Abstract
Ethanol consumption represents a significant public health problem, and excessive ethanol intake is a risk factor for cardiovascular disease (CVD), one of the leading causes of death and disability worldwide. The mechanisms underlying the effects of ethanol on the cardiovascular system are complex and not fully comprehended. The gut microbiota and their metabolites are indispensable symbionts essential for health and homeostasis and therefore, have emerged as potential contributors to ethanol-induced cardiovascular system dysfunction. By mechanisms that are not completely understood, the gut microbiota modulates the immune system and activates several signaling pathways that stimulate inflammatory responses, which in turn, contribute to the development and progression of CVD. This review summarizes preclinical and clinical evidence on the effects of ethanol in the gut microbiota and discusses the mechanisms by which ethanol-induced gut dysbiosis leads to the activation of the immune system and cardiovascular dysfunction. The cross talk between ethanol consumption and the gut microbiota and its implications are detailed. In summary, an imbalance in the symbiotic relationship between the host and the commensal microbiota in a holobiont, as seen with ethanol consumption, may contribute to CVD. Therefore, manipulating the gut microbiota, by using antibiotics, probiotics, prebiotics, and fecal microbiota transplantation might prove a valuable opportunity to prevent/mitigate the deleterious effects of ethanol and improve cardiovascular health and risk prevention.
Keywords: cardiovascular system, dysbiosis, ethanol, gut microbiota, immune system
STATING THE PROBLEM
Ethanol and the Cardiovascular System
Cardiovascular disease (CVD) is a group of disorders that affects the heart and blood vessels (1) and is among the leading causes of death and disability worldwide (1, 2). In 2016, 17.9 million deaths globally were attributed to CVD (3). Age, sex, genetic factors, unhealthy diet, sedentary lifestyle, metabolic diseases, tobacco use, and excessive consumption of ethyl alcohol (ethanol) are essential factors contributing to CVD development (4–6).
Excessive ethanol consumption itself is responsible for more than 3 million deaths every year or ∼5% of all deaths worldwide (3). In the United States, excessive alcohol intake is the third leading cause of premature death, behind only smoking and obesity (7). Although excessive ethanol consumption is a significant public health problem and contributes to elevated mortality, morbidity, and disability (8), the exact amount of ethanol that induces tissue/organ damage or contributes to the development of diseases is unclear. In addition, ethanol has distinctive effects that are modulated by several factors, including sex, age, genetic factors, ethnicity, body mass index, medications, diets, duration of ethanol use, drinking pattern behavior, type of alcoholic beverage consumed (fermented or distilled), and comorbidities (9–11).
The relationship between ethanol consumption and CVD is represented by a J-shaped curve (7, 12–14), indicating that low-to-moderate ethanol intake (1 drink/day for women or up to 2 drinks/day for men) poses a low risk for cardiovascular morbidity and mortality (12, 15). Low doses of ethanol regulate lipid metabolism and fibrinolysis, increase adiponectin and high-density lipoprotein (HDL) levels, decrease blood coagulation rate, modulate platelet activity and thrombogenic factors, and inhibit atheroma formation. Accordingly, low doses of ethanol benefit endothelial function, decrease inflammation, and improve insulin sensitivity and glucose metabolism (7, 12, 14, 16–20). In stark contrast, heavy (>4 drinks/day) and binge drinking (≥5 drinks within a few hours) are linked to adverse outcomes, increasing the risk of death and CVD (3, 7, 21).
CVD is a multifactorial disorder influenced by environmental and genetic factors. Adding to the complexity of CVD, growing evidence supports that the gut microbiota and its metabolites contribute to cardiovascular dysfunction (22–29). In addition, the gut microbiota also contributes to ethanol-induced cellular injury (30–34). However, although it is well established that long-term ethanol consumption is related to CVD (6, 7, 14), very few studies have investigated a causal role for the microbiota on ethanol-induced cardiovascular dysfunction. This review addresses the cross talk between ethanol consumption and gut microbiota diversity and composition and its implications to the cardiovascular system. Mechanisms underlying these interactions, with a focus on the activation of the innate and adaptive immune system, are discussed.
Potential Mechanisms Underlying Cardiovascular Dysfunction Induced by Ethanol
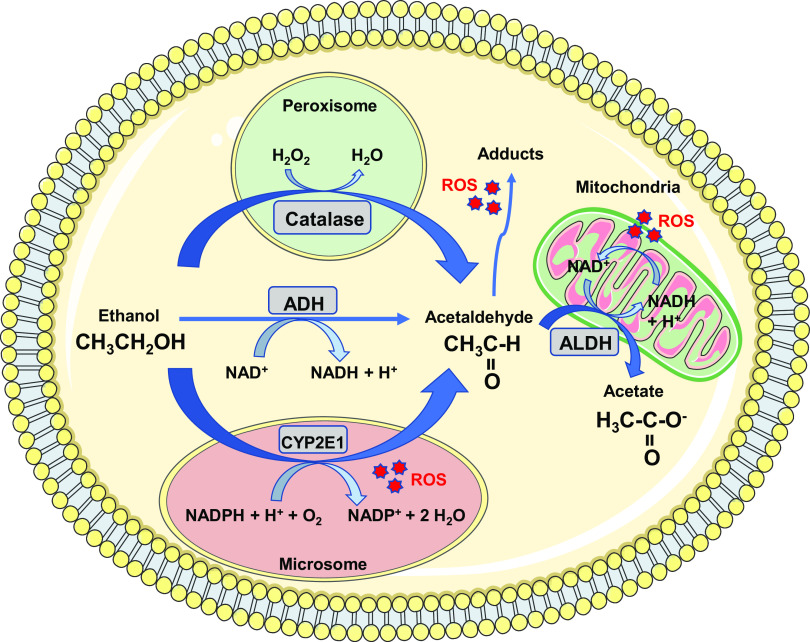
Alcohol (i.e., ethanol) is metabolized by oxidative and nonoxidative pathways. The primary enzymes involved in oxidative metabolism of ethanol include alcohol dehydrogenase (ADH), aldehyde dehydrogenase (ALDH), microsomal ethanol oxidizing system (MEOS) [i.e., cytochrome P450 2E1 (CYP2E1)], and catalase (35), as shown in Fig. 1. Ethanol metabolism increases reactive oxygen species (ROS) generation, changes the redox state (i.e., NADH:NAD+ ratio), and increases acetaldehyde production, a highly reactive and toxic byproduct that generates adducts (i.e., acetaldehyde-DNA or acetaldehyde-protein adducts) (35). Therefore, ethanol itself, as well as ethanol metabolites, produce deleterious effects on cells/tissues.
Figure 1.
Oxidative metabolism of alcohol. The enzymes alcohol dehydrogenase (ADH), cytochrome P450 2E1 (CYP2E1), and catalase convert alcohol (i.e., ethanol) to acetaldehyde, a highly reactive and toxic byproduct. Acetaldehyde is metabolized by aldehyde dehydrogenase (ALDH) in the mitochondria to form acetate and NADH. Increased reactive oxygen species (ROS) generation, NADH:NAD+ ratio and acetaldehyde adducts formation are observed during the metabolism of ethanol, which contributes to oxidative stress and organs/tissues damage. H+, hydrogen proton; H2O, water; H2O2, hydrogen peroxide; NAD+, nicotinamide adenine dinucleotide; NADH, reduced nicotinamide adenine dinucleotide; NADP+, nicotinamide adenine dinucleotide phosphate; NADPH, reduced nicotinamide adenine dinucleotide phosphate; O2, molecular oxygen.
In the cardiovascular system, the harmful effects of ethanol are more closely related to the drinking patterns than to the type of alcoholic drinks (i.e., wine, beer, and liquor) (15) and potential toxic effects of alcoholic beverage consumption are attributed to ethanol and to its metabolites (7, 18). Regular ethanol intake increases blood pressure in a dose-dependent manner (7). In addition, levels of ethanol above 90–120 g/day (∼7–15 standard drinks per day) during a 5- to 15-yr period are related to changes in cardiac structure and function (36). Toxic effects of high doses of ethanol include atherosclerosis, cardiac rhythm disturbances, heart failure, hypertension, atrial fibrillation, myocardial infarction, and hemorrhagic and other nonischemic strokes (3, 7, 14, 17, 18). The effects of ethanol on the cardiovascular system are complex (10) and are related, in part, to dysregulated levels of inflammatory cytokines and increased low-density lipoprotein (LDL) (15). Other potential mechanisms by which ethanol causes cardiovascular changes include increased ROS, reduced antioxidant defense, apoptotic cell death, mitochondrial stress, abnormalities in fatty acid metabolism and transport, and accelerated protein catabolism (10, 36). In the cardiovascular system, specifically, ethanol consumption increases oxidative stress, decreases nitric oxide (NO) bioavailability, stimulates the renin-angiotensin-aldosterone system (RAAS), increases sympathetic nervous system activity, causes insulin resistance, stimulates the hypothalamic-pituitary axis (HPA) with cortisol excess, alters Ca2+-Mg2+ handling, and stimulates the endothelin-1 system (10, 37–42). These processes may individually and synergistically contribute to cardiovascular remodeling and dysfunction, myocardial and vascular oxidative/nitrosative stress, vascular hyperresponsiveness, endothelial dysfunction, and vascular inflammation observed with excessive ethanol consumption (10, 38, 41–43).
Oxidative stress, defined as increased ROS that overwhelms antioxidant defense, is considered one of the primary mechanisms whereby ethanol initiates its adverse cardiovascular effects (36). Ethanol, directly (via stimulating the generation of free radicals) and indirectly (via increasing the cells’ susceptibility to other stressors), induces oxidative stress in many tissues and organs (42), including the gastrointestinal (GI) tract (44–46). Potential mechanisms for ethanol-induced ROS accumulation include activation of the enzymes involved in ethanol metabolism (as shown in Fig. 1), inhibition of antioxidant proteins (i.e., transport proteins responsible for glutathione transport from cytosol into the mitochondria), and antioxidant enzymes (i.e., superoxide dismutase, catalase, and glutathione peroxidase) as well as activation of neurohormonal systems (e.g., increased angiotensin II levels) (42). Other enzymatic systems, including xanthine oxidase, lipoxygenase, cyclooxygenase (COX), enzymes in the mitochondrial respiratory chains, and uncoupled endothelial NO synthase (eNOS), are linked to oxidative stress induced by ethanol (38, 42). Indeed, ethanol consumption induces the expression and activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (41, 47–50), which is the main source of ROS in the vasculature (10). Ethanol also upregulates the inducible isoform of NOS (iNOS) (47, 48, 51, 52), activates mitogen-activated protein kinases (MAPK) (41, 49), and increases proinflammatory cytokine levels [tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6] (47–49, 53, 54). Micro-RNAs (miRNAs) also contribute to chronic ethanol-induced oxidative stress and inflammation (55). Therefore, in addition to direct oxidative damage to macromolecules, including proteins, lipids, carbohydrates, and DNA, ethanol also induces oxidative stress by affecting redox-dependent signaling in several tissues and organs (10, 41), contributing to the development of diseases, including CVD. Despite our current understanding on ethanol-induced cardiovascular dysfunction, treatments are not effective in all alcoholic patients mainly due to patient noncompliance to alcohol abstinence and to the complex effects of ethanol on several tissues and organs. Perceiving the human body as a holobiont, i.e., recognizing the human body interactions with its associated communities of microorganisms, may lead to new therapeutic targets for ethanol-induced diseases.
GUT MICROBIOTA
Impact of the Gut Microbiota on the Immune System
More than 2,000 years ago, Hippocrates suggested that “All disease begins in the gut” (56). The microbiota of the GI tract consists of a wide diversity of species of bacteria, fungi, archaea, and protozoa, which live in a community, maintaining interactions with the host organism (57). Colonization of the GI tract by commensal microorganisms begins before birth (58), and the gut microbiota, as well as its products, increase the number of neutrophils, macrophages, and dendritic cells in neonates and adults (59, 60). In addition to its influence in hematopoiesis, the microbiota participates in the formation and maturation of the fetus’ immune system, helping to differentiate commensal bacteria from pathogenic bacteria (61). Immunological tolerance to antigens from commensal bacteria is mediated, in part, by Toll-like receptors (TLRs), expressed mainly in epithelial cells, macrophages, and dendritic cells (62). These receptors recognize pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), flagellin, peptidoglycan, dsRNA, which are present in pathogens and commensals, and damage-associated molecular patterns (DAMPs), such as heat shock proteins (63). By recognizing products derived from the gut microbiota, TLRs, instead of triggering an inflammatory response, contribute to intestinal homeostasis and increased immune function (64). Polysaccharides derived from the microbiota induce anti-inflammatory genes expression in murine intestinal macrophages (65). In addition, TLRs activation decreases in the first weeks of life, allowing commensal bacteria to steadily establish themselves in the intestine (66).
In addition to TLRs, NOD-like receptors (NLRs) also participate in the maintenance of intestinal homeostasis and modulate the occurrence of diabetes, inflammatory bowel diseases, and colorectal cancer (67, 68). NLRs are expressed in the cytosol, mainly in cells of innate and adaptive immunity, and are subdivided into the subfamilies NLRA, NLRB, NLRC, and NLRP (69). Colonization of germ-free mice with Lactobacillus plantarum or Escherichia coli strain Nissle 1917 increases the expression of NOD2 in the terminal ileum (70). In addition, the activation of NOD2 by commensal bacteria promotes the survival of intestinal stem cells and regeneration of the epithelium (71). On the other hand, translocation of gut microbiota to the pancreatic lymph nodes activates NOD2 and contributes to type 1 diabetes (72). NOD2-deficient mice fed a high-fat diet (HFD) show a reduction in Allobaculum, Lactobacillus, and an increase in the genus Bacteroides in feces compared with HFD-fed wild-type mice. This change in gut microbiota occurs concomitantly with a greater increase in adiposity, worsening insulin resistance, and increased M1 macrophages in adipose tissue and T helper 1 (Th1) lymphocytes in the mesenteric lymph nodes in the absence of NOD2 (73), all characteristics of metainflammation, defined as low-degree chronic inflammation due to immune system overactivation in metabolic tissues (74). The association between type 2 diabetes and metainflammation is a significant risk factor for the development of CVD (75). The administration of broad-spectrum antibiotics in mice results in gut dysbiosis and increased expression of NLRP3 and proinflammatory cytokines, such as IL-1β and IL-18 in the ileum (76). These strongly indicate that intestinal (or extraintestinal) NLRs homeostasis is finely regulated by the gut microbiota.
A balanced gut microbiota not only contributes to the metabolism of nutrients or drugs but also maintains the intestinal mucosal barrier, protects against pathogens, and induces protective immune cells (77). Colonization by commensal Bacteroides fragilis increases the number of regulatory T lymphocytes (Treg) in the large intestine of mice, promoting immunological tolerance (78), by TLR2-dependent mechanisms (79). Short-chain fatty acids (SCFAs), products of the gut microbiota fermentation, mainly butyrate, are known as classic inducers of Treg in the intestine (80). In addition, during intestinal homeostasis, the intestinal bacterium Akkermansia muciniphila induces specific antigenic follicular T lymphocyte (Tfh), showing an immunomodulatory role (81). Interestingly, commensal segmented filamentous bacteria (SFB) induce differentiation of Th17 lymphocytes that express reduced levels of proinflammatory genes in the small intestine compared with pathogen-induced Th17 lymphocytes, which have an inflammatory profile (82).
The interactions between the microbiota and immune cells may impact the development of CVD (75, 83). Endotoxins or metabolites derived from the gut microbiota can reach the systemic circulation and induce metabolic inflammation, especially in individuals with obesity, a risk factor for CVD (84). In this context, the differentiation of macrophages to a more inflammatory profile further aggravates metabolic inflammation (85, 86). Dietary l-carnitine, present in red meat, is converted by the microbiota into trimethylamine-N-oxide (TMAO), an inducer of atherosclerosis and thrombosis (87). In addition, supplementation of mice with TMAO or choline induces a significant increase in the expression of scavenger receptors on the surface of macrophages compared with mice that did not receive TMAO (22). In addition to potentially impacting the incidence of atherosclerosis, an imbalanced microbiota increases blood pressure. In mice, high amounts of salt in the diet changes gut microbiota diversity, mainly by reducing Lactobacillus murinus, which leads to an increase in Th17 lymphocytes number and blood pressure (88). Interestingly, patients with heart failure and a consequent reduction in blood flow in the mesenteric arteries and celiac trunk have high serum levels of anti-LPS immunoglobulin A (IgA), which is correlated with increased bacterial growth in colonic mucosa biopsies, but not in feces (89). The increased intestinal ischemia and edema formation present in most patients with heart failure has already been associated with bacterial translocation and leakage of microbiota products, culminating in increased proinflammatory cytokines in the circulation (90). Given these findings, it is clear that the microbiota-immune system axis impacts the onset and development/progression of CVD, including atherosclerosis, hypertension, and heart failure (29, 83).
Impact of the Gut Microbiota on the Cardiovascular System
Recent studies have suggested a strong association between gut microbiota and CVD (2, 23–26, 28, 29, 91–93). Abnormalities/changes in the gut microbiota diversity and microbial metabolism are implicated in the pathogenesis of metabolic and CVD (2, 23, 25, 28, 29, 94), including hypertension, atherosclerosis, heart failure, chronic kidney disease, obesity, and type 2 diabetes mellitus (95). Compared with healthy controls, hypertensive patients show gut dysbiosis characterized by reduced intestinal microbial richness and diversity (96, 97). In addition, studies on fecal microbiota transplantation demonstrate the impact of gut microbiota in the regulation of blood pressure and the development of hypertension (24, 91, 98, 99). Furthermore, the gut microbiome contributes to sex differences in blood pressure, possibly due to sexual dimorphism in immune system function, as well as to sex differences in the relationship between the microbiome and immune function (100). Sex differences on the gut microbiome are, in part, mediated by interactions with the host genotype and influenced by sex hormones levels (100). In this context, ethanol intake may differently impact the male and female gut microbiome, by differentially affecting male and female physiology and immune system activation, as well as by sex differences in the gut microbiota, which can be exacerbated by ethanol consumption (101).
Hypertensive patients exhibit dysfunction in the intestinal epithelial barrier, which is accompanied by increased systemic LPS levels and augmented proinflammatory Th17 cells, suggesting intestinal inflammation and increased intestinal permeability (94). The intestinal barrier integrity is essential to control gut permeability (102). Gut barrier function is highly efficient due to several mechanisms, including tight junction proteins, thickness and composition of the mucus layer, presence of antimicrobial molecules, intraepithelial lymphocytes, innate and adaptive immune cells, and production of IgA (103). Consistently, translocation of gut microbiota to the mesenteric lymph nodes is linked to intestinal barrier disruption and systemic inflammation (34). In this context, increased permeability of the gut epithelial barrier and an inflammatory state is associated with increased blood pressure (104).
Once in the blood, the gut microbiota and their metabolites modulate the function of organs and tissues that control the circulatory system homeostasis, including kidneys, blood vessels, and heart (105). Moreover, the gut microbiota also stimulates the enteric nervous system by affecting the activity of the brain centers that control the cardiovascular system (105). The gut microbiota activates molecular pathways, e.g., inflammatory cytokines [i.e., IL-17 and the interferon-γ (IFN-γ)] and enzymes (i.e., NAPDH oxidase and iNOS), that lead to systemic inflammation, vascular dysfunction, and hypertension-associated end-organ damage (23). Along with inflammation, changes in levels of microbial metabolites are also linked to gut dysbiosis that affects CVD pathogenesis (106).
To evaluate the direct effects of microbiota on blood pressure during homeostasis, we conventionalized germ-free (GF) rats with specific pathogen-free rats for ten days (107). The absence of microbiota resulted in relative hypotension in GF rats, compared with their conventionalized counterparts, suggesting an obligatory role of microbiota in blood pressure homeostasis. The decrease in blood pressure in GF rats is associated with a reduction in vascular contraction. Both blood pressure and vascular contractility are restored by introducing microbiota to GF rats, indicating that microbiota can impact blood pressure through a vascular-dependent mechanism. These data suggest that the vascular system senses the presence (or lack) of microbiota to maintain vascular tone. Overall, these results constitute a fundamental discovery of the essential nature of microbiota in blood pressure regulation (107). Corroborating evidence that microbiota is critical for vascular tone and blood pressure control, male and female GF mice also present a decrease in the contraction of resistance arteries (26). However, these changes are more pronounced in GF males than in GF female mice. Furthermore, resistance arteries from male GF mice exhibit increased stiffness and inward hypotrophic remodeling, a characteristic of chronic reduction in blood flow. Conversely, arteries from GF female mice exhibit outward hypertrophic remodeling, characteristic of aging. These observations indicate that commensal microbiota is central to maintain proper vascular function and structure homeostasis, in both males and females. Therefore, gut microbiota represents a potential source for novel therapeutic interventions (103).
Modulation of Gut Microbiota by Ethanol
Following consumption, ethanol is absorbed into the circulation predominantly in the small intestine and stomach. Accumulating evidence suggests direct toxicity of ethanol in the GI tract before it reaches the liver to be metabolized. Specifically, ethanol disrupts the intestinal protective mucus layer (108), decreases gastric acid secretion, increases bacterial growth, especially Gram-negative bacteria, increases intestinal permeability, and negatively impacts the immune response (109–111). Furthermore, ethanol induces perturbations in the gut microbiota, alters bile acids composition and circulation, causes nutrient malabsorption, and affects the function of mucosal immune cells (33, 112). Yan et al. (113) observed that bacterial translocation to the systemic circulation induced by long-term ethanol consumption occurs before the intestinal bacterial overgrowth. Therefore, increased intestinal permeability seems to be caused by ethanol itself, or ethanol oxidation into acetaldehyde, but not by quantitative dysbiosis induced by ethanol (113). In addition, reduced GI motility has been proposed as a key mechanism for fecal stasis and lumen bacterial proliferation associated with ethanol consumption (34). However, innate and adaptive immune responses also seem to contribute to changes in gut microbiota (34).
Ethanol also disrupts the circadian rhythm (33, 45, 114, 115), which may contribute to increased bacterial translocation from gut (33). In addition, ethanol-associated increased intestinal permeability may be related to high levels of LPS and other bacterial products (also termed PAMPs) that increase proinflammatory mediators, including ROS, proinflammatory cytokines (i.e., TNF-α, IL-6, IL-8, and IL-1β), chemokines (i.e., CXCL-1/KC), and leukotrienes, which, in turn, contribute to intestinal epithelial cell dysfunction and to changes in occlusion junctions (110, 116, 117), leading to leaky intestinal barrier (33, 118–120). Corroborating these findings, reduced expression and colocalization of tight junction proteins [i.e., zonula occludens-1 (ZO-1) and occludin (Ocln)] are observed in the ileum and proximal colon of animals following chronic, short-term or acute ethanol exposure (121). In addition, acetaldehyde also disrupts the epithelial barrier by interfering with the tight junctions, leading to increased permeability and endotoxemia (122). In this context, increased gut permeability and decreased tight junction proteins (i.e., Ocln, claudin 4, tight junction protein 1) lead to gut dysbiosis and hypertension in experimental models (104).
Several factors control the bacterial load of the gut, including host antimicrobial peptides and proteins that are secreted by epithelial and Paneth cells, including defensins, lysozyme, bactericidal lectin regenerating islet-derived 3 (Reg3), and cathelicidins (113). In this context, ethanol feeding downregulates gene and protein expression of Reg3-β (Reg3b) and Reg3-γ (Reg3g), which may contribute to intestinal bacterial overgrowth (113). Finally, chronic ethanol intake induces alterations in host gene expression and bacterial communities leading to disruptions of metabolism and protein trafficking (123).
Chronic ethanol consumption changes gut microbiota composition/diversity in experimental models (111, 113, 117) and humans (124–126). The gut dysbiosis induced by excessive ethanol consumption is characterized by increases in pathogenic Gram-negative bacteria (124). Differences in the gut microbiota at the level of specific genera are also observed in rats treated for short periods with moderate ethanol levels (122). In this study, the average species richness and phylogenetic diversity were both significantly lower in ethanol-treated rats compared with controls (122), suggesting that the rat microbiome is negatively affected by short-term and moderate levels of ethanol intake (122). Acute exposure to ethanol also causes gut dysbiosis, resulting in reduced intestinal Lactobacilli and increased plasma levels of LPS (127). These data suggest that different drinking patterns and duration of ethanol consumption impact the gut microbiota.
The association between α-diversity, which indicates the number of species between samples (100) and is correlated with health (122), and alcohol consumption was evaluated in experimental models (122, 128) and humans (122, 129). Decreased α-diversity has been described in experimental models of long-term (122) and short-term and low-dose (128) ethanol consumption, as well as in patients with alcohol-associated liver disease (129). In stark contrast, higher α-diversity was observed in the human gut of regular drinkers (individuals who reported drinking 3–5 times/wk) and daily drinkers (individuals who reported daily ethanol consumption) compared with nondrinkers (individuals who reported never drinking) (122). Interestingly, almost half (48%) of study participants reported consuming red wine (122). Therefore, favorable changes observed in the gut microbiota could be related to prebiotic effects associated with the red wine polyphenols, mainly resveratrol (130, 131).
Distinctive changes in the gut microbiota composition occur depending on the type of beverage consumed (i.e., gin, red wine, or nonalcoholic red wine) (130). Queipo-Ortuño et al. (130) observed an increase in the Bacteroides and Clostridium abundances in the gut microbiota of volunteers after ethanol intake (gin treatment). However, red wine polyphenols intake inhibited nonbeneficial bacteria from the human microbiota and potentiated the growth of probiotic bacteria, such as Bifidobacteria (130). Indeed, lower concentrations of C-reactive protein, a specific predictor of cardiovascular risk, was observed in study participants after nonalcoholic red wine and red wine treatment (130). Although these findings indicate that red wine intake has health benefits in the host along with changes in the gut microbiota, no studies have addressed the potential interaction between changes in the gut microbiota caused by ethanol itself and the beneficial cardiovascular effects associated with low-to-moderate ethanol consumption.
Previous studies demonstrated a lower abundance of the Bacteroidetes phylum and greater quantity of the Proteobacteria phylum in humans with alcohol use disorder (124, 126). The increased abundance of Proteobacteria has been associated with the development of diseases, particularly those related to the activation of proinflammatory immune responses (132, 133). In addition, gut microbiota mediates the exacerbated proinflammatory response observed in an experimental model of ethanol consumption (110). Together, these data suggest that ethanol alters the gut microbiota composition, contributing to ethanol-induced inflammatory diseases. Although the link between ethanol and CVD is well established, whether ethanol effects on the gut microbiota contributes to cardiovascular dysfunction remains elusive. Possible mechanisms whereby excessive ethanol consumption contributes to gut dysbiosis and, consequently, to dysfunction of the cardiovascular system are illustrated in Fig. 2.
Figure 2.
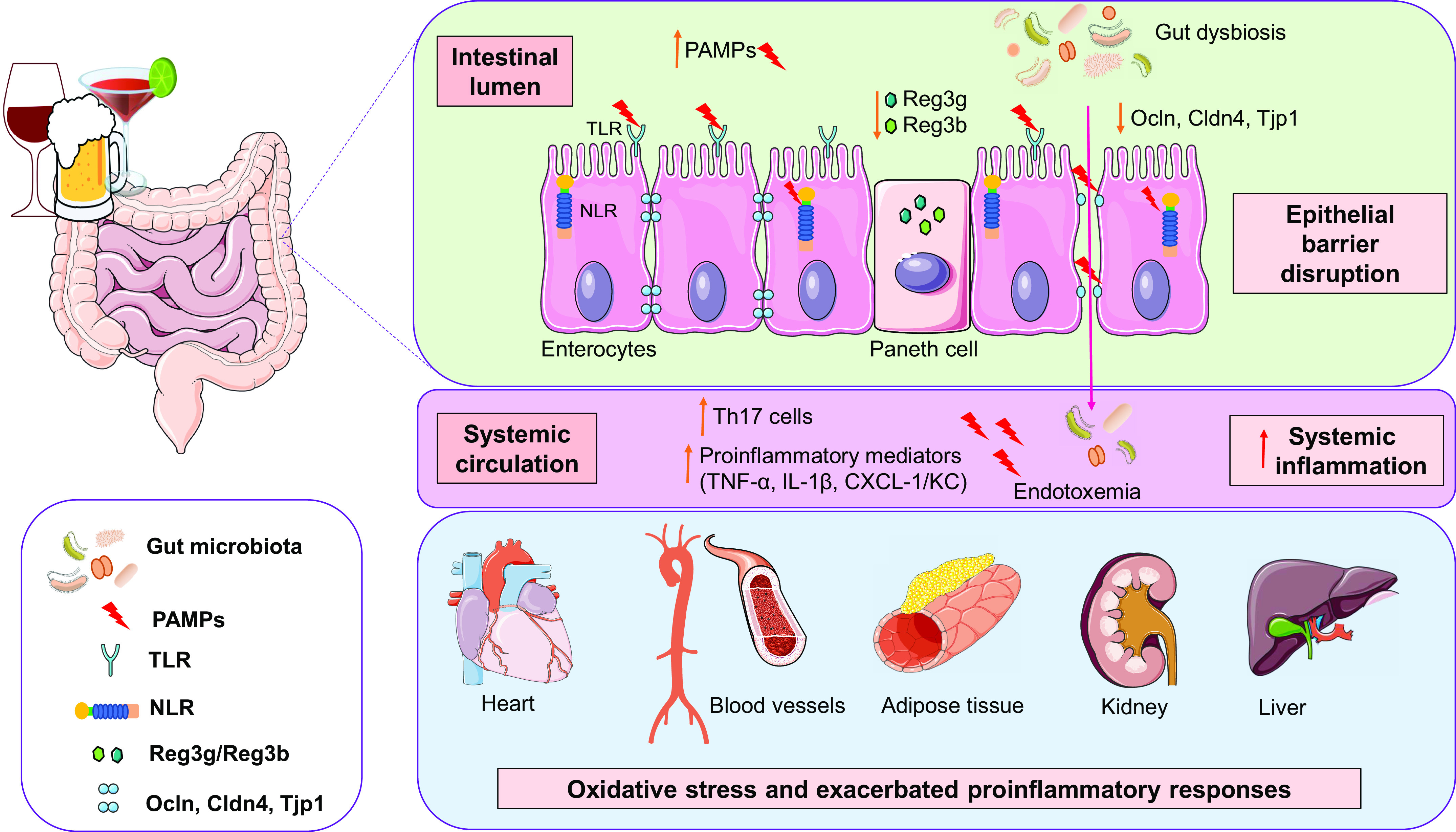
Schematic illustration of possible mechanisms whereby excessive ethanol consumption leads to gut dysbiosis and affects various organs and tissues. Ethanol-induced gut dysbiosis is related to decreased antimicrobial peptides (Reg3b and Reg3g) and increased levels of pathogen-associated molecular patterns (PAMPs), such as lipopolyssacaride (LPS), which are recognized by pattern recognition receptors (PRRs), including Toll-like receptors (TLRs) and NOD-like receptors (NLRs), leading to proinflammatory pathways activation. Furthermore, ethanol induces epithelial barrier disruption by decreasing tight junction protein (Ocln, Cldn4, and Tjp1) expression, promoting increased gut permeability and endotoxemia. These events are accompanied by enhancement in proinflammatory mediators, including cytokines and chemokines, and proinflammatory immune cells in the systemic circulation. Systemic inflammation, exacerbated proinflammatory and immune responses, and oxidative stress in different organs and tissues are linked to ethanol effects. Altogether, these mechanisms can contribute to cardiovascular dysfunction induced by excessive ethanol consumption. Cldn4, claudin 4; CXCL-1/KC, chemokine CXCL-1/KC; IL-1β, interleukin-1β; NLR, NOD-like receptor; Ocln, occludin; Reg3b/Reg3g, C-type regenerating islet derived-3; Th17, T helper 17 cells; Tjp1, tight junction protein 1; TLR, Toll-like receptor; TNF-α, tumor necrosis factor-α.
POSSIBLE MECHANISMS WHEREBY THE CROSS TALK BETWEEN ETHANOL AND GUT MICROBIOTA IMPACTS THE CARDIOVASCULAR SYSTEM
As already discussed, gut microbiota contributes to the pathogenesis of ethanol-related diseases (33, 110, 116, 117, 126). Possible mechanisms include translocation of bacteria and PAMPs, changes in microbial metabolites, inflammation, and changes in bile acids (33). Mechanisms by which ethanol consumption alters the gut microbiota remain poorly understood (116). Furthermore, although it is known that gut microbiota directly (via metabolites) and indirectly (via the immune system) contributes to CVD (25), a causal relationship on the interactions between ethanol and the gut microbiota and their impact on the cardiovascular system need to be explored. Possible pathways whereby ethanol interferes with the microbiota and, consequently, has a detrimental role in the cardiovascular system are discussed below. Figure 3 summarizes potential mechanisms linking ethanol-induced gut dysbiosis, activation of the immune system, and the development of CVD.
Figure 3.
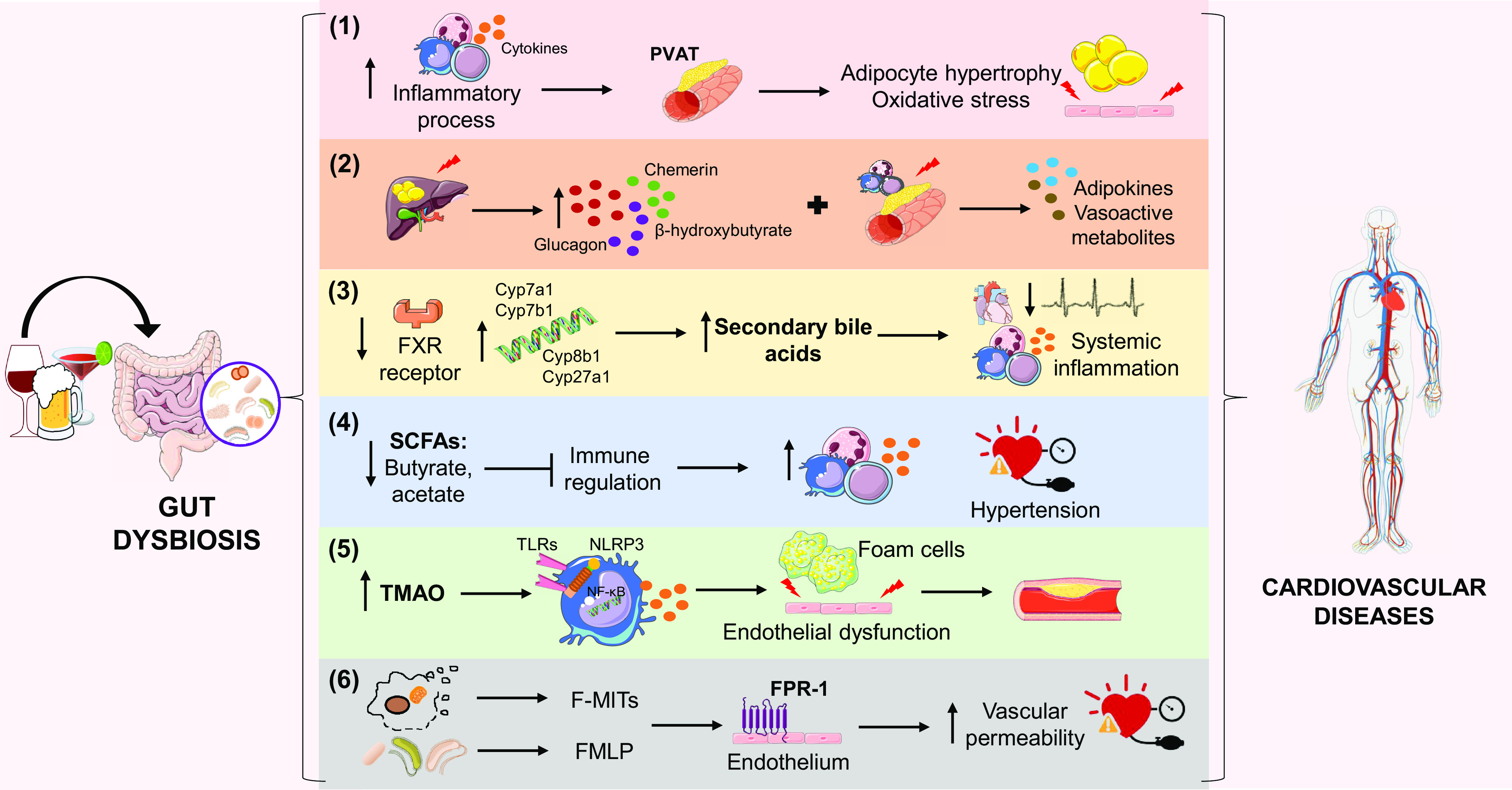
Potential mechanisms associated with ethanol-induced imbalances in the symbiotic relationship between the host and the gut microbiota, which can lead to the development and progression of cardiovascular disease (CVD). The immune system hypothesis (1) suggests that ethanol-induced dysbiosis increases inflammatory processes and immune cell infiltration, especially in the perivascular adipose tissue (PVAT), culminating in adipocyte hypertrophy and oxidative stress in the vascular endothelium. In addition, it is hypothesized that the interference of ethanol in the gut microbiota causes an imbalance in hepatic and adipose tissue homeostasis (2), increasing the levels of vasoactive metabolites. The increase in secondary bile acids (3) is associated with decreased expression of the farnesoid X-activated receptor (FXR) and increase of important genes in the synthesis of these acids, events associated with the consumption of ethanol. The consequences are decreased heart rate and systemic inflammation. The consumption of ethanol decreases the production of short-chain fatty acids (SCFAs), such as butyrate and acetate (4), by the gut microbiota and thus inhibits regulatory mechanisms of inflammation and favors hypertension. The excess of N-oxide-trimethylamine (TMAO) derived from the imbalanced microbiota (5) may activate Toll-like receptors (TLRs), nuclear factor-kappa B (NF-κB), and inflammasome, inducing inflammatory processes and endothelial dysfunction, in addition to favoring the accumulation of fat in macrophages. Ethanol consumption can also increase the levels of formylated peptides derived from the gut microbiota and mitochondria (6), which, when interacting with the formyl peptide receptor 1 (FPR-1), contribute to increased vascular permeability and the occurrence of hypertension.
The Immune System
The GI tract harbors probably the largest microbiota and immune cells load in the human body (103, 134). Interestingly, there is a symbiotic relationship between the gut and its microbiota to regulate both local gut and systemic immunity (104). Dendritic cells, lymphocytes, macrophages, and mast cells play a crucial role in regulating the interaction between the gut microbiota and its mucosal immune system (135). In addition, the gut microbiota modulates the function of neutrophils and T-cell differentiation into the different subpopulations (Th1, Th2, Th17, or Treg) (105). The link between the immune system and CVD is well established (136).
More than just our first line of host defense, the innate immune system consistently surveils for DAMPs from damaged tissues and specific PAMPs, including LPS, peptidoglycans, lipoteichoic acid, flagellin, muramyldipeptide (MDP), and bacterial DNA, that are recognized by conserved pattern recognition receptors (PRRs), such as TLR and NLR (30, 103). PAMPs play a crucial role in the interplay between the gut microbiota and CVD (106). In addition, gut bacteria and their products influence both the immune and vascular systems (104) and can directly engage PRRs in the intestine and in the vasculature, not only to trigger an antimicrobial response but also to regulate the host metabolism and inflammatory pathways associated with CVD (137).
Ethanol consumption modulates the expression of PRRs, including TLR and NLR, mainly on circulating monocytes and macrophages (138, 139). Monocytes from healthy humans, preincubated with 25 mM alcohol for 24 h, show reduced nuclear translocation of the nuclear factor-kappa B (NF-κB) in response to LPS, with a subsequent reduction in the release of proinflammatory cytokines (140). In general, acute alcohol consumption is related to decreased inflammatory responses induced by TLR2, TLR4, and TLR9 (141). The expression and responsiveness of TLR4 to LPS are reduced in macrophages from mice exposed to a single dose of alcohol (127, 142). This is associated with inhibition of IL-1 receptor-associated kinase (IRAK)-1 and ERK1/2, leading to decreased production of proinflammatory cytokines (143). The subsequent suppression of cytokines production culminates in impaired host defense, including decreased bacterial clearance, as shown in experimental models of sepsis (144) and pneumonia (145). In addition, acute alcohol consumption decreases the expression of tight junction proteins in the intestinal epithelium, leading to impaired intestinal barrier function, favoring the translocation of bacteria (146), which can potentially spread more easily, as the host’s defense is attenuated by the use of alcohol. On the other hand, monocytes from patients with alcoholic hepatitis show increased NF-κB activity compared with monocytes from healthy individuals due to increased hepatocyte injury and liver damage (147). Chronic alcohol consumption also increases TNF-α mRNA expression levels in immune cells and increases TLR4 expression in hepatic macrophages and ROS production (148, 149).
Chronic ethanol consumption also affects the host immune system (30). Decreased Th1-mediated immune responses, based on reduced antigen-specific T-cell proliferation and increased IgG and IgA antibody levels, as well as altered neutrophil, leukocyte, and macrophage functions are observed after chronic and acute ethanol consumption (150). Furthermore, cells of the innate and adaptive immune system reside in adipose tissues and in the intestine (151). The presence of inflammatory processes in the perivascular adipose tissue (PVAT) associated with exacerbated cytokine production and immune cell infiltration, maintains close relationship with adipocyte hypertrophy and oxidative stress, which contribute to vascular dysfunction, increased vascular tone, arterial stiffening, and increased peripheral resistance (152–155). The PVAT modulates vascular contractility and structure (156). In addition, the infiltration of immune cells in the PVAT contributes to the low-grade inflammation seen in multiple CVD (157). Ethanol affects the modulatory effects of PVAT on vascular function (40, 54). However, despite studies showing that both ethanol consumption and gut microbiota activate the immune system, and overactivation of the immune system is linked to cardiovascular dysfunction, the mechanisms underlying these events are not fully understood.
Hepatic and Adipose Tissue Homeostasis
Although it is well known that ethanol can have direct effects on lipid metabolism, particularly in the liver (i.e., alcoholic fatty liver disease) (158), ethanol-induced dysbiosis can also perturb hepatic and peripheral adipose tissue homeostasis. This disruption may then affect cardiovascular function through altered secretion of adipokines and vasoactive metabolites, particularly from the PVAT (159, 160). For example, dysbiosis of the gut microbiota during instances of nutritional excess can promote energy storage by decreasing thermogenesis in brown adipose tissue (BAT) and expanding the less thermogenic white adipose tissue (WAT) stores (161). Increases in WAT and loss of BAT can significantly increase vascular damage and cardiovascular risk (93). Furthermore, microbiota can regulate the inflammatory state of adipose tissue (162, 163).
Similarly, given the proximity of the gut and the liver, microbiota exerts an important influence on liver function (164) and are associated with many liver diseases (165). Therefore, we extrapolate that ethanol-induced dysbiosis may also contribute to liver dysfunction and injury, independent of alcoholic fatty liver disease, which could then impact the secretion of liver-derived vasoactive factors, such as chemerin, glucagon, or β-hydroxybutyrate.
Secondary Bile Acids
The liver synthesizes and secretes bile acids as conjugated metabolites, which participate in enterohepatic cycling upon changes in the gut microbiota and contribute to the digestion of lipids and fat-soluble vitamins (34, 106). The gut microbiota converts the primary bile acids into secondary bile acids, which initiate host physiological effects, including antimicrobial, anti-inflammatory, immunomodulatory, and metabolic downstream effects (166). The bile acids are recognized by receptor systems, such as muscarinic M2 and M3 receptors, G protein-coupled bile acid receptor 1 (GP-BAR1, also known as TGR5), vitamin D3 receptor (VDR), sphingosine 1-phosphate receptor 2 (S1PR2), pregnane X receptor [PXR, also known as nuclear receptor subfamily 1 group I member 2 (NR1I2)], and farnesoid X-activated receptor (FXR, also known as NR1H4) (106, 137, 167).
Secondary bile acids are linked to increased CVD risk (137, 168). Furthermore, the interaction of bile acids with its receptors contributes to regulation of cardiovascular function (167, 169), such as reduction of the heart rate by regulating channel conductance and calcium dynamics in sinoatrial and ventricular cardiomyocytes and regulation of vascular tone via both endothelium-dependent and endothelium-independent mechanisms (167). On the other hand, the modulation of bile acid receptors, such as FXR, PXR, TGR5, VDR, and S1PR2, also contribute to CVD (170). Bile acid receptors are expressed in several tissues, including cardiomyocytes (167, 169), endothelial cells, and vascular smooth muscle cells (VSMCs) (167). In addition, the genetic deletion of FXR, TGR5, PXR, or S1PR2 interferes with atherosclerosis progression and protection against atherosclerosis development (137).
Bile acids affect the cecal microbiota composition in rats (171). High levels of deoxycholic acid prevent the growth of beneficial members of Bacteroidetes and Firmicutes phylum (34). On the other hand, changes in the composition of intestinal bacteria alter the amount and type of metabolites produced, including bile acids. In this context, alterations in the microbial community are accompanied by higher secondary bile acids in alcoholics (34). Indeed, augmented bile acid-induced oxidative stress was reported in fetal rat liver after maternal exposure to ethanol (172). Increased bile acid levels may be partly explained by reduction of FXR expression (34, 173), upregulation of the expression of bile acid synthesis genes (i.e., Cyp7a1, Cyp7b1, Cyp8b1, and Cyp27a1) (173), as well as increasing intestinal bile acid reabsorption (174) induced by ethanol consumption. In addition, FXR is the primary receptor for bile acids in intestinal cells. It influences several bactericidal proteins, including angiogenin 1 and RNAse family member 4, whose reduction has been associated with bacterial overgrowth in the small intestine of mice (30). The agonist activity on the bile acid receptors may represent a link between gut microbiota and the cardiovascular system (169). In addition to the dose-dependent vasorelaxation, bile acids also modulate the transcription of vasoactive molecules by targeting transcription factor FXR, representing an additional mechanism for bile acids effects on cardiovascular function (175).
Altogether, these data show that bile acids are involved in CVD progression and in ethanol-induced gut microbiota alterations. In this context, a link between cholestasis and cardiovascular dysfunction has been proposed (175). Ethanol consumption leads to cholestasis (114, 172, 173, 176), characterized by increased bile salt retention (176). However, ethanol effects on bile secretion, the role of microbiota in this response, and their contribution to cardiovascular dysfunction remain elusive. Therefore, the gut microbiota-mediated effects on bile acid levels should be further studied in CVD associated with ethanol consumption.
Short-Chain Fatty Acids
SCFAs are metabolites derived from microbial metabolism that are formed due to the fermentation of complex carbohydrates (25). They influence the gut microbiota by stimulating Bifidobacteria growth while inhibiting gram-negative facultative and anaerobic bacteria (177). SCFAs exert many beneficial effects on intestinal epithelium, including inhibition of inflammation and modulation of oxidative stress (178). Once in the systemic circulation, SCFAs activate signaling cascades in distal organs (99). SCFAs are recognized by G protein-coupled receptors (GPRs) (103), including GPR41, GPR43, GPR109A, and olfactory receptor 78 (Olfr78) (137), which are a family of receptors that influences the functions of immune cells, particularly inflammatory leukocytes and Treg (179). Therefore, SCFAs can act as immune regulators in local and distal sites from the intestine (103, 179). In addition to their immunoregulatory functions, SCFAs’ effects on carbohydrate, lipid, and cholesterol metabolism and blood pressure also seem to modulate renal function, cardiovascular function, and CVD (180–183).
Previous studies show cardioprotective effects of SCFAs in experimental models of hypertension (94, 184, 185). The association between increased blood pressure and changes in gut microbiota composition, characterized by reduction of butyrate and acetate-producing bacteria, has been observed in both humans and experimental model studies (94, 96). Changes in SCFA levels have been reported in patients with long-term ethanol consumption (126) and rats following chronic ethanol administration (186). Reduced SCFAs levels induced by ethanol may be due to the impact of ethanol on content and composition of the gut microbiota (186), as well as to decreased absorption of fatty acids (115). In addition, reduced expression of a butyrate receptor (GPR109A) and butyrate transporter (SLC5A8) following chronic, short-term, and acute ethanol exposure has been reported in the ileum and proximal colon of rodents (121).
An increase in SCFAs-producing bacteria has been associated with reduced gut permeability and systemic inflammation (33). In addition, butyrate not only reduces paracellular permeability but also improves intestinal barrier function (115). In this context, butyrate through AMP-kinase-dependent signaling attenuates intestinal barrier dysfunction induced by ethanol (178). Furthermore, female mice treated with tributyrin, which is a lipid structured with three molecules of butyrate esterified in glycerol, are protected against blunted immune responses and proximal colon oxidative stress caused by chronic-binge ethanol exposure (187).
The inhibition of inflammatory responses induced by cytokines and NF-κB activation is a potential mechanism for the anti-inflammatory effects of butyrate (177). Consistent with these data, treatment with butyrate reduces the activation of NF-κB pathway induced by a high-fat diet, with a subsequent reduction of mRNA levels of proinflammatory cytokines (TNFα, IL-6, and IL-1β) and expression of inflammatory enzymes (COX-2 and iNOS) and TLRs (188). In addition, treatment with butyrate has been associated with a lower free radical release, which is related to reduced expression levels of NADPH oxidase and iNOS (189). Since the activation of these pathways is involved in ethanol effects in the cardiovascular system, as discussed above, decreased SCFAs levels may contribute to cardiovascular dysfunction induced by ethanol. Therefore, the modulation of gut microbiota-derived SCFAs may be one of the pathways linked to ethanol consumption-induced cardiovascular effects.
N-Oxide-Trimethylamine
Dietary lecithin, carnitine, and choline are metabolized by gut microbiota to trimethylamine (TMA). This metabolite is oxidized by the liver enzymes, called flavin-containing monooxygenase, predominantly the dimethylaniline monooxygenase (N-oxide-forming) 3 (FMO3) isoform, to N-oxide-trimethylamine (TMAO) (106, 137, 190), which seems to increase mortality and cardiovascular events (191). TMAO contributes to the accumulation of cholesterol in the liver, foam cell formation, platelet response, thrombus formation, vascular inflammation, cardiac remodeling, and CVD’s development and progression (2, 28). Moreover, TMAO enhances lipid accumulation in macrophages and initiates atherosclerosis (106). Therefore, inhibition of gut microbiota-mediated TMAO production as a therapeutic approach for treating cardiometabolic diseases has been suggested (192).
Activation of TLR, NF-κB, and inflammasomes, followed by increased cytokine release and oxidative stress, are mechanisms related to endothelial dysfunction and vascular inflammation induced by TMAO (190, 193, 194). The activation of mitogen-activated protein kinase (MAPK) also contributes to TMAO-induced vascular inflammation (195). In addition, nifedipine-sensitive calcium channels contribute to TMA-induced contraction, representing a mechanism by which this microbiota metabolite modulates vascular reactivity (196).
Although there are no studies on the effects of ethanol in TMAO levels and its relationship with cardiovascular dysfunction, specific signaling pathways are activated after exposure to ethanol or TMAO. Hoyt et al. (197) suggested that the activation of the NLRP3 inflammasome has an essential role in developing inflammation related to long-term exposure to ethanol. Of note, cardiac injury induced by ethanol consumption involves NF-κB activation (53). In addition, chronic ethanol exposure induces TLR4-mediated cytokine production (198).
Little is known regarding which bacteria species or broader communities are the main producers of TMA, the precursor of TMAO (137). However, members of the human gut microbiota responsible for the accumulation of TMA, including Anaerococcus hydrogenalis, Clostridium asparagiforme, Clostridium hathewayi, Clostridium sporogenes, Edwardsiella tarda, Escherichia fergusonii, Proteus penneri, and Providencia rettgeri have been identified (199). Higher relative abundance of the genera Clostridium was also observed in patients with chronic ethanol overconsumption (126). Altogether, these data suggest a possible association between ethanol intake and TMAO levels. However, additional studies are needed to determine whether TMAO is involved in ethanol-induced cardiovascular dysfunction.
Formyl Peptide Receptors
Immune system activation and inflammation have been proposed as a unifying mechanism linking the cardiovascular system, the kidneys, the autonomic nervous system (200, 201), and more recently, the gut (98) in CVD development, such as hypertension. However, it is unknown whether the activation of the innate immune system precedes CVD.
Formyl peptide receptors (FPRs) belong to the GPR family and are PRRs that recognize bacterial- and mitochondria-derived formylated peptides. FPR-1 and FPR-2 are expressed at high levels on leukocytes and mediate cell chemotaxis (202). Stimulation of FPR-1 in neutrophils causes polymerization of actin within 10 s and Ca2+ influx (203). The FPR family was originally identified by its ability to bind N-formyl peptides (NFPs) such as N-formylmethionine (FMLP), produced by bacterial degradation (202). Interestingly, mitochondria carry hallmarks of their bacterial ancestry, and one of these hallmarks is that these organelles use N-formyl-methionyl-tRNA as an initiator of protein synthesis (202, 204). Consequently, both mitochondrial and bacterial-produced peptides have a formyl group at their NH2-terminus. NFPs, regardless of their origin, are recognized by FPR-1 as pathogens and, thus, initiate inflammation. Any injury (e.g., high blood pressure, excessive alcohol intake) leading to cell damage promotes the release of DAMPS, including mitochondrial NFPs (F-MITs). We were the first to observe that F-MITs are increased in plasma from trauma patients and in animals that underwent sterile trauma. These peptides activate FPR-1 leading to cardiovascular collapse (204, 205). FPR-1 is also expressed in endothelial cells, VSMCs, and airways (204, 205). In vitro activation of FPR-1 induces a slow and sustained contraction in a concentration-dependent manner in airways and increases vascular permeability in arteries from rats (205). Further, FPR-1 activation leads to actin polymerization in VSMCs. Arteries that do not express FPR-1 (FPR-1−/−) also present a significant reduction in vascular contraction and loss of myogenic tone due to disruption of actin polymerization (206). Systemic FPR-1 activation causes sepsis-like symptoms, including lung inflammation and formation of neutrophil extracellular traps (NETs) (205).
Patients with hypertension and hypertensive animals have increased gut permeability due to immune system activation and cell death, with increasing circulating levels of bacterial and mitochondrial products, respectively. Based on these facts, we proposed that additional bacterial products, such as NFPs, leaking out of the gut are essential contributors to vascular dysfunction in hypertension (207). Furthermore, considering the dual-source of NFPs (from microbiota and mitochondria), we postulated that leaky gut-derived bacterial NFPs (from microbiota) and cell damage-derived F-MITs (from the host) could cause vascular dysfunction in hypertension. We observed that mitochondrial-derived peptides originating from the kidneys are responsible for FPR-1-induced vascular injury and premature elevation of blood pressure in inbred Dahl salt-sensitive (S) rats, independently of a high-salt diet. However, a high-salt diet leads to gut barrier disruption and, subsequently, a synergistic action of mitochondria and bacterial-derived leaky gut NFPs leads to severe and established hypertension (207). Inbred Dahl salt-sensitive rats, developed by Dr. John Rapp at the University of Toledo (208), mimics human salt-sensitive hypertension. Dr. Rapp observed that premature spontaneous elevation of blood pressure occurs in Dahl S rats fed a low salt diet, but not in Dahl salt resistance (R) rats, due to unknown genetic influences and independently of environmental factors (208). Specifically, premature spontaneous elevations in blood pressure begin in animals younger than 12 wk (208). A high-salt diet accelerates this process leading to malignant hypertension, similarly to humans. Therefore, unknown genetic influences rather than a high-salt diet trigger the vascular damage and genesis of elevated blood pressure. Consequently, mitochondrial and microbiota-derived NFPs are important for the premature spontaneous elevation of blood pressure and established hypertension in Dahl S rats, respectively (207). Given that ethanol leads to gut microbiota dysregulation and cell injury, FPR-1 activation may play a role in the cardiovascular dysfunction induced by ethanol consumption.
Other Potential Mechanisms
Other factors have been suggested as important mediators in gut microbiota-induced alterations in the cardiovascular system, including activation of sympathetic nervous system (94, 104), activation of RAAS (23, 99, 183), and dysregulation of miRNAs (209, 210). Indeed, these pathways are linked to damage and dysfunctions induced by ethanol (10, 38–40, 42, 55, 211–215). Further studies are needed to determine whether these mechanisms contribute to the causal relationship between the gut microbiota and ethanol-related CVD.
CONCLUSION
Although research involving the gut microbiota is still in the developing stage, accumulating evidence suggests that the gut microbiota is an essential mediator of host health and disease. Imbalances in the symbiotic relationship between the host and the commensal microbiota in a holobiont, as seen in chronic ethanol consumption, can lead to the development and progression of diseases. In this sense, the gut microbiota is central to maintain proper host homeostasis. Therefore, manipulating gut microbiota using antibiotics, probiotics, prebiotics, and fecal microbiota transplantation might prove a valuable opportunity to prevent/mitigate the deleterious effects of ethanol and improve cardiovascular health and risk prevention. Potential mechanisms underlying the cross talk among ethanol, gut microbiota, and immune responses discussed in this review may reveal new biomarkers and novel therapeutic approaches for ethanol-related CVD.
GRANTS
This study was supported by National Institutes of Health Grants K99HL151889 (to C. G. McCarthy), R00GM118885 (to C. F. Wenceslau), and R01HL149762 (to C. F. Wenceslau) and Sao Paulo Research Foundation (FAPESP) Grants 2018/14815-0 (to D. Carlos) and 2013/08216-2 (to R. C. Tostes), Center of Research in Inflammatory Diseases.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.B.P.S. and J.E-O prepared figures; C.B.P.S., J.E-O, C.G.M., C.F.W., D.C., and R.C.T. drafted manuscript; C.B.P.S., J.E-O, C.G.M., C.F.W., D.C., and R.C.T. edited and revised manuscript; C.B.P.S., J.E-O, C.G.M., C.F.W., D.C., and R.C.T. approved final version of manuscript.
REFERENCES
- 1.Bomfim GF, Cau SBA, Bruno AS, Fedoce AG, Carneiro FS. Hypertension: a new treatment for an old disease? Targeting the immune system. Br J Pharmacol 176: 2028–2048, 2019. doi: 10.1111/bph.14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad AF, Dwivedi G, O'Gara F, Caparros-Martin J, Ward NC. The gut microbiome and cardiovascular disease: current knowledge and clinical potential. Am J Physiol Heart Circ Physiol 317: H923–H938, 2019. doi: 10.1152/ajpheart.00376.2019. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global status report on alcohol and health, 2018.
- 4.Ortega KC, Ginani GF, Silva GV, Milon Jr. D. Prehypertension: concept, epidemiology and what the guidelines say. Hypertension 16: 83–86, 2009. 25606120 [Google Scholar]
- 5.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. 2017 ACC, / AHA, /Aapa / ABC, /Acpm / AGS, /APhA / ASH, /Aspc / NMA. /PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology. Hypertension 71: E13–E115, 2018. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 6.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol 16: 223–237, 2020. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Keefe EL, DiNicolantonio JJ, O'Keefe JH, Lavie CJ. Alcohol and CV health: Jekyll and Hyde J-Curves. Prog Cardiovas Dis 61: 68–75, 2018. doi: 10.1016/j.pcad.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Moos RH, Brennan PL, Schutte KK, Moos BS. High-risk alcohol consumption and late-life alcohol use problems. Am J Public Health 94: 1985–1991, 2004. doi: 10.2105/ajph.94.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minzer S, Losno RA, Casas R. The effect of alcohol on cardiovascular risk factors: is there new information? Nutrients 12: 912–922, 2020. doi: 10.3390/nu12040912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchi KC, Muniz JJ, Tirapelli CR. Hypertension and chronic ethanol consumption: what do we know after a century of study? World J Cardiol 6: 283–294, 2014. doi: 10.4330/wjc.v6.i5.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park B, Lee HR, Lee YJ. Alcoholic liver disease: focus on prodromal gut health. J Dig Dis 17: 493–500, 2016. doi: 10.1111/1751-2980.12375. [DOI] [PubMed] [Google Scholar]
- 12.Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Alcohol consumption and mortality in patients with cardiovascular disease. J Am Coll Cardiol 55: 1339–1347, 2010. doi: 10.1016/j.jacc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Goel S, Sharma A, Garg A. Effect of alcohol consumption on cardiovascular health. Curr Cardiol Rep 20: 19, 2018. doi: 10.1007/s11886-018-0962-2. [DOI] [PubMed] [Google Scholar]
- 14.Padro T, Muñoz-García N, Vilahur G, Chagas P, Deyà A, Antonijoan R, Badimon L. Moderate beer intake and cardiovascular health in overweight individuals. Nutrients 10: 1237, 2018. doi: 10.3390/nu10091237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Wiel A, de Lange DW. Cardiovascular risk is more related to drinking pattern than to the type of alcoholic drinks. Neth J Med 66: 467–473, 2008. [PubMed] [Google Scholar]
- 16.Mukherjee S, Das SK, Vasudevan DM. Effects of ethanol consumption on different organs—a brief overview. Asian J Biochem 2: 386–394, 2007. doi: 10.3923/ajb.2007.386.394. [DOI] [Google Scholar]
- 17.Saremi A, Arora R. The cardiovascular implications of alcohol and red wine. Am J Ther 15: 265–277, 2008. doi: 10.1097/MJT.0b013e3180a5e61a. [DOI] [PubMed] [Google Scholar]
- 18.Krenz M, Korthuis RJ. Moderate ethanol ingestion and cardiovascular protection: from epidemiologic associations to cellular mechanisms. J Mol Cell Cardiol 52: 93–104, 2012. doi: 10.1016/j.yjmcc.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nurmi K, Virkanen J, Rajamäki K, Niemi K, Kovanen PT, Eklund KK. Ethanol inhibits activation of NLRP3 and AIM2 inflammasomes in human macrophages–a novel anti-inflammatory action of alcohol. PLoS One 8: e78537, 2013. doi: 10.1371/journal.pone.0078537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernández-Solà J. Cardiovascular risks and benefits of moderate and heavy alcohol consumption. Nat Rev Cardiol 12: 576–587, 2015. doi: 10.1038/nrcardio.2015.91. [DOI] [PubMed] [Google Scholar]
- 21.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 373: 2223–2233, 2009. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WHW, Didonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57–65, 2011. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karbach SH, Schönfelder T, Brandão I, Wilms E, Hörmann N, Jäckel S, Schüler R, Finger S, Knorr M, Lagrange J, Brandt M, Waisman A, Kossmann S, Schäfer K, Münzel T, Reinhardt C, Wenzel P. Gut microbiota promote angiotensin II–induced arterial hypertension and vascular dysfunction. J Am Heart Assoc 5: e003698, 2016. doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B, Cai J. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5: 14, 2017. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease: opportunities and challenges. Circ Res 127: 553–570, 2020. doi: 10.1161/CIRCRESAHA.120.316242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards JM, Roy S, Tomcho JC, Schreckenberger ZJ, Chakraborty S, Bearss NR, Saha P, McCarthy CG, Vijay-Kumar M, Joe B, Wenceslau CF. Microbiota are critical for vascular physiology: Germ-free status weakens contractility and induces sex-specific vascular remodeling in mice. Vascul Pharmacol 125-126: 106633, 2020. doi: 10.1016/j.vph.2019.106633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding R. X, Goh WR, Wu R, Yue X, Qing Luo X, Khine WWT, Wu J, Rui Lee YK. Revisit gut microbiota and its impact on human health and disease. J Food Drug Anal 27: 623–631, 2019. doi: 10.1016/j.jfda.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu H, Wang X, Feng W, Liu Q, Zhou S, Liu Q, Cai L. The gut microbiota and its interactions with cardiovascular disease. Microb Biotechnol 13: 637–656, 2020. doi: 10.1111/1751-7915.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang WHW, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res 120: 1183–1196, 2017. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartmann P, Seebauer CT, Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin Exp Res 39: 763–775, 2015. doi: 10.1111/acer.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung H, Kim SW, Hong M, Suk KT. Microbiota-based treatments in alcoholic liver disease. World J Gastroenterol 22: 6673–6682, 2016. doi: 10.3748/wjg.v22.i29.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrere G, Wrzosek L, Cailleux F, Turpin W, Puchois V, Spatz M, Ciocan D, Rainteau D, Humbert L, Hugot C, Gaudin F, Noordine ML, Robert V, Berrebi D, Thomas M, Naveau S, Perlemuter G, Cassard AM. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol 66: 806–815, 2017. doi: 10.1016/j.jhep.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Sarin SK, Pande A, Schnabl B. Microbiome as a therapeutic target in alcohol-related liver disease. J Hepatol 70: 260–272, 2019. doi: 10.1016/j.jhep.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Meroni M, Longo M, Dongiovanni P. Alcohol or gut microbiota: who is the guilty? Int J Mol Sci 20: 4568, 2019. doi: 10.3390/ijms20184568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health 29: 245–254, 2006. [PMC free article] [PubMed] [Google Scholar]
- 36.Piano MR, Phillips SA. Alcoholic cardiomyopathy: pathophysiologic insights. Cardiovasc Toxicol 14: 291–308, 2014. doi: 10.1007/s12012-014-9252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kodavali L, Townsend RR. Alcohol and its relationship to blood pressure. Curr Hypertens Rep 8: 338–344, 2006. doi: 10.1007/s11906-006-0074-z. [DOI] [PubMed] [Google Scholar]
- 38.Ceron C, Marchi K, Muniz J, Tirapelli C. Vascular oxidative stress: a key factor in the development of hypertension associated with ethanol consumption. Curr Hypertens Rev 10: 213–222, 2014. doi: 10.2174/157340211004150319122736. [DOI] [PubMed] [Google Scholar]
- 39.Passaglia P, Ceron CS, Mecawi AS, Antunes-Rodrigues J, Coelho EB, Tirapelli CR. Angiotensin type 1 receptor mediates chronic ethanol consumption-induced hypertension and vascular oxidative stress. Vascul Pharmacol 74: 49–59, 2015. doi: 10.1016/j.vph.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 40.do Vale GT, Simplicio JA, Gonzaga NA, Yokota R, Ribeiro AA, Casarini DE, de Martinis BS, Tirapelli CR. Nebivolol prevents vascular oxidative stress and hypertension in rats chronically treated with ethanol. Atherosclerosis 274: 67–76, 2018. doi: 10.1016/j.atherosclerosis.2018.04.041. [DOI] [PubMed] [Google Scholar]
- 41.Marchi KC, Ceron CS, Muniz JJ, De Martinis BS, Tanus-Santos JE, Tirapelli CR. NADPH oxidase plays a role on ethanol-induced hypertension and reactive oxygen species generation in the vasculature. Alcohol Alcohol 51: 522–534, 2016. doi: 10.1093/alcalc/agw043. [DOI] [PubMed] [Google Scholar]
- 42.Phillips SA, Osborn K, Hwang C-L, Sabbahi A, Piano MR. Ethanol induced oxidative stress in the vasculature: friend or foe. Curr Hypertens Rev 16: 181–191, 2020. doi: 10.2174/1573402115666190325124622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matyas C, Varga ZV, Mukhopadhyay P, Paloczi J, Lajtos T, Erdelyi K, Nemeth BT, Nan M, Hasko G, Gao B, Pacher P. Chronic plus binge ethanol feeding induces myocardial oxidative stress, mitochondrial and cardiovascular dysfunction, and steatosis. Am J Physiol Heart Circ Physiol 310: H1658–H1670, 2016. doi: 10.1152/ajpheart.00214.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol 43: 163–172, 2009. doi: 10.1016/j.alcohol.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forsyth CB, Voigt RM, Burgess HJ, Swanson GR, Keshavarzian A. Circadian rhythms, alcohol and gut interactions. Alcohol 49: 389–398, 2015. doi: 10.1016/j.alcohol.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol 50: 538–547, 2009. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ceron CS, do Vale GT, Simplicio JA, Ricci ST, De Martinis BS, de Freitas A, Tirapelli CR. Chronic ethanol consumption increases vascular oxidative stress and the mortality induced by sub-lethal sepsis: potential role of iNOS. Eur J Pharmacol 825: 39–47, 2018. doi: 10.1016/j.ejphar.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Leite LN, do Vale GT, Simplicio JA, De Martinis BS, Carneiro FS, Tirapelli CR. Ethanol-induced erectile dysfunction and increased expression of pro-inflammatory proteins in the rat cavernosal smooth muscle are mediated by NADPH oxidase-derived reactive oxygen species. Eur J Pharmacol 804: 82–93, 2017. doi: 10.1016/j.ejphar.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 49.Simplicio JA, do Vale GT, Gonzaga NA, Leite LN, Hipólito UV, Pereira CA, Tostes RC, Tirapelli CR. Reactive oxygen species derived from NAD(P)H oxidase play a role on ethanol-induced hypertension and endothelial dysfunction in rat resistance arteries. J Physiol Biochem 73: 5–16, 2017. doi: 10.1007/s13105-016-0519-z. [DOI] [PubMed] [Google Scholar]
- 50.do Vale GT, da Silva CBP, Sousa AH, Gonzaga NA, Parente JM, Araújo KM, Castro MM, Tirapelli CR. Nebivolol prevents up-regulation of Nox2/NADPH oxidase and lipoperoxidation in the early stages of ethanol-induced cardiac toxicity. Cardiovasc Toxicol 21: 224–235, 2021. doi: 10.1007/s12012-020-09614-1. [DOI] [PubMed] [Google Scholar]
- 51.Tirapelli CR, Fukada SY, Yogi A, Chignalia AZ, Tostes RC, Bonaventura D, Lanchote VL, Cunha FQ, De Oliveira AM. Gender-specific vascular effects elicited by chronic ethanol consumption in rats: a role for inducible nitric oxide synthase. Br J Pharmacol 153: 468–479, 2008. doi: 10.1038/sj.bjp.0707589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang Y, Forsyth CB, Banan A, Fields JZ, Keshavarzian A. Oats supplementation prevents alcohol-induced gut leakiness in rats by preventing alcohol-induced oxidative tissue damage. J Pharmacol Exp Ther 329: 952–958, 2009. doi: 10.1124/jpet.108.148643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakashima MA, Silva CBP, Gonzaga NA, Simplicio JA, Omoto ACM, Tirapelli LF, Tanus-Santos JE, Tirapelli CR. Chronic ethanol consumption increases reactive oxygen species generation and the synthesis of pro-inflammatory proteins in the heart through TNFR1-dependent mechanisms. Cytokine 121: 154734, 2019. doi: 10.1016/j.cyto.2019.154734. [DOI] [PubMed] [Google Scholar]
- 54.Simplicio JA, Gonzaga NA, Nakashima MA, De Martinis BS, Cunha TM, Tirapelli LF, Tirapelli CR. Tumor necrosis factor-α receptor 1 contributes to ethanol-induced vascular reactive oxygen species generation and hypertension. J Am Soc Hypertens 11: 684–696, 2017. doi: 10.1016/j.jash.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 55.Bala S, Csak T, Saha B, Zatsiorsky J, Kodys K, Catalano D, Satishchandran A, Szabo G. The pro-inflammatory effects of miR-155 promote liver fibrosis and alcohol-induced steatohepatitis. J Hepatol 64: 1378–1387, 2016. doi: 10.1016/j.jhep.2016.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 9: 69, 2020. doi: 10.12688/f1000research.20510.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matijašić M, Meštrović T, Čipčić Paljetak H, Perić M, Barešić A, Verbanac D. Gut microbiota beyond bacteria—mycobiome, virome, archaeome, and eukaryotic parasites in IBD. Int J Mol Sci 21: 2668, 2020. doi: 10.3390/ijms21082668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med 6: 237ra65–237ra65, 2014. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo Y, Chen G-L, Hannemann N, Ipseiz N, Krönke G, Bäuerle T, Munos L, Wirtz S, Schett G, Bozec A. Microbiota from obese mice regulate hematopoietic stem cell differentiation by altering the bone niche. Cell Metab 22: 886–894, 2015. doi: 10.1016/j.cmet.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 60.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 16: 228–231, 2010. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al Nabhani Z, Eberl G. Imprinting of the immune system by the microbiota early in life. Mucosal Immunol 13: 183–189, 2020. doi: 10.1038/s41385-020-0257-y. [DOI] [PubMed] [Google Scholar]
- 62.McKernan DP; Pharmacology & Therapeutics, Human Biology Building, School of Medicine, National University of Ireland, Galway, H91 TK33, Republic of Ireland. Toll-like receptors and immune cell crosstalk in the intestinal epithelium. AIMS Allergy Immunol 3: 13–31, 2019. doi: 10.3934/Allergy.2019.1.13. [DOI] [Google Scholar]
- 63.Vidya MK, Kumar VG, Sejian V, Bagath M, Krishnan G, Bhatta R. Toll-like receptors: Significance, ligands, signaling pathways, and functions in mammals. Int Rev Immunol 37: 20–36, 2018. doi: 10.1080/08830185.2017.1380200. [DOI] [PubMed] [Google Scholar]
- 64.Chu H, Mazmanian SK. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat Immunol 14: 668–675, 2013. doi: 10.1038/ni.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Danne C, Ryzhakov G, Martínez-López M, Ilott NE, Franchini F, Cuskin F, Lowe EC, Bullers SJ, Arthur JSC, Powrie F. A large polysaccharide produced by Helicobacter hepaticus induces an anti-inflammatory gene signature in macrophages. Cell Host Microbe 22: 733–745.e5, 2017. doi: 10.1016/j.chom.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241, 2004. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 67.Bouskra D, Brézillon C, Bérard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456: 507–510, 2008. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 68.Elias-Oliveira J, Leite JA, Pereira ÍS, Guimarães JB, Manso GM, da C, Silva JS, Tostes RC, Carlos D. NLR and intestinal dysbiosis-associated inflammatory illness: drivers or dampers? Front Immunol 11: 1810, 2020. doi: 10.3389/fimmu.2020.01810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saxena M, Yeretssian G. NOD-Like receptors: master regulators of inflammation and cancer. Front Immunol 5: 327, 2014. doi: 10.3389/fimmu.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petnicki-Ocwieja T, Hrncir T, Liu YJ, Biswas A, Hudcovic T, Tlaskalova-Hogenova H, Kobayashi KS. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci USA 106: 15813–15818, 2009. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nigro G, Rossi R, Commere P-H, Jay P, Sansonetti PJ. The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell host Microbe 15: 792–798, 2014. doi: 10.1016/j.chom.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 72.Costa FRC, Francozo MCS, de Oliveira GG, Ignacio A, Castoldi A, Zamboni DS, Ramos SG, Camara NO, de Zoete MR, Palm NW, Flavell RA, Silva JS, Carlos D. Gut microbiota translocation to the pancreatic lymph nodes triggers NOD2 activation and contributes to T1D onset. J Exp Med 213: 1223–1239, 2016. doi: 10.1084/jem.20150744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carlos D, Pérez MM, Leite JA, Rocha FA, Martins LMS, Pereira CA, Fraga-Silva TFC, Pucci TA, Ramos SG, Câmara NOS, Bonato VLD, Tostes RC, Silva JS. NOD2 deficiency promotes intestinal CD4+ T lymphocyte imbalance, metainflammation, and aggravates type 2 diabetes in murine model. Front Immunol 11: 1265, 2020. doi: 10.3389/fimmu.2020.01265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li C, Xu MM, Wang K, Adler AJ, Vella AT, Zhou B. Macrophage polarization and meta-inflammation. Transl Res 191: 29–44, 2018. doi: 10.1016/j.trsl.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luz PL, da Haas EA, Favarato D. Intestinal microbiota and cardiovascular diseases. Int J Cardiovasc Sci 33: 462–471, 2020. doi: 10.36660/ijcs.20200043. [DOI] [Google Scholar]
- 76.Feng Y, Huang Y, Wang Y, Wang P, Song H, Wang F. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PloS One 14: e0218384, 2019. doi: 10.1371/journal.pone.0218384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol 21: 8787–8803, 2015. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Geuking MB, Cahenzli J, Lawson MAE, Ng DCK, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T Cell responses. Immunity 34: 794–806, 2011. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 79.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332: 974–977, 2011. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504: 446–450, 2013[Erratum inNature506: 254, 2014]. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 81.Ansaldo E, Slayden LC, Ching KL, Koch MA, Wolf NK, Plichta DR, Brown EM, Graham DB, Xavier RJ, Moon JJ, Barton GM. Akkermansia Muciniphila induces intestinal adaptive immune responses during homeostasis. Science 364: 1179–1184, 2019. doi: 10.1126/science.aaw7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Omenetti S, Bussi C, Metidji A, Iseppon A, Lee S, Tolaini M, Li Y, Kelly G, Chakravarty P, Shoaie S, Gutierrez MG, Stockinger B. The intestine harbors functionally distinct homeostatic tissue-resident and inflammatory Th17 cells. Immunity 51: 77–89.e6, 2019. doi: 10.1016/j.immuni.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kazemian N, Mahmoudi M, Halperin F, Wu JC, Pakpour S. Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome 8: 36, 2020. doi: 10.1186/s40168-020-00821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen X, Zheng L, Zheng Y-Q, Yang Q-G, Lin Y, Ni F-H, Li Z-H. The cardiovascular macrophage: a missing link between gut microbiota and cardiovascular diseases? Eur Rev Med Pharmacol Sci 22: 1860–1872, 2018. doi: 10.26355/eurrev_201803_14607. [DOI] [PubMed] [Google Scholar]
- 85.Geng S, Chen K, Yuan R, Peng L, Maitra U, Diao N, Chen C, Zhang Y, Hu Y, Qi C-F, Pierce S, Ling W, Xiong H, Li L. The persistence of low-grade inflammatory monocytes contributes to aggravated atherosclerosis. Nat Commun 7: 13436, 2016. doi: 10.1038/ncomms13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol 5: 514, 2014. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koeth RA, Lam-Galvez BR, Kirsop J, Wang Z, Levison BS, Gu X, Copeland MF, Bartlett D, Cody DB, Dai HJ, Culley MK, Li XS, Fu X, Wu Y, Li L, DiDonato JA, Tang WHW, Garcia-Garcia JC, Hazen SL. L-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J Clin Invest 129: 373–387, 2019. doi: 10.1172/JCI94601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551: 585–589, 2017. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sandek A, Swidsinski A, Schroedl W, Watson A, Valentova M, Herrmann R, Scherbakov N, Cramer L, Rauchhaus M, Grosse-Herrenthey A, Krueger M, von Haehling S, Doehner W, Anker SD, Bauditz J. Intestinal blood flow in patients with chronic heart failure. J Am Coll Cardiol 64: 1092–1102, 2014. doi: 10.1016/j.jacc.2014.06.1179. [DOI] [PubMed] [Google Scholar]
- 90.Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, Schroedl W, Karhausen T, Doehner W, Rauchhaus M, Poole-Wilson P, Volk H-D, Lochs H, Anker SD. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol 50: 1561–1569, 2007. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 91.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM, Durgan DJ. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics 49: 96–104, 2017. doi: 10.1152/physiolgenomics.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dan X, Mushi Z, Baili W, Han L, Enqi W, Huanhu Z, Shuchun L. Differential analysis of hypertension-associated intestinal microbiota. Int J Med Sci 16: 872–881, 2019. doi: 10.7150/ijms.29322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-γ deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation 126: 1067–1078, 2012. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim S, Goel R, Kumar A, Qi Y, Lobaton G, Hosaka K, Mohammed M, Handberg EM, Richards EM, Pepine CJ, Raizada MK. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci (Lond) 132: 701–718, 2018. doi: 10.1042/CS20180087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol 16: 137–154, 2019. doi: 10.1038/s41569-018-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut microbiota dysbiosis is linked to hypertension. Hypertension 65: 1331–1340, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05315.GUT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan Q, Gu Y, Li X, Yang W, Jia L, Chen C, Han X, Huang Y, Zhao L, Li P, Fang Z, Zhou J, Guan X, Ding Y, Wang S, Khan M, Xin Y, Li S, Ma Y. Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol 7: 381–389, 2017. doi: 10.3389/fcimb.2017.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics 47: 187–197, 2015. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jama HA, Kaye DM, Marques FZ. The gut microbiota and blood pressure in experimental models. Curr Opin Nephrol Hypertens 28: 97–104, 2019. doi: 10.1097/MNH.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 100.Beale AL, Kaye DM, Marques FZ. The role of the gut microbiome in sex differences in arterial pressure. Biol Sex Differ 10: 22, 2019. doi: 10.1186/s13293-019-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caslin B, Maguire C, Karmakar A, Mohler K, Wylie D, Melamed E. Alcohol shifts gut microbial networks and ameliorates a murine model of neuroinflammation in a sex-specific pattern. Proc Natl Acad Sci USA 116: 25808–25815, 2019. doi: 10.1073/pnas.1912359116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Santis S, Cavalcanti E, Mastronardi M, Jirillo E, Chieppa M. Nutritional keys for intestinal barrier modulation. Front Immunol 6: 612, 2015. doi: 10.3389/fimmu.2015.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cani PD. Human gut microbiome: hopes, threats and promises. Gut 67: 1716–1725, 2018. doi: 10.1136/gutjnl-2018-316723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, Cole-Jeffrey CT, Lobaton GO, Stewart DC, Rubiano A, Simmons CS, Garcia-Pereira F, Johnson RD, Pepine CJ, Raizada MK. Hypertension-linked pathophysiological alterations in the gut. Circ Res 120: 312–323, 2017. doi: 10.1161/CIRCRESAHA.116.309006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Amedei A, Morbidelli L. Circulating metabolites originating from gut microbiota control endothelial cell function. Molecules 24: 3992, 2019. doi: 10.3390/molecules24213992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jayachandran M, Chung SSM, Xu B. A critical review on diet-induced microbiota changes and cardiovascular diseases. Crit Rev Food Sci Nutr 60: 2914–2925, 2020. doi: 10.1080/10408398.2019.1666792. [DOI] [PubMed] [Google Scholar]
- 107.Joe B, McCarthy CG, Edwards JM, Cheng X, Chakraborty S, Yang T, Golonka RM, Mell B, Yeo J-Y, Bearss NR, Furtado J, Saha P, Yeoh BS, Vijay-Kumar M, Wenceslau CF. Microbiota introduced to germ-free rats restores vascular contractility and blood pressure. Hypertension 76: 1847–1855, 2020. doi: 10.1161/HYPERTENSIONAHA.120.15939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Giuffrè M, Campigotto M, Campisciano G, Comar M, Crocè LS. A story of liver and gut microbes: how does the intestinal flora affect liver disease? A review of the literature. Am J Physiol Gastrointest Liver Physiol 318: G889–G906, 2020. doi: 10.1152/ajpgi.00161.2019. [DOI] [PubMed] [Google Scholar]
- 109.Maio R, Dichi JB, Burini RC. Implicações do alcoolismo e da doença hepática crônica sobre o metabolismo de micronutrientes. Arq Gastroenterol 37: 120–124, 2000. doi: 10.1590/s0004-28032000000200009. [DOI] [PubMed] [Google Scholar]
- 110.Canesso MCC, Lacerda NL, Ferreira CM, Gonçalves JL, Almeida D, Gamba C, Cassali G, Pedroso SH, Moreira C, Martins FS, Nicoli JR, Teixeira MM, Godard ALB, Vieira AT. Comparing the effects of acute alcohol consumption in germ-free and conventional mice: the role of the gut microbiota. BMC Microbiol 14: 240–210, 2014. doi: 10.1186/s12866-014-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moreira Júnior RE, de Carvalho LM, Pedersen ASB, Damasceno S, Maioli TU, de Faria AMC, Godard ALB. Interaction between high-fat diet and ethanol intake leads to changes on the fecal microbiome. J Nutr Biochem 72: 108215, 2019. doi: 10.1016/j.jnutbio.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 112.Molina PE, Gardner JD, Souza-Smith FM, Whitaker AM. Alcohol abuse: critical pathophysiological processes and contribution to disease burden. Physiology (Bethesda) 29: 203–215, 2014. doi: 10.1152/physiol.00055.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 53: 96–105, 2011. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang Y, Zhang J. Bile acid metabolism and circadian rhythms. Am J Physiol Gastrointest Liver Physiol 319: G549–G563, 2020. doi: 10.1152/AJPGI.00152.2020. [DOI] [PubMed] [Google Scholar]
- 115.Bluemel S, Williams B, Knight R, Schnabl B. Precision medicine in alcoholic and nonalcoholic fatty liver disease via modulating the gut microbiota. Am J Physiol Gastrointest Liver Physiol 311: G1018–G1036, 2016. doi: 10.1152/ajpgi.00245.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vassallo G, Mirijello A, Ferrulli A, Antonelli M, Landolfi R, Gasbarrini A, Addolorato G. Review article: alcohol and gut microbiota—the possible role of gut microbiota modulation in the treatment of alcoholic liver disease. Aliment Pharmacol Ther 41: 917–927, 2015. doi: 10.1111/apt.13164. [DOI] [PubMed] [Google Scholar]
- 117.Fan Y, Ya-E Z, Ji-Dong W, Yu-Fan L, Ying Z, Ya-Lun S, Meng-Yu M, Rui-Ling Z. Comparison of microbial diversity and composition in the jejunum and colon of alcohol-dependent rats. J Microbiol Biotechnol 28: G1883–G1895, 2018. doi: 10.4014/jmb.1806.06050. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol 303: G32–G41, 2012. doi: 10.1152/ajpgi.00024.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Camilleri M, Lyle BJ, Madsen KL, Sonnenburg J, Verbeke K, Wu GD. Role for diet in normal gut barrier function: developing guidance within the framework of food-labeling regulations. Am J Physiol Gastrointest Liver Physiol 317: G17–G39, 2019. doi: 10.1152/ajpgi.00063.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ying W, Jing T, Bing C, Baifang W, Dai Z, Bingyuan W. Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction-associated proteins. Mol Med Rep 9: 2352–2356, 2014. doi: 10.3892/mmr.2014.2126. [DOI] [PubMed] [Google Scholar]
- 121.Cresci GA, Bush K, Nagy LE. Tributyrin supplementation protects mice from acute ethanol-induced gut injury. Alcohol Clin Exp Res 38: 1489–1501, 2014. doi: 10.1111/acer.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kosnicki KL, Penprase JC, Cintora P, Torres PJ, Harris GL, Brasser SM, Kelley ST. Effects of moderate, voluntary ethanol consumption on the rat and human gut microbiome. Addict Biol 24: 617–630, 2019. doi: 10.1111/adb.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barr T, Sureshchandra S, Ruegger P, Zhang J, Ma W, Borneman J, Grant K, Messaoudi I. Concurrent gut transcriptome and microbiota profiling following chronic ethanol consumption in nonhuman primates. Gut Microbes 9: 338–356, 2018. doi: 10.1080/19490976.2018.1441663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol 302: G966–G978, 2012. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Stärkel P, Windey K, Tremaroli V, Bäckhed F, Verbeke K, De Timary P, Delzenne NM. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci USA 111: E4485–E4493, 2014. doi: 10.1073/pnas.1415174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bjørkhaug ST, Aanes H, Neupane SP, Bramness JG, Malvik S, Henriksen C, Skar V, Medhus AW, Valeur J. Characterization of gut microbiota composition and functions in patients with chronic alcohol overconsumption. Gut Microbes 10: 663–675, 2019. doi: 10.1080/19490976.2019.1580097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou C, Zhao J, Li J, Wang H, Tang C. Acute ethanol administration inhibits Toll-like receptor 4 signaling pathway in rat intestinal epithelia. Alcohol 47: 231–239, 2013. doi: 10.1016/j.alcohol.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 128.Lee J-E, Ha JS, Park H-Y, Lee E. Alteration of gut microbiota composition by short-term low-dose alcohol intake is restored by fermented rice liquor in mice. Food Res Int 128: 108800, 2020. doi: 10.1016/j.foodres.2019.108800. [DOI] [PubMed] [Google Scholar]
- 129.Addolorato G, Ponziani FR, Dionisi T, Mosoni C, Vassallo GA, Sestito L, Petito V, Picca A, Marzetti E, Tarli C, Mirijello A, Zocco MA, Lopetuso LR, Antonelli M, Rando MM, Paroni Sterbini F, Posteraro B, Sanguinetti M, Gasbarrini A. Gut microbiota compositional and functional fingerprint in patients with alcohol use disorder and alcohol‐associated liver disease. Liver Int 40: 878–888, 2020. doi: 10.1111/liv.14383. [DOI] [PubMed] [Google Scholar]
- 130.Queipo-Ortuño MI, Boto-Ordóñez M, Murri M, Gomez-Zumaquero JM, Clemente-Postigo M, Estruch R, Cardona Diaz F, Andrés-Lacueva C, Tinahones FJ. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr 95: 1323–1334, 2012. doi: 10.3945/ajcn.111.027847. [DOI] [PubMed] [Google Scholar]
- 131.Kumar Singh A, Cabral C, Kumar R, Ganguly R, Kumar Rana H, Gupta A, Rosaria Lauro M, Carbone C, Reis F, Pandey AK. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 11: 2216, 2019. doi: 10.3390/nu11092216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 33: 496–503, 2015. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 133.Litvak Y, Byndloss MX, Tsolis RM, Bäumler AJ. Dysbiotic proteobacteria expansion: a microbial signature of epithelial dysfunction. Curr Opin Microbiol 39: 1–6, 2017. doi: 10.1016/j.mib.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 134.Montalban-Arques A, Chaparro M, Gisbert JP, Bernardo D. The innate immune system in the gastrointestinal tract: role of intraepithelial lymphocytes and lamina propria innate lymphoid cells in intestinal inflammation. Inflamm Bowel Dis 24: 1649–1659, 2018. doi: 10.1093/ibd/izy177. [DOI] [PubMed] [Google Scholar]
- 135.Celi P, Cowieson AJ, Fru-Nji F, Steinert RE, Kluenter AM, Verlhac V. Gastrointestinal functionality in animal nutrition and health: new opportunities for sustainable animal production. Anim Feed Sci Technol 234: 88–100, 2017. doi: 10.1016/j.anifeedsci.2017.09.012. [DOI] [Google Scholar]
- 136.Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KRC, Xiao L, Chen W, Mernaugh RL, Cai H, Bernstein KE, Goronzy JJ, Weyand CM, Curci JA, Barbaro NR, Moreno H, Davies SS, Roberts LJ, Madhur MS, Harrison DG. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest 126: 50–67, 2016. doi: 10.1172/JCI80761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brown JM, Hazen SL. Microbial modulation of cardiovascular disease. Nat Rev Microbiol 16: 171–181, 2018. doi: 10.1038/nrmicro.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leclercq S, de Timary P, Delzenne NM, Stärkel P. The link between inflammation, bugs, the intestine and the brain in alcohol dependence. Transl Psychiatry 7: e1048–e1048, 2017. doi: 10.1038/tp.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kany S, Janicova A, Relja B. Innate immunity and alcohol. J Clin Med 8: 1981, 2019. doi: 10.3390/jcm8111981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Muralidharan S, Ambade A, Fulham MA, Deshpande J, Catalano D, Mandrekar P. Moderate alcohol induces stress proteins HSF1 and hsp70 and inhibits proinflammatory cytokines resulting in endotoxin tolerance. J Immunol 193: 1975–1987, 2014. doi: 10.4049/jimmunol.1303468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Goral J, Kovacs EJ. In vivo ethanol exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. J Immunol 174: 456–463, 2005. doi: 10.4049/jimmunol.174.1.456. [DOI] [PubMed] [Google Scholar]
- 142.Nishiyama D, Ikejima K, Honda H, Hirose M, Takei Y, Sato N. Acute ethanol administration down-regulates toll-like receptor-4 in the murine liver. Hepatol Res 23: 130–137, 2002. doi: 10.1016/S1386-6346(01)00168-1. [DOI] [PubMed] [Google Scholar]
- 143.Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. TLR2- and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J Immunol 176: 7628–7635, 2006. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- 144.Bhatty M, Jan BL, Tan W, Pruett SB, Nanduri B. Role of acute ethanol exposure and TLR4 in early events of sepsis in a mouse model. Alcohol 45: 795–803, 2011. doi: 10.1016/j.alcohol.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Boé DM, Nelson S, Zhang P, Bagby GJ. Acute ethanol intoxication suppresses lung chemokine production following infection with Streptococcus pneumoniae. J Infect Dis 184: 1134–1142, 2001. doi: 10.1086/323661. [DOI] [PubMed] [Google Scholar]
- 146.Zhong W, Zhao Y, McClain CJ, Kang YJ, Zhou Z. Inactivation of hepatocyte nuclear factor-4{alpha} mediates alcohol-induced downregulation of intestinal tight junction proteins. Am J Physiol Gastrointest Liver Physiol 299: G643–G651, 2010. doi: 10.1152/ajpgi.00515.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hill DB, Barve S, Joshi-Barve S, McClain C. Increased monocyte nuclear factor-kappa B activation and tumor necrosis factor production in alcoholic hepatitis. J Lab Clin Med 135: 387–395, 2000. doi: 10.1067/mlc.2000.106451. [DOI] [PubMed] [Google Scholar]
- 148.Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, Franchimont D, Louis H, Devière J, Le Moine O. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology 43: 989–1000, 2006. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- 149.Pang M, Bala S, Kodys K, Catalano D, Szabo G. Inhibition of TLR8- and TLR4-induced Type I IFN induction by alcohol is different from its effects on inflammatory cytokine production in monocytes. BMC Immunol 12: 55, 2011. doi: 10.1186/1471-2172-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Szabo G. Consequences of alcohol consumption on host defence. Alcohol Alcohol 34: 830–841, 1999. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- 151.Nosalski R, McGinnigle E, Siedlinski M, Guzik TJ. Novel immune mechanisms in hypertension and cardiovascular risk. Curr Cardiovasc Risk Rep 11: 12, 2017. doi: 10.1007/s12170-017-0537-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Padilla J, Jenkins NT, Vieira-Potter VJ, Laughlin HM. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am J Physiol Regul Integr Comp Physiol 304: R543–R552, 2013. doi: 10.1152/ajpregu.00567.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu P, Huang G, Cao Z, Xie Q, Wei T, Huang C, Li Q, Sun M, Shen W, Gao P. Haematopoietic TLR4 deletion attenuates perivascular brown adipose tissue inflammation in atherosclerotic mice. Biochim Biophys Acta Mol Cell Biol Lipids 1862: 946–957, 2017. doi: 10.1016/j.bbalip.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 154.Withers SB, Forman R, Meza-Perez S, Sorobetea D, Sitnik K, Hopwood T, Lawrence CB, Agace WW, Else KJ, Heagerty AM, Svensson-Frej M, Cruickshank SM. Eosinophils are key regulators of perivascular adipose tissue and vascular functionality. Sci Rep 7: 44571–44512, 2017. doi: 10.1038/srep44571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Queiroz M, Sena CM. Perivascular adipose tissue in age-related vascular disease. Ageing Res Rev 59: 101040, 2020. doi: 10.1016/j.arr.2020.101040. [DOI] [PubMed] [Google Scholar]
- 156.Araujo HN, Victório JA, Valgas da Silva CP, Sponton ACS, Vettorazzi JF, de Moraes C, Davel AP, Zanesco A, Delbin MA. Anti-contractile effects of perivascular adipose tissue in thoracic aorta from rats fed a high-fat diet: role of aerobic exercise training. Clin Exp Pharmacol Physiol 45: 293–302, 2018. doi: 10.1111/1440-1681.12882. [DOI] [PubMed] [Google Scholar]
- 157.Martinez-Quinones P, McCarthy CG, Watts SW, Klee NS, Komic A, Calmasini FB, Priviero F, Warner A, Chenghao Y, Wenceslau CF. Hypertension induced morphological and physiological changes in cells of the arterial wall. Am J Hypertens 31: 1067–1078, 2018. doi: 10.1093/ajh/hpy083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 34: 9–19, 2004. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 159.Szasz T, Webb RC. Perivascular adipose tissue: more than just structural support. Clin Sci (Lond) 122: 1–12, 2012. doi: 10.1042/CS20110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A 13: 277–296, 1991. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 161.Suárez-Zamorano N, Fabbiano S, Chevalier C, Stojanović O, Colin DJ, Stevanović A, Veyrat-Durebex C, Tarallo V, Rigo D, Germain S, Ilievska M, Montet X, Seimbille Y, Hapfelmeier S, Trajkovski M. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med 21: 1497–1501, 2015. doi: 10.1038/nm.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Virtue AT, McCright SJ, Wright JM, Jimenez MT, Mowel WK, Kotzin JJ, Joannas L, Basavappa MG, Spencer SP, Clark ML, Eisennagel SH, Williams A, Levy M, Manne S, Henrickson SE, Wherry EJ, Thaiss CA, Elinav E, Henao-Mejia J. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci Transl Med 11: eaav1892, 2019. doi: 10.1126/scitranslmed.aav1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tran HQ, Bretin A, Adeshirlarijaney A, Yeoh BS, Vijay-Kumar M, Zou J, Denning TL, Chassaing B, Gewirtz AT. Western diet”-induced adipose inflammation requires a complex gut microbiota. Cell Mol Gastroenterol Hepatol 9: 313–333, 2020. doi: 10.1016/j.jcmgh.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Singh V, Yeoh BS, Chassaing B, Xiao X, Saha P, Aguilera Olvera R, Lapek JD, Zhang L, Wang W-B, Hao S, Flythe MD, Gonzalez DJ, Cani PD, Conejo-Garcia JR, Xiong N, Kennett MJ, Joe B, Patterson AD, Gewirtz AT, Vijay-Kumar M. Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Cell 175: 679–694.e22, 2018. doi: 10.1016/j.cell.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schwenger KJ, Clermont-Dejean N, Allard JP. The role of the gut microbiome in chronic liver disease: the clinical evidence revised. JHEP Rep 1: 214–226, 2019. doi: 10.1016/j.jhepr.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shanahan F, Sheehan D. Microbial contributions to chronic inflammation and metabolic disease. Curr Opin Clin Nutr Metab Care 19: 257–262, 2016. doi: 10.1097/MCO.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 167.Khurana S, Raufman JP, Pallone TL. Bile acids regulate cardiovascular function. Clin Transl Sci 4: 210–218, 2011. doi: 10.1111/j.1752-8062.2011.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Busnelli M, Manzini S, Chiesa G. The gut microbiota affects host pathophysiology as an endocrine organ: a focus on cardiovascular disease. Nutrients 12: 79, 2019. doi: 10.3390/nu12010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Trøseid M, Andersen GØ, Broch K, Hov JR. The gut microbiome in coronary artery disease and heart failure: current knowledge and future directions. EBioMedicine 52: 102649, 2020. doi: 10.1016/j.ebiom.2020.102649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ma J, Li H. The role of gut microbiota in atherosclerosis and hypertension. Front Pharmacol 9: 1082–1014, 2018. doi: 10.3389/fphar.2018.01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Islam KBMS, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 141: 1773–1781, 2011. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 172.Perez MJ, Velasco E, Monte MJ, Gonzalez-Buitrago JM, Marin JJG. Maternal ethanol consumption during pregnancy enhances bile acid-induced oxidative stress and apoptosis in fetal rat liver. Toxicology 225: 183–194, 2006. doi: 10.1016/j.tox.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 173.Manley S, Ding W. Role of farnesoid X receptor and bile acids in alcoholic liver disease. Acta Pharm Sin B 5: 158–167, 2015. doi: 10.1016/j.apsb.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chiang JYL, Ferrell JM. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am J Physiol Gastrointest Liver Physiol 318: G554–G573, 2020. doi: 10.1152/ajpgi.00223.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yuan YW, Wang L, Lu ZY, Long Y, Jiao YF, Xia Q, Wen DX, Yu WF. Overexcited MaxiK and KATP channels underlie obstructive jaundice-induced vasoconstrictor hyporeactivity of arterial smooth muscle. Sci Rep 6: 39246–39249, 2016. doi: 10.1038/srep39246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tung BY, Carithers RL. Cholestasis and alcoholic liver disease. Clin Liver Dis 3: 585–601, 1999. doi: 10.1016/s1089-3261(05)70086-6. [DOI] [PubMed] [Google Scholar]
- 177.Andoh A, Tsujikawa T, Fujiyama Y. Role of dietary fiber and short-chain fatty acids in the colon. Curr Pharm Des 9: 347–358, 2003. doi: 10.2174/1381612033391973. [DOI] [PubMed] [Google Scholar]
- 178.Elamin EE, Masclee AA, Dekker J, Pieters H-J, Jonkers DM. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J Nutr 143: 1872–1881, 2013. doi: 10.3945/jn.113.179549. [DOI] [PubMed] [Google Scholar]
- 179.Tan JK, McKenzie C, Mariño E, Macia L, Mackay CR. Metabolite-sensing G protein–coupled receptors—facilitators of diet-related immune regulation. Annu Rev Immunol 35: 371–402, 2017. doi: 10.1146/annurev-immunol-051116-052235. [DOI] [PubMed] [Google Scholar]
- 180.Richards LB, Li M, van Esch BCAM, Garssen J, Folkerts G. The effects of short-chain fatty acids on the cardiovascular system. PharmaNutrition 4: 68–111, 2016. doi: 10.1016/j.phanu.2016.02.001. [DOI] [Google Scholar]
- 181.Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes 5: 202–207, 2014. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Natarajan N, Pluznick JL. From microbe to man: the role of microbial short chain fatty acid metabolites in host cell biology. Am J Physiol Cell Physiol 307: C979–C985, 2014. doi: 10.1152/ajpcell.00228.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan L-X, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 110: 4410–4415, 2013. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang L, Zhu Q, Lu A, Liu X, Zhang L, Xu C, Liu X, Li H, Yang T. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin-angiotensin system. J Hypertens 35: 1899–1908, 2017. doi: 10.1097/HJH.0000000000001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S, Tsvetkov D, Krannich A, Wundersitz S, Avery EG, Haase N, Kräker K, Hering L, Maase M, Kusche-Vihrog K, Grandoch M, Fielitz J, Kempa S, Gollasch M, Zhumadilov Z, Kozhakhmetov S, Kushugulova A, Eckardt K-U, Dechend R, Rump LC, Forslund SK, Müller DN, Stegbauer J, Wilck N. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation 139: 1407–1421, 2019. doi: 10.1161/CIRCULATIONAHA.118.036652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xie G, Zhong W, Zheng X, Li Q, Qiu Y, Li H, Chen H, Zhou Z, Jia W. Chronic ethanol consumption alters mammalian gastrointestinal content metabolites. J Proteome Res 12: 3297–3306, 2013. doi: 10.1021/pr400362z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Glueck B, Han Y, Cresci GAM. Tributyrin supplementation protects immune responses and vasculature and reduces oxidative stress in the proximal colon of mice exposed to chronic-binge ethanol feeding. J Immunol Res 2018: 9671919–9671913, 2018. doi: 10.1155/2018/9671919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mattace Raso G, Simeoli R, Russo R, Iacono A, Santoro A, Paciello O, Ferrante MC, Canani RB, Calignano A, Meli R. Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS One 8: e68626, 2013. doi: 10.1371/journal.pone.0068626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Aguilar EC, dos SL, Leonel AJ, de Oliveira JS, Santos EA, Navia-Pelaez JM, da Silva JF, Mendes BP, Capettini LSA, Teixeira LG, Lemos VS, Alvarez-Leite JI. Oral butyrate reduces oxidative stress in atherosclerotic lesion sites by a mechanism involving NADPH oxidase down-regulation in endothelial cells. J Nutr Biochem 34: 99–105, 2016. doi: 10.1016/j.jnutbio.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 190.Liu Y, Dai M. Trimethylamine N-Oxide generated by the gut microbiota is associated with vascular inflammation: new insights into atherosclerosis. Mediators Inflamm 2020: 4634172–4634115, 2020. doi: 10.1155/2020/4634172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tang WHW, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 116: 448–455, 2015. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163: 1585–1595, 2015. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Singh GB, Zhang Y, Boini KM, Koka S. High mobility group Box 1 mediates TMAO-induced endothelial dysfunction. Int J Mol Sci 20: 3570, 2019. doi: 10.3390/ijms20143570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ma GH, Pan B, Chen Y, Guo CX, Zhao MM, Zheng LM, Chen BX. Trimethylamine N-oxide in atherogenesis: impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci Rep 37: 1–12, 2017. doi: 10.1042/BSR20160244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Seldin MM, Meng Y, Qi H, Zhu WF, Wang Z, Hazen SL, Lusis AJ, Shih DM. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κb. J Am Heart Assoc 5: 1–12, 2016. doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Restini CBA, Fink GD, Watts SW. Vascular reactivity stimulated by TMA and TMAO: are perivascular adipose tissue and endothelium involved? Pharmacol Res 163: 105273, 2021. doi: 10.1016/j.phrs.2020.105273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hoyt LR, Randall MJ, Ather JL, DePuccio DP, Landry CC, Qian X, Janssen-Heininger YM, van der Vliet A, Dixon AE, Amiel E, Poynter ME. Mitochondrial ROS induced by chronic ethanol exposure promote hyper-activation of the NLRP3 inflammasome. Redox Biol 12: 883–896, 2017. doi: 10.1016/j.redox.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol 183: 1320–1327, 2009. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Romano KA, Vivas EI, Amador-Noguez D, Rey FE. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 6: e02481, 2015. doi: 10.1128/mBio.02481-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Singh MV, Chapleau MW, Harwani SC, Abboud FM. The immune system and hypertension. Immunol Res 59: 243–253, 2014. doi: 10.1007/s12026-014-8548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.McCarthy CG, Wenceslau CF, Goulopoulou S, Ogbi S, Matsumoto T, Webb RC. Autoimmune therapeutic chloroquine lowers blood pressure and improves endothelial function in spontaneously hypertensive rats. Pharmacol Res 113: 384–394, 2016. doi: 10.1016/j.phrs.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol 23: 541–548, 2002. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- 203.Norgauer J, Krutmann J, Dobos GJ, Traynor-Kaplan AE, Oades ZG, Schraufstätter IU. Actin polymerization, calcium-transients, and phospholipid metabolism in human neutrophils after stimulation with interleukin-8 and N-formyl peptide. J Invest Dermatol 102: 310–314, 1994. doi: 10.1111/1523-1747.ep12371788. [DOI] [PubMed] [Google Scholar]
- 204.Wenceslau CF, McCarthy CG, Szasz T, Goulopoulou S, Webb RC. Mitochondrial N -formyl peptides induce cardiovascular collapse and sepsis-like syndrome. Am J Physiol Heart Circ Physiol 308: H768–H777, 2015. doi: 10.1152/ajpheart.00779.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wenceslau CF, Szasz T, McCarthy CG, Baban B, NeSmith E, Webb RC. Mitochondrial N-formyl peptides cause airway contraction and lung neutrophil infiltration via formyl peptide receptor activation. Pulm Pharmacol Ther 37: 49–56, 2016. doi: 10.1016/j.pupt.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wenceslau CF, McCarthy CG, Szasz T, Calmasini FB, Mamenko M, Webb RC. Formyl peptide receptor-1 activation exerts a critical role for the dynamic plasticity of arteries via actin polymerization. Pharmacol Res 141: 276–290, 2019. doi: 10.1016/j.phrs.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Edwards JM, Roy S, Galla SL, Tomcho JC, Bearss NR, Waigi EW, Mell B, Cheng X, Saha P, Vijay-Kumar M, McCarthy CG, Joe B, Wenceslau CF. FPR-1 (Formyl Peptide Receptor-1) activation promotes spontaneous, premature hypertension in Dahl salt-sensitive rats. Hypertension 77: 1191–1202, 2021. doi: 10.1161/HYPERTENSIONAHA.120.16237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rapp JP, Dene H. Development and characteristics of inbred strains of Dahl salt-sensitive and salt-resistant rats. Hypertension 7: 340–349, 1985. doi: 10.1161/01.HYP.7.3.340. [DOI] [PubMed] [Google Scholar]
- 209.Feng Q, Chen WD, Wang YD. Gut microbiota: an integral moderator in health and disease. Front Microbiol 9: 151–158, 2018. doi: 10.3389/fmicb.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vikram A, Kim YR, Kumar S, Li Q, Kassan M, Jacobs JS, Irani K. Vascular microRNA-204 is remotely governed by the microbiome and impairs endothelium-dependent vasorelaxation by downregulating Sirtuin1. Nat Commun 7: 12565–12569, 2016. doi: 10.1038/ncomms12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, Alao H, Kodys K, Szabo G. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology 56: 1946–1957, 2012. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Coleman LG, Zou J, Crews FT. Microglial-derived miRNA let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. J Neuroinflammation 14: 1–15, 2017. doi: 10.1186/s12974-017-0799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lippai D, Bala S, Catalano D, Kodys K, Szabo G. Micro-RNA-155 deficiency prevents alcohol-induced serum endotoxin increase and small bowel inflammation in mice. Alcohol Clin Exp Res 38: 2217–2224, 2014. doi: 10.1111/acer.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Natarajan SK, Pachunka JM, Mott JL. Role of microRNAs in alcohol-induced multi-organ injury. Biomolecules 5: 3309–3338, 2015. doi: 10.3390/biom5043309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Assis VO, Gonzaga NA, Silva CBP, Pereira LC, Padovan CM, Tirapelli CR. Ethanol withdrawal alters the oxidative state of the heart through AT1-dependent mechanisms. Alcohol Alcohol 55: 3–10, 2020. doi: 10.1093/alcalc/agz101. [DOI] [PubMed] [Google Scholar]