Abstract
The coordinated interplay of substrate adhesion and deadhesion is necessary for cell motility. Using MCF-7 cells, we found that insulin-like growth factor I (IGF-I) induces the adhesion of MCF-7 to vitronectin and collagen in a dose- and time-dependent manner, suggesting that IGF-I triggers the activation of different integrins. On the other hand, IGF-I promotes the association of insulin receptor substrate 1 with the focal adhesion kinase (FAK), paxillin, and the tyrosine phosphatase SHP-2, resulting in FAK and paxillin dephosphorylation. Abrogation of SHP-2 catalytic activity with a dominant-negative mutant (SHP2-C>S) abolishes IGF-I-induced FAK dephosphorylation, and cells expressing SHP2-C>S show reduced IGF-I-stimulated chemotaxis compared with either mock- or SHP-2 wild-type-transfected cells. This impairment of cell migration is recovered by reintroduction of a catalytically active SHP-2. Interestingly, SHP-2-C>S cells show a larger number of focal adhesion contacts than wild-type cells, suggesting that SHP-2 activity participates in the integrin deactivation process. Although SHP-2 regulates mitogen-activated protein kinase activity, the mitogen-activated protein kinase kinase inhibitor PD-98059 has only a marginal effect on MCF-7 cell migration. The role of SHP-2 as a general regulator of cell chemotaxis induced by other chemotactic agents and integrins is discussed.
Cell migration is one of the most important events contributing to tumor dissemination, and its prevention may arrest malignant evolution (23). Little is known, however, about the molecular events that regulate cell motility and invasion. The molecular characterization of invasion has led to the identification of two categories of checkpoints. The first category encompasses cell surface and secreted proteins; molecules falling into this group include cell adhesion receptors, degradative enzymes and their inhibitors, and motility-stimulating cytokines. The second checkpoint category involves regulatory proteins and pathways within the cell; molecules identified in this group regulate calcium-mediated signaling, G-protein activation, and tyrosine phosphorylation events.
Adhesion receptors, such as integrins, promote cellular migration and invasion (2, 13). Although integrin-mediated adhesion is necessary for tumor motility, it is not sufficient in itself. Cells expressing αvβ5 integrin require a tyrosine kinase-mediated signaling event for motility on vitronectin (21). In fact, several cytokines, such as insulin-like growth factor I (IGF-I), cooperate with integrins to promote tumor cell migration in vitro (12, 31, 48) and in vivo (5).
A functional link must therefore exist between chemotactic receptors and integrins. Taking the type 1 IGF receptor (IGF-1R) as an example, it has been shown that the insulin receptor substrate 1 (IRS-1), a pivotal molecule in insulin and IGF-1R signaling pathways, associates to αvβ3 integrin (54) and to the focal adhesion kinase (FAK). Functional cooperation is confirmed, since IGF-I-induced chemotaxis is achieved only when the integrin receptor is activated (12, 20); the mechanisms involved in this cooperation nonetheless remain confusing. The tyrosine kinase FAK is thought to coordinate integrin and growth factor signaling pathways (17). FAK is autophosphorylated immediately after integrin clustering and associates with different signaling proteins, including phosphatidylinositol-3-kinase, Grb2-Sos, p130cas, and paxillin (17). FAK-mediated signaling promotes cell migration; indeed, FAK−/− cells have a reduced migration rate compared to their FAK-expressing counterparts (19). Whereas other chemoattractants, such as platelet-derived growth factor (PDGF) or hepatocyte growth factor (scatter factor), induce FAK phosphorylation (10, 29), insulin and IGF-I promote either FAK phosphorylation (3, 26) or dephosphorylation (22, 40).
Although much research has focused on identifying the substrates whose phosphorylation is crucial for chemotaxis, protein tyrosine phosphorylation is a reversible, dynamic process in which the net level of phosphate observed in a substrate reflects the balance between the activity of a kinase enzyme and the phosphatase that catalyzes the dephosphorylation reaction. Tyrosine phosphatases may thus have a role in chemotactic and/or motility processes. In accordance with this view, inhibition of tyrosine phosphatases with orthovanadate was reported to suppress cell spreading and migration of lung carcinoma cells (49). More recently, it has been shown that fibroblasts derived from tyrosine phosphatase SHP-2−/− mice have lower levels of motility and spread more slowly than the wild-type cells (60). SHP-2 (previously called SH-PTP2, PTP2C, PTP1D, SHPTP3, and Syp) is widely expressed (1, 15) and participates in signaling events proximal to receptor protein tyrosine kinases, such as PDGF (14, 53), epidermal growth factor (14, 53), insulin (24), and IGF-I (46) receptors, as well as to hematopoietic receptors (reviewed in reference 34).
In this report, we attempt to elucidate the signaling pathways leading to cooperation between integrins and chemotactic receptors in cell migration. We used the human breast adenocarcinoma MCF-7 cell line as a model system, as these cells migrate in response to IGF-I but not in the absence of a stimulus, and integrin activation is required for this IGF-I-mediated chemotaxis (12). Here we show that IGF-I stimulation increases MCF-7 cell adhesion on vitronectin and collagen in a dose- and time-dependent manner and that it concurrently promotes the tyrosine dephosphorylation of FAK and paxillin through the recruitment of SHP-2 phosphatase. The neutralization of SHP-2 catalytic activity results in a diminished migration capability, which correlates with the absence of IGF-I-induced FAK dephosphorylation and with an increase in the number of focal adhesion contacts. Finally, our data support the view of SHP-2 as a general regulator of cell migration induced by different chemotactic agents and integrins.
MATERIALS AND METHODS
Materials.
Vitronectin and type IV collagen were purchased from Collaborative Biomedical (Bedford, Mass.). Human recombinant IGF-I was from Pharmacia and Upjohn (Stockholm, Sweden), transwell chambers (pore size, 8 μm) were from Corning Costar (Cambridge, Mass.), and polyclonal antisera to FAK and the type 1 IGF receptor β subunit, as well as peroxidase-labelled anti-phosphotyrosine monoclonal antibody (MAb) PY20, were from Santa Cruz Biochemicals (Santa Cruz, Calif.). Antibodies to IRS-1, SHP-2, and agarose-coupled anti-phosphotyrosine antibody 4G10 were from Upstate Biotechnology (Lake Placid, N.Y.) and the anti-integrin αvβ5 MAb was from Boehringer Ingelheim (Heidelberg, Germany). The anti-integrin αω chain MAb was made in our laboratory, and the anti-IGF-I MAb KM5A1 has been previously described (27). Cycloheximide (CHx) was from Sigma Chemicals (St. Louis, Mo.); PD-98059 and the anti-IGF-1R αIR3 MAb were from Calbiochem (San Diego, Calif.).
Cell transfections.
The human breast adenocarcinoma MCF-7 and the human prostatic adenocarcinoma DU-145 cells (ATCC HTB-81) were obtained from the American Type Culture Collection (Manassas, Va.) and cultured in Dulbecco’s modified Eagle medium (DMEM) (Gibco-BRL, Gaithersburg, Md.) supplemented with 10% fetal calf serum, l-glutamine, sodium pyruvate, and antibiotics.
Both cell lines were transfected with a catalytically inactive SHP-2-C>S dominant-negative mutant (61), the wild-type SHP-2 form, or the empty pRC vector (Invitrogen, San Diego, Calif.) using Lipofectamine reagent (Gibco-BRL) according to the manufacturer’s instructions and were selected with G418 (Gibco-BRL) used at 1.5 mg/ml. For MCF-7-transfected cells, cell clones were isolated; all transfected cells were maintained in DMEM containing G418 (0.5 mg/ml).
Cell migration assays.
MCF-7 and DU-145 cells were trypsinized and resuspended in serum-free DMEM with 0.01% bovine serum albumin (BSA) (DMEM-BSA), then seeded in the upper chamber of either type IV collagen- or vitronectin-coated transwells. The lower chamber was loaded with DMEM-BSA alone or with IGF-I (1 ng/ml) or RANTES (10 ng/ml; Peprotech, London, United Kingdom). In some experiments, the cells were incubated with inhibitory antibodies, PD-98059, or CHx for 30 min before seeding in the transwell. After 18 h of incubation at 37°C, the chamber was disassembled, and cells in the upper surface were removed. Filters were stained and cell numbers were calculated as described previously (12).
Cell proliferation assays.
For proliferation experiments, serum-starved MCF-7 cells were stimulated for 24 h with IGF-I in the presence or absence of the inhibitor PD-98059 or CHx and then pulsed for 8 h at 0.5 μCi/well with [3H]thymidine ([3H]TdR) (Amersham, Aylesbury, United Kingdom). Nuclei were harvested using a cell harvester (LKB-Wallac, Turku, Finland), and [3H]TdR incorporation was determined on a liquid scintillation counter.
Western blot analysis.
Subconfluent MCF-7 cells were cultured overnight in DMEM-BSA and then pulsed with IGF-I (1 nM/105 cells) for the times indicated at 37°C. After washing with ice-cold phosphate-buffered saline (PBS), cells were lysed (4°C, 20 min) using radioimmunoprecipitation assay buffer containing a proteinase inhibitor cocktail, 1 mM sodium orthovanadate, and 10 mM NaF. Lysates were centrifuged (12,000 × g, 25 min, 4°C), and their protein concentrations were determined by using the micro-bicinchoninic acid assay kit (Pierce, Rockford, Ill.). Cell lysates (50 to 100 μg) were immunoprecipitated and blotted with the indicated antibodies by following the manufacturer’s instructions.
Adhesion experiments.
Serum-starved MCF-7 cells were allowed to adhere to type IV collagen- or vitronectin-coated 96-well plates, alone or with IGF-I at different concentrations. After 10 min, nonadhered cells were removed by washing with PBS, and the cells were fixed with methanol and stained with crystal violet. Cell attachment to vitronectin was determined by measurement of the optical density at 570 nm. In time course experiments, nonattached cells were stimulated with IGF-I (10 ng/ml, 37°C). At the times indicated, cells were washed twice in ice-cold PBS, resuspended in DMEM-BSA, and added to plates as described above. An aliquot of nonattached cells was pelleted for each IGF-I treatment condition, and cell lysates were analyzed by immunoprecipitation with FAK antibodies.
Immunofluorescence analysis.
Cells were cultured on vitronectin-coated chamber slides, fixed in 3.7% formaldehyde, permeabilized in 0.5% Triton X-100 for 10 min, and stained with anti-paxillin antibody (Transduction Laboratories, Lexington, Ky.). The cells were further incubated with a Cy3-conjugated goat anti-mouse immunoglobulin G (Amersham) and visualized under a Leica fluorescence microscope (Leitz DMRD) at a ×1,000 magnification.
Determination of ectopic SHP-2 expression by reverse transcriptase PCR.
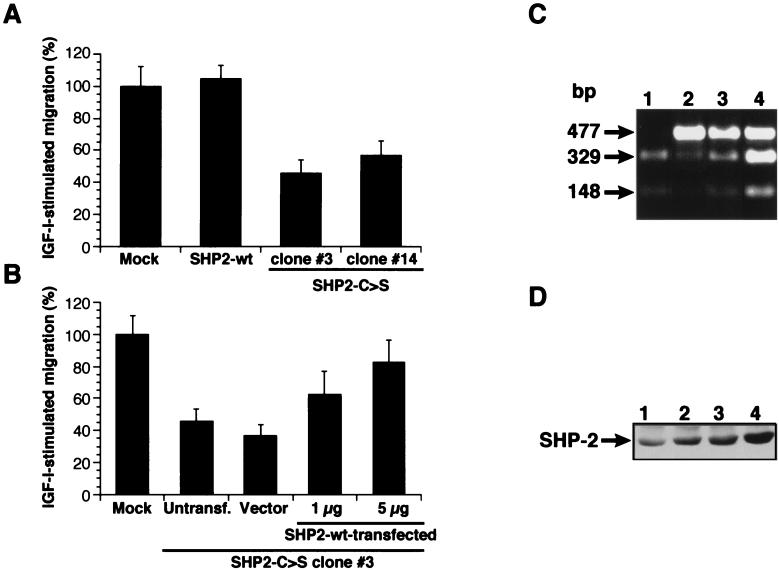
Total RNA was isolated by ultracentrifugation through a cesium chloride cushion. Cells were derived from cells transfected with the empty pRCvector, the wild-type SHP-2, mutant SHP-2-C>S, or wild-type retransfected SHP-2-C>S. This material (5 μg) was then reverse transcribed using the First-Strand cDNA Synthesis kit (Pharmacia AB, Stockholm, Sweden), and one-tenth of the transcription product was amplified for 20 PCR cycles with the SHP-2 specific primers for (5′-CGAGTGATTGTCATGACAACG-3′) and back (5′-TGCTTCTGTCTGGACCATCC-3′), rendering a 477-bp fragment. Finally, part (20 μl) of the PCR product was analyzed by digestion with the restriction enzyme PstI, which cleaves the endogenous and the ectopically expressed wild-type SHP-2 forms, but not the SHP-2-C>S mutant, rendering two fragments of 329 and 148 bp.
RESULTS
IGF-I induces adhesion of MCF-7 cells.
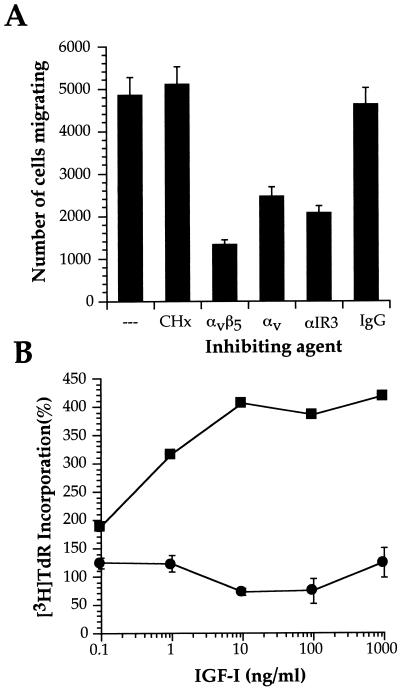
MCF-7 cells are known to migrate in response to IGF-I (12). We previously showed that the mechanism of IGF-I-triggered chemotaxis in MCF-7 involved αvβ5 interaction with an appropriate extracellular matrix (ECM) substrate, such as vitronectin, as well as the enzymatic activity of specific matrix metalloproteinases, such as MMP-9 (31). Cycloheximide treatment of MCF-7 does not block IGF-I-induced chemotaxis on vitronectin (Fig. 1A), although as expected, incubation of MCF-7 with antagonist antibodies to the IGF-1R or to αvβ5 integrin results in decreased cell migration. Cycloheximide nevertheless effectively blocks IGF-I-induced DNA synthesis in MCF-7 cells (Fig. 1B). The IGF-I- and integrin αvβ5-triggered signals for the induced invasion in MCF-7 cells thus do not require de novo protein synthesis.
FIG. 1.
IGF-I induces MCF-7 cell migration in the absence of de novo protein synthesis. (A) MCF-7 cells were incubated with αIR3 (0.2 μg/ml), anti-αvβ5, anti-αv, or immunoglobulin G as control (20 μg/ml in all cases) or CHx (5 μg/ml) and seeded in vitronectin-coated transwells, and IGF-I (1 ng/ml) was added to the lower chamber. The number of migrating cells represented is the mean ± standard deviation counted per filter. (B) Serum-starved MCF-7 cells were incubated with the indicated amounts of IGF-I in the presence (●) or absence (■) of CHx. [3H]TdR incorporation was determined after 24 h. The proliferation observed in the absence of IGF-I was considered 100%.
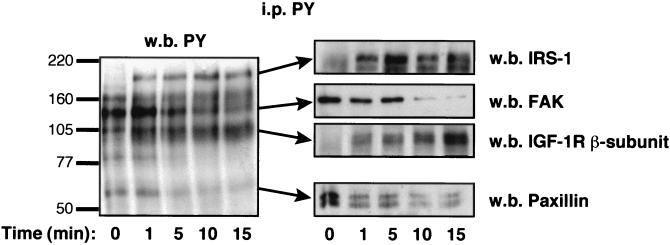
In vitronectin-attached, serum-starved MCF-7 cells, anti-phosphotyrosine antibody reveals five principal tyrosine-phosphorylated proteins: pp160; pp125, which has been identified as FAK; pp105; pp80; and pp68, which has been identified as paxillin (Fig. 2). In addition to rapid autophosphorylation of the IGF-1R β subunit and the IRS-1 substrate, IGF-I-stimulation of MCF-7 promotes dephosphorylation of FAK, paxillin, and pp80.
FIG. 2.
IGF-I induces tyrosine dephosphorylation of FAK and paxillin. Cell lysates from unstimulated (time 0) or IGF-I-stimulated MCF-7 cells seeded on vitronectin were immunoprecipitated with anti-phosphotyrosine antibody (PY) and resolved on polyacrylamide gels. The membranes were blotted with the indicated antibodies, and blotting was followed by enhanced chemiluminescence analysis. w.b., Western blot; i.p., immunoprecipitation.
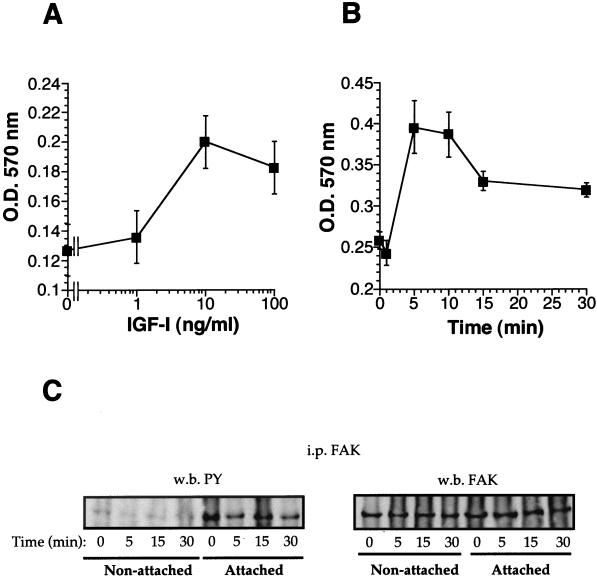
It is generally accepted that FAK is autophosphorylated following its interaction with the engaged integrins and that this phosphorylation helps to stabilize the focal adhesion contacts through integrin-cytoskeletal interaction (33). Since IGF-I promotes FAK dephosphorylation, IGF-I interference in MCF-7 cell adhesion to ECM substrates such as vitronectin and type IV collagen was therefore tested. IGF-I significantly increases MCF-7 cell adhesion to vitronectin in a dose-dependent manner (Fig. 3A). Subsequently, the effect of the IGF-I stimulation time on increased MCF-7 attachment to vitronectin was examined. IGF-I enhancement of MCF-7 cell adhesion shows a maximum effect after 10 min of IGF-I stimulation and a slight but consistent decline in MCF-7 adhesivity at longer times (Fig. 3B). Since similar results were obtained using type IV collagen (data not shown), these data suggest that signals provided by the IGF-1R promote the activation of integrins αvβ5 and α2β1, increasing cell adhesion to ECM substrates.
FIG. 3.
IGF-I enhances MCF-7 adhesion to vitronectin. (A) Serum-starved MCF-7 cells were detached with a trypsin-EDTA solution and, after two washes in PBS, incubated with the indicated doses of IGF-I in DMEM-BSA for 10 min. The mixture was then added to vitronectin-coated 96-well plates and incubated for an additional 10 min. After two washes, attached cells were assessed by crystal violet staining. (B) MCF-7 cells were detached and incubated in DMEM without BSA (30 min, 37°C). IGF-I (10 ng/ml) was added, and the cells were then incubated for the indicated times under the same conditions. IGF-I was removed by pelleting the cells and washing twice with PBS. Finally, cells were resuspended in DMEM-BSA and added to the vitronectin-coated plates. After washing with PBS, the attached cells were assessed by crystal violet staining. (C) Cells prepared as just described were lysed before (nonattached) or after (attached) adhesion to vitronectin plates. Cell lysates were immunoprecipitated (i.p.) with an FAK antibody, and the resolved proteins were analyzed by Western immunoblotting (w.b.) with anti-phosphotyrosine (PY) and anti-FAK antibodies. O.D., optical density.
It has been reported that IGF-1R may phosphorylate FAK in nonadhered cells (3). The analysis of FAK immunoprecipitates with anti-phosphotyrosine antibodies shows that IGF-I stimulation of nonadhered MCF-7 cells does not promote FAK phosphorylation, independent of the incubation time, although FAK is tyrosine phosphorylated upon cell attachment to vitronectin (Fig. 3C) or collagen (data not shown). These data indicate that IGF-I increases integrin-mediated MCF-7 cell adhesion independent of the FAK activation state.
IGF-1R activation induces FAK association with the phosphatase SHP-2.
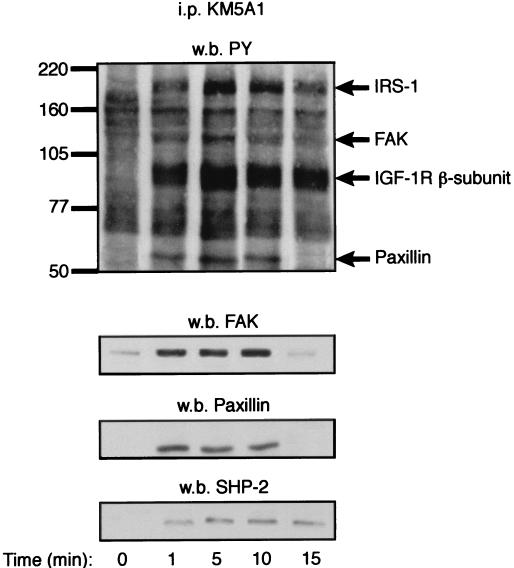
To analyze whether the FAK dephosphorylation observed in attached cells is a direct consequence of IGF-1R activation, we analyzed FAK association to the IGF-1R and the result of this association on FAK phosphorylation status. IGF-I-stimulated cell lysates were immunoprecipitated using the KM5A1 anti-IGF-I MAb that recognizes the IGF-I ligand when it is bound to the IGF-1R (27); this antibody thus enables specific analysis of proteins associated to the activated IGF-1R. IGF-I stimulation of MCF-7 cells triggers FAK and paxillin association to the IGF-1R, but this association results in a net dephosphorylation of FAK and paxillin (Fig. 4). Coprecipitation of the tyrosine phosphatase SHP-2 with the IGF-1R was also observed. After IGF-I stimulation, FAK and SHP-2 were also coprecipitated in experiments using anti-SHP-2 antibodies (data not shown). IGF-I may induce formation of a SHP-2, FAK, and paxillin complex; FAK dephosphorylation coincides with loss of this association.
FIG. 4.
IGF-1R-associated FAK is dephosphorylated. Vitronectin-attached MCF-7 cells were stimulated with IGF-I for the times indicated (time 0 represents no stimulation) at 37°C. Cells were then washed twice in PBS to remove unbound IGF-I, and cell lysates were immunoprecipitated (i.p.) with the anti-IGF-I antibody KM5A1. The resolved proteins were Western immunoblotted (w.b.) sequentially with the indicated antibodies.
SHP-2 catalytic activity is required for IGF-I-induced FAK dephosphorylation.
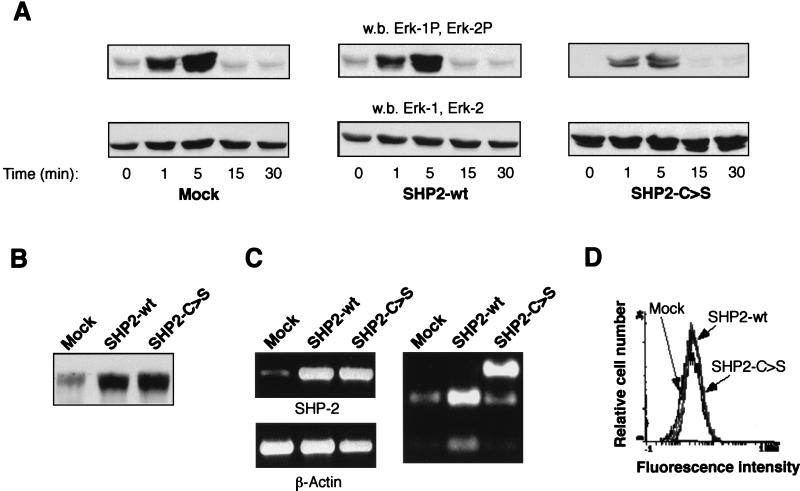
To show that SHP-2 catalytic activity is required for IGF-I-induced FAK dephosphorylation, MCF-7 cells were transfected with a dominant-negative SHP-2 (SHP2-C>S) catalytically inactive mutant. SHP-2 functions as part of a positive signaling pathway mediating insulin and IGF-I activation of mitogen-activated protein kinase (MAPK) (61). To analyze whether SHP2-C>S–MCF-7-transfected cells are in fact deficient in SHP-2 activity, we measured the MAPK phosphorylation level in response to IGF-I (Fig. 5A). MCF-7 cells transfected with either the empty vector or wild-type SHP-2 (SHP2-wt) show normal MAPK activation in response to IGF-I. Indeed, MAPK phosphorylation is also observed in the absence of IGF-I stimulation, probably as a consequence of cell attachment to the substrate (17). SHP2-C>S–MCF-7-transfected cells show, however, a substantial decrease in IGF-I-triggered MAPK phosphorylation. Moreover, the MAPK activation attributable to cell attachment disappeared completely in MCF-7 cells transfected with catalytically inactive SHP-2. Ectopic expression was also demonstrated by an increase in SHP-2 protein levels in total lysates from SHP-2-transfected cells (Fig. 5B), as well as by RT-PCR amplification and subsequent PstI restriction analysis (Fig. 5C).
FIG. 5.
Characterization of SHP-2-deficient clones. (A) Western immunoblot (w.b.) analysis of ERK kinases. Equal amounts of cell lysates (30 μg) of empty vector- (mock), wild-type SHP-2 (SHP2-wt), or mutant SHP2-C>S-transfected cells, stimulated with IGF-I at the times indicated, were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with anti-phospho-Erk-specific (upper panel) or anti-Erk antibodies (lower panel). These antibodies recognize both Erk-1 and Erk-2. (B and C) Determination of ectopically expressed SHP-2. In panel B, equal amounts of cell lysates (30 μg) were resolved by SDS-PAGE and immunoblotted with an anti-SHP-2 antibody. In panel C, total RNA was isolated from mock-, SHP2-wt-, and SHP2-C>S-transfected cells, and reverse transcriptase PCR was performed using specific primers for either SHP-2 (upper left panel) or β-actin (lower left panel). The primers used do not amplify nonreverse transcribed RNA (data not shown). Finally, a part of the PCR products was incubated with PstI (right panel), which digests the endogenous and the ectopically expressed SHP2-wt forms, but not the SHP2-C>S form. (D) Determination of integrin levels. MCF-7 cells, transfected as in panel A, were stained with an anti-αvβ5 antibody followed by a fluorescein-labelled second antibody and were then analyzed by FACS.
Although MAPK activation is clearly affected in SHP2-C>S–MCF-7 cells, the αvβ5 integrin level remains similar in all MCF-7 cell clones independently of the SHP-2 construction transfected, as analyzed by fluorescence-activated cell sorter (Fig. 5D) or Western blot (data not shown) analysis. Concurrent to MAPK activity, SHP2-C>S transfection also affects MCF-7 cell spreading (data not shown), as has recently been reported (60).
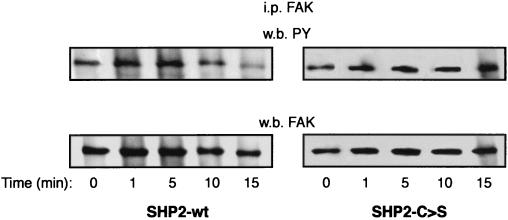
After establishing MCF-7 cell lines with decreased SHP-2 activity, we analyzed the consequences for FAK phosphorylation status (Fig. 6). IGF-I-induced FAK dephosphorylation is abolished in SHP2-C>S–MCF-7-transfected cells, whereas dephosphorylation occurs in cells transfected with wild-type SHP-2. This indicates that SHP-2 activity is required for IGF-I-induced FAK dephosphorylation.
FIG. 6.
IGF-I-induced FAK dephosphorylation is dependent on SHP-2 catalytic activity. MCF-7 cells transfected with wild-type SHP-2 (SHP2-wt) or the catalytically inactive mutant SHP-2-C>S were stimulated with IGF-I at the times indicated (time 0 represents the absence of stimulus). After a washing step, cell lysates were prepared and the proteins were immunoprecipitated (i.p.) with an anti-FAK antibody and resolved by SDS-PAGE. Western immunoblotting (w.b.) was performed sequentially with anti-phosphotyrosine (PY, upper panel) and, as a protein loading control, anti-FAK (lower panel) antibodies.
FAK phosphorylation status regulates MCF-7 invasivity.
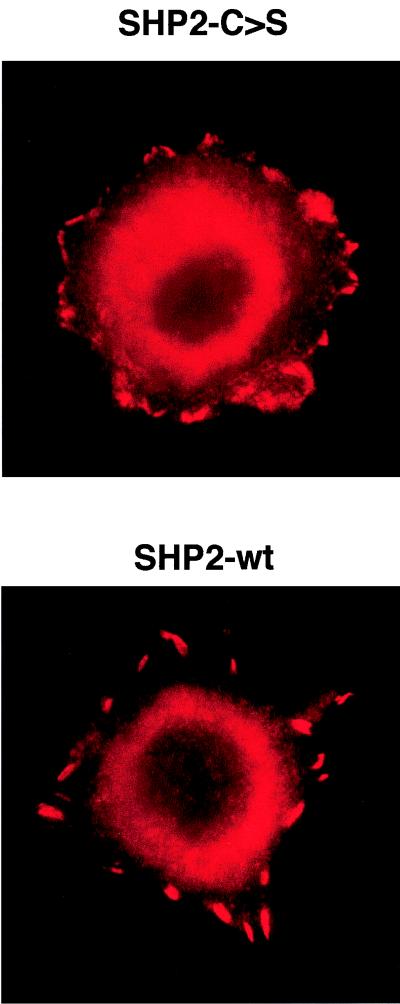
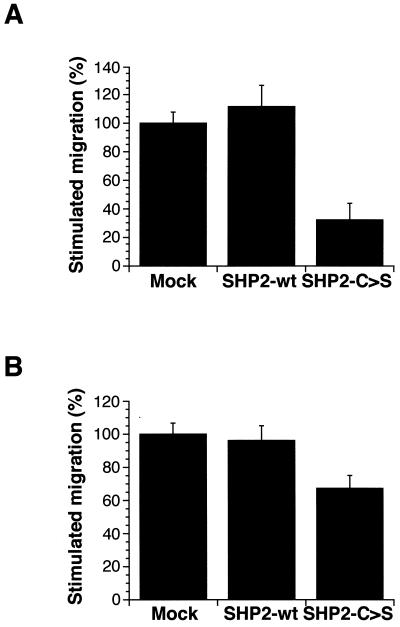
Using a transwell cell motility assay, we tested the relevance of FAK dephosphorylation to IGF-I-triggered MCF-7 invasivity. Compared with SHP2-wt- or mock-transfected cells, the ability of SHP2-C>S–MCF-7 cells to migrate on vitronectin is significantly reduced (Fig. 7A). In addition, SHP2-C>S–MCF-7 cells show a larger number of focal adhesion contacts than do MCF-7 cells transfected with empty vector or wild-type SHP-2 (Fig. 8), concurring with data obtained with SHP-2−/− mouse fibroblasts (60). To ensure that the reduced migration observed in SHP2-C>S–MCF-7-transfected cells is an effect of the catalytic inactivation of SHP-2, we transiently retransfected these cells with wild-type SHP-2 (Fig. 7C and D). Upon reintroduction of catalytically active SHP-2, the IGF-I-triggered chemotactic response of SHP2-C>S–MCF-7 cells was recovered in a manner dependent on the amount of retransfected SHP2-wt cDNA (Fig. 7B).
FIG. 7.
SHP-2 catalytic activity is required for IGF-I-triggered MCF-7 invasion. (A) MCF-7 transfected with empty vector (mock), wild-type SHP-2, or the mutant SHP-2-C>S were seeded on vitronectin-coated transwells, and IGF-I (1 ng/ml) was added to the lower chamber. The number of migrating mock-transfected cells was designated 100%. Two representative SHP-2-C>S-transfected cell clones are shown (#3 and #14). (B) The SHP2-C>S–MCF-7 clone #3 cell line was untransfected (untransf.) or transfected with the empty vector (vector) or with the vector coding for wild-type SHP-2 (SHP2-wt) at the indicated doses and then assayed for IGF-I-triggered invasion through vitronectin as in panel A. The number of migrating mock-transfected cells was designated 100%. (C) SHP2-wt mRNA expression in retransfected SHP2-C>S cells. As described in the legend to Fig. 5, total RNA was amplified by reverse transcriptase PCR using specific SHP-2 primers, and PCR products were digested with PstI. The figure shows the restriction analysis for mock-transfected cells (lane 1) and for SHP2-C>S cells alone (lane 2) transfected with empty vector (lane 3) or with SHP2-wt plasmid (5 μg) (lane 4). (D) Western blot analysis of SHP-2 levels in the cells used in panel C. Equal amounts of cell lysate were resolved by SDS-PAGE and blotted with an SHP-2-specific antibody. Data represent one of three similar independent experiments.
FIG. 8.
SHP-2 catalytic activity regulates the number of focal adhesions. MCF-7 cells transfected with wild-type (SHP2-wt) or inactive mutant (SHP2-C>S) constructs of SHP-2 were seeded on vitronectin-coated chamber slides, fixed, permeabilized with Triton X-100, and examined for focal adhesion plaques by using a paxillin antibody followed by a Cy3-conjugated secondary antibody.
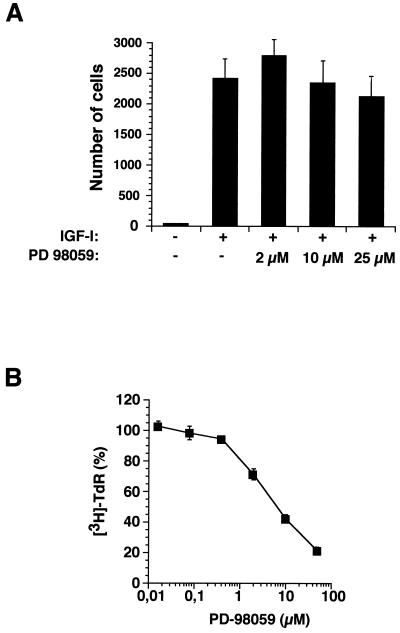
Since SHP-2 affects IGF-I-induced MAPK activation, we examined whether the effect of SHP-2 on cell motility is mediated through this signaling pathway. The migration of untransfected MCF-7 cells in response to IGF-I was unaffected by the MAPK inhibitor PD-98059 (Fig. 9A), although PD-98059 effectively blocks IGF-I-induced proliferation of MCF-7 cells (Fig. 9B). These results show that the role of SHP-2 on MCF-7 cell motility is independent of IGF-I-induced MAPK activation in these cells.
FIG. 9.
Effect of SHP-2 on MCF-7 cell invasion is independent of MAPK activation. (A) MCF-7 cells were incubated with the MEK inhibitor PD-98059 at the indicated concentrations for 30 min before seeding on vitronectin-coated transwells. The migration induced by IGF-I (1 ng/ml) added to the lower chamber was examined. The numbers of migrating cells are the means ± standard deviations of cells per filter. (B) Serum-starved MCF-7 cells were incubated with IGF-I (100 ng/ml) in the presence of the indicated amounts of PD-98059. [3H] TdR incorporation was determined after 24 h. The proliferation obtained in the absence of PD-98059 was considered 100%.
SHP-2 catalytic activity is a general requirement for cell chemotaxis.
To test whether the role of SHP-2 in IGF-I-induced MCF-7 migration is a general mechanism that can be extrapolated to other integrins, we tested the IGF-I-induced chemotactic response of MCF-7 cells seeded on type IV collagen. SHP2-C>S cells show a lower chemotactic response to IGF-I than mock- or SHP2-wt-transfected cells (Fig. 10A). Furthermore, expression of dominant-negative SHP2-C>S cDNA in the highly invasive human prostatic adenocarcinoma DU-145 cell line also resulted in decreased IGF-I-triggered migration on vitronectin-coated transwells compared to that in cells transfected with the empty vector or the SHP-2 wild-type form (Fig. 10B). These results thus show that catalytically active SHP-2 is required for the IGF-I-triggered invasivity in two different human cell lines, independent of the integrin activated.
FIG. 10.
SHP-2 catalytic activity is a general requirement for cell motility. (A) MCF-7 cells transfected with empty vector (mock), wild-type SHP-2, or the mutant SHP-2-C>S were seeded on type IV collagen-coated transwells and assayed for migration in response to IGF-I (1 ng/ml). The number of migrating mock-transfected cells was designated 100%. (B) DU-145 cells transfected with empty vector (mock), wild-type SHP-2 (SHP2-wt), or inactive SHP-2-C>S (SHP2-C>S) were seeded on vitronectin-coated transwells and assayed for migration in response to IGF-I. As for panel A, the number of migrating mock-transfected cells was designated 100%.
We further explored whether SHP-2 specifically affects IGF-I-induced MCF-7 migration or if it is also involved in other types of signals that trigger chemotaxis. The chemokines are a family of low-molecular-weight proteins implicated in leukocyte activation and migration that exert their effects by interaction with seven-transmembrane, G protein-coupled receptors (GPCR) present in the membrane of the target cell (55). Chemokines also trigger tyrosine phosphorylation and FAK association to the integrins (36). MCF-7 is reported to express receptors for and respond chemotactically to β-chemokines such as RANTES (59). Our data indicate that RANTES promotes migration of MCF-7 cells to a lesser extent than does IGF-I (Table 1). The ability of the dominant-negative SHP-2 mutant to affect the response to RANTES was therefore tested. The abrogation of SHP-2 catalytic activity results in a significant decrease in RANTES-induced chemotaxis (Table 1), thus indicating that SHP-2 is involved in the MCF-7 chemotactic response mediated by GPCR.
TABLE 1.
Chemotactic effect of RANTES on SHP-2 transfected cells
| Stimulus | Stimulation of migrationa
|
||
|---|---|---|---|
| Mock transfection | SHP2-C>S | SHP2-wt | |
| IGF-I | 13.83 ± 1.72 | 7.83 ± 0.94b | 16.22 ± 1.67 |
| RANTES | 2.44 ± 0.33 | 1.72 ± 0.28b | 2.94 ± 0.17 |
Fold stimulation of migration induced by IGF-I and RANTES over a negative control (absence of stimulus) in the different SHP-2-transfected cells.
P ≤ 0.05, as calculated by Mann-Whitney statistical analysis.
DISCUSSION
These data constitute the first direct demonstration that SHP-2 catalytic activity is involved in the crosstalk between the signals triggered by chemotactic factors and integrin receptors, leading to a cell motility response. Yu et al. (60) recently reported impaired cell spreading and migration in fibroblasts from SHP-2−/− mice, indicating that SHP-2 plays a role in cell motility. Since a hyperphosphorylated state of FAK is observed in SHP-2−/− compared with wild-type fibroblasts, these authors hypothesized that this phosphatase may mediate FAK turnover, thus affecting cell motility and spreading. Our results concur with this view and provide biochemical evidence of the manner in which SHP-2 is involved in the chemoattractant-induced turnover of focal adhesions, regulating the phosphorylation state of FAK, and probably of other focal adhesion-associated proteins, such as paxillin.
Cell movement across a two-dimensional substrate is generally viewed as a continuous, dynamic interplay of adhesions at the leading edge and deadhesions at the rear portion of the cells, combined with cell traction machinery (32). Indeed, “freezing” integrins in an activated state inhibits cell migration (25). A certain degree of cell attachment to ECM substrates is nevertheless necessary for cell motility. IGF-I-triggered MCF-7 chemotaxis requires cell adhesion through integrins to a specific ECM substrate. Incubation of MCF-7 cells with antibodies that block integrin binding to the ECM substrate results in inhibition of the IGF-I chemotactic response (12). IGF-I-stimulated MCF-7 chemotaxis involves this two-step process. IGF-1R activation initially promotes rapid cell adhesion to the substrate; activated IGF-1R then recruits and activates SHP-2, leading to FAK dephosphorylation and probably to deactivation of the focal adhesion formed.
FAK has been shown to be a convergence point between integrins and chemoattractant signaling pathways (reviewed in reference 17). FAK phosphorylation in response to IGF-I has also been reported in the SH-SY5Y cell line (26). In many instances, however, insulin and IGF-I have been described to induce FAK dephosphorylation (22, 40), which appears to be incompatible with chemotaxis promotion by IGF-I. Indeed, FAK phosphorylation and kinase activity have been found in migrating endothelial cells (43). Hepatocyte growth factor (scatter factor), which promotes migration and invasion of oral squamous carcinoma cells, initially induces FAK phosphorylation (29). Finally, mutation of the FAK autophosphorylation site Y397 was critical for the ability of FAK to stimulate cell migration (7, 8). Analysis of tyrosine-phosphorylated proteins in IGF-I-stimulated MCF-7 cells reveals that IGF-I induces dephosphorylation of FAK and paxillin.
Based on the observation that FAK autophosphorylates in tyrosine in response to integrin engagement (6), it has been proposed that FAK plays a role in integrin-mediated cell adhesion. In support of this view, the inhibition of FAK phosphorylation by different agents reduces cell adhesion (45). In addition, it has been proposed that FAK phosphorylation contributes to the stabilization of cell adhesion (33). Since IGF-I induces FAK dephosphorylation, we reasoned that IGF-I should negatively regulate MCF-7 cell adhesion. Nonetheless, IGF-I increases the adhesion of MCF-7 cells to vitronectin and type IV collagen, mediated by integrins αvβ5 and α2β1, respectively. Certain integrins are known to modulate their affinity for extracellular ligands in response to intracellular signals, a process termed activation or inside-out signaling (reviewed in reference 57). The time course experiments performed here suggest that activated IGF-1R provides a transient intracellular signal responsible for the enhanced adhesion.
Evidence suggesting that cell adhesion regulates the effect of insulin and IGF-I on FAK phosphorylation has been presented. FAK is dephosphorylated in insulin-stimulated attached cells but is cross-phosphorylated by the insulin and IGF-I receptors in nonattached cells (3). We thus analyzed whether IGF-1R could activate integrins by phosphorylating FAK. In vitronectin-attached MCF-7 cells, IGF-1R and IRS-1 associate to FAK rapidly following IGF-I activation, but the IGF-1R-associated FAK becomes dephosphorylated in time course experiments. Analysis of FAK immunoprecipitates from nonattached cells showed that IGF-1R does not promote FAK phosphorylation directly, although this phosphorylation occurs upon cell attachment to the ECM substrate. The enhancement of IGF-I-promoted cell adhesion therefore proceeds through a mechanism independent of FAK activation, concurring with previous data (19). It has been proposed that inside-out signaling involves the propagation of conformational changes in the cytoplasmic domains of integrins (38, 57). These changes may be mediated by integrin interaction with cytoskeletal proteins that serve to anchor and cluster integrins, thereby enhancing receptor avidity. Specific mutations in the IGF-1R carboxyl terminus have been reported to disrupt the actin cytoskeleton and the cellular localization of vinculin, a protein implicated in the control of adhesion in fibroblasts (42, 56), without affecting other known IGF-1R substrates (4).
As mentioned above, FAK associated to the activated IGF-1R is dephosphorylated over time. It has been proposed that FAK dephosphorylation in response to insulin occurs in two different ways, involving an indirect mechanism through the C-terminal Src kinase (51), which inhibits Src-mediated FAK phosphorylation (11), or through direct activity of the tyrosine phosphatase SHP-2 (39, 58). We found that the IGF-1R associates to SHP-2 with kinetics compatible with the FAK and paxillin dephosphorylation we observed. The overexpression of a catalytically inactive dominant-negative SHP-2 mutant in MCF-7 cells abolished IGF-I-induced FAK dephosphorylation, suggesting that SHP-2 may catalyze this reaction, although this point has not been formally demonstrated. The abrogation of IGF-I-induced FAK dephosphorylation in SHP2-C>S–MCF-7 cells correlates with decreased migration in response to IGF-I, compared to mock- or SHP2-wt-transfected cells; this phenotype is reversed by reintroducing catalytically active wild-type SHP-2. The reduced chemotaxis in cells overexpressing mutant SHP-2 correlates with an increase in the number of focal adhesion contacts per cell compared with wild-type counterparts, suggesting that SHP-2 catalytic activity contributes to the integrin deactivation process.
Recent reports suggest that integrin suppression could be mediated by transcription-independent signaling through the MAPK pathway (18). SHP-2 is generally a positive regulator of signals for proliferation, and expression of a catalytically inactive SHP-2 mutant, in fact, blocks MAPK activation in response to insulin (30), PDGF (41), epidermal growth factor (61), fibroblast growth factor (50), and IGF-I (47). An SHP-2 integrin suppression mechanism could thus be the stimulation of MAPK activity. In accordance with this view, SHP2-C>S-transfected cells have lower integrin-induced MAPK activity than the wild-type- or the SHP2-wt-transfected cells, and they show reduced cell spreading (reference 60 and our own results). It has been shown that SHP-2 associates to integrins (52). MCF-7 cell migration is not affected by the MAPK kinase (MEK) inhibitor PD-98059, however, which in fact diminishes IGF-I-induced MCF-7 cell proliferation. These results concur with those previously reported by Cary et al. (9) showing that FAK-promoted cell migration does not require MAPK activation and suggest that SHP-2 effects on cell motility are exerted mainly by regulating FAK dephosphorylation.
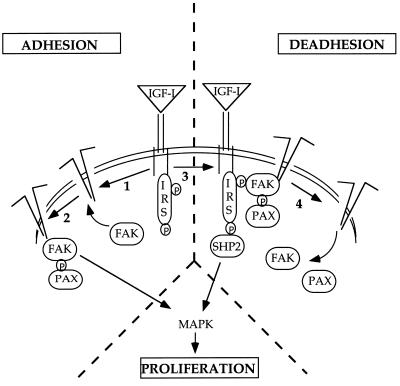
Together, our results suggest the following straightforward sequence of events that explains how IGF-I stimulates MCF-7 cell migration (Fig. 11). (i) Phosphorylated IGF-1R activates integrin αvβ5 at the leading cell edge, probably acting through cytoskeletal proteins such as vinculin. (ii) As a consequence of cell adhesion, FAK autophosphorylation takes place and focal adhesions are assembled, involving other proteins, such as paxillin and Src. (iii) Activated IGF-1R associates to integrins at the focal adhesion contacts, as reported for the insulin receptor (44), and then recruits the phosphatase SHP-2 (and probably C-terminal Src kinase as well). (iv) SHP-2 catalyzes the dephosphorylation of FAK, which could be a signal to turn off that particular focal adhesion contact, and the cell is locally detached. The fact that the SHP2-C>S mutant also diminishes IGF-I-triggered DU-145 cell invasion suggests that the mechanism described for MCF-7 cells may operate in other cell types. Since an IGF autocrine loop is operative in DU-145 cells (28), the results shown here may provide a valid hypothesis for the integration of signals in the control of cell growth and migration in highly invasive tumor cell lines.
FIG. 11.
Model for IGF-I-induced motility in MCF-7 cells. Schematic representation of the IGF-I-triggered signalling pathways leading to adhesion and deadhesion in MCF-7 cells. For details, see Discussion. PAX, paxillin; IRS, IRS-1; P, phosphotyrosine.
Since it has been demonstrated that FAK-mediated signal transduction plays a crucial role in cell migration (8, 16, 19), we further reasoned that SHP-2 activity may be a general requirement for cell chemotaxis promoted by integrins and chemotactic agents other than IGF-I and αvβ5. SHP2-C>S MCF-7 shows reduced IGF-I-triggered chemotaxis on type IV collagen, involving the integrin α2β1 (12). We also tested the effect of the SHP-2 mutant on β-chemokine-mediated MCF-7 invasivity. Chemokines are clearly implicated in the regulation of leukocyte motility and recruitment into inflamed tissue, acting through seven-transmembrane GPCR (55). A new surge of interest in chemokine research arose from the observation that many tumors produce a wide array of and respond chemotactically to chemokines (35, 37, 59). As in the case of IGF-I, the expression of the dominant-negative SHP-2 results in a decrease in RANTES-induced MCF-7 chemotaxis, suggesting that SHP-2 activity is also involved in the intracellular signals triggered by this chemokine. The mechanism by which RANTES induces SHP-2 activation requires further study, but SHP-2 phosphatase has been reported to be involved in GPCR-induced cell proliferation (41).
The initial process in the metastatic spread of tumor cells involves the invasion of malignant cells through the ECM of a basement membrane followed by their intravasation to lymph or blood vessels. Using MCF-7 as a model, we recently reported (31) that chemotactic agents such as IGF-I regulate matrix metalloprotease MMP-9 activity associated to the cell surface, as well as integrin levels. Evidence is provided here that chemotactic agents regulate FAK turnover through tyrosine phosphatase SHP-2 activity. Here we found that IGF-1R activation may trigger the attachment-detachment signaling pathways necessary for cell motility. On the one hand, IGF-I increases αvβ5 and α2β1 integrin-mediated cell adhesion which, in turn, results in FAK phosphorylation; on the other hand, IGF-I promotes FAK dephosphorylation, which requires an active SHP-2 tyrosine phosphatase. Furthermore, and perhaps more importantly, (i) SHP-2 catalytic activity is a general requirement for cell motility mediated through different integrins and induced by diverse chemotactic factors, and (ii) the SHP-2 effect on cell motility correlates with the control of FAK phosphorylation, which may in turn regulate the assembly and deassembly of focal adhesions, using a MAPK-independent mechanism. Our data therefore suggest that SHP-2 is at the crossroads of various signaling pathways triggered by growth factors, β-chemokines, and integrins to induce a chemotactic response, and they provide new objectives in the prevention of tumor dissemination.
ACKNOWLEDGMENTS
We thank P. Labrador for critical reading of the manuscript, M. Mellado and J. M. Rodríguez-Frade for the gift of RANTES, and C. Mark for editorial assistance.
The Department of Immunology and Oncology was founded and is supported by the Spanish Research Council (CSIC), Pharmacia, and Upjohn.
REFERENCES
- 1.Ahmad S, Banville D, Zhao Z, Fischer E, Shen S-H. A widely expressed human protein-tyrosine phosphatase containing src homology 2 domains. Proc Natl Acad Sci USA. 1993;90:2197–2201. doi: 10.1073/pnas.90.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyama S, Olden K, Yamada K. Fibronectin and integrins in invasion and metastasis. Cancer Metastasis Rev. 1995;14:173–189. doi: 10.1007/BF00690290. [DOI] [PubMed] [Google Scholar]
- 3.Baron V, Calléja V, Ferrari P, Alengrin F, Van Obberghen E. p125Fak focal adhesion kinase is a substrate for the insulin and insulin-like growth factor-I tyrosine kinase receptor. J Biol Chem. 1998;273:7162–7168. doi: 10.1074/jbc.273.12.7162. [DOI] [PubMed] [Google Scholar]
- 4.Blakesley V, Koval A, Stannard B, Scrimgeour A, LeRoith D. Replacement of tyrosine 1251 in the carboxyl terminus of the insulin-like growth factor-I receptor disrupts the actin cytoskeleton and inhibits proliferation and anchorage-independent growth. J Biol Chem. 1998;273:18411–18422. doi: 10.1074/jbc.273.29.18411. [DOI] [PubMed] [Google Scholar]
- 5.Brooks P, Klemke R, Schön S, Lewis J, Schwartz M, Cheresh D. Insulin-like growth factor receptor cooperates with integrin αvβ5 to promote tumor cell dissemination in vivo. J Clin Investig. 1997;99:1390–1398. doi: 10.1172/JCI119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burridge K, Turner C, Romer L. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calalb M B, Polte T R, Hanks S K. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cary L, Chang J, Guan J. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- 9.Cary L, Han D, Polte T, Hanks S, Guan J. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Guan J. Stimulation of phosphatidylinositol 3′-kinase association with focal adhesion kinase by platelet-derived growth factor. J Biol Chem. 1994;269:31229–31233. [PubMed] [Google Scholar]
- 11.Cobb B, Schaller M D, Leu T-H, Parsons J T. Stable association of pp60src and pp59fyn with the focal adhesion-associated protein tyrosine kinase, pp125FAK. Mol Cell Biol. 1994;14:147–155. doi: 10.1128/mcb.14.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doerr M, Jones J. The roles of integrins and extracellular matrix proteins in the insulin-like growth factor I-stimulated chemotaxis of human breast cancer cells. J Biol Chem. 1996;271:2443–2447. doi: 10.1074/jbc.271.5.2443. [DOI] [PubMed] [Google Scholar]
- 13.Felding-Habermann B, Mueller B, Romerdahl C, Cheresh D. Involvement of integrin αv gene expression in human melanoma tumorgenicity. J Clin Investig. 1992;89:2018–2022. doi: 10.1172/JCI115811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng G, Hui C, Pawson T. SH2-containing phosphotyrosine phosphatases as a target of protein-tyrosine kinases. Science. 1993;259:1607–1610. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- 15.Feng G, Pawson T. Phosphotyrosine phosphatases with SH2 domains: regulators of signal transduction. Trends Genet. 1994;10:54–58. doi: 10.1016/0168-9525(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 16.Gilmore A, Romer L. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 1996;7:1209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan J. Role of focal adhesion kinase in integrin signaling. Int J Biochem Cell Biol. 1997;29:1085–1096. doi: 10.1016/s1357-2725(97)00051-4. [DOI] [PubMed] [Google Scholar]
- 18.Hughes P, Renshaw M, Pfaff M, Forsyth J, Keivens V, Schwartz M, Ginsberg M. Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell. 1997;88:521–530. doi: 10.1016/s0092-8674(00)81892-9. [DOI] [PubMed] [Google Scholar]
- 19.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T, Aizawa S. Reduced cell motility and enhanced focal contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 20.Jones J, Prevette T, Gockerman A, Clemmons D. Ligand occupancy of the αvβ3 integrin is necessary for smooth muscle cells to migrate in response to insulin-like growth factor-I. Proc Natl Acad Sci USA. 1996;93:2482–2487. doi: 10.1073/pnas.93.6.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klemke R, Yebra E, Bayna E, Cheresh D. Receptor tyrosine kinase signaling required for integrin αvβ5-directed cell motility but not adhesion on vitronectin. J Cell Biol. 1994;127:850–866. doi: 10.1083/jcb.127.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight J, Yamauchi K, Pessin J. Divergent insulin and platelet-derived growth factor regulation of focal adhesion kinase (pp125FAK) tyrosine phosphorylation, and rearrangement of actin stress fibers. J Biol Chem. 1995;270:10199–10203. doi: 10.1074/jbc.270.17.10199. [DOI] [PubMed] [Google Scholar]
- 23.Kohn E, Liotta L. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995;55:1856–1862. [PubMed] [Google Scholar]
- 24.Kuhné M, Pawson T, Lienhard G, Feng G. The insulin-receptor substrate 1 associates with the SH2-containing phosphotyrosine phosphatase Syp. J Biol Chem. 1993;268:11479–11481. [PubMed] [Google Scholar]
- 25.Kuijpers T, Mul E, Blom M, Kovach N, Gaeta F, Tollefson V, Elices M, Harlan J. Freezing adhesion molecules in a state of high-avidity binding blocks eosinophil migration. J Exp Med. 1993;178:279–284. doi: 10.1084/jem.178.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leventhal P, Shelden E, Kim B, Feldman E. Tyrosine phosphorylation of paxillin and focal adhesion kinase during insulin-like growth factor-I-stimulated lamellipodial advance. J Biol Chem. 1997;272:5214–5218. doi: 10.1074/jbc.272.8.5214. [DOI] [PubMed] [Google Scholar]
- 27.Mañes S, Kremer L, Albar J P, Mark C, Llopis R, Martínez-A C. Functional epitope mapping of insulin-like growth factor-I by anti-IGF-I monoclonal antibodies. Endocrinology. 1997;138:905–915. doi: 10.1210/endo.138.3.4965. [DOI] [PubMed] [Google Scholar]
- 28.Mañes, S., M. Llorente, R. Lacalle, L. Kremer, E. Mira, and C. Martínez-A. The insulin-like growth factor (IGF)-triggered autocrine response in DU-145 carcinoma cells is regulated by IGF-binding protein-3 and the matrix metalloproteinase-9. J. Biol. Chem., in press. [DOI] [PubMed]
- 29.Matsumoto K, Matsumoto K, Nakamura T, Kramer R. Hepatocyte growth factor/scatter factor induces tyrosine phosphorylation of focal adhesion kinase (p125FAK) and promotes migration and invasion by oral squamous cell carcinoma cells. J Biol Chem. 1994;269:31807–31813. [PubMed] [Google Scholar]
- 30.Milarski K, Saltiel A. Expression of catalytically inactive Syp phosphatase in 3T3 cells blocks stimulation of mitogen-activated protein kinase by insulin. J Biol Chem. 1994;269:21239–21243. [PubMed] [Google Scholar]
- 31.Mira, E., S. Mañes, R. Lacalle, G. Márquez, and C. Martínez-A. IGF-I-triggered cell migration and invasion is mediated by the matrix metalloproteinase MMP-9. Endocrinology, in press. [DOI] [PubMed]
- 32.Mitchison T, Cramer L. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 33.Miyamoto S, Teramoto H, Coso O, Gutkind J, Burbelo P, Akiyama S, Yamada K. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neel B, Tonks N. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 35.Negus R, Allavena P, Sozzani S, Mantovani A, Balkwill F. The detection and localisation of monocyte chemoattractant protein 1 (MCP-1) in human ovarian cancer. J Clin Investig. 1995;95:2391–2396. doi: 10.1172/JCI117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nieto M, Frade J, Sancho D, Mellado M, Martínez-A C, Sánchez-Madrid F. Polarization of the chemokine receptors to the leading edge during lymphocyte migration. J Exp Med. 1997;186:153–158. doi: 10.1084/jem.186.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Opdenakker G, van Damme J. Chemotactic factors, passive invasion and metastasis of cancer cells. Immunol Today. 1992;13:463–464. doi: 10.1016/0167-5699(92)90079-M. [DOI] [PubMed] [Google Scholar]
- 38.O’Toole T, Katagiri Y, Faull R, Peter K, Tamura R, Quaranta V, Loftus J, Shattil S, Ginsberg M. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouwens D, Mikkers H, van der Zon G, Stein-Gerlach M, Ullrich A, Maasen J. Insulin-induced tyrosine dephosphorylation of paxillin and focal adhesion kinase requires active phosphotyrosine phosphatase 1D. Biochem J. 1996;318:609–614. doi: 10.1042/bj3180609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pillay T, Sasaoka T, Olefsky J. Insulin stimulates the tyrosine dephosphorylation of pp125 focal adhesion kinase. J Biol Chem. 1995;270:991–994. doi: 10.1074/jbc.270.3.991. [DOI] [PubMed] [Google Scholar]
- 41.Rivard N, McKenzie F, Brondello J, Pouysségur J. The phosphotyrosine phosphatase PTP1D, but not PTP1C, is an essential mediator of fibroblast proliferation induced by tyrosine kinase and G protein-coupled receptors. J Biol Chem. 1995;270:11017–11024. doi: 10.1074/jbc.270.18.11017. [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez-Fernandez J, Geiger B, Salomon D, Ben-Ze’ev A. Suppression of vinculin expression by antisense transfection confers changes in cell morphology, motility and anchorage-dependent growth of 3T3 cells. J Cell Biol. 1993;122:1285–1294. doi: 10.1083/jcb.122.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romer L, McLean N, Turner C, Burridge K. Tyrosine kinase activity, cytoskeleton organization, and motility in human vascular endothelial cells. Mol Biol Cell. 1994;5:349–361. doi: 10.1091/mbc.5.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneller M, Vuori K, Ruoslahti E. Alphavbeta3 integrin associates with activated insulin and PDGFbeta receptors and potentiates the biological activity of PDGF. EMBO J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz M, Schaller M, Ginsberg M. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 46.Seely B, Reichart D, Staubs P, Jhun B, Hsu D, Maegawa H, Milarski K, Saltiel A, Olefsky J. Localization of the insulin-like growth factor I receptor binding sites for the SH2 domain proteins p85, Syp and GTPase activating protein. J Biol Chem. 1995;270:19151–19157. doi: 10.1074/jbc.270.32.19151. [DOI] [PubMed] [Google Scholar]
- 47.Shi Z, Lu W, Feng G. The SHP-2 tyrosine phosphatase has opposite effects in mediating the activation of extracellular signal-regulated and c-Jun NH2-terminal mitogen-activated protein kinases. J Biol Chem. 1998;273:4904–4908. doi: 10.1074/jbc.273.9.4904. [DOI] [PubMed] [Google Scholar]
- 48.Stracke M, Engel J, Wilson L, Rechler M, Liotta L, Schiffmann E. The type-1 insulin-like growth factor receptor is a motility receptor in human melanoma cells. J Biol Chem. 1989;264:21544–21549. [PubMed] [Google Scholar]
- 49.Takenaga K. Suppression of metastatic potential of high-metastatic Lewis lung carcinoma cells by vanadate, an inhibitor of tyrosine phosphatase, through inhibiting cell-substrate adhesion. Invasion Metastasis. 1996;16:97–106. [PubMed] [Google Scholar]
- 50.Tang T, Freeman R, O’Reilly A, Neel B, Sokol S. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 51.Tobe K, Sabe H, Yamamoto T, Yamauchi T, Asai S, Kaburagi Y, Tamemoto H, Ueki K, Kimura H, Akanuma Y, Yazaki Y, Hanafusa H, Kadowaki T. Csk enhances insulin-stimulated dephosphorylation of focal adhesion proteins. Mol Cell Biol. 1996;16:4765–4772. doi: 10.1128/mcb.16.9.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsuda M, Matozaki T, Fukunaga K, Fujioka Y, Imamoto A, Noguchi T, Takada T, Yamao T, Takeda H, Ochi F, Yamamoto T, Kasuga M. Integrin-mediated tyrosine phosphorylation of SHPS-1 and its association with SHP-2. J Biol Chem. 1998;273:13223–13229. doi: 10.1074/jbc.273.21.13223. [DOI] [PubMed] [Google Scholar]
- 53.Vogel W, Lammers R, Huang J, Ullrich A. Activation of a phosphotyrosine phosphatase by tyrosine phosphorylation. Science. 1993;259:1611–1614. doi: 10.1126/science.7681217. [DOI] [PubMed] [Google Scholar]
- 54.Vuori K, Ruoslahti E. Association of insulin receptor substrate-1 with integrins. Science. 1994;266:1576–1578. doi: 10.1126/science.7527156. [DOI] [PubMed] [Google Scholar]
- 55.Ward S, Bacon K, Westwick J. Chemokines and T lymphocytes: more than an attraction. Immunity. 1998;9:1–11. doi: 10.1016/s1074-7613(00)80583-x. [DOI] [PubMed] [Google Scholar]
- 56.Westmeyer A, Ruhnau K, Wegner A, Jockusch B. Antibody mapping of functional domains in vinculin. EMBO J. 1990;9:2071–2078. doi: 10.1002/j.1460-2075.1990.tb07374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams M, Hughes P, O’Toole T, Ginsberg M. The inner world of cell adhesion: integrin cytoplasmic domains. Trends Cell Biol. 1994;4:109–112. doi: 10.1016/0962-8924(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 58.Yamauchi K, Milarski K, Saltiel A, Pessin J. Protein-tyrosine-phosphatase SHPTP2 is a required positive effector for insulin downstream signaling. Proc Natl Acad Sci USA. 1995;92:664–668. doi: 10.1073/pnas.92.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Youngs S, Ali S, Taub D, Rees R. Chemokines induce migrational responses in human breast carcinoma cell lines. Int J Cancer. 1997;71:257–266. doi: 10.1002/(sici)1097-0215(19970410)71:2<257::aid-ijc22>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 60.Yu D, Qu C, Henegariu O, Lu X, Feng G. Protein-tyrosine phosphatase SHP-2 regulates cell spreading, migration and focal adhesion. J Biol Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- 61.Zhao Z, Tan Z, Wright J, Diltz C, Shen S, Krebs E, Fischer E. Altered expression of protein-tyrosine phosphatase 2C in 293 cells affects protein tyrosine phosphorylation and mitogen-activated protein kinase activation. J Biol Chem. 1995;270:11765–11769. doi: 10.1074/jbc.270.20.11765. [DOI] [PubMed] [Google Scholar]