Abstract
The molecular mechanisms underlying myogenic induction by insulin-like growth factor I (IGF-I) are distinct from its proliferative effects on myoblasts. To determine the postmitotic role of IGF-I on muscle cell differentiation, we derived L6E9 muscle cell lines carrying a stably transfected rat IGF-I gene under the control of a myosin light chain (MLC) promoter-enhancer cassette. Expression of MLC–IGF-I exclusively in differentiated L6E9 myotubes, which express the embryonic form of myosin heavy chain (MyHC) and no endogenous IGF-I, resulted in pronounced myotube hypertrophy, accompanied by activation of the neonatal MyHC isoform. The hypertrophic myotubes dramatically increased expression of myogenin, muscle creatine kinase, β-enolase, and IGF binding protein 5 and activated the myocyte enhancer factor 2C gene which is normally silent in this cell line. MLC–IGF-I induction in differentiated L6E9 cells also increased the expression of a transiently transfected LacZ reporter driven by the myogenin promoter, demonstrating activation of the differentiation program at the transcriptional level. Nuclear reorganization, accumulation of skeletal actin protein, and an increased expression of β1D integrin were also observed. Inhibition of the phosphatidyl inositol (PI) 3-kinase intermediate in IGF-I-mediated signal transduction confirmed that the PI 3-kinase pathway is required only at early stages for IGF-I-mediated hypertrophy and neonatal MyHC induction in these cells. Expression of IGF-I in postmitotic muscle may therefore play an important role in the maturation of the myogenic program.
During mammalian embryogenesis, skeletal muscle undergoes a series of developmental changes that are reflected in a progression of structural gene expression patterns, such that the specific contractile protein composition of a myocyte is considered a measure of its maturity. The orchestration of this progression presumably involves a complex interplay between intrinsic programs of lineage specification and extrinsic controls such as innervation, extracellular milieu, and humoral factors in the embryonic environment (46). In the adult, muscle injury and subsequent regeneration result in a partial recapitulation of these embryonic programs of maturation, underscoring the enduring plasticity of the muscle phenotype in the face of changing external conditions.
The insulin-like growth factors (IGFs) have been implicated in the control of skeletal muscle growth and differentiation in both embryonic development and in the muscle regeneration process (20, 22, 29, 36). Recent studies of the effects of IGFs on the myogenic program in muscle cell culture have focused specifically on IGF-I, since it is the predominant form in mature muscle tissue. In cultured myoblasts, as in other cell types, IGF-I induces cell proliferation (14, 37). However, unlike other growth factors, IGF-I also stimulates myogenic differentiation and generates pronounced myocyte hypertrophy (14, 18, 37), suggesting that this growth factor can regulate both proliferative and differentiative responses in muscle cells.
In previous reports, we and others have established that proliferation precedes differentiation in IGF-I-stimulated myogenesis in culture (14, 41). From these studies, it is clear that the effects of IGF-I on proliferation and differentiation are temporally separated. IGF-I plays its roles step by step, first acting upon myoblast replication, increasing the expression pattern of factors involved in the cell cycle progression (14, 41), and subsequently promoting myogenic differentiation by induction of myogenic regulatory factors (MRFs). The effects of IGF-I are also modulated by a family of six IGF binding proteins (IGFBPs) (25), which can either inhibit or potentiate the IGF-stimulated actions in vivo and in vitro.
Recent experimental evidence suggests that IGF-I exerts its functions by activating two different intracellular signal transduction pathways, conveying either proliferative or differentiative signals (10, 26). The proliferative response is mediated by the mitogen-activated protein (MAP) kinase pathway, whereas the pathway leading to differentiation involves the activation of phosphatidylinositol (PI) 3-kinase. The phosphorylated products of this lipid kinase, PI 3,4-bisphosphate and PI 3,4,5-trisphosphate activate downstream effectors, ultimately leading to induction of muscle gene expression. The alternate activation of these two pathways presumably underlies the observed pleiotropic effects of IGF-I on muscle cells.
To determine whether IGF-I can stimulate myogenesis in the absence of proliferation, we established an experimental cell culture system where the action of IGF-I on proliferation and on differentiation could be uncoupled. The neonatal rat L6E9 myogenic line has been used extensively to study the role of IGF-I in myogenesis (33, 47), since it does not express this growth factor itself and can respond to IGF-I stimulation through presentation of IGF-I receptors (39). Although the L6 cells fail to express a number of characteristic myogenic regulators such as MyoD (5, 38) or myocyte enhancer factor (MEF) 2C (30), they are still able to undergo fusion and to activate a subset of muscle structural genes in response to exogenous stimuli (19). Treatment of L6E9 myoblasts with exogenous IGF-I in defined serum-free medium induces a transient increase in cell cycle markers and cell proliferation (14, 41). This response is rapidly followed by withdrawal from the cell cycle and activation of myogenic factors, resulting in a net increase in structural gene expression and larger myotubes (14).
To bypass the proliferative effects of IGF-I, we stably transfected L6E9 cultures with a muscle-specific IGF-I expression vector, myosin light chain (MLC)–IGF-I, which is activated only after myoblasts have withdrawn from the cell cycle and have committed to differentiation. In this way, the influence of IGF-I on myogenic differentiation could be dissected and manipulated independently of its role in myoblast proliferation. Studies of the MLC–IGF-I transfectants suggest that postmitotic expression of IGF-I potentiates and accelerates the myogenic program, induces dramatic myotube hypertrophy, activates a new battery of genes, and initiates a shift towards a more mature fiber type. Moreover, disruption of specific signal transduction pathways in these cells reveals a potential third PI 3-kinase-independent signal transduction pathway that presumably participates in later stages of myogenic differentiation. These studies in cell culture support a role for IGF-I in the establishment and maintenance of the mature muscle phenotype in normal and regenerating muscle tissue.
MATERIALS AND METHODS
Cell culture and transfection.
L6E9 cell cultures (33, 47) were maintained in growth medium (GM) consisting of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% fetal bovine serum (FBS).
For stable transfection, L6E9 cells were grown in 100-mm-diameter plates to 70% confluence and then washed with serum- and antibiotic-free medium. Cells were transfected with an MLC–IGF-I plasmid (pMLC/IGF-I) and with a puromycin-selectable vector (pPUR; Clontech) (10:1) according to the Lipofectamine transfection method (Gibco-BRL). Briefly, DNA (10 μg of pMLC/IGF-I and 1 μg of pPUR), 50 μl of Lipofectamine, and 1.6 ml of serum- and antibiotic-free medium were incubated at room temperature for 45 min and then added to the plate for 5 h. Following incubation, 10 ml of GM was added. At 24 h following the start of transfection, the transfection mixture was removed and replaced with DMEM plus 20% FBS. At 48 h after transfection, the cells were passaged at a 1:10 dilution into selection medium containing 20% FBS and 3 μg of puromycin per ml. Stable myoblasts were maintained in DMEM selection medium, which was replaced every 2 days, for 12 days. Fifteen drug-resistant clones (L6MLC/IGF-I) were isolated by using cloning rings and characterized morphologically and by Northern blot analysis for IGF-I expression.
For transient transfections, L6E9 and L6MLC/IGF-I cells were transfected with Lipofectamine by using either a myogenin–LacZ–β-Gal (Myo1565LacZ) or a CMV–β-Gal plasmid. The CMV-β-Gal plasmid was included to assess transfection efficiency in both L6E9 and L6MLC/IGF-I cells. To activate myogenic differentiation, and the expression of MLC–IGF-I, myoblasts were switched to differentiation medium (DM; DMEM plus 1% bovine serum albumin). Myogenin promoter activity was analyzed, by determining relative numbers of LacZ-positive cells, after either 0 (80% confluence) or 3 days in DM.
RNA preparation, Northern blotting, and hybridization.
Total RNA from L6E9 and L6MLC/IGF-I cells was obtained by RNA-TRIzol extraction (Gibco-BRL). Fifteen micrograms of total RNA was separated on 1.3% agarose gels containing 2.2 M formaldehyde and transferred directly from the gel to Nytran membranes (GeneScreen Plus; NEN) in 10× SSC (1× SSC is 0.15 M NaCl plus 1.5 mM sodium citrate) overnight. After transfer, RNA was UV cross-linked (120,000 μJ of UV) and the membranes were baked at 80°C for 2 h.
Hybridization was carried out at 42°C in a mixture containing 50% formamide, 10% dextran sulfate, 1% sodium dodecyl sulfate (SDS), and 100 μg of salmon sperm DNA (Sigma) per ml. The cDNA probes were 32P labeled by random priming (16) to a specific activity of 108 cpm/mg of DNA. Filters were washed at high stringency with 0.2× SSC–0.5% SDS at 65°C and exposed for autoradiography on RP-X-OMAT film (Kodak).
Immunohystochemical analysis.
L6E9 and L6MLC/IGF-I myotubes (after 4 days in DM) were fixed in 4% paraformaldehyde and then preincubated in phosphate-buffered saline (PBS) containing 1% BSA and a 1:30 dilution of goat serum for 30 min. Myotubes were treated with monoclonal antibody (MAb) against either skeletal actin (Sigma) (1:6), the myosin heavy chain (MyHC) embryonic subunit (MAb BF-45) (1) (1:100), or the MyHC neonatal subunit (MAb BF-34) (1) (1:100) overnight at 4°C.
For actin analysis, after primary antibody incubation, cells were rapidly washed twice with PBS and once with PBS containing 1% BSA for 15 min and incubated with biotinylated goat anti-mouse antibody (Sigma) at a 1:800 dilution for 1 h at room temperature, washed again as described above, and incubated with avidin-alkaline phosphatase conjugate at a 1:100 dilution for 1 h at room temperature. The cells were washed with PBS containing 1% BSA and processed for alkaline phosphatase activity (Sigma kit B5655).
After incubation with the MyHC embryonic subunit or the MyHC neonatal subunit primary antibody, the myotubes were rapidly washed twice with PBS and once with PBS containing 1% BSA for 15 min and incubated with goat anti-mouse rhodamine-conjugated antibody. The cells were washed again as described above and mounted in 90% glycerol in PBS (pH 8). The nuclei of myogenic cells were visualized by Hoechst staining.
Biotinylation and immunoprecipitation.
Control L6E9 and L6MLC/IGF-I myoblasts were grown to approximately 80% confluence in GM and then switched to DM (day 0) and cultured for 3 days. The membrane proteins of myoblasts and myotubes were labeled with 0.5 mg of Sulfo-NHS-biotin (Pierce) in buffer A (1.3 mM CaCl2, 0.4 mM MgSO4, 5 mM KCl, 138 mM NaCl, 5.6 mM d-glucose, 25 mM HEPES [pH 7.4]) at 4°C for 30 min on a rocker. The labeled reaction mixture was blocked by washing with DMEM containing 0.6% BSA and 20 mM HEPES (pH 7.4).
For integrin immunoprecipitation, L6E9 and L6MLC/IGF-I myoblasts and myotubes were extracted with 0.5% Triton X-100 in 50 mM HEPES (pH 7.5)–150 mM NaCl containing protease and phosphatase inhibitors (2 mM phenylmethylsulfonyl fluoride, 100 U of aprotinin per ml, 10 μg of leupeptin and pepstatin per ml, 20 mM sodium vanadate) and centrifuged at 12,000 × g for 30 min at 4°C to remove cellular debris. The supernatant was incubated with protein A-Sepharose beads to effect a preclearing for 1 h at 4°C on a rocker. The protein concentration was determined by using a protein assay reagent kit (Bio-Rad). Samples containing 1 mg of total protein extract were immunoprecipitated with rabbit polyclonal anti-β1D integrin antibody (kindly provided by G. Tarone) for 2 h at 4°C on a rocker and then incubated with protein A-Sepharose beads at 4°C for 1 h. The beads were extensively washed with 0.5% Triton X-100 in 50 mM HEPES (pH 7.5)–150 mM NaCl.
For electrophoresis, proteins were separated by use of a 7% polyacrylamide gel (SDS-polyacrylamide gel electrophoresis [PAGE]) (28) and transferred onto an Immobilon membrane (Amersham) as described by Towbin et al. (44). The blot was blocked with 5% nonfat dry milk–0.1% Tween 20 in Tris-buffered saline (TBS; 50 mM Tris-HCl, 150 mM NaCl [pH 7.5]) for 1 h at room temperature and probed with ExtrAvidin-peroxidase-conjugated (dilution, 1:2,000). The blots were washed extensively with TBS plus 0.1% Tween 20 and then with TBS and finally developed by using enhanced chemiluminescence ECL reagents (Amersham).
LY294002 treatment.
Cells were grown as described above and treated with a 10 μM concentration of a PI 3-kinase inhibitor, LY294002, at different times in the differentiation program: during growth in GM, at 80% of confluence (day 0), and at days 1, 2, and 3 in DM. Cells that were treated during growth in GM with LY294002 in dimethyl sulfoxide were switched to DM without PI 3-kinase inhibitor when they were at 80% confluence. Cells that did not receive inhibitor received the control vehicle dimethyl sulfoxide. After 4 days in DM, the cells were analyzed morphologically by eosin-Wright’s stain or analyzed by Northern blotting or immunohistochemistry analysis.
RESULTS
Postmitotic expression of IGF-I in L6E9 cultures stimulates the myogenic program.
To uncouple the proliferative and differentiative effects of IGF-I on muscle cells, we generated a myogenic cell line in which the IGF-I expression was forced only after myoblast withdrawal from the cell cycle and induction of differentiation. The L6E9 line is a subclone of the parental rat neonatal myogenic line which does not express IGF-I but which presents IGF-I receptors and is therefore responsive to the growth factor. L6E9 myoblasts were stably transfected with an expression vector in which rat IGF-I cDNA was driven by regulatory elements from the MLC1/3 locus. These elements (an MLC1 promoter and the downstream MLC enhancer) have been extensively characterized both in cell culture and in transgenic mice (13, 40) and are activated only after differentiation has been initiated. Therefore, the transfected MLC–IGF-I transcription unit was expressed only in L6E9 cultures that have permanently withdrawn from the cell cycle and that have committed to myogenic differentiation.
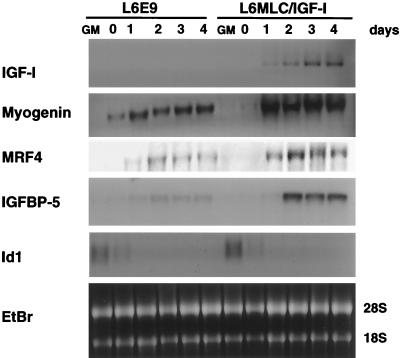
MLC–IGF-I expression was evaluated by Northern blot analysis of total RNA isolated both from untransfected L6E9 cultures and from MLC–IGF-I transfectants (L6MLC/IGF-I). An IGF-I cDNA probe confirmed that IGF-I expression was undetectable in control L6E9 cells, whereas it accumulated to high levels in L6MLC/IGF-I cells after removal of growth medium and induction of differentiation (Fig. 1).
FIG. 1.
Postmitotic expression of IGF-I enhances expression of the markers of muscle terminal differentiation. Control L6E9 myoblasts and L6E9 myoblasts stably transfected with the MLC–IGF-I expression vector (L6MLC/IGF-I) were grown to approximately 80% confluence in GM and then switched to DM (day 0). Total RNA was isolated from proliferative myoblasts (lanes GM) and at 0, 1, 2, 3, and 4 days after switching to DM. Northern blots of total RNA samples (15 μg) were analyzed with the indicated 32P-labeled probes. Ethidium bromide (EtBr) staining was used to verify equal loading of the RNA sample.
It has been previously demonstrated that myogenin expression increases in L6 cultures treated with IGF-I (14, 19). To verify whether the induction of transfected MLC–IGF-I in postmitotic myotubes correlated with an increase in myogenin levels, we analyzed the same RNA samples with a myogenin probe. Similar levels of myogenin transcript were observed in proliferative (GM) myoblasts of both control L6E9 and L6MLC/IGF-I cell lines (Fig. 1). However, a marked increase in myogenin transcripts was observed in L6MLC/IGF-I cells as early as 1 day after serum withdrawal (Fig. 1, day 1). In addition, the levels of MRF4 transcripts were modulated by postmitotic expression of IGF-I (Fig. 1).
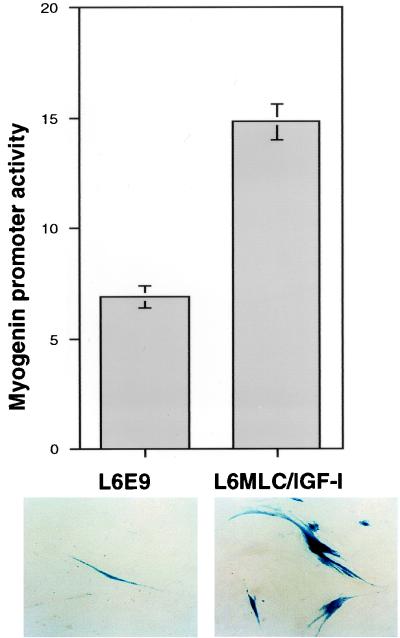
To verify whether the increased levels of myogenin transcript accumulation correlated with an increase of myogenin promoter activity, we transfected both L6E9 and L6MLC/IGF-I myoblasts with a myogenin promoter-driven LacZ reporter construct (myogenin-LacZ). As shown in Fig. 2, MLC–IGF-I expression resulted in a dramatic increase in myogenin promoter activity, as determined by the numbers of LacZ-positive cells relative to those of parental L6E9 cells. In contrast, analysis of the myogenin promoter activity at day 0 (80% confluence), before MLC–IGF-I expression was induced, did not show any significant difference between the numbers of LacZ-positive cells in L6E9 and L6MLC/IGF-I cultures (data not shown).
FIG. 2.
IGF-I enhances myogenin promoter activity. L6E9 and L6MLC/IGF-I myoblasts were transiently transfected with a myogenin–LacZ–β-Gal vector. Cell cultures were shifted 24 h after transfection to serum-free medium and cultured for an additional 72 h. Fifty separate randomly selected fields per each cell line were counted to quantitate LacZ-expressing cells. The numbers of total cells in each field per each cell line were approximately the same. The number of β-Gal-positive cells per field are indicated on the y axis.
The effects of IGF-I, both in cell culture and in vivo, are modulated by a family of serum binding proteins, IGFBPs, with different roles (25). Specifically, IGFBP4 expression is associated with myoblast proliferation (31), IGFBP6 plays a role in quiescence (15), whereas the positive modulator of the differentiative action of IGF-I, IGFBP5, is expressed during myoblast differentiation (32). Analysis of the IGFBP5 transcript in L6E9 and L6MLC/IGF-I cultures during differentiation revealed an increase during myoblast differentiation in response to IGF-I induction (Fig. 1), suggesting that the IGFBP5 gene is itself controlled by IGF-I and that IGFBP5 positively modulates the differentiative effect of IGF-I.
To determine whether expression of MLC–IGF-I affected only those genes expressed after differentiation, we analyzed genes expressed in proliferating L6E9 myoblasts. The myogenic regulatory factor Myf-5 (6) was expressed at very low levels, only in the proliferative L6E9 myoblasts, and this expression pattern was maintained in both L6E9 and L6MLC/IGF-I cells (data not shown). Expression of another member of the helix-loop-helix family of transcriptional regulators, Id1 (4), was repressed similarly during the differentiation of both L6E9 and L6MLC/IGF-I cells (Fig. 1). Thus, the expression of the early genes myf-5 and Id1, which are presumably involved in myoblast proliferation, is not affected by postmitotic expression of IGF-I.
IGF-I potentiates the myogenic program by induction of MEF-2C expression.
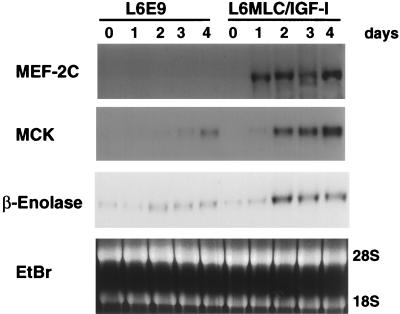
It has been demonstrated that myogenin expression is necessary but clearly not sufficient for the enhanced myogenesis caused by IGF-I (21). In fact, the induction of myogenic differentiation by the MRFs is achieved by interaction with multiple additional transcription factors. Among these, the MEF-2 family is involved in a complex regulatory network with the MRFs (24, 30). MEF-2A and MEF-2D are active during proliferation, whereas MEF-2C expression is accumulated during myogenic differentiation (30). The L6E9 line lacks MEF-2C transcripts (Fig. 3), confirming that the MEF-2C gene is not activated in these cells.
FIG. 3.
IGF-I promotes the myogenic program by induction of MEF-2C expression. Northern blots of total RNA (15 μg) isolated from L6E9 and L6MLC/IGF-I myogenic cultures at 0, 1, 2, 3, and 4 days after switching to DM and probed with MEF-2C (30), MCK (8), and β-enolase (35) 32P-labeled cDNA probes are shown. Ethidium bromide (EtBr) staining was used to verify equal loading of the RNA sample.
We analyzed the expression pattern of MEF-2C in L6MLC/IGF-I transfectants to determine whether IGF-I could activate the expression of silent genes, which are normally involved in regulating the differentiation program in other muscle cells. Northern blot analysis revealed that MEF-2C expression was undetectable in control L6E9 proliferating myoblasts (data not shown) and quiescent myoblasts (Fig. 3, day 0), as well as during all stages of differentiation (Fig. 3, 1 to 4 days after serum withdrawal). In contrast L6MLC/IGF-I cultures activated MEF-2C transcription after only 1 day of serum withdrawal (Fig. 3). This result establishes a link between the action of IGF-I and the MEF-2C gene, whose expression it activates.
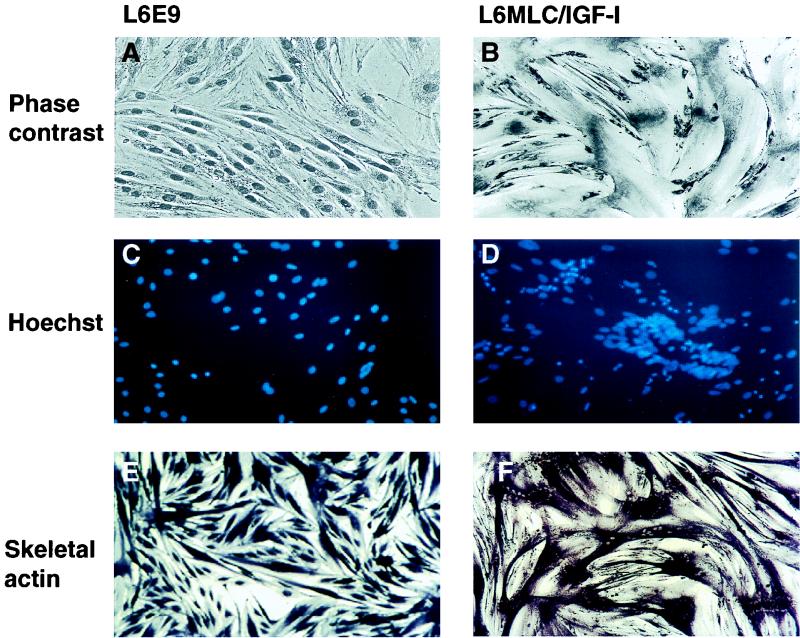
FIG. 4.
Postmitotic expression of IGF-I induces myotube hypertrophy and cytoskeletal reorganization. L6E9 and L6MLC/IGF-I cell lines were grown to approximately 80% confluence in GM and then switched to DM and cultured for 4 days. (A and B) Eosin-Wright’s stain (phase contrast) of L6E9 and L6MLC/IGF-I differentiated muscle cells; (C and D) Hoechst nuclear staining; (E and F) differentiated cultures immunostained with mouse anti-α actin MAb, processed for alkaline phosphatase activity, and then photographed at the same magnification.
To verify whether known targets of MEF-2C were also activated during differentiation of L6MLC/IGF-I cells, we examined expression of the muscle creatine kinase (MCK) and β-enolase genes, which are normally activated to very low levels in the parent L6E9 line. The MCK gene promoter contains, in addition to several MRF E-box targets, a MEF-2 binding site which is essential for its activity (12), and β-enolase presents in its enhancer a G-rich box that interacts with ubiquitous factors and a MEF-2 binding site (35). MCK and β-enolase expression levels were dramatically enhanced in L6MLC/IGF-I differentiating cultures, in contrast to expression levels found in the control L6E9 cultures (Fig. 3). This suggests that the activation of MEF-2C gene expression by IGF-I reinforces and amplifies its effect on the myogenic program.
Morphological changes induced by postmitotic expression of IGF-I.
We and others have previously reported that exogenous IGF-I induces hypertrophy of skeletal myofibers in muscle cell cultures (14, 45). To examine whether transfected MLC–IGF-I expression in L6E9 cells could induce a similar hypertrophy, we analyzed the morphological phenotype of control L6E9 and L6MLC/IGF-I cultures as they differentiated. As shown in Fig. 4B, expression of MLC–IGF-I generated pronounced myotube hypertrophy. More detailed morphological analysis revealed an unusual nuclear organization in L6MLC/IGF-I cells, grouped in characteristic rings located within the middle of the myofibers (Fig. 4D). That this phenomenon may be related to sarcomeric reorganization in the hypertrophied fibers is suggested by the localization of skeletal actin in these cells. Immunohistochemical analysis (Fig. 4E and F) revealed that skeletal actin protein was uniformly distributed in control L6E9 myotubes, whereas it accumulated in a perinuclear pattern in L6MLC/IGF-I myotubes.
Patterns of integrin expression are altered by postmitotic IGF-I expression.
Integrins play a decisive role in cell adhesion, modulating many cellular functions as well as cell growth, differentiation, programmed cell death, and tissue repair. Recently, a new muscle-specific isoform of β1 integrin β1D, has been characterized (3); the expression of this isoform is undetectable in proliferative myoblasts but is induced upon myoblast fusion. The β1D integrin is associated with α7A and α7B subunits and localizes to various adhesive structures of muscle cells, as well as to costameres, and to myotendinous neuromuscular junctions (3).
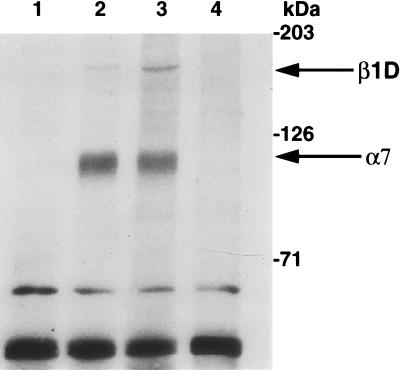
To determine whether IGF-I modulates integrin expression, we analyzed the expression pattern of β1D integrin which is associated with myogenic differentiation, as well as its α7 heterodimeric partner, which in contrast is expressed during muscle proliferation and differentiation. Immunoprecipitation analysis with anti-β1D integrin antibody (Fig. 5) revealed that β1D integrin was undetectable in L6E9 (data not shown) and in L6MLC/IGF-I myoblasts (Fig. 5, lane 1), was expressed at low levels in control L6E9 myotubes (Fig. 5, lane 2), but was conspicuously accumulated in L6MLC/IGF-I myotubes (Fig. 5, lane 3). In contrast, levels of α7 subunits were not affected by IGF-I expression, since they remained unchanged in L6MLC/IGF-I myotubes (Fig. 5). This result suggests that the β1D integrin, a marker of terminal myogenic differentiation, is induced by IGF-I, potentially to regulate cytoskeleton reorganization and to mediate the IGF-I activity on muscle-specific gene expression and hypertrophy.
FIG. 5.
IGF-I induces accumulation of β1D integrin. Biotinylated total cell extracts from L6E9 and L6MLC/IGF-I proliferative myoblasts and differentiated myotubes were immunoprecipitated with anti-β1D integrin antibody. Proteins were resolved by SDS-PAGE, blotted onto nitrocellulose membrane, and probed with ExtrAvidin-peroxidase. Lane 1, L6MLC/IGF-I proliferative myoblasts plus antibody (Ab); lane 2, L6E9 myotubes plus Ab; lane 3, L6MLC/IGF-I myotubes plus Ab; lane 4, L6MLC/IGF-I myotubes without Ab. Arrows labeled β1D and α7 indicate the bands corresponding to β1D and α7 integrins, respectively.
Postmitotic expression of IGF-I induces a structural marker of muscle maturation in L6E9 cells.
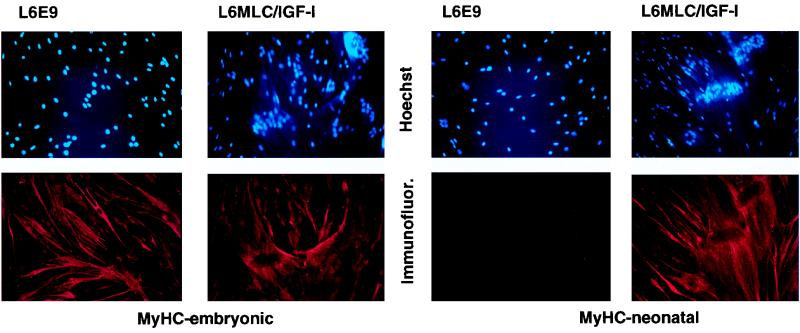
During development, skeletal muscle tissue undergoes a succession of changes to more mature phenotypes (7). This is accomplished by shifts in gene expression patterns, which includes a switch from embryonic MyHC to more mature MyHC isoforms. The L6E9 line most closely represents an immature myogenic stage, in which the embryonic form of MyHC is still expressed. To explore whether the hypertrophic muscle phenotype induced by IGF-I in vitro mimics the muscle maturation process in vivo, we scored the expression of embryonic and neonatal MyHC isoforms in L6E9 and L6MLC/IGF-I myotubes by immunohistochemical analysis. The data in Fig. 6 show that whereas L6E9 myotubes express only the embryonic MyHC isoform, L6MLC/IGF-I myotubes express both embryonic and neonatal MyHCs. This confirms that in L6E9 myotubes, expression of IGF-I induces a shift towards a more mature muscle type.
FIG. 6.
IGF-I activates expression of the neonatal isoform of MyHC. L6E9 and L6MLC/IGF-I cell lines were grown to approximately 80% confluence in GM and then switched to DM and cultured for 4 days. Differentiated cultures were immunostained with MAb against either the embryonic or neonatal isoform of MyHC (Immunofluor) or were subjected to nuclear staining (Hoechst).
IGF-I-mediated muscle hypertrophy is only transiently dependent on the PI 3-kinase pathway.
The activation of a specific developmental program requires the integration of multiple extrinsic signals from the cell membrane that culminate in changes of nuclear gene expression patterns. Among the known signal transduction intermediates in muscle cells, the PI 3-kinase pathway has been shown to modulate myogenic differentiation (10, 26).
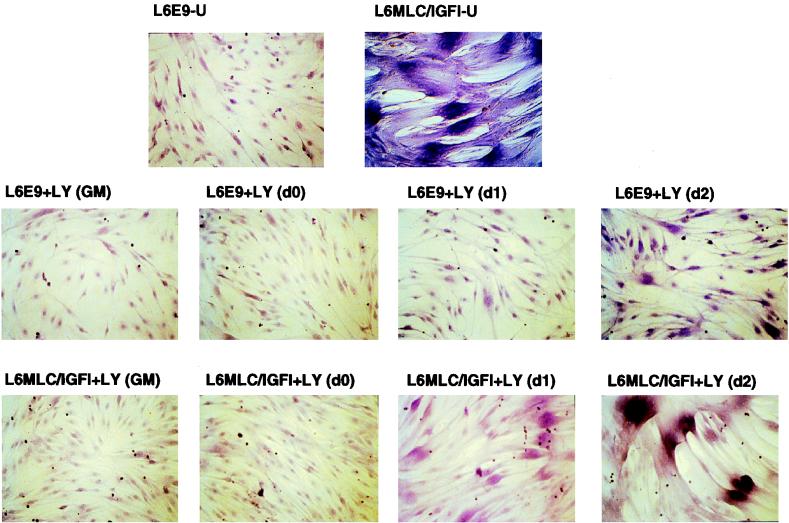
To define the specific signal transduction pathway through which IGF-I elicits the morphological and molecular changes observed in differentiated L6MLC/IGF-I cultures, we analyzed the requirement for PI 3-kinase in myogenic differentiation of this line. Using a specific PI 3-kinase inhibitor, LY294002, we blocked kinase activity in proliferating L6E9 and L6LMLC/IGF-I myoblast cultures and then challenged them to differentiate in the absence of inhibitor. Proliferating L6E9 and L6MLC/IGF-I myoblasts cultured in GM were sensitive to LY294002 and were not able to form myotubes, as analyzed with eosin-Wright’s stain, even after 4 days in serum-free medium without the inhibitor (Fig. 7, GM panels). This indicates a requirement for PI 3-kinase in the transition from the proliferative to the myogenic program.
FIG. 7.
Morphological effect of PI 3-kinase inhibitor on IGF-I-induced hypertrophy. Cells were grown as described in the legend to Fig. 1 and treated with a 10 μM concentration of the PI 3-kinase inhibitor LY294002 at different times of culture: during growth in GM, at 80% of confluence (d0), and at days 1, 2, and 3 in DM. Cells treated with LY294002 in GM were switched to DM without PI 3-kinase inhibitor when they were at 80% of confluence. After 4 days, the cultures were washed with PBS, fixed with methanol, and stained with eosin–Wright’s stain.
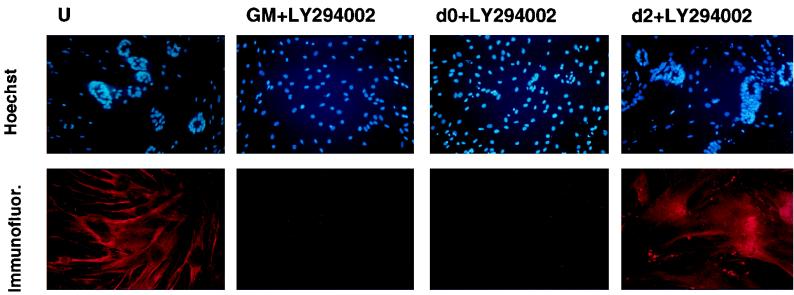
To determine whether later stages of MLC–IGF-I-induced differentiation were equally dependent upon the PI 3-kinase signal transduction pathway, we blocked kinase activity in differentiating L6E9 and L6MLC/IGF-I cultures with LY294002 at progressively later times after serum withdrawal and then analyzed their morphology with eosin-Wright’s stain after 4 days in these differentiation-promoting conditions, in the continuous presence of inhibitor. As expected, both L6E9 and L6MLC/IGF-I cultures treated with LY294002 immediately upon serum withdrawal (Fig. 7, day 0 panels) failed to differentiate and remained myoblastic even after 4 days in serum-free medium. However, when LY294002 was added to cells already cultured in serum-free medium for 1 or 2 days (Fig. 7, day 1 and day 2 panels), myogenic differentiation proceeded and the hypertrophic morphology of L6MLC/IGF-I myotubes was similar to that of myotubes differentiated in serum-free medium without inhibitor (Fig. 7, upper two panels). As shown in the Hoechst-stained cultures (see Fig. 9, upper panels), characteristic circular localization of nuclei induced by IGF-I occurred both in untreated cultures (panel U) and in cultures treated with inhibitor 2 days after serum withdrawal (panel d2+LY294002). In contrast, cultures treated with inhibitor as myoblasts (see Fig. 9, upper GM+LY294002 panel) or immediately upon serum withdrawal (see Fig. 9, upper d0+LY294002 panel) did not exhibit the same characteristic nuclear pattern. These results indicate that PI 3-kinase is required for the initiation but not for the maintenance of the hypertrophic phenotype induced by IGF-I.
FIG. 9.
PI 3-kinase is not required in maintenance of muscle phenotype induced by MLC–IGF-I. (Lower panels) Immunofluorescence analysis of the neonatal isoform of MyHC in L6MLC/IGF-I cultures treated with 10 μM LY294002 inhibitor as myoblasts (panels GM) or differentiated in the presence of inhibitor added immediately upon serum withdrawal (panels d0+LY294002) or 2 days after serum withdrawal (panels d2+LY294002) is shown. U, untreated cultures. Upper panels show Hoechst nuclear staining. All cultures were analyzed 4 days after serum withdrawal.
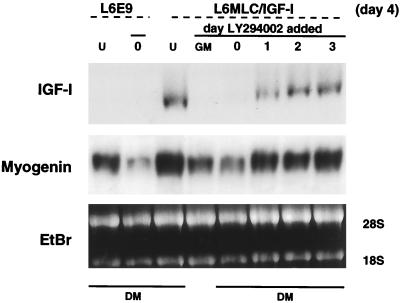
Since the activity of the transfected IGF-I gene in the L6MLC/IGF-I line is under the control of differentiation-specific regulatory elements with target sites for myogenic factors, MLC–IGF-I expression as well as myogenin gene expression should be sensitive to the inhibitory effects of LY294002. This was verified by analyzing the prevalence of IGF-I and myogenin transcripts in L6MLC/IGF-I cultures untreated with LY294002 and differentiated in serum-free medium for 4 days (Fig. 8, lanes U) in comparison to L6MLC/IGF-I cultures treated with inhibitor as myoblasts (Fig. 8, lane GM) or differentiated in the presence of inhibitor after 0, 1, 2, or 3 days in serum-free medium (Fig. 8, lanes 0, 1, 2, and 3). IGF-I expression was undetectable in L6MLC/IGF-I cultures treated during growth in GM or at day 0, although myogenin transcripts were present, suggesting that the levels of myogenin were not sufficient to activate MLC–IGF-I expression. By contrast, IGF-I transcripts as well as myogenin transcripts were present at high expression levels in L6MLC/IGF-I cultures subjected to LY294002 even after only 1 day in differentiating conditions. This accounts for the morphology of L6MLC/IGF-I cultures in Fig. 7, which are capable of activating the MLC–IGF-I gene and thereby respond to the hypertrophic action of IGF-I in the absence of PI 3-kinase signaling, once the cells have withdrawn from the cell cycle.
FIG. 8.
Late inhibition of PI 3-kinase does not affect myogenic differentiation. Northern blots of total RNA samples (15 μg) isolated from untreated (lanes L6E9 or L6MLC/IGF-I cells (lanes U) or from cells treated with 10 μM LY294002 at different times of differentiation are shown: during growth in GM, at 80% of confluence (day 0), and at days 1, 2, and 3 in DM. RNAs were probed with IGF-I or myogenin 32P-labeled probes. Ethidium bromide (EtBr) staining was used to verify equal loading of the RNA samples.
To determine whether the molecular changes elicited by postmitotic expression of IGF-I in the L6E9 background could also be sustained in the absence of PI 3-kinase, we analyzed the profile of MyHC isoforms in L6MLC/IGF-I cultures treated with inhibitor as myoblasts (Fig. 9, panels GM+LY294002) or differentiated in the presence of inhibitor added immediately upon serum withdrawal (panels d0+LY294002) or 2 days in serum-free medium (panels d2+LY294002). All cultures were analyzed 4 days after serum withdrawal, and the effects of PI 3-kinase inhibition on the IGF-I-mediated shift in MyHC isoforms was assessed by immunohistochemical analysis. As shown in Fig. 9 (lower panels), accumulation of the neonatal MyHC isoform was inhibited by LY294002 when added at the time of serum withdrawal (panels d0+LY294002) but not by LY294002 when added after 2 days in serum-free medium (panels d2+LY294002). Thus, the ability of IGF-I to maintain a more mature muscle phenotype in differentiated L6E9 cultures is independent of PI 3-kinase activity.
DISCUSSION
The IGFs play an important and persistent role in skeletal muscle development and homeostasis. In this study, we focused on the role of IGF-I in the regulation of the differentiated muscle phenotype. The confounding effects of this growth factor on myoblast proliferation were eliminated from the test system by placing an IGF-I transcription unit under the control of muscle-specific regulatory elements from the MLC1/3 locus (13). By uncoupling the proliferative and differentiative roles of IGF-I in muscle cell cultures that are deficient in IGF-I themselves, we were able to determine the extent to which this factor affects the myogenic differentiation program in the postmitotic milieu and to manipulate the signal transduction pathways induced in response to IGF-I. Our findings support a model wherein postmitotic expression of IGF-I facilitates maturation of the myogenic program, through the induction of novel genetic pathways in the IGF-deficient L6E9 background.
Cell morphology affected by IGF-I.
The postmitotic expression of IGF-I in differentiated L6E9 cultures induced dramatic morphological changes, generating pronounced hypertrophic myotubes accompanied by accumulation of skeletal actin at juxtaposed myotubes, and increased expression of β1D integrin. This suggests that the effects of IGF-I include cyotoskeletal reorganization leading to a hypertrophic phenotype. The formation of nuclear rings within the body of hypertrophic fibers observed in these cultures may be due to cytoskeletal disruption in a microtubule-deficient setting, since similar nuclear arrangements are observed in muscle cultures treated with cytocalasin B (43a).
Molecular mechanisms of IGF-I action.
It has been previously demonstrated that enhancement of differentiation in cell cultures by exogenous IGF-I application is mediated by myogenin (19). In the present study, we demonstrated that the postmitotic expression of a stably transfected IGF-I gene had similar effects, inducing hypertrophy and displaying dramatic increases in the expression of myogenic differentiation markers, such as myogenin, MRF4, MCK, and IGFBP5. Moreover, the elevation of myogenin transcript in L6MLC/IGF-I cells was associated with a dramatic increase in myogenin promoter activity, suggesting that IGF-I modulates the myogenin expression at the transcriptional level. This conclusion is supported by our ability to generate the L6MLC/IGF-I experimental model itself. In L6E9 cells, endogenous MLC1 transcription is normally undetectable (23, 34). The fact that the transfected IGF-I mRNA is expressed at high levels during L6MLC/IGF-I differentiation suggests that the initial elevated expression of myogenin and/or MEF-2C is sufficient to activate transcriptional activity of the MLC promoter-enhancer, which in the absence of IGF-I remains undetectable. Activation of MLC–IGF-I expression presumably catalyzes a positive feedback loop that maintains elevated myogenin expression levels, which in turn guarantees persistent expression of MLC–IGF-I. Although myogenin is the major IGF-I-stimulated differentiation mediator, it is not sufficient to promote the formation of larger myotubes (14, 15). In contrast to these previous studies, the postmitotic expression of a stably transfected IGF-I gene analyzed here induced the expression of novel myogenic markers, such as MEF-2C, which appears to amplify and reinforce the differentiative effects of the growth factor, possibly by contributing to the sustained activity of MLC–IGF-I. Our results support previous models of IGF action (43) in which the IGF system participates in a feed-forward loop that may modulate the rate and extent of terminal differentiation and may be part of the molecular mechanism leading to skeletal muscle hypertrophy.
Postmitotic expression of IGF-I shifts differentiated muscle cells to a more mature phenotype.
Skeletal muscle development is characterized by an orderly progression of molecular signals leading to generation of heterogeneous muscle fibers. The orchestration of this program involves different populations of myoblasts that, expressing different combination of muscle-specific genes, define the embryonic and fetal phenotype (11, 42). L6E9 cells recapitulate some aspects of immature muscles which express the embryonic, but not neonatal, isoform of MyHC and support very low expression levels of MCK and β-enolase. In MLC–IGF-I transfectants, the postmitotic expression of IGF-I induces the expression of the neonatal isoform of MyHC, normally silent in L6E9 cells (Fig. 6), up-regulation of MCK, normally silent in embryonic muscle (17), and up-regulation of β-enolase, normally expressed at very low levels in embryonic stages (2, 27), suggesting that postmitotic IGF-I expression promotes a maturation of the myogenic program. This view is consistent with the phenotype of knockout mice nullizygous either for IGF-I or the IGF-I receptor, each of which suffer a decline in growth rate beginning at embryonic day 13.5 and exhibit marked muscle hypoplasia at birth (29, 36).
IGF-I-mediated signal transduction pathways.
In differentiation of muscle, as in other tissues, the effects of extracellular stimuli are mediated by signal molecules, such as hormone and growth factors, that transduce the information from cell membrane to the nucleus, eliciting changes in gene expression. It has been recently demonstrated that the proliferative and differentiative effects of IGF-I are mediated by MAP kinase and PI 3-kinase pathways, respectively (10, 26). In the present study, we sought to define further the role of PI 3-kinase in the hypertrophic response to postmitotic expression of IGF-I in differentiating muscle cultures. From the results presented here, it appears that PI 3-kinase is transiently involved in MLC–IGF-I-mediated signal transduction, leading to enhanced cell differentiation as well as the hypertrophic response. However, after myoblast commitment to the differentiation program, PI 3-kinase is not required for the maintenance of MLC–IGF-I-mediated phenotype changes, as indicated by activation of the MLC1 promoter and accumulation of neonatal MyHC protein in the presence of PI 3-kinase inhibitors.
Several possible scenarios can be invoked to account for the transient dependence of the IGF-I-mediated response upon PI 3-kinase in this system. A positive-feedback loop may be established downstream of the PI 3-kinase action in the signal transduction pathway that perpetuates the initial response to IGF-I once it is set in motion. Alternatively, a third, PI 3-kinase-independent signal transduction pathway comes into play after the initial phases of commitment to the differentiated phenotype, which mediates the hypertrophic action of IGF-I in postmitotic skeletal muscle cells. Current studies are aimed at distinguishing between these possibilities.
Prospects for IGF-I as a therapeutic agent.
One of the most severe characteristics of muscular dystrophies is the progressive loss of muscle tissue due to chronic degeneration, accompanied by the exhaustion of satellite cells necessary to replace damaged fibers. The persistent protein degradation observed in neuromuscular diseases is considered an accelerated form of the progressive atrophy which occurs in skeletal muscles during normal aging and in both cases reflects an increase in muscle catabolism. Designing approaches directed towards the attenuation of muscle degeneration in both aging and neuromuscular pathologies therefore requires the identification of factors involved in normal anabolic pathways, in order to promote stabilization and maintenance of muscle tissues.
The present study establishes IGF-I as an important regulator of muscle cell differentiation, through its ability to potentiate maturation of the myogenic program, induce myotube hypertrophy, and increase expression of muscle-specific genes. The pleiotropic actions of IGF-I in normal muscle growth and regeneration suggest a crucial role for this growth factor in multiple steps of the myogenic program. In mature muscle, IGF-I is transiently induced to orchestrate the necessary responses of muscle cells to changing functional requirements after injury or atrophy, or during aging, making it an attractive candidate for gene therapeutic approaches to the attenuation of muscle degeneration.
The fact that postmitotic expression of IGF-I enhances rather than inhibits myogenic differentiation in cell culture indicates that undesirable hyperplastic side effects may be circumvented in therapeutic settings by restricting the expression of exogenously administered IGF-I genes to postmitotic cells. Evidence from transgenic mice expressing IGF-I localized to skeletal muscle suggests this to be the case, since to date no neoplastic growth has been observed in these animals, which undergo varying degrees of muscle hypertrophy (9, 32a). It is also possible that persistent forced expression of IGF-I in mature postmitotic muscle may provide a milieu which serves to maintain or expand the stem cell population, increasing the regeneration potential of atrophied or degenerating muscle. Preliminary results from our laboratory support this idea, as dramatic increases in satellite cell proliferation have been documented in cell cultures from the hypertrophic muscles of mice expressing the MLC–IGF-I cassette as a transgene (32a). Our recent demonstration that virus-mediated expression of IGF-1 prevents age-related loss of muscle mass and function, accompanied by a relative increase in DNA content (2a), lends additional support to a role for IGF-1 in satellite cell proliferation. Further elucidation of the mechanisms by which IGF-1 augments the regenerative capacity of adult muscle fibers will be necessary to determine the efficacy of IGF-I in the maintenance of muscle metabolism, morphology, and function and to define specific therapeutic courses for the treatment of neuromuscular diseases.
ACKNOWLEDGMENTS
We thank A. Musacchio, members of the Rosenthal laboratory, and the reviewers for critical evaluation of the manuscript. We also thank G. Tarone and F. Balzac for providing the β1D antibody, S. Schiaffino for MyHC antibodies, and A. Giallongo, W. Wright, H. Arnold, E. Olson, and J. Florini for providing the β-enolase, myogenin, myf-5, MEF-2C, myogenin-LacZ, and IGFBP5 probes, respectively. We are grateful to L. Sweeney and J. Florini for helpful discussion. We acknowledge José Gonzalez for generating MLC–IGF-I transgenic mice and Esfir Slonimsky and Serafima Zaltsman for managing the mouse colony.
This work was supported by grants to N.R. from the National Institute on Aging.
REFERENCES
- 1.Azzarello G, Sartore S, Saggin L, Gorza L, D’Andrea E, Chieco-Bianchi L, Schiaffino S. Myosin isoform expression in rat rhabdomyosarcoma induced by moloney murine sarcoma virus. J Cancer Res Clin Oncol. 1987;113:417–429. doi: 10.1007/BF00390035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbieri G, De Angelis L, Feo S, Cossu G, Giallongo A. Differential expression of muscle-specific enolase in embryonic and fetal myogenic cells during mouse development. Differentiation. 1990;45:179–184. doi: 10.1111/j.1432-0436.1990.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 2a.Barton-Davis E R, Shoturma D I, Musarò A, Rosenthal N, Sweeney L H. Viral mediated expression of insulin-like growth factor I blocks the aging related loss of skeletal muscle function. Proc Natl Acad Sci USA. 1998;91:15603–15607. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belkin A M, Zhidkova N I, Balzac F, Altruda F, Tomatis D, Maier A, Tarone G, Koteliansky V E, Burridge K. β1D integrin displaces the β1A isoform in striated muscle: localization at junctional structures and signaling potential in nonmuscle cells. J Cell Biol. 1996;132:211–226. doi: 10.1083/jcb.132.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benezra R, Davis R L, Lockshon D, Turner D L, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding protein. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- 5.Braun T, Bober E, Buschhausen-Denker G, Kohtz S, Grzeschik K H, Arnold H H. Differential expression of myogenic determination genes in muscle cells: possible autoactivation by the Myf gene products. EMBO J. 1989;8:3617–3625. doi: 10.1002/j.1460-2075.1989.tb08535.x. . (Erratum 13:358.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun T, Buschhausen-Denker G, Bober E, Arnold H H. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversation in 10T1/2 fibroblasts. EMBO J. 1989;8:701–708. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buonanno A, Rosenthal N. Molecular control of muscle diversity and plasticity. Dev Gen. 1996;19:95–107. doi: 10.1002/(SICI)1520-6408(1996)19:2<95::AID-DVG1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain J F, Jaynes J B, Hauschka S D. Regulation of creatine kinase induction in differentiating mouse myoblasts. Mol Cell Biol. 1985;5:487–492. doi: 10.1128/mcb.5.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman M E, DeMayo F, Yin K C, Lee H M, Geske R, Montgomery C, Schwartz R J. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem. 1995;270:12109–12116. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- 10.Coolican S A, Samuel D S, Ewton D Z, McWade F J, Florini J R. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem. 1997;272:6653–6662. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- 11.Cossu G, Molinaro M. Cell heterogeneity in the myogenic lineage. Curr Top Dev Biol. 1987;23:185–208. doi: 10.1016/s0070-2153(08)60625-0. [DOI] [PubMed] [Google Scholar]
- 12.Csejesi P, Lilly B, Hinkley C, Perry M, Olson E N. Homeodomain protein Mhox and MADS protein myocyte enhancer-binding factor-2 converge on a common element in the muscle creatine kinase enhancer. J Biol Chem. 1994;269:16740–16745. [PubMed] [Google Scholar]
- 13.Donoghue M, Ernst H, Wentworth B, Nadal-Ginard B, Rosenthal N. A muscle-specific enhancer is located at the 3′ end of the myosin light-chain 1/3 gene locus. Genes Dev. 1988;2:1779–1790. doi: 10.1101/gad.2.12b.1779. [DOI] [PubMed] [Google Scholar]
- 14.Engert J C, Berglund E B, Rosenthal N. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol. 1996;135:431–440. doi: 10.1083/jcb.135.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ewton D Z, Florini J R. Insulin-like growth factor binding proteins -4, -5, and -6 may play specialized roles during L6 myoblast proliferation and differentiation. J Endocrinol. 1995;144:539–553. doi: 10.1677/joe.0.1440539. [DOI] [PubMed] [Google Scholar]
- 16.Feinber A P, Volgelstein B. A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari S, Molinari S, Melchionna R, Cusella-De Angelis M G, Battini R, De Angelis L, Kelly R, Cossu G. Absence of MEF2 binding to the A/T-rich element in the muscle creatine kinase (MCK) enhancer correlates with lack of early expression of the MCK gene in embryonic mammalian muscle. Cell Growth Differ. 1997;8:23–34. [PubMed] [Google Scholar]
- 18.Florini J R, Ewton D Z, Falen S L, Van Wyk J J. Biphasic concentration dependency of the stimulation of myoblast differentiation by somatomedins. Am J Physiol. 1986;250:771–778. doi: 10.1152/ajpcell.1986.250.5.C771. [DOI] [PubMed] [Google Scholar]
- 19.Florini J R, Ewton D Z. Highly specific inhibition of IGF-I stimulated differentiation by an antisense oligodeoxyribonucleotide to myogenin mRNA. No effects on other actions of IGF-T. J Biol Chem. 1990;265:13435–13437. [PubMed] [Google Scholar]
- 20.Florini J R, Ewton D Z, Magri K A. Hormones, growth factors, and myogenic differentiation. Annu Rev Physiol. 1991;53:201–216. doi: 10.1146/annurev.ph.53.030191.001221. [DOI] [PubMed] [Google Scholar]
- 21.Florini J R, Ewton D Z, Magri K A, Mangiacapra F J. IGFs and muscle differentiation. In: Raizada M K, LeRoith D, editors. Current directions in insulin-like growth factor research. New York, N.Y: Plenum Press; 1994. p. 319. [Google Scholar]
- 22.Florini J R, Ewton D Z, Coolican S A. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev. 1996;17:481–516. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- 23.Garfinkel L I, Periasamy M, Nadal-Ginard B. Cloning and characterization of cDNA sequences corresponding to myosin light chains 1, 2, and 3, troponin-C, troponin-T, alpha-tropomyosin, and alpha-actin. J Biol Chem. 1982;257:11078–11086. [PubMed] [Google Scholar]
- 24.Gosset L A, Kelvin D J, Stenberg E A, Olson E N. A new myocyte-specific enhancer-binding factor that recognizes a conserved element associated with multiple muscle-specific genes. Mol Cell Biol. 1989;9:5022–5033. doi: 10.1128/mcb.9.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones J I, Clemmons D R. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 26.Kaliman P, Viñals F, Testar X, Palacín M, Zorzano A. Phosphatidylinositol 3-kinase inhibitors block differentiation of skeletal muscle cells. J Biol Chem. 1996;271:19146–19151. doi: 10.1074/jbc.271.32.19146. [DOI] [PubMed] [Google Scholar]
- 27.Keller A, Ott M O, Lamande N, Lucas M, Gros F, Buckingham M, Lazar M. Activation of the gene encoding the glycolytic enzyme beta-enolase during early myogenesis precedes an increased expression during fetal muscle development. Mech Dev. 1992;38:41–54. doi: 10.1016/0925-4773(92)90037-k. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Liu J-L, Baker J, Perkins A S, Robertson E J, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factors I (IGF-I) and type 1 IGF receptor (IGF1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 30.Martin J F, Schwarz J J, Olson E N. Myocyte enhancer factor (MEF) 2C: a tissue-restricted member of the MEF-2 family of transcription factors. Proc Natl Acad Sci USA. 1993;90:5282–5286. doi: 10.1073/pnas.90.11.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCusker R H, Clemmons D R. Insulin-like growth factor binding protein secretion by muscle cells: effects of cellular differentiation and proliferation. J Cell Physiol. 1988;137:505–512. doi: 10.1002/jcp.1041370316. [DOI] [PubMed] [Google Scholar]
- 32.McCusker R H, Camacho-Hubner C, Clemmons D R. Identification of the types of insulin-like growth factor-binding proteins that are secreted by muscle cells in vitro. J Biol Chem. 1989;264:7795–7800. [PubMed] [Google Scholar]
- 32a.Musarò, A., and N. Rosenthal. Unpublished data.
- 33.Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978;15:855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- 34.Pajak L, Mariappan M, Wieczorek D F. Reprogramming of myosin light chain 1/3 expression in muscle heterokaryons. Dev Biol. 1991;145:28–39. doi: 10.1016/0012-1606(91)90210-t. [DOI] [PubMed] [Google Scholar]
- 35.Passantino R, Antona V, Barbieri G, Rubino P, Melchionna R, Cossu G, Feo S, Giallongo A. Negative regulation of β enolase gene transcription in embryonic muscle is dependent upon a zinc finger factor that binds to the G-rich box within the muscle-specific enhancer. J Biol Chem. 1998;273:484–494. doi: 10.1074/jbc.273.1.484. [DOI] [PubMed] [Google Scholar]
- 36.Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillet N, Stewart T A. IGF-I is required for normal embryonic growth in mice. Genes Dev. 1993;7:2609–2617. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- 37.Quinn L S, Steinmetz B, Maas A, Ong L, Kaleko M. Type-1 insulin-like growth factor receptor overexpression produces dual effects on myoblast proliferation and differentiation. J Cell Physiol. 1994;159:387–398. doi: 10.1002/jcp.1041590302. [DOI] [PubMed] [Google Scholar]
- 38.Rhodes S J, Konieczny S F. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev. 1989;3:2050–2061. doi: 10.1101/gad.3.12b.2050. [DOI] [PubMed] [Google Scholar]
- 39.Rosen K M, Wentworth B M, Rosenthal N, Villa-Komaroff L. Specific, temporally regulated expression of the insulin-like growth factor II gene during muscle cell differentiation. Endocrinology. 1993;133:474–481. doi: 10.1210/endo.133.2.8393762. [DOI] [PubMed] [Google Scholar]
- 40.Rosenthal N, Berglund E B, Wentworth B M, Donoghue M, Winter B, Bober E, Braun T, Arnold H H. A highly conserved enhancer downstream of the human MLC1/3 locus is a target for multiple myogenic determination factors. Nucleic Acids Res. 1990;18:6239–6246. doi: 10.1093/nar/18.21.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenthal S M, Cheng Z-Q. Opposing early and late effects of insulin-like growth factor I on differentiation and the cell cycle regulatory retinoblastoma protein in skeletal myoblasts. Proc Natl Acad Sci USA. 1995;92:10307–10311. doi: 10.1073/pnas.92.22.10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stockdale F E, Miller J B. The cellular basis of myosin heavy chain heterogeneity during development of avian skeletal muscle. Dev Biol. 1987;123:1–9. doi: 10.1016/0012-1606(87)90420-9. [DOI] [PubMed] [Google Scholar]
- 43.Stewart C E, Rotwein P. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiol Rev. 1996;76:1005–1026. doi: 10.1152/physrev.1996.76.4.1005. [DOI] [PubMed] [Google Scholar]
- 43a.Sweeney, L. Personal communication.
- 44.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vandenburg H H, Karlish P, Shansky J, Feldstein R. Insulin and IGF-I induce pronounced hypertrophy of skeletal myofibers in tissue culture. Am J Physiol. 1991;260:C475–C484. doi: 10.1152/ajpcell.1991.260.3.C475. [DOI] [PubMed] [Google Scholar]
- 46.Weintraub H. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell. 1993;75:1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- 47.Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci USA. 1968;61:477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]