Keywords: bile acid, C-fiber, esophagus, nociceptive, vagal
Abstract
Bile acid reflux in the esophagus plays a role in the pathogenesis of certain esophageal disorders, where it can induce esophageal pain and heartburn. The present study aimed to determine whether bile acid, deoxycholic acid (DCA), directly activates and sensitizes esophageal vagal nociceptive afferent C-fiber subtypes. DCA-elicited effects on vagal nodose and jugular neurons were studied by calcium imaging. Its effects on esophageal-labeled nodose and jugular neurons were then determined by patch-clamp recording. At nodose and jugular C-fiber nerve endings in the esophagus, DCA-evoked action potentials (APs) were compared by extracellular single-unit recordings in ex vivo esophageal-vagal preparations. DCA application induced calcium influxes in nodose and jugular neurons and elicited inward currents in esophageal-labeled nodose and jugular neurons. In the presence of DCA, the current densities elicited by capsaicin were enhanced in those labeled neurons. Consistently, DCA perfusion at nerve terminals in the esophagus evoked APs in about 50% of esophageal nodose and jugular C-fibers. In DCA-sensitive C-fibers, DCA perfusion also sensitized the fibers such that the subsequent response to capsaicin was amplified. Collectively, these results provide new evidence that DCA directly activates and sensitizes nociceptive nodose and jugular C-fibers in the esophagus. Such activation and sensitization effects may contribute to bile acid-induced esophageal nociceptive symptoms that are refractory to proton-pump inhibitor therapy.
NEW & NOTEWORTHY Bile acid reflux in the esophagus can induce pain and heartburn in certain esophageal disorders, but the underlying neuronal mechanism is still unclear. The present study demonstrated that bile acid, deoxycholic acid (DCA), directly activates esophageal vagal afferent nodose and jugular nociceptive C-fibers and sensitizes their response to capsaicin. Such effects may contribute to bile acid-induced esophageal nociceptive symptoms that refractory to proton-pump inhibitors (PPIs) therapy.
INTRODUCTION
Heartburn and noncardiac chest pain are the predominant symptoms in many esophageal disorders. It is likely the case that reflux acid stimulates nociceptive afferent nerve terminals in the esophagus leading to action potential (AP) discharge that is conducted ultimately to pain centers in the central nervous system (1). Thus, drugs that aim to reduce the refluxed protons are effective in inhibiting reflux-associated heartburn and pain. Even though acid inhibition by proton pump inhibitors (PPIs) relieves the heartburn associated with gastroesophageal reflux disease (GERD) in most patients, there are still 30%–40% of patients whose nociceptive symptoms are refractory to PPIs therapy (2). This indicates that other factors than acid may also contribute to the induction of esophageal nociception.
Bile acid (BA) is a potentially noxious substance that is often present in the esophageal refluxate. Refluxed bile acids are known to cause esophageal mucosal injury and inflammation, which could subsequently induce esophagitis, Barrett’s esophagus, and neoplasia (3–5). Tissue inflammation can lead to the production of inflammatory mediators that are capable of activating and/or sensitizing esophageal nociceptors. Previous studies have shown that bile acid infusion can induce GERD-like symptoms, including heartburn, discomfort, and pain (6). Recent studies demonstrated that bile reflux contributes significantly to the persistent reflux symptoms in refractory GERD and is associated with more severe heartburn symptom in patients with nonerosive reflux disease (NERD) (6, 7). These strongly indicate that refluxed bile acid has a direct impact on esophageal nociceptive afferent nerves and may play an important role in the induction of esophageal sensation and nociception. At present, it is not known whether bile acids can directly activate or sensitize esophageal nociceptive nerve terminals, independently of tissue inflammation.
Our previous studies have demonstrated that in addition to the spinal dorsal root ganglion (DRG) C-fibers, the esophagus is richly innervated by two fundamentally distinct types of nociceptive vagal C-fibers: one subtype derived from neural crest neurons situated in the vagal jugular ganglia and the other subtype placodal in origin situated in the vagal nodose ganglia (8). These two subtypes of vagal afferent C-fibers have high threshold to esophageal mechanical distension and their activation responses are not saturated in the noxious range. Moreover, these C-fibers are also responsive to noxious chemical stimulations (such as capsaicin and acid). In contrast, esophageal vagal nodose Aδ-fibers are low-threshold mechanosensors. They cannot discriminate between in-noxious and noxious mechanical distensions and do not respond to noxious chemical stimulations (i.e., capsaicin insensitive). In the present study, we aimed to determine the effects of bile acids on esophageal nociceptive vagal nodose and jugular C-fiber subtypes. We addressed the hypothesis that bile acids component deoxycholic acid (DCA) directly activates/sensitizes esophageal nociceptive vagal afferent C-fibers.
METHODS
Male Hartley guinea pigs (150–200 g) were purchased from Hilltop Laboratory Animals (Scottsdale, PA). All animals were kept in pathogen-free conditions and fed with standard guinea pig food and clean water. All experiments were approved by the Johns Hopkins University Animal Care and Use Committee. When tissues were harvested, the animal was put into a chamber and euthanized by inhalation of compressed CO2 gas from a cylinder.
Calcium Imaging in Dissociated Nodose and Jugular Neurons
Nodose and jugular neurons were prepared as described previously (9). Briefly, after animals were killed by CO2 asphyxiation, nodose and jugular ganglia were first dissected and collected in an ice-cold Krebs bicarbonate solution (118 mM NaCl, 1.0 mM NaH2PO4, 25.0 mM NaHCO3, 5.4 mM KCl, 1.9 mM CaCl2, 1.2 mM MgSO4, and 11.1 mM dextrose, pH 7.4, gassed with 95% O2–5% CO2) and then treated with enzyme [2 mg/mL collagenase and 2 mg/mL dispase in Ca2+/Mg2+-free Hank’s balanced salt solution (HBSS) buffer] at 37°C for 2 h. During the incubation, neurons were dissociated by mild trituration and then harvested by centrifugation. After being resuspended in fresh L15 media, these neurons were transferred onto coverslips (Warner Instrument) precoated with poly-d-lysine (0.1 mg/mL) and laminin (5 µg/mL), and allowed to adhere for 2 h in an incubator at 37°C. Then, the cells were washed with fresh media and cultured in fresh L15 media overnight in the incubator at 37°C and used within 24 h.
Calcium imaging studies were performed as described previously (9). Briefly, cultured vagal sensory neurons were loaded with 2 mM fura-2-AM and 0.05% Pluronic F-127 dissolved in normal extracellular solution (ECS, in mM: 140 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose, adjusted to pH 7.4 with NaOH) in a dark environment at 37°C for 45 min. After being washed three times with ECS, these neurons were allowed to de-esterify for at least 30 min before use. Fluorescence changes were measured with a Zeiss Upright Scope equipped with PTI-RatioMaster. Chemicals were applied with a custom-built perfusion system as follows: DCA (10 µM, 60 s), allyl isothiocyanate (AITC, 100 µM, 60 s), and capsaicin (1 µM, 60 s), each separated by a 60-s wash with ECS.
At the end of each experiment, a 50 mM KCl buffer (95 mM NaCl, 50 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, and 10 mM glucose, adjusted to pH 7.4 with NaOH) was applied to distinguish excitable cells. Only KCl-responsive cells were considered to be excitable cells and used for analysis.
Patch-Clamp Recording in Dil-Labeled Esophageal Nodose and Jugular Neurons
Retrograde labeling of nodose and jugular neurons from the esophagus with Dil (1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine perchlorate) (Molecular Probe, Eugene, OR) were performed in eight guinea pigs according to our previously described method (9). Briefly, under ketamine (50 mg/kg) and xylazine (5 mg/kg) anesthesia, the cervical esophagus was surgically exposed. Dil solution (1–2 µL, 1% in dimethyl sulfoxide and normal saline mixture) was injected in the wall of the esophagus at 50–60 mm above the gastric-esophageal junction (the injection site was confirmed at the time of dissecting the ganglia). Each esophagus received two to three injections. Postoperatively, animals were carefully monitored, and if necessary treated for pain until total recovery. After 2 wk, animals were killed by CO2 asphyxiation and both nodose and jugular ganglia (2 of each per animal) were collected and disassociated (see above) for whole cell patch-clamp recordings.
Whole cell patch-clamp recordings in Dil-labeled esophageal nodose and jugular neurons were performed according to our previously described methods (9). Briefly, borosilicate glass (WPI, Sarasota, FL) electrodes were 2–3 MΩ when filled with the pipette solution (in mM): 140 CsCl, 1 MgCl2, 5 MgATP, 2 EGTA, 10 HEPES (pH 7.2 with CsOH). Whole cell patch-clamp was performed using an Axo-patch 200B patch-clamp amplifier and Axograph software (Axon Instruments, Foster City, CA). Currents were typically digitized at 10 kHz and filtered at 2 kHz. The whole cell currents were recorded using a voltage ramp from −100 mV to 100 mV in 100-ms duration whereas cells were patched with a holding potential of 0 mV.
During the experiments, the cells were continuously superfused with extracellular solution (140 mM NaCl, 5 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, and 10 mM glucose, adjusted to pH 7.4 with NaOH) at room temperature. Drugs were delivered in the superfusion solution as follows: DCA (10 µM, 60 s), allyl isothiocyanate (AITC, 100 µM, 60 s), and capsaicin (1 µM, 60 s). In some experiments, the response to capsaicin (1 µM) and a combination of DCA (10 µM) and capsaicin (1 µM) for 60 s was obtained. The data are presented as means ± SE of inward current density (inward current normalized for the cell capacitance, pA/pF).
Extracellular Single-Unit Recording in Ex Vivo Esophageal-Vagal Preparation
Extracellular single-unit recordings from nodose or jugular neurons were performed in ex vivo esophageal-vagal preparations with intact nerve endings in the esophagus according to our previous studies (8, 10). Briefly, guinea pigs were killed by CO2 asphyxiation, and the esophagus and trachea with intact bilateral extrinsic vagal innervation (including jugular and nodose ganglia) were dissected. The tissue was pinned in a small Sylgard-lined Perspex chamber filled with Krebs bicarbonate buffer. The two compartments were separately superfused with KBS (pH 7.4, 35°C, 4–6 mL/min). Polyethylene tubing was inserted 3–5 mm into the cranial and caudal esophagus and secured for esophageal distension. Isobaric (constant pressure) distension of the esophagus was achieved by increasing intraluminal esophageal pressure to 10, 30, and 60 mmHg. The pressure was generated by a calibrated device using fluid (KBS) columns.
Extracellular recordings were performed using an aluminosilicate glass microelectrode (pulled with a Flaming-Brown micropipette puller, Sutter Instrument Company, Novato, CA) and filled with 3 M sodium chloride (electrode resistance 2 MΩ). The electrode was placed into an electrode holder connected directly to the headstage (A-M Systems, Everett, WA). A return electrode of silver-silver chloride wire and earthed silver-silver chloride pellet were placed in the perfusion fluid of the recording compartment. The recorded signal was amplified (Microelectrode AC amplifier 1800, A-M Systems) and filtered (low cut-off, 0.3 kHz; high cut-off, 1 kHz) and the resultant activity was displayed on an oscilloscope (TDS 340, Tektronix, Beaverton, OR) and a model TA240 chart recorder (Gould, Cleveland, OH). The data were stored and analyzed on a Macintosh computer using the software TheNerveOfIt (sampling frequency 33 kHz; PHOCIS, Baltimore, MD) and further processed using spreadsheet software (Microsoft Excel).
The recording electrode was micromanipulated into either the nodose or jugular ganglion (left or right). A distension-sensitive unit was identified when esophageal distension (with a rapid increase in intraluminal pressure to 60 mmHg for 5 s) evoked action potential discharges. The serosal surface of the esophagus was then searched with a stimulate electrode (pulse duration 1.5 ms, frequency 1 Hz) applied to the tissue. A mechanosensitive receptive field was located when the electronic stimulus evoked discharge of action potentials with waveforms identical to the action potentials evoked by distension. Conduction time was measured as the time between the stimulation pulse and the action potential (visualized by oscilloscope). Conduction velocity was calculated by dividing the length of the approximated nerve pathway by conduction time. Isobaric esophageal distension for 20 s with an intraluminal pressure of 10, 30, and 60 mmHg separated by at least 60 s was used to determine the distension pressure-nerve activity relationship of an esophageal afferent fiber. To assess the reproducibility of distension-evoked activation, this distension protocol was repeated after at least 5 min. The distension-evoked response was quantified as the peak frequency of action potentials discharged during the 20 s of distension from which the spontaneous activity (if present) was subtracted. The peak frequency (Hz) was defined as the maximal frequency of action potential discharge.
After recording the baseline spontaneous activity and mechanical excitability (esophageal distension under the pressure of 10, 30, and 60 mmHg) of esophageal vagal C-fibers, DCA (100 µM) was perfused to the serosal surface of the esophagus for 30 min. The action potential discharges (if activated) of esophageal nodose or jugular C-fibers induced by DCA were monitored continuously for 30 min and analyzed in 1-s bins (yielding the number of action potentials in each second, Hz). The esophageal distension-evoked responses of the same fibers were also detected before and after DCA perfusion. Then, DCA was washed out with fresh KBS (pH = 7.4) for 60 min, AITC, and/or capsaicin-evoked action potential discharges were recorded (60-min washing with fresh KBS between each chemical). Similarly, in the separated studies, the effect of low-dose capsaicin (0.1 µM)-induced responses before and after DCA perfusion in esophageal vagal afferent C-fiber subtypes were recorded and compared.
Chemicals
The chemicals used in the experiments were purchased from Sigma-Aldrich (St. Louis, MO), including deoxycholic acid (DCA), capsaicin, and allyl isothiocyanate (AITC). The stock solution of capsaicin (10 mM) was prepared in ethanol. The stock solution was separated into small aliquots and stored in −20°C, and working solution was prepared freshly by the day of use. DCA and AITC were prepared freshly with KBS.
Collagenase/dispase was purchased from Roche Applied Science (Indianapolis, IN). Fetal bovine serum, HBSS, and Pluronic(R) F-127 were purchased from Life Technologies (Grand Island, NY). Collagenase/dispase (2 mg/mL), laminin (5 μg/mL) were prepared in sterile Ca2+/Mg2+-free HBSS, Fura-2-AM (2M) was prepared in acetone, and Poly-l-lysine (1 mg/mL) was diluted in sterile water.
Data Analysis
In the extracellular study, we only analyzed the results from either capsaicin- or AITC-responsive C-fibers, which were confirmed by the end of each recording to indicate that the nerve terminals were exposed to chemical perfusion. The agonist-evoked nerve response was quantified as the peak frequency of the action potential discharges within a 5-min period, and averaged from six recording periods for a total of 30-min. The peak frequency (Hz) of the action potential discharges were presented as means ± SE and compared by paired Student’s t test or one-way ANOVA. For all experiments, significance was defined as P < 0.05.
In calcium imaging studies, neurons were defined as “responders” to a given compound if the mean response was greater than the mean baseline plus 2 times the SD using unpaired t test. Patch-clamp data were analyzed with Sigmaplot 11.0 (SPSS). All data are presented as means ± SE statistical comparisons were made with unpaired student’s t test and Wilcoxon rank-sum test, and differences were considered significant at P < 0.05.
RESULTS
DCA-Evoked Calcium Influxes in Nodose and Jugular Neurons
We used DCA as a prototypical bile acid for our study. We performed calcium imaging studies in nodose or jugular ganglia neurons dissociated from guinea pigs. As shown in Fig. 1, A and B, DCA, AITC, and capsaicin each elicited calcium response in a portion of nodose and jugular neurons. Interestingly, only a relatively small population of AITC-sensitive (TRPA1) neurons and capsaicin-sensitive (TRPV1) neurons were also activated by DCA (Fig. 1, C and D), suggesting that DCA may not elicit calcium response via directly activating TRPA1 or TRPV1 channels. Moreover, the overlapping of DCA-sensitive, AITC-sensitive, and capsaicin-sensitive population showed distinct differences between nodose and jugular neurons (Fig. 1, E and F). We point out that the nodose and jugular neurons in this assay were not labeled from the esophagus. They included those that innervated all other vagally innervated visceral organs.
Figure 1.
DCA evoked calcium influxes in vagal nodose and jugular neurons. Representative traces of calcium influxes that were evoked by DCA (10 µM, 60 s), allyl isothiocyanate (AITC, 100 µM, 60 s), capsaicin (1 µM, 60 s), and KCl (50 mM, 60 s), each separated by 60-s wash with ECS, in nodose (A) and jugular neurons (B), respectively. The percentage of responsive neurons to DCA, AITC, and capsaicin in KCl-sensitive nodose (C) and jugular neurons (D), respectively. DCA-sensitive neurons were shown in black, whereas DCA-insensitive neurons in white. Venn diagrams showing responsive overlaps of DCA with the agonists of TRPA1 and TRPV1 in nodose (E) and jugular neurons (F). DCA, deoxycholic acid; ECS, extracellular solution. n represents the number of esophageal-labeled neurons.
DCA-Elicited Inward Currents in Dil-Labeled Esophageal Nodose and Jugular C-Fiber Neurons
To address the effect of DCA on esophageal-specific afferent neurons, we performed patch-clamp recordings in 22 nodose and 17 jugular C-fiber neurons that were retrogradely labeled from the esophagus, respectively. Among them, DCA elicited inward currents in 10 nodose neurons (45.45%) and 8 jugular neurons (40.06%), whereas 9 nodose (40.91%) and 7 jugular (41.18%) neurons were sensitive to capsaicin. As shown in Fig. 2, A and B, DCA directly evoked inward currents in both nodose and jugular neurons that were very close to the amplitudes with the capsaicin-activated TRPV1 current and AITC-activated TRPA1 currents (Fig. 2, C and D).
Figure 2.
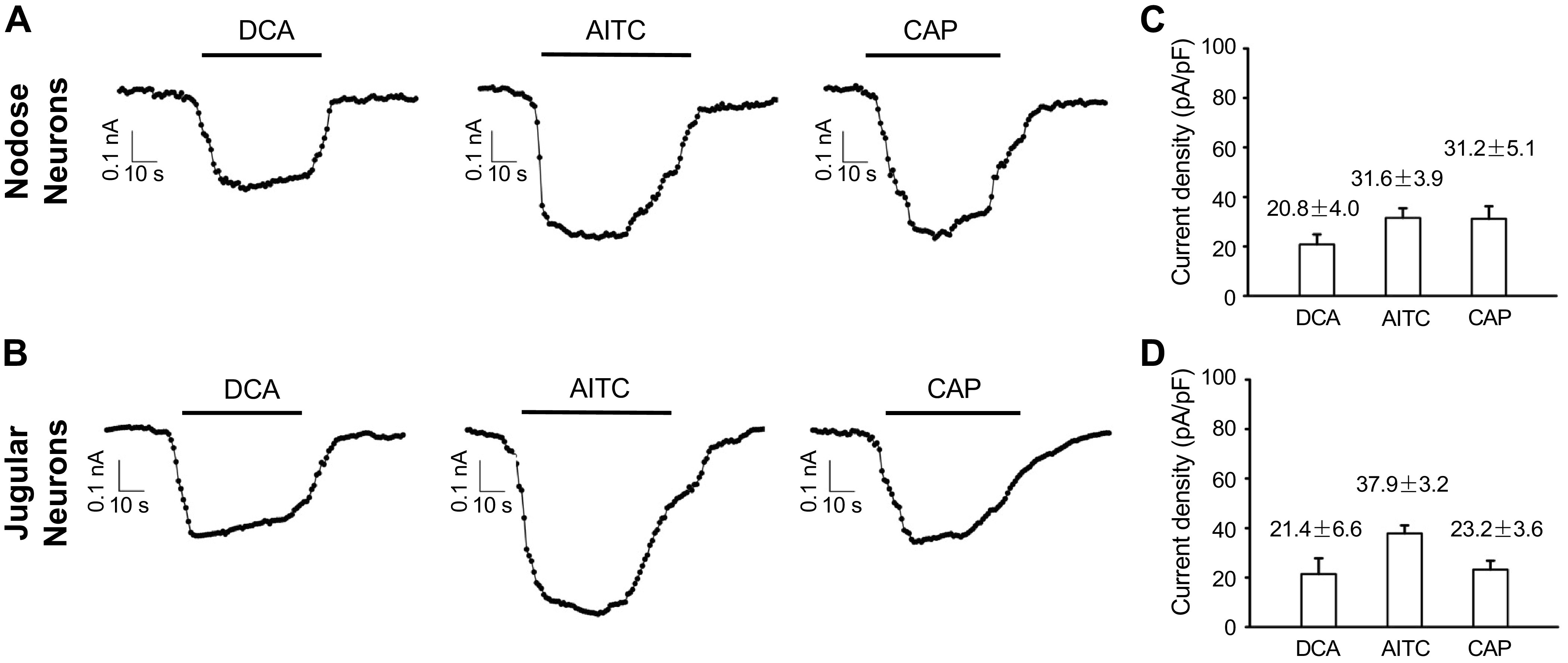
DCA elicited inward currents in Dil-labeled esophageal nodose and jugular C-fiber neurons. Representative traces of inward currents elicited by DCA (10 µM, 60 s), allyl isothiocyanate (AITC, 100 µM, 60 s), and capsaicin (1 µM, 60 s), each separated by 60-s wash with ECS, in Dil-labeled esophageal nodose (A) and jugular neurons (B), respectively. C: summary of current densities elicited by DCA (20.8 ± 4 pA/pF, n = 10), AITC (31.6 ± 3.9 pA/pF, n = 10), and capsaicin (31.8 ± 5.1 pA/pF, n = 9) in Dil-labeled esophageal nodose neurons. D: summary of current densities elicited by DCA (21.4 ± 6.6 pA/pF, n = 8), AITC (37.9 ± 3.2 pA/pF, n = 7), and capsaicin (23.2 ± 3.6 pA/pF, n = 7) in Dil-labeled esophageal jugular neurons. CAP, capsaicin; DCA, deoxycholic acid; ECS, extracellular solution. n represents the number of esophageal-labeled neurons.
We also investigated whether DCA potentiated TRPV1 agonist capsaicin-induced activation responses in esophageal-labeled nodose and jugular C-fiber neurons. In Dil-labeled nodose neurons, capsaicin (1 µM) activated neurons have an average current density of 31.2 ± 5.1 pA/pF. In the presence of DCA, capsaicin-elicited response was significantly increased with current density of 60.6 ± 7.9 pA/pF (n = 4, P < 0.01). Similarly, in DiI-labeled jugular neurons, capsaicin activated studied neurons with current density of 23.2 ± 6.6 pA/pF. In the presence of DCA, capsaicin-elicited current density was significantly increased to 36.8 ± 6.3 pA/pF (n = 4, P < 0.05; Fig. 3).
Figure 3.
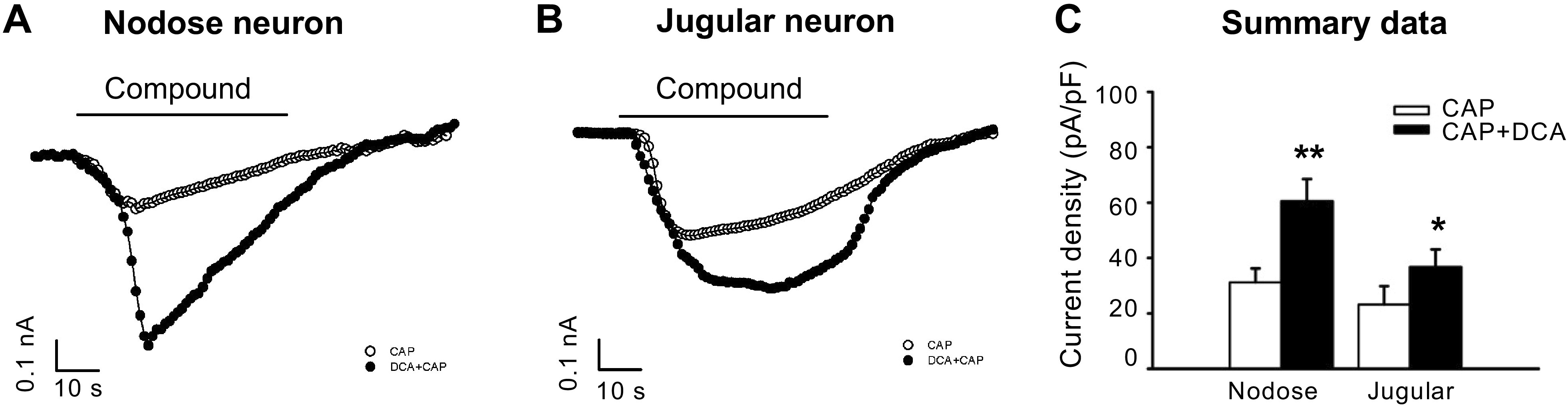
DCA increases capsaicin-elicited current densities in esophageal nodose and jugular neurons. Representative traces of esophageal-labeled nodose (A) and jugular neurons (B) that were activated by capsaicin (1 μM, 60 s) with or without DCA (10 μM, 60 s). C: summary data of current densities in capsaicin-elicited currents in the presence or absence of DCA in nodose and jugular neurons. In nodose neurons, DCA significantly enhanced capsaicin-induced currents from 31.2 ± 5.1 to 60.6 ± 7.9 pA/pF (n = 4, **P < 0.01, unpaired t test). Similarly, in jugular neurons, the enhancement was from 23.2 ± 6.6 to 36.8 ± 6.3 pA/pF (n = 4, *P < 0.05, unpaired t test). CAP, capsaicin; DCA, deoxycholic acid. n represents the number of esophageal-labeled neurons.
DCA-Evoked Action Potential Discharges in Vagal Nodose and Jugular C-Fiber Nerve Endings in the Esophagus
Using extracellular recording, we studied one afferent C-fiber (either nodose or jugular) from each animal per day. So, the total number of recorded C-fibers (n) was equal to the number of animals (N) used in the experiments. The recorded nerve fiber was identified as an esophageal afferent nerve by its positive response to esophageal distension. The esophageal afferent fiber was defined as C-fiber by its slow conduction velocity, unsaturated response to esophageal distension over 30 mmHg, and activation response to capsaicin. The average conduction velocity of studied esophageal nodose C-fibers was 0.68 ± 0.05 m/s (N = 11) and that of jugular C-fibers was 1.01 ± 0.06 m/s (N = 19).
Perfusion with DCA (100 µM) for 30 min evoked AP discharge in 5 of 11 studied esophageal nodose C-fibers. The overall peak discharge rate of action potentials averaged 2.2 ± 0.3 Hz after perfusion with DCA (compared with baseline of 0.73 ± 0.19 Hz, P < 0.01, N = 11). This AP discharge was not as large as that observed with capsaicin. After washing out DCA for 60 min, TRPV1 agonist capsaicin (1 µM) was perfused. Capsaicin activated most of studied nodose C-fibers (N = 8/11), including those DCA-responsive fibers. The peak discharge rates averaged 6.9 ± 1.7 Hz after capsaicin perfusion (compared with that before capsaicin at 1.5 ± 0.7 Hz, P < 0.05, N = 11) (Fig. 4, A and C).
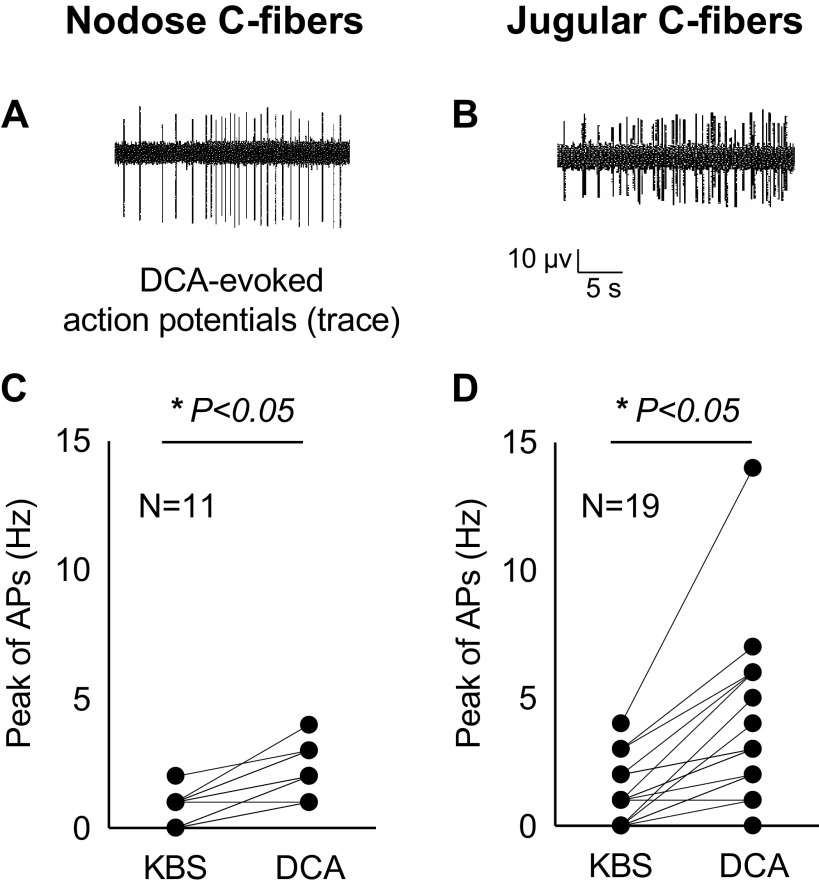
Figure 4.
DCA-activated esophageal nodose and jugular C-fibers. Typical traces of DCA-evoked action potentials (APs) in esophageal nodose (A) and jugular (B) C-fibers, respectively. C: DCA perfusion (100 μM, 30-min) evoked APs in 5/11 of nodose C-fibers. The overall peaks of APs significantly increased from baseline (labeled as KBS) of 0.73 ± 0.19 Hz to that of 2.18 ± 0.3 Hz after DCA perfusion (*P < 0.05, N = 11). D: DCA-evoked APs in 12/19 of jugular C-fibers. The overall peaks of APs significantly increased from baseline (labeled as KBS) of 1.45 ± 0.71 Hz to that of 6.91 ± 1.66 Hz after DCA perfusion (*P < 0.05, N = 19). APs, action potentials; DCA, deoxycholic acid. N represents the number of animals, which equals to the number of C-fibers.
DCA (100 µM) perfusion for 30 min also activated most of the jugular C-fiber terminals (N = 12/19). The overall peak discharge rate of action potentials averaged 3.95 ± 0.74 Hz after perfusion with DCA (compared with baseline of 1.21 ± 0.27 Hz, P < 0.01, N = 19). As with the nodose C-fibers, the jugular C-fibers responded more strongly to capsaicin than DCA. After DCA was washed out for 60 min, TRPV1 agonist capsaicin (1 µM) was perfused. Capsaicin activated most of studied jugular C-fibers (N = 17/19), including those DCA-responsive fibers. The peak discharge rates averaged 8.68 ± 1.4 Hz after capsaicin perfusion (compared with that before capsaicin at 1.26 ± 0.33 Hz, P < 0.05, n = 19; Fig. 4, B and D).
DCA-Enhanced Capsaicin Responses in Esophageal Nodose and Jugular C-Fibers
DCA has been shown to sensitize TRP channels in the DRG neurons and elicit nocifensive behavior (11, 12). Here, we specifically focused on its possible effect on capsaicin sensitivities in esophageal nodose and jugular C-fibers. Our previous studies have demonstrated that capsaicin-sensitive nodose and jugular C-fibers, via TRPV1, play important roles in sensing acid in the esophagus (10). Clarifying whether DCA could enhance their capsaicin-sensitivity is helpful to elucidate the role of bile acid in esophageal acid-sensing process. To minimize capsaicin-induced desensitization effects on C-fibers, we chose to compare lower dose (0.1 µM) capsaicin. After initial response to capsaicin had terminated by washing with fresh KBS for 30 min, the tissue was treated with DCA. After DCA-induced AP discharge stopped, the tissue was again treated with capsaicin. These data are illustrated in Fig. 5.
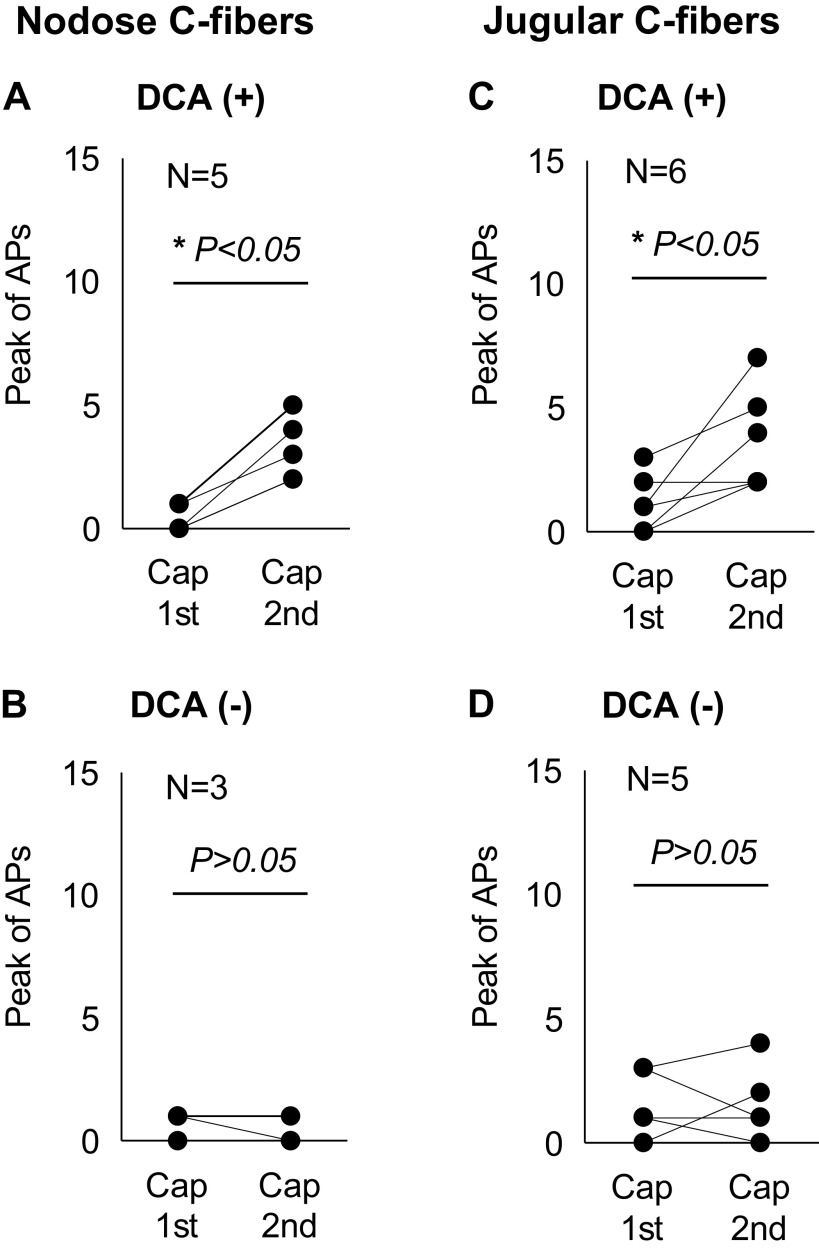
Figure 5.
DCA-enhanced esophageal nodose and jugular C-fibers’ responses to TRPV1 agonist capsaicin. A: in DCA-sensitive esophageal nodose C-fibers, DCA sensitized their lower dose capsaicin (0.1 μM)-evoked responses before and after DCA (100 μM, 30-min) were compared. Capsaicin-evoked APs significantly increased after DCA perfusion (1st vs. 2nd capsaicin: 0.4 ± 0.25 Hz vs. 3.2 ± 0.58 Hz, *P < 0.05, N = 5). B: in contrast, in DCA-insensitive esophageal nodose C-fibers, DCA did not sensitize their responses to capsaicin. Capsaicin-evoked APs did not change after DCA perfusion (1st vs. 2nd capsaicin: 0.67 ± 0.33 vs. 0.33 ± 0.33 Hz, P > 0.05, N = 3). C: in DCA-sensitive esophageal jugular C-fibers, DCA sensitized their responses to lower dose capsaicin (0.1 μM). Capsaicin-evoked APs significantly increased after DCA perfusion (1st vs. 2nd capsaicin: 1.17 ± 0.48 Hz vs. 3.67 ± 0.84 Hz, *P < 0.05, N = 6). D: in DCA-insensitive jugular C-fibers, DCA perfusion did not change capsaicin-evoked action potential discharges (1st vs. 2nd capsaicin: 1.6 ± 0.6 vs. 1.6 ± 0.68 Hz, P > 0.05, N = 5). APs, action potentials; Cap, capsaicin; DCA, deoxycholic acid. N represents the number of animals, which equals to the number of C-fibers.
In DCA-sensitive esophageal nodose C-fibers, DCA perfusion (100 µM, 30 min) significantly increased capsaicin-induced activation responses. The capsaicin-evoked APs increased from 0.4 ± 0.2 Hz (before DCA) to 3.2 ± 0.6 Hz (after DCA) (P < 0.05, N = 6). In contrast, in DCA-insensitive nodose C-fibers, DCA perfusion did not change capsaicin-evoked APs (before versus after DCA: 0.7 ± 0.3 versus 0.3 ± 0.3 Hz, P > 0.05, N = 3; Fig. 5, A and B).
In jugular DCA-sensitive C-fibers, DCA perfusion (100 µM, 30 min) significantly increased capsaicin-induced activation responses. The capsaicin-evoked APs increased from 1.2 ± 0.48 Hz (before DCA) to 3.7 ± 0.84 Hz (after DCA) (P < 0.05, N = 6). In DCA-insensitive jugular C-fibers, DCA perfusion did not change capsaicin-evoked APs (before versus after DCA: 1.6 ± 0.6 versus1.6 ± 0.68 Hz, P > 0.05, N = 5; Fig. 5, C and D).
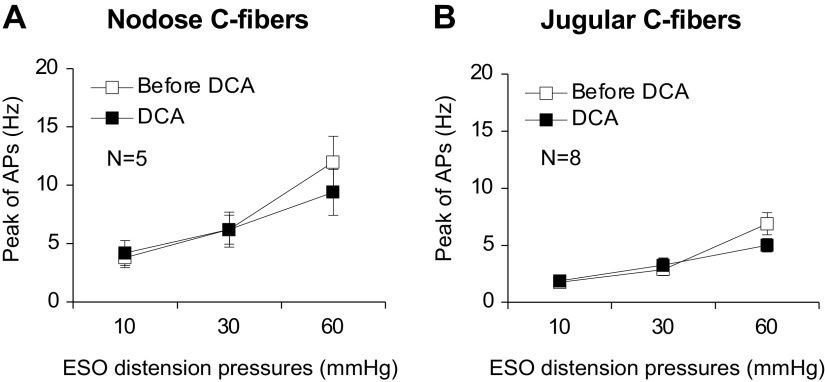
In contrast to its sensitization effect on chemical activation with capsaicin, DCA had no effect on the response of either nodose or jugular C-fibers to mechanical distention (Fig. 6). Only DCA-sensitive fibers were studied in this analysis.
Figure 6.
DCA did not change esophageal distension-evoked APs in esophageal (ESO) nodose and jugular C-fibers. A: in DCA-responsive nodose C-fibers, DCA treatment did not change esophageal distension-evoked APs at pressures of 10, 30, and 60 mmHg, respectively (before vs. after DCA: 3.8 ± 0.86, 6.2 ± 1.24 and 12.0 ± 2.2 Hz vs. 4.2 ± 1.07, 6.2 ± 1.5, and 9.4 ± 1.97 Hz, P > 0.05, N = 5). B: similarly, in DCA-responsive jugular C-fibers, DCA perfusion did not change esophageal distension-evoked APs at pressures of 10, 30, and 60 mmHg, respectively (before vs. after DCA: 3.29 ± 0.71, 6.29 ± 1.15 and 11.14 ± 1.94 Hz vs. 3.43 ± 0.9, 5.29 ± 1.19, and 7.86 ± 1.81 Hz, P > 0.05, one-way ANOVA, N = 8). APs, action potentials; DCA, deoxycholic acid. N represents the number of animals, which equals to the number of C-fibers.
DISCUSSION
Recent studies have revealed that BA reflux is associated with severe heartburn symptoms in patients with NERD and refractory GERD (6, 7). Bile acid sequestrant significantly reduces heartburn severity in refractory GERD (13). These suggest that BAs may participate in regulation of nociceptive afferent nerve functions in the esophagus. The primary BAs in humans include chenodeoxycholic acid (CDCA) and cholic acid (CA). They are converted into secondary bile acids lithocholic acid (LCA) and deoxycholic acid (DCA), respectively, through bacterial dehydroxylation in the intestine (14). Both primary and secondary BAs have been identified to be present in the refluxates in the esophagus and each of them may display variable effects depending on acidic pH levels in the tissues (15).
The results of the present study indicate that BA can both overtly activate nociceptive C-fiber terminals in the esophagus and sensitize the terminals to disparate chemical activators. We have previously found that the esophagus is innervated by two distinct subtypes of vagal nociceptive C-fibers. One type is derived from the neural crest with neurons situated in the jugular or supranodose ganglia and the other is placodally derived with neurons situated in the nodose ganglia (8). The relative consequence of the two subtypes of C-fibers with respect to sensations and reflex is still to be determined, but it is known that they terminate in distinct nuclei within the brainstem. The nodose fibers terminate mainly in the nucleus of the solitary tract, whereas the jugular fibers terminate largely in the paratrigeminal ganglia (a location more commonly associated with somatosensory pathways such as pain) (16, 17). We found BA was effective at stimulating both subtypes of vagal C-fibers in the esophagus.
BA in the esophagus was also able to potentiate the response of C-fibers to the TRPV1 activator capsaicin in both vagal C-fiber subtypes. Since we know that TRPV1 is also involved with acid activation of C-fibers (10), it follows that BA in the refluxate may not only overtly activate C-fibers, but also potentiate the response to a given concentration of acid and other TRPV1-relevant stimuli.
Refluxed BA may evoke inflammatory response in the esophagus. The inflammation, in turn, could lead to the production of C-fiber-activating autacoids. That BA acutely activated both nodose and jugular C-fiber terminals in our ex vivo vagal-innervated esophageal preparation, indicates that inflammation is not a necessary prerequisite for BA-induced nociception. It is more likely that the BA-induced AP discharge is due to a direct effect on the nerve terminal in the esophagus. This conclusion of a direct action on the nerve terminal is further supported by the strong inward current evoked by BA in isolated esophageal-specific patch-clamped vagal sensory neurons. Our findings are consistent with previous reports showing that bile collected from ferret gallbladder activated 60%–100% of gastroesophageal vagal afferent fibers in mice (18) and that DCA activated mouse colonic spinal afferents (19, 20).
The mechanism of the overt activation or sensitization cannot be deduced from the data presented. It is unlikely that DCA activated the fibers by directly gating opening TRPV1 or TRPA1 channels. This conclusion is based on the calcium imaging studies that show BA is incapable of evoking a calcium influx in most TRPV1- and TRPA1-expressing vagal sensory neurons. It is more likely that DCA activates the C-fibers by interacting with a BA receptor, for example, TGR5, but this hypothesis at present is difficult to convincingly address in guinea pigs. The DCA-induced sensitization observed from subsequent capsaicin challenge is unlikely due to a nonspecific increase in membrane excitability (i.e., as might be seen with inhibition of certain potassium channels), as there was no increase in sensitivity to mechanical distention. Since the sensitization occurred only in those fibers where DCA evoked AP discharge, it is likely that the proximal event for activation and sensitization may be similar, that is, through activating a BA receptor such as TGR5. Indeed, recent studies demonstrated that bile acid activated TGR5 in cutaneous DRG neurons, sensitized TRPA1, and induced itch sensation in wild-type but not TGR5 knockout mice (11, 12).
Collectively, our studies support the hypothesis that refluxed BA may be very relevant to the activation and sensitization of esophageal pain- and nocifensive reflex-causing sensory C-fibers. A better understanding of the mechanism of these BA-elicited effects on esophageal nociceptive afferent nerves may help to develop new treatments to relieve bile acid-induced esophageal nociceptive symptoms that are refractory to PPIs therapy.
GRANTS
This work is supported by National Institutes of Health Grant DK110366 (to S.Y.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.Y., Y.H., M.Y., B.J.U., and S.Y. conceived and designed research; X.Y. and Y.H. performed experiments; X.Y., Y.H., M.Y., and S.Y. analyzed data; X.Y., Y.H., B.J.U., and S.Y. interpreted results of experiments; X.Y., Y.H., M.Y., and S.Y. prepared figures; X.Y., Y.H., M.Y., B.J.U., and S.Y. drafted manuscript; X.Y., Y.H., M.Y., B.J.U. and S.Y. edited and revised manuscript; X.Y., Y.H., M.Y., B.J.U., and S.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Crystal Chang (JHU) for proofreading the manuscript.
REFERENCES
- 1.Page AJ, Blackshaw LA. Roles of gastro-oesophageal afferents in the mechanisms and symptoms of reflux disease. Handb Exp Pharmacol: 227–257, 2009. doi: 10.1007/978-3-540-79090-7_7. [DOI] [PubMed] [Google Scholar]
- 2.Spechler SJ. Evaluation and treatment of patients with persistent reflux symptoms despite proton pump inhibitor treatment. Gastroenterol Clin North Am 49: 437–450, 2020. doi: 10.1016/j.gtc.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Vaezi MF, Singh S, Richter JE. Role of acid and duodenogastric reflux in esophageal mucosal injury: a review of animal and human studies. Gastroenterology 108: 1897–1907, 1995. doi: 10.1016/0016-5085(95)90156-6. [DOI] [PubMed] [Google Scholar]
- 4.Souza RF, Krishnan K, Spechler SJ. Acid, bile, and CDX: the ABCs of making Barrett's metaplasia. Am J Physiol Gastrointest Liver Physiol 295: G211–G218, 2008. doi: 10.1152/ajpgi.90250.2008. [DOI] [PubMed] [Google Scholar]
- 5.Hong J, Behar J, Wands J, Resnick M, Wang LJ, DeLellis RA, Lambeth D, Souza RF, Spechler SJ, Cao W. Role of a novel bile acid receptor TGR5 in the development of oesophageal adenocarcinoma. Gut 59: 170–180, 2010. doi: 10.1136/gut.2009.188375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McQuaid KR, Laine L, Fennerty MB, Souza R, Spechler SJ. Systematic review: the role of bile acids in the pathogenesis of gastro-oesophageal reflux disease and related neoplasia. Aliment Pharmacol Ther 34: 146–165, 2011. doi: 10.1111/j.1365-2036.2011.04709.x. [DOI] [PubMed] [Google Scholar]
- 7.de Bortoli N, Gyawali CP, Frazzoni M, Tolone S, Frazzoni L, Vichi E, Visaggi P, Bellini M, Marabotto E, Penagini R, Savarino V, Marchi S, Savarino EV. Bile reflux in patients with nerd is associated with more severe heartburn and lower values of mean nocturnal baseline impedance and chemical clearance. Neurogastroenterol Motil 32: e13919, 2020. doi: 10.1111/nmo.13919. [DOI] [PubMed] [Google Scholar]
- 8.Yu S, Undem BJ, Kollarik M. Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J Physiol 563: 831–842, 2005. doi: 10.1113/jphysiol.2004.079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu Y, Liu Z, Yu X, Pasricha PJ, Undem BJ, Yu S. Increased acid responsiveness in vagal sensory neurons in a guinea pig model of eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol 307: G149–G157, 2014. doi: 10.1152/ajpgi.00097.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu X, Hu Y, Yu S. Effects of acid on vagal nociceptive afferent subtypes in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 307: G471–G478, 2014. doi: 10.1152/ajpgi.00156.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alemi F, Kwon E, Poole DP, Lieu T, Lyo V, Cattaruzza F, Cevikbas F, Steinhoff M, Nassini R, Materazzi S, Guerrero-Alba R, Valdez-Morales E, Cottrell GS, Schoonjans K, Geppetti P, Vanner SJ, Bunnett NW, Corvera CU. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest 123: 1513–1530, 2013. doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieu T, Jayaweera G, Zhao P, Poole DP, Jensen D, Grace M, McIntyre P, Bron R, Wilson YM, Krappitz M, Haerteis S, Korbmacher C, Steinhoff MS, Nassini R, Materazzi S, Geppetti P, Corvera CU, Bunnett NW. The bile acid receptor TGR5 activates the TRPA1 channel to induce itch in mice. Gastroenterology 147: 1417–1428, 2014. doi: 10.1053/j.gastro.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaezi MF, Fass R, Vakil N, Reasner DS, Mittleman RS, Hall M, Shao JZ, Chen Y, Lane L, Gates AM, Currie MG. IW-3718 reduces heartburn severity in patients with refractory gastroesophageal reflux disease in a randomized trial. Gastroenterology 158: 2093–2103, 2020. doi: 10.1053/j.gastro.2020.02.031. [DOI] [PubMed] [Google Scholar]
- 14.Bunnett NW. Neuro-humoral signalling by bile acids and the TGR5 receptor in the gastrointestinal tract. J Physiol 592: 2943–2950, 2014. doi: 10.1113/jphysiol.2014.271155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nehra D, Howell P, Williams CP, Pye JK, Beynon J. Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut 44: 598–602,1999. doi: 10.1136/gut.44.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Driessen AK, Farrell MJ, Mazzone SB, McGovern AE. The role of the paratrigeminal nucleus in vagal afferent evoked respiratory reflexes: a neuroanatomical and functional study in guinea pigs. Front Physiol 6: 378,.2015. doi: 10.3389/fphys.2015.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SH, Hadley SH, Maddison M, Patil M, Cha B, Kollarik M, Taylor-Clark TE. Mapping of sensory nerve subsets within the vagal ganglia and the brainstem using reporter mice for Pirt, TRPV1, 5-HT3, and Tac1 expression. eNeuro 7: ENEURO.0494-19.2020, 2020. doi: 10.1523/ENEURO.0494-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol 87: 2095–2103, 2002. doi: 10.1152/jn.00785.2001. [DOI] [PubMed] [Google Scholar]
- 19.Castro J, Harrington AM, Lieu T, Garcia-Caraballo S, Maddern J, Schober G, O'Donnell T, Grundy L, Lumsden AL, Miller P, Ghetti A, Steinhoff MS, Poole DP, Dong X, Chang L, Bunnett NW, Brierley SM. Activation of pruritogenic TGR5, MrgprA3, and MrgprC11 on colon-innervating afferents induces visceral hypersensitivity. JCI Insight 4: e131712, 2019. doi: 10.1172/jci.insight.131712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Y, Villalobos-Hernandez EC, Pradhananga S, Baker CC, Keating C, Grundy D, Lomax AE, Reed DE. Deoxycholic acid activates colonic afferent nerves via 5-HT(3) receptor-dependent and -independent mechanisms. Am J Physiol Gastrointest Liver Physiol 317: G275–G284, 2019. doi: 10.1152/ajpgi.00016.2019. [DOI] [PubMed] [Google Scholar]