Abstract
Esophageal cancer is one of the most commonly diagnosed malignant tumors, especially in north China. Surgery is one of the major treatments. However, for locally advanced cases, surgery alone does not achieve an ideal prognosis. As a result of rapid development in recent years, neoadjuvant chemotherapy, neoadjuvant radiotherapy or neoadjuvant chemoradiotherapy followed by surgery are becoming the “standard treatment pattern” for patients with locally advanced esophageal cancer, and an improvement in prognosis is evident. With the gradual application of immunotherapy in esophageal cancer, neoadjuvant immunotherapy has also shown an important role. This article mainly focuses on the history and current status of neoadjuvant treatment and its future role in the treatment of esophageal cancer.
Keywords: locally advanced esophageal cancer, neoadjuvant chemoradiotherapy, neoadjuvant chemotherapy, neoadjuvant immunotherapy, neoadjuvant radiotherapy
Comprehensive treatment of esophageal cancer.
INTRODUCTION
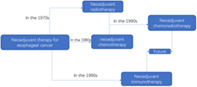
Esophageal cancer is one of the common malignant tumors. China is a high‐risk area for esophageal cancer. In 2015, the national incidence rate of esophageal cancer ranked fifth in all malignant tumors, and mortality rate ranked fourth.1 The disease seriously affects peoples’ health. Surgery, chemotherapy and radiotherapy are still the main treatments for esophageal cancer. With the advancement of oncology and advances in surgical techniques, the 5‐year survival rate of esophageal cancer has increased from 19% in the 1970s2 to 47% today.3 This large‐scale increase in survival rate is more dependent on the wide application of neoadjuvant radiotherapy and chemotherapy in locally advanced esophageal cancer. In addition, since 2018, immunotherapy for esophageal cancer has gradually emerged, and neoadjuvant immunotherapy combined with neoadjuvant chemotherapy or chemoradiotherapy has been successively launched. A number of studies at home and abroad have shown that neoadjuvant immunotherapy combined with neoadjuvant chemoradiotherapy can achieve better tumor regression in patients with locally advanced esophageal cancer. This article reviews the history of neoadjuvant therapy in patients with locally advanced esophageal cancer and explores the value of neoadjuvant radiotherapy, chemotherapy and neoadjuvant immunotherapy.
ADJUVANT CHEMOTHERAPY AND RADIOTHERAPY
The purpose of adjuvant chemotherapy is to control and eliminate potential micrometastases in the body. Adjuvant radiotherapy is designed to control non‐R0 resected local lesions and suspicious scattered tumor cells. A combination of both aims is to improve the long‐term survival rate in patients with locally advanced esophageal cancer after surgery. Platinum‐based two‐ or three‐drug chemotherapy can improve survival rate to a certain extent, and is related to the degree of tumor differentiation; that is, patients with poorly differentiated tumors benefit greatly from adjuvant chemotherapy.4, 5 However, meta‐analyses indicate that adjuvant chemotherapy remains controversial. In a study by Huang et al., although the survival rate of the adjuvant chemotherapy group improved, the results in most studies were not statistically significant.6 Indications for adjuvant radiotherapy are narrower, most are used for R1 or R2 resection, and the meaning of the results is therefore limited.7 Due to the considerable difference in the dietary rehabilitation process after esophageal cancer surgery, most patients have low PS scores. Postoperative radiotherapy and chemotherapy are often difficult to implement as scheduled. Neoadjuvant therapy for esophageal cancer has gradually become an important exploration direction. It has been previously reported that the effects of neoadjuvant radiotherapy and chemotherapy are better than adjuvant radiotherapy and chemotherapy.8
NEOADJUVANT CHEMOTHERAPY
Compared with adjuvant chemotherapy, neoadjuvant chemotherapy has the following theoretical advantages: (i) Preoperative patients have better PS scores, better tolerance and compliance to chemotherapy. (ii) There is a downstaging effect on locally advanced esophageal cancer, which can improve the R0 resection rate. (iii) It can eliminate potential micrometastasis in the blood and occult distant metastatic lesions. (iv) It can sensitize preoperative radiotherapy to further improve the R0 resection rate. (v) It enables objective evaluation of the sensitivity of chemotherapeutic drugs in vivo, and (vi) facilitates the screening of appropriate surgical patients based on chemotherapy response.
In the 1980s, clinical trials of neoadjuvant chemotherapy in esophageal cancer had begun to be implemented. At that time, the chemotherapy regimen was basically a two‐ or three‐drug regimen based on cisplatin (cisplatin +5‐FU/methotrexate/vincristine/bleomycin, etc. The most commonly used regimen was cisplatin combined with 5‐FU two‐drug regimen). Initially, whether it was a one‐arm or randomized controlled trial, the conclusion of several studies was that neoadjuvant chemotherapy did not play a role in the treatment of esophageal cancer with no improvement in long‐term patient survival.9, 10, 11 A study by Schlag reported that neoadjuvant chemotherapy increased the risk of perioperative death.12 The famous American INT 113 trial also concluded that neoadjuvant chemotherapy was ineffective. It randomly included 452 patients with stages I–III esophageal cancer, of which 46% had adenocarcinoma (ADC) and 54% had squamous cell carcinoma (SCC). Patients in the experimental group received three‐cycles of cisplatin +5‐FU chemotherapy before surgery, and received two‐cycles of cisplatin +5‐FU chemotherapy after surgery. There was no significant difference in R0 resection rate between the two groups. The pathological complete response (pCR) of the preoperative chemotherapy group was 2.5%. There was no significant difference in 5‐year survival between the two groups. Although most trials have failed to support the potential of neoadjuvant chemotherapy to improve the long‐term efficacy of esophageal cancer, the studies concluded that patients with better lesion response after chemotherapy (pCR patients) achieved significantly longer survival. Even the patient survival rate has been previously reported to double compared with those patients who underwent direct surgery.13, 14
It is precisely because of the good long‐term survival of patients with pCR after neoadjuvant chemotherapy that the exploration of neoadjuvant chemotherapy continues. In 2002, the Medical Research Council Esophageal Cancer Working Group (MRC)15 reported the preliminary results of a randomized controlled trial of MRC‐OEO2. From 1990 to 1998, 802 patients with stage I–III esophageal cancer were included, of which 70% were adenocarcinoma and 30% were squamous cell carcinoma. The patients in the experimental group received two cycles of cisplatin +5‐FU chemotherapy before surgery. The R0 resection rate of the experimental group was higher than that of the control group (60% vs. 54%, p < 0.0001). The median follow‐up time was 17 months and observations as follows: preoperative chemotherapy improved the 2‐year survival rate (43% vs. 34%, p < 0.05) of experimental group patients, and the median survival of the experimental group was also higher than the control group (16.8 months vs. 13.3 months, p = 0.004). Further stratified analysis found that preoperative chemotherapy could prolong the survival of patients with adenocarcinoma and squamous cell carcinoma. After the trial stopped in 1998, follow‐up work was still ongoing. The study extended the follow‐up time to a median follow‐up of six years. In 2009, the final data of long‐term survival comparison was given: 400 in the experimental group, 402 in the surgical group. There were 655 deaths, including 320 in the experimental group and 335 in the surgical group. Survival benefit risk ratio (HR) was 0.84, The 5‐year survival rate of the experimental group was 23.0%, while the 5‐year survival rate of the surgical group was 17.1%. There were significant differences, and the therapeutic effect was consistent in patients with both adenocarcinoma and squamous cell carcinoma. In the analysis of disease‐free survival (DFS), positive events often occurred in R2 resected or unresected patients, with an incidence of 26.4% in the surgical group and 14.3% in the experimental group (p < 001). The three‐year survival rate by resection type was 42.4% for R0, 18.0% for R1, and 8.6% for R2.16
In 2007, eight randomized controlled clinical trials (n = 1724) including preoperative (neoadjuvant) chemoradiotherapy or chemotherapy, or surgery alone for meta‐analysis were reported by Gebski et al.17 Preoperative chemotherapy was found to reduce the relative risk of death in patients with esophageal cancer by 10%, and increase the 2‐year survival rate of patients with esophageal cancer by 7%. However, unlike the OEO2 results, meta‐analysis showed that preoperative chemotherapy was only effective for patients with adenocarcinoma and not squamous cell carcinoma. In the same year, a large‐scale randomized controlled trial in the United States, reported an opposing view.18 A total of 216 patients received chemotherapy before surgery and 227 patients underwent direct surgery. Fifty‐nine percent of patients were in the direct surgery group and 63% of patients underwent chemotherapy plus surgery and received R0 resection (p = 0.5137). Patients with non‐R0 resection had a poor prognosis; 32% of patients who underwent R0 resection were alive at five years without disease progression, and only 5% of patients who underwent R1 resection survived for more than five years. There was no significant difference in median survival for patients with R1, R2 or no resection. Although the overall survival (OS) rate of patients undergoing perioperative chemotherapy did not differ from those undergoing surgery alone, patients who achieved objective tumor regression after preoperative chemotherapy were found to have improved survival. This highlights that for patients with localized esophageal cancer, regardless of whether or not chemotherapy is performed before surgery, only R0 resection can achieve substantial long‐term survival. Even a histologically‐confirmed positive margin is an independent risk factor for prognosis. With the passage of time, a randomized controlled trial of squamous cell carcinoma in Japan in 2008 obtained positive results. In this trial, 330 patients with stages II–III esophageal squamous cell carcinoma were treated with cisplatin +5‐FU regimen for two courses. The 5‐year survival rate was significantly higher in the preoperative chemotherapy group than in the postoperative chemotherapy group (60% vs. 38%; p = 0.013).19 Two subsequent randomized controlled trials in 2011 demonstrated the benefits of neoadjuvant chemotherapy in squamous cell carcinoma and adenocarcinoma patients, respectively. Boonstra et al.20 in the Netherlands reported 169 patients with esophageal squamous cell carcinoma, of which 85 received preoperative chemotherapy (etoposide + cisplatin) (CS group) and 84 underwent immediate surgery (S group). There were 148 deaths, including 71 in the CS group and 77 in the S group. The median OS time in the CS group was 16 months, while the S group was 12 months; the 2‐year survival rates were 42% and 30% respectively; the 5‐year survival rates were 26% and 17% respectively, with significant differences. Ychou et al.21reported that 224 patients with resectable adenocarcinoma of the lower esophagus and adenocarcinoma at the gastroesophageal junction were randomly assigned to perioperative chemotherapy and surgery (CS group; n = 113) or surgery alone (S group; n = 111). The chemotherapy regimen was cisplatin +5FU. The results showed that the CS group had a better OS (5‐year rate of 38% vs. 24%) compared with the S group; and better DFS (5‐year ratio: 34% vs. 19%).
In summary, compared with direct surgery, surgery after neoadjuvant chemotherapy can increase the R0 resection rate, thereby increasing the long‐term survival rate. However, due to the different pathological types of esophageal cancer in the East and West, chemotherapy regimens and baseline characteristics of different randomized controlled trials, it remains difficult to conclude whether neoadjuvant chemotherapy can improve the long‐term survival rate of patients with esophageal cancer. A large sample size randomized controlled trial of a single pathological type and the same chemotherapy regimen would therefore be beneficial to verify these findings.
NEOADJUVANT RADIOTHERAPY
Neoadjuvant radiotherapy is a topical treatment designed to control tumor growth, or to reduce tumor volume and increase R0 resection rate. Theoretically after radiotherapy it will affect tissue blood supply, resulting in fibrous scar formation and difficult dissection, and increased difficulties during surgery, in particular with postoperative anastomosis healing. However, clinical trial results have shown that preoperative radiotherapy does not increase difficulties during surgery and perioperative mortality, and that survival time is prolonged. A review of 200 cases of esophageal squamous cell carcinoma by Nakayama et al.2 reported that the 4‐year survival rate of patients with upper thoracic esophageal cancer treated with neoadjuvant radiotherapy plus surgery was 31.8%, and the 5‐year survival rate was 37.5%. The 4‐year survival rate of patients who underwent surgery only was 15.4% and the 5‐year survival rate was 19.1%. In 2000, Arnott et al.22 conducted a meta‐analysis of preoperative radiotherapy, which included five studies, with a total of 1147 patients, with a median follow‐up of nine years. In a group of patients with squamous cell carcinoma, the preoperative radiotherapy risk ratio (HR) was 0.89, the overall mortality risk was reduced by 11%, the 2‐year absolute survival benefit was 3%, and the 5‐year absolute survival benefit was 4%. This highlights that neoadjuvant radiotherapy is safe, but that the long‐term survival benefits are minimal. Therefore, the improvement of R0 resection rate while pursuing long‐term efficacy has become the focus of recent studies. In addition, the MRC‐OEO2 test shows the absolute advantage of the 2‐year survival rate of neoadjuvant chemotherapy after two years,15 and the sensitization of chemotherapy to radiotherapy. The role of preoperative chemotherapy and preoperative radiotherapy has gradually become the mainstream mode of multidisciplinary treatment of esophageal cancer, with a decline in studies of preoperative radiotherapy alone.
NEOADJUVANT CHEMORADIATION
Preoperative chemotherapy or preoperative radiotherapy have a certain degree of related side effects, especially blood toxicity. The combination of the two appears to increase side effects, leading to a decline in surgical safety. In a one‐arm study of 43 patients in 1990, Orringer et al.23 found that III–IV° myelosuppression occurred in 93% of patients after neoadjuvant chemoradiotherapy, two of which were treatment‐related deaths. A similar study was also included in the randomized controlled trial by Bosset et al.24 in 1997, which found more deaths in the neoadjuvant chemoradiation group and a significant difference compared with the direct surgery group. However, more studies point to the safety side of neoadjuvant chemoradiation. In 1994, Le Prise10 reported the results of a randomized controlled trial which included 86 patients, confirming that neoadjuvant chemoradiation does not increase the risk of postoperative death, but long‐term survival benefits are small. Supporting the results of the Le Prise study are the results of a randomized controlled trial by Apinop et al.11 in 1994 and the meta‐analysis of Urschel and Vasan25 in 2003. In 2007, Gebski et al.17included eight randomized controlled trials of neoadjuvant radiotherapy and conducted a meta‐analysis. The results not only confirmed the safety of neoadjuvant chemotherapy and neoadjuvant chemoradiation, but further proved that neoadjuvant therapy does not increase the risk of perioperative death and causes it to decline. A total of 1724 patients were collected. The analysis showed that preoperative chemotherapy reduced the relative risk of death in patients with esophageal cancer by 10% and increased the 2‐year survival rate of patients with esophageal cancer by 7%. Preoperative chemoradiation could reduce the risk of death in patients with esophageal cancer (HR = 0.81, p = 0.002), reducing the relative risk of death by 19%, and increasing the 2‐year survival rate of patients with esophageal cancer by 13%. Since then, neoadjuvant chemoradiotherapy has become the standard model for multidisciplinary treatment of esophageal cancer. More research has focused on long‐term survival and the combination of thoracoscopic minimally invasive esophageal cancer radicalization techniques.
Whether preoperative chemotherapy, preoperative radiotherapy or preoperative chemoradiotherapy are used, the improvement of long‐term efficacy depends on the response of patients to neoadjuvant therapy. That is, R0 resection rate and pCR rate, both of which are independent risk factors for postoperative prognosis of esophageal cancer.18 A one‐arm study by Stahl et al.26 showed that 90 patients underwent neoadjuvant chemoradiation, 72 of whom underwent surgery, of which 44 had R0 resection and 16 had postoperative pCR. The 3‐year survival rate was 33% in the whole group, 42% in R0 resection, and 68% in pCR, which strongly demonstrated the important contribution of R0 resection and pCR to long‐term survival after neoadjuvant therapy. In patients with pCR, the survival rate can therefore be doubled. From the perspective of tumor‐free survival, pCR is also very important. Studies have shown that the 5‐year DFS rate of preoperative chemoradiotherapy pCR is significantly higher than non‐pCR (62% vs. 31%).27 In 2010, Vallböhmer et al.28 retrospectively analyzed 229 cases of esophageal cancer after neoadjuvant therapy, including 118 ADC, 118 SCC (284 neoadjuvant chemoradiation, 15 neoadjuvant chemotherapy). The 5‐year survival rate was 55%, disease‐specific 5‐year survival rate was 68%, recurrence rate was 3.4% (n = 70), local recurrence rate was 3.3%, and distant recurrence rate was 20.1%. Cox regression analysis determined that age was the only independent predictor of survival, and gender, histology, type of esophagectomy, type of neoadjuvant therapy, and number of resected lymph nodes had no effect on prognosis. It can be seen that the response rate after neoadjuvant chemoradiotherapy; that is, the effect of clinical imaging on the atrophy of the lesion is a good indication for the follow‐up of surgery.
The safety of neoadjuvant radiotherapy and chemotherapy has been affirmed, and R0 resection and pCR are important targets for neoadjuvant therapy. However, not all neoadjuvant treatments can achieve pCR. The pCR rate of neoadjuvant chemotherapy has been reported to be 2.5%–5.0%,15, 25 and the pCR rate of neoadjuvant chemoradiation to reach 20%–25%.27 The vast majority of patients still fail to achieve pCR after neoadjuvant chemoradiotherapy. Although long‐term survival is inferior to pCR patients, this group of patients can still obtain satisfactory distance survival effect from subsequent surgery compared with direct surgery. As mentioned previously, in a meta‐analysis by Gebski et al.17 preoperative chemotherapy has been reported to reduce the relative risk of death in patients with esophageal cancer by 10% and increase the 2‐year survival rate of patients with esophageal cancer by 7%. Preoperative radiotherapy and chemotherapy can reduce the risk of death in patients with esophageal cancer by 19%, and increase the 2‐year survival rate of patients with esophageal cancer by 13%. However, stratified analysis showed that preoperative chemotherapy can only benefit patients with esophageal adenocarcinoma. Preoperative radiotherapy and chemotherapy can benefit patients with adenocarcinoma and squamous cell carcinoma. Although the long‐term survival benefit of this meta‐analysis is not as significant as the benefit of pCR, a 2‐year improvement in the 2‐year survival rate of neoadjuvant chemoradiation is also encouraging.
The epoch‐making randomized controlled multicenter trial of neoadjuvant chemoradiation for esophageal cancer was reported in the 2008 CROSS study29 whereby neoadjuvant chemoradiotherapy in patients with potentially curable esophageal cancer was compared with surgery alone, with 175 patients in each group. The neoadjuvant chemoradiation group received concurrent treatment for more than five weeks (paclitaxel 50 mg/m2 and carboplatin AUC 2 mg/ml/min infusion on days 1, 8, 15, 22 and 29). The total external dose was 41.4 Gy, five times a week, 1.8 Gy each time, a total of 23 times. The study endpoints were compared with median survival and quality of life (pretreatment, during and after treatment), pathological response, progression‐free survival, and number of R0 resections. The 2012 CROSS study30 reported preliminary results: from 2004 to December 2008, a total of 368 patients were enrolled, of which 366 were included in the analysis: 275 (75%) adenocarcinoma, 84 (23%) with squamous cell carcinoma, and seven large cell undifferentiated carcinoma (2%). Of the 366 patients, 178 patients were randomly assigned to surgery after chemoradiotherapy, and 188 patients underwent surgery alone. The most common hematological toxicity in the chemoradiotherapy‐operative group was leukopenia (6%) and neutropenia (2%); the most common nonhematological toxicities were anorexia (5%) and fatigue (3%). A total of 92% of patients in the chemoradiotherapy group underwent complete resection, compared with 69% in the surgical group (p < 0.001). Of the 161 patients who underwent chemoradiotherapy, 47 (29%) had pCR. Postoperative complications were similar in both groups, with a hospital mortality rate of 4%. The median OS of the chemoradiotherapy‐operative group was 49.4 months, compared with 24.0 months in the surgical group. The OS rate of the chemoradiotherapy‐operative group was significantly improved (HR = 0.657, p = 0.003). In the 2015 CROSS study3 after a median of 45 months of follow‐up, a total of 366 patients were analyzed (178 in the neoadjuvant chemoradiation group and 188 in the surgery alone group). The median survival of the neoadjuvant chemoradiation group and the surgery alone group was 48.6 months and 24.0 months, respectively (p = 0.003). The stratified analysis showed that in the squamous cell carcinoma group, the median survival time of patients with neoadjuvant chemoradiation plus surgery was 81.6 months, and that of the surgery alone group was 21.1 months (p = 0.008). The median survival of patients with neoadjuvant chemoradiotherapy plus surgery in the adenocarcinoma group was 43.2 months, compared with 27.1 months in the surgery alone group (p = 0.038). Long‐term follow‐up in the CROSS study confirmed a significant benefit in the OS of neoadjuvant chemoradiotherapy in patients with resectable esophageal or esophagogastric junctional cancer. This benefit has clinical implications for both squamous and adenocarcinoma subtypes.
SELECTION OF CHEMOTHERAPY REGIMENS IN NEOADJUVANT CHEMOTHERAPY/NEOADJUVANT CHEMORADIATION
After the 1990s, a new generation of chemotherapy drugs such as paclitaxel, docetaxel, and rituximab have been used in neoadjuvant radiotherapy and chemotherapy for esophageal cancer. The meta‐analysis of Thirion et al.31 in 2008 suggested that there was no difference in histology, performance status, age, or treatment regimen, both in cumulative and tumor‐free survival. However, in 2015, Huang et al.32 published a systematic review which specifically outlined the chemotherapy regimens in the neoadjuvant treatment of esophageal cancer. Based on the OS rate, the efficacy of paclitaxel plus platinum and platinum plus 5‐fluorouracil was compared. The review concluded that neoadjuvant chemoradiotherapy with paclitaxel plus platinum was a better treatment for locally advanced esophageal cancer than platinum plus 5‐FU, especially in patients with squamous cell carcinoma. However, esophageal cancer site, patient age, pathological type, radiotherapy plan, surgical procedure, dose of chemotherapy, number of chemotherapy cycles, neoadjuvant therapy and surgery interval, etc will have an impact on the evaluation of the efficacy of neoadjuvant chemotherapy. A large randomized controlled trial is needed for confirmation of these findings.
NEOADJUVANT CHEMORADIOTHERAPY COMBINED WITH NEOADJUVANT IMMUNOTHERAPY
Although neoadjuvant chemoradiotherapy has brought more significant long‐term survival benefits for locally advanced esophageal cancer patients, the increased difficulty of surgery caused by neoadjuvant chemoradiotherapy and the poor prognosis of non‐PCR patients still urge us to search for better neoadjuvant regimens.
Programmed death factor receptor‐1 (PD‐1), discovered in 1992, is a negative regulatory immune checkpoint expressed in T, B, and NK cells and consists of two ligands: PD‐L1 and PD‐L2 that combine to suppress the local immune response.33, 34, 35 Immune checkpoint inhibitors (ICIs), including PD‐1, PD‐L1 and CTLA‐4, are monoclonal antibodies that exert antitumor effects by blocking the negative immune regulation of immune checkpoints and enhance the body's antitumor immunity. Immunotherapy has enabled new breakthroughs in various tumor species, among which a large number of clinical studies on immune checkpoint inhibitors have been conducted in esophageal cancer and positive results have been achieved. For patients with resectable locally advanced esophageal cancer, immunotherapy has shown better results than that of chemotherapy in esophageal cancer second‐line treatment effect in overcoming the problem of how to improve the R0 resection rate, reduce the rate of recurrence and prolong overall survival. The addition of immunotherapy to neoadjuvant therapy regimens for esophageal cancer may also yield an OS benefit.
Pembrolizumab, developed by Merck, was the first PD‐1 inhibitor and approved by the FDA in 2014 to treat advanced or unresectable melanoma. In 2019, ASCO reported the preliminary results of a phase II study of pembrolizumab combined with neoadjuvant chemoradiotherapy (paclitaxel + carboplatin) in patients with resectable esophageal squamous cell carcinoma.33, 34, 35 A total of 28 patients with locally advanced esophageal squamous cell carcinoma underwent neoadjuvant therapy, of which 26 underwent surgery. Among the 26 patients, two patients died in hospital after surgery as a result of acute lung injury. Among the patients who underwent surgical resection, the pathological complete response (pCR) rate reached 46.1%, and the OS rate at six and 12 months was 89.3% and 82.1%, respectively. In addition, the study confirmed that combination therapy did not increase the toxic side effects associated with chemoradiotherapy and immunotherapy.36
Nivolumab is a fully humanized IgG4 monoclonal antibody against PD‐1 developed by Bristol‐Myers Squibb, which has a high affinity for PD‐1 and can inhibit the binding of PD‐L1 / PD‐L2 and PD‐1.37 In 2019, ASCO GI reported the results of a pretrial of nivolumab combined with neoadjuvant chemoradiotherapy.38 In this study, a total of 16 patients with stage II and III esophageal or gastroesophageal junction cancer were enrolled, with two cases of ESCC. Nivolumab 240 mg was first given for two cycles of induction, followed by sequential nivolumab combined with chemoradiotherapy. Carboplatin combined with paclitaxel was selected as the chemotherapy regimen. Among the 16 patients in the pre‐experiment, five cases were in pathological complete response, nine cases achieved pathological “decline stage,” and 15 cases underwent surgical resection with R0 margins. The results showed that nivolumab combined with chemoradiotherapy is a safe and feasible neoadjuvant therapy for stage II and III esophageal or gastroesophageal junction cancer after induction therapy.
PD‐L1 is the ligand of PD‐1 mAb. Currently, the three types of PD‐L1 mAb approved by the FDA are durvalumab, atezolizumab and avelumab. Research data on PD‐L1 in esophageal cancer are limited, and a number of clinical studies are currently underway. The safety and efficacy of neoadjuvant chemoradiotherapy combined with avelumab in resectable esophageal cancer and gastroesophageal junction tumors was reported in the 2019 ASCO Conference.39 No grade ≥3 adverse events were observed in the six patients enrolled in this study, of which five patients achieved R0 resection and two patients achieved pathological complete response. No additional surgical complications occurred. It has good tolerability and a favorable safety profile. However, this study was a stage I/II randomized clinical study with a small sample size. The efficacy of neoadjuvant chemoradiotherapy combined with avelumab in resectable esophageal cancer and gastroesophageal junction tumors should be confirmed with a larger sample size.
A phase II trial of atezolizumab, a PD‐L1 inhibitor, in combination with neoadjuvant chemoradiotherapy has also been reported by ASCO in 2019.40 A total of 39 patients were enrolled into this study, of which 24 completed all neoadjuvant therapy. The results revealed that 39% of patients achieved PCR, higher than 23% in the CROSS study,3 and treatment‐related adverse reactions were manageable.
At present, immunotherapy has been advanced from the back line of advanced esophageal cancer to the first‐, and second‐line, and even the perioperative treatment of locally advanced esophageal cancer. In the immunotherapy regimen, scholars have explored the use of single drugs to combination drugs. However, some key immunotherapy questions remain unanswered such as how to comprehensively evaluate the immune status of patients and determine the biomarkers which predict the efficacy of immunotherapy, and how to determine the dose, intensity and duration of chemoradiotherapy when immunotherapy is combined with chemoradiotherapy. Also, how to manage the toxicity of immunotherapy and so on. Therefore, more phase III clinical trials and more convincing research results are urgently required to establish a new treatment model for esophageal cancer patients to provide new hope for more patients.
CONFLICT OF INTEREST
There are no conflicts of interest.
ACKNOWLEDGMENTS
We thank the Professors of Department of Oncology, Department of Gastroenterology, Department of Pathology, Peking University Third Hospital for providing guidance and coaching. This study was supported by the Development Center for Medical Science & Technology National Health Commission of the People's Republic of China (grant no. W2013R75). The authors disclose receipt of the following financial support for the research of this article. This work was supported by the Capital Development Fund key project, randomized, open, multicenter, parallel control evaluation of thoracoscopic and open three‐incision surgery mode in the treatment of esophageal cancer prospective clinical study (grant no. 2014‐1‐4021).
Li J, Ma S. History and current situation of neoadjuvant treatment for locally advanced esophageal cancer. Thorac Cancer. 2021;12:2293–2299. 10.1111/1759-7714.14069
REFERENCES
- 1.Chen W. Cancer statistics: updated cancer burden in China. Chin J Cancer Res. 2015;27:1. 10.3978/j.issn.1000-9604.2015.02.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakayama K, Orihata H, Yamaguchi K. Surgical treatment combined with preoperative concentrated irradiation for esophageal cancer. Cancer. 1967;20:778–88. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro J, van Lanschot J, Hulshof M, van Hagen P, van Berge Henegouwen M, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long‐term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–8. 10.1016/S1470-2045(15)00040-6 [DOI] [PubMed] [Google Scholar]
- 4.Liu HC, Hung SK, Huang CJ, Chen CC, Chen MJ, Chang CC, et al. Esophagectomy for locally advanced esophageal cancer, followed by chemoradiotherapy and adjuvant chemotherapy. World J Gastroenterol. 2005;11:5367–72. 10.3748/wjg.v11.i34.5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyu X, Huang J, Mao Y, Liu Y, Feng Q, Shao K, et al. Adjuvant chemotherapy after esophagectomy: is there a role in the treatment of the lymph node positive thoracic esophageal squamous cell carcinoma? J Surg Oncol. 2014;110:864–8. 10.1002/jso.23716 [DOI] [PubMed] [Google Scholar]
- 6.Huang WZ, Fu JH, Hu Y, Zhang X, Yang H. Meta‐analysis of postoperative adjuvant chemotherapy for localized esophageal carcinoma. Chin J Cancer. 2006;10:1303–6. [PubMed] [Google Scholar]
- 7.Tachibana M, Yoshimura H, Kinugasa S, Shibakita M, Dhar DK, Ueda S, et al. Postoperative chemotherapy vs chemoradiotherapy for thoracic esophageal cancer: a prospective randomized clinical trial. Eur J Surg Oncol. 2003;29:580–7. 10.1016/S0748-7983(03)00111-2 [DOI] [PubMed] [Google Scholar]
- 8.Fujita H, Sueyoshi S, Tanaka T, Tanaka Y, Sasahara H, Shirouzu K, et al. Prospective non‐randomized trial comparing esophagectomy‐followed‐by‐chemoradiotherapy versus chemoradiotherapy‐followed‐by‐esophagectomy for T4 esophageal cancers. J Surg Oncol. 2005;90:209–19. 10.1002/jso.20259 [DOI] [PubMed] [Google Scholar]
- 9.Nygaard K, Hagen S, Hansen HS, Hatlevoll R, Hultborn R, Jakobsen A, et al. Pre‐operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre‐operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg. 1992;16:1104–9. 10.1007/BF02067069 [DOI] [PubMed] [Google Scholar]
- 10.Le Prise E, Etienne PL, Meunier B, Maddern G, Hassel MB, Gedouin D, et al. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer. 1994;73:1779–84. [DOI] [PubMed] [Google Scholar]
- 11.Apinop C, Puttisak P, Preecha N. A prospective study of combined therapy in esophageal cancer. Hepatogastroenterology. 1994;41:391–3. [PubMed] [Google Scholar]
- 12.Schlag PM. Randomized trial of preoperative chemotherapy for squamous cell cancer of the esophagus. The Chirurgische Arbeitsgemeinschaft Fuer Onkologie der Deutschen Gesellschaft Fuer Chirurgie Study Group. Arch Surg. 1992;127:1446–50. 10.1001/archsurg.1992.01420120080015 [DOI] [PubMed] [Google Scholar]
- 13.Vogel SB, Mendenhall WM, Sombeck MD, Marsh R, Woodward ER. Downstaging of esophageal cancer after preoperative radiation and chemotherapy. Ann Surg. 1995;221:685–93. 10.1097/00000658-199506000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–7. 10.1056/NEJM199608153350702 [DOI] [PubMed] [Google Scholar]
- 15.Bancewicz J, Clark PI, Smith DB, Donnelly RJ, Fayers P, Weeden S, et al. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727–33. 10.1016/S0140-6736(02)08651-8 [DOI] [PubMed] [Google Scholar]
- 16.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long‐term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–7. 10.1200/JCO.2009.22.2083 [DOI] [PubMed] [Google Scholar]
- 17.Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta‐analysis. Lancet Oncol. 2007;8:226–34. 10.1016/S1470-2045(07)70039-6 [DOI] [PubMed] [Google Scholar]
- 18.Kelsen DP, Winter KA, Gunderson LL, Mortimer J, Estes NC, Haller DG, et al. Long‐term results of RTOG trial 8911 (USA intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25:3719–25. 10.1200/JCO.2006.10.4760 [DOI] [PubMed] [Google Scholar]
- 19.Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5‐fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68–74. 10.1245/s10434-011-2049-9 [DOI] [PubMed] [Google Scholar]
- 20.Boonstra JJ, Kok TC, Wijnhoven BP, van Heijl M, van Berge Henegouwen MI, Ten Kate FJ, et al. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: long‐term results of a randomized controlled trial. BMC Cancer. 2011;11:181. 10.1186/1471-2407-11-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–21. 10.1200/JCO.2010.33.0597 [DOI] [PubMed] [Google Scholar]
- 22.Arnott SJ, Duncan W, Gignoux M, Hansen HS, Launois B, Nygaard K, et al. Preoperative radiotherapy for esophageal carcinoma. Cochrane Database Syst Rev. 2005;4:CD001799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forastiere AA, Orringer MB, Perez‐Tamayo C, Urba SG, Husted S, Takasugi BJ, et al. Concurrent chemotherapy and radiation therapy followed by transhiatal esophagectomy for local‐regional cancer of the esophagus. J Clin Oncol. 1990;8:119–27. 10.1200/JCO.1990.8.1.119 [DOI] [PubMed] [Google Scholar]
- 24.Bosset JF, Gignoux M, Triboulet JP, Tiret E, Mantion G, Elias D, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous‐cell cancer of the esophagus. N Engl J Med. 1997;337:161–7. 10.1056/NEJM199707173370304 [DOI] [PubMed] [Google Scholar]
- 25.Urschel JD, Vasan H. A meta‐analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185:538–43. 10.1016/S0002-9610(03)00066-7 [DOI] [PubMed] [Google Scholar]
- 26.Stahl M, Wilke H, Fink U, Stuschke M, Walz MK, Siewert JR, et al. Combined preoperative chemotherapy and radiotherapy in patients with locally advanced esophageal cancer. Interim analysis of a phase II trial. J Clin Oncol. 1996;14:829–37. 10.1200/JCO.1996.14.3.829 [DOI] [PubMed] [Google Scholar]
- 27.Berger A, Scott W, Freedman G, Konski A, Weiner L, Cheng J, et al. Morbidity and mortality are not increased after induction chemoradiotherapy followed by esophagectomy in patients with esophageal cancer. Semin Oncol. 2005;32:16–20. 10.1053/j.seminoncol.2005.04.017 [DOI] [PubMed] [Google Scholar]
- 28.Vallbohmer D, Holscher AH, DeMeester S, DeMeester T, Salo J, Peters J, et al. A multicenter study of survival after neoadjuvant radiotherapy/chemotherapy and esophagectomy for ypT0N0M0R0 esophageal cancer. Ann Surg. 2010;252:744–9. 10.1097/SLA.0b013e3181fb8dde [DOI] [PubMed] [Google Scholar]
- 29.van Heijl M, van Lanschot JJB, Koppert LB, van Berge Henegouwen M, Muller K, Steyerberg EW, et al. Neoadjuvant chemoradiation followed by surgery versus surgery alone for patients with adenocarcinoma or squamous cell carcinoma of the esophagus (CROSS). BMC Surg. 2008;8:21. 10.1186/1471-2482-8-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Hagen P, Hulshof MCCM, van Lanschot JJB, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BPL, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. [DOI] [PubMed] [Google Scholar]
- 31.Thirion P, Maillard E, Pignon J. Individual patient data‐based meta‐analysis assessing the effect of preoperative chemo‐radiotherapy in resectable oesophageal carcinoma. Int J Radiat Oncol Biol Phys. 2008;72:S71–2. 10.1016/j.ijrobp.2008.06.929 [DOI] [Google Scholar]
- 32.Huang TC, Hsu CH, Lin CC, Tu YK. Systematic review and network meta‐analysis: neoadjuvant chemoradiotherapy for locoregional esophageal cancer. Jpn J Clin Oncol. 2015;45:1023–8. 10.1093/jjco/hyv119 [DOI] [PubMed] [Google Scholar]
- 33.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD‐1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pico DCY, Choudhury A, Kiessling R. Checkpoint blockade for cancer therapy: revitalizing a suppressed immune system. Trends Mol Med. 2015;21:482–91. 10.1016/j.molmed.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 36.Hong MH, Kim HR, Park SY, Kim DJ, Lee CG, Cho J, et al. A phase II trial of preoperative chemoradiotherapy and pembrolizumab for locally advanced esophageal squamous cell carcinoma (ESCC). J Clin Oncol. 2019;37:4027. [Google Scholar]
- 37.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single‐agent anti‐programmed death‐1 (MDX‐1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. 10.1200/JCO.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly RJ, Smith KN, Anagnostou V, Thompson E, Hales RK, Battafarano RJJ, et al. Neoadjuvant nivolumab plus concurrent chemoradiation in stage II/III esophageal/gastroesophageal junction cancer. J Clin Oncol. 2019;37:142. [Google Scholar]
- 39.Uboha NV, Maloney JD, Mccarthy D, Deming DA, LoConte NK, Matkowskyj K, et al. Safety of neoadjuvant chemoradiation (CRT) in combination with avelumab (A) in the treatment of resectable esophageal and gastroesophageal junction (E/GEJ) cancer. J Clin Oncol. 2019;37:4041. [Google Scholar]
- 40.van den Ende T, de Clercq NC, van Berge Henegouwen MI, Gisbertz SS, Meijer SL, Schokker S, et al. A phase II feasibility trial of neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: the PERFECT trial. J Clin Oncol. 2019;37:4045. [DOI] [PubMed] [Google Scholar]


