Abstract
Millions of people who survive sepsis each year are rehospitalized and die due to late pulmonary complications. To prevent and treat these complications, biomarkers and molecular mediators must be identified. Persistent immune reprogramming in the form of immunoparalysis and impaired host defense is proposed to mediate late pulmonary complications after sepsis, particularly new pulmonary infections. However, immune reprogramming may also involve enhanced/primed responses to secondary stimuli, although their contribution to long-term sepsis complications remains understudied. We hypothesize that enhanced/primed immune responses in the lungs of sepsis survivors are associated with late pulmonary complications. To this end, we developed a murine sepsis model using cecal ligation and puncture (CLP) followed 3 wk later by administration of intranasal lipopolysaccharide to induce inflammatory lung injury. Mice surviving sepsis exhibit enhanced lung injury with increased alveolar permeability, neutrophil recruitment, and enhanced Ly6Chi monocyte Tnf expression. To determine the mediators of enhanced lung injury, we performed flow cytometry and RNA sequencing of lungs 3 wk after CLP, prior to lipopolysaccharide. Sepsis survivor mice showed expanded Ly6Chi monocytes populations and increased expression of many inflammatory genes. Of these, S100A8/A9 was also elevated in the circulation of human sepsis survivors for months after sepsis, validating our model and identifying S100A8/A9 as a potential biomarker and therapeutic target for long-term pulmonary complications after sepsis. These data provide new insight into the importance of enhanced/primed immune responses in survivors of sepsis and establish a foundation for additional investigation into the mechanisms mediating this response.
Keywords: lung injury, monocyte, priming, sepsis, sepsis survival
INTRODUCTION
The incidence of acute sepsis is increasing and with it the number of sepsis survivors, estimated at nearly 25 million people each year worldwide (1). Sepsis survivors remain at risk for long-term health care needs and complications following discharge when compared with age- and comorbidity-matched controls (2–4). The mean 1-yr hospitalization and mortality rates of adult sepsis survivors approach 40% and 16%, respectively (5, 6). Pulmonary diagnoses are commonly associated with rehospitalization and include pneumonia, pneumonitis, exacerbation of chronic respiratory disease, and/or respiratory failure (2, 5). Current strategies to prevent long-term sequelae of sepsis are focused on high-quality acute care in the hospital and posthospital follow-up (7). Unfortunately, despite these measures, patients continue to experience pulmonary-related rehospitalization. To identify those at risk for pulmonary complications and develop treatments, we need to understand the underlying mechanisms that drive long-term sequelae experienced by sepsis survivor patients (8–10). Our understanding of how and why these long-term pulmonary complications occur in sepsis survivors is limited due to lack of observational human studies and relevant animal models focusing on late time points (>7 days after sepsis) (9, 10).
Acute sepsis is associated with reorganization of transcriptional and metabolic processes in immune cells leading to altered functional responses to secondary inflammatory stimuli, a phenomenon referred to as immune reprogramming (8, 11). Several immune programs have been defined following acute infection, including tolerance (also immunoparalysis), priming, and trained immunity (12). Tolerance/immunoparalysis is defined as the inability of immune cells to activate gene transcription upon secondary stimulation, leading to a reduced/hyporesponsive functional state (12–14). In contrast, both priming and trained immunity result in enhanced or synergistic responses to secondary stimuli, leading to an enhanced functional state; however, the latter is more specifically defined by lasting epigenetic alterations that drive this functional change (12). Prior research in patients with sepsis has largely focused on tolerance/immunoparalysis, characterized by reduced Toll-like receptor (TLR) responses and phagocytosis among innate immune populations (15). Since it reduces host defense, tolerance/immunoparalysis is hypothesized to contribute to health care-associated infections during hospitalization for sepsis (15–19). Though well characterized in acute sepsis (20), observational studies have yet to draw substantial connections between persistent immunoparalysis and long-term outcomes in sepsis survivors weeks to months after hospitalization (21–25). Moreover, even if persistent immunoparalysis impairs host defense and predisposes to more severe infection, it is also possible that tissue injury may arise through enhanced cytokine responses to secondary stimuli. Importantly, it has been shown that following an initial immune challenge, the host defense response to secondary infection and injurious cytokine responses to secondary stimuli are not predictive of each other (26). In addition, murine models of long-term sepsis survival have shown enhanced/primed TNFα production in innate immune cells, including microglia and monocytes, in response to ex vivo lipopolysaccharide (LPS) administration (27–29). Even in murine studies of late sepsis survival showing persistent immunoparalysis with reduced phagocytic capacity and increased susceptibility to secondary bacterial pneumonia, the impaired innate immune cells still maintain their ability to produce TNFα in response to LPS ex vivo at or above the level of control (19, 30). These findings suggest that long-term consequences of sepsis on the lung are governed by a more complex interaction of host response to infection and cytokine response to inflammatory stimuli leading to tissue injury. Enhanced/primed immune responses arising after sepsis may therefore directly contribute to long-term pulmonary complications independent of or in conjunction with impaired host defense response to infection. Enhanced/primed immune responses after acute sepsis remain understudied in vivo and difficult to examine in models using secondary challenge with live microbes, which are confounded by the host defense response to infection.
To determine whether immune priming and its molecular mediators contribute to late pulmonary complications after sepsis, we developed a murine model of late postsepsis sterile inflammatory lung injury. We induced sepsis by cecal ligation and puncture (CLP) followed 3 wk later by intranasal LPS-induced lung injury. Given our prior work with this sepsis survival model demonstrating enhanced/primed responses to LPS in innate immune cells outside the lung (27), we hypothesized that post-CLP mice would have amplified lung injury due to functionally enhanced cytokine responses. We found that post-CLP mice experience enhanced lung injury associated with enhanced alveolar neutrophil recruitment, cytokine, and chemokine production, and Tnf expression in Ly6Chi monocytes. Prior to the administration of LPS, post-CLP mice demonstrate expanded lung leukocyte populations, including increased Ly6Chi monocytes, and whole lung gene expression consistent with a failure of sepsis survivors to return to immune homeostasis. We identify several potential molecular mediators contributing to enhanced lung injury, including the damage-associated molecular pattern (DAMP) S100A8/A9, which remains persistently elevated in mice and humans surviving sepsis. Our findings highlight a complex and multifactorial predisposition to pulmonary complications after sepsis survival occurring through primed immune responses, a shift from the paradigm that postsepsis pulmonary complications are due to reduced host defense or immunoparalysis alone.
METHODS
Mouse Studies
Male 8- to 12-wk-old C57BL/6J mice (Jackson Laboratory) were used in all experiments. All procedures involving animals were undertaken in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health. The study protocol was approved by the Institutional Animal Care and Use Committee of the University of Michigan (protocol no. PRO00008999).
Human Plasma Studies
All participants were patients enrolled in the Acute Lung Injury Specialized Center of Clinically Oriented Research multicenter, randomized, placebo-controlled trial of granulocyte-macrophage colony stimulating factor conducted at three academic medical centers between July 2004 and October 2007; the study protocol was previously described (31). All patients were randomized within 3–7 days of meeting the Berlin criteria for acute respiratory distress syndrome (ARDS) (32), and all time points were defined relative to day of randomization. Plasma samples from patients in the placebo or observational arms with a primary diagnosis of sepsis or pneumonia at the time of study inclusion were analyzed. As all patients with sepsis or pneumonia had suspected infection and required mechanical ventilation, all patients were presumed to have sepsis. These clinical criteria approximate the rigorously evaluated adult sepsis event definition from the CDC, which defines sepsis as 1) presumed infection and 2) organ dysfunction (33). Patient characteristics can be found in Supplemental Table S1 (all Supplemental material is available at https://doi.org/10.6084/m9.figshare.14749296.v3). For healthy controls, a single blood sample was collected from three healthy subjects following written informed consent. All healthy control subjects were less than 55 years of age, without chronic medical illness, and not taking chronic medications. Protocols were approved by the institutional review boards at each site, and the trials were registered in the National Clinical Trials registration database (NCT00201409).
Cecal Ligation and Puncture
Low-mortality (∼10%–20%) CLP was performed as previously described (27, 34–36). Animal suffering and distress were minimized with local lidocaine infiltration as well as anesthesia with ketamine and xylazine. Briefly, under aseptic conditions, a 1- to 2-cm laparotomy was performed. The cecum was ligated with a silk suture and punctured through and through with a 19-gauge needle. The incision was closed with surgical clips. Sham operation consisted of laparotomy with mobilization of the cecum, but without ligation-puncture procedure. Imipenem/cilastatin (Merck, 0.5 mg/mouse in 200 µL of normal saline) and normal saline (0.5 mL) were administered subcutaneously to all CLP or sham mice immediately following abdominal closure. All deaths occurred within 5 days of CLP, were attributed to the procedure, and were considered expected.
Lipopolysaccharide-Induced Lung Injury
Three weeks post-CLP (range 19–25 days postprocedure) and age-matched unoperated or sham control mice underwent lung injury by administering Escherichia coli LPS (O111:B4, Sigma) intranasally. Mice were anesthetized using ketamine and xylazine. LPS was dissolved in sterile normal saline and administered intranasal 25 µL per nostril (50 µg, 1 µg/µL). Mice were observed and allowed to recover from anesthesia. All mice within a cage were euthanized at the same time point and examined 24, 48, and 72 h after LPS administration. The University of Michigan policy for humane endpoints was followed.
Serum Collection
Following CO2 euthanasia, whole blood was obtained by puncture of the right ventricle using a heparinized 1-mL syringe and a 26-gauge needle. Needles were removed before ejection of blood from the syringe to minimize cell lysis. Blood was placed on ice for no more than 1 h. Samples were centrifuged at 2,000 g for 10 min and stored at −80°C until time of use.
Bronchoalveolar Lavage and Alveolar Leukocyte Count
Mice were euthanized with CO2 and bronchoalveolar lavage (BAL) was performed as described previously (37). Briefly, the trachea was exposed and intubated using a 1.7-mm outer diameter polyethylene catheter. BAL was performed by instilling PBS containing 5 mM EDTA in 1-mL aliquots; 3 mL PBS were instilled per mouse. BAL was then centrifuged to pellet cells and remove supernatant, which was aliquoted and stored at −80°C until further use. Cell counts and viability were determined using Trypan blue exclusion counting on a hemacytometer. Cytospins were prepared on BAL fluid and stained with Diff-Quick (Baxter) to determine differential for polymorphonuclear (PMN) neutrophils and mononuclear cells.
Lung Single-Cell Suspension Preparation
Lung single-cell suspensions were prepared as previously described (38). All mice were perfused with 10 mL cold PBS. Lungs were removed and minced with scissors in 15 mL of digestion buffer [RPMI, 10% fetal bovine serum, 1 mg/mL collagenase (Roche Diagnostics), 30 μg/mL DNase (Sigma-Aldrich)]. Lung slurries were enzymatically digested for 30 min at 37°C. Undigested fragments were dispersed by drawing the solution through a 10-mL syringe and passed through a 100-µm filter. The lung cell suspension was pelleted, resuspended, and spun through a 40% Percoll gradient to enrich for leukocytes. Cell counts and viability were determined using Trypan blue exclusion counting on a hemacytometer.
Flow Cytometry and Cell Sorting
Lung single-cell suspensions were washed, blocked for nonspecific staining with anti-CD16/32 Fc receptor block (clone 2.4G2, BD), and stained with fluorophore-conjugated antibodies before analysis on a FACS Aria II (BD) or MA900 (Sony) flow cytometer and cell sorter. Antibodies included: anti-CD45 (clone 30-F11, Biolegend), anti-Ly6G (clone 1A8, Biolegend), anti-CD11b (clone M1/70, Biolegend), anti-CD11c (clone N418, Biolegend), anti-CD64 (clone X54-5/7.1.1, BD), anti-CD24 (clone M1/69, BD), anti-Ly6C (clone HK1.4, Biolegend), and Anti MHCII/anti-I-A/E (clone M5/144.15.2, Biolegend). Whole lung leukocyte populations, including monocyte and macrophage subpopulations, were identified using an established gating strategy for mouse lung (Supplemental Fig. S1) (39). Selected populations were isolated by cell sorting (5,000–100,000 cells) directly into TRIzol (Invitrogen) for RNA isolation and gene expression analysis.
Quantification of BAL, Serum, and Whole Lung Proteins
BAL and serum were isolated from euthanized mice as previously mentioned. Whole lungs were isolated and frozen in liquid nitrogen until use. Lungs were homogenized in sterile PBS supplemented with protease inhibitor (Roche) on ice, using a rotor-stator homogenizer (Tissue-Tearor; BioSpec Products). Particulates were cleared by centrifugation, filtered at 0.45 µm, and lysates frozen at −80°C until use. Lung lysate samples were diluted 10-fold for ELISA and normalized to total protein, as measured by bicinchoninic acid assay (Thermo Scientific). All serum samples were diluted twofold for ELISA. All baseline BAL samples before LPS administration were run without dilution. For BAL samples above standard assay range, appropriate dilutions were determined based on the specific time point and protein being examined. The following ELISAs were used: TNFα, IL-1β, IL-6, IL-10, MIP-2, MCP-1, KC, S100A8/A9, RAGE (Mouse Duoset, R&D Systems), Pierce BCA assay (Thermo Scientific), and IgM (Invitrogen or Bethyl Laboratories).
Peritoneal Lavage and Aerobic Culture
Peritoneal lavage was performed by closed injection and withdrawal of 3–5 mL sterile PBS with 5 mM EDTA into the abdominal cavity. Aerobic culture of serial peritoneal fluid dilutions was performed at 37°C on tryptic soy agar plates.
RNA Isolation
RNA was isolated from whole lung tissue or monocyte/macrophage populations using TRIzol (Invitrogen) as described by the manufacturer.
Gene Expression Analysis by Quantitative PCR
Quantitative PCR was performed on a StepOnePlus thermocycler (Applied Biosystems) as previously described (40). Primers for Tnf and Il1b were purchased from Integrated DNA Technologies.
QuantSeq 3′ mRNA Sequencing for RNA Quantification
RNA was isolated from whole lung using TRIzol and reprecipitated using ethanol and sodium acetate. Contaminating DNA was removed with DNase I (Qiagen). RNA quality was assessed using a bioanalyzer (Agilent) and all RINs were greater than 8. Library preparation, sequencing, and identification of transcript frequency were performed by the University of Michigan Advanced Genomics Core. The QuantSeq 3' mRNA-seq Library Prep Kid FWD (Lexogen) kit was used with the UMI Second Strand Synthesis Module (Lexogen) to prepare cDNA libraries corresponding to polyadenylated mRNA. Libraries were sequenced using the NovaSeq platform. Reads were then trimmed using TrimGalore (v 0.5.0, https://github.com/FelixKrueger/TrimGalore), aligned using STAR (v 2.6.0) (41), and unique molecular identifiers collapsed using Lexogen’s supplied software, per manufacturer recommendations. Gene reference and annotation were performed using the Genome Reference Consortium Mouse Build 38. Transcripts were counted using the R package Rsubread and the featureCounts function (42).
Weighted Gene Correlation Network Analysis and Gene Enrichment Analysis
First, raw count data were filtered and then transformed using the variance stabilizing transformation within the DEseq2 (v 1.22.2) R package (43). Weighted gene correlation network analysis (WGCNA) was performed using the WGCNA (v 1.68) R package per the recommendation for RNAseq data (44). Modules, intramodular hub genes, and module trait relationships were identified. Gene enrichment analysis for Gene Ontology (GO) biologic process terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were performed using the ShinyGO (v 0.61) web application (45). Full methods and statistical analyses are available in the Supplemental Methods. Access to raw data can be found at the Gene Expression Omnibus database (GSE168796; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE168796).
Data Analysis
Analyses included ANOVA followed by post hoc testing when ANOVA was significant or unpaired t testing as indicated in the text. To minimize spurious comparisons, we prespecified post hoc comparisons only between CLP/unoperated or healthy/sepsis conditions. All figures show mean and standard error unless otherwise specified. Statistical analyses were carried out in Prism (v7, Graphpad).
RESULTS
Weight Loss and Infection Are Resolved in 3-wk Post-CLP Mice
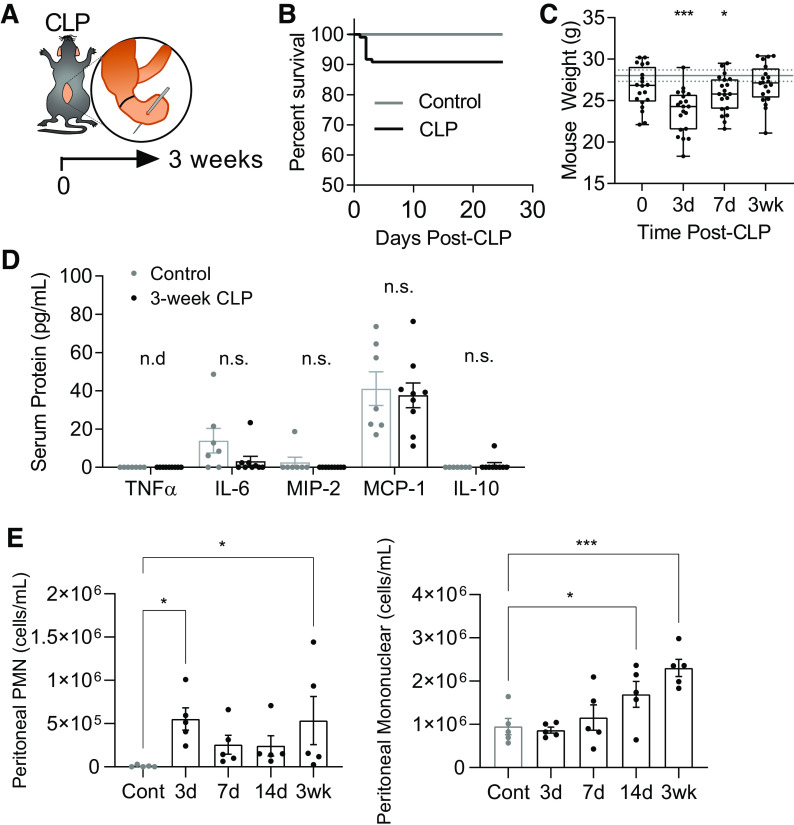
The majority of murine sepsis studies have focused on early responses during the first 1–7 days after infection but do not characterize the late recovery period following this. To gain insight into the mechanisms of late complications after sepsis, we studied mice 3 wk after CLP. To ensure we are studying mice that have both survived and recovered from acute sepsis, we evaluated our model (Fig. 1A) for signs of persistent infection and weight loss during late time points after CLP. Our model exhibits ∼10% mortality across multiple experiments (range 0%–20%, median 10%, n = 109, 9 cohorts) without any death after day 5 (Fig. 1B). Surviving post-CLP mice recover acute weight loss over 3 wk to the level of age-matched unoperated controls (Fig. 1C). Several serum cytokines and chemokines associated with acute sepsis (46) were not significantly elevated in 3-wk post-CLP mice as compared with unoperated control (Fig. 1D). We have previously shown in this model that peritoneal infection resolves by 5–7 days after CLP (27, 34). We confirmed the absence of bacterial growth in peritoneal lavage cultures 3 wk after CLP (data not shown, n = 4 CLP). However, despite initial improvement in acute neutrophilic exudation following CLP at day 3, there was evidence of mononuclear cell predominant aseptic peritonitis evolving by 3 wk (Fig. 1E). These data suggest that acute sepsis has resolved by 3 wk after CLP, supporting the use of this model to study survivors of acute sepsis with resolved peritoneal infection at a time point relevant to patients who are at risk for rehospitalization due to pulmonary complications.
Figure 1.
Acute sepsis resolves in post-CLP mice. Sepsis was induced through CLP with antibiotic treatment and fluid resuscitation; mice are allowed to recover for 3 wk (A). CLP mortality, range 0%–20%, median 10% mortality n = 109 across nine cohorts (B). Weight loss profile following CLP, total n = 14–19 per group (box and whisker plot showing median, 25th percentile, 75th percentile, max and min), solid and dashed lines represent mean ± SE weight for age-matched unoperated controls at 3 wk (C). Typical pro- and anti-inflammatory cytokines and chemokines associated with acute sepsis in serum of 3-wk post-CLP mice (n = 9) and unoperated control (n = 7; D). Peritoneal lavage PMN and mononuclear cells in post-CLP mice (days 3, 7, 14, and 3 wk) and unoperated control (n = 5 per group; E). Mean ± SE shown. One-way ANOVA unoperated versus CLP post hoc Fisher’s least significant difference P-value (C, E) or unpaired t test (D) shown *P < 0.05, ***P < 0.001. CLP, cecal ligation and puncture; Cont, control; MCP-1, monocyte chemotactic protein-1; MIP-2, macrophage inhibitory protein-2; n.d., below the level of detection; n.s., not significant; PMN, polymorphonuclear.
LPS-Induced Lung Injury Is Enhanced in Surviving Post-CLP Mice 3 wk after Sepsis
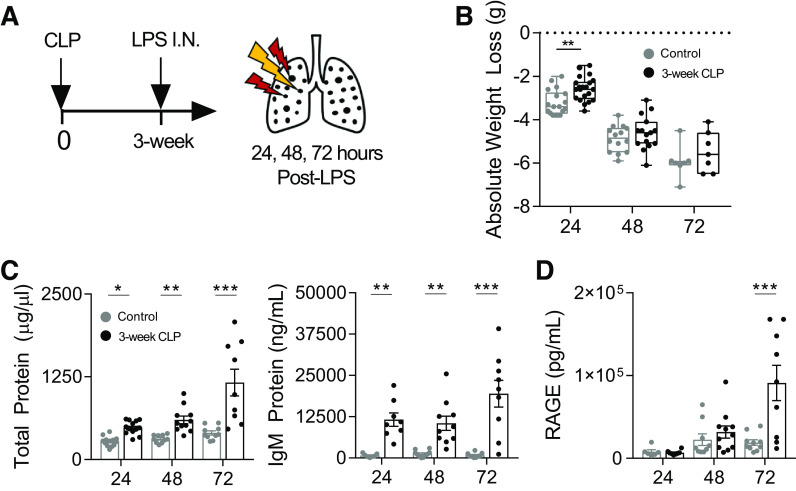
To determine the long-term effects of sepsis on pulmonary inflammatory responses, without confounding antimicrobial host response, we developed a model of CLP survival followed by sterile lung injury with intranasal LPS administration. Unoperated age-matched control mice and 3-wk post-CLP mice underwent administration of intranasal LPS (Fig. 2A). Although sham surgery alone may alter inflammatory responses to secondary stimuli (47), we found no difference in leukocyte recruitment or alveolar leak in response to intranasal LPS in unoperated as compared with 3-wk postsham operation controls (Supplemental Fig. S2). We therefore used unoperated age-matched controls to assess the long-term effects of CLP for all experiments.
Figure 2.
LPS-induced lung injury is enhanced in mice surviving CLP. 3-wk post-CLP mice and age-matched unoperated controls underwent intranasal LPS (50 µg) administration. Lung injury was measured in BAL at 24, 48, and 72 h after administration (A). Weight loss trajectories after intranasal LPS in unoperated (24, 48, and 72 h post-LPS; n = 18, 14, 7, respectively) and 3 wk post-CLP (24, 48, and 72 h post-LPS; n = 21, 15, 7, respectively; box and whisker plot showing median, 25th percentile, 75th percentile, max and min; B). Lung permeability measured by total protein and IgM (C) and type I alveolar epithelial cell injury by soluble RAGE (D) in BAL by ELISA. Data represent two to three independent experiments per time point, total n = 7–21 mice per group. Mean ± SE shown. Two-way ANOVA all P < 0.01 for effect of CLP (B–D), post hoc Sidak (B) or Fisher’s least significant difference (C, D) P-values shown *P < 0.05, **P < 0.01, ***P < 0.001. BAL, bronchoalveolar lavage; CLP, cecal ligation and puncture; I.N., intranasal; LPS, lipopolysaccharide; RAGE, receptor for advanced glycation end-products.
To determine if post-CLP mice have enhanced lung injury responses, we measured BAL markers of injury and inflammation 24–72 h following intranasal LPS. Importantly, before LPS administration, there were no significant differences in alveolar permeability or inflammation 3 wk after CLP as compared with control (Supplemental Fig. S3), indicating that CLP alone does not cause a long-term alveolitis or tissue injury. Post-CLP mice and controls exhibited similar weight loss trajectory after LPS (Fig. 2B). However, mortality through 72 h after LPS-induced lung injury tended to be higher post-CLP (2 out of 11 total mice) as compared with control (0 out of 10 total mice), suggesting higher severity of illness in post-CLP mice. In response to LPS, lung injury in post-CLP mice was associated with enhanced lung permeability as measured by BAL total protein and IgM as compared with control at all time points (Fig. 2C). To determine if enhanced alveolar leak was associated with biomarkers of alveolar epithelial injury, we measured the soluble receptor for advanced glycation end-products (sRAGE) in BAL fluid, which is a marker of type I alveolar epithelial cell (AEC) injury (48). BAL sRAGE was significantly elevated in post-CLP mice at 72 h after intranasal LPS as compared with controls (Fig. 2D). Taken together, these data indicate that post-CLP mice exhibited enhanced alveolar leak and injury of type I AECs in response to sterile lung injury with intranasal LPS. Importantly, these findings demonstrate enhanced lung injury in response to LPS in long-term sepsis survival, in contrast to reduced/hypoinflammatory responses, which might be expected with immunoparalysis.
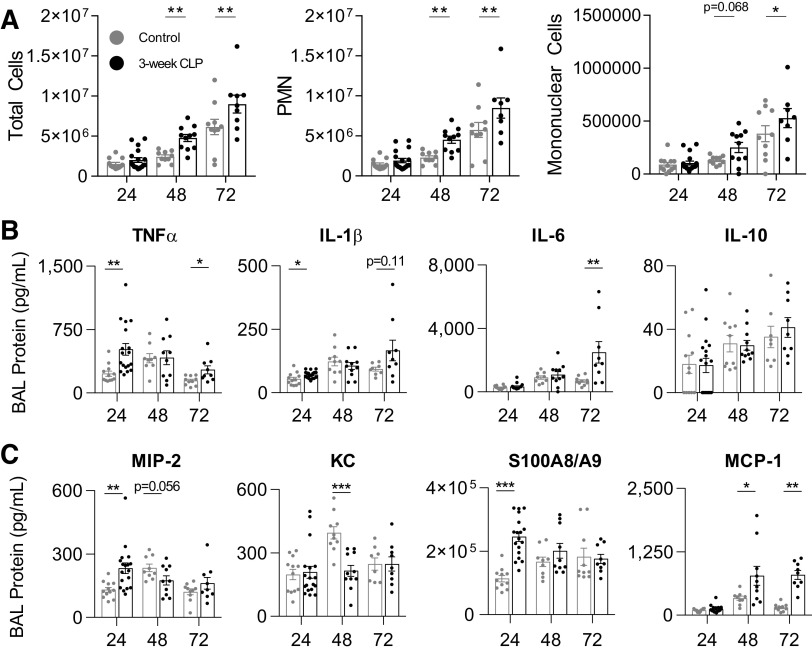
Intranasal LPS induces inflammatory, rather than direct, lung injury (49). To determine if enhanced tissue injury after intranasal LPS administration was due to enhanced inflammation in post-CLP mice, we measured several key mediators of acute lung injury in BAL fluid (50). We found that enhanced lung injury in post-CLP mice was associated with increased BAL total cells, PMN, and mononuclear cells after intranasal LPS as compared with controls (Fig. 3A). Twenty-four hours after intranasal LPS, we found statistically significant increases in BAL TNFα and IL-1β in post-CLP mice as compared with control (Fig. 3B). TNFα levels declined but remained significantly higher in post-CLP mice whereas IL-6 increased significantly in post-CLP mice over 72 h. There was no significant difference in BAL IL-10 at any time point. Neutrophil chemotactic proteins macrophage inhibitory protein-2 (MIP-2/CXCL2) and S100A8/A9, but not keratinocytes-derived chemokine (KC/CXCL1), were significantly increased early after intranasal LPS (Fig. 3C). We additionally found BAL levels of monocyte chemotactic protein-1 (MCP-1) were significantly increased after intranasal LPS in post-CLP mice as compared with control. These data support our hypothesis that sterile lung injury would be associated with enhanced/primed pulmonary inflammatory responses to LPS.
Figure 3.
LPS induces enhanced alveolar inflammation in the BAL fluid of CLP mice. BAL leukocytes (A); cytokines (B); neutrophil chemokines (MIP-2, KC), neutrophil chemoattractant (S100A8/A9), and monocyte chemokine (MCP-1; C) 24–72 h after intranasal LPS administration in 3-wk post-CLP mice and unoperated control. Data represent two to three independent experiments per time point, total n = 8–18 per group. Mean ± SE shown. Two-way ANOVA all P < 0.01 for effect of CLP (A). Post hoc Fisher’s least significant difference (A) and individual unpaired t test (B, C) P-values shown *P < 0.05, **P < 0.01, ***P < 0.001. BAL, bronchoalveolar lavage; CLP, cecal ligation and puncture; KC, keratinocytes-derived chemokine; LPS, lipopolysaccharide; MCP-1, monocyte chemotactic protein-1; MIP-2, macrophage inhibitory protein-2; PMN, polymorphonuclear.
Ly6Chi Monocytes of Post-CLP Mice Have Enhanced Tnf Expression in Response to Intranasal LPS
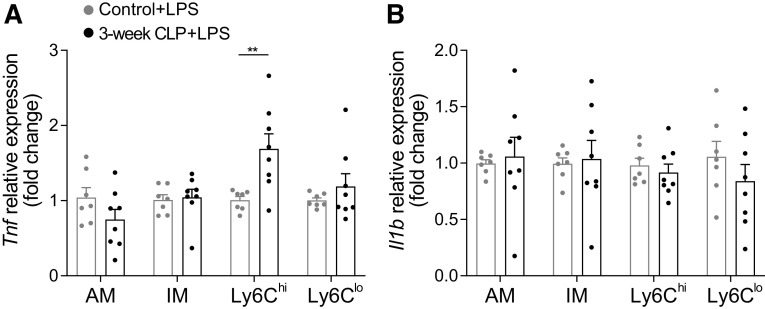
To determine if enhanced cytokine production and lung injury was due to enhanced/primed gene expression among innate immune cells in post-CLP mice, we evaluated gene expression among several innate immune populations of the lung 24 h after intranasal LPS isolated by flow cytometry and cell sorting. These populations included alveolar macrophage (AM), interstitial macrophage (IM), Ly6Chi monocytes, and Ly6Clo monocytes (Supplemental Fig. S1). We found that Ly6Chi monocytes in post-CLP mice express significantly more Tnf relative to unoperated control in response to intranasal LPS (Fig. 4A). In contrast, Il1b expression was not significantly enhanced among the examined cell populations (Fig. 4B). This suggests that Ly6Chi monocytes in the lung 24 h after intranasal LPS are reprogrammed by CLP to possess enhanced gene expression responses to a sterile inflammatory stimulus and may participate in enhanced lung injury in the post-CLP mice.
Figure 4.
Ly6Chi monocytes in post-CLP mice express more Tnf in response to intranasal LPS. Tnf (A) and Il1b (B) gene expression in alveolar macrophage (AM), interstitial macrophage (IM), Ly6Chi monocytes (Ly6Chi) and Ly6Clo monocytes (Ly6Clo) 24 h after intranasal LPS sorted from lung digests and analyzed by qPCR. Data represent two independent experiments, total n = 7–8 per group. Mean ± SE shown. Two-way ANOVA for effect of cell type P < 0.01 (A). Post hoc Sidak P-value **P < 0.01. CLP, cecal ligation and puncture; LPS, lipopolysaccharide.
Post-CLP Mice Demonstrate Altered Lung Leukocyte Populations Prior to the Administration of LPS
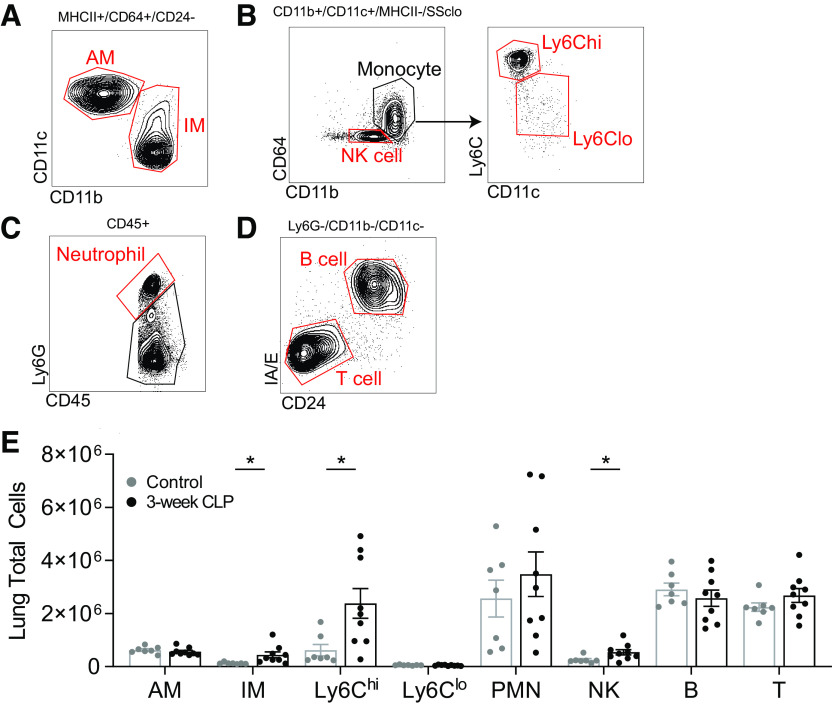
The proportions of leukocytes in the lung before LPS administration, including primed Ly6Chi monocytes, may directly contribute to enhanced lung injury after LPS. To determine if lung leukocyte populations were altered before LPS administration, we performed flow cytometry using various cell surface markers (Fig. 5, A–D) (39). We found that post-CLP mice had expanded Ly6Chi monocyte, interstitial macrophage (IM), and natural killer (NK) cell populations in the lungs before LPS administration (Fig. 5E). In contrast, we did not observe an increase in numbers of other cell types including PMN, B, or T cells. These data suggest an expansion of multiple cell types in the lung of post-CLP mice before LPS, which may have additive or synergistic effects on the inflammatory and tissue injury responses to LPS.
Figure 5.
Prior to LPS administration, Ly6Chi monocyte, interstitial macrophage, and NK cell populations are expanded in post-CLP mouse lungs. Flow cytometry gating scheme shown, populations identified alveolar macrophage (AM) and interstitial macrophage (IM; A); NK cell (NK), Ly6Chi monocyte (Ly6Chi), and Ly6clo monocyte (Ly6Clo; B); neutrophil (PMN; C); B cell and T cell (D). Lung leukocyte populations measured by multiparameter flow cytometry in unoperated (n = 7) and 3-wk post-CLP (n = 9; E). Total cells per population = (lung homogenate cell count × %[CD45]) × %[gated population]. Mean ± SE shown, unpaired t test P-value *P < 0.05. CLP, cecal ligation and puncture; LPS, lipopolysaccharide; NK cell, natural killer cell; PMN, polymorphonuclear.
Whole Lung Gene Expression Reveals Persistent Immune Reprograming and Altered Immune Function in the Lungs of Post-CLP Mice
Having identified several cell types associated with vulnerability to lung injury in post-CLP mice, we also wanted to determine the potential molecular contributors. To perform an unbiased assessment of mediators of enhanced lung injury, we evaluated gene expression in whole lung 3 wk after CLP using 3'-end sequencing of RNA and weighted gene correlation network analysis (WGCNA). WGCNA allows for the unbiased identification of modules (clusters) of genes that covary across samples and highly connected hub genes within modules (44). Hub genes within modules represent genes with the highest level of intramodular connectivity and therefore potential biologic importance. WGCNA revealed 17 modules of covarying genes which were significantly correlated with CLP (P < 0.05), each module was randomly assigned a color identifier by the WGCNA R package (Supplemental Fig. S4). Of these 17 modules, we evaluated 11 modules with the strongest correlation with CLP (|R2| ≥ 0.70, P ≤ 0.01) for hub genes (Table 1) and subjected them to gene ontology and pathway enrichment analysis.
Table 1.
WGCNA of whole lung gene expression
| Module Name | Module Association with CLP, R2 | P-Value | Total Genes in Module (n) | Hub Genes |
|---|---|---|---|---|
| Purple | 0.90 | 1.0E-05 | 352 | Ifi209, Lmo2, Nfatc2, Lyn, Klri2, Rasa3, Marchf1, Gmip, Dach1, Plek |
| Darkturqoise | 0.83 | 2.0E-03 | 210 | Camk1d, Jchain, Gm42759, Gm15987, Arhgdib, Ighg2b, Ighm, Iglc2, Gm49660, Ndc80 |
| Coral4 | 0.81 | 3.0E-03 | 63 | Cenpe, Gm43292, Gm45244, Cotl1, Ttk, Rap1gap2, A930006K02Rik, Prss34, Treml4, Pold4 |
| Skyblue4 | 0.79 | 4.0E-03 | 83 | Gm37936, Ankle2, Gm13782, Lpcat3, Specc1l, Lsm11, Myo18a, Endou, Tekt5, Tiam1 |
| Darkred | 0.77 | 6.0E-03 | 193 | Cxcr2, Spi1, S100a8, S100a9, Pilra, Clec4e, Itgam, Ifitm6, Camp, Slfn4 |
| Thistle | 0.73 | 1.0E-02 | 92 | Rapgef4os3, a, 3110067C02Rik, Ripk2, Elp1, Popdc3, Ankfy1, Metrnl, Gm31282, Abra |
| Light cyan | −0.73 | 1.0E-02 | 232 | Fam98b, Slc7a3, Rpl36a-ps2, Mcm9, Exosc7, Meox2, Sdf2l1, Smoc2, Pcyox1l, mt-Nd1 |
| Darkolivegreen4 | −0.73 | 1.0E-02 | 94 | Mlph, Cdk9, Tmem215, Med18, Fras1, Brap, Gm9967, Pcsk4, Zrsr1, Gm20517 |
| Lavendarblush1 | −0.73 | 1.0E-02 | 67 | Efcab2, Dipk1b, Thbs3, Gstm2, Fam205c, Slc25a51, Me3, Gm45684, Osbpl7, Ahnak2 |
| Plum | −0.79 | 4.0E-03 | 102 | Tiprl, Hspa12b, Cct3, H3c15, Hspb1, Rbm15b, Srsf1, Hsp90aa1, Mta1, Gm49706 |
| Salmon1 | −0.93 | 4.0E-05 | 69 | Aox1, Wfdc12, Prickle3, Maob, Gm43490, Zfp551, Unc45a, Gm45693, Gm7589, Gm12346 |
Modules of covarying genes and their association with CLP as measured by R2 and correlation P value. Module size and hub genes, which have the highest level of intramodular connectivity, are listed. CLP, cecal ligation and puncture; WGCNA, weighted gene correlation network analysis.
We hypothesized that whole lung gene expression in post-CLP mice would reveal increased expression of inflammatory mediators, particularly markers of cell types identified in our flow cytometry and markers of activation of monocyte/macrophage inflammatory responses. Among modules positively correlated with CLP, several hub genes associated with enhanced monocyte/macrophage cytokine responses and proinflammatory function were identified including Clec4e (51) and Slfn4 (52, 53) (darkred module), Treml4 (54) (coral4 module), and Metrnl (55, 56) (thistle module). Among cell types expanded in the post-CLP lung on flow cytometry, we identified the NK cell-specific hub gene Klri2 (57) (purple module). Hub genes specific to other cell types, which were not expanded in the lungs on flow cytometry, included markers of B cells (Jchain, Ighg2b, Ighm, Iglc2; darkturquoise module) and neutrophils (Cxcr2; darkred module).
Functional enrichment analysis using gene ontology (GO) terms was performed to identify biological processes enriched among all genes within each module. Representative GO terms were selected from the top 20 significant terms identified with enrichment false discovery rate (FDR) P-value ≤ 0.05. Enriched GO terms in modules associated with CLP were represented predominantly by immune cells and their activation. These included specific terms related to myeloid/B cell/T cell activation, adhesion, and migration (Supplemental Fig. S5A). Enriched terms also included those for cytokine production and release, with one specific module containing interferon-β production (coral4 module). Terms for biologic processes in modules that were negatively correlated with CLP included cellular responses to alcohols and dicarboxylic acid metabolism (Supplemental Fig. S5B).
Pathway enrichment analysis was then performed using KEGG terms among all genes within each module (Table 2). Representative KEGG pathways were selected from the top 10 significant terms identified with an enrichment FDR P-value ≤ 0.05. Of modules positively correlated with CLP, several terms for inflammatory pathways were identified including natural killer cell-mediated cytotoxicity and chemokine signaling pathway (purple module). Similarly, the NOD-like receptor signaling pathway (darkred module) was significantly enriched, containing key members of the NLR family pyrin domain containing 3 (NLRP3) inflammasome, along with the pro-inflammatory IL-17 signaling pathway. In both the purple and darkred modules, we found enrichment of selectin family genes including Sell, Selp, and Selplg, in the terms cell adhesion molecules (CAMs) and Staphylococcus aureus infection. In modules negatively associated with CLP, enriched pathways included Fc-γ R-mediated phagocytosis, protein processing in the endoplasmic reticulum, and tryptophan metabolism. Taken together, these data confirm the presence of multiple proinflammatory pathways enriched within the lung before LPS administration.
Table 2.
KEGG pathway enrichment analysis of modules significantly associated with CLP
| Module Name | EGG Term | FDR | Genes in Module (n) | Genes in Term (n) | Gene Names |
|---|---|---|---|---|---|
| Modules positively correlated with CLP | |||||
| Purple | Natural killer cell-mediated cytotoxicity | 7.89E-05 | 11 | 113 | Ifng, Itgb2, Klra4, Lcp2, Klrb1c, Ncr1, Nfatc2, Vav1, Plcg2, Sh3bp2, Fcgr4 |
| Purple | Hematopoietic cell lineage | 7.89E-05 | 10 | 92 | Cd1d1, Cd34, Cd38, Cd4, Gp1ba, Itga2, Itga4, Itgb3, Kit, Kitl |
| Purple | Cell adhesion molecules (CAMs) | 4.04E-03 | 10 | 159 | Cd34, Cd4, H2-t24, Itga4, Itgb2, Sell, Lrrc4c, Icosl, Cadm1, Nectin3 |
| Purple | Chemokine signaling pathway | 1.39E-02 | 10 | 197 | Ccr3, Cx3cr1, Gng7, Hck, Lyn, Ncf1, Vav1, Grk6, Pik3r5, Ppbp |
| Darkred | Osteoclast differentiation | 6.91E-05 | 9 | 123 | Sirpb1c, Csf1r, Fosb, Il1b, Lilra6, Pirb, Spi1, Tyrobp, Sirpb1b |
| Darkred | NOD-like receptor signaling pathway | 6.91E-05 | 11 | 197 | Casp1, Casp4, Camp, Ifi204, Il1b, Naip6, Pstpip1, Nlrp3, Trpm2, Nlrp12, Mefv |
| Darkred | Staphylococcus aureus infection | 1.84E-02 | 5 | 87 | Camp, Itgam, Ptafr, Selp, Selplg |
| Darkred | IL-17 signaling pathway | 1.84E-02 | 5 | 90 | Fosb, Il1b, Mmp9, S100a8, S100a9 |
| Modules negatively correlated with CLP | |||||
| Plum | Protein processing in endoplasmic reticulum | 1.44E-02 | 5 | 162 | Hsph1, Hsp90ab1, Hsp90aa1, Ubqln1, Lman1 |
| Plum | IL-17 signaling pathway | 1.44E-02 | 4 | 90 | Srsf1, Fadd, Hsp90ab1, Hsp90aa1 |
| Salmon1 | Tryptophan metabolism | 4.35E-03 | 3 | 47 | Maob, Aox3, Aoc1 |
| Salmon1 | Fc-γ R-mediated phagocytosis | 4.53E-02 | 2 | 85 | Pld2, Amph |
Representative pathways are shown. CLP, cecal ligation and puncture; FDR, false discovery rate P-value; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Validation of Lung Gene Expression and Biomarker Identification in Mice and Humans
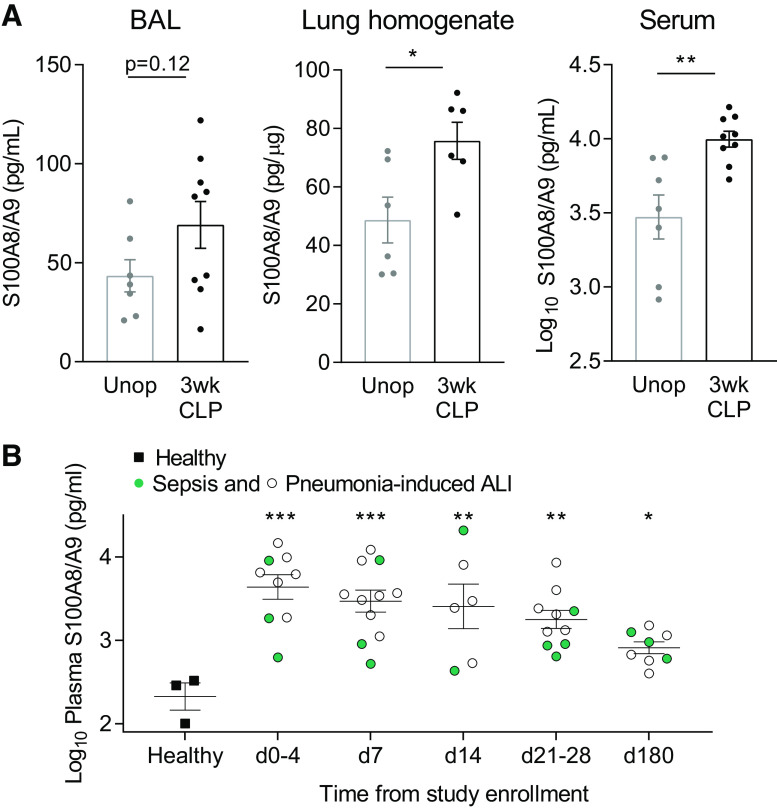
Thus, we have developed a mouse model of sepsis survival associated with late enhanced lung injury responses. To validate the clinical relevance of this model, we determined whether the many molecular mediators identified here are associated with human pathology. We selected biomarkers from highly connected hub genes to further evaluate in humans. Among hub genes, S100a8 and S100a9 were identified as being positively correlated with post-CLP mice and encode the components of the S100A8/A9 heterodimer, which has been associated with lung injury responses in murine models (58, 59). To confirm that S100A8/A9 is persistently elevated at late time points after sepsis in mice, we evaluated the level of this protein in the BAL, lung, and serum 3 wk after CLP, but before LPS. We found that S100A8/A9 is significantly elevated in the serum and lungs and tended to be elevated in the BAL of 3-wk post-CLP mice as compared with control (Fig. 6A). To determine if S100A8/A9 is also elevated in the circulation of human survivors of sepsis, we evaluated S100A8/A9 in the plasma of patients during the course of recovery from acute lung injury (ALI) due to sepsis or pneumonia (31). Survivors of sepsis or pneumonia-induced ALI demonstrated significantly elevated plasma S100A8/A9 levels through 180 days after acute illness as compared with healthy controls (Fig. 6B). Taken together, these data both validate our mouse model as a clinically relevant model and identify S100A8/A9 as a candidate biomarker to evaluate further in clinical studies of long-term pulmonary complications in patients who have survived sepsis.
Figure 6.
DAMP S100A8/A9 is persistently elevated in post-CLP mice and humans after sepsis-associated ALI. BAL, serum, and lung homogenate S100A8/A9 levels in 3-wk post-CLP mice and unoperated controls (A). Lung homogenate levels are normalized to total protein and expressed as picogram S100A8/A9 per µg total protein. Log10 plasma S100A8/A9 levels in human survivors (n = 12 patients) of sepsis (non-pulmonary; green) or pneumonia-induced ALI (white) as compared with healthy control (B). Mean ± SE shown, unpaired t test (A) or one-way ANOVA, post hoc Holm–Sidak P-value (B) shown *P < 0.05, **P < 0.01, ***P < 0.001. ALI, acute lung injury; BAL, bronchoalveolar lavage; CLP, cecal ligation and puncture; DAMP, damage-associated molecular pattern; Unop, unoperated.
DISCUSSION
As the number of patients surviving sepsis grows each year, millions of patients will be rehospitalized with pulmonary complications (5). Though hospital and patient-level characteristics may play roles in predicting long-term outcomes, there is growing evidence suggesting that long-term immune trajectories after hospitalization for sepsis are associated with poor outcome (21, 60). Prior models have focused on reduced host defense to infection as a potential mechanism; however, we postulated that enhanced tissue injury as a result of enhanced/primed immune responses may also contribute. To better understand inflammatory mechanisms predisposing to pulmonary complications after sepsis, we developed a murine model of long-term pulmonary complications using inflammatory lung injury at late time points after CLP-induced sepsis. We then sought to identify potential biomarkers, which might predispose the lung toward enhanced/primed immune responses promoting tissue injury for further use in sepsis survivor patients.
Our antibiotic-treated low-mortality CLP model led to disseminated infection (27, 34), weight loss, and recovery from acute infection over the course of 3 wk. Though peritoneal cultures were negative 3 wk after CLP, post-CLP mice demonstrated evidence of an evolving aseptic mononuclear cell predominant peritonitis. When challenged with intranasal LPS 3 wk after CLP, we found that post-CLP mice have enhanced alveolar epithelial injury. This is associated with enhanced leukocyte recruitment and early enhancement of cytokine, chemokine, and DAMP production in BAL fluid including TNFα and IL-1β. This is followed by downstream enhancement of IL-6 and MCP-1 production in a feedforward proinflammatory cascade. To begin to understand factors contributing to this process, we evaluated lung leukocyte populations in post-CLP mice and controls before LPS administration. We found that Ly6Chi monocytes accumulate in the lung after CLP and that these monocytes also have functionally enhanced/primed Tnf expression in response to intranasal LPS. We found that other leukocyte populations including NK cells and IM are also expanded in the lung before LPS administration, indicating an ongoing and complex multicellular inflammatory process in the lungs after CLP. This was supported by WGCNA of whole lung, which revealed significant enrichment of NK cell genes while also revealing B cell and neutrophil genes. We highlighted the presence of S100a8 and S100a9 as potential biomarkers and found the heterodimer protein S100A8/A9 to be persistently elevated in the serum and lungs of post-CLP mice and the plasma of patients surviving sepsis or pneumonia-induced ALI. Identification of these cellular and molecular mediators sets the foundation for future mechanistic and clinical research to determine their association with and direct contribution to long-term pulmonary complications after sepsis leading to rehospitalization.
Our observations support a potential role for Ly6Chi monocytes contributing to enhanced lung injury in post-CLP mice. Monocytes are plastic cells that may acquire new effector functions under pathologic conditions including proinflammatory activity, antigen presentation, tissue remodeling, or anti-inflammatory activity (61, 62). At steady state, monocytes egress from the bone marrow expressing high levels of Ly6C and C-C chemokine receptor type 2 (CCR2) and serve primarily to differentiate into vascular patrolling (Ly6Clo) monocytes and tissue phagocytes, where they participate in endothelial repair and/or tissue remodeling/homeostasis (61, 62). During acute infection/inflammation, however, Ly6Chi monocytes play various roles in host defense and tissue injury (61, 62). Ly6Chi monocytes are known to be key mediators of acute lung injury (63–67). As such, accumulation of proinflammatory Ly6Chi monocytes with enhanced/primed LPS responses in the lung may be a primary mediator of enhanced lung injury in this model. We hypothesize that these Ly6Chi monocytes are likely to represent an expansion of the vascular marginated pool. This population exists in the lung at baseline and as has been previously observed to be expanded for weeks after sterile inflammation and sepsis due to CLP in mice (30, 66, 68–71). We and others have also previously observed persistent monocyte margination in the brain perivasculature days to weeks after experimental sepsis (27, 36, 72, 73). This is the first report associating sterile lung injury many weeks after CLP with the presence of lung accumulating monocytes. These monocytes display enhanced transcriptional responses to secondary LPS stimulation suggesting immune reprogramming due to either priming or trained immunity (12, 74, 75). Although both priming and trained immunity refer to enhanced responses to secondary stimulation following an initial immune stimulus, trained immunity is a distinct form of innate immune memory where long-term epigenetic and metabolic alterations persist despite return to a basal transcriptional state (74–80). Trained immunity has been implicated in enhancing cytokine responses in monocytes in other models of post-CLP immune reprogramming through the identification of parallel metabolic derangements (29). However, given evidence of broad transcriptional changes in the lung in 3-wk post-CLP mice, we hypothesize that the cellular program present in this model is more representative of priming, rather than solely the epigenetic immune memory observed in trained immunity. Additional work is required to determine the transcriptional and epigenetic mechanisms of enhanced monocyte responses to LPS observed in this model. In addition, whether monocyte accumulation in the lungs post-CLP or monocyte priming contribute directly to pulmonary complications after sepsis in humans or mice requires further investigation.
Our WGCNA identified the DAMP genes S100a8 and S100a9 as hub genes in a module highly correlated with CLP. We subsequently confirmed that the heterodimer S100A8/A9 remains elevated in the circulation of mice 3 wk after CLP. Importantly, we discovered that S100A8/A9 also remains persistently elevated in humans surviving sepsis-induced ALI. These findings validate our model and identify S100A8/A9 as a candidate biomarker to examine in future studies of patients surviving sepsis. Moreover, it establishes S100A8/A9 as a target for mechanistic research to determine its role in mediating enhanced lung injury. S100A8/A9 is an acute inflammatory protein released by neutrophils, monocytes, and macrophage during acute inflammation or infection (81). It is elevated in the circulation of patients during acute sepsis and higher levels are associated with increased mortality (82), though its persistent elevation following survival of sepsis has not been well defined. S100A8/A9 has been associated with acute lung injury through enhancing recruitment and transepithelial migration of neutrophils to the alveolus in murine models of sterile and infectious lung injury (58, 59, 81, 83–86). Although S100A8/A9 binds to both the RAGE and TLR4 receptors, its proinflammatory actions occur predominantly through TLR4 (81). S100A8 and S100A9 bind the TLR4/myeloid differentiation factor-2 (MD2) complex acting as important upstream enhancers of the production of TNFα in phagocytes responding to LPS (87, 88). However, complexities exist in the function of S100A8/A9 as it has also been shown to perform autoinhibitory regulation to reduce local inflammation through heterotetramer formation and obscuration of TLR4 binding (88). The role of S100A8/A9 in this model requires further investigation, and ongoing work is being done to develop the experimental tools necessary to accomplish this task. Importantly, we validated our findings in mice and show persistent plasma S100 proteins in patients surviving sepsis-induced ALI, identifying S100A8/A9 as a potential biomarker worthy of further investigation in future sepsis survival cohort studies.
We also found broad proinflammatory derangements in leukocyte gene expression within the lungs of post-CLP mice before LPS administration. Interestingly, the NK cell-mediated cytotoxicity pathway was highly enriched in our WGCNA analysis. This correlated with expanded NK cell populations in the lungs as measured by flow cytometry. NK cells have a significant role in cytokine secretion and activation of the innate immune response in acute sepsis and lung injury (89, 90). Recent observations suggest NK cells are also subject to epigenetic immune reprogramming similar to trained immunity, with enhanced interferon gamma (IFN-γ) secretion following remote inflammatory exposures (91, 92). NK cell-derived IFN-γ may further propagate the enhanced cytokine and chemokine secretion observed in our model. As such, a multicellular crosstalk including reprogrammed monocytes may be contributory. Additional studies are needed on NK cells and other inflammatory populations using techniques such as single-cell sequencing to further define the contributions of specific populations and their responses to LPS-induced lung injury in this model. In addition, we found enrichment of the NOD-like receptor signaling pathway in post-CLP lungs, which contained several important members of the NLRP3 inflammasome including Nlrp3, Il1b, Casp1, and Casp4. This was associated with a modest but significant increase in IL-1β production in the BAL of post-CLP mice 24 h after intranasal LPS. Though monocyte and macrophage populations did not have enhanced Il1b expression after intranasal LPS, IL-1β production is often regulated at the protein processing level. Therefore, activation of the NLRP3 inflammasome may still lead to enhanced IL-1β production and enhanced lung injury in this model (58, 93, 94). The importance of this finding and whether it represents an upregulation of NLRP3 genes within specific populations or just an increase in NLRP3 expressing leukocytes in the lungs before LPS administration requires further investigation.
This study provides several important insights into the pathophysiology of postsepsis lung immune responses and also how modeling sepsis survival can be reexamined. Primarily, we find that sterile lung injury in this model of CLP is enhanced at late time points, and prior work has not previously identified enhanced tissue injury as a potential mediator of long-term pulmonary complications of sepsis. This novel finding, which diverges from the prominent literature, may be due to multiple factors. First, a focus on immunoparalysis using singular infectious challenges with death, bacterial dissemination and phagocytosis as primary endpoints may limit our understanding of how infectious insults and the inflammatory cascade respectively damage the alveolar epithelial barrier leading to poor outcome. Using noninfectious or antibiotic-treated infectious challenges, with a focus on alveolar barrier function and injury may complement our understanding of how postsepsis immune reprogramming contributes to long-term pulmonary complications. Second, the response to sepsis is compartmental (95) and the primary source of sepsis may further influence the long-term inflammatory response in the lung. Whether nonpulmonary sepsis as a primary insult is unique in its long-term effect on the lung remains to be seen. Third, model specifics such as severity of initial sepsis, survivor bias, time point examined, and presence of persistent infection are likely to have a substantial effect on the long-term immune program detected. For example, it can be hypothesized that models with elevated late mortality and persistent peritoneal infection will be more likely to identify immunoparalysis. Specific factors such as evolving aseptic peritonitis in this model need to be further explored including analyses with culture-independent microbial evaluation to determine the relative influence of microbes and/or tissue injury due to cecal ligation on peritoneal inflammation and lung immune reprogramming. In addition, a comparison across CLP models with differing long-term immunophenotypes is warranted to determine which of these factors influences the secondary response to inflammation/infection. Finally, we recognize there is heterogeneity in the LPS inflammatory and lung injury responses, particularly at 72 h, observed in post-CLP mice. Model specifics such as the uniformity of LPS dose delivery via intranasal route, as well as, uniformity/severity of sepsis induction among CLP mice are suspected to contribute to this observation. Heterogeneity in lung injury response remains a focus of future investigation, particularly how initial CLP severity and characteristics of illness recovery (e.g., ongoing peritoneal inflammatory responses) contribute to the enhanced lung injury response.
This study has several limitations. Our observations are in a single model of sepsis induced by fecal peritonitis. We chose to study a nonpulmonary source of sepsis to avoid confounding lung immune responses to LPS by recent pulmonary infection. Differential effects on lung injury may be expected when the primary septic insult is pneumonia, as long-term negative effects on AM have been observed following bacterial pneumonia in mice and humans (19, 96). Using models of long-term survival with primary antibiotic-treated pneumosepsis followed by sterile lung injury would be required to further explore this. Furthermore, we have limited our evaluation to young male mice, as sex (97, 98) and age (99, 100) are known to influence the acute response to sepsis and therefore may have independent consequences on long-term pulmonary inflammatory response after sepsis. Future studies are needed examining the effect of variables such as sex and age on long-term pulmonary immune responses in this model. In addition, our secondary inflammatory challenge in this model is a single dose of LPS, whereas pulmonary complications in patients triggered by bacteria, aspiration, or viral infections are likely to be induced in a more complex manner. Additional work in this model to explore the effects of secondary bacterial pneumonia and/or aspiration after sepsis is required. Finally, we noted that circulating S100A8/A9 is known to be elevated in both acute and chronic medical conditions unrelated to sepsis (81). Thus, our observations using young healthy controls are more likely to be biased toward detecting a difference between groups. Future studies with a larger sample size in subjects matched for age, comorbidity, and severity of illness among hospitalized patients with and without sepsis will be helpful to further define the sepsis-specific association of S100A8/A9 levels and long-term outcome.
In conclusion, we utilized a model of murine sepsis survival following CLP to uncover the novel finding of enhanced lung injury responses at time points later than the predominant literature has examined. Post-CLP mice demonstrate pro-inflammatory immune reprogramming associated with enhanced/primed Ly6Chi monocyte responses to LPS. Broad abnormalities in immune populations of the lung were detected and a potential role for the interactions of multiple cell types including NK cells was identified. S100a8 and S100a9 gene expression was increased in the lungs before LPS administration and the S100A8/A9 protein was found to be persistently elevated in the circulation of mice and humans surviving sepsis. These findings suggest that immune priming may contribute to late pulmonary complications of sepsis and that tissue injury responses may be an important outcome of interest. The cellular and molecular mediators identified in this model may serve as biomarkers and/or targets for intervention to prevent long-term pulmonary complications after sepsis.
SUPPLEMENTAL DATA
Supplemental Table S1, Supplemental Figs. S1–S5, and Supplemental Methods: https://doi.org/10.6084/m9.figshare.14749296.v3.
GRANTS
This work was supported by National Institutes of Health Grants T32HL00774921, F32HL149182, K08HL153799 (to S.J.D.), T32HL00774921 (to A.C.B.), K08NS101054 (to B.H.S.), R01HL123515 (to T.J.S.), R01HL147920, and R01HL131608 (to R.L.Z.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.J.D., T.J.S., and B.H.S. conceived and designed research; S.J.D. and M.W.N. performed experiments; S.J.D., A.C.B., and M.W.N. analyzed data; S.J.D., A.C.B., B.B.M., T.J.S., R.L.Z., and B.H.S. interpreted results of experiments; S.J.D., A.C.B., and R.L.Z. prepared figures; S.J.D. and R.L.Z. drafted manuscript; S.J.D., A.C.B., M.W.N., B.B.M., T.J.S., R.L.Z., and B.H.S. edited and revised manuscript; S.J.D., T.J.S., R.L.Z., and B.H.S. approved final version of manuscript.
ACKNOWLEDGMENTS
S. J. Denstaedt acknowledges the peer mentoring of the Multi-Disciplinary Intensive Care Research Workgroup (MICReW) and Dr. Hallie Prescott at the University of Michigan for their thoughtful discussions and contributions to this work.
REFERENCES
- 1.Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K. Assessment of global incidence and mortality of hospital-treated sepsis—current estimates and limitations. Am J Respir Crit Care Med 193: 1–61, 2015. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 2.Prescott HC, Langa KM, Iwashyna TJ. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA 313: 1055–1057, 2015. doi: 10.1001/jama.2015.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prescott HC, Dickson RP, Rogers MAM, Langa KM, Iwashyna TJ. Hospitalization type and subsequent severe sepsis. Am J Respir Crit Care Med 192: 581–588, 2015. doi: 10.1164/rccm.201503-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ. Late mortality after sepsis: propensity matched cohort study. BMJ 353: i2375, 2016. doi: 10.1136/bmj.i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shankar-Hari M, Saha R, Wilson J, Prescott HC, Harrison D, Rowan K, Rubenfeld GD, Adhikari NKJ. Rate and risk factors for rehospitalisation in sepsis survivors: systematic review and meta-analysis. Intensive Care Med 46: 619–636, 2020. doi: 10.1007/s00134-019-05908-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shankar-Hari M, Ambler M, Mahalingasivam V, Jones A, Rowan K, Rubenfeld GD. Evidence for a causal link between sepsis and long-term mortality: a systematic review of epidemiologic studies. Crit Care 20: 101, 2016. doi: 10.1186/s13054-016-1276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. J Am Med Assoc 319: 62–75, 2018. doi: 10.1001/jama.2017.17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Der Poll T, Van De Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol 17: 407–420, 2017. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 9.Rubio I, Osuchowski MF, Shankar-Hari M, Skirecki T, Winkler MS, Lachmann G, Rosée PL, Monneret G, Venet F, Bauer M, Brunkhorst FM, Kox M, Cavaillon J-M, Uhle F, Weigand MA, Flohé SB, Wiersinga J, Martin-Fernandez M, Almansa R, Martin-Loeches I, Torres A. Current gaps in sepsis immunology: new opportunities for translational research. Lancet Infect Dis 19: e422–e436, 2019. doi: 10.1016/s1473-3099(19)30567-5. [DOI] [PubMed] [Google Scholar]
- 10.Prescott HC, Iwashyna TJ, Blackwood B, Calandra T, Chlan LL, Choong K, Connolly B, Dark P, Ferrucci L, Finfer S, Girard TD, Hodgson C, Hopkins RO, Hough CL, Jackson JC, Machado FR, Marshall JC, Misak C, Needham DM, Panigrahi P, Reinhart K, Yende S, Zafonte R, Rowan KM, Angus DC. Understanding and enhancing sepsis survivorship. Priorities for research and practice. Am J Respir Crit Care Med 200: 972–981, 2019. doi: 10.1164/rccm.201812-2383CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent J-L, Angus DC. The third international consensus definitions for sepsis and septic shock (sepsis-3). J Am Med Assoc 315: 801–810, 2016. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Divangahi M, Aaby P, Abdul Khader S, Barreiro LB, Bekkering S, Chavakis T, et al. Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat Immunol 22: 2–6, 2021. doi: 10.1038/s41590-020-00845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol 17: 407–420, 2017. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 14.Denstaedt SJ, Spencer-Segal JL, Newstead M, Laborc K, Zeng X, Standiford TJ, Singer BH. Persistent neuroinflammation and brain specific immune priming in a novel survival model of murine pneumosepsis. Shock 54: 78–86, 2020. doi: 10.1097/shk.0000000000001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denstaedt SJ, Singer BH, Standiford TJ. Sepsis and nosocomial infection: patient characteristics, mechanisms, and modulation. Front Immunol 9: 2446, 2018. doi: 10.3389/fimmu.2018.02446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muenzer JT, Davis CG, Chang K, Schmidt RE, Dunne WM, Coopersmith CM, Hotchkiss RS. Characterization and modulation of the immunosuppressive phase of sepsis. Infect Immun 78: 1582–1592, 2010. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 348: 138–150, 2003. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 18.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci USA 96: 14541–14546, 1999. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roquilly A, Jacqueline C, Davieau M, Mollé A, Sadek A, Fourgeux C, Rooze P, Broquet A, Misme-Aucouturier B, Chaumette T, Vourc’h M, Cinotti R, Marec N, Gauttier V, McWilliam HEG, Altare F, Poschmann J, Villadangos JA, Asehnoune K. Alveolar macrophages are epigenetically altered after inflammation, leading to long-term lung immunoparalysis. Nat Immunol 21: 636–648, 2020. doi: 10.1038/s41590-020-0673-x. [DOI] [PubMed] [Google Scholar]
- 20.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent J-L. Sepsis and septic shock. Nat Rev Dis Primers 2: 16045, 2016. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yende S, Kellum JA, Talisa VB, Peck Palmer OM, Chang C-CH, Filbin MR, Shapiro NI, Hou PC, Venkat A, LoVecchio F, Hawkins K, Crouser ED, Newman AB, Angus DC. Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open 2: e198686, 2019. doi: 10.1001/jamanetworkopen.2019.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shalova IN, Lim JY, Chittezhath M, Zinkernagel AS, Beasley F, Hernández-Jiménez E, Toledano V, Cubillos-Zapata C, Rapisarda A, Chen J, Duan K, Yang H, Poidinger M, Melillo G, Nizet V, Arnalich F, López-Collazo E, Biswas SK. Human monocytes undergo functional re-programming during sepsis mediated by hypoxia-inducible factor-1α. Immunity 42: 484–498, 2015. doi: 10.1016/j.immuni.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Riché F, Chousterman BG, Valleur P, Mebazaa A, Launay JM, Gayat E. Protracted immune disorders at one year after ICU discharge in patients with septic shock. Crit Care 22: 42, 2018. doi: 10.1186/s13054-017-1934-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borken F, Markwart R, Requardt RP, Schubert K, Spacek M, Verner M, Rückriem S, Scherag A, Oehmichen F, Brunkhorst FM, Rubio I. Chronic critical illness from sepsis is associated with an enhanced TCR response. J Immunol 198: 4781–4791, 2017. doi: 10.4049/jimmunol.1700142. [DOI] [PubMed] [Google Scholar]
- 25.Zorio V, Venet F, Delwarde B, Floccard B, Marcotte G, Textoris J, Monneret G, Rimmele T. Assessment of sepsis-induced immunosuppression at ICU discharge and 6 months after ICU discharge. Ann Intensive Care 7: 80, 2017. doi: 10.1186/s13613-017-0304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fensterheim BA, Guo Y, Sherwood ER, Bohannon JK. The cytokine response to lipopolysaccharide does not predict the host response to infection. J Immunol 198: 3264–3273, 2017. doi: 10.4049/jimmunol.1602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denstaedt SJ, Spencer-Segal JL, Newstead MW, Laborc K, Zhao AP, Hjelmaas A, Zeng X, Akil H, Standiford TJ, Singer BH. S100A8/A9 drives neuroinflammatory priming and protects against anxiety-like behavior after sepsis. J Immunol 200: 3188–3200, 2018. doi: 10.4049/jimmunol.1700834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valdés-Ferrer SI, Rosas-Ballina M, Olofsson PS, Lu B, Dancho ME, Ochani M, Li JH, Scheinerman JA, Katz DA, Levine YA, Hudson LK, Yang H, Pavlov VA, Roth J, Blanc L, Antoine DJ, Chavan SS, Andersson U, Diamond B, Tracey KJ. HMGB1 mediates splenomegaly and expansion of splenic CD11b+ Ly-6C(high) inflammatory monocytes in murine sepsis survivors. J Intern Med 274: 381–390, 2013[Erratum inJ Intern Med41: 266, 2014]. doi: 10.1111/joim.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bomans K, Schenz J, Sztwiertnia I, Schaack D, Weigand MA, Uhle F. Sepsis induces a long-lasting state of trained immunity in bone marrow monocytes. Front Immunol 9: 2685, 2018. doi: 10.3389/fimmu.2018.02685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baudesson de Chanville C, Chousterman BG, Hamon P, Laviron M, Guillou N, Loyher PL, Meghraoui-Kheddar A, Barthelemy S, Deterre P, Boissonnas A, Combadière C. Sepsis triggers a late expansion of functionally impaired tissue-vascular inflammatory monocytes during clinical recovery. Front Immunol 11: 675, 2020. doi: 10.3389/fimmu.2020.00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paine R, Standiford TJ, Dechert RE, Moss M, Martin GS, Rosenberg AL, Thannickal VJ, Burnham EL, Brown MB, Hyzy RC. A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury. Crit Care Med 40: 90–97, 2012. doi: 10.1097/CCM.0b013e31822d7bf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS; ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. J Am Med Assoc 307: 2526–2533, 2012. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 33.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, Jernigan JA, Martin GS, Septimus E, Warren DK, Karcz A, Chan C, Menchaca JT, Wang R, Gruber S, Klompas M; the CDC Prevention Epicenter Program. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. J Am Med Assoc 318: 1241–1249, 2017. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer BH, Dickson RP, Denstaedt SJ, Newstead MW, Kim K, Falkowski NR, Erb-Downward JR, Schmidt TM, Huffnagle GB, Standiford TJ. Bacterial dissemination to the brain in sepsis. Am J Respir Crit Care Med 197: 747–756, 2018. doi: 10.1164/rccm.201708-1559OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickson RP, Singer BH, Newstead MW, Falkowski NR, Erb-Downward JR, Standiford TJ, Huffnagle GB. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol 1: 16113, 2016. doi: 10.1038/nmicrobiol.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singer BH, Newstead MW, Zeng X, Cooke CL, Thompson RC, Singer K, Ghantasala R, Parent JM, Murphy GG, Iwashyna TJ, Standiford TJ. Cecal ligation and puncture results in long-term central nervous system myeloid inflammation. PLoS One 11: e0149136–21, 2016. doi: 10.1371/journal.pone.0149136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, Standiford TJ. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest 116: 2532–2542, 2006. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai WC, Strieter RM, Mehrad B, Newstead MW, Zeng X, Standiford TJ. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia (Online). http://iai.asm.org/[2021 Feb 25] [DOI] [PMC free article] [PubMed]
- 39.Yu Y-RA, O'Koren EG, Hotten DF, Kan MJ, Kopin D, Nelson ER, Que L, Gunn MD. A Protocol for the comprehensive flow cytometric analysis of immune cells in normal and inflamed murine non-lymphoid tissues. PLoS One 11: e0150606, 2016. doi: 10.1371/journal.pone.0150606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballinger MN, Newstead MW, Zeng X, Bhan U, Horowitz JC, Moore BB, Pinsky DJ, Flavell RA, Standiford TJ, Theodore J. TLR signaling prevents hyperoxia-induced lung injury by protecting the alveolar epithelium from oxidant-mediated death. J Immunol 189: 356–364, 2012. doi: 10.4049/jimmunol.1103124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao Y, Smyth GK, Shi W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res 47: e47, 2019. doi: 10.1093/nar/gkz114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9: 559, 2008. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xijin Ge S, Jung D, Yao R. ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics 6: 2628–2629, 2020. doi: 10.1093/bioinformatics/btz931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walley KR, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun 64: 4733–4738, 1996. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shankar Hari M, Summers C. Major surgery and the immune system: from pathophysiology to treatment. Curr Opin Crit Care 24: 588–593, 2018. doi: 10.1097/MCC.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 48.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med 173: 1008–1015, 2006. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chignard M, Balloy V. Neutrophil recruitment and increased permeability during acute lung injury induced by lipopolysaccharide. Am J Physiol Lung Cell Mol Physiol 279: L1083–L1090, 2000. doi: 10.1152/ajplung.2000.279.6.l1083. [DOI] [PubMed] [Google Scholar]
- 50.Cross M, Matthay MA. Biomarkers in acute lung injury: insights into the pathogenesis of acute lung injury. Crit Care Clin 27: 355–377, 2011. doi: 10.1016/j.ccc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patin EC, Orr SJ, Schaible UE. Macrophage inducible C-type lectin as a multifunctional player in immunity. Front Immunol 8: 861, 2017. doi: 10.3389/fimmu.2017.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding L, Hayes MM, Photenhauer A, Eaton KA, Li Q, Ocadiz-Ruiz R, Merchant JL. Schlafen 4-expressing myeloid-derived suppressor cells are induced during murine gastric metaplasia. J Clin Invest 126: 2867–2880, 2016. doi: 10.1172/JCI82529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Zuylen WJ, Garceau V, Idris A, Schroder K, Irvine KM, Lattin JE, Ovchinnikov DA, Perkins AC, Cook AD, Hamilton JA, Hertzog PJ, Stacey KJ, Kellie S, Hume DA, Sweet MJ. Macrophage activation and differentiation signals regulate Schlafen-4 gene expression: evidence for Schlafen-4 as a modulator of myelopoiesis. PLoS One 6: e15723, 2011. doi: 10.1371/journal.pone.0015723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nedeva C, Menassa J, Duan M, Liu C, Doerflinger M, Kueh AJ, Herold MJ, Fonseka P, Phan TK, Faou P, Rajapaksha H, Chen W, Hulett MD, Puthalakath H. TREML4 receptor regulates inflammation and innate immune cell death during polymicrobial sepsis. Nat Immunol 21: 1585–1596, 2020. doi: 10.1038/s41590-020-0789-z. [DOI] [PubMed] [Google Scholar]
- 55.Ushach I, Arrevillaga-Boni G, Heller GN, Pone E, Hernandez-Ruiz M, Catalan-Dibene J, Hevezi P, Zlotnik A. Meteorin-like/meteorin-β is a novel immunoregulatory cytokine associated with inflammation. J Immunol 201: 3669–3676, 2018. doi: 10.4049/jimmunol.1800435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ushach I, Burkhardt AM, Martinez C, Hevezi PA, Gerber PA, Buhren BA, Schrumpf H, Valle-Rios R, Vazquez MI, Homey B, Zlotnik A. METEORIN-LIKE is a cytokine associated with barrier tissues and alternatively activated macrophages. Clin Immunol 156: 119–127, 2015. doi: 10.1016/j.clim.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saether PC, Westgaard IH, Flornes LM, Hoelsbrekken SE, Ryan JC, Fossum S, Dissen E. Molecular cloning of KLRI1 and KLRI2, a novel pair of lectin-like natural killer-cell receptors with opposing signalling motifs. Immunogenetics 56: 833–839, 2005. doi: 10.1007/s00251-004-0759-x. [DOI] [PubMed] [Google Scholar]
- 58.Zhao B, Lu R, Chen J, Xie M, Zhao X, Kong L. S100A9 blockade prevents lipopolysaccharide-induced lung injury via suppressing the NLRP3 pathway. Respir Res 22: 45, 2021. doi: 10.1186/s12931-021-01641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chakraborty D, Zenker S, Rossaint J, Hölscher A, Pohlen M, Zarbock A, Roth J, Vogl T. Alarmin S100A8 activates alveolar epithelial cells in the context of acute lung injury in a TLR4-dependent manner. Front Immunol 8: 1493, 2017. doi: 10.3389/fimmu.2017.01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yende S, D'Angelo G, Kellum JA, Weissfeld L, Fine J, Welch RD, Kong L, Carter M, Angus DC. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med 177: 1242–1247, 2008. doi: 10.1164/rccm.200712-1777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kratofil RM, Kubes P, Deniset JF. Monocyte conversion during inflammation and injury. Arterioscler Thromb Vasc Biol 37: 35–42, 2017. doi: 10.1161/ATVBAHA.116.308198. [DOI] [PubMed] [Google Scholar]
- 62.Guilliams M, Mildner A, Yona S. Developmental and functional heterogeneity of monocytes. Immunity 49: 595–613, 2018. doi: 10.1016/j.immuni.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Xing Z, Han J, Hao X, Wang J, Jiang C, Hao Y, Wang H, Wu X, Shen L, Dong X, Li T, Li G, Zhang J, Hou X, Zeng H. Immature monocytes contribute to cardiopulmonary bypass-induced acute lung injury by generating inflammatory descendants. Thorax 72: 245–255, 2017. doi: 10.1136/thoraxjnl-2015-208023. [DOI] [PubMed] [Google Scholar]
- 64.Herold S, von Wulffen W, Steinmueller M, Pleschka S, Kuziel WA, Mack M, Srivastava M, Seeger W, Maus UA, Lohmeyer J. Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. J Immunol 177: 1817–1824, 2006. doi: 10.4049/jimmunol.177.3.1817. [DOI] [PubMed] [Google Scholar]
- 65.Dhaliwal K, Scholefield E, Ferenbach D, Gibbons M, Duffin R, Dorward DA, Morris AC, Humphries D, MacKinnon A, Wilkinson TS, Wallace WAH, van Rooijen N, Mack M, Rossi AG, Davidson DJ, Hirani N, Hughes J, Haslett C, Simpson AJ. Monocytes control second-phase neutrophil emigration in established lipopolysaccharide-induced murine lung injury. Am J Respir Crit Care Med 186: 514–524, 2012. doi: 10.1164/rccm.201112-2132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O'Dea KP, Young AJ, Yamamoto H, Robotham JL, Brennan FM, Takata M. Lung-marginated monocytes modulate pulmonary microvascular injury during early endotoxemia. Am J Respir Crit Care Med 172: 1119–1127, 2005. doi: 10.1164/rccm.200504-605OC. [DOI] [PubMed] [Google Scholar]
- 67.O'Dea KP, Dokpesi JO, Tatham KC, Wilson MR, Takata M. Regulation of monocyte subset proinflammatory responses within the lung microvasculature by the p38 MAPK/MK2 pathway. Am J Physiol Cell Mol Physiol 301: L812–L821, 2011. doi: 10.1152/ajplung.00092.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chong SZ, Evrard M, Devi S, Chen J, Lim JY, See P, et al. CXCR4 identifies transitional bone marrow premonocytes that replenish the mature monocyte pool for peripheral responses. J Exp Med 213: 2293–2314, 2016. doi: 10.1084/jem.20160800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hamon P, Loyher P-L, Chanville C. D, Licata F, Combadière C, Boissonnas A. CX3CR1-dependent endothelial margination modulates Ly6Chigh monocyte systemic deployment upon inflammation in mice. Blood 129: 1296–1307, 2017. doi: 10.1182/blood-2016-08-732164. [DOI] [PubMed] [Google Scholar]
- 70.O'Dea KP, Wilson MR, Dokpesi JO, Wakabayashi K, Tatton L, van Rooijen N, Takata M. Mobilization and margination of bone marrow Gr-1high monocytes during subclinical endotoxemia predisposes the lungs toward acute injury. J Immunol 182: 1155–1166, 2009. doi: 10.4049/jimmunol.182.2.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodero MP, Poupel L, Loyher P-L, Hamon P, Licata F, Pessel C, Hume DA, Combadière C, Boissonnas A. Immune surveillance of the lung by migrating tissue monocytes. eLife 4: e07847, 2015. doi: 10.7554/eLife.07847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andonegui G, Zelinski EL, Schubert CL, Knight D, Craig LA, Winston BW, Spanswick SC, Petri B, Jenne CN, Sutherland JC, Nguyen R, Jayawardena N, Kelly MM, Doig CJ, Sutherland RJ, Kubes P. Targeting inflammatory monocytes in sepsis-associated encephalopathy and long-term cognitive impairment. JCI Insight 3: e99364, 2018. doi: 10.1172/jci.insight.99364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Denstaedt SJ, Spencer-Segal JL, Newstead M, Laborc K, Zeng X, Standiford TJ, Singer BH. Persistent neuroinflammation and brain-specific immune priming in a novel survival model of murine pneumosepsis. Shock 54: 78–86, 2020. doi: 10.1097/SHK.0000000000001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, Jacobs L, Jansen T, Kullberg BJ, Wijmenga C, Joosten LAB, Xavier RJ, Van Der Meer JWM, Stunnenberg HG, Netea MG. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12: 223–232, 2012. doi: 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ifrim DC, Quintin J, Joosten LAB, Jacobs C, Jansen T, Jacobs L, Gow NAR, Williams DL, Van Der Meer JWM, Netea MG. Trained immunity or tolerance: Opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clin Vaccine Immunol 21: 534–545, 2014. doi: 10.1128/CVI.00688-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dominguez-Andres J, Netea MG. Long-term reprogramming of the innate immune system. J Leukoc Biol 105: 329–338, 2018. doi: 10.1002/JLB.MR0318-104R. [DOI] [PubMed] [Google Scholar]
- 77.Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JHA, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJW, van der Veer BMJW, van der Meer BMJW, Deen PMT, Logie C, O'Neill LA, Willems P, van de Veerdonk FL, van der Meer JWM, Ng A, Joosten LAB, Wijmenga C, Stunnenberg HG, Xavier RJ, Netea MG. mTOR- and HIF-1 -mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345: 1250684–1250684, 2014[Erratum inScience346: aaa1503, 2014]. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Novakovic B, Habibi E, Wang SY, Arts RJW, Davar R, Megchelenbrink W, Kim B, Kuznetsova T, Kox M, Zwaag J, Matarese F, van Heeringen SJ, Janssen-Megens EM, Sharifi N, Wang C, Keramati F, Schoonenberg V, Flicek P, Clarke L, Pickkers P, Heath S, Gut I, Netea MG, Martens JHA, Logie C, Stunnenberg HG. β-glucan reverses the epigenetic state of LPS-induced immunological tolerance. Cell 167: 1354–1368.e14, 2016. doi: 10.1016/j.cell.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arts RJW, Novakovic B, ter Horst R, Carvalho A, Bekkering S, Lachmandas E, Rodrigues F, Silvestre R, Cheng SC, Wang SY, Habibi E, Gonçalves LG, Mesquita I, Cunha C, van Laarhoven A, van de Veerdonk FL, Williams DL, van der Meer JWM, Logie C, O’Neill LA, Dinarello CA, Riksen NP, van Crevel R, Clish C, Notebaart RA, Joosten LAB, Stunnenberg HG, Xavier RJ, Netea MG. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab 24: 807–819, 2016. doi: 10.1016/j.cmet.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Divangahi M, Aaby P, Khader SA, Barreiro LB, Bekkering S, Chavakis T, et al. Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat Immunol 22: 2–6, 2021. doi: 10.1038/s41590-020-00845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pruenster M, Vogl T, Roth J, Sperandio M. S100A8/A9: from basic science to clinical application. Pharmacol Ther 167: 120–131, 2016. doi: 10.1016/j.pharmthera.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 82.Dubois C, Marcé D, Faivre V, Lukaszewicz AC, Junot C, Fenaille F, Simon S, Becher F, Morel N, Payen D. High plasma level of S100A8/S100A9 and S100A12 at admission indicates a higher risk of death in septic shock patients. Sci Rep 9: 15660, 2019. doi: 10.1038/s41598-019-52184-8.[31666644] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuipers MT, Vogl T, Aslami H, Jongsma G, van den Berg E, Vlaar APJ, Roelofs JJTH, Juffermans NP, Schultz MJ, van der Poll T, Roth J, Wieland CW. High levels of S100A8/A9 proteins aggravate ventilator-induced lung injury via TLR4 signaling. PLoS One 8: e68694, 2013. doi: 10.1371/journal.pone.0068694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raquil M-A, Anceriz N, Rouleau P, Tessier PA. Blockade of antimicrobial proteins S100A8 and S100A9 inhibits phagocyte migration to the alveoli in streptococcal pneumonia. J Immunol 180: 3366–3374, 2008. doi: 10.4049/jimmunol.180.5.3366. [DOI] [PubMed] [Google Scholar]
- 85.Pruenster M, Kurz ARM, Chung K-J, Cao-Ehlker X, Bieber S, Nussbaum CF, Bierschenk S, Eggersmann TK, Rohwedder I, Heinig K, Immler R, Moser M, Koedel U, Gran S, McEver RP, Vestweber D, Verschoor A, Leanderson T, Chavakis T, Roth J, Vogl T, Sperandio M. Extracellular MRP8/14 is a regulator of β2 integrin-dependent neutrophil slow rolling and adhesion. Nat Commun 6: 6915, 2015. doi: 10.1038/ncomms7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bouma G, Lam-Tse WK, Wierenga-Wolf AF, Drexhage HA, Versnel MA. Increased serum levels of MRP-8/14 in type 1 diabetes induce an increased expression of CD11b and an enhanced adhesion of circulating monocytes to fibronectin. Diabetes 53: 1979–1986, 2004. doi: 10.2337/diabetes.53.8.1979. [DOI] [PubMed] [Google Scholar]
- 87.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen M A D, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 13: 1042–1049, 2007. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 88.Vogl T, Pap T, Roth J, Vogl T, Stratis A, Wixler V, Völler T, Thurainayagam S, Jorch SK, Zenker S, Dreiling A, Chakraborty D, Fröhling M, Paruzel P, Wehmeyer C, Hermann S, Papantonopoulou O, Geyer C, Loser K, Schäfers M, Ludwig S, Stoll M. Autoinhibitory regulation of S100A8/S100A9 alarmin activity locally restricts sterile inflammation. J Clin Invest 128: 1852–1866, 2018. doi: 10.1172/JCI89867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo Y, Patil NK, Luan L, Bohannon JK, Sherwood ER. The biology of natural killer cells during sepsis. Immunology 153: 190–202, 2018. doi: 10.1111/imm.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoegl S, Ehrentraut H, Brodsky KS, Victorino F, Golden-Mason L, Eltzschig HK, McNamee EN. NK cells regulate CXCR2 + neutrophil recruitment during acute lung injury. J Leukoc Biol 101: 471–480, 2017. doi: 10.1189/jlb.3A0516-227R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rasid O, Chevalier C, Camarasa TM, Fitting C, Cavaillon JM, Hamon MA. H3K4me1 supports memory-like NK cells induced by systemic inflammation. Cell Rep 29: 3933–3945.e3, 2019. doi: 10.1016/j.celrep.2019.11.043. [DOI] [PubMed] [Google Scholar]
- 92.Rasid O, Chevalier C, Camarasa TMN, Fitting C, Cavaillon JM, Hamon MA. H3K4me1 supports memory-like NK cells induced by systemic inflammation. Cell Rep 29: 3933–3945.e3, 2019. doi: 10.1016/j.celrep.2019.11.043. [DOI] [PubMed] [Google Scholar]
- 93.Grailer JJ, Canning BA, Kalbitz M, Haggadone MD, Dhond RM, Andjelkovic AV, Zetoune FS, Ward PA. Critical role for the NLRP3 inflammasome during acute lung injury. J Immunol 192: 5974–5983, 2014. doi: 10.4049/jimmunol.1400368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mcvey MJ, Steinberg BE, Goldenberg NM. Inflammasome activation in acute lung injury. Am J Physiol Lung Cell Mol Physiol 320: L165–L178, 2021. doi: 10.1152/ajplung.00303.2020. [DOI] [PubMed] [Google Scholar]
- 95.Cavaillon J-M, Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res 12: 151–170, 2006. doi: 10.1179/096805106X102246. [DOI] [PubMed] [Google Scholar]
- 96.Roquilly A, McWilliam HEG, Jacqueline C, Tian Z, Cinotti R, Rimbert M, Wakim L, Caminschi I, Lahoud MH, Belz GT, Kallies A, Mintern JD, Asehnoune K, Villadangos JA. Local modulation of antigen-presenting cell development after resolution of pneumonia induces long-term susceptibility to secondary infections. Immunity 47: 135–147.e5, 2017. doi: 10.1016/j.immuni.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 97.Diodato MD, Knöferl MW, Schwacha MG, Bland KI, Chaudry IH. Gender differences in the inflammatory response and survival following haemorrhage and subsequent sepsis. Cytokine 14: 162–169, 2001. doi: 10.1006/cyto.2001.0861. [DOI] [PubMed] [Google Scholar]
- 98.Schröder J, Kahlke V, Staubach KH, Zabel P, Stüber F. Gender differences in human sepsis. Arch Surg 133: 1200–1205, 1998. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 99.Turnbull IR, Wlzorek JJ, Osborne D, Hotchkiss RS, Coopersmith CM, Buchman TG. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock 19: 310–313, 2003. doi: 10.1097/00024382-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 100.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med 34: 15–21, 2006. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]