Abstract
Infectious bronchitis virus (IBV) has a variety of serotypes with relatively limited cross-protection leading the disease to be a major problem in the poultry industry. The IBV 793/B strain has identified to circulate in Iran; therefore, the development of a specific vaccine to protect against the virulent virus has received attention. In this regard, the live IB 793/B vaccine (793/B.08IR) was developed in the Razi Vaccine and Serum Research Institute. In this study, the immunogenicity of 793/B.08IR vaccine via different routes of vaccination and efficacy of the vaccine were determined in specific-pathogen-free (SPF) chickens. Three treatment groups of 10 SPF chickens received the vaccine via eye drops, spray, and drinking water. The sera were collected from the chicks at 3 and 6 weeks after the vaccination, and IBV specific antibody was measured using enzyme-linked immunosorbent assay (ELISA) and serum neutralization (SN) test. To evaluate 793/B.08IR vaccine efficacy, 10 SPF chickens were vaccinated using eye drops. Moreover, 10 unvaccinated chickens were separately retained as negative controls. The birds were challenged with the virulent virus 3 weeks following the vaccination. Five days after the challenge, the tracheal swab was taken for virus reisolation. In the immunogenicity test, the ELISA titers of three vaccinated groups were significantly higher than the background values obtained in the control group (p<0.0001). The mean value of ELISA titer in the spray vaccinated group was higher than the spray and drinking water vaccinated groups 3 weeks following the vaccination; however, the difference was not statistically significant. No differences were observed in antibody titers among the three vaccinated groups 6 weeks after the vaccination. The results of the SN test confirmed the data obtained from the ELISA. The results of antibody titer and its increasing trend in chickens showed that 793/B.08IR vaccine induce proper immunity against the virus. In the efficacy test, IBV was isolated from 90% of the unvaccinated controls and 10% of vaccinated groups. The results of the recovery of the virus after the challenge showed that 793/B.08IR vaccine can provide a significantly improved protection against the pathogen in SPF vaccinated chickens.
Keywords: IBV vaccine, 793/B strain, Immunogenicity, Efficacy, SPF
Introduction
Infectious bronchitis (IB) is an important highly contagious acute viral disease in the upper respiratory tract of chickens ( Raj and Jones, 1997 ). Infectious bronchitis virus (IBV) as an etiologic agent replicates in respiratory and other epithelial organ surfaces, such as the kidneys, oviduct, and gonads. The disease poses a major economic threat to the poultry industry worldwide, which is associated with poor weight gain, loss of feed efficiency in broilers, as well as drop in the quantity and quality of egg production in layers ( Cavanagh and Naqi, 2003 ; Boltz et al., 2004 ; Cavanagh, 2005 ). Some IBV strains also cause high rate mortality due to nephritis ( Cook et al., 2012 ). The IBV is a member of the Coronaviridae family. The genome of the virus is RNA that encodes four structural proteins, including spike (S), membrane glycoprotein (M), small membrane protein (E), and nucleocapsid protein (N). The S glycoprotein is post-translationally cleaved into S1 and S2 subunits ( Cavanagh and Naqi, 2003 ). The S1 is responsible for cellular receptor binding, antibody neutralization, and hemagglutination inhibition ( Casais et al., 2003 ; Cavanagh, 2007 ). The subunit is highly variable in both nucleotide sequence and primary protein structure, as a result of point mutation or recombination ( Bochkov et al., 2007 ; Ammayappan et al., 2008 ; Lee et al., 2008 ). The high rates of mutation in the S1 fragment caused the emergence of several different serotypes and/or antigenic variants of IBV in many countries ( Cavanagh, 2007 ). The IBV strains of the 4/91, which are also known as 793/B, were firstly recognized in Britain in 1990/1991 and then have been dominant serotypes in Europe ( De Wit, 2000 ). Serological analysis has demonstrated the worldwide distribution of IBV 793/B serotype infection in broiler and layer chickens ( Sjaak de Wit et al., 2011 ). In Iran, the presence of the serotype was confirmed by serum neutralization (SN) assay following the involvement of the poultry respiration complex in 1999 ( Momayez et al., 2002 ). Since that time, several studies have reported the isolation and detection of IBV 793/B strain in some provinces of Iran ( Shapouri et al., 2002 ; Seyfi Abad Shapouri et al., 2004 ; Poorbaghi et al., 2012 ; Hosseini et al., 2013 ). The live attenuated IBV vaccine is one of the most important preventive IBV diseases. However, the presence of the different antigenically serotypes and newly emerged variants may cause vaccine failure ( Boltz et al., 2004 ). Vaccination against circulating strains would provide immunity against the serotypes in industrial poultry and prevent the complications of the disease associated with the broiler or laying flocks ( Azad et al., 2005 ). It has been revealed that the immunity against IBV is serotype- specific, and low cross-protection can be observed among IBV genotypes. These will require changing in the vaccination strategy against the virus in which the vaccine strain is matched to the circulating strains ( Sjaak de Wit et al., 2011 ). The use of heterologous vaccine strains has broadened the protection spectrum in some cases ( Malo et al., 1998 ). Due to the existence of different IBV variants in Iran, the development of a live 793/B IBV vaccine could improve the protection levels against IBV. To reach this goal, in the Razi Vaccine and Serum Research Institute, IBV 793/B.08IR vaccine has been developed in the experimental scale (Masoudi, unpublished data), and an approval test for this vaccine has been in progress. The drinking water, spray, and eye drops are the common administration routes of IBV live vaccines. The wrong method of immunization will often lead to the failure of vaccines to develop proper immunity ( Cavanagh and Naqi, 2003 ; Franzo et al., 2016 ). The assessment of these methods and determination of the best technique is one of the requirements for immunization. In the present study, immunogenicity with different routes of administration and efficacy profile of the newly developed live IBV 793/B.08IR vaccine were analyzed in specific-pathogen-free (SPF) chickens. Most tests described in this study were carried out in accordance with the requirements of the World Organisation for Animal Health ( OIE, 2013 ).
Material and Methods
Infectious bronchitis virus vaccine
The 793/B IBV (793/B.08IR) was injected into the allantoic cavities of 9-11 day-old embryonated SPF eggs (Vanky´s, India) and incubated at 37°C for 2 days. The allantoic fluid was harvested and titrated by Karber’s method. The IBV live attenuated vaccines would have a minimum titer of 103.0-103.5 EID50 per dose ( OIE, 2013 ).
Immunization trial
The immunogenicity of the IBV 793/B.08IR vaccine via three recommended routes of vaccination was determined in SPF chickens. For this purpose, a total of 40 SPF chickens were randomly divided into four groups (n=10). Each treatment group at 5-day-old received the IBV 793/B.08IR vaccine via eye drops, spray, and drinking water separately. The vaccine was diluted in a way that each dose contained at least 10 3.5 EID50 of IBV. The fourth group was used as negative controls without any vaccination. The chickens were placed in separate rooms, and food and water were provided ad libitum. Three and six weeks after the vaccination, the blood samples were taken from the chickens, and the level of specific antibody against IBV was determined using enzyme-linked immunosorbent assay (ELISA) and SN test.
Efficacy determination
The efficacy of the IBV 793/B.08IR vaccine was evaluated through the recovery of the virus from tracheal swab according to OIE (2013). Ten SPF chickens aged three weeks of were vaccinated via eye drops with one dose of the 793/B.08IR vaccine. The unvaccinated negative controls with similar age and source of chicks were also chosen for the study. The blood samples were taken 3 weeks following the Post-vaccination, and the antibody was detected by the SN test. The birds of both groups were challenged via eye drops with 103.0–103.5 EID50 of the IBV 793/B virulent virus (IR/773/2001) ( Momayez, et al. 2002 ). The chickens were daily observed for clinical signs attributed to IB infection, such as respiratory signs and mortality, up to the end of the experiment. A swab of the trachea was taken for the reisolation of the virus 5 days after the challenge. The tracheal swab was placed in 3 ml of tryptose phosphate buffer containing streptomycin (100 mg/ ml) and penicillin (100 units/ml). Then, 0.2 ml of the suspension was inoculated into the allantoic cavity of five 9-11 day embryonated eggs. The allantoic fluid was harvested from the two eggs of each group 2 days after the incubation at 37°C. The fluid was used for the three consequent passages. Other eggs were incubated up to 7 days after the inoculation. The dead embryo was recorded during the incubation period, and the egg containing live embryo was examined for lesions characteristic of avian IB, such as stunting (dwarfing) and curling of the embryo, as well as its feet ( Cavanagh et al., 1992 ).
Serological tests:
Serum neutralization test
The specific antibody against IBV was determined by SN, alpha method ( OIE, 2013 ). Briefly, tenfold dilution of the 793/B virus was prepared. The mixture of an equal volume of each dilution of the virus and serum of the chicks was incubated at room temperature for 45 min. Each mixture was inoculated into the group of 5 10-day-old SPF embryonated eggs. The virus alone was titrated in parallel. The embryos were examined for IBV lesions, such as stunting and curling, 7 days after the inoculation. The results were expressed as the neutralization index (NI) that represents the log10 difference in the titers of the virus alone and virus/antiserum mixtures.
Enzyme-linked immunosorbent assay
A commercial ELISA kit (BioCheck, Netherland) was used to determine IBV antibodies in the individual sera of chickens according to the manufacturer’s instruction. The geometric mean of antibody titers (GMT) was calculated at each bleeding time.
Statistical analysis
GraphPad Prism software (version 5) was used for plotting graphs and statistical analysis. Statistical differences were evaluated using a one-way analysis of variance with Tukey’s post hoc Honest Significant Difference test. P-value less than 0.05 was considered statistically significant.
Results
Immunogenicity assessment of live infectious bronchitis virus 793/B.08IR vaccine
To introduce the best method of IBV 793/B.08IR vaccination, antibody responses were determined in chickens vaccinated through the routes of drinking water, eye drops, and spray. No respiratory signs and mortality were observed in all vaccinated chickens groups. As shown in Figure 1, the NI in the eye drops and spray vaccinated groups were higher than drinking water group (4 and 4.2 vs. 2.6) at 3 weeks following the Post-vaccination. However, the index was similar in all groups at 6 weeks after the vaccination. The GMT of ELISA titer of three immunized groups with IBV 793/B.08IR vaccine was shown in Figure 2. As depicted in Figure 2, the titer is significantly higher at 3 weeks following the Post-vaccination in the treatment groups, compared to that reported for the control group (p<0.0001). The mean of ELISA antibody in the spray vaccinated group was higher than other groups (945±212 vs. 857±217 and 763±233); however, the difference was not statistically significant. The amount of IBV antibody trended to increase, especially in drinking water and eye drop groups. The significant differences were shown between these groups at the end of the experiment (p<0.0001). The mean antibody titers recorded for the spray, eye drop, and drinking water vaccinated groups were recorded as 991±325, 1157±332, and 1057±347, respectively.
Figure 1.
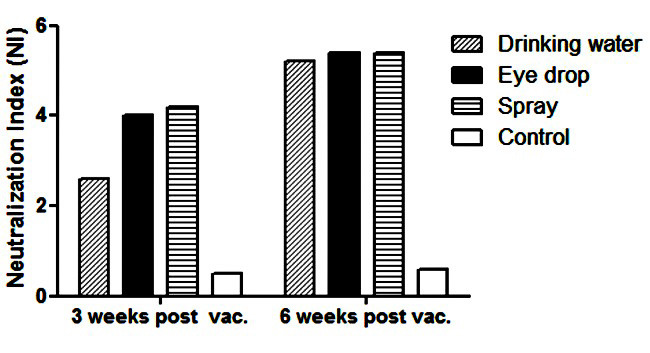
Serum neutralization data of chickens vaccinated with infectious bronchitis virus 793/B.08IR via three different vaccination methods at 3 and 6 weeks after vaccination; neutralization index ≥ 1.5 as positive.
Figure 2.
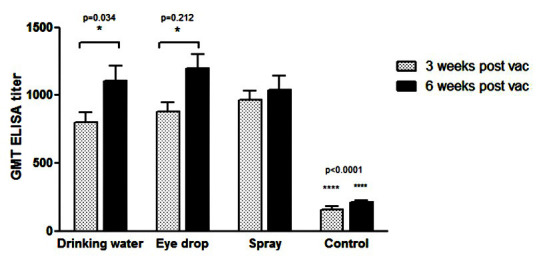
Enzyme-linked immunosorbent assay antibody in groups of chickens vaccinated by live infectious bronchitis virus 793/B.08IR strain via drinking water, eye drops, and spray methods; bars representing mean values and standard errors of mean obtained for each group; asterisks indicating statistically significant differences: * P<0.05 and **** P<0.0001; asterisks directly above bars demonstrating statistically significant differences between vaccinated groups and control group of chickens.
Efficacy of infectious bronchitis virus 793/B.08IR vaccine
The efficacy of the 793/B.08IR vaccine was determined in a group of 10 21-day-old chickens, compared to that reported among the unvaccinated chickens. The estimated NIs for these groups as 5.2 and 0.6 indicated that the vaccine could increase the antibody titer in chickens. Table 1 tabulates the results of virus isolation from tracheal swabs 5 days after the challenge. At the end of the experiment, IBV was isolated from 90% of the unvaccinated and 10% of vaccinated chickens. In tracheal swab culture, IBV was detected in 5 of 10 control chickens in the first passage. In the second and third passages, the challenge virus was reisolated from one vaccinated chicken; however, it was recovered from nine control chickens. The vaccination challenge test showed that 793/B.08IR vaccine could provide protection against the challenge with a virulent strain, IR/773/2001 IB virus, with no mortality. The obtained results showed that 793/B.08IR vaccine was able to trigger significantly strong humoral immune responses, compared to those by the negative controls. After the challenge, the vaccine could induce a high rate of protection against the virulent virus. The present experiment indicated that nine vaccinated chickens were without the evidence of the recovery of the virus on the fifth day after the challenge.
Table 1.
Reisolation of virus in vaccinated and unvaccinated control groups 5 days after challenge
| Group | Chicken embryo passage | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Vaccinated with 793/B | 0/10 * | 1/10 | 1/10 |
| Unvaccinated control | 5/10 | 9/10 | 9/10 |
Number of affected embryos/total number of embryos
Discussion
Vaccination is a powerful strategy to save farming efficacy and improve the protection of the flocks against virulence IBV strains. The disease is widely controlled by live attenuated and inactivated vaccines; however, a broad variety of circulating serotypes and genotypes contributes to the low cross-protection acquired by these vaccine strains ( Boltz et al., 2004 ). The distribution of IBV serotypes should influence the choice of vaccine to use in each geographic region ( OIE, 2013 ). Since 1999, the isolation of IBV 793/B strain has been reported from some provinces of Iran ( Aghakhan et al., 1994 , Momayez et al., 2002 , Shapouri et al., 2002 , Seyfi Abad Shapouri et al., 2004 , Jackwood et al., 2005 , Poorbaghi et al., 2012 , Hosseini et al., 2013 ). For the improvement of the immune responses and protection against IB virulent virus, IBV 793/B (793/B.08IR), the vaccine is developed in the Razi Vaccine and Serum Research Institute at the experimental scale. The present study examined the immunogenicity of the vaccine and its efficacy in SPF chicks via different vaccination routes. As shown in the results, the antibody titer in the spray vaccinated group was the highest among the groups at 3 weeks after the vaccination. However, the amount of antibodies in all three vaccinated groups was approximately similar in the sixth week after the vaccination, and no significant differences were observed between them. The results of the present study revealed that at 3 weeks following the post-vaccination, induced immune response via drinking water was lower than those by spray and eye drops. At older age, there were no differences between the antibody titer of the three vaccinated groups. It seems that in chicks at lower early stages, immune responses are stimulated faster via eye drops and spray, compared to drinking water. The poultry vaccination via drinking water is a great labor-saving procedure and designed to simultaneously vaccinate large numbers of chickens. However, the method of vaccination via drinking water cannot cause to establish a good immune response in chickens (http://www. poultryhub.org/health/health-management/vaccination, 2019). It was explained that the intraocular administration of the vaccine stimulates both humoral and cell-mediated immune responses via the Harderian gland just behind the eye socket to provide a strong immune defense ( Jeurissen et al., 2000 ; van Ginkel et al., 2008 ). The protective stimulation using spray vaccination is restricted to the nasal mucosa or also linked to the oral and eye surfaces which can cause a difference in the immunological response ( Purswell et al., 2010 ). In the present study, serum antibody levels increased in all three groups at 6 weeks after the vaccination, compared to those at 3 weeks before that. Antibody titer and its increasing trend in SPF chickens under controlled conditions showed that the vaccine had the appropriate potency against the IBV. It is recommended to vaccinate chicks at an early age through spray or eye drops. Furthermore, the current study investigated the efficiency of the 793/B.08IR IBV if it could provide protection against the virulent strain. The assessment of the efficacy of the IBV vaccine is commonly performed by the reisolation of the virulent virus from the culture of the tracheal swab. It is also conducted by the microscopical quantification of the tracheal rings in ciliary activity assay ( OIE, 2013 ). In accordance with the present study, Tarpy ( Tarpey et al., 2006 ) investigated the efficacy of the Beaudette-derived viruses expressing spike protein of virulent M41 strain, as potential vaccines using tracheal ciliary activity. They demonstrated that the vaccine candidate was safe for delivery by in ovo vaccination. In another study, the efficacy of an attenuated live QX-like IBV vaccine candidate was tested in SPF chickens and broilers with maternally derived antibodies ( Geerligs et al., 2011 ). Their results showed that the protection against virus challenge was higher in SPF chickens, compared to that reported for broilers. Terregino et al. (2008) used virus isolation from the tracheas of vaccinated SPF chickens after the challenge to show the efficacy of the IB vaccines. They observed that the vaccination with the Ma5 IB vaccine at 1 day and 4/91 IB vaccine at 14 days of age fully protected the vaccinated birds with no virus detection following the challenge. The present experiment showed that 793/B.08IR vaccine could trigger significantly strong humoral immune responses and provide protection against the challenge with IR/773/2001 IBV strain. The challenge strain virus was reisolated from 10% of the vaccinated birds at 5 days following the post-challenge, indicating the induction of 90% protection against IBV. These results are totally in accordance with the OIE guidelines for the evaluation of vaccine efficacy. The IBV live vaccine is suitable to use if no evidence of IBV could be shown in the trachea of at least 90% of the challenge vaccinated birds; nevertheless, 90% or more of the control birds should have the evidence of the IBV presence ( OIE, 2013 ). Based on the obtained data in the present study, it can be concluded that under experimental conditions, the 793/B.08IR vaccine could protect SPF chickens from infection following the challenge with IBV 793/B virulent strain. Despite the long-established IB vaccination, the relatively weak protection and existence of new IBV serotypes highlighted the need for change in vaccination programs by adding the circulating strain. Based on the data presented in several studies, vaccination with both H120 and 793/B live vaccines cause a good protection against virulent IBV ( Malo et al., 1998 ; Cook et al., 1999 ; Terregino et al., 2008 ; Sjaak de Wit et al., 2011 ; Shirzad et al., 2012 ; Mohammadi et al., 2014 ; Chhabra et al., 2015 ). Malo et al. (1998) showed that the administration of 4-91 and Ma5 IBV vaccines together considerably broaden the protection against challenge with a variety of antigenically different IBVs. A combination of the Mass and 4/91 vaccine regimen have prevented nephropathogenic IBV infection ( Terregino et al., 2008 ). Mohammadi et al. (2014) indicated that the vaccination with both H120 and 793/B strains could be helpful for the reduction of economic losses caused by newly evolving IBV variants. Chhabra et al. (2015) showed the administration of live H120 and CR88 vaccines at the same time in one-day-old chickens followed by CR88 vaccine at 14 days-old provided more than 80% tracheal ciliary protection from virulent variant isolates. Further studies are required to establish the underlying immune mechanisms for such higher and broader protection conferred by this vaccination program. These data indicated that such a change in vaccination program with these two vaccines could provide good protection in poultry flocks that can prevent the economic losses caused by the IBV.
In conclusion, the obtained results of the present experiment revealed that the 793/B.08IR is a potent and efficient vaccine with the ability to induce proper immune responses in SPF chickens. Due to the predominance of the H120 serotype in Iran, the efficacy of combined 793/B.08IR with H120 should be evaluated among commercial broiler chickens in future studies.
Ethics
We hereby declare all ethical standards have been respected in preparation of the submitted article.
Conflict of Interest
The authors declare that they have no conflict of interest.
Grant Support
This study was financially supported by the Razi Vaccine and Serum Research Institute, Agricultural Research, Education and Extension Organization in Karaj, Iran (No. 12-18-18-9453-94005).
Authors’ Contribution
Study concept and design: Masoudi S
Acquisition of data: Masoudi S, Pishraft-Sabet L, Shahsavandi S
Analysis and interpretation of data: Masoudi S, Pishraft-Sabet L
Drafting of the manuscript: Pishraft-Sabet L
Critical revision of the manuscript for important intellectual content: Shahsavandi S, Masoudi S
Statistical analysis: Pishraft-Sabet L
Administrative, technical, and material support: Masoudi S, Pishraft-Sabet L
References
- 1.Aghakhan SM, Abshar N, Fereidouni SR, Marunesi C, Khodashenas M. Studies on avian viral infections in Iran. Arch Razi Ins. 1994 ;44:45, 1–10. [Google Scholar]
- 2.Ammayappan A, Upadhyay C, Gelb J, Vakharia VN. Complete genomic sequence analysis of infectious bronchitis virus Ark DPI strain and its evolution by recombination. Virol J. 2008;5(1):1–7. doi: 10.1186/1743-422X-5-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azad GA, Marandi MV, Aminae HK. Detection and molecular characterisation of infectious bronchitis viruses by RT-PCR/RFLP and sequencing. Abstract Book of 14th WVP Congress; 2005 p.136 [Google Scholar]
- 4.Bochkov YA, Tosi G, Massi P, Drygin VV. Phylogenetic analysis of partial S1 and N gene sequences of infectious bronchitis virus isolates from Italy revealed genetic diversity and recombination. Virus Genes. 2007;35(1):65–71. doi: 10.1007/s11262-006-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boltz DA, Nakai M, Bahr JM. Avian infectious bronchitis virus: a possible cause of reduced fertility in the rooster. Avian Dis. 2004;48(4):909–15. doi: 10.1637/7192-040808R1. [DOI] [PubMed] [Google Scholar]
- 6.Casais R, Dove B, Cavanagh D, Britton P. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J Virol. 2003;77(16):9084–9. doi: 10.1128/JVI.77.16.9084-9089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavanagh D. Coronaviruses in poultry and other birds. Avian Pathol. 2005; 34: 439–448. doi: 10.1080/03079450500367682. [DOI] [PubMed] [Google Scholar]
- 8.Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet Res. 2007; 38: 281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- 9.Cavanagh D, Davis PJ, Cook JK, Li D, Kant A, Koch G. Location of the amino acid differences in the S1 spike glycoprotein subunit of closely related serotypes of infectious bronchitis virus. Avian Pathol. 1992;21(1):33–43. doi: 10.1080/03079459208418816. [DOI] [PubMed] [Google Scholar]
- 10.Cavanagh D, Naqi S. Infectious bronchitis. Dis Poultry. 2003; 11: 101–119. [Google Scholar]
- 11.Chhabra R, Forrester A, Lemiere S, Awad F, Chantrey J, Ganapathy K. Mucosal, Cellular, and Humoral Immune Responses Induced by Different Live Infectious Bronchitis Virus Vaccination Regimes and Protection Conferred against Infectious Bronchitis Virus Q1 Strain. Clin Vaccine Immunol. 2015;22(9):1050–9. doi: 10.1128/CVI.00368-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook JK, Jackwood M, Jones RC. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012;41(3):239–50. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- 13.Cook JK, Jackwood M, Jones RC. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012;41(3):239–50. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- 14.De Wit JJ. Detection of infectious bronchitis virus. Avian Pathol. 2000; 29: 71–93. doi: 10.1080/03079450094108. [DOI] [PubMed] [Google Scholar]
- 15.Franzo G, Tucciarone CM, Blanco A, Nofrarías M, Biarnés M, Cortey M, Majó N, Catelli E, Cecchinato M. Effect of different vaccination strategies on IBV QX population dynamics and clinical outbreaks. Vaccine. 2016 ;34(46):5670–5676. doi: 10.1016/j.vaccine.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geerligs HJ, Boelm GJ, Meinders CA, Stuurman BG, Symons J, Tarres-Call J, Bru T, Vila R, Mombarg M, Karaca K, Wijmenga W. Efficacy and safety of an attenuated live QX-like infectious bronchitis virus strain as a vaccine for chickens. Avian Pathol. 2011;40(1):93–102. doi: 10.1080/03079457.2010.542742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosseini AS, Momayez R, Mahmodzadeh M, Yosefi AA. Detection of 793/B serotype of infection bronchitis virus from broiler flocks with respiratory signs in west of Mazandran province. J Vet Clin Res. 2013; 4: 91–97. [Google Scholar]
- 18.Jackwood MW, Hilt DA, Lee CW, Kwon HM, Callison SA, Moore KM, Moscoso H, Sellers H, Thayer S. Data from 11 years of molecular typing infectious bronchitis virus field isolates. Avian Dis. 2005;49(4):614–8. doi: 10.1637/7389-052905R.1. [DOI] [PubMed] [Google Scholar]
- 19.Jeurissen SH, Boonstra‐Blom AG, Al‐Garib SO, Hartog L, Koch G. Defence mechanisms against viral infection in poultry: a review. Vet Q. 2000;22(4):204–8. doi: 10.1080/01652176.2000.9695059. [DOI] [PubMed] [Google Scholar]
- 20.Lee EK, Jeon WJ, Lee YJ, Jeong OM, Choi JG, Kwon JH, Choi KS. Genetic diversity of avian infectious bronchitis virus isolates in Korea between 2003 and 2006. Avian Dis. 2008;52(2):332–7. doi: 10.1637/8117-092707-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 21.Malo A, Orbell S, Huggins M, Woods M, Cook J. Cross protection studies after the use of live-attenuated IBV vaccines Nobilis® IB 4-91 and Nobilis® IB Ma5 (Massachusetts type) Intervet VSD Newsl. 1998;5:1–6. [Google Scholar]
- 22.Mohammadi P, Karimi V, Hashemzadeh M, Ghalyanchi LA, Ghafouri S, Khaltabadi FR, et al. Combination of H120 and 793/B Types of Infectious Bronchitis Virus Vaccine Protects Chickens against Challenge with OX Like Strain of the Virus. Iran J Virol. 2014; 8: 20–24. [Google Scholar]
- 23.Momayez R, Pourbakhsh SA, Khodashenas M, Banani M. Isolation and identification of infectious bronchitis virus from commericial chickens. Arch Razi Ins. 2002; 53: 1–10. [Google Scholar]
- 24.OIE A. Manual of diagnostic tests and vaccines for terrestrial animals. Office international des epizooties, Paris, France; 2013 [Google Scholar]
- 25.Poorbaghi SL, Mohammadi A, Asasi K. Molecular detection of avian infectious bronchitis virus serotypes from clinically suspected broiler chicken flocks in Fars province of Iran. Pak Vet J. 2012;32(1):93–6. [Google Scholar]
- 26.Purswell JL, Mayer JJ, Evans JD, Branton SL, Davis JD. Eye surface area and dosage rates for spray vaccination. Avian Dis. 2010;54(4):1310–5. doi: 10.1637/9478-072110-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 27.Raj GD, Jones RC. Infectious bronchitis virus: Immunopathogenesis of infection in the chicken. Avian Pathol. 1997;26: 677–706. doi: 10.1080/03079459708419246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seyfi Abad Shapouri MR, Mayahi M, Assasi K, Charkhkar S. A survey of the prevalence of infectious bronchitis virus type 4/91 in Iran. Acta Vet Hung. 2004;52(2):163–6. doi: 10.1556/AVet.52.2004.2.4. [DOI] [PubMed] [Google Scholar]
- 29.Shapouri S, Mayahi M, Charkhkar S, Assasi K. Serotype identification of recent iranian isolates of infectious bronchitis virus by type-specific multiplex RT-PCR. Arch Razi Ins. 2002; 53: 79–85. [Google Scholar]
- 30.Shirzad MR, Asasi K, Mohammadi A. Efficacy of vaccination programmes using two commercial live infectious bronchitis vaccines against a field IRFIB 32 strain. Bulgarian J Vet Med. 2012 ;15(4): 260–272. [Google Scholar]
- 31.Sjaak de Wit JJ, Cook JK, van der Heijden HM. Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathol. 2011;40(3):223–35. doi: 10.1080/03079457.2011.566260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarpey I, Orbell SJ, Britton P, Casais R, Hodgson T, Lin F, et al. Safety and efficacy of an infectious bronchitis virus used for chicken embryo vaccination. Vaccine. 2006;24(47-48):6830–8. doi: 10.1016/j.vaccine.2006.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terregino C, Toffan A, Beato MS, De Nardi R, Vascellari M, Meini A, Ortali G, et al. Pathogenicity of a QX strain of infectious bronchitis virus in specific pathogen free and commercial broiler chickens, and evaluation of protection induced by a vaccination programme based on the Ma5 and 4/91 serotypes. Avian Pathol. 2008;37(5):487–93. doi: 10.1080/03079450802356938. [DOI] [PubMed] [Google Scholar]
- 34.Vaccination. PoultryHub. 2019. Available at: http://www.poultryhub.org/health/health-management/ vaccination/ [Google Scholar]
- 35.van Ginkel FW, van Santen VL, Gulley SL, Toro H. Infectious bronchitis virus in the chicken Harderian gland and lachrymal fluid: viral load, infectivity, immune cell responses, and effects of viral immunodeficiency. Avian Dis. 2008;52(4):608–17. doi: 10.1637/8349-050908-Reg.1. [DOI] [PubMed] [Google Scholar]


