Dudeja PK. The probiotic Lactobacillus plantarum counteracts TNF-α-induced downregulation of SMCT1 expression and function. Am J Physiol Gastrointest Liver Physiol 299: G928–G934, 2010. doi: 10.1152/ajpgi.00279.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17: 662–671, 2015. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadjiagapiou C, Schmidt L, Dudeja PK, Layden TJ, Ramaswamy K. Mechanism(s) of butyrate transport in Caco-2 cells: role of monocarboxylate transporter 1. Am J Physiol Gastrointest Liver Physiol 279: G775–G780, 2000. doi: 10.1152/ajpgi.2000.279.4.G775. [DOI] [PubMed] [Google Scholar]
- 23.Ritzhaupt A, Wood IS, Ellis A, Hosie KB, Shirazi-Beechey SP. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: its potential to transport L-lactate as well as butyrate. J Physiol 513: 719–732, 1998. doi: 10.1111/j.1469-7793.1998.719ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta N, Martin PM, Prasad PD, Ganapathy V. SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter. Life Sci 78: 2419–2425, 2006. doi: 10.1016/j.lfs.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 25.Thangaraju M, Cresci G, Itagaki S, Mellinger J, Browning DD, Berger FG, Prasad PD, Ganapathy V. Sodium-coupled transport of the short chain fatty acid butyrate by SLC5A8 and its relevance to colon cancer. J Gastrointest Surg 12: 1773–1781, 2008. doi: 10.1007/s11605-008-0573-0. [DOI] [PubMed] [Google Scholar]
- 26.Gurav A, Sivaprakasam S, Bhutia YD, Boettger T, Singh N, Ganapathy V. Slc5a8, a Na+-coupled high-affinity transporter for short-chain fatty acids, is a conditional tumour suppressor in colon that protects against colitis and colon cancer under low-fibre dietary conditions. Biochem J 469: 267–278, 2015. doi: 10.1042/BJ20150242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwiecinski M, Elfimova N, Noetel A, Tox U, Steffen HM, Hacker U, Nischt R, Dienes HP, Odenthal M. Expression of platelet-derived growth factor-C and insulin-like growth factor I in hepatic stellate cells is inhibited by miR-29. Lab Invest 92: 978–987, 2012. doi: 10.1038/labinvest.2012.70. [DOI] [PubMed] [Google Scholar]
- 28.Dalal SR, Kwon JH. The role of microRNA in inflammatory bowel disease. Gastroenterol Hepatol (NY) 6: 714–722, 2010. [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 59: 775–784, 2010. doi: 10.1136/gut.2009.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doench JG, Petersen CP, Sharp PA. siRNAs can function as miRNAs. Genes Dev 17: 438–442, 2003. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng Y, Yi R, Cullen BR. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc Natl Acad Sci USA 100: 9779–9784, 2003. doi: 10.1073/pnas.1630797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao X, Tyagi R, Magrini V, Ly A, Jasmer DP, Mitreva M. Compartmentalization of functions and predicted miRNA regulation among contiguous regions of the nematode intestine. RNA Biol 14: 1335–1352, 2017. doi: 10.1080/15476286.2016.1166333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonneau E, Neveu B, Kostantin E, Tsongalis GJ, De Guire V. How close are miRNAs from clinical practice? A perspective on the diagnostic and therapeutic market. EJIFCC 30: 114–127, 2019. [PMC free article] [PubMed] [Google Scholar]
- 34.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of HCV infection by targeting microRNA. N Engl J Med 368: 1685–1694, 2013. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]