Abstract
Background
Adipocyte-derived adiponectin may play a role in the host inflammatory response to cancer. We examined the association of plasma adiponectin with the density of tumor-infiltrating lymphocytes (TILs) in colon cancers and with vitamin D, clinicopathological features, and patient survival.
Methods
Plasma adiponectin and 25-hydroxyvitamin D [25(OH)D] were analyzed by radioimmunoassay in 600 patients with stage III colon cancer who received FOLFOX-based adjuvant chemotherapy (NCCTG N0147 [Alliance]). TIL densities were determined in histopathological sections. Associations with disease-free survival (DFS), time to recurrence, and overall survival were evaluated by multivariable Cox regression adjusting for potential confounders (ie, body mass index, race, TILs, and N stage). All statistical tests were 2-sided.
Results
We found a statistically significant reduction in adiponectin, but not 25(OH)D, levels in tumors with high vs low TIL densities (median = 6845 vs 8984 ng/mL; P = .04). A statistically significant reduction in adiponectin was also observed in obese (body mass index >30 kg/m2) vs nonobese patients (median = 6608 vs 12 351 ng/mL; P < .001), in men vs women (median = 8185 vs 11 567 ng/mL; P < .001), in Blacks vs Whites or Asians (median = 6412 vs 8847 vs 7858 ng/mL; P < .03), and in those with fewer lymph node metastases (N1 vs N2: median = 7768 vs 9253 ng/mL; P = .01). Insufficiency of 25(OH)D (<30 ng/mL) was detected in 291 (48.5%) patients. In multivariable analyses, neither adiponectin nor 25(OH)D were associated with a statistically significant difference in DFS, overall survival , or time to recurrence in models adjusted for potential confounders. We found a statistically significant association of TILs with prognosis, yet no such interaction was observed for the association of adiponectin with TILs for DFS.
Conclusions
Lower circulating adiponectin levels were associated with a statistically significant increase in TIL densities in colon cancers, indicating an enhanced antitumor immune response. In contrast to TILs, neither adiponectin nor 25(OH)D was independently prognostic.
Colorectal cancer (CRC) is the third most common cancer and is second only to lung cancer as a cause of cancer-related mortality in the United States (1). Obesity is an established risk factor in this malignancy, and it is postulated that adiponectin may mediate the biological link between obesity and CRC (2). Although the mechanism for this potential link is unknown, adipocyte-derived adiponectin may play a role in immune regulation and in the host inflammatory response to cancer (3,4). Adiponectin, the most abundant hormone secreted by adipose tissue, senses metabolic stress and modulates metabolic adaption by targeting the innate immune system under physiological and pathological conditions (5). Epidemiological studies suggest an inverse association between plasma adiponectin levels and risk of developing CRC (6,7). This inverse relationship was observed among men, but not women, in the Nurses’ Health Study and Health Professionals Follow-up Study (6). In a meta-analysis of patients with established CRC, a statistically significant decrease in adiponectin levels was observed compared with non CRC controls (8). Limited data suggest an association between prediagnostic plasma adiponectin and risk of CRC-specific and overall mortality (9). However, the only prospective study of adiponectin measured at the time of diagnosis of CRC was not prognostic (10), and postdiagnosis data are lacking.
Plasma adiponectin was found to be associated with vitamin D levels in non-CRC patients (11). Vitamin D insufficiency (<30 ng/mL) is relatively common in healthy individuals and in CRC populations (12). Serum 25-hydroxyvitamin D [25(OH)D] was inversely associated with CRC risk (13), and some studies suggest that 25(OH)D insufficiency in patients with established CRC may be associated with worse clinical outcome (14-16). Individuals deficient in vitamin D had statistically significantly higher levels of the serum inflammatory biomarkers interleukin-6 (IL-6) and C-reactive protein (17). Interestingly, a prior study found that a high 25(OH)D level was associated with a lower risk of developing CRC with an intense immune reaction (18), suggesting that vitamin D may influence the tumor-host interaction. In a study of the 25(OH)D score measured post-CRC diagnosis, its association with CRC-specific mortality differed by the extent of peritumoral lymphocytic reaction (19).
To date, adiponectin has not been analyzed in relationship to the tumor immune microenvironment. We sought to test the hypothesis that adiponectin may play a role in the host inflammatory response to colon cancer, indicated by tumor-infiltrating lymphocytes (TILs). In patients with CRC, studies indicate that TILs can independently predict recurrence and survival (20-22). However, only limited data exist for adiponectin or vitamin D in patients with established CRC, and their impact on survival is largely unknown. We determined the association of postsurgical plasma adiponectin levels with TIL densities, 25(OH)D, clinicopathological features, and clinical outcome in patients with stage III colon cancers from a phase III adjuvant trial of FOLFOX-based chemotherapy (NCCTG N0147 [Alliance]) (23).
Methods
Study Population
Among 2686 patients with resected stage III colon cancer who had been randomly assigned to adjuvant FOLFOX alone or combined with cetuximab, we randomly selected 600 patients (300 per arm) for analysis of adiponectin and 25(OH)D (NCCTG N0147; ClinicalTrials.gov Identifier: NCT00079274). Right-sided tumors were defined as located proximal to the splenic flexure. N1 tumors had 1-3 metastatic regional lymph nodes; N2 tumors had at least 4 nodes. All patients provided written informed consent at the time of clinical trial enrollment. All data analyses shown here were approved by Mayo Clinic Institution Review Board.
Adiponectin and Vitamin D Assays
Plasma adiponectin and 25(OH)D were analyzed in processed and stored blood obtained at adjuvant study registration. Biomarker concentrations were measured by radioimmunoassay (laboratory of M. Pollak, McGill University, Montreal, QC, Canada) as previously described (24). Circulating levels of total and high molecular weight adiponectin were measured in duplicate using standard enzyme-linked immunosorbent assay methods. Because total and high molecular weight adiponectin levels were almost perfectly correlated (Spearman P = .99), we report total adiponectin. 25(OH)D was extracted from serum or plasma with acetonitrile, and samples were then assayed using an equilibrium radioimmunoassay procedure using an antibody specific for 25(OH)D (24). Blinded quality control (QC) samples were included with test samples, and pooled QC specimens were added to each of the batches. For both assays, masked QC samples were interspersed among case samples. All laboratory personnel were blinded to patient outcomes. The mean coefficient of variation of the assay was 8%.
Histologic Examination of TILs
A representative hematoxylin and eosin-stained tumor section from each patient was scanned at low power to identify areas with the most intraepithelial TILs. Once identified, 5 consecutive 40X fields were counted, and mean TILs per high power field (HPF) were calculated by dividing the total number of TILs by 5. All cases were scored independently by 2 gastrointestinal pathologists blinded to clinical and molecular data (25). Cutoffs for TIL densities were previously determined in N0147 tumors based on their association with patient (disease-free survival [DFS]) and categorized as low (≤3 per HPF) vs high (>3 per HPF) TILs (25).
DNA Mismatch Repair (MMR) Status and KRAS/BRAF Mutation Analysis
Prospectively collected tumor tissues were analyzed for MMR status by analysis of MLH1, MSH2, and MSH6 proteins using immunohistochemistry. Tumors with deficient MMR were defined as having absent expression of 1 or more MMR protein. Testing for a BRAF (c.1799T>A V600E) mutation in exon 15 and KRAS mutations in codons 12 and 13 of exon 2 was previously described (26).
Statistical Analysis
Adiponectin and 25(OH)D were analyzed as continuous variables in the primary analysis. As binary variables, adiponectin was dichotomized at the median, and 25(OH)D was dichotomized at 30 ng/ml with lower levels regarded as insufficiency (12). The association of adiponectin with TILs was prespecified as the primary analysis and was analyzed by the Kruskal Wallis test. Associations between adiponectin and 25(OH)D and with clinicopathological features were assessed by Fisher exact, Pearson χ2, t test, and Kruskal-Wallis tests as appropriate. The association between adiponectin and clinical outcomes were as follows: DFS, time from randomization to recurrence or death from any cause; overall survival (OS), time from randomization to death from all causes; and time to recurrence (TTR) as time from randomization to recurrence. Distributions of DFS, OS, and TTR were estimated by the Kaplan-Meier method. Biomarkers were analyzed in relationship to clinical outcome as continuous variables using a relative risk model with cubic splines and as binary variables in multivariable Cox regression. Multivariable models included variables that were considered to be potential confounders. Interaction tests were performed. A 2-sided P value of less than .05 was considered statistically significant and was not adjusted for multiple comparisons. SAS software version 9.4 and R version 3.6.2 were used (SAS Institute, Cary, NC).
Results
Association of Plasma Adiponectin and 25(OH)D With Patient Characteristics and TILs
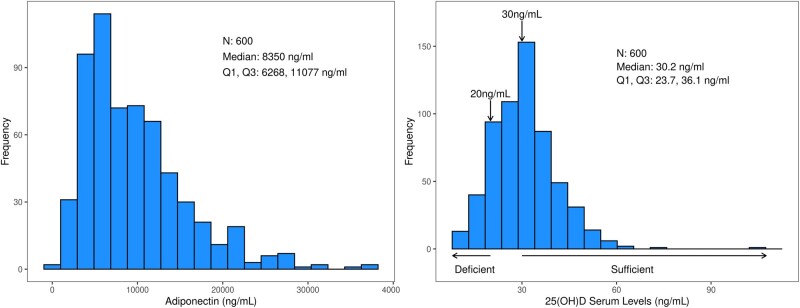
The frequency distribution and median levels of plasma adiponectin and 25(OH)D are shown in Figure 1. We found a statistically significant decrease in the level of adiponectin in patients whose tumors had high vs low TIL densities (median = 6845 vs 8984 ng/mL; P = .04) (Table 1). Furthermore, a statistically significant and inverse association between adiponectin level and body mass index (BMI) category was observed whereby obese patients had a lower median adiponectin level (median = 6608 ng/mL) than did those of normal weight (median = 10 693 ng/mL) or underweight (median = 18 010 ng/mL; P < .001). A statistically significant decrease in adiponectin was observed in men vs women (median = 8185 vs 11 567 ng/mL; P < .001), in those aged 65 years or younger (median = 7663 vs 9279 ng/mL; P = .007), and in Blacks vs Whites or Asians (median = 6412 vs 8847 vs 7858 ng/mL; P < .03) (Table 1). Findings by race were not explained by differences in BMI, which was similar by race. Adiponectin levels differed by N stage in that a lower level was associated with fewer regional lymph node metastases (N1 vs N2: median = 7768 vs 9253 ng/mL; P = .01). Adiponectin was not associated with a statistically significant correlation with 25(OH)D (Spearman r = −.0038; P = .93) or with T stage, DNA MMR, or the mutational status of KRAS or BRAF genes.
Figure 1.
Frequency distributions of postsurgical plasma adiponectin (left) and 25-hydroxyvitamin D (right) in 600 patients with surgically resected stage III colon cancers treated with adjuvant FOLFOX-based chemotherapy.
Table 1.
Association between plasma adiponectin level and clinicopathological variables
| Variable | No. | Plasma adiponectin level, ng/mL |
P a | ||
|---|---|---|---|---|---|
| Mean (SD) | Median | Q 1, Q3 | |||
| Age, y | .007 | ||||
| ≤65 | 420 | 9372.2 (5877.4) | 7663.0 | 5106.0, 12 419.5 | |
| >65 | 180 | 10 751.7 (6382.7) | 9279.0 | 6268.8, 13 581.8 | |
| Race | .002 | ||||
| Asian | 28 | 9503.2 (5247.7) | 7858.0 | 5110.2, 13 653.0 | |
| Black | 53 | 7245.5 (4791.3) | 6412.0 | 4086.0, 8988.0 | |
| White | 502 | 10 065.3 (6115.2) | 8847.0 | 5472.5, 12 842.8 | |
| Sex | <.001 | ||||
| Female | 284 | 11 567.9 (6528.8) | 10 439.0 | 6420.2, 14 978.5 | |
| Male | 316 | 8184.7 (5111.8) | 6816.5 | 4466.2, 10 694.8 | |
| BMI | <.001 | ||||
| Underweight | 10 | 16944.1 (9775.4) | 18009.5 | 8890.0, 25 879.0 | |
| Normal | 159 | 12 218.2 (6908.1) | 10693.0 | 6906.0, 15 800.0 | |
| Overweight | 210 | 9454.6 (5508.4) | 8350.0 | 52 38.5, 12 040.8 | |
| Obese | 218 | 7938.8 (4759.6) | 6608.0 | 45 96.0, 10 348.0 | |
| T stage | .48 | ||||
| T1 or T2 | 96 | 9348.5 (6397.1) | 6961.0 | 5084.0, 12 162.8 | |
| T3 | 441 | 9896.8 (6013.4) | 8580.0 | 5328.0, 12 729.0 | |
| T4 | 63 | 9677.5 (5933.8) | 8741.0 | 5411.5, 12 695.5 | |
| N stage | .01 | ||||
| N1 | 365 | 9372.3 (6008.2) | 7768.0 | 4959.0, 12 069.0 | |
| N2 | 235 | 10 428.7 (6099.8) | 9253.0 | 5733.0, 13 262.0 | |
| Performance status | .93 | ||||
| 0 | 453 | 9791.9 (6015.2) | 8435.0 | 5308.0, 12 729.0 | |
| 1 | 141 | 9706.5 (6178.2) | 8145.0 | 5215.0, 11 977.0 | |
| 2 | 6 | 11 212.0 (7671.0) | 10 068.0 | 4993.0, 17 817.5 | |
| Tumor location | .74 | ||||
| Left | 286 | 9822.6 (5996.2) | 8390.0 | 5331.2, 13 020.8 | |
| Right | 303 | 9721.2 (5957.8) | 8354.0 | 5250.0, 12 285.5 | |
| TILs | .04 | ||||
| Low (≤3) | 248 | 10 163.4 (5881.1) | 8983.5 | 5575.5, 13 467.0 | |
| High (>3) | 100 | 9294.3 (6612.9) | 6844.5 | 4945.5, 11 389.8 | |
| BRAF | .85 | ||||
| Mutant | 198 | 9548.7 (5729.9) | 8147.0 | 5501.8, 12 046.0 | |
| Wild type | 357 | 9829.8 (6070.0) | 8434.0 | 5215.0, 12 729.0 | |
| KRAS | .61 | ||||
| Mutant | 73 | 10 287.8 (6416.9) | 8492.0 | 5443.0, 12 729.0 | |
| Wild type | 482 | 9645.0 (5875.3) | 8350.0 | 5278.5, 12 552.5 | |
| MMR | .96 | ||||
| dMMR | 73 | 9784.9 (5995.4) | 8580.0 | 5443.0, 11 972.0 | |
| pMMR | 504 | 9762.8 (5955.6) | 8303.0 | 5279.5, 12 687.5 | |
Kruskal-Wallis rank sum test. All statistical tests were 2-sided. BMI = body mass index; dMMR = deficient mismatch repair; MMR = mismatch repair; pMMR = proficient mismatch repair; TILs = tumor-infiltrating lymphocytes; Q = Quartile.
A statistically significant reduction in plasma 25(OH)D, as a continuous variable, was found in Blacks vs Whites or Asians (P < .001) (Table 2). Differences by sex were statistically, but not clinically significant. A statistically significant decrease in 25(OH)D levels was found in the relatively few patients with poor performance status. Insufficiency of 25(OH)D (<0 ng/mL) was detected in 49% (291 of 600) of study participants. Consistent results were found for the dichotomous 25(OH)D by patient race (P < .001) and sex (P < .03) (Supplementary Table 1, available online). Specifically, insufficiency of 25(OH)D was more common in Blacks vs Whites or Asians (73.6% vs 45% vs 53.6%) and in women vs men (53.2% vs 44.3%), with both achieving statistical significance. 25(OH)D was not statistically significantly associated with BMI or adiponectin level. Neither the association of the continuous nor of the dichotomous 25(OH)D level with TIL density achieved statistical significance (Table 2; Supplementary Table 1, available online).
Table 2.
Association between continuous vitamin D level and clinicopathological variables
| Variable | No. | Vitamin D level, ng/mL |
P a | ||
|---|---|---|---|---|---|
| Mean (SD) | Median | Q1, Q3 | |||
| Age, y | .74 | ||||
| ≤65 | 420 | 30.9 (10.2) | 30.3 | 24.0, 35.9 | |
| >65 | 180 | 30.7 (10.3) | 30.0 | 23.2, 36.5 | |
| Race | <.001 | ||||
| Asian | 28 | 30.7 (9.9) | 29.6 | 25.0, 35.7 | |
| Black | 53 | 25.2 (8.7) | 23.1 | 18.9, 30.3 | |
| White | 502 | 31.5 (10.3) | 31.0 | 24.2, 36.7 | |
| Sex | .03 | ||||
| Female | 284 | 30.0 (10.9) | 29.2 | 22.7, 35.7 | |
| Male | 316 | 31.5 (9.5) | 30.8 | 25.6, 36.4 | |
| BMI | .09 | ||||
| Underweight | 10 | 30.7 (8.8) | 32.4 | 26.2, 35.6 | |
| Normal | 159 | 31.8 (10.2) | 30.6 | 24.9, 36.6 | |
| Overweight | 210 | 31.3 (9.9) | 31.5 | 23.4, 37.5 | |
| Obese | 218 | 29.6 (10.5) | 29.3 | 23.1, 34.0 | |
| T stage | .51 | ||||
| T1 or T2 | 96 | 31.9 (10.9) | 31.0 | 24.6, 38.3 | |
| T3 | 441 | 30.7 (10.1) | 30.2 | 23.5, 35.9 | |
| T4 | 63 | 29.9 (9.3) | 29.8 | 23.5, 34.5 | |
| N stage | .60 | ||||
| 1-3 | 365 | 31.1 (11.0) | 30.2 | 23.7, 36.6 | |
| 4 | 235 | 30.3 (8.8) | 30.2 | 23.7, 35.2 | |
| Performance status | .047 | ||||
| 0 | 453 | 31.3 (10.4) | 30.8 | 24.2, 36.7 | |
| 1 | 141 | 29.3 (9.5) | 29.2 | 22.7, 34.9 | |
| 2 | 6 | 26.0 (4.5) | 25.8 | 22.7, 28.1 | |
| Tumor location | .34 | ||||
| Left | 286 | 31.4 (10.8) | 30.7 | 24.2, 36.9 | |
| Right | 303 | 30.4 (9.6) | 29.8 | 23.6, 35.7 | |
| TILs | .07 | ||||
| Low (≤3) | 248 | 31.4 (10.7) | 30.2 | 24.2, 37.6 | |
| High (>3) | 100 | 29.1 (9.4) | 29.2 | 22.6, 34.2 | |
| BRAF | .82 | ||||
| Mutant | 198 | 31.4 (11.5) | 30.1 | 23.7, 36.5 | |
| Wild type | 357 | 30.5 (9.6) | 30.3 | 23.4, 36.0 | |
| KRAS | .47 | ||||
| Mutant | 73 | 29.8 (10.1) | 30.2 | 22.5, 36.1 | |
| Wild type | 482 | 31.0 (10.3) | 30.2 | 23.7, 36.1 | |
| MMR | .90 | ||||
| dMMR | 73 | 30.6 (10.2) | 31.2 | 22.7, 36.1 | |
| pMMR | 504 | 30.8 (10.3) | 30.1 | 23.7, 35.9 | |
Kruskal-Wallis rank sum test. All statistical tests were 2-sided. BMI = body mass index; dMMR = deficient mismatch repair; MMR = mismatch repair; pMMR = proficient mismatch repair; TILs = tumor-infiltrating lymphocytes; Q = Quartile.
Association of Plasma Adiponectin and 25(OH)D Levels With Patient Clinical Outcome
Univariately, plasma adiponectin was not statistically significantly associated with DFS as a dichotomous variable (cut at median; hazard ratio [HR] = 0.98, 95% confidence interval [CI] = 0.74 to 1.29; P = .88) shown in a Kaplan Meier plot (Figure 2, A) or a continuous variable shown by cubic spline (Figure 2, C). No association was found between adiponectin and OS (HR = 0.90, 95% CI = 0.66 to 1.22; P = .49) or TTR (HR = 1.03, 95% CI = 0.75 to 1.34; P = 1.00) (data not shown). We then constructed a multivariable model that included adiponectin and potential confounding variables based on observed associations (Table 1) and the literature. The association of adiponectin with patient DFS did not achieve statistical significance as a continuous (adjusted HR [HRadj] = 1.01, 95% CI = 0.98 to 1.04; adjusted P [Padj] = .57) or a dichotomous (data not shown) variable in a multivariable model adjusted for treatment arm, race, BMI, TILs, and N stage (Table 3). In this model, statistically significantly poorer DFS was observed for tumors with low vs high TILs (HRadj = 1.77, 95% CI = 1.11 to 2.81; Padj = .02) and N2 vs N1 stage (HRadj = 2.08, 95% CI = 1.44 to 2.99; Padj < .001) (Table 3). Adiponectin was not statistically significantly associated with OS or TTR as either a continuous or dichotomous variable.
Figure 2.
Univariate association of postsurgical plasma adiponectin and 25(OH)D with DFS in patients with stage III colon cancer. Variables are analyzed as dichotomous using Kaplan-Meier plots (A, B) or as continuous using a relative risk model with cubic splines (C, D). Adiponectin level was dichotomized at the median value (A), and 25(OH)D level was dichotomized as insufficient (<30 ng/mL) vs sufficient (>30 ng/mL) (B). All statistical tests were 2-sided. 25(OH)D = 25-hydroxyvitamin D; CI = confidence interval; d.f. = degrees of freedom; DFS = disease-free survival; HR = hazard ratio.
Table 3.
Multivariable analysis of plasma adiponectin and patient disease-free survival
| Variable | Events/Total | HR (95% CI) | P |
|---|---|---|---|
| Adiponectin (step size: 1000) | 1.01 (0.98 to 1.04) | .57a | |
| Treatment arm | |||
| FOLFOX | 56/169 | Referent | |
| FOLFOX + cetuximab | 66/169 | 1.09 (0.76 to 1.58) | .63a |
| Race | .34b | ||
| Asian | 3/14 | 0.47 (0.15 to 1.51) | .20a |
| Black | 9/22 | 1.09 (0.55 to 2.18) | .81a |
| White | 110/302 | Referent | |
| BMI | .41b | ||
| Normal | 28/88 | Referent | |
| Obese | 54/133 | 1.50 (0.93 to 2.41) | .10a |
| Overweight | 38/112 | 1.26 (0.77 to 2.07) | .36a |
| Underweight | 2/5 | 1.02 (0.23 to 4.49) | .97a |
| TILs | |||
| Low (≤3) | 99/241 | 1.77 (1.11 to 2.81) | .02a |
| High (>3) | 23/97 | Referent | |
| N Stage | |||
| N1 | 59/214 | Referent | |
| N2 | 63/124 | 2.08 (1.44 to 2.99) | <.001a |
Covariate Wald P value. All tests were 2-sided. BMI = body mass index; CI = confidence interval; HR = hazard ratio; TILs = tumor-infiltrating lymphocytes.
Type 3 likelihood-ratio P value. All tests were 2-sided.
Univariately, insufficient vs sufficient 25(OH)D was not statistically significantly associated with DFS (HR = 1.12, 95% CI = 0.85 to 1.47; P = .44) (Figure 2, B), OS (HR = 1.20, 95% CI = 0.88 to 1.63; P = .25), or TTR (HR = 1.11, 95% CI = 0.83 to 1.49; P = .47). Similarly, the continuous 25(OH)D was not statistically significantly associated with DFS (Figure 2, D) or with other outcome variables. In contrast, low vs high TIL density was associated with poorer patient DFS (HR = 1.89, 95% CI = 1.20 to 2.94; P = .005) that achieved statistical significance with 3-year survival rates of 65.4% vs 80.5%, respectively. In a multivariable model that included potential confounders, 25(OH)D as a continuous variable was not associated with patient DFS (HRadj = 1.00, 95% CI = 0.98 to 1.02; Padj = 1.00) (Table 4) or with OS or TTR (data not shown). In this model, TILs (low vs high: HR = 1.77, 95% CI = 1.11 to 2.83; P = .02) and N stage (HR = 2.10, 95% CI = 1.46 to 3.02; P < .001) were statistically significantly associated with DFS (Table 4). We also examined 25(OH)D as a dichotomous variable whereby insufficiency (<30 ng/mL) was not associated with a statistically significant difference in DFS, OS, or TTR (data not shown). Furthermore, we dichotomized 25(OH)D according to the World Health Organization definition of vitamin D deficiency (<20 ng/mL) and found that it was again not statistically significantly prognostic for DFS (data not shown).
Table 4.
Multivariate analysis of plasma 25-hydroxyvitamin D and patient disease-free survival
| Variable | Events/Total | HR (95% CI) | P |
|---|---|---|---|
| Vitamin D ng/mL (step size: 1) | 1.00 (0.98 to 1.02) | 1.00a | |
| Treatment arm | |||
| FOLFOX | 56/169 | Referent | |
| FOLFOX + cetuximab | 66/169 | 1.11 (0.77 to 1.59) | .58a |
| Race | .32b | ||
| Asian | 3/14 | 0.46 (0.14 to 1.46) | .19a |
| Black | 9/22 | 1.07 (0.53 to 2.16) | .85a |
| White | 110/302 | Referent | |
| BMI | .46b | ||
| Normal | 28/88 | Referent | |
| Obese | 54/133 | 1.45 (0.91 to 2.30) | .12a |
| Overweight | 38/112 | 1.24 (0.76 to 2.03) | .39a |
| Underweight | 2/5 | 1.13 (0.27 to 4.78) | .87a |
| TILs | |||
| Low (≤3) | 99/241 | 1.77 (1.11 to 2.83) | .02a |
| High (>3) | 23/97 | Referent | |
| N Stage | |||
| N1 | 59/214 | Referent | |
| N2 | 63/124 | 2.10 (1.46 to 3.02) | <.001a |
Covariate Wald P value. All tests were 2-sided. BMI = body mass index; CI = confidence interval; HR = hazard ratio; TILs = tumor-infiltrating lymphocytes.
Type 3 likelihood-ratio P value. All tests were 2-sided.
We constructed 3-way interaction models that examined the association of adiponectin and 25(OH)D (adjusting for race and BMI) by TILs and N stage subgroups. No statistically significant relationships were found indicating that the observed associations were not interdependent. Of note, TIL density did not differ by BMI category (P = .50) (data not shown). Furthermore and given observed differences in adiponectin and 25(OH)D levels by patient sex (Tables 1 and 2), analysis of 25(OH)D with clinical outcome variables (TTR, DFS, OS) by sex or TIL density failed to reveal any statistically significant associations after adjustment for covariates.
Discussion
We analyzed adiponectin and 25(OH)D in postsurgical blood samples from patients with stage III colon cancer treated in a phase III trial of adjuvant chemotherapy. Based on preclinical data suggesting that adiponectin may influence the host inflammatory response to cancer, we determined the association of adiponectin with TIL density in the tumor immune microenvironment. Lower adiponectin levels were associated with a statistically significant increase in TIL density, indicating an inverse relationship between adiponectin and antitumor immunity. This result is consistent with data in a murine model where adiponectin deficiency was associated with tumors with an increased inflammatory infiltrate compared with tumors in nonadiponectin-deficient mice (27). Adiponectin has been shown to reduce T-cell and B-cell recruitment, induce production of anti-inflammatory cytokines such as IL-10 and an inhibitor of metalloproteinase-1, and inhibit pro-inflammatory chemokines such as IL-6 and TNF-α (5,28). Furthermore, adiponectin was shown to impede inflammation-induced tumorigenesis (29). In contrast to adiponectin, we did not find a statistically significant association between the level of plasma 25(OH)D and TIL density. In another report, higher plasma 25(OH)D levels were associated with a lower risk of CRCs with a high density of CD3+ T cells (18). We found that lower adiponectin levels were statistically significantly associated with cancers showing fewer regional lymph node metastases (N1 vs N2 stage). Relevant to this finding is our prior observation of statistically significantly higher TIL densities in N1 vs N2 stage colon cancers (P = .0001) (25), yet no interaction was found between TIL densities, N stage, and adiponectin in the current study.
Importantly, we confirmed the reported inverse relationship of adiponectin level to BMI in our dataset (30,31). A statistically significant and inverse relationship between adiponectin and BMI category was observed, with the lowest adiponectin levels found among obese patients. Although adiponectin is exclusively secreted by adipocytes, its paradoxical reduction in obesity may reflect reduced secretion from visceral fat rather than subcutaneous fat. Hypertrophic adipocytes synthesize monocyte chemotactic protein-1 leading to infiltration of macrophages in white adipose tissue accompanied by elevated local TNF-α and increased free fatty acid concentrations that suppress adiponectin secretion (32). Other factors may include dysregulation of the adiponectin gene (ADIPOQ) in obesity (33).
We observed differences in adiponectin by sex whereby men had statistically significantly lower levels compared with women. This observation may be explained by higher serum androgens in men (34), and the finding of increased adiponectin levels in older vs younger men may be due to an age-related decline in testosterone levels. In this regard, adiponectin levels were shown to decrease after testosterone administration in hypogonadal men (35). In women, adiponectin has been shown to decrease with the transition to menopause (36). We found statistically significantly lower adiponectin levels among Blacks compared with Whites or Asians, which was not explained by differences in BMI by race. However, lower adiponectin levels among Blacks vs Whites was attributed to adiponectin’s association with BMI in the population-based race-ethnic Northern Manhattan Study (37). Differences in adiponectin levels may also have a hereditary component, and further analysis of single nucleotide polymorphisms in adiponectin-related genes may provide further insight (38).
Whereas plasma adiponectin was associated with vitamin D levels in nonCRC patients (11), no such relationship was observed in our CRC cohort. We observed that nearly one-half of patients were vitamin D insufficient (<30 ng/mL), which far exceeds the 8% prevalence of vitamin D insufficiency in the US general population (39). We found a higher rate of vitamin D insufficiency among Black vs White or Asian. In addition to dietary factors and sunlight exposure, race can influence vitamin D status in that melanin in skin reduces the rate of vitamin D biosynthesis (12,40). Furthermore, racial differences in the prevalence of common genetic polymorphisms can influence vitamin D levels; for example, Blacks are more likely to have the T allele at rs7041 and less likely to have the A allele at rs4588 vs Whites (41). Differences by sex for the continuous vitamin D variable were modest; however, vitamin D insufficiency was statistically significantly more common in women vs men, which is consistent with an increased prevalence of hypovitaminosis D in women especially after menopause (42).
Despite the inverse association of adiponectin levels and TIL densities that we observed, adiponectin was not statistically significantly associated with patient DFS, OS, or TTR univariately or after adjustment for potential confounders. To our knowledge, these are the first data for postsurgical adiponectin and clinical outcome in CRC patients. Adiponectin at diagnosis of CRC was also not prognostic in a prospective study in 344 consecutive cases (10). Regarding prediagnostic adiponectin, 2 large observational cohorts found a statistically significant and independent association of higher levels with reduced CRC-specific and OS that was more evident in patients with metastatic disease (9). A potential explanation may be an elevation of adiponectin during weight loss, which is a known predictor of poor prognosis in cancer patients. Adiponectin is an insulin-sensitizing hormone, and low levels are associated with an increased risk of type 2 diabetes independent of other risk factors (43). Adiponectin mediates its insulin-sensitizing effect through activation of AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor–alpha pathways with resultant suppression of tumor growth in animal models (44). As with adiponectin, plasma 25(OH)D was not statistically significantly associated with clinical outcome variables in our cohort. However, a study of resected stage III colon cancer patients found that a higher predicted 25(OH)D score was associated with improved survival outcomes (16). Of note, 25(OH)D scores were calculated based on serial patient questionnaire data, but not measurements in blood samples. Another study of 1598 patients with stage I to III CRC found that patients with the highest vs lowest tertile of postoperative plasma 25(OH)D levels had lower CRC-specific and all-cause mortality that achieved statistical significance (45). 25(OH)D levels, however, were not prognostic in patients with stage IV CRC (n = 515) from a clinical trial cohort (46). We interpret our results and those in stage IV cancers to suggest that any effect of vitamin D on prognosis is attenuated or lost once the disease has metastasized either to regional lymph nodes (stage III) or to distant sites (stage IV). In contrast, a statistically significant association of TILs with prognosis was found in our cohort with low vs high TILs being associated with poorer patient survival as we previously reported (22,25).
Strengths of our study include same stage patients with uniform treatment in a clinical trial with long-term and meticulous follow-up data. Blood was collected from all patients prior to initiation of chemotherapy, such that the time period between biomarker sampling and initiation of adjuvant treatment was relatively constant. Whereas other studies have used cancer prediagnosis measurements (47), our study is the first to examine postdiagnostic plasma adiponectin levels in relation to survival outcomes in CRC patients. Study limitations include retrospective biomarker analysis that was not prespecified and the single assay timepoint. Importantly, measurement of 25(OH)D at a single timepoint was shown to provide an accurate assessment of an individual’s long-term vitamin D status (48). Similarly, adiponectin levels were shown to remain stable over time (49). We cannot exclude the possibility that the modest sample size of our study may have contributed to the failure to detect a prognostic effect for either biomarker.
In conclusion, we identified a novel inverse association of adiponectin with the intratumoral antitumor immune response indicated by TIL density. Validation of this association in an independent cohort of patients with CRC is warranted. In contrast to TILs, neither adiponectin nor 25(OH)D was found to be prognostic.
Funding
Research reported in this publication was supported by the National Cancer Institute (NCI) of the National Institutes of Health (NIH) under award numbers U10CA180821, U10CA180882; U10CA180863, CCSRI 021039 (CCTG); U10CA180868 (NRG); U10CA180888, and UG1CA233163 (SWOG). NCCTG N0147 received funds from Sanofi Aventis. The study was also supported by NCI R01CA210509 (to FAS).
Notes
Role of the funders: The funders had no role in the design of the study or in the data collection, analysis, or interpretation. Furthermore, the funders did not participate in the writing of the manuscript or in the decision to submit the manuscript for publication.
Disclosures: FAS is a co-inventor of intellectual property with Roche Ventana Medical Systems (Tucson, AZ) and may receive royalties paid to Mayo Clinic. No other relevant conflicts related to the subject matter of this manuscript are reported by the study authors.
Author contributions: Study conceptualization: FAS. Data curation: QS. Formal analysis: SBJ, NRF, QS. Funding acquisition: FAS, SRA. Investigation: TCS, RMG, SJC, SG, MSK, SN, AFS, BNJ, MNP, SRA. Methodology: MNP. Project administration/supervision: FAS. Writing: original draft: FAS. Writing-review and editing: all authors.
Data Availability
Data from published Alliance or North Central Cancer Treatment Group (NCCTG) trials can be accessed by submission of an Alliance Data Sharing Request Form (concepts@allianceNCTN.org). Requests to utilize biomarker data published in this manuscript should also be directed to the corresponding author.
Supplementary Material
References
- 1.Gupta S, Coronado GD, Argenbright K, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(4):283–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanyuda A, Lee DH, Ogino S, et al. Long-term status of predicted body fat percentage, body mass index and other anthropometric factors with risk of colorectal carcinoma: two large prospective cohort studies in the US. Int J Cancer. 2020;146(9):2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folco EJ, Rocha VZ, López-Ilasaca M, Libby P.. Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10. J Biol Chem. 2009;284(38):25569–25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim KY, Kim JK, Han SH, et al. Adiponectin is a negative regulator of NK cell cytotoxicity. J Immunol. 2006;176(10):5958–5964. [DOI] [PubMed] [Google Scholar]
- 5.Luo Y, Liu M.. Adiponectin: a versatile player of innate immunity. J Mol Cell Biol. 2016;8(2):120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song M, Zhang X, Wu K, et al. Plasma adiponectin and soluble leptin receptor and risk of colorectal cancer: a prospective study. Cancer Prev Res (Phila). 2013;6(9):875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.An W, Bai Y, Deng SX, et al. Adiponectin levels in patients with colorectal cancer and adenoma: a meta-analysis. Eur J Cancer Prev. 2012;21(2):126–133. [DOI] [PubMed] [Google Scholar]
- 8.Lu W, Huang Z, Li N, Liu H.. Low circulating total adiponectin, especially its non-high-molecular weight fraction, represents a promising risk factor for colorectal cancer: a meta-analysis. Onco Targets Ther. 2018;11:2519–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong DQ, Mehta RS, Song M, et al. Prediagnostic plasma adiponectin and survival among patients with colorectal cancer. Cancer Prev Res (Phila). 2015;8(12):1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volkova E, Willis JA, Wells JE, et al. Association of angiopoietin-2, C-reactive protein and markers of obesity and insulin resistance with survival outcome in colorectal cancer. Br J Cancer. 2011;104(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaidya A, Williams JS, Forman JP.. The independent association between 25-hydroxyvitamin D and adiponectin and its relation with BMI in two large cohorts: the NHS and the HPFS. Obesity (Silver Spring). 2012;20(1):186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holick MF.Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. [DOI] [PubMed] [Google Scholar]
- 13.Gorham ED, Garland CF, Garland FC, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 2007;32(3):210–216. [DOI] [PubMed] [Google Scholar]
- 14.Ng K, Wolpin BM, Meyerhardt JA, et al. Prospective study of predictors of vitamin D status and survival in patients with colorectal cancer. Br J Cancer. 2009;101(6):916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maalmi H, Walter V, Jansen L, et al. Association between blood 25-hydroxyvitamin D levels and survival in colorectal cancer patients: an updated systematic review and meta-analysis. Nutrients. 2018;10(7):896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs MA, Yuan C, Sato K, et al. Predicted vitamin D status and colon cancer recurrence and mortality in CALGB 89803 (Alliance). Ann Oncol. 2017;28(6):1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wesselink E, Balvers M, Bours MJL, et al. The association between circulating levels of vitamin D and inflammatory markers in the first 2 years after colorectal cancer diagnosis. Therap Adv Gastroenterol. 2020;13:1756284820923922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song M, Nishihara R, Wang M, et al. Plasma 25-hydroxyvitamin D and colorectal cancer risk according to tumour immunity status. Gut. 2016;65(2):296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamada T, Liu L, Nowak JA, et al. Vitamin D status after colorectal cancer diagnosis and patient survival according to immune response to tumour. Eur J Cancer. 2018;103:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rozek LS, Schmit SL, Greenson JK, et al. Tumor-infiltrating lymphocytes, Crohn’s-like lymphoid reaction, and survival from colorectal cancer. J Natl Cancer Inst. 2016;108(8):djw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pages F, Mlecnik B, Marliot F, et al. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391(10135):2128–2139. [DOI] [PubMed] [Google Scholar]
- 22.Sinicrope FA, Shi Q, Hermitte F, et al. Contribution of immunoscore and molecular features to survival prediction in stage III colon cancer. JNCI Cancer Spectr. 2020;4(3):pkaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin DW, Kim MA, Lee J-C, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. BMC Res Notes. 2021;14(1):272–1393.34266478 [Google Scholar]
- 24.Hollis BW.Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–186. [DOI] [PubMed] [Google Scholar]
- 25.Lee H, Sha D, Foster NR, et al. Analysis of tumor microenvironmental features to refine prognosis by T, N risk group in patients with stage III colon cancer (NCCTG N0147) (Alliance). Ann Oncol. 2020;31(4):487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon HH, Tougeron D, Shi Q, et al. ; for the Alliance for Clinical Trials in Oncology. KRAS codon 12 and 13 mutations in relation to disease-free survival in BRAF-wild-type stage III colon cancers from an adjuvant chemotherapy trial (N0147 alliance). Clin Cancer Res. 2014;20(11):3033–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saxena A, Chumanevich A, Fletcher E, et al. Adiponectin deficiency: role in chronic inflammation induced colon cancer. Biochim Biophys Acta. 2012;1822(4):527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fenton JI, Birmingham JM.. Adipokine regulation of colon cancer: adiponectin attenuates interleukin-6-induced colon carcinoma cell proliferation via STAT-3. Mol Carcinog. 2010;49(7):700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yehuda-Shnaidman E, Schwartz B.. Mechanisms linking obesity, inflammation and altered metabolism to colon carcinogenesis. Obes Rev. 2012;13(12):1083–1095. [DOI] [PubMed] [Google Scholar]
- 30.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun . 1999;257(1):79–83. [DOI] [PubMed] [Google Scholar]
- 31.Pais R, Silaghi H, Silaghi AC, et al. Metabolic syndrome and risk of subsequent colorectal cancer. World J Gastroenterol. 2009;15(41):5141–5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makki K, Froguel P, Wolowczuk I.. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hivert MF, Manning AK, McAteer JB, et al. Common variants in the adiponectin gene (ADIPOQ) associated with plasma adiponectin levels, type 2 diabetes, and diabetes-related quantitative traits: the Framingham Offspring Study. Diabetes. 2008;57(12):3353–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Böttner A, Kratzsch JRGEN, MüLler G, et al. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab. 2004;89(8):4053–4061. [DOI] [PubMed] [Google Scholar]
- 35.Lanfranco F, Zitzmann M, Simoni M, Nieschlag E.. Serum adiponectin levels in hypogonadal males: influence of testosterone replacement therapy. Clin Endocrinol. 2004;60(4):500–507. [DOI] [PubMed] [Google Scholar]
- 36.Jürimäe J, Jürimäe T.. Plasma adiponectin concentration in healthy pre- and postmenopausal women: relationship with body composition, bone mineral, and metabolic variables. Am J Physiol Endocrinol Metab. 2007;293(1):E42–47. [DOI] [PubMed] [Google Scholar]
- 37.Gardener H, Crisby M, Sjoberg C, et al. Serum adiponectin in relation to race-ethnicity and vascular risk factors in the Northern Manhattan Study. Metab Syndr Relat Disord. 2013;11(1):46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen SS, Gammon MD, Signorello LB, et al. Serum adiponectin in relation to body mass index and other correlates in Black and White women. Ann Epidemiol. 2011;21(2):86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Looker AC, Johnson CL, Lacher DA, et al. Vitamin D status: United States. NCHS Data Brief 2011. 2011;(59):1–8. [PubMed] [Google Scholar]
- 40.Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and White women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. 2002;76(1):187–192. [DOI] [PubMed] [Google Scholar]
- 41.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of Black Americans and White Americans. N Engl J Med. 2013;369(21):1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verdoia M, Schaffer A, Barbieri L, et al. Impact of gender difference on vitamin D status and its relationship with the extent of coronary artery disease. Nutr Metabol Cardiovasc Dis. 2015;25(5):464–470. [DOI] [PubMed] [Google Scholar]
- 43.Lindberg S, Jensen JS, Pedersen SH, et al. Low adiponectin levels and increased risk of type 2 diabetes in patients with myocardial infarction. Dia Care. 2014;37(11):3003–3008. [DOI] [PubMed] [Google Scholar]
- 44.Moon HS, Liu X, Nagel JM, et al. Salutary effects of adiponectin on colon cancer: in vivo and in vitro studies in mice. Gut. 2013;62(4):561–570. [DOI] [PubMed] [Google Scholar]
- 45.Zgaga L, Theodoratou E, Farrington SM, et al. Plasma vitamin D concentration influences survival outcome after a diagnosis of colorectal cancer. J Clin Oncol. 2014;32(23):2430–2439. [DOI] [PubMed] [Google Scholar]
- 46.Ng K, Sargent DJ, Goldberg RM, et al. Vitamin D status in patients with stage IV colorectal cancer: findings from intergroup trial N9741. J Clin Oncol. 2011;29(12):1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ulrich CM, Holmes RS.. Shedding light on colorectal cancer prognosis: vitamin D and beyond. J Clin Oncol. 2008;26(18):2937–2939. [DOI] [PubMed] [Google Scholar]
- 48.Hofmann JN, Yu K, Horst RL, et al. Long-term variation in serum 25-hydroxyvitamin D concentration among participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2010;19(4):927–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu K, Feskanich D, Fuchs CS, et al. Interactions between plasma levels of 25-hydroxyvitamin D, insulin-like growth factor (IGF)-1 and C-peptide with risk of colorectal cancer. PLoS One. 2011;6(12):e28520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from published Alliance or North Central Cancer Treatment Group (NCCTG) trials can be accessed by submission of an Alliance Data Sharing Request Form (concepts@allianceNCTN.org). Requests to utilize biomarker data published in this manuscript should also be directed to the corresponding author.