Abstract
Introduction:
Neighborhood socioeconomic status is associated with health outcomes. Cardiac rehabilitation (CR) provides a cost-effective, multidisciplinary approach to improve outcomes in cardiovascular disease. We aimed to evaluate the association of Area Deprivation Index (ADI), a marker of neighborhood social composition, with risk of recurrent cardiovascular outcomes and assessed the modifying effect of CR.
Methods:
We identified patients with a primary diagnosis of (1) myocardial infarction or (2) incident heart failure (HF) admitted 2010–2018 to a large-sized regional health center. We derived ADI from home addresses and categorized it into quartiles (higher quartiles indicating increased deprivation). We obtained number of CR visits and covariates from the health record. We compared rehospitalization (cardiovascular, acute coronary syndrome [ACS], and HF) and mortality rates across ADI quartiles.
Results:
We included 6957 patients (age 69.2±13.4 yr, 38% women, 89% white race). After covariate adjustment, ADI was significantly associated with higher incidence rates (IR) per 100 person-yr of cardiovascular rehospitalization (Quartile 1, IR 34.6 [95% CI, 31.2–38.2]; Quartile 4, 41.5 [95% CI, 39.1–44.1, P < .001). In addition, ADI was significantly associated with higher rates of rehospitalization for HF (P < .001), and ACS (P <.012), and all-cause mortality (P < .04). These differences in rehospitalization and mortality rates by ADI were no longer significant in those who attended CR.
Conclusions:
We found increased ADI was adversely associated with rehospitalizations and mortality. However, in individuals with CR, outcomes were significantly improved compared with those with no CR. Our findings suggest that CR participation has the potential to improve outcomes in disadvantaged neighborhoods.
Keywords: Myocardial Infarction, Heart Failure, Cardiac Rehabilitation, Area Deprivation Index, Neighborhood Socioeconomic Factors
Neighborhood socioeconomic status is associated with cardiovascular disease (CVD) risk and outcomes. In addition to increased CVD incidence, poorer neighborhood socioeconomic status is associated with worse 6-mo all-cause rehospitalization in heart failure (HF)1 and worse long-term survival post-myocardial infarction (MI).2 Neighborhood socioeconomic vulnerability has been summarized at the census-level by the area deprivation index (ADI). ADI is a census block-group based index which uses 17 indicators of poverty, education, housing and employment to measure neighborhood socioeconomic status. Even after consideration of individual socioeconomic factors, living in a disadvantaged neighborhood by measures such as ADI is associated with increased incidence of coronary artery disease3 and HF4. Additionally, individuals living in the most disadvantaged neighborhoods, as defined by ADI, have significantly higher all-cause readmission rates.5
Cardiac rehabilitation (CR) offers a cost-effective, multidisciplinary approach to cardiac care that has been shown to significantly reduce adverse outcomes in CVD.6 Previous work has shown CR participation is associated with decreased mortality and MI in a dose-dependent fashion in patients with coronary artery disease.7,8 as well as decreased mortality following percutaneous coronary intervention.9 In addition to being a class I indication for patients who have history of cardiac surgery, MI, coronary intervention, stable angina, or peripheral artery disease, the 2014 guidelines for the management of HF added the recommendation for CR as a class IIa recommendation for HF patients with an ejection fraction <35% and New York Heart Association class II-IV symptoms.10 Despite clear benefits, enrollment in CR for HF and MI patients occurs at suboptimal rates.11,12
The negative effect of neighborhood deprivation on CVD may be attenuated by participation in CR. Access to a structured supervised cardiovascular (CV) exercise program, such as CR, may provide an alternative to the decreased resources experienced in deprived neighborhoods. In the present study, we evaluated the association of ADI with CV events in individuals with incident HF or MI in a large, regional healthcare system from 2010–2018. We further evaluated if the association of ADI on CV events is modified by level of participation in CR. We hypothesized 1) that higher ADI would be associated with a higher rate of CV events and 2) that participation in CR would mitigate the adverse association of higher ADI on adverse CV events.
Methods
The present study is an observational cohort study conducted in a large, regional health care system from January 1, 2010 to December 31, 2018. We evaluated inpatient admissions for adult patients (age >18 yr) with a primary diagnosis of either (1) MI with receipt of percutaneous coronary intervention or (2) incident HF with an ejection fraction <35%. Individuals were included in either subgroup if the index hospitalization included the relevant International Classification of Diseases (ICD), Ninth Revision and Tenth Revision codes in any order of priority. The SDC lists the ICD-9 and ICD-10 codes used to define the HF and MI individuals. We defined incident HF by determining that cohort participants had no prior hospitalizations for HF in the 2 yr prior to the incident diagnosis. The University of Pittsburgh Institutional Review Board approved the analyses described here. Informed consent was waived because of the retrospective nature of this study. Given the observational nature of the study we did not have patient or public involvement.
The ADI is a census block-group based index which uses 17 indicators of poverty, education, housing and employment to measure neighborhood socioeconomic status.13 Higher ADI scores represent increased neighborhood deprivation and worse neighborhood socioeconomic status. For this analysis we used participant addresses on the date of admission as recorded in the electronic health record. The ADI dataset is published by the University of Wisconsin and organized at the geography of census block group. Each patient’s ZIP+4 and census block group was obtained from the US Postal Service geocoding Application Programming Interface. We computed the composite score using the weighted ADI coefficients. We queried a national database of ADI values and, using those cutoffs for quartiles, grouped our database into national quartiles.14
Demographic factors were collected from the electronic health record. Age, recorded in yr, sex, and race (White, Black, or other) were derived from the health record. We categorized tobacco use into current, former, never, or unknown based on electronic health record documentation. Insurance status was categorized into commercial, Medicaid, Medicare, or self-pay/other. Clinical covariates were obtained by extracting ICD-9 and ICD-10 codes, Current Procedural Terminology, and Healthcare Common Procedure Coding System codes, and billing data for each individual patient. Comorbid disease status was classified as present or absent for hypertension, diabetes, coronary artery disease, atrial fibrillation, liver disease, obesity and stroke. Obesity was determined using documented body mass index at the time of admission with a cut-point of body mass index ≥30 kg/m2. Glomerular filtration rate was obtained from clinical lab data.
Cardiac rehabilitation participation was obtained from the electronic health record using relevant administrative coding. Cardiac rehabilitation visits for individuals were quantified using diagnosis codes from any subsequent visit within the health care system during the study period after the index hospitalization.
Cardiac rehospitalization was defined as a subsequent readmission for any CVD. Acute coronary syndrome (ACS) rehospitalization was defined as any admission with a primary diagnosis of ACS treated with percutaneous coronary intervention. HF rehospitalization was defined as any admission with a primary diagnosis of HF. Rehospitalization outcomes were obtained using ICD coding. All‐cause mortality over the 3-yr following index hospitalization was derived from routine access to the social security death index via the Social Security Administration Death Master file.
Statistical Analysis
Descriptive statistics were used to summarize categorical variables by frequencies and proportions and continuous variables by the mean ± SD. Distribution differences among groups were compared using the chi-square test for categorical variables and one-way ANOVA for continuous variables. Multicollinearity was explored by examining the correlation matrix with a correlation coefficient threshold of ±0.8. We further examined multicollinearity through the variance inflation factor and tolerance in regression analysis. There was no significant collinearity present among variables. Incidence rates for different events are expressed as the number of patients having events divided by the total number of subgroup patients in the entire cohort. We used the incidence rate and 95% CI to describe the relation between ADI quartile or CR participation and survival or rehospitalizations. Analyses were multivariable-adjusted for age, sex, race, body mass index, smoking status, type of insurance, glomerular filtration rate and medical history including hypertension, coronary artery disease, atrial fibrillation, liver disease, obesity, and stroke. The covariate-adjusted survival curves were then generated for 3-yr rehospitalization rate and mortality. Cardiac rehabilitation was quantified using diagnosis codes from any subsequent visit with the hospital system during the study period after the index hospitalization and it was dichotomized as either attending or not, because separating by distribution of CR attendance resulted in limited sample sizes. We evaluated the combined cohort as well as subgroup analyses of the MI and HF cohorts. All statistical analyses were performed using SAS Version 9.4 (SAS). Statistical significance was established at the 2-sided 5% alpha-level.
Results
Table 1 summarizes baseline characteristics by quartile of ADI. Our final analysis included 6957 patients and consisted of 4215 with an MI and 3343 individuals with a HF hospitalization. The final cohort was 69.2±13.4 yr, 38% women, and 89% white race. Of the cohort, 61% had hypertension and 32% diabetes. Individuals with residences in areas of higher ADI were more likely to be younger, female sex, non-white race, higher body mass index, increased current tobacco use, and have diagnoses of hypertension, diabetes, or liver disease. Over half of the patients in the cohort lived in neighborhoods that were above the median deprivation index. The majority of individuals had no CR with 5685 (82%) of patients with 0 visits.
Table 1.
Baseline Characteristics, by National Area Deprivation Index quartile
| Total | 1st Quartile | 2nd Quartile | 3rd Quartile | fourth Quartile | |
|---|---|---|---|---|---|
| (n=6957) | (n=696) | (n=1801) | (n=2558) | (n=1902) | |
| Age, yr | 69.2 ± 13.4 | 70.5 ± 13.4 | 70.8 ± 12.7 | 69.8 ± 13.0 | 66.4 ± 14.3 |
| Women | 2654 (38) | 230 (33) | 644 (36) | 955 (37) | 825 (43) |
| Race | |||||
| White | 6172 (89) | 658 (94) | 1747 (97) | 2399 (94) | 1368 (72) |
| Black | 718 (10) | 30 (4) | 37 (2) | 139 (5) | 512 (27) |
| Other | 67 (1) | 8 (1) | 17 (1) | 20 (1) | 22 (1) |
| Body Mass Index, kg/m 2 | 29.9±6.5 | 29.0±5.9 | 29.3±6.1 | 30.0±6.4 | 30.4±7.2 |
| Tobacco Use | |||||
| Current | 1339 (19) | 69 (10) | 275 (15) | 499 (20) | 496 (26) |
| Former | 3011 (43) | 312 (45) | 790 (44) | 1113 (43) | 796 (42) |
| Never | 2502 (36) | 307 (44) | 713 (40) | 900 (35) | 582 (31) |
| Unknown | 105 (2) | 8 (1) | 23 (1) | 46 (2) | 28 (1) |
| Insurance | |||||
| Commercial | 1603 (23) | 221 (32) | 493 (27) | 542 (21) | 347 (18) |
| Medicaid | 4786 (69) | 452 (65) | 1253 (70) | 1833 (72) | 1248 (66) |
| Medicare | 467 (7) | 15 (2) | 33 (2) | 149 (6) | 270 (14) |
| Self-pay/other | 101 (1) | 8 (1) | 22 (1) | 34 (1) | 37 (2) |
| Hypertension | 4213 (61) | 411 (59) | 1065 (59) | 1576 (62) | 1161 (61) |
| Diabetes | 2193(32) | 185 (27) | 508 (28) | 830 (32) | 670 (35) |
| Coronary Artery Disease | 3040 (44) | 296 (43) | 743 (41) | 1189 (46) | 812 (43) |
| Atrial Fibrillation | 1361 (20) | 172 (25) | 355 (20) | 486 (19) | 348 (18) |
| Liver Disease | 214 (3) | 16 (2) | 58 (3.2%) | 63 (2) | 77 (4) |
| Glomerular filtration Rate | 67.6±30.3 | 65.6±26.4 | 68.4±29.7 | 67.2±30.1 | 68.0±32.5 |
| Obesity | 709 (10) | 54 (8) | 143 (8) | 279 (11) | 233 (12) |
| Stroke | 613 (9) | 52 (8) | 160 (9) | 258 (10) | 143 (8) |
| Cardiac Rehabilitation Indicator | |||||
| Myocardial Infarction | 4215 (61) | 412 (59) | 1130 (63) | 1604 (63) | 1069 (56) |
| Heart Failure | 3343 (48) | 328 (47) | 826 (46) | 1191 (47) | 998 (52) |
| Cardiac Rehabilitation Participation | |||||
| 0 visit | 5685 (82) | 516 (74) | 1411 (78) | 2120 (83) | 1638 (86) |
| 1 – 12 visits | 408 (6) | 55 (8) | 121 (7) | 141 (5) | 91 (5) |
| 13 – 24 visits | 347 (5) | 63 (9) | 130 (7) | 96 (4) | 58 (3) |
| 25+ visits | 517 (7) | 62 (9) | 139 (8) | 201 (8) | 115 (6) |
Data presented as mean ± SD or n (%).
Overall, 1272 individuals or 18.3% of the cohort participated in any CR. Individuals who participated in CR had significantly lower rates of CV rehospitalization, ACS rehospitalization, HF rehospitalization, and mortality when compared to those who did not participate in CR.
In the overall cohort, including both MI and HF subgroups, we observed differences in incidence rates when comparing the 4th (highest, most severe) quartile to the 1st (lowest, least severe) quartile. First, individuals residing in the 4th quartile of ADI had incident rates for CV rehospitalization of 41.5 [95% CI, 39.1–44.1] compared to 34.6 [95% CI, 31.2–38.2]) per 100 person-yr. Similarly, for the outcome of ACS rehospitalization, we observed incidence rates for the fourth quartile of ADI of 5.3 [95% CI, 4.7–6.1] compared to 3.7 [95% CI, 2.9–4.8] for the first quartile. For HF rehospitalization, we observed incidence rates for the fourth quartile of ADI of 9.5 [95% CI, 8.6–10.5] compared to 7.3 [95% CI, 6.1–8.8] for the first quartile. Finally, when looking at mortality there was a significantly higher rate of mortality for the fourth quartile of ADI 13.1 [95% CI, 10.1–14.2] compared to the first 12.3 [95% CI, 10.8–14.1]. We summarize these data in Table 2a. We present the difference in events across ADI quartiles graphically in Figure 1 and Figure 2.
Table 2.
Incidence rate (per 100 person-yr) of cardiovascular rehospitalization and mortality, by Area Deprivation Index quartile.
| Table 2a. All Participants (n=6957) | |||||
|---|---|---|---|---|---|
| 1st quartile | 2nd quartile | 3rd quartile | fourth quartile | P -value | |
| Cardiovascular Rehospitalization | 34.6 (31.2–38.2) | 34.6 (32.5–36.8) | 36.8 (35.0–38.8) | 41.5 (39.1–44.0) | <.001 |
|
| |||||
| ACS Rehospitalization | 3.7 (2.9–4.8) | 4.5 (4.0–5.3) | 5.5 (4.9–6.1) | 5.3 (4.7–6.1) | .012 |
|
| |||||
| Heart Failure Rehospitalization | 7.3 (6.1–8.8) | 6.6 (5.9–7.5) | 7 (6.4–7.8) | 9.5 (8.6–10.5) | <.001 |
|
| |||||
| All-Cause Mortality | 12.3 (10.8–14.1) | 11.2 (10.3–12.2) | 12.8(11.9–13.7) | 13.1 (10.1–14.2) | .044 |
| Table 2b. Heart Failure Indication (n=3343) | |||||
| 1st quartile | 2nd quartile | 3rd quartile | fourth quartile | P -value | |
|
| |||||
| Cardiovascular Rehospitalization | 62.9 (55.2–71.7) | 72.5 (66.8–78.5) | 68.3 (63.8–73.1) | 75.3 (70.1–81.0) | .059 |
|
| |||||
| ACS Rehospitalization | 3.06 (2.0–4.8) | 4.16 (3.3–5.3) | 4.67 (3.9–5.6) | 4.27 (3.46–5.3) | .34 |
|
| |||||
| Heart Failure Rehospitalization | 15.7(12.7–19.3) | 16 (14.1–18.3) | 15.4 (13.8–17.2) | 19.6 (17.6–21.6) | .013 |
|
| |||||
| All-Cause Mortality | 23.9 (20.5–27.8) | 23.3 (21.1–25.7) | 23 (21.2–24.9) | 22.5 (20.5–24.6) | .91 |
| Table 2c. Myocardial Infarction Indication (n=4215) | |||||
| 1st quartile | 2nd quartile | 3rd quartile | fourth quartile | P -value | |
|
| |||||
| Cardiovascular Rehospitalization | 22.7 (19.6–26.2) | 22.1 (20.2–24.2) | 25.6 (23.9–27.5) | 26.4 (24.2–28.8) | .013 |
|
| |||||
| ACS Rehospitalization | 4.3 (3.2–5.8) | 5.4 (4.6–6.4) | 6.7 (5.9–7.6) | 6.9 (5.9–8.0) | .004 |
|
| |||||
| Heart Failure Rehospitalization | 2.8 (2.0–4.1) | 2.5 (2.1–3.4) | 3.0 (2.6–3.8) | 3.7 (3.3–4.8) | .06 |
|
| |||||
| All-Cause Mortality | 5.5 (4.3–7.1) | 5.4 (4.6–6.2) | 7.3 (6.5–8.1) | 6.7 (5.8–7.8) | .007 |
Data presented as IR (95% CI).
Abbreviation: ACS, acute coronary syndrome.
Figure 1.
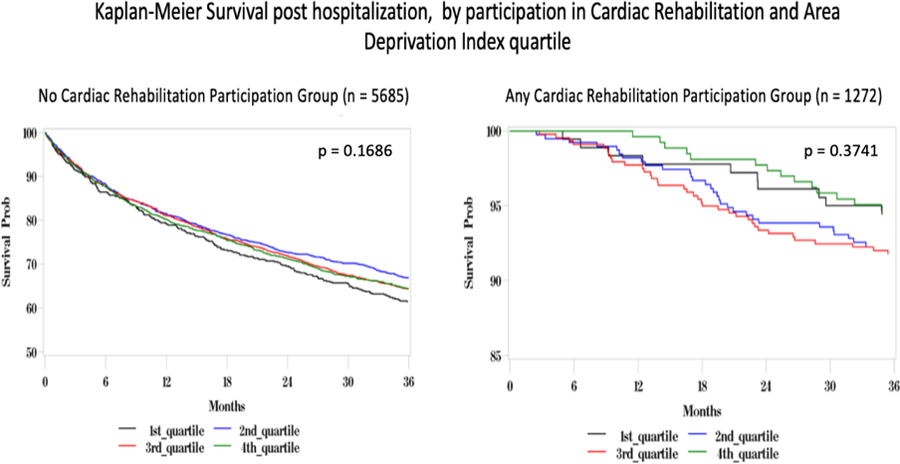
shows 36-mo all-cause mortality across Area Deprivation Index (ADI), stratified by cardiac rehabilitation (CR) participation. In a cohort of 6957 patients with heart failure and myocardial infarction patients there was increased mortality in the no CR participation group (n=5685) when compared to any CR participation group (n=1272). There was no significant effect of ADI on survival.
Figure 2.
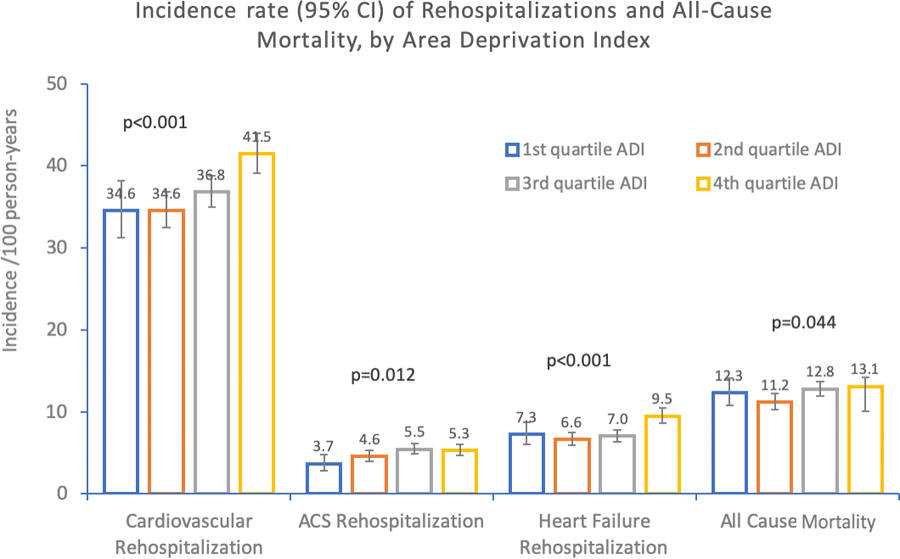
shows incidence rates (per 100 person-years) of rehospitalizations and all-cause mortality, by Area Deprivation Index (ADI). When compared across ADI, those with increased ADI had higher rates of CV rehospitalization (P <.001), acute coronary syndrome Rehospitalization (P=.012), heart failure rehospitalization (P <.001), and all-cause mortality (P =.044).
Table 2b show that when we examined the HF subgroup (n=3343) we identified a significantly increased rate of HF hospitalization for patients with higher ADI, but did not observe such a difference for CV rehospitalization, ACS, or 3-yr all-cause mortality. Table 2c shows that when evaluating the MI subgroup (n=4215), there remained significantly increased rates of CV rehospitalization, ACS rehospitalization, and HF rehospitalization with increasing area deprivation. Additionally, in the MI subgroup there was a significantly increased association between ADI and all-cause mortality.
Table 3 summarizes stratification by participation in CR (0 visits compared to any visits). Individuals who participated in CR had significantly lower rates of cardiac rehospitalization, ACS rehospitalization, and HF rehospitalization, as well as improved all-cause mortality, when compared with those who did not participate in CR (Table 3a). Figure 3 shows the gradient of increasing rates of CV rehospitalization by ADI in those without CR. In contrast, such a gradient by ADI is not present in those with CR. Figure 1 shows the Kaplan-Meier survival curve for morality by ADI and CR.
Table 3.
Incidence rate (per 100 person-yr) of cardiovascular rehospitalization and mortality, by Area Deprivation Index quartile and stratified by Cardiac Rehabilitation Participation.
| Table 3a. All Participants (n = 6957) | ||||||
|---|---|---|---|---|---|---|
| 1st quartile | 2nd quartile | 3rd quartile | 4th quartile | P-value | ||
| Cardiovascular Rehospitalization | CR=0 | 42.6 (38.1–47.7) | 41.4 (38.7–44.3) | 42.0 (39.8–44.4) | 47.1 (44.3–50.1) | .018 |
| CR>0 | 19.3 (15.4–24.3) | 17.7 (15.0–20.8) | 19.5 (16.8–22.6) | 19.3 (15.9–23.3) | .82 | |
|
| ||||||
| ACS Rehospitalization | CR=0 | 4.2 (3.1–5.6) | 4.8 (4.1–5.7) | 5.7 (5.0–6.4) | 5.7 (4.9–6.5) | .11 |
| CR>0 | 2.6 (1.5–4.5) | 3.8 (2.7–5.1) | 4.7 (3.6–6.1) | 3.7 (2.6–5.4) | .23 | |
|
| ||||||
| Heart Failure Rehospitalization | CR=0 | 9.5 (7.8–11.6) | 8.5 (7.5–9.6) | 8.4 (7.6–9.3) | 11.1 (10.0–12.3) | <.001 |
| CR>0 | 2.6 (1.5–4.5) | 1.6 (1.0–2.6) | 2.1 (1.4–3.1) | 2.6 (1.6–4.0) | .49 | |
|
| ||||||
| All-Cause Mortality | CR=0 | 17.0 (14.8–19.6) | 14.1 (12.9–14.4) | 15.3 (14.3–16.5) | 15.4 (14.2–16.7) | .14 |
| CR>0 | 1.9 (1.0–3.5) | 2.7 (1.8–3.8) | 2.9 (2.1–4.0) | 1.8 (1.1–3.0) | .36 | |
| Table 3b. Heart Failure Indication (n = 3343) | ||||||
| 1 st quartile | 2 nd quartile | 3 rd quartile | 4 th quartile | P -value | ||
|
| ||||||
| Cardiovascular Rehospitalization | CR=0 | 67.3 (58.7–77.3) | 74.6 (68.6–81.1) | 69.8 (65.1–74.9) | 77.2 (71.8–83.2) | .15 |
| CR>0 | 39.3 (25.8–59.7) | 52.8 (38.8–71.0) | 54.2 (42.5–68.7) | 49.3 (35.0–69.3) | .60 | |
|
| ||||||
| ACS Rehospitalization | CR=0 | 3.2 (2.0–5.1) | 4.0 (3.1–5.1) | 4.7 (3.8–5.7) | 4.3 (3.5–5.5) | .47 |
| CR>0 | 2.1 (0.5–8.4) | 6.0 (3.1–11.6) | 4.8 (2.7–8.5) | 3.9 (1.6–9.3) | .50 | |
|
| ||||||
| Heart Failure Rehospitalization | CR=0 | 17.0 (13.7–21.2) | 17.2 (15.0–19.6) | 16.3 (14.5–18.3) | 20.5 (18.3–22.9) | .033 |
| CR>0 | 8.0 (3.8–16.9) | 6.7 (3.6–12.4) | 8.0 (5.0–12.7) | 8.0 (4.1–15.3) | .97 | |
|
| ||||||
| All-Cause Mortality | CR=0 | 27.1 (23.2–31.7) | (22.4–27.4) | 25.0 (23.0–27.1) | 23.7 (21.7–26.0) | .55 |
| CR>0 | 5.1 (2.1–12.3) | 9.5 (5.8–15.5) | 6.4 (4.0–10.3) | 3.8 (1.6–9.1) | .25 | |
|
Table 3c Myocardial Infarction Indication (n = 4215)
| ||||||
| 1 st quartile | 2 nd quartile | 3 rd quartile | 4 th quartile | P -value | ||
| Cardiovascular Rehospitalization | CR=0 | 27.3 (22.9–32.5) | 25.8 (23.2–28.5) | 29.5 (27.3–31.9) | 29.9 (27.2–32.9) | .12 |
| CR>0 | 16.5 (12.7–21.4) | 15.4 (12.9–18.4) | 15.9 (13.4–18.8) | 16.8 (13.6–20.8) | .93 | |
|
| ||||||
| ACS Rehospitalization | CR=0 | 5.5 (3.9–7.7) | 6.2 (5.2–7.5) | 7.2 (6.3–8.3) | 7.9 (6.8–9.3) | .10 |
| CR>0 | 2.5 (1.4–4.6) | 3.9 (2.9–5.4) | 5.2 (3.9–6.8) | 3.9 (2.6–5.7) | .13 | |
|
| ||||||
| Heart Failure Rehospitalization | CR=0 | 3.8 (2.5–5.6) | 3.4 (2.7–4.3) | 3.6 (3.0–4.4) | 4.3 (3.5–5.3) | .52 |
| CR>0 | 1.4 (0.6–3.0) | 0.9 (0.8–1.6) | 1.1 (0.6–1.9) | 2.1 (1.2–3.5) | .16 | |
|
| ||||||
| All-Cause Mortality | CR=0 | 8.4 (6.4–10.9) | 6.9 (5.8–8.1) | 9.0 (8.0–10.1) | 8.5 (7.3–9.8) | .07 |
| CR>0 | 1.3 (0.6–2.9) | 2.3 (1.5–3.4) | 2.3 (1.6–3.0) | 1.7 (1.0–3.0) | .49 | |
Data presented as IR (95% CI.
Abbreviation: ACS, acute coronary syndrome.
Figure 3.
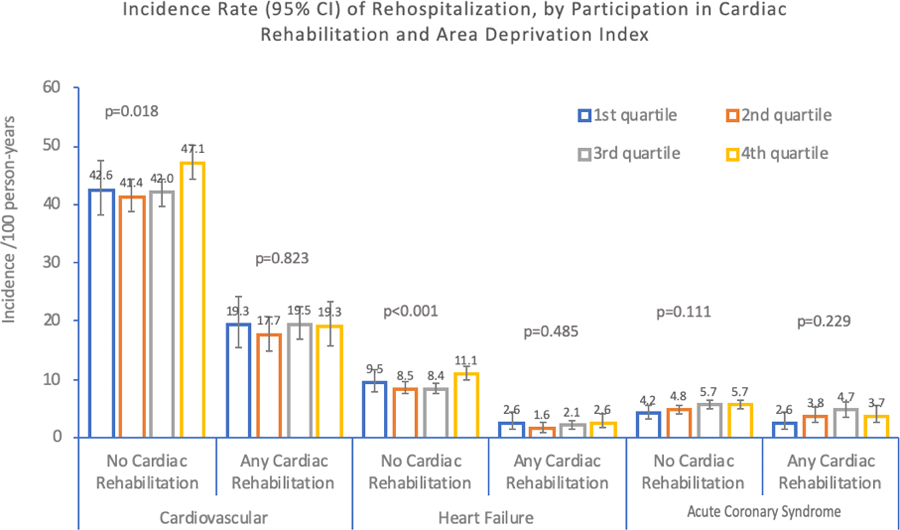
shows incidence rate (per 100 person-years) of rehospitalization, by participation in cardiac rehabilitation (CR) and Area Deprivation Index (ADI). Those with CR participation had lower CV rehospitalizations, acute coronary syndrome rehospitalizations, heart failure rehospitalizations, and all-cause mortality than those without CR participation in all ADI groups. Additionally, there was a graded effect of ADI on CV (P =.018) and heart failure rehospitalization (P <.001) in the no CR group but not in the CR participation group.
Discussion
In a cohort of patients receiving care from a large regional health system, we found that higher area deprivation was associated with increased rates of rehospitalization and mortality following adjustment for clinical and sociodemographic covariates. The association between ADI and the outcomes studied here was attenuated in those individuals who attended CR. Individuals in our health care system who attended CR had significantly lower incidence of rehospitalization and mortality compared to those who did not.
Previous studies have related neighborhood factors to CR participation. Distance to CR and lower neighborhood socioeconomic status have been associated with decreased rates of participation in CR. In a study of > 1800 individuals in rural and urban settings from hospitals in Ontario, those with a greater than a 30-min drive from a rehabilitation center attended significantly fewer sessions.15 In a cohort of > 4000 CR participants in the Southern Community Cohort Study, lower neighborhood socioeconomic context was associated with decreased CR participation even after accounting for individual socioeconomic status; individuals residing in the most deprived communities were less than half as likely to participate in CR.16 Our study adds to this body of literature by evaluating the effect of ADI on outcomes by CR. Our findings suggest that CR participation may mitigate the adverse effect of increased ADI on CV outcomes.
Previous work has identified multiple factors that are associated with limited referral and enrollment in CR including female sex, older age, non-white race, lack of insurance, low socioeconomic status, lack of perceived need for CR, limited social support, and absence of referral or programs that service a geographic area.15,16 In a survey-based study, participation rates in CR following MI were associated with younger age, male sex, having been seen by a cardiologist during hospitalization, no prior history of MI, having received procedural treatment for the MI, presentation with ST-segment elevation, and referral to CR while still in the hospital.17 In addition to differences in referral rates, there are also differences noted in enrollment and completion rates of CR. In a study of 822 patients referred to CR, white individuals were 78% more likely to initiate CR than non-white minorities.18 While many of factors impact CR participation and are correlated with area deprivation, our study found that ADI was independently associated with worse CV outcomes. Our study adds to this literature by focusing on the aggregate socioeconomic status effect using ADI while controlling for individual-level factors. It is possible that the individual factors provide independent or compounded impacts on these outcomes which has been explored in other studies.
Our findings suggest that individuals residing in neighborhoods with high deprivation may have benefit from increased access to CR. Our results suggest that ADI is highly relevant towards CV risk and merits consideration when evaluating and referring patients to CR. Individuals residing in neighborhoods with higher ADI should be heavily recruited for rehabilitation including consideration for additional interventions to mitigate obstacles to their participation. While geographic distance and access are major obstacles for CR attendance, diverse contemporary strategies may alleviate or reduce such challenges. For example, mobile health-based interventions, home-based CR19, community-based CR programs, or interventions to increase access to current programs may all improve access in individuals residing in high ADI neighborhoods.
The strengths of our study are that we utilized a uniform electronic health record to identify a large-sized cohort of individuals with CVD. We were thereby able to capture clinical and sociodemographic factors relevant to the relation of area deprivation and CR. Our study also has noteworthy limitations. First, as the data were extracted from an electronic health record, we cannot discern if individuals lacking any record of CR participated in programs outside of our health system. We note that our CR participation rate is similar to nationally reported data14 and as such we suspect that misclassification of CR participation is minimal. Similarly, we were only able to identify hospitalization events within the regional health care system in which we conducted this study. Second, although we performed multivariable analysis adjusting for several sociodemographic and clinical variables, there remains the potential for unmeasured confounders and factors not captured by the electronic health record. Third, we relied on ICD coding to identify HF, MI, and subsequent events. We neither adjudicated cases nor included imaging and laboratory data that could support ascertainment of these diagnoses. We note that an extensive array of prior studies has used ICD coding to identify CVD. Fourth, ADI may be linked to factors that impact detection of disease such as access to medical services. If there is lower detection of CVD in areas with high deprivation we may be underestimating the impact of ADI on CVD outcomes. Fifth, CR participation was defined as any participation in CR and thus our study was unable to elucidate any dose effects of CR. It is notable, however, that individuals with even only 1 session of CR had significantly improved outcomes compared to no CR. Finally, we used participant address at the time of hospitalization but we were unable to include data regarding participants moving or how long they resided at that address.
CONCLUSION
Our findings are consistent with prior studies that have demonstrated the contribution of ADI to health outcomes. Importantly, however, we demonstrated that CR attenuates the association between ADI and adverse events in a cohort of patients with CVD. Our findings support the rationale for interventions to increase CR participation and particularly the need for targeting vulnerable individuals residing in neighborhoods with higher social deprivation.
Supplementary Material
Sources of Funding:
NIH T32HL083825
Footnotes
Conflict of Interests: None
REFERENCES
- 1.Bikdeli B, Wayda B, Bao H, et al. Place of Residence and Outcomes of Patients with Heart Failure: An Analysis from the TELE-HF Trial Participants. J Am Coll Cardiol 2013;61(10):E783. [Google Scholar]
- 2.Gerber Y, Benyamini Y, Goldbourt U, Drory Y, Infarction ISG on FAM. Neighborhood Socioeconomic Context and Long-Term Survival After Myocardial Infarction. Circulation. 2010;121(3):375–383. [DOI] [PubMed] [Google Scholar]
- 3.Roux AVD, Merkin SS, Arnett D, et al. Neighborhood of Residence and Incidence of Coronary Heart Disease. New Engl J Med 2001;345(2):99–106. [DOI] [PubMed] [Google Scholar]
- 4.Akwo EA, Kabagambe EK, Harrell FE, et al. Neighborhood Deprivation Predicts Heart Failure Risk in a Low-Income Population of Blacks and Whites in the Southeastern United States. Circ Cardiovasc Qual Outcomes. 2018;11(1):e004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kind AJH, Jencks S, Brock J, et al. Neighborhood Socioeconomic Disadvantage and 30-Day Rehospitalization: A Retrospective Cohort Study. Ann Intern Med 2014;161(11):765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arena R, Williams M, Forman DE, et al. Increasing Referral and Participation Rates to Outpatient Cardiac Rehabilitation. Circulation. 2012;125(10):1321–1329. [DOI] [PubMed] [Google Scholar]
- 7.Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship Between Cardiac Rehabilitation and Long-Term Risks of Death and Myocardial Infarction Among Elderly Medicare Beneficiaries. Circulation. 2010;121(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suaya JA, Stason WB, Ades PA, Normand S-LT, Shepard DS. Cardiac Rehabilitation and Survival in Older Coronary Patients. J Am Coll Cardiol 2009;54(1):25–33. [DOI] [PubMed] [Google Scholar]
- 9.Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of Cardiac Rehabilitation on Mortality and Cardiovascular Events After Percutaneous Coronary Intervention in the Community. Circulation. 2011;123(21):2344–2352. [DOI] [PubMed] [Google Scholar]
- 10.. MEMBERS WC, Yancy CW, Jessup M, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure. Circulation. 2013;128(16):e240–e327. [DOI] [PubMed] [Google Scholar]
- 11.Ades PA, Keteyian SJ, Wright JS, et al. Increasing Cardiac Rehabilitation Participation From 20% to 70%: A Road Map From the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc 2017;92(2):234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wall HK, Stolp H, Wright JS, et al. The Million Hearts Initiative. J Cardiopulm Rehabil 2020;40(5):290–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kind AJH, Buckingham WR. Making Neighborhood-Disadvantage Metrics Accessible — The Neighborhood Atlas. New Engl J Med 2018;378(26):2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neighborhood Atlas University of Wisconsin. https://www.neighborhoodatlas.medicine.wisc.edu. Date of Access7/15/2020
- 15.Shanmugasegaram S, Oh P, Reid RD, McCumber T, Grace SL. Cardiac rehabilitation barriers by rurality and socioeconomic status: a cross-sectional study. Int J Equity Health. 2013;12(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachmann JM, Huang S, Gupta DK, et al. Association of Neighborhood Socioeconomic Context With Participation in Cardiac Rehabilitation. J Am Heart Assoc 2017;6(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunlay SM, Witt BJ, Allison TG, et al. Barriers to participation in cardiac rehabilitation. Am Heart J 2009;158(5):852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prince DZ, Sobolev M, Gao J, Taub CC. Racial Disparities in Cardiac Rehabilitation Initiation and the Effect on Survival. PM&R 2014;6(6):486–492. [DOI] [PubMed] [Google Scholar]
- 19.Thomas RJ, Beatty AL, Beckie TM, et al. Home-Based Cardiac Rehabilitation. J Cardiopulm Rehabil 2019; 39(4):208–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


