Abstract
Antigen-specific T cell-derived induced pluripotent stem cells (iPSCs) have been shown to re-differentiate into functional T cells and thus provide a potential source of T cells that could be useful for cancer immunotherapy. Human Vα24+ invariant natural killer T (Vα24+iNKT) cells are subset of T cells that are characterized by the expression of an invariant Vα24-Jα18 paired with Vβ11, that recognize glycolipids, such as α-galactosylceramide (α-GalCer), presented by the MHC class I-like molecule CD1d. Vα24+iNKT cells capable of producing IFN-γ are reported to augment anti-tumor responses, which affects both NK cells and CD8+ cytotoxic T lymphocytes to eliminate MHC- and MHC+ tumor cells, respectively. Here we describe a robust protocol to reprogram human Vα24+iNKT cells into iPSC, and then to re-differentiate them into Vα24+iNKT cells (iPS-Vα24+iNKT). We further provide a protocol to measure the activity of iPS-Vα24+iNKT cells.
Keywords: Induced pluripotent stem cell, iPSC, Vα24+invariant natural killer T cell , Vα24+iNKT , Anti-tumor activity, IFN-γ production, Tumor immunotherapy
Background
It was previously reported that clinical trials of Vα24+iNKT cell cancer immunotherapy targeting advanced non-small cell lung cancer (NSCLC) and head and neck cancer showed efficacy and were well-tolerated ( Motohashi et al., 2009 ; Yamasaki et al., 2011 ). However, it has been known that the cell yield from ex vivo expansion of Vα24+iNKT cells from peripheral blood mononuclear cells (PBMCs) is often low ( Motohashi et al., 2006 ). Reprogramming of somatic cells into induced pluripotent stem cells (iPSCs) using Yamanaka factors (Oct4, Sox2, Klf4 and c-Myc) has contributed greatly to the goals of regenerative medicine. The technology has recently been used to regenerate tumor-specific cytotoxic T lymphocytes and murine invariant natural killer T (iNKT) cells from iPSCs, thus opening up a new approach for cancer immunotherapy. Here, we have established a robust protocol to reprogram human Vα24+iNKT cells. We showed that iPS-derived Vα24+iNKT cells acted as cellular adjuvants and exerted anti-tumor activity, further extending their therapeutic potential. The complementation of other therapies with functionally validated Vα24+iNKT cells derived from iPSC could be valuable for cancer patients.
Materials and Reagents
Pipette tips
24-well plate (Corning, Falcon®, catalog number: 353047)
15 ml centrifuge tube (Corning, Falcon®, catalog number: 352196)
G27 needle (Terumo, catalog number: NN-2719S)
12-well plate (Corning, Falcon®, catalog number: 353043)
Cell strainer (size: 100 μm) (Greiner Bio One International, catalog number: 542000)
10 cm dish (Corning, Falcon®, catalog number: 353003)
6-well plate (Corning, Falcon®, catalog number: 353046)
6 cm dish (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 150288)
96-well round-bottomed plate (Corning, Falcon®, catalog number: 351190)
96-well round-bottomed plate for Procedures C and D (Corning, Falcon®, catalog number: 353077)
StemPro EZ passage tool (Thermo Fisher Scientific, GibcoTM, catalog number: 23181010)
Cell scraper (IWAKI, catalog number: 9000-220)
0.22 μm bottle top filter (EMD Millipore, catalog number: SCGVU05RE)
Human Vα24+iNKT cells
K562 (ATCC, catalog number: CCL-243)
OP9 feeder cells (obtained from RIKEN BRC Cell No. RCB2926)
SeV-KOS and SeV-c-MYC from CytoTune-iPS 2.0 (MEDICAL & BIOLOGICAL LABORATORIES, catalog number: DV-0305-3A)
OP9DLL1 feeder cells (obtained from RIKEN BRC Cell No. RCB2927)
Peripheral blood mononuclear cell (PBMC)
Cord blood mononuclear cell (CBMC)
Trypan blue stain 0.4% (Thermo Fisher Scientific, InvitrogenTM, catalog number: T10282)
SeV vector with a SV40 large T antigen (T) insertion (SeV-SV40) (ID Pharma, custom order)
Mitomycin-C (Sigma-Aldrich, catalog number: M4287)
iMatrix-511 (Nippi, catalog number: 892 012)
StemFit AK02N (ReproCELL, catalog number: RCAK02N)
Freezing medium for human ES/iPS cells (DAP213) (ReproCELL, catalog number: RCHEFM001)
Stem-cell banker GMP grade (Nippon Zenyaku Kogyo, Zenoaq, catalog number: CB045)
0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) (Thermo Fisher Scientific, GibcoTM, catalog number: 25200056)
TripLE select (Thermo Fisher Scientific, GibcoTM, catalog number: 12563011)
Y-27632, inhibitor of Rho-associated, coiled-coil containing protein kinase (ROCK) (Wako Pure Chemical Industries, catalog number: 253-00513)
Hanks’ balanced salt solution without phenol red (HBSS+) (Wako Pure Chemical Industries, catalog number: 084-08965)
Collagenase (Wako Pure Chemical Industries, catalog number: 036-23141)
Dulbecco’s phosphate buffered saline (D-PBS) (Wako Pure Chemical Industries, catalog number: 045-29795)
Stem cell factor (SCF) (R&D Systems, catalog number: 255-SC-050)
Recombinant human interleukin-7 (IL-7) (PeproTech, catalog number: 200-07)
Fms-related tyrosine kinase 3 (Flt-3) ligand (R&D Systems, catalog number: 3008-FK-025)
Recombinant human IL-15 (PeproTech, catalog number: 200-15)
7-AAD staining solution (BD, BD Biosciences, catalog number: 559925)
V450 mouse anti-human CD3 clone UCHT1 (BD, BD Biosciences, catalog number: 560365)
Anti-TCR Vβ11-APC (Beckman Coulter, catalog number: A66905)
Anti-TCR Vα24-PE (Beckman Coulter, catalog number: IM2283)
Lactate dehydrogenase (LDH) cytotoxicity detection kit (Takara Bio, catalog number: MK401)
BD OptELISA human IFN-γ enzyme-linked immunosorbent assay (ELISA) set (BD, BD Biosciences, catalog number: 555142)
BD OptELISA Human IL-4 ELISA Set (BD, BD Biosciences, catalog number: 555194)
Triton X-100 (Sigma-Aldrich, catalog number: T8787-100ML)
RPMI1640 (Sigma-Aldrich, catalog number: R8758)
Fetal bovine serum (Sigma-Aldrich, catalog number: 172012-500ML)
Penicillin-streptomycin (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122)
Hanks’ balanced salt solution without phenol red, without calcium, without magnesium (HBSS-) (Wako Pure Chemical Industries, catalog number: 085-09355)
2-[4-(2-Hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (HEPES) (Sigma-Aldrich, catalog number: H3375-250G)
Human IL-2 (Shionogi, catalog number: 4987058697900)
Primate ES Cell Medium (ReproCELL, catalog number: RCHEMD001)
Fibroblast growth factor basic (bFGF) (Wako Pure Chemical Industries, catalog number: 062-06661)
MEMα (Thermo Fisher Scientific, GibcoTM, catalog number: 11900-073)
Sodium hydrogen carbonate (NaHCO3) (Nacalai Tesque, catalog number: 31213-15)
Recombinant mouse GM-CSF (PeproTech, catalog number: 315-03)
α-galactosylceramide (α-GalCer) (Funakoshi, catalog number: KRN7000)
Lipopolysaccharide (LPS) (Thermo Fisher Scientific, InvitrogenTM, catalog number: 00-4976-93)
Dulbecco’s modified Eagle’s medium (D-MEM) (Wako Pure Chemical Industries, catalog number: 044-29765)
R10 medium (see Recipes)
NKT Media (see Recipes)
Human pluripotent stem cell medium (see Recipes)
OP9 medium (see Recipes)
DC/Gal (see Recipes)
MEF medium (see Recipes)
Equipment
CO2 incubator (35 °C, 37 °C, 38 °C) (Thermo Fisher Scientific, Thermo ScientificTM, model: HeracellTM 150i)
Inverted microscope (Leica Microsystems, model: DM IL LED)
Stereomicroscope (Leica, model: MZ75)
P200-pipette (Gilson, catalog number: FA10005P)
Flow cytometers (BD, BD Biosciences, model: FACSCanto II)
Centrifuge (KUBOTA, model: 2800)
Cell counter (Thermo Fisher Scientific, InvitrogenTM, catalog number: C10227)
Controlled rate freezer (Grant Instruments, model: EF600M)
OptEIA (BD, BD Biosciences, San Jose, CA)
Microplate reader (Molecular Devices, model: SpectraMax 190)
Software
FlowJo software (FlowJo, LLC)
Procedure
-
iPSC induction from human Vα24+iNKT cells
Day -3
Human Vα24+iNKT cells are seeded at 1 x 106 cells/ml in NKT medium into a 24-well plate (1 x 106 cells/well) and stimulated with 1 x 105 DC/Gal as previously described ( Shimizu et al., 2006 ; Yamada et al., 2016 ).
Day 0
Collect stimulated human Vα24+iNKT cells (Figure 1) into a 15 ml centrifuge tube by pipetting and count the viable cell number by trypan blue staining. Resuspend the cells in 1 ml R10 medium without antibiotics and seed at 1 x 106 cells/ml into a 24-well plate.
Add SeV (MOI: KOS, c-MYC, SV40 = 30, 3, 30) into the 24-well plate with the human Vα24+iNKT cells and mix gently.
Incubate at 35 °C in an incubator with 5% CO2.
Day 1
Replace the medium with 1 ml complete R10 medium.
Incubate at 35 °C in an incubator with 5% CO2.
Day 6
Collect the cells and count the viable cell number by trypan blue staining.
Resuspend the cells with complete R10 medium and seed at 3 x 105 cells on mitomycin C-treated MEFs or iMatrix-511 coated 6 cm dish.
Incubate at 35 °C in an incubator with 5% CO2.
Day 7
Replace the medium with human pluripotent stem cell medium (on mitomycin C-treated MEF) or StemFit AK02N (on iMatrix-511 coated).
Incubate at 35 °C in an incubator with 5% CO2.
Replace the medium every other day until human ESC-like colonies (Figure 2) appear (Takahashi and Yamanaka, 2006; Takahashi et al., 2007 ).
Day 21-28
Divide each human ESC-like colony (the diameter will be about 1 to 1.5 mm) into two clumps using a G27 needle under a stereomicroscope. Collect one clump (about 500 cells) into a tube by pipetting and subject to PCR for human Vα24+iNKT cell-specific TRAV (Vα24-Jα18) recombination detection ( Yamada et al., 2016 ).
Transfer the other clump onto mitomycin C-treated MEFs or iMatrix-511 coated 12 well-plate for cell proliferation after dissociation using gentle pipetting.
Incubate at 37 °C in an incubator with 5% CO2.
Maintain the cells using a StemPro EZ passage tool (on mitomycin C-treated MEF) or TripLE select (on iMatrix-511 coated) plates as previously described ( Vizcardo et al., 2013 ; Nakagawa et al., 2014 ). Store the cells using freezing medium for human ES/iPS cells (for cells on mitomycin C-treated MEF) or Stem cell banker (for cells on iMatrix-511 coated plates).
-
Differentiation of Vα24+iNKT cells from Vα24+iNKT-iPSC (iPS-NKT) (Figure 3)
Day -7
Dissociate OP9 cells (around 90% confluent) with 0.25% trypsin-EDTA.
Collect dissociated OP9 cells by passing through a cell strainer (size: 100 μm), and seed at 6 x 105 cells in a 10 cm dish in 10 ml OP9 medium.
Incubate at 37 °C in an incubator with 5% CO2.
Day -5
Add 10 ml OP9 medium.
Incubate at 37 °C in an incubator with 5% CO2.
Day 0
Replace OP9 medium with fresh OP9 medium supplemented with 10 μM Y-27632.
Dissociate human iPS cells (80% confluent) into clumps using a StemPro EZ passage tool under a stereomicroscope (Figure 4A).
Collect about 100 clumps (Figure 4B) using a P200-pipette under a stereomicroscope and plate them onto an OP9-seeded 10 cm dish.
Incubate at 37 °C in an incubator with 5% CO2.
Day 1
Replace the medium with 20 ml OP9 medium.
Incubate at 37 °C in an incubator with 5% CO2.
Day 9
Discard half of the supernatant (media).
Add 10 ml OP9 medium.
Incubate at 37 °C in an incubator with 5% CO2.
Day 12
Dissociate OP9DLL1 cells (around 90% confluent) with 0.25% trypsin-EDTA.
Collect dissociated OP9DLL1 cells by passing through a cell strainer (size: 100 μm), and seed at 2 x 106 cells on a 10 cm dish in 10 ml OP9 medium.
Incubate at 37 °C in an incubator with 5% CO2.
Day 13
Discard medium and rinse the cells with 10 ml HBSS+.
Add 6 ml collagenase (20 mg/ml).
Incubate at 37 °C, 45 min in a CO2 incubator.
Discard collagenase and rinse the cells with 10 ml D-PBS.
Add 6 ml 0.25% trypsin-EDTA.
Incubate at 37 °C, 30 min in a CO2 incubator.
Add 4 ml OP9 medium and collect dissociated cells passing through a cell strainer (size: 100 μm).
Resuspend the collected cells with 10 ml OP9 medium supplemented with 10 ng/ml of SCF, 5 ng/ml human IL-7 and 5 ng/ml Flt3-ligand and plate on an OP9DLL1 cell-seeded 10 cm dish (prepared at Day 12).
Day 15
Dissociate OP9DLL1 cells (around 90% confluent) with 0.25% trypsin-EDTA.
Collect the dissociated OP9DLL1 cells by passing through a cell strainer (size: 100 μm), and seed at 2 x 106 cells in a 10 cm dish in 10 ml OP9 medium.
Incubate at 37 °C in an incubator with 5% CO2.
Day 16
-
Preparation of mitomycin-C treated OP9DLL1 cells
Replace medium of OP9DLL1 cells with 6 ml OP9 medium supplemented with 1 μg/ml of mitomycin-C.
Incubate for 2 h, at 37 °C, 5% CO2 in a CO2 incubator.
Rinse mitomycin-C treated OP9DLL1 cells with D-PBS twice, and add 6 ml OP9 medium.
Incubate at 37 °C in an incubator with 5% CO2.
-
Passage of differentiating iPS-NKT cells.
Collect the cells (prepared at Day 13) by pipetting and dissociate by passing through a cell strainer (size: 100 μm).
Resuspend collected cells with 10 ml OP9 medium supplemented with10 ng/ml SCF, 5 ng/ml human IL-7 and 5 ng/ml Flt3-ligand and plate onto a mitomycin-C treated OP9DLL1 cell-seeded 10 cm dish.
Incubate at 37 °C in an incubator with 5% CO2.
Day 24
Dissociate OP9DLL1 cells (around 90% confluent) with 0.25% trypsin-EDTA.
Collect the dissociated cells by passing through a cell strainer (size: 100 μm), and seed at 2 x 106 cells on 10 cm dish in 10 ml OP9 medium.
Incubate at 37 °C in an incubator with 5% CO2.
Day 25
-
Preparation of mitomycin-C treated OP9DLL1 cells
Replace medium of OP9DLL1 cells with 6 ml OP9 medium supplemented with 1 μg/ml of mitomycin-C.
Incubate for 2 h at 37 °C, 5% CO2 in a CO2 incubator.
Rinse mitomycin-C treated OP9DLL1 cells with D-PBS twice, and add 6 ml OP9 medium.
Incubate at 37 °C in an incubator with 5% CO2.
-
Passage of differentiating iPS-NKT cells
Collect the cells (prepared at Day 16) by pipetting and dissociate by passing through a cell strainer (size: 100 μm).
Resuspend collected cells with 10 ml OP9 medium supplemented with 10 ng/ml SCF, 5 ng/ml human IL-7 and 5 ng/ml Flt3-ligand and plate onto a mitomycin-C treated OP9DLL1 cell-seeded 10 cm dish.
Incubate at 37 °C in an incubator with 5% CO2.
Day 30
Add 5 ml OP9 medium supplemented with10 ng/ml SCF, 5 ng/ml human IL-7 and 5 ng/ml Flt3-ligand (total 15 ml).
Incubate at 37 °C in an incubator with 5% CO2.
Day 33
Collect the cells by passing through a cell strainer (size: 100 μm).
Count the viable cell number by trypan blue staining and use 1 x 105 cells to analyze iPS-NKT marker (CD3, TRAV24 and TRBV11) expression by flow cytometry.
Seed the iPS-NKT cells at 5 x 105 cells/well into a 6 well-plate in 2 ml OP9 medium supplemented with 5 ng/ml human IL-7 and 10 ng/ml human IL-15.
Incubate at 37 °C in an incubator with 5% CO2.
Day 35, 37, 39 and 41
Add 1 ml OP9 medium supplemented with 5 ng/ml human IL-7 and 10 ng/ml human IL-15.
Incubate at 37 °C in an incubator with 5% CO2.
Day 43
Collect iPS-NKT cells (Figure 5) by pipetting and passing through a cell strainer (size: 100 μm).
Count the viable iPS-NKT cell number by trypan blue staining and use 1 x 105 cells analyze iPS-NKT marker (CD3, TRAV24 and TRBV11) expression by flow cytometry (Figure 6).
iPS-NKT cells can be used at this point for functional assays such as anti-tumor cell line assay or cytokine production assay.
-
Cytokine production assay
iPS-NKT cells are seeded on a 96-well round-bottomed plate (1 x 105 cells/well/200 μl).
Cells are co-cultured with or without murine DC/Gal (1 x 105 cells/well) for 24 h at 37 °C at 37 °C, 5% CO2 in a CO2 incubator. The amount of IFN-γ and IL-4 in the culture supernatants is measured with OptEIA.
-
Anti-tumor cell line assay
Effector cells are cultured with 1 x 104 of target cells at an E/T ratio of 5 or 10 in a 96-well round-bottomed plate.
-
Six hours after incubation at 37 °C, 5% CO2 in a CO2 incubator, 100 μl of supernatant is collected and LDH activity is measured with an LDH cytotoxicity detection kit in a 96-well flat-bottomed plate.
As a positive control, target cells lysed with 2% of Triton X-100 are used.
As background controls, effector cells or target cells alone are used.
3. In anti-tumor analysis using the LDH kit, O.D. at 490 nm and 600 nm is measured with a SpectraMax 190. O.D. at 490 nm is subtracted from the O.D. at 600 nm as noise. Killing activity is calculated with following formula: Killing activity = [{(O.D. of ‘effector + target’ - O.D. of medium) - (O.D. of target alone - O.D. of medium)} - (O.D. of effector alone - O.D. of medium)]/(O.D. of positive control - O.D. of target alone) x 100 (%).
Figure 1. Representative microscopic image for the stimulated human Va24+iNKT cells.
Human Va24+iNKT cells were stimulated with DC/Gal for several days.
Figure 2. Representative ESC-like colony shape.
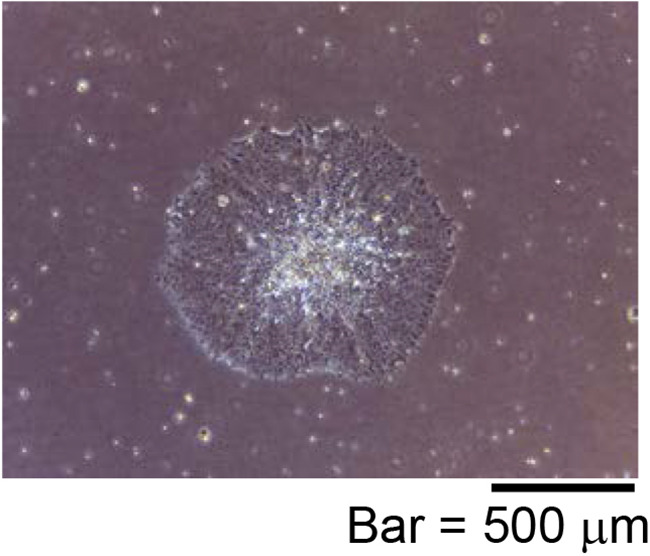
A diameter of ESC-like colony is about 1 to 1.5 mm.
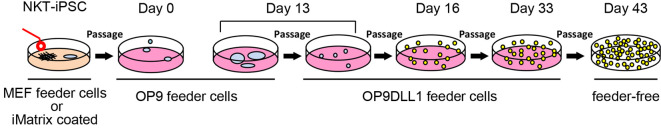
Figure 3. Schematic representation of iPS-NKT differentiation from NKT-iPSCs.
NKT derived iPSCs (NKT-iPSC) were separated into small clumps and seeded on OP9 feeder cells at day 0. On Day 13, cells were transferred to co-culture with OP9DLL1 feeder cells. On Day 33, cells were collected for feeder-free culture another 10 days.
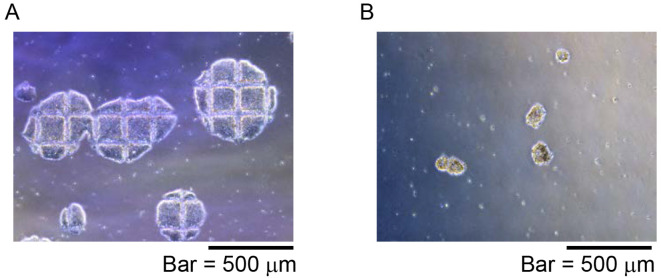
Figure 4. Representative NKT-iPSC morphology for iPS-NKT differentiation.
NKT-iPSC were divided into a few clumps using EZ-passage tool and pipetting. A. Colony shape of NKT-iPSC after cut using StemPro EZ-passage tool; B. Small NKT-iPSC clumps were prepared and collected by pipetting and passage into OP9 feeder cells.
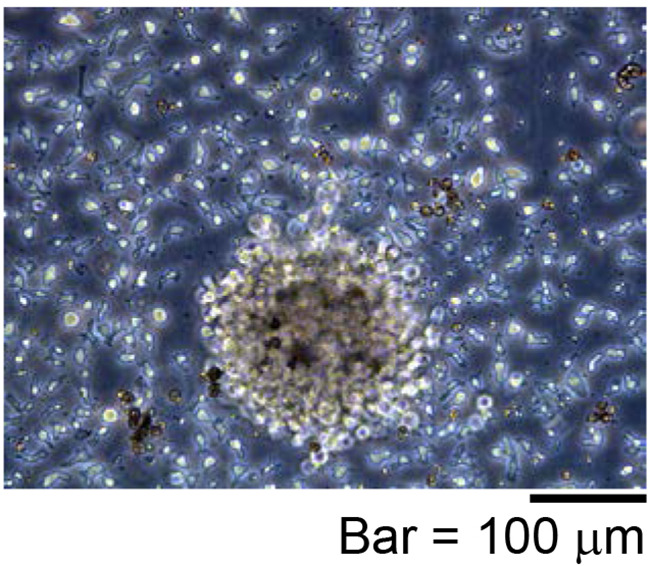
Figure 5. Representative proliferating iPS-NKT cells.
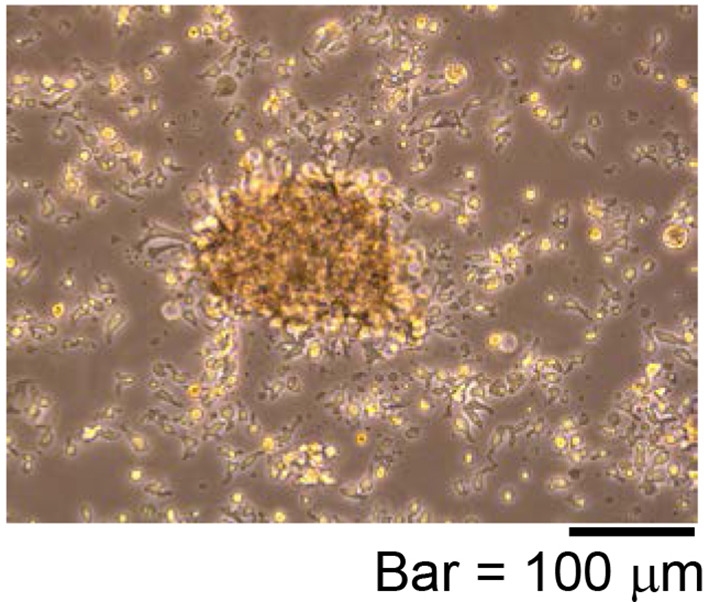
Activated T cell-like morphology is observed.
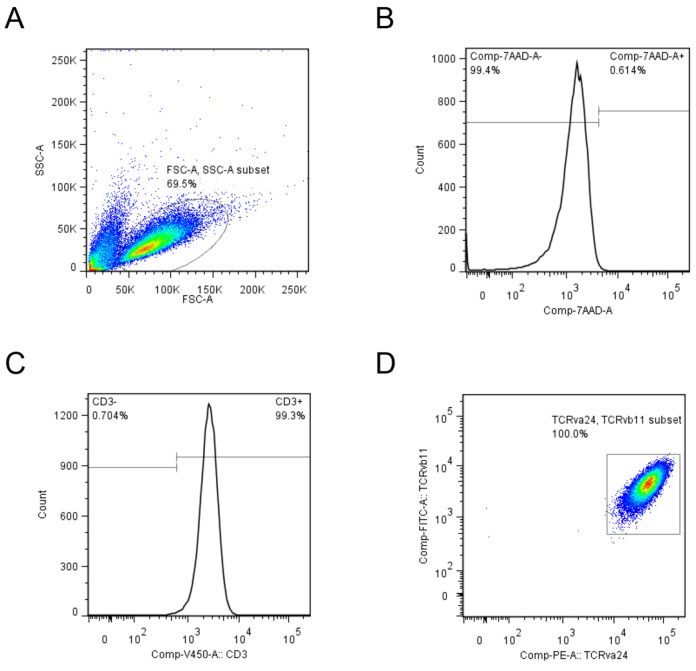
Figure 6. Representative iPS-NKT cells analysis using flow cytometer.
A. Lymphocyte fraction was gated using FSC and SSC as indicated by ellipse. B. Live cells were selected by 7-AAD negative fraction. C. CD3 positive fraction was selected and D. TRAV24 and TRBV11 positive cells were indicated as square.
Data analysis
We performed reprogramming of Vα24+iNKT cells into iPSCs from 4 donors and used karyotype stability for iPSC line selection. For assessing the phenotypes, we use FlowJo software (FlowJo, LLC) to process flow cytometry data of iPS-NKT cells obtained using a FACSCanto II. In the cytokine producing assay, we performed 4 to 7 independent experiments with 2 to 3-well replicates and pooled results were statistically analyzed with the Student’s t-test. In the anti-tumor cell line assay, we performed 8 independent experiments with triplicate replicates and statistically analyzed the data with the Student’s t-test. We describe details of replicates in the cytokine production assay in the Figure 2A legend in our original paper ( Yamada et al., 2016 ) and those of the anti-tumor cell line assay in Figure 2B ( Yamada et al., 2016 ).
Notes
We verified the reproducibility of our iPS-NKT cell flow cytometry data, cytokine producing assay and anti-tumor effects in at least 4 independent experiments in all studies. In all, we successfully reprogrammed four different Vα24+iNKT cells into iPSCs and regenerated three of them into Vα24+iNKT cells. These iPS-NKT cells always showed high IFN-γ production activity (more than 30 ng/ml) and low IL-4 production (lower than 0.3 ng/ml) upon stimulation with DC/Gal. After stimulation with DC/Gal or cytokines (IL-7+IL-15), iPS-NKT cells always show high cytotoxic activity against tumor cell lines (more than 50% against the K562 leukemia cell line). These results are very reproducible. In our hands, we observed high killing activity of iPS-NKT cells, i.e., 50-70%, against K562 leukemia cells.
As extra notes and technical tips, we addressed the following important items. Since the cell proliferation may depend on the lot of FCS, we have tested different lots for culturing OP9 feeder cells (Figure 7). In the anti-tumor cell line assay, we paid attention to removing dead cells before culturing iPS-NKT cells and target tumor cells since the dead cells may lead to a high background.
At Day 6 of iPSC induction, there are 1 x 106 to 1.5 x 106 cells in a 24-well plate. We usually observe 200-300 ES-like colonies by Day 21-28.
At Day 33, about 1 x 106 iPS-NKT cells are obtained from about 5 x 104 NKT-iPSC and finally these cells can proliferate around 10-fold 8 days after being stimulated with IL-7 and IL-15. For clinical use, we can increase the OP9DLL1-seeded 10 cm dish to ten dishes, yielding ~5 x 107 iPS-NKT cells.
-
Mitomycin-C treated MEFs are prepared as previously described (Conner, 2001) with a small modification.
Replace medium of the MEFs with 6 ml MEF medium supplemented with 1 μg/ml of mitomycin-C.
Incubate for 2 h at 37 °C, 5% CO2 in a CO2 incubator.
Rinse mitomycin-C treated MEF with D-PBS twice, and add 6 ml MEF medium.
Incubate at 37 °C in an incubator with 5% CO2.
Mitomycin C-treated MEFs purchased from ReproCELL can also be used.
We usually start with 2-4 x 107 mononuclear cells from peripheral blood or cord blood that can be stored in liquid nitrogen until use. PBMCs or CBMCs are cultured in RPMI supplemented with 10% FBS and 100 U/ml of hIL-2 and stimulated with α-GalCer (100 ng/ml) for 10-14 days. Cells are stained with FITC-conjugated anti-human Va24 antibody followed by anti-FITC MACS beads (Miltenyi Biotech, 10 μl beads to 107 cells). According to manufacturer’s protocol, Vα24+iNKT cells are positively purified by using an LS column (Miltenyi Biotech, purity > 95%). Purified Vα24+iNKT cells are cultured in complete NKT medium. We usually obtain around 2 million Vα24+iNKT cells from one donor.
Figure 7. Representative OP9 feeder cells morphology.
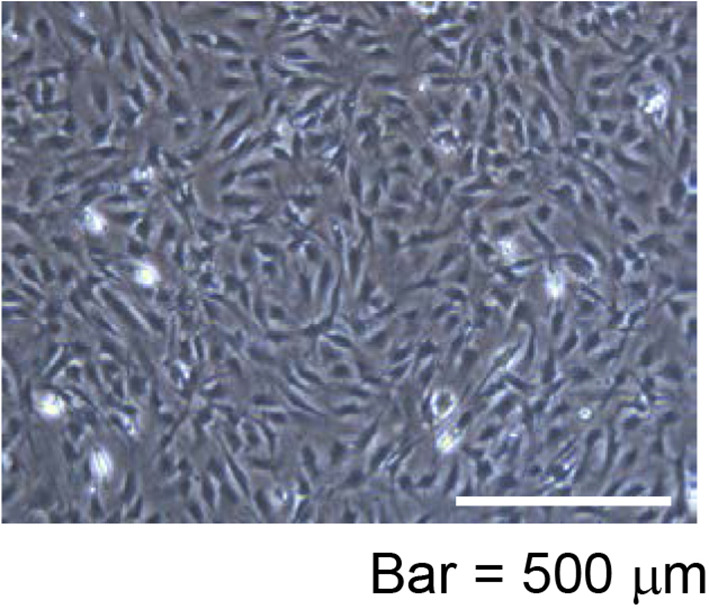
After 48 h cultivation (seeded at 6 x 105 cells in a 10 cm dish), more than 3 x 106 OP9 feeder cells (about 90% confluent) in the 10 cm dish were observed.
Recipes
-
R10 medium
RPMI1640 containing the following supplements:
10% fetal bovine serum (FBS)
100 U/ml penicillin
100 μg/ml streptomycin
10 mM HEPES
-
NKT medium
R10 medium containing human IL-7 (5 ng/ml), human IL-15 (10 ng/ml) and human IL-2 (100 U/ml)
-
Human pluripotent stem cell medium
Primate ES Cell Medium containing bFGF (10 ng/ml)
-
OP9 medium
MEMα powder and 2.2 g of NaHCO3 are dissolved into 1 L of distilled water and sterilized using a 0.22 μm bottle top filter
Finally, the following supplements are added:
100 U/ml penicillin
100 μg/ml streptomycin
20% fetal bovine serum (FBS)
-
DC/Gal
Mouse bone marrow cells which are depleted of CD4, CD8, Class II and B220 positive cells are cultured in a 24-well plate in the presence of recombinant GM-CSF (20 ng/ml)
Bone marrow-derived DCs are pulsed with 100 ng/ml α-GalCer for 48 h on Day 6 and stimulated by adding LPS (100 ng/ml) for the last 24 h
-
MEF medium
D-MEM containing the following supplements:
15% fetal bovine serum (FBS)
100 U/ml penicillin
100 μg/ml streptomycin
Acknowledgments
We are grateful to prof. P.D. Burrows for the critical reading of the manuscript. We would like to thank Genta Kitahara, Momoko Okoshi, Midori Kobayashi, Maki Sakurai for their technical assistance. This work was supported by the Research Center Network for Realization of Regenerative Medicine from Japan Agency for Medical Research and Development (AMED) and CREST, Japan Science and Technology Agency. This protocol was modified from previous works that we had done with murine iPS-NKT cells and human iPS-T cells ( Watarai et al., 2010 ; Vizcardo et al., 2013 ).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Conner D. A.(2001). Mouse embryo fibroblast(MEF) feeder cell preparation. Curr Protoc Mol Biol 23(2): Unit 23.2. [DOI] [PubMed] [Google Scholar]
- 2.Motohashi S., Ishikawa A., Ishikawa E., Otsuji M., Iizasa T., Hanaoka H., Shimizu N., Horiguchi S., Okamoto Y., Fujii S., Taniguchi M., Fujisawa T. and Nakayama T.(2006). A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer . Clin Cancer Res 12(20Pt 1): 6079-6086. [DOI] [PubMed] [Google Scholar]
- 3.Motohashi S., Nagato K., Kunii N., Yamamoto H., Yamasaki K., Okita K., Hanaoka H., Shimizu N., Suzuki M., Yoshino I., Taniguchi M., Fujisawa T. and Nakayama T.(2009). A phase I-II study of alpha-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol 182(4): 2492-2501. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa M., Taniguchi Y., Senda S., Takizawa N., Ichisaka T., Asano K., Morizane A., Doi D., Takahashi J., Nishizawa M., Yoshida Y., Toyoda T., Osafune K., Sekiguchi K. and Yamanaka S.(2014). A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci Rep 4: 3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimizu K., Hidaka M., Kadowaki N., Makita N., Konishi N., Fujimoto K., Uchiyama T., Kawano F., Taniguchi M. and Fujii S.(2006). Evaluation of the function of human invariant NKT cells from cancer patients using alpha-galactosylceramide-loaded murine dendritic cells. J Immunol 177(5): 3484-3492. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. and Yamanaka S.(2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5): 861-872. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K. and Yamanaka S.(2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4): 663-676. [DOI] [PubMed] [Google Scholar]
- 8.Vizcardo R., Masuda K., Yamada D., Ikawa T., Shimizu K., Fujii S., Koseki H. and Kawamoto H.(2013). Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8+ T cells . Cell Stem Cell 12(1): 31-36. [DOI] [PubMed] [Google Scholar]
- 9.Watarai H., Fujii S., Yamada D., Rybouchkin A., Sakata S., Nagata Y., Iida-Kobayashi M., Sekine-Kondo E., Shimizu K., Shozaki Y., Sharif J., Matsuda M., Mochiduki S., Hasegawa T., Kitahara G., Endo T. A., Toyoda T., Ohara O., Harigaya K., Koseki H. and Taniguchi M.(2010). Murine induced pluripotent stem cells can be derived from and differentiate into natural killer T cells. J Clin Invest 120(7): 2610-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamasaki K., Horiguchi S., Kurosaki M., Kunii N., Nagato K., Hanaoka H., Shimizu N., Ueno N., Yamamoto S., Taniguchi M., Motohashi S., Nakayama T. and Okamoto Y.(2011). Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy. Clin Immunol 138(3): 255-265. [DOI] [PubMed] [Google Scholar]
- 11.Yamada D., Iyoda T., Vizcardo R., Shimizu K., Sato Y,, Endo T. A., Kitahara G., Okoshi M., Kobayashi M., Sakurai M., Ohara O., Taniguchi M., Koseki H. and Fujii S. I.(2016). Efficient regeneration of human Vα24+ invariant natural killer T cells and their anti-tumor activity in vivo . Stem Cells 34(12): 2852-2860. [DOI] [PubMed] [Google Scholar]