Abstract
Background
tRNA‐derived fragments (tRFs) have been found to play a regulatory role in the occurrence and development of many tumors. The aim of this study was to identify the expression of tRFs in breast cancer and their ability to serve as diagnostic markers for breast cancer.
Methods
Total RNA was extracted from breast cancer and paracancerous tissues (n = 83), as well as from the sera of breast cancer patients (n = 214) and healthy donors (n = 113) using trizol reagents. Expression of tRFs was then detected by q‐PCR, and analyzed using t‐test and ROC to illuminate their potential as biomarkers for breast cancer.
Results
Our results demonstrated that tRFs: tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 were downregulated in both tissues and sera from breast cancer patients as well as early‐stage patients compared with those in the healthy donors. More importantly, the three tRFs were capable of serving as circulating biomarkers of diagnostics and early diagnosis of breast cancer, possessing areas under the curve (AUC) of 0.7871 and 0.7987, respectively.
Conclusions
tRFs: tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 are downregulated in breast cancer and early breast cancer and act as new potential biomarkers for the diagnosis and early diagnosis of breast cancer.
Keywords: breast cancer, tRF‐Gly‐CCC‐046, tRF‐Pro‐TGG‐001, tRF‐Tyr‐GTA‐010, tRNA‐derived fragments (tRFs)
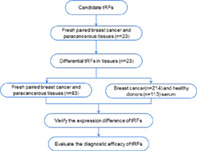
As shown in the technology roadmap, due to the low primer specificity and expression levels, some tRFs were ruled out and others were subjected to validation in fresh tissues, including 23 breast cancer tissues and paired paracancerous tissues. After that, tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 were observed with significant decrease in breast cancer tissue and selected as the candidates. The three differential tRFs were further verified in expanded tissue (n = 83) and serum cohorts (n = 214). Finally, ROC curves were used to evaluate the diagnostic efficacy of the three tRFs for breast cancer and early breast cancer.
INTRODUCTION
Breast cancer, the most common solid tumor among women,1, 2 has surpassed lung cancer as the most common cancer worldwide. New cases of breast cancer annually reach 1.7 million globally.3 Despite continuous improvement in diagnosis and treatment, breast cancer‐related morbidity and mortality are relatively high, particularly in developing countries. Patients with breast cancer often present at late or metastatic stages when diagnosed due to lack of access to early screening and diagnosis. Therefore, the development of an efficient biomarker for the early diagnosis of breast cancer and the monitoring of its spatial and temporal progression is urgently needed.
tRNA‐derived small RNAs (tDRs) are short RNAs with sizes ranging from 14 to 50 nt and are derived from tRNAs, the RNA molecules abundant in cells with important functions of transporting amino acids and assisting in protein synthesis.4 According to the location of biogenesis, tDRs can be generally grouped into tRNA halves and tRNA‐derived small RNA fragments (tRFs).5 tRFs, the new type of non‐coding RNA found in many organisms, have been found to play an important regulatory role in a variety of biological processes in humans.6, 7, 8 The cutting of tRNA or pre‐tRNA in different positions by different enzymes results in various kinds of tRFs,9 which are mainly divided into three categories: tRF‐5, tRF‐3 and tRF‐1. Among them, tRF‐5 is derived from the 5 'end of mature tRNA and is cut from D loop in the manner of Dicer‐dependent; tRF‐3 is decomposed by the T loop of mature tRNA by Dicer enzyme or angiopoietin10; meanwhile tRF‐1 is formed by cutting pre‐tRNA with RNase Z.4
Recently, as high‐throughput techniques evolve and develop, novel tRFs have been gradually discovered and have gained more and more attention from researchers, and their roles in a variety of diseases, especially in cancer, have also been gradually revealed. Many studies have demonstrated that tRFs are involved in the tumorigenesis and development of tumors. They work mainly through binding with RNA binding proteins (RBPs),11 or targeting 3′ untranslated regions (UTRs) of mRNA directly like microRNAs, such as tRFs derived from tRF‐Asp‐GTC, tRF‐Glu‐YTC, and tRF‐Gly‐TCC. They can replace 3′ UTRs of multiple carcinogenic transcripts from YBX1, a multifunctional RNA‐binding protein overexpressed in a variety of cancers and enhances expression of oncogene via stabilizing their transcripts, thereby inhibiting the progression of breast cancer.9 Another example is tRF‐3019 which directly regulates the tumor suppressor gene FBXO47 by binding with its 3′ UTR, thus promoting the proliferation, metastasis and invasion of gastric cancer cells;12 moreover, tRF is also closely related with breast cancer patient stratification. The differential expression of tRFdb‐5024a has been observed in breast ductal cancer, lobular cancer, and others, indicating its association with histological type in breast cancer patients.
tRFs are stable and abundant in body fluids including the blood plasma, serum,13 and urine of cancer patients, empowering them with the potential as noninvasive biomarkers for malignancies. For example, in a recent study, tRNA‐ValTAC‐3, tRNA‐GlyTCC‐5, tRNA‐ValAAC‐5 and tRNA‐GluCTC‐5 in plasma exosomes from liver cancer patients were found to be significantly upregulated, serving as diagnostic biomarkers for liver cancer.14 The downregulation of tDR‐7816 in nontriple negative breast cancer (non‐TNBC) provided the potential for diagnosis of patients with early non‐TNBC;15 whereas exosomal tRF‐25, tRF‐38 and tRF‐18 possessed a favorable diagnostic efficiency, not only for the diagnosis but also prognosis of gastric cancer.13 All these suggest circulating tRFs can serve as diagnostic biomarker for cancers. Furthermore, tRFs can also be applied as biomarkers to predict cancer metastasis. Londin et al. investigated the relationship between tRFs and metastasis in patients with uveal melanoma (UVM). The expression abundance of tRF‐22‐BP4MJYSZH and tRF‐21‐45dBNIB9b was significantly lower in metastatic patients, illustrating their potential of tRFs to monitor metastastatic cancer.16 Moreover, there have also been previous studies highlighting tRFs as prognostic biomarkers. For example, the expression of tDR‐000620 in TNBC cancer stem cells and sera has been reported to be relatively decreased, and its low expression correlated with shorter recurrence‐free survival17; Consistently, the expression levels of another five tRFs (tRFDB‐5024a, 5p_tRNA‐LeU‐CAA‐4‐1, ts‐49, ts‐34, and ts‐58) were all correlated with the overall survival of breast cancer patients, reflecting the prognostic value of tRFs.18 Taken together, these findings apparently suggest tRFs might be promising biomarkers for cancer, including breast cancer.
In the current study, we identified that three tRFs: tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 were downregulated in both tissues and sera from breast cancer patients, as well as early‐stage patients compared with those in healthy donors. More importantly, these three tRFs were capable of serving as circulating biomarkers of diagnostics and the early diagnosis of breast cancer, possessing favorable diagnostic efficiency, and revealing the crucial role of tRFs as diagnostic biomarkers for breast cancer.
METHODS
Patients and healthy donors
A total of 83 pairs of fresh tissue samples (from September 2017 to September 2019) as well as 214 serum samples (from July 2020 to December 2020) from breast cancer patients admitted to Shandong Cancer Hospital and Institute were enrolled in the current study. Patients included in the validation cohort met the following conditions: (i) they had been diagnosed with breast cancer as a result of a combination of clinical symptoms, imaging, and pathological findings; and (ii) none of the patients had received any antitumor therapy. Breast cancer patients who received any anticancer treatment or had suffered from other types of cancer or suffered any other endocrine, immune, or metabolic diseases at the same time were excluded from the study. TNM stage was determined based on the American Joint Committee on Cancer (AJCC) eighth edition. Fifty‐seven healthy volunteers from the above hospital and 56 from Shandong Provincial Third Hospital, who were excluded from having any malignant tumor after examination, were enrolled in this study. All participants gave their informed consents for specimen and clinical information collection.
RNA extraction
For fresh tissue samples, tissues weighing 80–100 mg were cut into small sections and thoroughly ground in a dedicated mortar and transferred into a 1.5 ml centrifuge tube. For serum samples, sera were centrifuged at 13 000 g for 10 min at 4°C to remove the residual cellular sediment and transferred into a 1.5 ml centrifuge tube. Then, 1 ml trizol or 750 μl trizol LS reagent (Thermo Fisher Scientific) was added to each centrifuge tube to extract RNA according to the procedure.
Reverse transcription and qPCR
The above extracted RNAs were reverse‐transcribed into cDNA in a 10 μl system using the Mix‐X miRNA First‐Strand Synthesis Kit (TaKaRa Bio) according to the protocol. LightCycler 480 qPCR system (Roche Diagnostics) was used for qPCR with a 20‐μl reaction system, including 10 μl of TB‐Green Premix Ex Taq II Reagent (TaKaRa), 7.2 μl of RNase‐free water, 0.4 μl of upstream and 0.4 μl of downstream primers, and 2 μl of cDNA template. U6 was used as an internal reference gene, and the relative gene expression was calculated as ΔCT = CTtRF‐CTU6, as previously described.19 The primer sequences involved are detailed in Table 1.
TABLE 1.
Primer sequences involved
| Gene | Sequence (5′‐3′) |
|---|---|
| U6‐F | TGGAACGCTTCACGAATTTGCG |
| U6‐R | GGAACGATACAGAGAAGATTAGC |
| tRF‐Gly‐CCC‐046‐F | TATATATTCCCGGGCGGCGC |
| tRF‐Tyr‐GTA‐010‐F | ATCCGGCTCGAAGGACCA |
| tRF‐Pro‐TGG‐001‐F | CGCGCAAAGACTTTTTCTCTGACCA |
Statistical analysis
IBM SPSS Statistics 19 software and GraphPad Prism 8.0.1 software were used for statistical analysis of the data. Kolmogorov–Smirnov test was performed to verify whether the data were normally distributed. If the data followed normal analysis, an unpaired t‐test was used, and if not, a Mann–Whitney test was used. Multigroup analysis was tested by one‐way ANOVA or Kruskal‐Wallis test. In paired data, the normally distributed numeric variables were evaluated by paired t‐test, whereas non‐normally distributed variables were analyzed by Wilcoxon rank‐test. A receiver operating characteristic (ROC) curve was used to evaluate diagnostic efficiency, including calculation of the area under the curve (AUC), and specificity and sensitivity. All the values were mean ± SD (standard deviation) and two‐sided p < 0.05 was considered to be statistically significant.
RESULTS
tRFs: tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 downregulated in breast cancer tissue
To investigate the differential expression of tRFs in breast cancer, we first validated the expression of several candidate tRFs based on previous laboratory studies in 23 breast cancer and paired paracancerous tissues. tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 were significantly downregulated in breast cancer tissue compared to the control, whereas tRF‐Gly‐GCC‐020 and tRF‐Lys‐TTT‐027 showed no significant difference (data not shown). Therefore, these three differential tRFs were verified in an expanded cohort (n = 83). tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 were consistently obviously decreased in breast cancer tissues (p = 0.0002, p < 0.0001 and p < 0.0001, respectively) compared with those in paracancerous tissue (Figure 1(a)–(c)). Moreover, the relationship between the expression of the three tRFs and clinical characteristics was also analyzed, and all three tRFs were obviously associated with human epidermal growth factor receptor‐2 (HER‐2) status. tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 were also correlated with T stage and menstrual status, but irrelevant with other characteristics (Table 2).
FIGURE 1.

tRFs: tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 were downregulated in breast cancer tissue. (a)–(c) tRF‐Gly‐CCC‐046 (a), tRF‐Tyr‐GTA‐010 (b) and tRF‐Pro‐TGG‐001 (c) were downregulated in breast cancer tissue compared with paracarcinoma tissue (n = 83); (d)–(f) tRF‐Gly‐CCC‐046 (d), tRF‐Tyr‐GTA‐010 (e) and tRF‐pro‐TGG‐001 (f) were downregulated in early‐stage breast cancer tissue compared with paracarcinoma tissue (n = 58); **p < 0.005; ***p < 0.001; ****p < 0.0001
TABLE 2.
Correlation between expression of tRFs in tissue and clinicopathological characteristics of breast cancer patients
| Characteristics | Cases | tRF‐Gly‐CCC‐046 | Tyr‐GTA‐010 | tRF‐Pro‐TGG‐001 | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | p | Mean ± SD | p | Mean ± SD | p | ||
| Age | |||||||
| <=50 | 49 | −5.052 ± 3.088 | 0.334 | −0.986 ± 2.195 | 0.052 | −1.504 ± 2.183 | 0.065 |
| >50 | 34 | −4.358 ± 2.883 | −0.095 ± 1.952 | −0.642 ± 1.888 | |||
| T stage | |||||||
| T1 T2 | 71 | −4.517 ± 2.947 | 0.052 | −0.417 ± 2.105 | 0.036 * | −0.951 ± 2.088 | 0.038 * |
| T3 T4 | 10 | −6.507 ± 3.113 | −0.392 ± 2.163 | −2.426 ± 1.923 | |||
| Unknown | 2 | ||||||
| Lymph node metastasis | |||||||
| N0 | 34 | −5.17 ± 3.078 | 0.307 | −0.951 ± 2.076 | 0.217 | −1.496 ± 2.037 | 0.177 |
| N1 | 49 | −4.488 ± 2.957 | −0.392 ± 3.113 | −0.912 ± 2.128 | |||
| TNM stage | |||||||
| Stage I | 17 | −4.442 ± 2.129 | 0.837 | −0.541 ± 1.927 | 0.804 | −1.118 ± 1.874 | 0.776 |
| Stage II | 41 | −4.996 ± 3.172 | −0.773 ± 2.242 | −1.304 ± 2.34 | |||
| Stage III | 25 | −4.615 ± 3.304 | −0.424 ± 2.146 | −0.923 ± 2.074 | |||
| Pathological type | |||||||
| IDC II | 38 | −4.530 ± 3.051 | 0.612 | −0.390 ± 2.163 | 0.572 | −0.933 ± 2.132 | 0.587 |
| IDC III | 35 | −4.900 ± 3.229 | −0.684 ± 2.262 | −1.212 ± 2.227 | |||
| Others | 10 | ||||||
| ER | |||||||
| − | 10 | −5.434 ± 2.598 | 0.295 | −0.912 ± 2.009 | 0.669 | −1.433 ± 1.879 | 0.608 |
| + | 71 | −4.706 ± 3.096 | −0.596 ± 2.185 | −1.127 ± 2.162 | |||
| Unknown | 2 | ||||||
| PR | |||||||
| − | 28 | −4.490 ± 3.117 | 0.513 | −0.227 ± 2.068 | 0.215 | −0.745 ± 1.984 | 0.232 |
| + | 53 | −4.958 ± 3.007 | −0.851 ± 2.188 | −1.387 ± 2.174 | |||
| Unknown | 2 | ||||||
| HER‐2 | |||||||
| − | 47 | −5.897 ± 2.934 | 0.0001 * | −1.314 ± 2.005 | 0.0002 * | −1.824 ± 1.981 | 0.0002 * |
| + | 31 | −3.160 ± 2.368 | −0.442 ± 1.824 | −0.115 ± 1.794 | |||
| Unknown | 5 | ||||||
| Menstrual status | |||||||
| + | 47 | −5.267 ± 3.194 | 0.128 | −1.042 ± 2.173 | 0.039 * | −1.544 ± 2.161 | 0.045 * |
| − | 36 | −4.116 ± 2.648 | −0.072 ± 1.976 | −0.637 ± 1.925 | |||
| Ki‐67 | |||||||
| <14 | 21 | −4.879 ± 2.732 | 0.678 | −0.894 ± 2.029 | 0.535 | −1.415 ± 2.000 | 0.595 |
| >14 | 58 | −4.686 ± 3.177 | −0.490 ± 2.223 | −1.022 ± 2.186 | |||
| Unknown | 4 | ||||||
| Subtype | |||||||
| Triple‐negative | 3 | −7.188 ± 2.269 | 0.243 | −1.945 ± 2.014 | 0.413 | −2.402 ± 1.975 | 0.471 |
| HER2‐enriched | 8 | −4.333 ± 2.508 | −0.233 ± 1.956 | −0.778 ± 1.818 | |||
| Luminal | 67 | −4.759 ± 3.092 | −0.603 ± 2.135 | −1.132 ± 2.114 | |||
| Unknown | 5 | ||||||
Abbreviation: IDC, invasive ductal carcinoma.
Bold value, p < 0.05.
We then analyzed the differential expression of the three tRFs in 58 early‐stage breast cancer patients, as well as their paired paracancerous tissues. The three tRFs were consistently also dramatically decreased in early‐stage breast cancer patients (p = 0.0042, p < 0.0001 and p = 0.0003, respectively) compared with those in healthy subjects (Figure 1(d)–(f)). Taken together, our data demonstrated tRFs: tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 were downregulated in breast cancer patients and early‐stage cancer patients, implying they might be involved in tumorigenesis of breast cancer.
tRFs: tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 as noninvasive diagnostic biomarkers for breast cancer
To investigate the potential of the three tRFs as biomarkers for breast cancer, we examined their expression in serum from 214 breast cancer patients and 113 healthy controls. As expected, tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 were significantly downregulated (all, p < 0.0001) in sera like in tissues from breast cancer patients compared to those from healthy donors (Figure 2(a)–(c)), thereby possessing favorable diagnostic efficiency. The AUC of tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 was 0.7223 with 80.4% sensitivity and 55.8% specificity, 0.7809 with 83.6% sensitivity and 61.9% specificity, 0.7085 with 82.2% sensitivity and 53.1% specificity, respectively. When combined, the AUC reached 0.7871 with 83.6% sensitivity and 56.6% specificity, suggesting the great potential of these three tRFs as noninvasive circulating biomarkers for breast cancer (Figure 2(d)–(g)).
FIGURE 2.

tRFs: tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 as noninvasive diagnostic biomarkers for breast cancer. (a)–(c) tRF‐Gly‐CCC‐046 (a), tRF‐Tyr‐GTA‐010 (b) and tRF‐Pro‐TGG‐001 (c) were downregulated in the serum of breast cancer patients (n = 214) compared with healthy controls (n = 113); (d)–(g) Receiver operating characteristic (ROC) curve analysis of tRF‐Gly‐CCC‐046 (d), tRF‐Tyr‐GTA‐010 (e), tRF‐Pro‐TGG‐001 (f) and their combination (g) for breast cancer. HD, healthy donors; BC, breast cancer patients; ****p < 0.0001
We also evaluated the relationship between expression of the three tRFs and clinical characteristics in 214 breast cancer patients. As shown in Table 3, tRF‐Gly‐CCC‐046 and tRF‐Pro‐TGG‐001 were obviously associated with pathological types of breast cancer, while tRF‐Tyr‐GTA‐010 was correlated with pathological type and lymph node metastasis, but this was irrelevant when compared with other clinical features (Table 3).
TABLE 3.
Correlation between expression of tRFs in sera and clinicopathological characteristics of breast cancer patients
| Characteristics | Cases | tRF‐Gly‐CCC‐046 | Tyr‐GTA‐010 | tRF‐Pro‐TGG‐001 | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | p | Mean ± SD | p | Mean ± SD | p | ||
| Age | |||||||
| <=50 | 122 | −8.148 ± 1.691 | 0.170 | −3.231 ± 1.735 | 0.356 | −3.297 ± 1.765 | 0.704 |
| >50 | 92 | −7.800 ± 1.993 | −2.957 ± 1.981 | −3.013 ± 2.137 | |||
| T stage | |||||||
| Tis | 14 | −8.401 ± 1.465 | 0.397 | −3.449 ± 1.567 | 0.741 | −3.778 ± 1.413 | 0.136 |
| T1 | 92 | −7.948 ± 1.887 | −3.145 ± 1.851 | −3.196 ± 1.871 | |||
| T2 | 79 | −8.802 ± 1.820 | −3.078 ± 1.854 | −3.172 ± 2.159 | |||
| T3 | 11 | −7.964 ± 1.649 | −3.082 ± 1.933 | −2.969 ± 1.725 | |||
| T4 | 15 | −7.161 ± 1.695 | −2.494 ± 1.982 | −2.435 ± 1.641 | |||
| Unknown | 3 | ||||||
| Lymph node metastasis | |||||||
| N0 | 97 | −8.132 ± 1.729 | 0.055 | −3.372 ± 1.581 | 0.017 * | −3.497 ± 1.541 | 0.074 |
| N1 | 68 | −7.735 ± 1.664 | −2.690 ± 1.844 | −2.835 ± 2.111 | |||
| N2 | 25 | −8.551 ± 2.179 | −3.655 ± 1.988 | −3.451 ± 1.941 | |||
| N3 | 20 | −7.256 ± 2.048 | −2.323 ± 2.399 | −2.189 ± 2.625 | |||
| Unknown | 4 | ||||||
| TNM stage | |||||||
| Stage 0 | 12 | −8.571 ± 1.303 | 0.614 | −3.738 ± 1.187 | 0.574 | −3.935 ± 1.294 | 0.241 |
| Stage I | 59 | −7.892 ± 1.799 | −3.106 ± 1.781 | −3.158 ± 1.714 | |||
| Stage II | 76 | −8.022 ± 1.744 | −3.020 ± 1.774 | −3.164 ± 2.080 | |||
| Stage III | 36 | −7.776 ± 2.414 | −2.859 ± 2.429 | −2.825 ± 2.339 | |||
| Stage IV | 23 | −8.064 ± 1.252 | −3.337 ± 1.409 | −3.128 ± 1.625 | |||
| Unknown | 8 | ||||||
| Pathological type | |||||||
| MC | 4 | −6.244 ± 1.177 | 0.043 * | −0.960 ± 1.432 | 0.008 * | −1.224 ± 2.254 | 0.004 |
| IDC | 185 | −7.938 ± 1.850 | −3.058 ± 1.856 | −3.116 ± 1.968 | |||
| DCIS | 19 | −8.663 ± 1.618 | −3.745 ± 1.541 | −4.038 ± 1.338 | |||
| Unknown | 6 | ||||||
| ER | |||||||
| − | 48 | −8.060 ± 1.828 | 0.630 | −3.426 ± 1.692 | 0.204 | −3.497 ± 1.698 | 0.136 |
| + | 149 | −7.912 ± 1.845 | −2.936 ± 1.920 | −2.977 ± 2.048 | |||
| Unknown | 17 | ||||||
| PR | |||||||
| − | 51 | −7.900 ± 1.956 | 0.806 | −3.161 ± 1.904 | 0.604 | −3.084 ± 1.989 | 0.964 |
| + | 145 | −7.989 ± 1.784 | −3.044 ± 1.850 | −3.146 ± 1.941 | |||
| Unknown | 18 | ||||||
| HER‐2 | |||||||
| − | 142 | −8.006 ± 1.832 | 0.269 | −3.118 ± 1.872 | 0.266 | −3.157 ± 1.735 | 0.891 |
| + | 51 | −7.811 ± 1.857 | −2.887 ± 1.864 | −3.102 ± 2.037 | |||
| Unknown | 21 | ||||||
| Menstrual status | |||||||
| + | 112 | −8.048 ± 1.583 | 0.883 | −3.136 ± 1.589 | 0.969 | −3.209 ± 1.541 | 0.385 |
| − | 84 | −8.010 ± 2.047 | −3.152 ± 2.008 | −3.199 ± 2.212 | |||
| Unknown | 18 | ||||||
| Ki‐67 | |||||||
| <14 | 49 | −7.992 ± 1.754 | 0.913 | −3.070 ± 1.792 | 0.466 | −3.179 ± 1.564 | 0.512 |
| >14 | 146 | −7.959 ± 1.861 | −3.077 ± 1.895 | −3.116 ± 2.072 | |||
| Unknown | 19 | ||||||
| Subtype | |||||||
| Triple‐negative | 18 | −8.442 ± 1.941 | 0.407 | −3.480 ± 1.854 | 0.469 | −3.471 ± 1.804 | 0.432 |
| HER2‐enriched | 18 | −7.643 ± 1.968 | −3.313 ± 1.785 | −3.112 ± 1.764 | |||
| Luminal | 157 | −7.934 ± 1.810 | −2.979 ± 1.881 | −3.076 ± 2.001 | |||
| Unknown | 21 | ||||||
Abbreviations: DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; MC, mucinous carcinoma.
Bold value, p < 0.05.
tRFs: tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 as biomarkers for early diagnosis of breast cancer
To investigate the diagnostic value of the three tRFs in the diagnosis of early breast cancer, we further analyzed the expression of tRFs in 147 patients with early breast cancer (Tis stage = 12, I stage = 59, IIA stage = 76) and 113 healthy subjects. As shown in Figure 3(a)–(c), tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 were significantly reduced in the sera of patients with early‐stage breast cancer (all, p < 0.0001) compared with healthy donors. Subsequently, when comparing the patients with early‐stage breast cancer to healthy controls, ROC curves demonstrated favorable diagnostic efficiencies of tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001, processing AUCs of 0.7254 with 85% sensitivity and 52.2% specificity, 0.7937 with 84.4% sensitivity and 68.1% specificity, 0.7075 with 78.9% sensitivity and 56.6% specificity, respectively, as well as 0.7987 with 84.4% sensitivity and 67.3% specificity for their combination (Figure 3(d)–(g)). Taken together, these data suggest that the three tRFs are promising biomarkers for the early diagnosis of breast cancer.
FIGURE 3.

tRFs: tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 as biomarkers for the early diagnosis of breast cancer. (a)–(c) tRF‐Gly‐CCC‐046 (a), tRF‐Tyr‐GTA‐010 (b) and tRF‐Pro‐TGG‐001 (c) were downregulated in the serum of early‐stage breast cancer patients (n = 147) compared with healthy controls (n = 113). (d)–(g) Receiver operating characteristic (ROC) curve analysis of tRF‐Gly‐CCC‐046 (d), tRF‐Tyr‐GTA‐010 (e), tRF‐Pro‐TGG‐001 (f) and their combination (g) for early‐stage breast cancer; ****p < 0.0001
tRFs: tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 facilitate monitoring breast cancer progression
Next, we investigated the role of the three tRFs in monitoring breast cancer progression. Unexpectedly, they demonstrated an unsatisfactory efficiency to diagnose early or advanced breast cancer, possessing AUC of 0.6055 with 75.5% sensitivity and 46.2% specificity for the three combinations (Figure 4(a)). Subsequently, we investigated the role of several traditional breast cancer‐related biomarkers: carcinoembryonic antigen (CEA), carbohydrate antigen125 (CA125), and carbohydrate antigen153 (CA153) in monitoring disease progression, which demonstrated diagnostic performance with an AUC of 0.6936 with 83% sensitivity and 49.6% specificity, 0.6550 with 54.7% sensitivity and 70.1% specificity, 0.7401 with 71.7% sensitivity and 61.5% specificity, respectively (Figure 4(b)–(d)). When combined with the three tRFs, AUC increased to 0.8012 with a sensitivity of 73.6% and a specificity of 70.9% (Figure 4(e)), indicating that they significantly enhance the ability of traditional biomarkers to predict breast cancer progression.
FIGURE 4.

tRFs: tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 facilitate the monitoring of breast cancer progression. (a) Receiver operating characteristic (ROC) curve analysis of combination of tRFs for breast cancer progression; (b)–(d) ROC curve analysis of CEA (b), CA125 (c), CA153 (d) for breast cancer progression; (e) ROC curve analysis of CEA, CA125, CA153 for breast cancer progression combined with tRFs
DISCUSSION
Despite continuous improvement in diagnosis and treatment, the incidence and mortality rate of breast cancer still remains high, ranking first in incidence and fourth in the mortality spectrum of female malignant tumors in China. A large proportion of deaths might be attributable to a delay in diagnosis and treatment, patients presenting with advanced or metastatic stage cancer when diagnosed, or those losing the opportunity of surgical cure. Hence, a diagnostic tool with high sensitivity and specificity is urgently needed to remedy the deficiency of early diagnosis.20
Recently, accumulating evidence has demonstrated tRFs serve as biomarkers in various cancer types, such as non‐small cell lung cancer (NSCLC),21 colorectal cancer (CRC),22 gastric,13, 23 pancreatic,24, 25 breast,26, 27 ovarian28 and prostate cancers.29 Many studies have revealed alterations in the level of tRFs in various cancers, some of which are cancer type‐specific.30 They can regulate the proliferation, metastasis and invasion of cancer and are related to drug resistance,12, 31 indicating their crucial role in tumorigenesis and tumor development. More importantly, tRFs are expressed and measurable in body fluids including the blood plasma, serum, and urine of cancer patients. Due to their short length, tRFs are difficult to be degraded by RNase, empowering them with the potential as noninvasive biomarkers for the diagnosis of malignancies. In the current study, we demonstrated tRFs: tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 acted as novel diagnostic biomarkers for breast cancer. First, we identified three tRFs: tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 were downregulated in both tissues and sera from breast cancer patients, as well as in early‐stage patients compared with those in healthy donors. Second, the three tRFs were capable of serving as circulating biomarkers for the diagnosis and early diagnostics of breast cancer, thereby possessing considerable diagnostic efficiency. We also found that the combination of the three tRFs effectively promoted the role of CEA, CA125, and CA153 in monitoring breast cancer progression via the analysis of early and advanced patients, thus revealing the crucial role of tRFs in the diagnosis of breast cancer.
Notably, our data demonstrated the three tRFs were significantly correlated with HER‐2 status. HER‐2, the second member of the human epidermal growth factor receptor (HER) family,32 acts as an important prognostic and curative effect biomarker for breast cancer.33, 34 Its amplification or protein overexpression is present in 20% of invasive breast cancers,35 implying poor prognosis in patients. In previous studies, it has been reported that tRF‐Glu‐CTC‐003 in the plasma of patients with early breast cancer was significantly lower than that in normal controls, and its low expression was closely associated with shorter disease‐free survival (DFS) and overall survival (OS) in patients with HER‐2 positive breast cancer27; whereas tRF‐30‐JZOYJE22RR33 and TRF‐27‐ZDXPHO53KN were increased in trastuzumab‐resistant patients compared to sensitive individuals, and associated with significantly shorter progression‐free survival (PFS) in patients with metastatic HER‐2 positive breast cancer.36 In our study, tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 were significantly associated with HER‐2 status, and they were downregulated in the HER‐2 positive cohort compared with the HER‐2 negative, thereby implying these three tRFs may have the potential as prognostic biomarkers for breast cancer, although further studies are needed to confirm these findings.
Nevertheless, some limitations in our study should be carefully taken into consideration. First, the total sample sizes in current study were small. Only 83 pairs of tissue samples, as well as sera from 214 patients and from 113 healthy donors were included, which might result in the lack of statistically vigorous power. Consequently, we failed to analyze the different roles of tRFs in different molecular types of breast cancer. Second, long‐term clinical follow‐up data were also absent due to time constraints, which currently limit the ability to explore the prognostic values of the three tRFs. Third, we failed to obtain the information of traditional tumor markers such as CEA, CA125 and CA153 from healthy donors, so we were unable to analyze the combined performance of tRFs and traditional tumor biomarkers in the diagnosis, or early diagnostics of breast cancer.
In conclusion, our study identified that tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 were downregulated in breast cancer tissues as well as serum, thereby acting as a new potential biomarker for the diagnosis and early diagnosis of breast cancer.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81972014); Shandong Provincial Medicine and Health Science Technology Development Program (2019WS198); Jinan Clinical Medical Science and Technology Innovation Program (202019054).
Zhang Y, Bi Z, Dong X, Yu M, Wang K, Song X, et al. tRNA‐derived fragments: tRF‐Gly‐CCC‐046, tRF‐Tyr‐GTA‐010 and tRF‐Pro‐TGG‐001 as novel diagnostic biomarkers for breast cancer. Thorac Cancer. 2021;12:2314–2323. 10.1111/1759-7714.14072
Funding information Jinan Clinical Medical Science and Technology Innovation Program, Grant/Award Number: 202019054; Shandong Provincial Medicine and Health Science Technology Development Program, Grant/Award Number: 2019WS198; National Natural Science Foundation of China, Grant/Award Number: 81972014
REFERENCES
- 1.Wang M, Liao J, Tan C, Zhou H, Wang J, Wang K, et al. Integrated study of miR‐215 promoting breast cancer cell apoptosis by targeting RAD54B. J Cell Mol Med. 2021;25:3327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adhikary S, Chakravarti D, Terranova C, Sengupta I, Maitituoheti M, Dasgupta A, et al. Atypical plant homeodomain of UBR7 functions as an H2BK120Ub ligase and breast tumor suppressor. Nat Commun. 2019;10:1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mo D, Jiang P, Yang Y, Mao X, Tan X, Tang X, et al. A tRNA fragment, 5'‐tiRNA (Val), suppresses the Wnt/beta‐catenin signaling pathway by targeting FZD3 in breast cancer. Cancer Lett. 2019;457:60–73. [DOI] [PubMed] [Google Scholar]
- 4.Sun C, Fu Z, Wang S, Li J, Li Y, Zhang Y, et al. Roles of tRNA‐derived fragments in human cancers. Cancer Lett. 2018;414:16–25. [DOI] [PubMed] [Google Scholar]
- 5.Falconi M, Giangrossi M, Zabaleta ME, Wang J, Gambini V, Tilio M, et al. A novel 3'‐tRNA (Glu)‐derived fragment acts as a tumor suppressor in breast cancer by targeting nucleolin. FASEB J. 2019;33:13228–40. [DOI] [PubMed] [Google Scholar]
- 6.Raina M, Ibba M. tRNAs as regulators of biological processes. Front Genet. 2014;5:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson P, Ivanov P. tRNA fragments in human health and disease. FEBS Lett. 2014;588:4297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romano G, Veneziano D, Acunzo M, Croce CM. Small non‐coding RNA and cancer. Carcinogenesis. 2017;38:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodarzi H, Liu X, Nguyen HCB, Zhang S, Fish L, Tavazoie SF. Endogenous tRNA‐derived fragments suppress breast cancer progression via YBX1 displacement. Cell. 2015;161:790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Ender C, Meister G, Moore PS, Chang Y, John B. Extensive terminal and asymmetric processing of small RNAs from rRNAs, snoRNAs, snRNAs, and tRNAs. Nucleic Acids Res. 2012;40:6787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu L, Ge J, Li T, Shen Y, Guo J. tRNA‐derived fragments and tRNA halves: the new players in cancers. Cancer Lett. 2019;452:31–7. [DOI] [PubMed] [Google Scholar]
- 12.Zhang F, Shi J, Wu Z, Gao P, Zhang W, Qu B, et al. A 3'‐tRNA‐derived fragment enhances cell proliferation, migration and invasion in gastric cancer by targeting FBXO47. Arch Biochem Biophys. 2020;690:108467. [DOI] [PubMed] [Google Scholar]
- 13.Lin C, Zheng L, Huang R, Yang G, Chen J, Li H. tRFs as potential exosome tRNA‐derived fragment biomarkers for gastric carcinoma. Clin Lab. 2020;66:961–969. [DOI] [PubMed] [Google Scholar]
- 14.Zhu L, Li J, Gong Y, Wu Q, Tan S, Sun D, et al. Exosomal tRNA‐derived small RNA as a promising biomarker for cancer diagnosis. Mol Cancer. 2019;18:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Ge H, Zheng M, Cui Y, Fu Z, Wu X, et al. Serum tRNA‐derived fragments (tRFs) as potential candidates for diagnosis of nontriple negative breast cancer. J Cell Physiol. 2020;235:2809–24. [DOI] [PubMed] [Google Scholar]
- 16.Londin E, Magee R, Shields CL, Lally SE, Sato T, Rigoutsos I. IsomiRs and tRNA‐derived fragments are associated with metastasis and patient survival in uveal melanoma. Pigment Cell Melanoma Res. 2020;33:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng W, Li Y, Chu J, Li J, Zhang Y, Ding X, et al. Identification of tRNA‐derived small noncoding RNAs as potential biomarkers for prediction of recurrence in triple‐negative breast cancer. Cancer Med. 2018;7:5130–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shan N, Li N, Dai Q, Hou L, Yan X, Amei A, et al. Interplay of tRNA‐derived fragments and T cell activation in breast cancer patient survival. Cancers (Basel). 2020;12:2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko HH, Lee JJ, Chen HM, Kok SH, Yen‐Ping Kuo M, Cheng SJ, et al. Upregulation of vascular endothelial growth factor mRNA level is significantly related to progression and prognosis of oral squamous cell carcinomas. J Formos Med Assoc. 2015;114:605–11. [DOI] [PubMed] [Google Scholar]
- 20.Mishra S, Srivastava AK, Suman S, Kumar V, Shukla Y. Circulating miRNAs revealed as surrogate molecular signatures for the early detection of breast cancer. Cancer Lett. 2015;369:67–75. [DOI] [PubMed] [Google Scholar]
- 21.Shao Y, Sun Q, Liu X, Wang P, Wu R, Ma Z. tRF‐Leu‐CAG promotes cell proliferation and cell cycle in non‐small cell lung cancer. Chem Biol Drug Des. 2017;90:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Yang X, Jiang G, Zhang H, Ge L, Chen F, et al. 5'‐tRF‐GlyGCC: a tRNA‐derived small RNA as a novel biomarker for colorectal cancer diagnosis. Genome Med. 2021;13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong X, Fan X, He X, Chen S, Huang W, Gao J, et al. Comprehensively identifying the key tRNA‐derived fragments and investigating their function in gastric cancer processes. Onco Targets Ther. 2020;13:10931–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Jin L, Gao Y, Gao P, Ma L, Zhu B, et al. Low expression of tRF‐Pro‐CGG predicts poor prognosis in pancreatic ductal adenocarcinoma. J Clin Lab Anal. 2021;35:e23742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin L, Zhu C, Qin X. Expression profile of tRNA‐derived fragments in pancreatic cancer. Oncol Lett. 2019;18:3104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Yang Y, Tan X, Mao X, Wei D, Yao Y, et al. Identification of tRNA‐derived fragments expression profile in breast cancer tissues. Curr Genomics. 2019;20:199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Ma G, Li M, Han X, Xu J, Liang M, et al. Plasma tRNA fragments derived from 5′ends as novel diagnostic biomarkers for early‐stage breast cancer. Mol Ther Nucleic Acids. 2020;21:954–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang M, Li F, Wang J, He W, Li Y, Li H, et al. tRNA‐derived fragment tRF‐03357 promotes cell proliferation, migration and invasion in high‐grade serous ovarian cancer. Onco Targets Ther. 2019;12:6371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olvedy M, Scaravilli M, Hoogstrate Y, Visakorpi T, Jenster G, Martens‐Uzunova ES. A comprehensive repertoire of tRNA‐derived fragments in prostate cancer. Oncotarget. 2016;7:24766–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee YS, Shibata Y, Malhotra A, Dutta A. A novel class of small RNAs: tRNA‐derived RNA fragments (tRFs). Genes Dev. 2009;23:2639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang B, Yang H, Cheng X, Wang D, Fu S, Shen W, et al. tRF/miR‐1280 suppresses stem cell‐like cells and metastasis in colorectal cancer. Cancer Res. 2017;77:3194–206. [DOI] [PubMed] [Google Scholar]
- 32.Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim MH, Kim GM, Kim JH, Kim JY, Park HS, Park S, et al. Intermediate HER2 expression is associated with poor prognosis in estrogen receptor‐positive breast cancer patients aged 55 years and older. Breast Cancer Res Treat. 2020;179:687–97. [DOI] [PubMed] [Google Scholar]
- 34.Kreutzfeldt J, Rozeboom B, Dey N, De P. The trastuzumab era: current and upcoming targeted HER2+ breast cancer therapies. Am J Cancer Res. 2020;10:1045–67. [PMC free article] [PubMed] [Google Scholar]
- 35.Ahn S, Woo JW, Lee K, Park SY. HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation. J Pathol Transl Med. 2020;54:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun C, Yang F, Zhang Y, Chu J, Wang J, Wang Y, et al. tRNA‐derived fragments as novel predictive biomarkers for Trastuzumab‐resistant breast cancer. Cell Physiol Biochem. 2018;49:419–31. [DOI] [PubMed] [Google Scholar]


