Abstract
Neuropathic pain is one of the highly debilitating chronic pain conditions, for which, currently, there is no therapeutic treatment. In order to reveal the underlying mechanism for neuropathic pain, various animal models have been established ( Burma et al., 2016 ). This protocol describes how to prepare spinal nerve injury model (Kim and Chung, 1992; Rigaud et al., 2008 ; Masuda et al., 2016 ), one of the most frequently-used and highly reproducible models in which multiple alterations occur both in the peripheral and central nervous system.
Keywords: Neuropathic pain, Nerve injury, Spinal cord, Mouse, Dorsal root ganglion (DRG), Allodynia
Background
Revealing the underlying mechanism of neuropathic pain is necessary to develop effective therapy for its optimal management. Therefore, various animal models for neuropathic pain have been developed. In particularly, rodent models have been frequently used because they are highly reproducible and exhibit pain hypersensitivity that is also observed in patients with neuropathic pain. In this protocol, we describe how to prepare the mouse spinal nerve injury model.
Materials and Reagents
5-0 silk suture (Alfresa Pharma, catalog number: GA05SW)
Sterile scalpel blades (FATHER Safety Razor, catalog number: No.10)
Wild-type C57BL/6 mice (6-15 weeks old) (Japan Clea)
Isoflurane (Mylan)
75 % ethanol (Takasugi Pharmaceutical)
Equipment
-
Electric clippers (Daito Electric Machine Industry, catalog number: 605AD, Special C)
Note: This product has been discontinued.
Agricola retractor (Fine Science Tools, catalog number: 17005-04)
Forceps (NATSUME SEISAKUSHO, catalog number: A-14)
Vannas spring scissors (Fine Science Tools, catalog number: 15070-08)
Electric drill (URAWA Kogyo, model: MINITORJET UC210)
Scalpel holder (NATSUME SEISAKUSHO, catalog number: D-11)
Heating pad (VIVARIA, catalog number: MP-916-NV)
Double sided micro spatula (Fine Science Tools, catalog number: 10091-12)
Isoflurane dispenser (FORWICK, MURACO Medical)
Procedure
Ethic Local animal ethics regarding animal housing and animal experiment need to be followed.
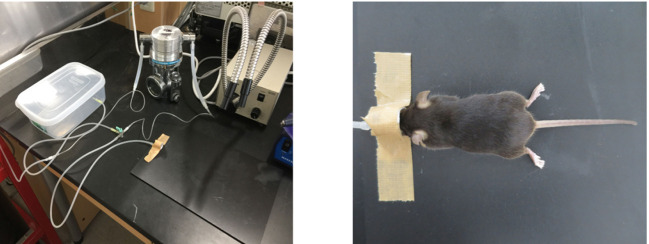
In order to induce anesthesia, put the mouse in the plastic chamber where 5% isoflurane is provided through a tube to the dispenser (Figure 1). It takes approximately 30 sec.
Anesthetize the mouse with 2-3% inhaled isoflurane by setting on the board with the head placed into a mask connected to the dispenser (Figure 1).
Make sure that the mouse is adequately anesthetized by applying pressure on the mouse toe with forceps (toe pinch).
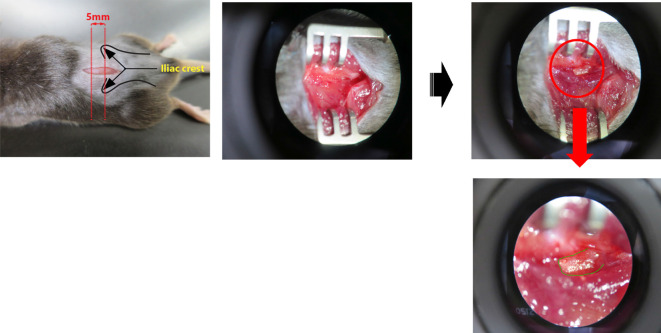
Remove hair from the proper region of the back of the mouse with electric clippers, and make a small incision with a fine scalpel from the lumbar L3 to the sacral S1 vertebra after cleaning with 75% ethanol (Figure 2).
Spread the tissue underneath the skin with an agricola retractor, and scratch off the muscle lying on the transverse process of the lumbar L5 vertebra (highlighted in green) with micro spatula to keep it visible (Figure 3, Video 1). Normally the transverse process of the lumbar L5 vertebra is located underneath the muscle within 5 mm from the tip of the iliac crest (Figure 3, left).
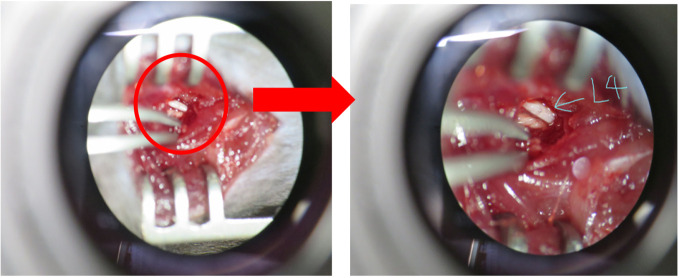
Drill the root of the transverse process of the lumbar vertebra at the blue line at a speed of 3,000 rpm (Figure 4) and remove it.
Expose the parallel-lying L3 and L4 spinal nerves by removing the paraspinal muscle and fat from the L5 transverse process with forceps (Figure 5).
Carefully isolate the L4 spinal nerve with fine forceps and cut it with spring scissors without injuring the L3 nerve (Figure 6).
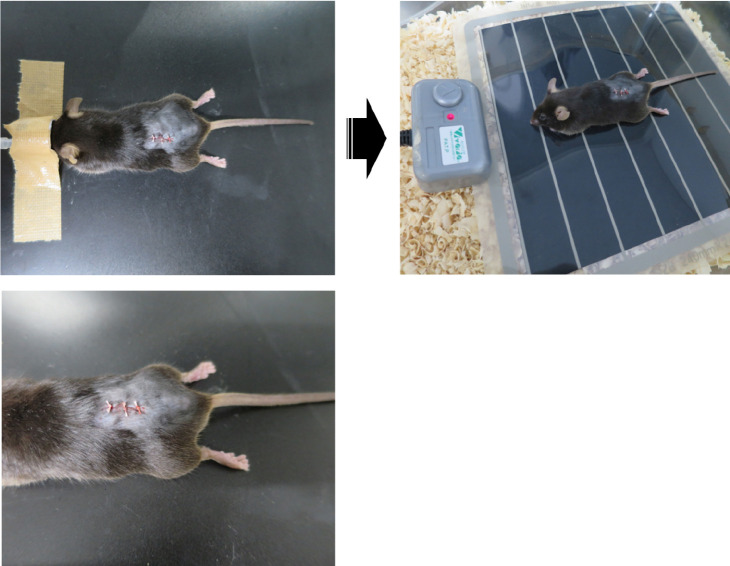
After making sure there is no bleeding from the lesion site (see Notes), suture the wound and the surrounding skin with 5-0 silk sutures (Figure 7). Keep the mouse on a heating pad after the operation until the mouse wakes up (Figure 7).
Figure 1. Inhalational anesthesia with isoflurane.
The mouse was anesthetized via an inhalation mask using 2-3% isoflurane.
Figure 2. Small incision on the back skin.
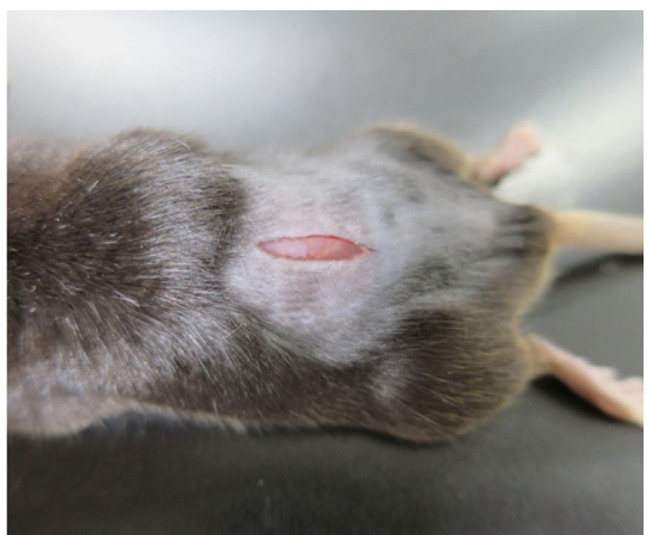
A small incision from the lumbar L3 to the sacral S1 vertebra was made with a fine scalpel after removing the hair.
Figure 3. Exposure of the transverse process of the lumbar L5 vertebra.
The transverse process of the lumbar L5 vertebra was exposed by scratching off the muscle.
Video 1. Spinal nerve injury.
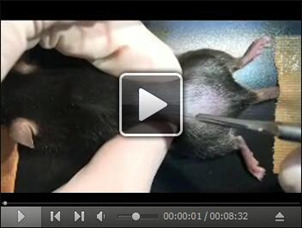
This video shows how the L4 spinal nerve injury is performed.
Figure 4. Removal of the transverse process of the lumbar L5 vertebra.
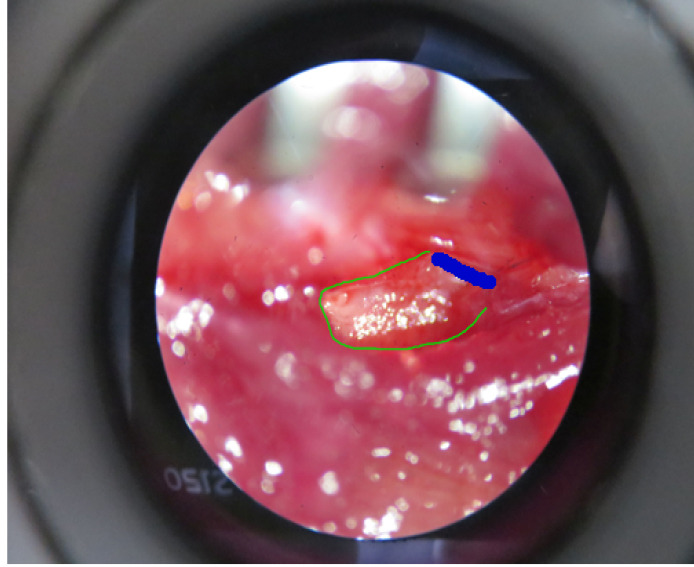
The transverse process of the lumbar L5 vertebra (highlight in green) needs to be removed after drilling the root of it (highlight in blue).
Figure 5. Exposure of L4 spinal nerve.
L4 spinal nerve was exposed by removing the paraspinal muscle and fat from the L5 transverse process.
Figure 6. Cut of L4 spinal nerve.
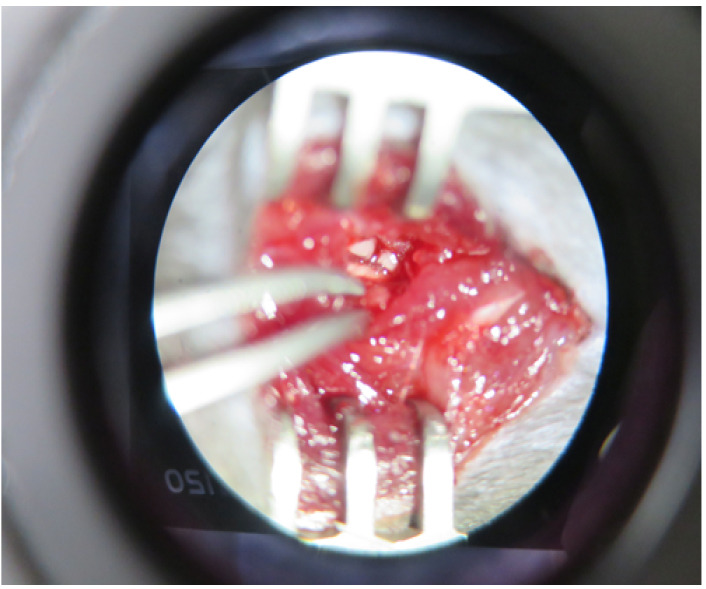
L4 spinal nerve was cut with spring scissors.
Figure 7. Suturing the wound.
The wound and the surrounding skin were sutured, and the mouse was placed on a heating pad.
Notes
To avoid causing undesirable tissue inflammation at the operation site, it is important to try to keep the operative time as short as possible; normally this procedure takes approximately 7 min to complete in our laboratory.
If there is accidental bleeding from the operation site, apply proper pressure with a cotton bud. If the bleeding does not stop, the mouse should not be used for further experiments.
Since there are strain-dependent variations in the number of lumbar vertebrae in mice ( Rigaud et al., 2008 ), careful identification and isolation of the L4 spinal nerve is necessary.
As a control, sham surgery could be performed by exposing and drilling the root of the transverse process of the lumbar vertebra without removing the process to keep the L4 spinal nerve intact.
Acknowledgments
This protocol has been developed based on a previously published procedure (Kim and Chung, 1992; Rigaud et al., 2008 ) with some modifications. T.M. is granted as a JSPS postdoctoral fellowship for research abroad. This work was supported by Grant-in Aid of for Scientific Research (KAKEN 25117013, 15H02522, 16K18885) from JSPS (to K.I., M.T., Y.K.).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Burma N. E., Leduc-Pessah H., Fan C. Y. and Trang T.(2016). Animal models of chronic pain: Advances and challenges for clinical translation. J Neurosci Res 95(6):1242-1256. [DOI] [PubMed] [Google Scholar]
- 2.Kim S. H. and Chung J. M.(1992). An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50(3): 355-363. [DOI] [PubMed] [Google Scholar]
- 3.Masuda T., Ozono Y., Mikuriya S., Kohro Y., Tozaki-Saitoh H., Iwatsuki K., Uneyama H., Ichikawa R., Salter M. W., Tsuda M. and Inoue K.(2016). Dorsal horn neurons release extracellular ATP in a VNUT-dependent manner that underlies neuropathic pain. Nat Commun 7: 12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rigaud M., Gemes G., Barabas M. E., Chernoff D. I., Abram S. E., Stucky C. L. and Hogan Q. H.(2008). Species and strain differences in rodent sciatic nerve anatomy: implications for studies of neuropathic pain. Pain 136(1-2): 188-201. [DOI] [PMC free article] [PubMed] [Google Scholar]