Abstract
A vast challenge within neuropsychiatric research has been the development of animal models that accurately reflect symptoms associated with affective disorders. An ethologically valid model that has been shown to be effective in studying depression is the chronic social defeat stress model. In this model, C57BL/6J mice are subjected to chronic social defeat stress induced by CD-1 aggressor mice for 10 consecutive days. Discussed here is a protocol describing the screening process of the CD-1 aggressor mice, the confrontations between the C57BL/6J and CD-1 aggressor mice, and analysis of social avoidance scores as an indication of depression-like behaviors.
Keywords: Social defeat, Depression, Chronic stress, Social interaction, Mouse model
Background
Depressive disorders are recurring and life-threatening conditions that affect approximately 120 million people worldwide. Of significant interest within the field of neuropsychiatric research is the development of valid animal models for depression to aid the understanding of the neurobiological and molecular mechanisms underlying depressive disorders. Various models of chronic stress have been used to induce symptoms in mice that are relevant to depression, among those are chronic unpredictable stress, foot-shock stress, immobilization stress followed by measurements of anhedonia or despair-like behaviors (e.g., sucrose preference test, forced swim test and tail suspension test), but they are not well validated as models of human depression (Krishnan and Nestler, 2011). Currently, chronic social defeat stress has been widely accepted as a well-validated model for depression, because of its reliability and reproducibility ( Berton et al., 2006 ; Krishnan et al., 2007 ; Golden et al., 2011 ; Krishnan and Nestler, 2011; Russo and Nestler, 2013). The social defeat paradigm has been validated as a good model of human depression because of following reasons: 1) The depression phenotypes of susceptible mice (social avoidance, anhedonia, metabolic changes, etc.) are reversed by chronic treatment of anti-depressant drugs but not by acute administration. 2) Social defeat stress produces both a susceptible and resilient phenotype and this can be used to explain individual differences of human stress susceptibility in depression pathophysiology. 3) The susceptible phenotypes induced by social defeat stress are long lasting with concomitant genetic and epigenetic changes in the brain reward circuitry.
Materials and Reagents
-
C57BL/6J (THE JACKSON LABORATORY): 7-8 weeks old mice are used in social defeat (6-7 weeks mice are ordered for a week acclimation period in the vivarium)
Note: Mice are housed in standard cages with 4 to 5 mice per cage.
-
CD-1 mice (Charles River): Retired male breeder mice were 4-6 months of age and singly housed in standard mouse cages
Note: Social defeat paradigms use a consistent subordination of subject mice as a social stressor and a forced subordination strategy is employed in this protocol with the most widely used strains, C57BL/6J: CD-1 pair.
Cleaning solution (for cleaning arena and custom Plexiglas social interaction arena/divider in between trials): 0.5% hydrogen peroxide
Equipment
Clear rat cages (disposable rat cage with bedding) (Innovive, catalog number: RS1-H-C8)
Standard water bottle (mouse water bottle) (Innovive, catalog number: M-WB-300)
Water bottle stopper with sipper tube (Neoprene stopper, size: #8.5; Ancare, 1” bend tubes – open tip) (Ancare, catalog number: OT-199 - 3”)
Cylinder (custom made, plastic holder for additional water spout; Figure 2B)
Divider (custom made, clear perforated Plexiglas plate; Figure 2B)
Removable enclosure (custom made, clear Plexiglas, top and bottom sides are open; Figure 3B)
Stopwatch
Open field chamber (custom made, 44 [w] x 44 [d] x 30 [h] cm)
Video tracking system for social interaction test (e.g., Noldus Information Technology, model: EthoVision system, CCD camera & computer)
Figure 2. Standard cage setup in chronic social defeat stress.
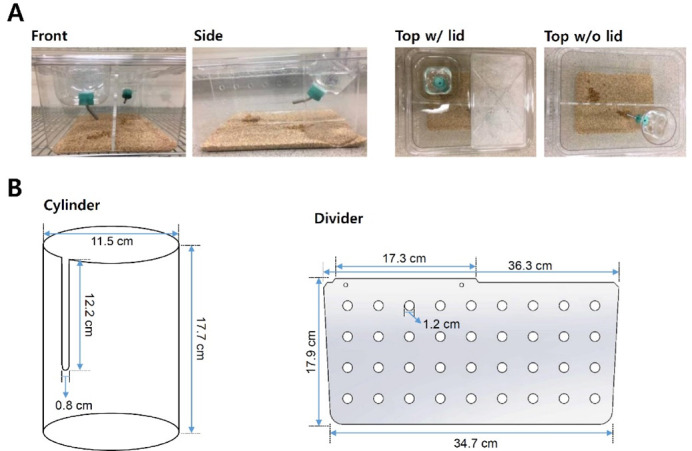
A. Standard rat cage used in chronic social defeat stress sessions. The resident aggressor CD-1 mouse is permanently housed on one side of the perforated divider, while C57BL/6J mice are rotated each day following defeat session to avoid habituation to aggressor mouse. B. Dimensions of additional parts for standard rat cage (custom made). The Cylinder holds an additional water bottle on side opposite of standard water bottle side. The perforated Plexiglas divider physically separates mice during non-social defeat periods (perforations allow for sensory interaction).
Figure 3. Schematics of the social interaction test arena.
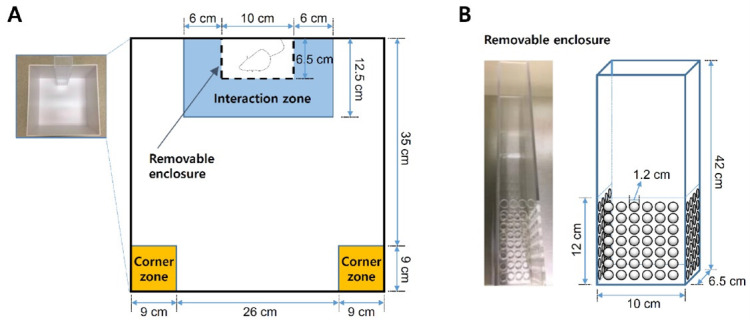
A. Social defeat open field arena and zones for social interaction analysis. B. Removable, perforated Plexiglas enclosure for ‘target’ CD-1 aggressor mice.
Software
Tracking software: EthoVision XT (Noldus Information Technology)
Procedure
Note: Chronic social defeat stress experiments must comply with governmental and institutional guidelines for care and use of laboratory animals.
-
Screening of CD-1 aggressor mice
Order male CD-1 retired breeder mice at 4-6 months of age (order at least 2-3 times more than required, as less than half of CD-1 mice will be aggressive enough). CD-1 mice should be singly housed in standard mouse cages with access to food and water ad libitum. Allow CD-1 mice to habituate to their new environment for a week prior to initiating screening process.
C57BL/6J ‘screener’ male mice are used only to screen CD-1 mice. The screener mice can range in age (8-20 weeks) and can be used for subsequent screening processes. Remove any aggressive ‘screener’ mice (rare).
Screening of CD-1 mice is performed in the cage of the resident CD-1 mouse. C57BL/6J ‘screener’ mouse should be placed directly into home cage with aggressor CD-1 mouse for 5 min. Latency to aggression should be noted for each CD-1. Perform three screening sessions for 3 consecutive days (once daily) with different screeners.
CD-1 aggressor mice should be selected based on two criteria: During three 5-min screening sessions, the latency to initial aggression must be less than 60 sec during two consecutive sessions. And the CD-1 must continuously attack the C57BL/6J screener mouse for at least 10 sec without interruption for all the three 5-min sessions.
House CD-1 aggressor mice selected for experimentation with access to food and water ad libitum (ready to use). Aggressor mice can be used for up to 3 months after initial screening (CD-1 aggressor mice can habituate to social defeat sessions, and thus need to be rescreened prior to the next social defeat experiment).
-
Chronic social defeat stress (Figure 1)
-
To assemble social defeat cages (Figure 2A), take standard rat cage and fill the bottom of cage with woodchip bedding. Place the perforated Plexiglas divider in the center of cage. Divider will physically separate the mice after defeat sessions and allow for sensory interaction during non-defeat housing periods. Place cylinder (additional water bottle holder) on side opposite of standard water bottle side. Insert assembled water bottle with cylindrical holder. Ensure that water is easily accessible. Place food on bottom of each side (water and food should be available ad libitum). Place lid of standard rat cage securely onto cage. At least 10 social defeat cages with CD-1 aggressors are needed for the 10-day social defeat protocol.
Note: The divider should be firmly positioned to prevent escapes.
-
Place resident CD-1 aggressor mouse on one side of the divided home cage overnight prior to the start of social defeat sessions for habituation in the new cage.
Note: The cylinder side is preferred for efficient defeats in smaller space.
-
On the first day of social defeat sessions, place intruder C57BL/6J mouse directly into the cage side of CD-1 aggressor mouse. After 5-10 min of social defeat (Video 1), transfer the intruder mouse to opposite side of perforated divider and house within compartment for the remainder of the 24-h period.
Note: Placing intruder mouse close to the aggressor at session start is helpful to initiate aggressive behaviors. Social defeat stress may be done with up to 10 subject mice at a time to allow close observation.
For the control group, place C57BL/6J mice pairs in an identical social defeat cage set up with one mouse on each side of perforated Plexiglas divider for the duration of the 10-day social defeat test without any physical contact. The mice are rotated daily between control cages (‘intruder’ side only).
Repeat daily social defeat sessions in a novel CD-1 aggressors’ homecage for 10 days.
Immediately following the last social defeat session, singly house all C57BL/6J intruder mice. Provide animals with ad libitum access to food and water. All CD-1 aggressor mice are returned to their singly-housed cages and used for future social defeat experiments (need to be rescreened).
-
-
Social interaction test
Social interaction tests can be performed 24 h after the last social defeat session. In the social interaction test, the CD-1 aggressor mouse should be completely novel to the defeated C57BL/6J mouse being tested. Screen the CD-1 aggressor mouse prior to performing social interaction test to ensure mouse meets criterion for aggression.
Assemble social interaction arenas as shown in Figure 3. Set up video-tracking system (e.g., EthoVision XT) to collect all data automatically by tracking software.
Habituate defeated C57BL/6J mice to behavioral testing suite at least 1 h prior to social interaction test (under red light condition, isolated from external noise sources and animals).
-
Each social interaction test is composed of two 2.5 min phases under red light condition. In the first phase (‘target’ absent), place the C57BL/6J defeated mouse into the social interaction arena (at the center of opposite side to the enclosure) and allow to explore freely without a ‘target’ CD-1 mouse.
Note: Do not clean the arena after the first phase.
-
After the first phase, return the defeated C57BL/6J mouse back to its home cage until the second phase. Then, place the ‘target’ CD-1 mouse directly into the removable enclosure.
Note: Drop the CD-1 mouse gently from top of the enclosure.
-
In the second phase (‘target’ present), reintroduce the defeated C57BL/6J mouse and let it move freely around in the presence of the ‘target’ CD-1 mouse.
Note: Place the defeated mice in a consistent manner between phases.
At the end of each test session, remove the target and defeated mouse and place them into their home cage. Sanitize all equipment using cleaning solution.
Data can be analyzed as discussed in Data analysis section.
Figure 1. Schematic of social defeat and social interaction test.
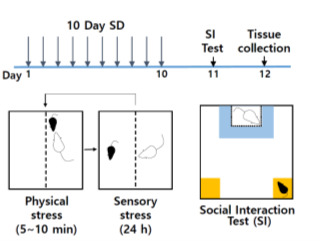
Chronic social defeat scheme uses 10-days of consecutive social defeat using CD-1 aggressor mice in the divided cages, followed by social interaction test and subsequent analysis.
Video 1. A bout of aggression in social defeat by CD-1 aggressor.
Approximately 3 to 5 bouts of consecutive aggressive behaviors can be observed during a social defeat period.
Data analysis
Using an automated video tracking system, cumulative times in interaction zone and corner zone can be calculated from video data of each trial. Control mice usually spend more time in the interaction zone in ‘target’ present trial, but in defeated mice this kind of social interaction time with the ‘target’ mice is decreased (Figure 4). Thus, social avoidance of mice can be quantified by comparing interaction zone times with or without a ‘target’ mouse (CD-1 aggressor) in the enclosure. Social interaction ratio (SI ratio) is calculated from both interaction zone times as follows:
Figure 4. Statistic analysis of the social interaction test.
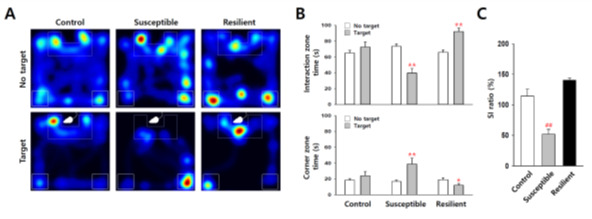
A. Representative heatmap of social interaction data for control, susceptible and resilient mice. B and C. Statistical analysis show decreased social interaction with ‘target’ CD-1 mice in susceptible group (n = 18) after social defeat stress, but not in control (n = 23) and resilient groups (n = 13). *P < 0.05, **P < 0.01 vs. ‘no target’, ##P < 0.01 vs. control, ANOVA. Data are represented as the mean ± SEM.
SI ratio (%) =
Mice showing less than 100% of SI ratio are grouped as depression-susceptible mice, and, on the other hand, mice showing over 100% SI ratio belong to depression-resilient group. In most cases, more than half of C57BL/6J mice show susceptible phenotype when they are subjected to chronic social defeat stress. Corner zone time is another useful index of social avoidance, which tends to inversely correlate with the interaction zone time (Figure 4B, lower panel).
Notes
The cage setup for social defeat stress can be varied, and additional equipment like divider and cylinder can be modified or substituted according to the cage shape and dimension.
Social defeat duration (5-10 min) can be adjusted according to the aggressiveness of CD-1 aggressor mice for appropriate physical stress without severe wounding. Observe each session carefully and replace CD-1 mice that are too aggressive (consistently make open wounds), or not aggressive enough during social defeat sessions. Euthanize severely injured defeated mice.
-
Variations of social defeat protocol: To shorten the period of stress (e.g., coincidence with viral expression period, such as HSV) or to minimize damage to implanted cannula (e.g., optogenetics), mice can be subjected to shortened social defeat stress paradigms.
Submaximal social defeat stress (Subthreshold or micro-defeat; 1 day protocol): Mice are placed into the CD-1 aggressor’s cage for 5 min, followed by a 15 min break. This defeat session is repeated two more times with novel CD-1 aggressors ( Sun et al., 2015 ; Kim et al., 2016 ). Pro-susceptibility of drug treatment or gene modulation can be tested with this method, but the protocol has no significant effect on wild-type mice.
Accelerated social defeat stress (4~5 day protocol): Mice are subjected to an accelerated social defeat stress paradigm (two daily defeats for 4~5 days), and the shortened protocol is equally robust in inducing susceptibility as 10-day chronic social defeat protocol ( Vialou et al., 2014 ; Sun et al., 2015 ; Heller et al., 2016 ).
Acknowledgments
This work was supported by grants from NIMH and NARSAD (NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation). This protocol is modified from previous social defeat procedures ( Berton et al., 2006 ; Krishnan et al., 2007 ; Golden et al., 2011 ).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Berton O., McClung C. A., Dileone R. J., Krishnan V., Renthal W., Russo S. J., Graham D., Tsankova N. M., Bolanos C. A., Rios M., Monteggia L. M., Self D. W. and Nestler E. J.(2006). Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311(5762): 864-868. [DOI] [PubMed] [Google Scholar]
- 2.Golden S. A., Covington H. E. 3rd Berton O. and Russo S. J.(2011). A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6(8): 1183-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heller E. A., Hamilton P. J., Burek D. D., Lombroso S. I., Pena C. J., Neve R. L. and Nestler E. J.(2016). Targeted epigenetic remodeling of the Cdk5 gene in nucleus accumbens regulates cocaine- and stress-evoked behavior . J Neurosci 36(17): 4690-4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim H. D., Hesterman J., Call T., Magazu S., Keeley E., Armenta K., Kronman H., Neve R. L., Nestler E. J. and Ferguson D.(2016). SIRT1 mediates depression-like behaviors in the nucleus accumbens. J Neurosci 36(32): 8441-8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnan V., Han M. H., Graham D. L., Berton O., Renthal W., Russo S. J., Laplant Q., Graham A., Lutter M., Lagace D. C., Ghose S., Reister R., Tannous P., Green T. A., Neve R. L., Chakravarty S., Kumar A., Eisch A. J., Self D. W., Lee F. S., Tamminga C. A., Cooper D. C., Gershenfeld H. K. and Nestler E. J.(2007). Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131(2): 391-404. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan V. and Nestler E. J.(2011). Animal models of depression: molecular perspectives. Curr Top Behav Neurosci 7: 121-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo S. J. and Nestler E. J.(2013). The brain reward circuitry in mood disorders. Nat Rev Neurosci 14(9): 609-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun H., Damez-Werno D. M., Scobie K. N., Shao N. Y., Dias C., Rabkin J., Koo J. W., Korb E., Bagot R. C., Ahn F. H., Cahill M. E., Labonte B., Mouzon E., Heller E. A., Cates H., Golden S. A., Gleason K., Russo S. J., Andrews S., Neve R., Kennedy P. J., Maze I., Dietz D. M., Allis C. D., Turecki G., Varga-Weisz P., Tamminga C., Shen L. and Nestler E. J.(2015). ACF chromatin-remodeling complex mediates stress-induced depressive-like behavior. Nat Med 21(10): 1146-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vialou V., Bagot R. C., Cahill M. E., Ferguson D., Robison A. J., Dietz D. M., Fallon B., Mazei-Robison M., Ku S. M., Harrigan E., Winstanley C. A., Joshi T., Feng J., Berton O. and Nestler E. J.(2014). Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of DeltaFosB. J Neurosci 34(11): 3878-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]


