Abstract
Tuberculin skin test, also known as the tuberculin or purified protein derivative (PPD) test, is an extensively applied diagnostic test for the detection of primary infection with Mycobacterium Tuberculosis (Mtb). The production of PPD is accompanied by some difficulties that require a series of modifications in the production and purification processes. The present study aimed to determine the facilitation level of the manufacturing process by modifying evaluation methods for the production of PPD tuberculin. Mtb strains were cultured in Lowenstein-Jensen media, and the cultured strains were inoculated into the Dorset-Henley liquid medium by the biphasic medium of potato-Dorset-Henley. After incubation, flasks containing cultured strain were selected for bacterial inactivation, and the optimal gamma radiation dose(s) was determined. Tuberculoproteins were precipitated by ammonium sulfate (AS) and Trichloroacetic acid (TCA). Protein concentration was determined using the Bradford and Kjeldahl protein assay methods. Finally, the lymphocyte transformation test and potency test were performed. Based on the results, the Dorset-Henley liquid medium is suitable for the massive growth of the bacterium. The transferal of Mtb from solid to liquid medium was directly carried out without intermediate culture. It was found that during tuberculoprotein production, heating at 100°C for 3 h would be safe for killing mycobacterium. Furthermore, the simultaneous use of heating and gamma irradiation (8 kGgy) killed all of the mycobacteria, while doses of 1, 1.5, and 7 kGy decreased a significant number of bacterial cells. The results also indicated that the concentration of tuberculoprotein extracted by TCA precipitation method was higher than that obtained by AS precipitation. The tuberculoproteins which were produced by these two methods in the lymphocyte transformation test were not significantly different in terms of potency (P>0.05). Moreover, due to the high volume of produced protein, the protein measurement was more efficiently carried out by the Kjeldahl method, compared to the Bradford method. Finally, the results of the present study demonstrated that in addition to the novel approach of gamma irradiation, optimum methods are efficient and applicable in the production of PPD tuberculin.
Keywords: Mycobacterium Tuberculosis, PPD, Gamma radiation, Lymphocyte transformation test
Introduction
Tuberculosis (TB) as one of the top 10 causes of global death worldwide is an infectious disease usually caused by Mycobacterium tuberculosis (Mtb). World health organization (WHO) estimates that 33% of the world population is infected with Mtb ( Dye et al., 1999 ; Corbett et al., 2003 ). The large reservoir of TB-infected individuals and its antigen diversity are recognized as the major obstacle to the global control of tuberculosis ( Fogel, 2015 ).
In 2017, an estimated 10 million people fell ill with TB, and 1.6 million others died from this disease (including 0.3 million HIV-infected patients). Approximately 95% of deaths caused by Tuberculosis (TB) occur in low-and middle-income countries ( WHO, 2018 ). Today, there has been a growing concern over the HIV/AIDS pandemic, which increases the global burden of tuberculosis, and its impact on Multidrug-resistant TB (MDR-TB) ( Corbett et al., 2003 ; Sterling et al., 2003 ).
TB is one of the most serious public health problems in Iran, being most prevalent among patients with low socioeconomic status. The main reasons for this infection include population density (TB is spread through the air from one person to another), malnutrition, the poor immune response to infection, and transmission from family members infected with the disease ( Khazaei et al., 2005 ). In addition, the immigrants and pilgrims from Pakistan, Afghanistan, and Iraq increase the incidence of this disease ( Hassan Zadeh et al., 2013 ).
Tuberculin skin test (TST) is a reliable tool for the detection of early Mtb infection. Skin test interpretation depends on two factors, including the measurement of induration in millimeters (mm) and the risk of TB infection in a person and its progression if the person is infected. In the tuberculin purified protein derivative (PPD) test, an extract of Mtb is injected into the inner surface of the forearm ( Marcdante and Kliegman, 2014 , Prevention, 2016 ). As a valuable product, tuberculin is still used in human and animal diagnostic programs.
Moreover, Bacillus Calmette-Guérin (BCG) vaccine is used for the prevention and control of TB and its epidemiological impact.
The methods of PPD production have undergone no modifications since the early 1940s. Therefore, updated technologies are required to improve production in this area. PPD production is a crudely prepared protein suspension precipitated by trichloroacetic acid from autoclaved culture supernatants of Mtb in the liquid medium. The traditional production process requires extensive incubation durations for sufficient culture growth. The absence of a standard production protocol for detailing incubation times and specific strains for use results in serious inconsistencies between PPD suspensions ( Wynne et al., 2012 ).
Improvements in production methods, such as modification of growth condition and heat killing of Mtb in cultured cells, can produce consistent in-vivo and in-vitro testing results. Nonetheless, the breakdown of protein components due to the autoclaving process complicates the use of PPD ( Capsel, 2015 ). Removing the autoclaving step, replacing heat-killing with gamma irradiation, and utilizing sterile filtration will ensure the removal of alive bacterial cells from the final product. The absence of consistent PPD production methods leads to some differences among the production lots in terms of potency, challenging the specificity of skin tests ( Capsel, 2015 ).
Nowadays, gamma irradiation is more common in medicine, and Co60, a radioactive metal that emits γ-rays, is widely used as a source of gamma radiation. Gamma rays are intensively used in radiotherapy, sterilization of health products, and production of radio-vaccine ( Wales and Kusel, 1992 ). Furthermore, gamma irradiation holds great promise for the efficient control of microbial contamination of laboratory animal diets ( Shahhosseini et al., 2018.). Gamma irradiation weakens and kills a wide range of pathogens and induces a high level of immunity by destroying pre-pathogenesis bacteria ( Demicheli et al., 2007 ).
The production of tuberculin is accompanied by some difficulties which require a series of modifications in the production and purification process. This paper directly addressed this need through the improvement of the current PPD production process. It aimed to evaluate optimal methods, in addition to the novel approach of gamma irradiation, in the production of PPD tuberculin.
Material and Methods
Culture conditions and growth of Mtb
8-week-old cultures of M. tuberculosis strains C (ATCC 35808, TMC116), DT (ATCC 35810, TMC119), and PN (ATCC 35809, TMC117) in modified Lowenstein Jensen (LJ) medium supplemented with glycerol were separately sub-cultured in modified Sauton and Dorset-Henley medium with 10 replications. Cultured strains in the LJ medium were inoculated into the Dorset-Henley liquid medium by the biphasic medium of potato-Dorset-Henley. Thereafter, the floating colonies of strains were collected and transferred to 500 ml flasks containing a liquid medium.
Determination of the optimal gamma radiation dose(s) for the inactivation of the bacterium
The administration of cobalt-60 irradiation was performed at the Nuclear Science and Technology Research Institute. After 8 weeks of incubation at 35°C, two flasks containing cultured DT strain were subjected to bacterial inactivation. One flask was exposed to steam at 100°C for 3 h without complete closure of the autoclave door. The other flask was incubated in a water bath at 80°C for 1 h. Subsequently, the contents of both flasks were cultured in the LJ medium supplemented with glycerol to determine the suitable method for the killing of the bacterium.
At this stage, 44 bottles with screw caps (including 36 bottles with 100 ml and 8 bottles with 250 ml) containing cultured strain in the Dorset-Henley medium were exposed to a gamma irradiator (SVST-Co60/B) with a cobalt-60 source that emits gamma rays. Following the irradiation process, the bottles were studied for 12 months. The bacterium in each bottle was sub-cultured by the Mycobacteria growth indicator tube (MGIT) system to determine optimum bactericidal dose(s) of gamma irradiation.
Protein sedimentation
Three Erlenmeyer flasks containing a 2-month-old culture of C, DT, and PN strains (each strain in individual Erlenmeyer flasks) in 1 lit of Dorset-Henley medium were subjected to steam at 100°C for 3 h to kill the bacterium. Thereafter, the extracts were separated from bacterial debris using Whatman cellulose filter papers with 1, 0.8, 0.45, and 0.22 μm pore sizes. In the next stage, the extracts (secreted proteins) of three cultured strains were mixed and divided into two parts (each container with 900 ml of filtered solution).
The saturated ammonium sulfate (AS) was gently added to extract in order to precipitate tuberculin (Table 1). The AS was added for 30 min, followed by placing the solution on the magnetic stirrer at 4°C for 24 h to obtain tuberculoprotein precipitation. The supernatant was discarded, and the precipitated residue was re-suspended in one-third of its initial volume with phosphate buffer (6.9±0.1). Thereafter, it was poured into a dialysis bag boiled in 2% ethylenediaminetetraacetic acid for 1 min and was then gently stirred in distilled water on a magnetic mixer at 4°C for 72 h. The final volume of tuberculin using AS precipitation was 490 ml.
Table 1.
Saturated solutions of ammonium sulfate in water at several temperatures
| Temp (°C) | Grams (NH4)2SO4 required to saturate 1000 g of H2O a,b | Grams (NH4)2SO4 added per liter of saturated solution c | Moles (NH4)2SO4 in 1000 g of H2O | Percentage by weight | Molarity of saturated (NH4)2SO4 solution | Apparent specific volume in saturated solution c | VG/1000 c |
|---|---|---|---|---|---|---|---|
| 0 | 706.86 | 514.72 | 5.35 | 41.42 | 3.90 | 0.5262 | 0.271 |
| 10 | 730.53 | 525.05 | 5.53 | 42.22 | 3.97 | 0.5357 | 0.281 |
| 20 | 755.82 | 536.34 | 5.73 | 43.09 | 4.06 | 0.5414 | 0.290 |
| 25 | 766.80 | 541.24 | 5.82 | 43.47 | 4.10 | 0.5435 | 0.294 |
| 30 | 777.55 | 545.88 | 5.91 | 43.85 | 4.13 | 0.5458 | 0.298 |
Note that the volume changes upon addition of solid (NH4)2SO4.
The degree of saturation is usually considered to be that which is calculated for the addition of a saturated solution without allowing for a volume change upon mixing. For example, 1 volume of water plus 1 volume of saturated (NH4)2SO4 is considered to yield a solution that is 0.5 (50%) saturated. According to this convention, the volume (V) of saturated (NH4)2SO4, which has to be added to 100 ml of solution of initial saturation S1 to produce a final saturation S2 is given by the equation: V=100(S2-S1)/(1-S2), where S1 and S2 are expressed as fractions of the saturated solution. For example, if S1=0.5 and S2=0.7, then V=66.67 ml.
When preparing more concentrated solutions of (NH4)2SO4, it is more economical and often more convenient to add the solid salt instead of a stock solution of saturated (NH4)2SO4. The weight in grams, X, to be added to 100 ml of solution of saturation S1 to yield a solution of saturation S2 may be calculated as follows: X=0.1G(S2-S1)/(1-(VG/1000)S2), where G=grams of (NH4)2SO4 in 1000 ml of saturated solution, and V=apparent specific volume of (NH4)2SO4 in a saturated solution. Values of G, V, and VG/1000 at different temperatures are given in columns 3, 7, and 8, respectively. For example, if S1 = 0.5 and S2=0.7, at 0°C, G=514.72 and VG/1000=0.271, then X=20.2 g.
Other tuberculins were precipitated by adding 40% trichloroacetic acid (TCA) at the ratio of 9:1 for 24 h. This solution was allowed to stand overnight at room temperature without stirring. The next day, most of the supernatant was removed by aspiration, and the precipitated residue was used to resuspend the precipitate and transfer to centrifuge bottles. The bottles were centrifuged at 2500 g for 10 min to remove residual supernatant. The precipitate was washed once with 1% TCA and once with 10% NaCl.
Finally, the volumes obtained by two separate methods were unified and the diluents solution of tuberculin (containing 3.5 mM Na2HPO4.7H2O, 10% Glycerol, 5 mM Phenol, 8.5 mM NaCl and D.W up to one liter) was then added to the extract. The pH was adjusted to 6.9±0.1 with 10 N NaOH.
Protein extraction analysis using Bradford and Kjeldahl assay methods
After filtration, the isolated bacterium mass was distorted using sonication (Hielscher, UP400S) at a 2-cm probe depth and 100 or 150 amplitudes on an ice bath at three-minute intervals. After centrifuging at 10000 rpm, the resulting supernatant was filtered with 0.22 and 0.8-micron pore sizes. Subsequently, the extracted protein was measured by the Kjeldahl method (this stage was once performed on bacterium mass, which was killed by water steam at 100°C for 3 h, and once on live bacterium).
Two extracted tuberculin levels in step three were analyzed using the Bradford and Kjeldahl methods. It is noteworthy that the Bradford method is more sensitive, rapid, and accurate, compared to other methods, and is able to detect even 1 µg of protein. In this process, bovine serum albumin (BSA) was used as a standard. In total, 20 tubes were used as standard, whereas two tubes were utilized for control. In addition, a sufficient number of unknown samples were used. The absorbance of samples was read by spectrophotometer at 595 nm from 2 min to 1 h. For protein measurement by Bradford and Kjeldahl methods, the protocol was used as described ( Horwitz and Latimer, 2005 , Babaie et al., 2020 ).
Skin sensitization test
Skin sensitization of 100 albino male guinea pigs with a mean weight of 350-500 g was carried out using a suspension of the sensitizer (killed Mtb powder). After two months, the potency test was performed on 25 guinea pigs to determine the survival rate of vaccinated guinea pigs.
Lymphocyte transformation test (LTT)
The LTT was performed at the end of the seventh week of sensitization. To this end, intracardiac blood samples were collected in heparinized tubes (5 IU/ml). In this respect, the plasma was isolated from the whole blood by centrifugation at 300 g for 15 min. In addition, 3 ml ficoll/histopaque (Sigma-H8889) and 7 ml blood were added to 10 ml tubes and centrifuged at 200 g for 20 min. Buffy coat was gently collected and washed twice in RPMI-1640 medium (Sigma) and once more in RPMI-1640 medium supplemented with 5% FBS at 250 g for 15 min. Subsequently, the supernatant was discarded, and cell viability was determined. In the next stage, 6×106 cells were seeded in 96-well plates, and 200 µl RPMI-1640 (5 mM/ml 1-glutamine, 100 IU/ML penicillin, 100 µg/ml streptomycin, and 20% FBS) was added to the wells. The cells were exposed to 5 µg/ml and 10 µg/ml TCA and AS precipitated proteins and standard reference PPD (Serum Institute, Denmark PPD-M Sub Lot B-1, institute Copenhagen), respectively. At the end of the fifth day, the cells were subjected to 1 μ/Ci 3H-thymidine (Amersham, England) and incubated. After 16 h, the cells were harvested onto glass fiber filters, and the samples were counted in a beta-counter (Pharmacia Wallack Beta Counter).
Potency test
In total, 2µ g/ml precipitated tuberculin solution was prepared in phosphate buffer, followed by the preparation of 1/200, 1/1000, and 1/5000 dilutions. After preparing the 5000 IU/mL reference standard for tuberculin (PPD-81 Sub Lot B-1), 500 IU/m, 100 IU/mL, and 20 IU/mL dilutions were prepared from the solution. In general, a phosphate buffer solution without AS control and nine different tuberculin dilutions were used for injection as displayed in Table 2. Different tuberculin dilutions were administered based on an eight-point assay, in which four intradermal injections were carried out on each flank of the animal. Intradermal injection sites were pointed as A, B, C, and D from head to tail on each lateral and contralateral sides. Moreover, the injection volume was 0.2 ml, and different diameters of erythema were measured and recorded 24 h after the injection. The mean diameter of two perpendicular lines in the erythema site was recorded after determining the erythema sites. The identification code for each injection site is presented in Table 2. Furthermore, potency was determined by comparing the process with a standard preparation of PPD in sensitized guinea pigs. The results of the tests were reported as described by standard operating procedures.
Table 2.
Identification codes for injected solutions
| Trichloroacetic acetic acid | Ammonium sulfate | Standard reference | Phosphate buffer |
|---|---|---|---|
| 1: T1 | 4: AS1 | 7: S1 | 10: 9 |
| 2: T2 | 5: AS2 | 8: S2 | |
| 3: T3 | 6: AS3 | 9: S3 |
Statistical analysis
Data analysis was performed in SPSS statistics (version 21) using Chi-square and Fisher’s exact tests (to compare the results of LTT [positive/negative]), as well as ANOVA (to compare the mean responses of PPD skin test with guinea pig potency test). A p-value less than 0.05 was considered statistically significant.
Results
Based on the results of the present study, the modified Dorset-Henley liquid medium was more appropriate for the massive growth of Mtb in the liquid medium, compared to the modified Sauton medium. One gentle and meticulous direct inoculation of Mtb from LJ into Dorset-Henley liquid medium may lead to massive production. The procedure used in the current research reduced two months of the production planning process. It was demonstrated that 100°C for 3 h was more reliable for bactericidal purposes. According to the obtained results, gamma radiation doses of 1, 1, and 7 kGy significantly decreased the bacterial count; nonetheless, 8 kGy completely eliminated the bacterium.
After the final filtration in the third stage, the tuberculin extracted by TCA precipitation was greater than AS precipitation. Moreover, the level of protein extracted by the TCA method before the final filtration was higher, compared to the AS method. Total protein concentration values by precipitation in TCA and AS were obtained at 0.75 and 0.12, respectively. Moreover, these values for pure protein concentration were reported as 1 and 0.16 mg/ml, respectively. The addition of the diluent solution of tuberculin and re-filtration by 0.22 micron yielded 2.78 and 0.44 mg/ml of total and pure protein in TCA precipitation. On the other hand, in AS precipitation, the total and pure protein values were obtained at 1.78 and 0.28 mg/ml, respectively.
In killing bacteria via heating, the level of total protein, the protein of filtrated solution without germs, and protein in sonicated bacillus microorganisms were 5, 1.87, and 0.34 mg/ml, respectively. In terms of the non-killed bacterium (without elimination of bacteria), the obtained protein levels in sonicated microorganisms and the filtrated solution were 0.8 and 0.9 mg/ml, respectively, pointing to its unsuitability for massive production of tuberculoprotein. It was determined that due to the high concentration of the extracted protein, the Kjeldahl method gave higher protein estimates, compared to the Bradford method (figures 1 and 2).
Figure 1.
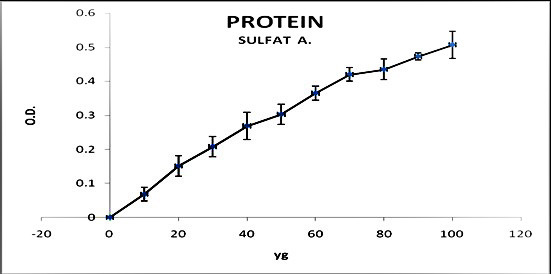
Protein concentration in ammonium sulfate precipitation approach
Figure 2.
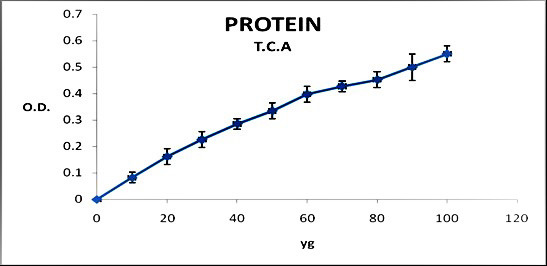
Protein concentration in trichloroacetic acid precipitation approach
According to LTT results, there was no significant difference in the stimulation coefficient in groups receiving AS precipitation-derived antigens (proteins), Phytohe-magglutinin (PHA), standard reference PPD, and precipitated PPD by TCA (P>0.05). Regarding the highest stimulation index achieved in TCA precipitation-derived antigens and PPD-S (SI=2), the results demonstrated no significant difference (P>0.05).
The potency test on guinea pigs indicated that the reactions achieved by PPD-S and TCA precipitation-derived antigens were higher, compared to the AS precipitation-derived antigens; however, the difference was not significant (P>0.05).
Evaluation of tuberculin potency
Comparative analysis results from calibration of TCA and AS precipitation-derived tuberculoprotein with standard reference tuberculin on sensitized guinea pigs demonstrated that the potency of TCA precipitation-derived tuberculoprotein coincidence with standard reference tuberculin was in the accepted range of 85%-135%. Nevertheless, the AS precipitation-derived tuberculoprotein potency was out of the standard range (167%). Injection codes and reaction sizes pertaining to each of the ten solutions are listed in Table 3.
Table 3.
Summary of reaction results of various types of injected tuberculin in 12 guinea pigs
| No | Standard reference (S) | Ammonium Sulfate (AS) | Trichloroacetic Acid (TCA) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | AS1 | AS2 | AS3 | T1 | T2 | T3 | |
| 1 | 20.5 | 7 | 6 | - | 10 | - | 18.5 | 10 | - |
| 2 | 24 | 17 | 8.5 | - | 10 | 10 | 12.5 | - | - |
| 3 | 10 | 9 | - | 4.5 | - | 8.5 | 9.5 | 9 | 11 |
| 4 | 19 | 10.5 | 11.5 | 11.5 | 11.5 | 10.5 | - | 13.5 | 16 |
| 5 | 15.5 | 10 | 9.5 | 10.5 | - | 9 | 11 | 9.5 | - |
| 6 | - | 10 | 8 | 9.5 | 11.5 | 9 | 13.5 | - | 10.5 |
| 7 | - | 9.5 | 10 | 14.5 | 8 | 10.5 | 13 | - | 8.5 |
| 8 | 15 | 9.5 | 8.5 | 19 | 11.5 | 12 | - | 10 | 9 |
| 9 | 12.5 | 10.5 | - | 20.5 | 11 | 9.5 | - | 14.5 | 9 |
| 10 | 14 | - | - | 10 | 10 | 9.5 | 10.5 | 10.5 | 6.5 |
| 11 | - | 12 | 9.5 | 15 | 9 | - | 20.5 | 12.5 | 10 |
| 12 | 21 | - | 12 | 14.5 | 10 | - | 17 | 11 | 14 |
| Sum | 151.5 | 105 | 83.5 | 129.5 | 102.5 | 88.5 | 126 | 100.5 | 94.5 |
| Mean | 16.83 | 10.5 | 9.27 | 12.95 | 10.25 | 9.83 | 14 | 11.21 | 10.5 |
| Total (TCA) | - | - | - | - | - | - | 321 | ||
| Total (AS) | - | - | - | 320.5 | - | - | - | ||
| Total (S) | 340 | - | - | - | - | - | - | ||
Discussion
The PPD production process from Mtb has been historically problematic due to the unique culture requirements of floating cultures and maintaining floating colonies in long incubation periods. The floating cultures are used to extract expressed surface proteins and the proteins that are secreted by the growth media. Numerous efforts have been made to improve the production process, such as the modification of growth media. The production methods and incubation time are associated with difficulties that affect the quality of produced PPD. Early production methods focus on the modification of heating and protein precipitation methods. Nevertheless, early production methods using heat-concentrated culture media were later modified to produce PPDs applying the protein precipitation methods. Research has been conducted on the complicating issues on immunogenic components of PPD preparations using proteomic analysis of PPD for improving the specificity of the product ( Santema et al., 2009 , Wynne et al., 2012 ). In this regard, we modified culture growth characteristics and used gamma radiation along with the traditional heating method to kill mycobacterium. In addition, we evaluated the biological activity of the extracted protein.
Based on the results of the present study, the long-term process of Mtb culture can be shortened in a modified Dorset-Henley medium, rather than the Sauton medium. It signifies that the Dorset-Henley medium may supply Mtb requirements for better growth. The inoculation of the bacterium from LJ into Dorset Henley medium in Erlenmeyer flask will lead to a better estimation by gentle contact of the inoculating loop to the wall of Erlenmeyer flask. It causes the floating of bacterial cells on the medium surface, rather than being precipitated. Therefore, without the growth of the bacterium in a biphasic medium of potato-Dorset-Henley, the production time could be shortened by two months.
In the current research, attempts were made to kill bacterial cells in a way that the antigenic sites somehow remain intact, and the spatial shape of antigens is left unchanged to the greatest extent possible. One session of heating at 80°C was used in the current research. There is a possibility that temperature does not completely affect alive bacterial; consequently, the procedure of tuberculin production may be hazardous for individuals involved. Some studies have reported that the autoclaving process may not be necessary for PPD production. Nevertheless, the potency of the PPD is under question ( Capsel, 2015 ). Therefore, it is possible to use other methods, instead of the traditional production method involving heat-killing.
In the present study, 8 kGy gamma radiation completely eliminated bacterium. Since small cultures were used for this purpose, it was impossible to obtain tuberculin from little bottles. Since the issue of economics was not discussed in the present study, it is suggested that irradiation and heating-derived tuberculin be compared in a comprehensive study so that the issue could be economically determined.
Based on previously performed studies ( ICMSF, 1980 , Garcia et al., 1987 ), gamma irradiation doses of 1, 1.5, 7 kGy, and below 1 kGy ( Zack et al., 1974 ) were suitable for Mtb elimination. Moreover, consistent with the findings of the present research, a recent study has demonstrated that the application of gamma irradiation at 7.5 kGy eliminated Mtb in infected cattle carcasses ( Shaltout et al., 2017 ).
In the current research, different doses of γ-irradiation were subjected to two different bacterial species at various time intervals. According to the results, the application of doses below 8 kGy could be hazardous in the tuberculin production process. In the third stage, as other studies have reported, TCA precipitation yielded more protein, compared to AS precipitation. Similar results were obtained in the present study, ( Kiran et al., 1985 ; Abou-Zeid et al., 1988 ).
In the current study, the extraction of tuberculoprotein by sonication was recognized as a time-consuming process requiring the addition of a lysozyme treatment to the sonication to improve the method ( Rezwan et al., 2007 ). Moreover, the sonication of the alive bacillus was determined to be dangerous for the people involved despite obtaining an intact high-quality extracted protein. It is worth noting that the protein obtained from the sonication of alive bacillus showed better results in latex agglutination in TB cases, compared to PPD (data not presented).
In the protein measurement process using different methods, there was uncertainty around the initial concentration of protein measured by the Bradford method due to the presence of TCA in one of the obtained tuberculins after preparing dilutions from extracted protein. Finally, it was demonstrated that the traditional Kjeldahl method had high precision and efficiency and was still suitable in this respect.
The results of LTT on sensitized guinea pigs with an equal amount of both obtained tuberculins demonstrated that TCA precipitation-derived protein had better results, compared to the standard reference. Nevertheless, no significant difference was observed between these proteins and the standard reference. In the potency test, the result of TCA precipitation-derived tuberculin was within the allowed range (130%), whereas AS precipitation-derived tuberculin required modification in concentration and potency in order to be applicable in the allowed range (85%-135%).
Moreover, TCA precipitation yielded a higher level of protein, compared to the AS precipitation. Regardless of the concentration of extracted protein, AS provided better results, in comparison with TCA. Since the extracted protein is a pool of various proteins, further research is required on the use of the guinea pig potency test with identified proteins to determine the proper candidates to be applied in cell-mediated immune response assays and in vitro IFN-γ stimulation assay.
The AS precipitation method is safe since ammonium sulfate is the only agent applied for precipitation, and no waste substances are produced. On the other hand, the TCA precipitation process is unsafe due to the carcinogenicity of the trichloroacetic acid vapors. Moreover, in the TCA method, there is a higher presence of nucleic acids in the final product, compared to the AS technique ( Bendinelli and Friedman, 1988 ). The obtained tuberculin and standard reference tuberculin were subjected to electrophoresis, demonstrating the same range of protein size (18-68 kD) on gel electrophoresis (data not shown).
As a conclusion, time, cost, and precipitation possess of the natural proteins from Dorset-Henley liquid medium were decreased in the present research. Since gamma radiation completely eliminated bacterium, it can be a novel safe technique in the killing process during the production of PPD and elimination of the autoclaving step in the production process. It seems that this method would not be detrimental to the immunological characteristics. There are no standard criteria for determining optimal incubation and harvest times for Mtb cultures during PPD production. The production process would be facilitated by developing a well-characterized PPD via the elimination of the autoclave step and assessing its sensitivity and specificity using both in-vivo and in-vitro testing.
Authors’ Contribution
Study concept and design: N. M. and Gh. Sh.
Acquisition of data: A. K., K. T. and A. Z. H.
Analysis and interpretation of data: N. M. and M. B.
Drafting of the manuscript: A. K., M. B. and N. M.
Critical revision of the manuscript for important intellectual content: N. M., Gh. Sh. and M. B.
Statistical analysis: N. M., A. K. and M. B.
Administrative, technical, and material support: N. M. and Gh. Sh.
Ethics
We hereby declare all ethical standards have been respected in preparation of the submitted article.
Grant Support
This study was funded by Nuclear Agriculture Research School, Nuclear Science, and Technology Research Institute, Karaj, Iran.
Conflict of Interest:
The authors declare that they have no conflict of interest
Acknowledgement
Hereby, we extend our gratitude to the personnel of the Razi Vaccine and Serum Research Institute, especially Tuberculin and Mallein production and research department, for assisting us in this research. In addition, we would like to thank the contributions of nuclear agriculture research school, nuclear science and technology research institute. Finally, we would like to thank the Dr. Ali akbar Mohammadi, previous director from Razi Vaccine and Serum Research Institute. This manuscript mainly was written by Gholamreza Shahhosseini and Abbas Karimi. However, they have equal contributions in this project.
References
- 1.Abou-Zeid C, Smith I, Grange JM, Ratliff TL, Steele J, Rook GA. The secreted antigens of Mycobacterium tuberculosis and their relationship to those recognized by the available antibodies. Microbiology. 1988; 134: 531–538. doi: 10.1099/00221287-134-2-531. [DOI] [PubMed] [Google Scholar]
- 2.Babaie M, Ghaem panah A, Mehrabi Z, Mollaei A. Partial purification and characterization of antimicrobial effects from snake (Echis carinatus), scorpion (Mesosobuthus epues) and bee (Apis mellifera) venoms. Iran J Med Microbiol. 2020; 14(5): 460–477. [Google Scholar]
- 3.Bendinelli M, Friedman H. Mycobacterium Tuberculosis: Interactions with the Immune System, Springer Science & Business Media; 1988 [Google Scholar]
- 4.Capsel RT. Development of an improved production method, determination of protein composition, and potency characterization of Mycobacterium avium subsp. paratuberculosis purified protein derivative; 2015. doi: 10.1371/journal.pone.0154685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163(9):1009–21. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 6.Demicheli MC, Goes AM, de Andrade AS. Ultrastructural changes in Paracoccidioides brasiliensis yeast cells attenuated by gamma irradiation. Mycoses. 2007;50(5):397–402. doi: 10.1111/j.1439-0507.2007.01389.x. [DOI] [PubMed] [Google Scholar]
- 7.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282(7):677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 8.Fogel N. Tuberculosis: A disease without boundaries. Tuberculosis. 2015; 95: 527–531. doi: 10.1016/j.tube.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Garcia MM, Brooks BW, Stewart RB, Dion W, Trudel JR, Ouwerkerk T. Evaluation of gamma radiation levels for reducing pathogenic bacteria and fungi in animal sewage and laboratory effluents. Can J Vet Res. 1987;51(3):285–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan Zadeh J, Nasehi M, Rezaianzadeh A, Tabatabaee H, Rajaeifard A, Ghaderi E. Pattern of reported tuberculosis cases in iran 2009-2010. Iran J Public Health. 2013;42(1):72–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Horwitz W, Latimer GW. Official methods of analysis of AOAC International, AOAC International, Gaithersburg, Md; 2005 [Google Scholar]
- 12.ICMSF. 3-Ionizing Irradiation Microbial Ecology of Foods, Academic Press; 1980 pp. 46-69 [Google Scholar]
- 13.Khazaei HA, Rezaei N, Bagheri GR, Dankoub MA, Shahryari K, Tahai A, Mahmoudi M. Epidemiology of tuberculosis in the Southeastern Iran. Eur J Epidemiol. 2005;20(10):879–83. doi: 10.1007/s10654-005-2152-y. [DOI] [PubMed] [Google Scholar]
- 14.Kiran U, Shriniwas, Kumar R, Sharma A. Efficacy of three mycobacterial antigens in the serodiagnosis of tuberculosis. Eur J Respir Dis. 1985;66(3):187–95. [PubMed] [Google Scholar]
- 15.Marcdante K, Kliegman RM. Nelson Essentials of Pediatrics E-Book, Elsevier Health Sciences; 2014 [Google Scholar]
- 16.Prevention CfDCa. TB Elimination Tuberculin Skin Testing. 2016. https://www.cdc.gov/tb .
- 17.Rezwan M, Lanéelle MA, Sander P, Daffé M. Breaking down the wall: fractionation of mycobacteria. J Microbiol Methods. 2007;68(1):32–9. doi: 10.1016/j.mimet.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Santema W, Overdijk M, Barends J, Krijgsveld J, Rutten V, Koets A. Searching for proteins of Mycobacterium avium subspecies paratuberculosis with diagnostic potential by comparative qualitative proteomic analysis of mycobacterial tuberculins. Vet Microbiol. 2009;138(1-2):191–6. doi: 10.1016/j.vetmic.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Shahhosseini G, Karimi A, Amanpour S, Mansouri MA. Effect of Gamma Irradiation on Microbial Decontamination, Crude Nutrient Content, and Mineral Nutrient Composition of Laboratory Animal Diets. Arch Razi Inst. 2019;74(2):175–182. doi: 10.22092/ari.2017.116153.1160. [DOI] [PubMed] [Google Scholar]
- 20.Shaltout F, Em R, Ahmed T, Asmaa A. Studying the Effect of Gamma Irradiation on Bovine Offal's Infected with Mycobacterium tuberculosis Bovine Type. J Food Biotechnol Res. 2017; 1: 1–5. [Google Scholar]
- 21.Sterling TR, Lehmann HP, Frieden TR. Impact of DOTS compared with DOTS-plus on multidrug resistant tuberculosis and tuberculosis deaths: decision analysis. BMJ. 2003;326(7389) doi: 10.1136/bmj.326.7389.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wales A, Kusel J. Biochemistry of irradiated parasite vaccines: suggested models for their mode of action. Parasitol Today. 1992; 8: 358–363. doi: 10.1016/0169-4758(92)90167-z. [DOI] [PubMed] [Google Scholar]
- 23.WHO. Tuberculosis fact sheet. 2018. http://www.who.int/en/news-room/fact-sheets/detail/tuberculosis .
- 24.Wynne JW, Shiell BJ, Colgrave ML, Vaughan JA, Beddome G, Michalski WP. Production and proteomic characterisation of purified protein derivative from Mycobacterium avium subsp. paratuberculosis. Proteome Sci. 2012;10(1):22. doi: 10.1186/1477-5956-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zack MB, Stottmeier K, Berg G, Kazemi H. The effect of radiation on microbiologic characteristics of M tuberculosis. Chest. 1974;66(3):240–3. doi: 10.1378/chest.66.3.240. [DOI] [PubMed] [Google Scholar]


